Comproportionation of a dialuminyne with alane or dialane dihalides as a clean route to dialuminenes†
Joshua D. Queen and
Philip P. Power
and
Philip P. Power *
*
Department of Chemistry, University of California, One Shields Avenue, Davis, California, USA. E-mail: pppower@ucdavis.edu
First published on 9th November 2022
Abstract
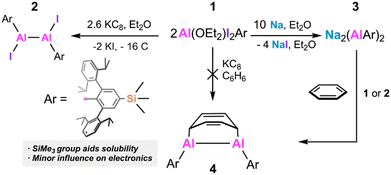
Dialuminenes RAlAlR (R = m-terphenyl or bulky aryl) react with the aromatic solvents (e.g. benzene or toluene) in which they dissolve. We synthesized –SiMe3 substituted derivatives of known terphenyl ligands to increase their solubility in alkanes which have lower reactivity than arenes. The new dialuminene was synthesized via the comproportionation reaction of Na2(AlAriPr4-4-SiMe3)2 (3) (AriPr4-4-SiMe3 = 2,6-(2,6-iPr2C6H3)2-4-SiMe3C6H2) with either the diiodide Al(Et2O)I2AriPr4-4-SiMe3 (1) or the 1,2-diiododialane 4-SiMe3AriPr4(I)Al–Al(I)AriPr4-4-SiMe3 (2). This cleanly generates the dialuminene 4-SiMe3AriPr4AlAlAriPr4-4-SiMe3 which was trapped as its cycloaddition product (4) with benzene. Even in non-aromatic, essentially inert, solvents red 4 decomposes to colorless solutions. This indicates that the instability of the free dialuminene is an inherent property rather than arising from of the method of synthesis, solvent employed, or the presence of impurities.
The recognition in the 1970s and 80s that the heavier p-block elements can form multiple bonds to each other1–3 led to the synthesis of series of alkene and alkyne analogues derived from the heavier members of the group 13, 14, and 15 elements.4 The compounds with multiple bonds between aluminium atoms are of special interest due to their very high reactivity5–9 and their calculated diradical character.10 However this apparent diradical character makes them difficult to isolate as neutral species. Thus, many structurally characterized Al–Al multiply bonded molecules (Scheme 1) are anionic11–13 and derived from the reduction of a bonding π-type LUMO in a neutral species. Alternatively, complexation of the aluminium atoms by Lewis bases confers stability on the multiple bonded compounds.14–16 Another important compound class related to the multiple bonded species are the cycloaddition derivatives of Al
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Al bonded moieties and solvent arenes.17–21 This is a reversible process in some cases.18–20
Al bonded moieties and solvent arenes.17–21 This is a reversible process in some cases.18–20
 | ||
| Scheme 1 Representative Al–Al multiple bonded species. AriPr4 = 2,6-(2,6-iPr2C6H3)2C6H3, ArMe6 = 2,6-Mes2C6H3, Bbt = 2,6-{CH(SiMe3)2}2C6H3, Tbt = 2,6-{CH(SiMe3)2}2-4-tBuC6H3. | ||
The first isolated Al–Al π-bonded compounds resulted from one electron reduction of the tetraorganodialanes R2Al–AlR2 (R = CH(SiMe3)2, C6H3-2,4,6-iPr3).12,22 Their EPR spectra showed the unpaired electron to be located predominantly in the π-type SOMO between the two Al atoms thereby generating multiple bond character and shortened Al–Al bonds.11,12
While the sterically demanding terphenyl ligand AriPr4 (AriPr4 = 2,6-(2,6-iPr2C6H3)2C6H3) allowed the isolation of the neutral dimetallenes AriPr4EEAriPr4 (E = Ga–Tl)23–25 which dissociate to :EAriPr4 monomers in solution, the Al congener AriPr4AlAlAriPr4 could not be isolated from hexanes or ether, due to its poor solubility.17 Rather, it could only be trapped and characterized as its cycloaddition products with toluene or Me3SiCCSiMe3 following the reduction of the halide derivative AlI2AriPr4 in Et2O.17,26 However, the dianionic dialuminyne Na2(AlAriPr4)2 and the trimeric Na2(AlArMe6)3 (ArMe6 = 2,6-(2,4,6-Me3C6H2)2C6H3) were isolable by reduction of AlI2AriPr4 and AlI2ArMe6 with excess Na metal.13 The anions contained in these salts are analogues of the corresponding Ga species Na2(GaAriPr6)227 (AriPr6 = 2,6-(2,4,6-iPr3C6H2)2C6H3) and Na2(GaArMe6)328 isolated earlier by Robinson and coworkers. By increasing the steric demand of the terphenyl ligand on the group 13 elements, the crystalline metallanediyls :EAriPr6 (E = In, Tl),29,30 :GaAriPr8 (AriPr8 = 2,6-(2,4,6-iPr3C6H2)2-3,5-iPr2C6H) and :Ga(2,6-(2,6-iPr2C6H3)2-3,5-iPr2C6H)31 were isolated. Following these results, we recently reported the synthesis of a stable one-coordinate aluminium species :AlAriPr8.32 A favorable dimerization energy of :AlAriPr8 to yield AriPr8AlAlAriPr8 (ΔG = −20 kJ mol−1) was calculated although this dimer was not isolated and only the monomer :AlAriPr8 was obtained from solution. Nonetheless, calculations suggested AriPr8AlAlAriPr8 as the reactive species with H2 (cf. monomeric :GaAriPr8 does not react with H2 although the dimer AriPr4GaGaAriPr4 is highly reactive).33
Tokitoh and coworkers isolated dialuminene–benzene cycloaddition products by reduction of the 1,2-dibromodialanes Bbt(Br)Al–Al(Br)Bbt (Bbt = 2,6-{CH(SiMe3)2}2C6H3) and Tbt(Br)Al–Al(Br)Tbt (Tbt = 2,6-{CH(SiMe3)2}2-4-tBuC6H2) with KC8 in benzene.18,34 They further showed that these complexes act as synthetic equivalents to dialuminenes by the reversible dissociation of the benzene to yield ArAlAlAr in solution. The complexed benzene could also be irreversibly exchanged with naphthalene, anthracene, or Me3SiCCSiMe3 while the reaction with H2 to give {AlH(μ-H)Ar}2 (Ar = Bbt, Tbt) was also observed under ambient conditions.19
As mentioned above, stable compounds containing Al![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Al double bonds were only recently isolated through coordination of Lewis bases to the reactive Al centers and are so far limited to a few examples. Thus Inoue and coworkers reported the carbene complexed dialuminenes (NHC)RAl
Al double bonds were only recently isolated through coordination of Lewis bases to the reactive Al centers and are so far limited to a few examples. Thus Inoue and coworkers reported the carbene complexed dialuminenes (NHC)RAl![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) AlR(NHC) (R = SitBu2Me, 2,4,6-iPr3C6H2; NHC = 1,3-diisopropyl-4,5-dimethyl-imidazolin-2-ylidene)14,15 and Cowley and coworkers reported the synthesis of an amidophosphine supported dialuminene which reversibly dissociates to monomers in solution.16 However the coordination of Lewis bases to the aluminium atoms does not guarantee stability of the Al
AlR(NHC) (R = SitBu2Me, 2,4,6-iPr3C6H2; NHC = 1,3-diisopropyl-4,5-dimethyl-imidazolin-2-ylidene)14,15 and Cowley and coworkers reported the synthesis of an amidophosphine supported dialuminene which reversibly dissociates to monomers in solution.16 However the coordination of Lewis bases to the aluminium atoms does not guarantee stability of the Al![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Al bonded moiety. The amidinate supported dialuminene (AmDipp)Al
Al bonded moiety. The amidinate supported dialuminene (AmDipp)Al![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Al(AmDipp) (AmDipp = C{N(2,6-iPr2C6H3)}2(4-MeC6H4)) of Bakewell and coworkers, generated by hydrogen abstraction from AlH2AmDipp by Al(BDIDipp) (BDIDipp = C{C(Me)N(2,6-iPr2C6H3)}2) at 80 °C, went on to react with the benzene solvent to give the respective cycloaddition product.21 Braunschweig and coworkers reported that the reduction of the carbene supported terphenyl aluminium diiodide Al(NHC′)I2ArMe6 (NHC′ = 1,3,4,5-tetramethylimidazol-2-ylidene) apparently gave ArMe6(NHC′)AlAl(NHC′)ArMe6 which added across a C
Al(AmDipp) (AmDipp = C{N(2,6-iPr2C6H3)}2(4-MeC6H4)) of Bakewell and coworkers, generated by hydrogen abstraction from AlH2AmDipp by Al(BDIDipp) (BDIDipp = C{C(Me)N(2,6-iPr2C6H3)}2) at 80 °C, went on to react with the benzene solvent to give the respective cycloaddition product.21 Braunschweig and coworkers reported that the reduction of the carbene supported terphenyl aluminium diiodide Al(NHC′)I2ArMe6 (NHC′ = 1,3,4,5-tetramethylimidazol-2-ylidene) apparently gave ArMe6(NHC′)AlAl(NHC′)ArMe6 which added across a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of a flanking aryl ring, and the monomer Al(NHC′)ArMe6 which ring opened the benzene or toluene solvent to give dihydropentalene type structures.20
C bond of a flanking aryl ring, and the monomer Al(NHC′)ArMe6 which ring opened the benzene or toluene solvent to give dihydropentalene type structures.20
Despite these advances, compounds of the type RAlAlR free of complexing ligands remain unisolated. Recent reports on the synthesis of the one-coordinate Al species32,35,36 have illustrated the importance of reducing agent selection to access low oxidation state Al compounds—the products are obtained only when Na metal dispersed on NaCl powder is used. As the chemistry of dialuminenes remains underdeveloped, we revisited their synthesis with the objective of developing isolable ArAlAlAr species that are soluble in solvents with which they do not react. The direct stoichiometric reduction of the aluminium iodide precursors with alkali metals did not yield the targeted dialuminene, instead decomposition occured with elimination of the terphenyl arene. This led us to investigate alternative methods of their preparation free of alkali metal reductants. We found that the comproportionation reaction between a dialuminyne salt and aluminium iodides proceeded rapidly in benzene to cleanly afford a dialuminene-benzene complex. However, the comproportionation reaction in nonreactive ether or alkane solvents led to decomposition of the mixture, indicating an inherent instability of the dialuminene. Despite this, the reaction offers a new synthetic route to reactive Al–Al multiple bonded species.
Using the -SiMe3 modified ligand AriPr4-4-SiMe3 (AriPr4-4-SiMe3 = 2,6-(2,6-iPr2C6H3)2-4-SiMe3C6H2), we anticipated that the extra aliphatic groups would increase the solubility of ArAlAlAr sufficiently to permit its handling in non-aromatic solvents with which it does not react (e.g. hexane, ether). The Hammett constant σpara = −0.07 for –SiMe337 also suggested it would have minimal electronic effects on the Al![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Al moiety in comparison to the AriPr4 derivative (σpara for H = 0). Addition of LiAriPr4-4-SiMe3 to AlH3NMe3 in Et2O afforded the aluminate salt Li(Et2O)AlH3AriPr4-4-SiMe3 as a white solid. Subsequent treatment with CH3I (5 eq.) gave the iodide Al(Et2O)I2AriPr4-4-SiMe3 (1). The Al-I distances in 1 (Fig. 1) are 2.5288(7) Å and 2.5578(7) Å, an Al–C bond length of 1.9936(19) Å and Al–O bond being 1.8703(15) Å. Treating 1 with KC8 (1.3 eq.) gave the yellow 1,2-diiododialane 4-SiMe3AriPr4(I)Al–Al(I)AriPr4-4-SiMe3 (2) (Scheme 2). The Al–Al distance of 2.604(2) Å in 2 is nearly identical to that of AriPr4(I)Al–Al(I)AriPr4 (2.609(2) Å). The I atoms are disordered over two sites with Al-I distances of 2.5083(11) and 2.5354(13) Å but in each case the coordination around the Al atom is trigonal planar. The UV-Visible spectrum of 2 showed a single absorbance at 386 nm.
Al moiety in comparison to the AriPr4 derivative (σpara for H = 0). Addition of LiAriPr4-4-SiMe3 to AlH3NMe3 in Et2O afforded the aluminate salt Li(Et2O)AlH3AriPr4-4-SiMe3 as a white solid. Subsequent treatment with CH3I (5 eq.) gave the iodide Al(Et2O)I2AriPr4-4-SiMe3 (1). The Al-I distances in 1 (Fig. 1) are 2.5288(7) Å and 2.5578(7) Å, an Al–C bond length of 1.9936(19) Å and Al–O bond being 1.8703(15) Å. Treating 1 with KC8 (1.3 eq.) gave the yellow 1,2-diiododialane 4-SiMe3AriPr4(I)Al–Al(I)AriPr4-4-SiMe3 (2) (Scheme 2). The Al–Al distance of 2.604(2) Å in 2 is nearly identical to that of AriPr4(I)Al–Al(I)AriPr4 (2.609(2) Å). The I atoms are disordered over two sites with Al-I distances of 2.5083(11) and 2.5354(13) Å but in each case the coordination around the Al atom is trigonal planar. The UV-Visible spectrum of 2 showed a single absorbance at 386 nm.
The reduction of 1 with excess Na in the form of either Na mirror or 5% w/w Na/NaCl in Et2O gave a dark green-brown solution. Removal of the volatile components and extraction of the residue with hexanes and then toluene gave two fractions: the hexanes extract, despite having a dark red color, contained 4-SiMe3-AriPr4H as the only identifiable product (isolated yield 62% based on 1), indicating a significant amount of decomposition under reducing conditions. From the dark green toluene extract the anionic dialuminyne complex Na2(4-SiMe3-AriPr4AlAlAriPr4-4-SiMe3) 3 was isolated as dark green crystals in ca. 30% yield. The structure of 3 shows two crystallographically independent molecules with Al–Al distances of ca. 2.43 Å which is near to the 2.428(1) Å in Na2(AriPr4AlAlAriPr4).13 The Na–Al distances are in the range 3.1016(8) to 3.1403(8) Å, and the average Na-C distance is 2.968 Å. The UV-Visible spectrum of 3 displays absorbances at 344 nm, 470 nm, and at 612 nm with a shoulder at 660 nm (cf. 354, 456, and 600 nm in Na2(AriPr4Al-AlAriPr4). Overall, the new compounds showed little deviation from their AriPr4 substituted counterparts while having the desired increased solubility properties. However, attempts to reduce 1 or 2 with alkali metals to the dialuminene 4-SiMe3-AriPr4AlAlAriPr4-4-SiMe3 under a variety of conditions were unsuccessful.
As a result of the difficulty with direct reduction to the dialuminene (see above), we tested the comproportionation reaction of the dialuminyne 3 with the aluminium iodides 1 or 2. Treating 3 with excess 1 in C6D6 resulted in the formation of 2 with a trace amount of a new product that was later determined to be the dialuminene-benzene cycloaddition species 4. Mixtures of 1 or 2 with a slight excess of 3 in C6D6 gave dark brown solutions that indicated consumption of the iodide materials along with formation of 4 in the 1H NMR spectra. On a preparative scale an equimolar mixture of 1 and 3 was dissolved in benzene, resulting in rapid formation of a red solution. Filtration and concentration of the solution to ca. 1 mL followed by storage at ca. 8 °C afforded red crystals of the dialuminene-benzene cycloaddition complex 4 (Fig. 2). 1H NMR monitoring of the reaction indicated the formation of no other AriPr4-4-SiMe3 substituted species except 4.
The Al–Al bond length in 4 is 2.5585(6) Å with Al–Cipso distances of 1.9681(14) and 1.9743(13) Å while the Al–C distances to the bridging cyclohexadiene moiety are 2.0000(15) and 1.9976(14) Å. The Al atoms are almost planar coordinated (∑°Al = 358.57(9)°, 359.57(9)°) and the C(1)–Al(1)–Al(2)–C(34) torsion angle is 34.18(9)°. UV-Visible spectroscopy in hexanes showed an absorbance at 323 nm with a weaker shoulder spanning the range ca. 430 to 530 nm. A broad absorbance in the same region was found earlier for the BbtAlAlBbt benzene complex mentioned above.18
The isolation of 4 is consistent with the growing number of cycloaddition products that have been isolated from the reaction of arenes and Al![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Al bonded species.17,18,20,21 Its formation indicates that the dialuminene ArAlAlAr is obtained cleanly from the reaction between 3 and 1 or 2. The direct reduction of 1 in benzene with ≥2 eq. of alkali metal reducing agents did not afford the dialuminene but instead gave red solutions for which 1H NMR spectroscopy showed the generation of the free arene 4-SiMe3-AriPr4H as the major product with no signals assignable to 4.
Al bonded species.17,18,20,21 Its formation indicates that the dialuminene ArAlAlAr is obtained cleanly from the reaction between 3 and 1 or 2. The direct reduction of 1 in benzene with ≥2 eq. of alkali metal reducing agents did not afford the dialuminene but instead gave red solutions for which 1H NMR spectroscopy showed the generation of the free arene 4-SiMe3-AriPr4H as the major product with no signals assignable to 4.
Complex 4 was observed to be soluble in benzene and cyclohexane as well as being sparingly soluble in hexanes or ether. While 4 showed no signs of decomposition in benzene after several days, solutions of 4 in hexane or cyclohexane gradually lost their color overnight. This is probably due to reversible dissociation of 4 and a shift of the equilibrium toward benzene and the free dialuminene, which subsequently decomposes or rearranges. Attempts to comproportionate 1 or 2 with 3 in Et2O or hexanes afforded a dark purple solution that also rapidly faded to colorless. We conclude that the instability of the dialuminene 4-SiMe3-AriPr4AlAlAriPr4-4-SiMe3 is an inherent property of the compound rather than an artifact of the synthetic route employed or impurities in the system. It is possible that in the future suitable electronic modification of the ligand may yield a less reactive, isolable dialuminene species.
In summary, we have shown that the dialuminene 4-SiMe3-AriPr4AlAlAriPr4-4-SiMe3 is cleanly generated stoichiometrically in solution via the reaction of 1 or 2 with 3. Its decomposition in hexanes or ether indicates that it is inherently unstable, probably as a result of its singlet diradical character.10,38 However, it can be trapped as 4 by its reaction with benzene. Further investigations into the use of 3 as a reducing agent to generate Al–Al multiply bonded species are underway.
This work is dedicated to the memory of Robert West. We thank the U.S. Department of Energy (DE-FG02-07ER46475) for supporting this work.
Note added after first publication: This article replaces the version published on 22nd November 2022, which contained errors in the display of chemical formulae throughout.
Conflicts of interest
There are no conflicts to declare.Notes and references
- D. E. Goldberg, D. H. Harris, M. F. Lappert and K. M. Thomas, J. Chem. Soc., Chem. Commun., 1976, 261–262 RSC.
- R. West, M. J. Fink and J. Michl, Science, 1981, 214, 1343–1344 CrossRef CAS PubMed.
- M. Yoshifuji, I. Shima, N. Inamoto, K. Hirotsu and T. Higuchi, J. Am. Chem. Soc., 1981, 103, 4587–4589 CrossRef CAS.
- R. C. Fischer and P. P. Power, Chem. Rev., 2010, 110, 3877–3923 CrossRef CAS.
- A. J. Downs, Coord. Chem. Rev., 1999, 189, 59–100 CrossRef CAS.
- G. H. Robinson, Adv. Organomet. Chem., 2001, 47, 283–294 CrossRef CAS.
- Y. Wang and G. H. Robinson, Organometallics, 2007, 26, 2–11 CrossRef CAS.
- Y. Wang and G. H. Robinson, Chem. Commun., 2009, 5201–5213 RSC.
- P. Bag, C. Weetman and S. Inoue, Angew. Chem., Int. Ed., 2018, 57, 14394–14413 CrossRef CAS PubMed.
- J. Moilanen, P. P. Power and H. M. Tuononen, Inorg. Chem., 2010, 49, 10992–11000 CrossRef CAS PubMed.
- W. Uhl, A. Vester, W. Kaim and J. Poppe, J. Organomet. Chem., 1993, 454, 9–13 CrossRef CAS.
- R. J. Wehmschulte, K. Ruhlandt-Senge, M. M. Olmstead, H. Hope, B. E. Sturgeon and P. P. Power, Inorg. Chem., 1993, 32, 2983–2984 CrossRef CAS.
- R. J. Wright, M. Brynda and P. P. Power, Angew. Chem., Int. Ed., 2006, 45, 5953–5956 CrossRef CAS PubMed.
- P. Bag, A. Porzelt, P. J. Altmann and S. Inoue, J. Am. Chem. Soc., 2017, 139, 14384–14387 CrossRef CAS PubMed.
- C. Weetman, A. Porzelt, P. Bag, F. Hanusch and S. Inoue, Chem. Sci., 2020, 11, 4817–4827 RSC.
- R. L. Falconer, K. M. Byrne, G. S. Nichol, T. Krämer and M. J. Cowley, Angew. Chem., Int. Ed., 2021, 60, 24702–24708 CrossRef CAS.
- R. J. Wright, A. D. Phillips and P. P. Power, J. Am. Chem. Soc., 2003, 125, 10784–10785 CrossRef CAS PubMed.
- T. Agou, K. Nagata and N. Tokitoh, Angew. Chem., Int. Ed., 2013, 52, 10818–10821 CrossRef CAS PubMed.
- K. Nagata, T. Murosaki, T. Agou, T. Sasamori, T. Matsuo and N. Tokitoh, Angew. Chem., Int. Ed., 2016, 55, 12877–12880 CrossRef CAS PubMed.
- D. Dhara, A. Jayaraman, M. Härterich, R. D. Dewhurst and H. Braunschweig, Chem. Sci., 2022, 13, 5631–5638 RSC.
- C. Bakewell, K. Hobson and C. J. Carmalt, Angew. Chem., Int. Ed., 2022, 61, e202205901 CrossRef CAS PubMed.
- W. Uhl, Z. Naturforsch., B: J. Chem. Sci., 1988, 43, 1113–1118 CrossRef CAS.
- N. J. Hardman, R. J. Wright, A. D. Phillips and P. P. Power, Angew. Chem., Int. Ed., 2002, 41, 2842–2844 CrossRef CAS PubMed.
- R. J. Wright, A. D. Phillips, N. J. Hardman and P. P. Power, J. Am. Chem. Soc., 2002, 124, 8538–8539 CrossRef CAS PubMed.
- R. J. Wright, A. D. Phillips, S. Hino and P. P. Power, J. Am. Chem. Soc., 2005, 127, 4794–4799 CrossRef CAS PubMed.
- C. Cui, X. Li, C. Wang, J. Zhang, J. Cheng and X. Zhu, Angew. Chem., Int. Ed., 2006, 45, 2245–2247 CrossRef CAS PubMed.
- J. Su, X.-W. Li, R. C. Crittendon and G. H. Robinson, J. Am. Chem. Soc., 1997, 119, 5471–5472 CrossRef CAS.
- X.-W. Li, W. T. Pennington and G. H. Robinson, J. Am. Chem. Soc., 1995, 117, 7578–7579 CrossRef CAS.
- S. T. Haubrich and P. P. Power, J. Am. Chem. Soc., 1998, 120, 2202–2203 CrossRef CAS.
- M. Niemeyer and P. P. Power, Angew. Chem., Int. Ed., 1998, 37, 1277–1279 CrossRef CAS PubMed.
- Z. Zhu, R. C. Fischer, B. D. Ellis, E. Rivard, W. A. Merrill, M. M. Olmstead, P. P. Power, J. D. Guo, S. Nagase and L. Pu, Chem. – Eur. J., 2009, 15, 5263–5272 CrossRef CAS PubMed.
- J. Queen, A. Lehmann, J. Fettinger, H. Tuononen and P. Power, J. Am. Chem. Soc., 2020, 142, 20554–20559 CrossRef CAS.
- C. A. Caputo, J. Koivistoinen, J. Moilanen, J. N. Boynton, H. M. Tuononen and P. P. Power, J. Am. Chem. Soc., 2013, 135, 1952–1960 CrossRef CAS PubMed.
- K. Nagata, T. Agou, T. Sasamori and N. Tokitoh, Chem. Lett., 2015, 44, 1610–1612 CrossRef CAS.
- X. Zhang and L. L. Liu, Angew. Chem., Int. Ed., 2021, 60, 27062–27069 CrossRef CAS PubMed.
- A. Hinz and M. P. Müller, Chem. Commun., 2021, 57, 12532–12535 RSC.
- C. Hansch, A. Leo and R. W. Taft, Chem. Rev., 1991, 91, 165–195 CrossRef CAS.
- Y. Jung, M. Brynda, P. P. Power and M. Head-Gordon, J. Am. Chem. Soc., 2006, 128, 7185–7192 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2212327–2212330. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2cc05646a |
| This journal is © The Royal Society of Chemistry 2023 |