 Open Access Article
Open Access ArticleMultifunctional conductive hyaluronic acid hydrogels for wound care and skin regeneration
Víctor
Castrejón-Comas
 ab,
Carlos
Alemán
ab,
Carlos
Alemán
 *abc and
Maria M.
Pérez-Madrigal
*abc and
Maria M.
Pérez-Madrigal
 *ab
*ab
aDepartament d'Enginyeria Química (EQ), Campus Diagonal Besòs (EEBE), Universitat Politècnica de Catalunya · BarcelonaTech (UPC), C/Eduard Maristany, 10-14, 08019, Barcelona, Spain. E-mail: carlos.aleman@upc.edu; m.mar.perez@upc.edu
bBarcelona Research Center for Multiscale Science and Engineering, Campus Diagonal Besòs (EEBE), Universitat Politècnica de Catalunya · BarcelonaTech (UPC), C/Eduard Maristany, 10-14, 08019, Barcelona, Spain
cInstitute for Bioengineering of Catalonia (IBEC), The Barcelona Institute of Science and Technology, Baldiri Reixac 10-12, 08028 Barcelona, Spain
First published on 13th March 2023
Abstract
Although the main function of skin is to act as a protective barrier against external factors, it is indeed an extremely vulnerable tissue. Skincare, regardless of the wound type, requires effective treatments to prevent bacterial infection and local inflammation. The complex biological roles displayed by hyaluronic acid (HA) during the wound healing process have made this multifaceted polysaccharide an alternative biomaterial to prepare wound dressings. Therefore, herein, we present the most advanced research undertaken to engineer conductive and interactive hydrogels based on HA as wound dressings that enhance skin tissue regeneration either through electrical stimulation (ES) or by displaying multifunctional performance. First, we briefly introduce to the reader the effect of ES on promoting wound healing and why HA has become a vogue as a wound healing agent. Then, a selection of systems, chosen according to their multifunctional relevance, is presented. Special care has been taken to highlight those recently reported works (mainly from the last 3 years) with enhanced scalability and biomimicry. By doing that, we have turned a critical eye on the field considering what major challenges must be overcome for these systems to have real commercial, clinical, or other translational impact.
Introduction
The recent rapid development of stretchable and flexible electrodes for soft electronics1 has opened the door to exploit hydrogel-based systems as implantable biomedical devices, i.e. bioelectronics.2–5 Indeed, designing conductive hydrogels, which represent the dominant soft material in Materials Engineering applied to biomedicine, with a combination of advanced features, makes them ideal platforms to promote the regeneration of those tissues sensitive to electrical signals, such as cardiac, skin and nerve tissue.6,7 Not only that but also the integration of multifunctionality, as well as prolonged performance, results in promising opportunities, not yet achieved, for long-term clinical applications in terms of tissue restoration.Skin, which is the largest and most exposed organ in the human body, weighs ca. 4.5 kg with the thickness varying between 0.5 and 4 mm. It is sensitive to electrical signals and, therefore, is an obvious target for electro-stimulation using conductive functional materials.8,9 Composed of several layers (i.e. epidermis, dermis and hypodermis), the skin structure includes a wide range of elements (cells, extracellular matrix (ECM), collagen fibres, nerves, blood vessels, subcutaneous glands, fat tissue, hair, and nails, among others), whose main function is to protect the body against the external environment, acting as a barrier towards mechanical forces, UV light, temperature, etc., as well as being the first line of our immunological defence in front of pathogens. However, despite this, skin is also an extremely vulnerable tissue affected by aging, injuries from trauma, surgical procedures, or burns, for instance, and diseases, such as venous leg ulcers.
Research over the last 50 years on several areas (skin cell biology, polymer scaffolds, or tissue regeneration, for instance) has produced commercial medical products for the engineering of skin tissue.10 More specifically, a wide range of soft dressings have been recently developed to treat skin wounds by exploiting the peculiar and advantageous features of hydrogels: hydrophilic nature, soft tissue-like water content, and adequate biocompatibility and flexibility. Overall, if adequately designed, hydrogels have been reported as excellent candidates that are able to fulfil additional requirements and, more recently, highly advanced biotechnological features.
Skincare: hyaluronic acid hydrogels and electrical stimulation
Although wound healing and skin repair are complex and slow processes, which include four interactive phases (i.e. hemostasis, inflammation, proliferation, and remodelling), most skin defects are effectively healed within 2 weeks.11 However, more severe skin damage, which includes acute (over 8–12 week healing period) or chronic wounds (months), may require prolonged time. For instance, extensive full-thickness skin wounds, such as diabetic foot ulcers (DFUs), are hard-healing chronic conditions susceptible to bacterial infection and local inflammation, which ultimately have a severe negative impact on the patient's health and induce associated costs. Indeed, the figures recently reviewed by Laurano et al.12 justify the current urge to develop cost-effective wound care treatments.13Electrical stimulation and skin repair
Back in 2013, G. Thakral et al. concluded that wound healing was accelerated by electrical stimulation (ES) with few adverse effects after identifying 21 randomized clinical trials.14 A similar conclusion was reached by Rajendran et al. a few years later.15 Indeed, the wound healing process involves electric fields that act as an overriding guidance cue for directional cell migration16 and, hence, electroactive scaffolds have been designed to maximize skin tissue engineering (Fig. 1a).17 In contrast to other techniques, such as debridement, negative-pressure therapy, or flap repair,18 ES-based therapies display high efficacy, low cost, and safety while accelerating wound closure. Indeed, for removing nonviable wound tissue (i.e. debridement), the surgical method is the most widely used, which requires a skilled practitioner and local anesthesia, conditions also necessary for flap surgery. Regarding negative-pressure therapy, the exact mechanism of action is unknown and sufficient data have not been collected to support general use yet. For instance, Y. Wang and co-workers have shown with in vivo ES experiments using diabetic rats how the wound healing rate is faster when a conductive scaffold is used as a wound dressing.19,20 In another example, a flexible electrical patch (ePatch) was prepared with silver nanowires and methacrylated alginate, and both in vitro and in vivo ES studies showed promising results.21 Further considering the technical requirements of power supply and treatment limitations (time and place), an all-in-one self-powered electronic stimulated wound dressing has been recently designed,22 where sodium hyaluronate acts as an active substance to accelerate wound healing under ES. In addition, using natural bio-based monomers, a combination of pyrrole, gelatin and alginate was manufactured to produce a 3D-printable hydrogel cytocompatible and electrically conductive for electrical-stimulation-assisted tissue engineering.23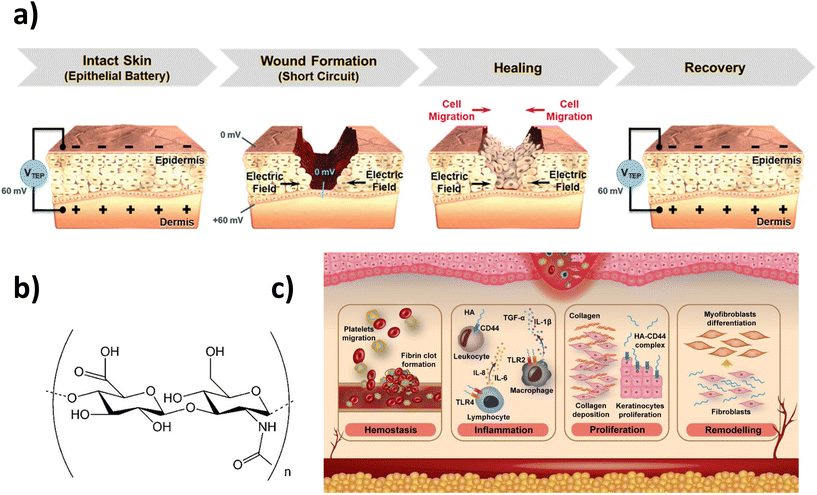 | ||
| Fig. 1 Skincare: HA hydrogels and electrical stimulation: (a) trans-epithelial potential and electric field at the wound site before and after the healing process (reprinted with permission from reference Adv. Healthcare Mater., 2021, 10, 2001384.17 Copyright 2020 John Wiley and Sons). (b) Molecular structure of HA and (c) illustration of HA main roles in the wound healing process. (b) and (c) Reprinted with permission from the reference Carbohydr. Polym., 2020, 241, 116364.33 Copyright 2020 Elsevier. | ||
Role of hyaluronic acid in wound healing
Hyaluronic acid (HA), which is a natural linear anionic polysaccharide (Fig. 1b), is a primary component of the ECM and is widely distributed in the human body.24,25 Why has HA become a vogue recently as a wound healing agent? Simply put, HA displays several unexpected complex biological roles,26 among which we find that HA has been considered one of the key players and, more recently, an electro-regulatory mediator,9 in the tissue regeneration process.27 Specifically, HA enhances collagen deposition, epithelialization and wound vascularization (Fig. 1c).28–30 Specifically, during the proliferation phase, HA facilitates the migration and proliferation of fibroblasts and keratinocytes.28 In addition, the ability of HA to absorb water maintains wound moisture while being non-antigenic. Such biological functions, which result in distinctive features with respect to other materials and their hydrogels, result in an advantageous acceleration of wound healing.However, as a drawback, HA does not form hydrogels on its own, but requires chemical functionalization to introduce crosslinking sites.31 Despite this, on account of the singularities of HA,32 which include biocompatibility, biodegradability, native biofunctionality, hydrophilicity, and non-immunoreactivity, HA-based hydrogels have attracted so much attention recently as wound dressings.33 Considering other biomedical applications, Wang et al. gave a complete up-to-date overview of those systems synthesized by exploiting dynamic-covalent coupling chemistry,34 while Ding et al. reviewed novel 3D printing manufacturing techniques for their fabrication.35 On the market, commercial HA-based wound dressings are already available, such as Hyalofill® and Hyalosafe® (Anika Therapeutics, Bedford, MA) and Hyalo Regen® (Fidia Pharma USA, NJ).12
Bearing all these in mind, herein, we survey the recent advances made in the research area of skin tissue engineering to produce electro-responsive hydrogels made of HA. To the best of our knowledge, although some recent reviews displayed a similar scope,17,36,37 none has brought into focus the relevance of HA as a multifaceted polysaccharide. Therefore, we aim to fill such a gap with this minireview by first looking into how HA hydrogels have been made electroactive by exploiting several conductive moieties; then, selected multifunctional systems, which are prepared to handle the complexity of the skin regeneration, are highlighted; and, finally, interactivity, which is the future direction this line of research is taking, is discussed. Overall, we present a summary of important research developments that exploit the combined benefits of HA and ES to treat skin wounds.
Rendering HA hydrogels electroactive
In general, conductive hydrogels have been achieved mainly by using in the formulation various conductive materials,38 such as metal/metal oxide nanoparticles (NPs; e.g. Au, Ag, Pt, FeO and ZnO), carbon-based moieties (e.g. graphene, graphene oxide, carbon nanotubes and nanowires), and conducting polymers (CPs; e.g. polypyrrole (PPy), polyaniline (PAni), polythiophene (PT), poly(3,4-ethylene dioxythiophene (PEDOT), etc.) (Fig. 2). Besides, to show the reader the versatility and scope of application of multifunctional conductive HA-based hydrogels for skincare treatments, Table 1 summarizes selected works described in the following sections.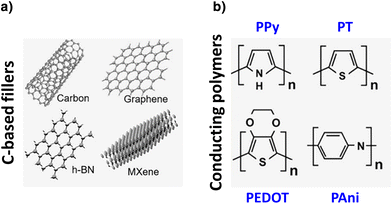 | ||
| Fig. 2 Some conductive materials used to render hydrogels electroactive: (a) carbon-based moieties and (b) conducting polymers. Adapted with permission from the reference ACS Mater. Lett., 2020, 2, 1287–1301.7 Copyright 2020 American Chemical Society. | ||
| Aim | Approach | Details | Ref. |
|---|---|---|---|
| Rendering HA hydrogels electroactive | Metal/metal oxide particles | Ag+ ions reduced to silver NPs; microgels based on HA modified with both methacrylates and gallols; microfluidic channel/water-in-oil droplet formation. | 39 |
| Au–Pt alloy NPs. | 48 | ||
| Combination of Ag nanoclusters with hollow mesoporous manganese dioxide NPs in an adipic acid dihydrazide/tannic acid-grafted HA hydrogel. | 49 | ||
| ZnO NPs/cinnamon essential oil mixture in HA-based nanofiber scaffolds. | 51 | ||
| Zn organic framework into methacrylate HA-based degradable microneedles. | 52 | ||
| Carbon-based moieties | Reduced graphene oxide in HA-graft-dopamine platforms. | 53 | |
| Graphene oxide loaded in a natural polymer network (HA grafted with tyramine and gelatin grafted with gallic acid). | 54 | ||
| Graphene oxide + Ag NPs in a topical hydrogel based on gelatin, PVA and HA. | 55 | ||
| GO into a hydrogel wound dressing composed of HA and chitosan. | 56 | ||
| Light-responsive carbon dots embedded in soft HA hydrogels. | 59 | ||
| Ti3C2 MXene nanosheets in a HA/alginate bioink formulation. | 60 | ||
| Conducting polymers | PPy in HA-based hydrogels. | 62 and 63 | |
| Aniline oligomers and PAni to produce conductive HA hydrogels. | 66–68 | ||
| PEDOT:PSS in aldehyde (ALD)-modified HA hydrogels. | 70 | ||
| Achieving multifunctionality in HA hydrogels (distinctive features) | Bioadhesiveness | Introduction of protein–catechol groups (PDA NPs or by chemical modification) | 73–75 and 77 |
| Antibacterial | Bactericidal effect of doped PAni; aniline oligomers. | 20, 66 and 68 | |
| Hypoxia-inducing capability | Hyperbranched poly(β-amino ester)-tetra-aniline cross-linked with thiolated HA via a thiol–ene click reaction; vanillin-grafted gelatin and laccase created a hypoxic microenvironment. | 67 | |
| UV-blocking ability; on-demand removability | Complex system where the rapid cleavage of the disulfide bonds results in on-demand removability, while the UV-blocking ability was ascribed to the melanin-inspired PDA@PPy nanocomposite. | 63 | |
| Antioxidant | Glucose responsiveness to scavenge reactive oxygen species. | 81 and 82 | |
| Photothermal antibacterial | Cuttlefish melanin NPs into HA hydrogels for photothermal antibacterial therapy. | 63 and 85 | |
With metal/metal oxide nanoparticles
In an effort to build a percolation network of 0D nature with metal NPs within a HA hydrogel, which is a challenging task, Shin et al. prepared microgels based on HA modified with both methacrylates and gallols (MeHA–Ga) using a microfluidic channel and water-in-oil droplet formation.39 Later, the microgels were polymerized by UV light and added to a AgNO3 solution, where gallol moieties reduced Ag+ ions to silver NPs. The resulting injectable system enabled 3D printing of conductive hydrogel patterns that display electroactivity for tissue engineering (electroconductivity on the order of 0.05 ± 0.003 S cm−1).39 Other recent works also reported the use of silver NPs within HA-based hydrogels; however, they mostly focus on their antibacterial properties instead of a conductive platform.40–46 Hence, the novel approach by Shin et al. presented a versatile system for numerous applications, such as 3D printing and electroactive tissue conduction, which enhances cell viability and allows for the control of electrical conductivity. Similarly, even though the biopolymer of choice was cationic guar gum, the same approach was recently applied to synthesize silver NPs onto polydopamine (PDA) NPs that were subsequently encapsulated in the hydrogel network for prompt wound healing.47In the context of diabetic chronic wound management, Au–Pt alloy NPs were embedded in a self-healing hydrogel dressing composed through a Schiff-base reaction of oxidized hyaluronic acid and carboxymethyl chitosan.48 However, the main role of these NPs was to introduce relevant biofunctions, such as lowering blood glucose, alleviating oxidative damage from reactive oxygen species (ROS), and providing O2 by simulating glucose oxidase and catalase. Other systems exploited similar functions by combining Ag nanoclusters with hollow mesoporous manganese dioxide NPs in an adipic acid dihydrazide/tannic acid-grafted HA-based click-hydrogel platform.49
Zinc oxide (ZnO) has also been included in the formulation of wound dressings because it simulates the action of certain growth factors, although the exact wound healing mechanism requires further investigation,50 while displaying an anti-inflammatory and antimicrobial effect. Indeed, ZnO NP/cinnamon essential oil mixtures have been added as antibacterial healing promoters in HA-based nanofiber scaffolds for wound treatment.51 With that goal in mind, a Zn organic framework (Zn-MOF) was added into methacrylate HA-based degradable microneedles (MeHA) prepared with antibacterial activity by ultraviolet (UV) crosslinking. Despite no conductive performance being reported, a continuous and stable release of the metal cations (Zn2+) was achieved at the wound site, which promoted healing, with little secondary damage.52
With carbon-based materials
Carbon-based fillers display mechanical robustness and excellent electrical conductivity, which makes them ideal materials to turn hydrogels into conductive networks.1 As a common concern when applying them for biotechnological purposes, both their limited integration/dispensability, as well as their cytotoxic performance, need to be taken into account.For instance, Liang et al. developed injectable nanocomposite conductive hydrogel dressings for wound healing based on HA-graft-dopamine and reduced graphene oxide (rGO) using a H2O2/HPR (horseradish peroxidase) system (Fig. 3).53 In addition to high swelling, degradability, and tunable rheological performance, the presence of PDA induced antioxidant activity and tissue adhesiveness, as well as hemostatic and self-healing ability. Overall, the multifunctional system acted as a sustained drug release platform with photothermal antibacterial activity. Graphene oxide (GO) was also loaded as a conductive filler in a natural polymer network consisting of HA grafted with tyramine (HT) and gelatin grafted with gallic acid,54 whereas it was also used as a support for dispersing and stabilizing Ag NPs in a topical hydrogel based on gelatin, PVA and HA.55
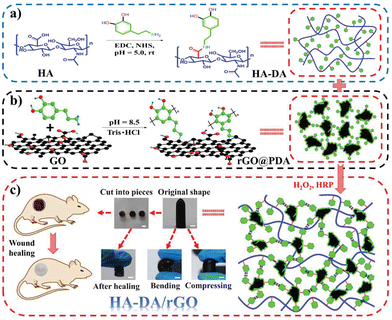 | ||
| Fig. 3 Diagrammatic sketch of HA-dopamine/rGO hydrogel preparation. (a) Preparation scheme of the HA-dopamine polymer and (b) rGO@PDA, (c) scheme of the HA-dopamine/rGO hydrogel and the original, bending, compressing, and self-healing representations and the application in wound healing. Scale bar: 5 mm. Reprinted with permission from the reference Small, 2019, 15, 1900046.53 Copyright 2019 John Wiley and Sons. | ||
Moreover, Ou et al. introduced GO into a hydrogel wound dressing with a double-fold strategy.56 On the one hand, GO enhanced the mechanical properties of the hydrogel, which was synthesized by the dynamic Schiff base reaction of the aldehyde of oxidized HA with the amino group of N-carboxyethyl chitosan. On the other hand, GO imparted to the hydrogel not only excellent conductivity but also immune regulation. Hence, such a combination of features endowed the multifunctional system with the possibility of directing endogenous currents and regulating immunity.
Finally, for treating infected chronic wounds, rGO was combined with rare-earth terbium ions (Tb3+) into a poly(vinyl alcohol) (PVA)–alginate hydrogel that exhibited an antibacterial effect without containing any antibiotic drug,57 an approach that could be adapted to HA-based platforms.
Among carbon-based materials, we find carbon dots (CDs) standing out in having unique optical, electrochemical, biocompatible and photoluminescence properties. In addition to that, other features, such as low toxicity, stability at physiological pH and good dispersion in water, allow them to be used as biomaterials within systems containing HA, for instance.58 In a recent work, the photodynamic capabilities of light-responsive CDs, which were embedded in soft HA hydrogels, were exploited to render a platform able to fight infectious bacteria.59
Interestingly, the outstanding properties of Ti3C2 MXene nanosheets (large specific surface area, high electrical conductivity, low toxicity, and biodegradability) were exploited in a HA/alginate formulation to render electroconductive cell-laden bioink for extrusion-based 3D bioprinting.60
With conducting polymers and other fillers
However, as Min et al. reviewed,38 even though metal NPs and carbon-based materials are widely reported for tissue engineering applications, their long-term cytotoxicity and low stability in some cases have restricted their further use. In contrast, among other advantages, CPs offer excellent electro-optical properties, high stability and versatile doping chemistry with adequate performance in biological environments.61 For instance, PPy has been exploited to enhance both the mechanical and conductive properties of HA-based hydrogels,62,63 as well as chondroitin sulphate films64 and hydrogels that promoted diabetic wound repair by enhancing local neurovascular regeneration.65 Besides, aniline oligomers66,67 and PAni20,68 have also produced conductive HA hydrogels.For instance, PEDOT, a polythiophene derivative with excellent conductivity, stands out because of its biocompatibility and water dispersibility when doped with polystyrene sulfonate (PSS).69 Indeed, PEDOT:PSS was used as an electroconductive network in an aldehyde (ALD)-modified HA hydrogel.70 The dynamism of the system, in terms of noncovalent interactions, as well as Schiff-base bonds, endow the system with self-healing, shear-thinning, and adhesive abilities (Fig. 4).
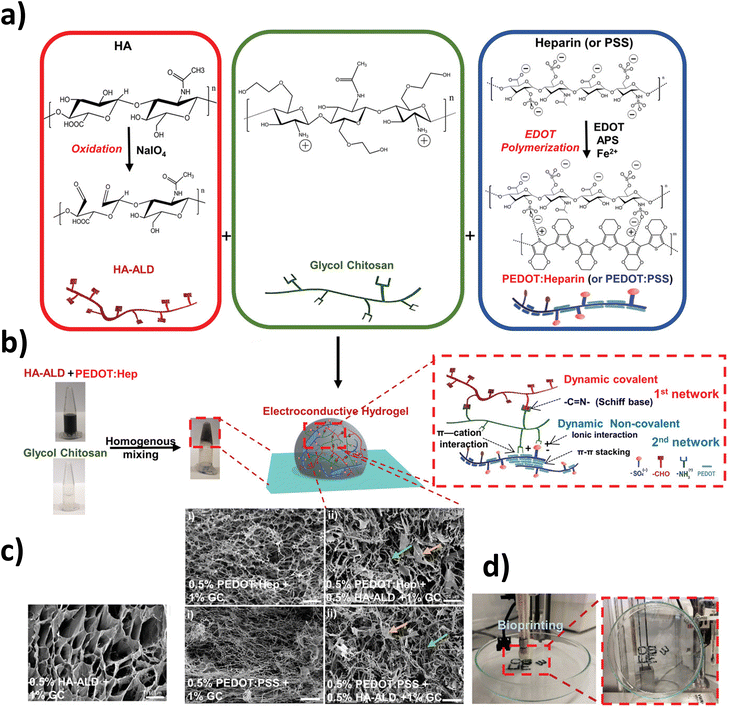 | ||
| Fig. 4 Characteristics of the dual cross-linked electroconductive PEDOT:Heparin/HA-ALD/GC hydrogels. (a) Oxidation of HA by NaIO4, resulting in the formation of HA-ALD with the characteristic presence of aldehyde groups (left box); structure of glycol chitosan (middle box); PEDOT:Heparin and PEDOT:PSS syntheses by polymerizing EDOT to form PEDOT particles using heparin or PSS as the dopant (right box). (b) Representative hydrogel formation by homogeneous mixing of HA-ALD + PEDOT:Heparin with GC solutions in microtubes; schemes of the 3D structure of the dual cross-linked hydrogel network and the interactions involved in the formation of the double network. (c) Scanning electron microscopy (SEM) images of hydrogel networks. The arrows in the dual cross-linked sample (ii) indicate features, such as lamellar-like structures (yellow arrow) or fibers (green arrow). Scale bar: 10 μm. (d) Bioprinting of a PEDOT:Heparin + HA-ALD + GC hydrogel, which can adhere to the surface of a glass Petri dish, when holding the dish vertically. Reprinted from the reference Adv. Sci., 2019, 6, 1802077 under a Creative Commons license (CC BY 4.0).70 | ||
Finally, being aware of the importance of treating diabetic wounds and the positive effect of ES on promoting diabetic wound healing, Liu et al. turned HA hydrogels into conductive platforms after functionalization with an ionic liquid (i.e. a diamino imidazolium ionic liquid, 1,1′-(ethyl-1-bis-(3-(3-aminopropyl)))-1H imidazole tetrafluoroborate, PBAimBF4) through Schiff reactions, which also induced antibacterial properties.71 Most notably, the resulting system, coupled with exogenous ES on a covered diabetic wound, induced enhanced healing in comparison with a commercial Tegaderm™ film.
Achieving multifunctional performance
The therapeutic effect of single-function wound dressings is limited in front of the complex pathological mechanism and underlying physiological conditions in a wound healing process. Hence, multifunctionality, which implies having several different uses so as to make a system versatile and multifaceted, has been recently pursued. Specifically, functional hydrogels encompass an almost endless list of features, such as antimicrobial response, adhesion and hemostasis, anti-inflammatory and anti-oxidation performance, substance delivery, self-healing, stimulus response, and conductivity, among others.72 Even though most of the works cited already in the previous segment do conform to such a meaning, in this section we bring into focus those conductive HA-based hydrogels that present a distinctive feature which grabbed our attention in that they could revolutionize the field of skin regeneration and actually prompt a transition to the clinic (Table 1).Bioadhesiveness in HA hydrogels, which facilities the reconnection of skin tissue in a fast and efficient way, has been acquired by taking inspiration from mussels and, thus, introducing protein–catechol groups, either as PDA NPs73 or by chemical modification of the HA backbone.74,75 In fact, the tendency now is to prepare smart bioadhesive materials, in that they incorporate a biosensing function (in some cases wireless) that allows for real-time and precise evaluation of the healing stage.76 Within this context, by using Li+ and Na+ as conductive ions, Lv et al. designed mussel-inspired conductive HA hydrogels by employing borax as a dynamic cross-linking agent.77 Although the final goal was to obtain strain sensors for electronic skin (e-skin) applications, the overall performance of the system (i.e. excellent stretchability (up to 2800%), high tensile toughness (42.4 kPa), self-adhesiveness (adhesion strength to porcine skin of 49.6 kPa), and good self-healing properties) could be exploited in the design of smart wound dressings with ionic conductivity.
As e-skin devices, as well as any wound electronic patch in contact with skin, might be prone to bacterial infections, antibacterial properties are also highly desirable.78,79 In 2019, Qu et al. reported the use of an aniline oligomer for the first time to yield multifunctional HA-based wound dressings.66 In addition to conductivity, other features, such as antibacterial and anti-oxidant effective responses, as well as degradability and injectability, were obtained. Specifically, amoxicillin was the encapsulated antibiotic, whereas the aniline tetramer significantly accelerated the wound healing rate in a full-thickness skin defect. In fact, recently, the bactericidal effect of doped PAni, which carries a high density of positive charges, against Gram-positive bacteria, has been verified.20 Wu et al. coupled PAni with a macromolecular dopant sulfonated HA to construct a conductive hydrogel dressing whose potential for curing intractable infected chronic wounds was investigated in vivo with ES (Fig. 5). In a later work, a similar hydrogel platform (i.e. PAni covalently grafted on quaternized chitosan) plus ES accelerated chronic diabetic wound healing while displaying enhanced electrical conductivity and intrinsic antibacterial response.68
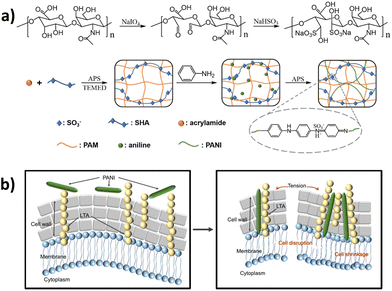 | ||
| Fig. 5 (a) Preparation of the polyacrylamide-sulfonated hyaluronic acid–polyaniline (PAM-SHA-PAni, PSP) hydrogel; (b) scheme of the damage of Gram-positive bacteria induced by the specific interaction between PAni and lipoteichoic acid. Adapted with permission from the reference ACS Appl. Mater. Interfaces, 2021, 13, 52308–52320.20 Copyright 2021 American Chemical Society. | ||
Tetra-aniline was also exploited to produce an injectable conductive hydrogel with a novel additional function, which is sustainable hypoxia-inducing capability.67 Here, a diabetic wound was chosen as the representative injury model onto which the hydrogel was injected. Concretely, hyperbranched poly(β-amino ester)-tetra-aniline was cross-linked with thiolated HA via a thiol–ene click reaction. The presence of vanillin-grafted gelatin and laccase was responsible for casting a hypoxic microenvironment. Other CPs, such as PPy nanotubes embedded in N-isopropylacrylamide (NIPAm), adenine, and quaternized chitosan-graft-β-cyclodextrin hydrogels, produced systems with superior multifunctionality to induce skin tissue regeneration,80 which could be a source of inspiration and be translated to HA-based platforms.
Yan et al. designed a complex system (hydrogels composed of cystamine-modified HA, benzaldehyde-functionalized poly(ethylene glycol)-co-poly(glycerol sebacate), and PDA@PPy nanocomposite; HA-CYS/PFA/PDA@PPy hydrogels) that displayed distinctive features not reported before, such as UV-blocking ability, photothermal anti-infection, and on-demand removability, among other capabilities (i.e. injectability, self-healing, tissue adhesion, etc.).63 The exhaustive characterization of the platform proved its multifunctionality, which was focused on the improved treatment of wounds infected with methicillin-resistant Staphylococcus aureus (MRSA). Specifically, the rapid cleavage of the disulfide bonds present in the network by dithiothreitol (DTT) was responsible for its collapse (on-demand removability), while the UV-blocking ability was ascribed to the melanin-inspired PDA@PPy nanocomposite (Fig. 6).
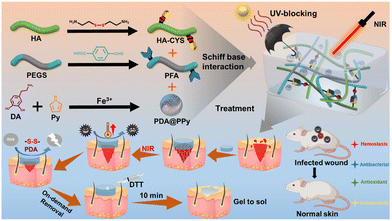 | ||
| Fig. 6 Fabrication, function, and application of HA-CYS/PFA/PDA@PPy hydrogels. Reprinted with permission from reference ACS Appl. Mater. Interfaces, 2022, 14, 41726–41741.63 Copyright 2022 American Chemical Society. | ||
Even though not necessarily conductive, it is worth mentioning a couple of works where antioxidant HA hydrogels have been designed with glucose responsiveness in an effort to scavenge reactive oxygen species (ROS), which cause the delayed closure rate of diabetic chronic wounds.81,82
As a final note, melanin derivatives have been reported to be highly conductive if adequately treated via simple thermal annealing under vacuum.83 For instance, melanin-inspired PDA–Fe NPs were successfully incorporated into a synthetic hydrogel sensor.84 To promote wound healing, Li et al. introduced cuttlefish melanin NPs with excellent photothermal capacity into HA hydrogels for photothermal antibacterial therapy,85 completing a long list of additional features, which include anti-oxidation, hemostasis, exudate absorption and sustained release property, among others (i.e. injectable, stretchable and self-healing systems).
Next step: interactive HA hydrogels
By electrotaxis, the wound-induced electric current favours wound healing and promotes tissue regeneration. Indeed, this endogenous ES guides the motion of biological cells to close the wound.86 As mentioned earlier, when an external electric current is additionally applied, i.e. exogenous ES, this healing process can be further accelerated. However, the state-of-the-art conductive wound dressings are designed not only to facilitate the homogeneous application of ES but also to act as interactive platforms by monitoring and analysing the healing stage and, thus, be applied appropriately and, if possible, remotely.As a first approach to achieve a certain degree of interactivity, a wide variety of stimuli-responsive nanocomposite hydrogels, among which we find HA-based systems, have been engineered to dynamically react to a wide plethora of either internal (e.g. glucose, pH, electrical signalling, enzymes, etc.) or external stimuli (e.g. temperature, ultrasound, light, magnetic and mechanical stress, etc.).87 However, current trends are focusing more on personal healthcare diagnosis and care by smart medical devices that allow for motion detection, temperature control and, even, wireless control of other appliances.88 Thus, what would the next-generation of skin wound dressings look like? Would they overcome the demanding combination of real-time monitoring of human health while promoting skin wound healing?
For instance, Yao and co-workers have reported several systems, based on polyvinyl alcohol-sodium alginate-g-dopamine-silver nanowire-borax89 and chitosan,90 which do provide a preliminary answer to these questions. Specifically, in addition to a correct in vivo wound-healing performance, the epidermal strain sensor did record microscale human activities at various scales, such as pulse and respiration, blinking of the eyes, movement of fingers and wrists, as well as the movement of hand clenching and opening, with fast response and good stability.90 Similarly, in the search for interactive functionality, conductive hydrogels with antibacterial ability were developed for use as epidermal sensors and diabetic foot wound dressings in an effort to mimic the functions of the dermis tissue (Fig. 7).91 Although polyvinyl alcohol was used as a biocompatible polymer, in such a skin-inspired device, PDA-decorated silver NPs acted as the antibacterial component, while PAni was the conductive one. In another example, Tian et al. reported a hydrogel with multifunctional properties for health detection (i.e. detection of the degree of compression on the wound in real-time) and wound healing.92 The distinctive note in this device was given by the addition of glycerin into the alginate-based dressing, which improved the resilience and frost resistance of the hydrogel to ensure its correct functioning as a sensor in cold environments.
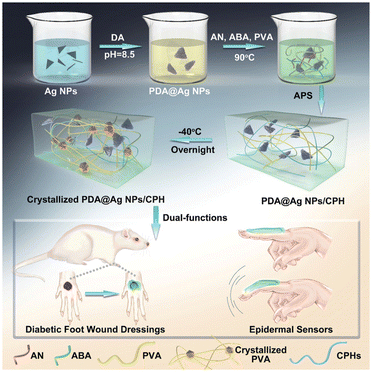 | ||
| Fig. 7 Synthesis of PDA@Ag NPs/conductive polymer-based hydrogels and further applications as epidermal sensors for diabetic foot wound dressing. Reprinted with permission from the reference Adv. Funct. Mater., 2019, 29, 1901474.91 Copyright 2019 John Wiley and Sons. | ||
Therefore, it is evident that there is a great challenge ahead: the manufacture of conductive multifunctional HA-based hydrogel skin dressings that not only promote skin restoration through passive features but also interact with patients by monitoring in real-time their health condition and, later, transfer this information to the medical team. In this regard, by taking advantage of the excellent properties of HA, we expect the reporting of future devices that ultimately enhance skin tissue regeneration through ES while displaying bacterial growth control. Indeed, other advanced features might include the rapid detection of infections coupled with the electrically controlled release of antibiotic drugs.93,94
Conclusions
The remaining challenges ahead in the field of skin regeneration, and in particular, considering chronic wound healing, require innovative solutions based on all the factors and complex processes involved. Hence, for preparing the ultimate and best method, among the valuable ingredients in hand, HA and ES are extremely convenient. Indeed, we have come to understand them as key factors in the pursuit of a cost-effective solution.The works summarized in this contribution evidence the recent advances in designing conductive and interactive hydrogels based on HA as wound dressings, where skincare and tissue repair are achieved by ES and/or multifunctional features. Among the conductive materials used to render HA hydrogels electroactive, the most relevant ones include metal/metal oxide nanoparticles, carbon-based moieties, and conducting polymers. Still, though, we expect even more advanced developments in the following years when other conductive materials, such as PEDOT derivatives, will be applied to HA-based hydrogels.
To address the skin regeneration process and its multiple aspects, the hydrogels are conceived as multifunctional systems, thus combining superior characteristics, such as self-healing, adhesive, antimicrobial, injectable, and anti-oxidative characteristics, among others. In fact, all-in-one multifunctional wound dressings with longer performance result in cost savings as the use of several single-use monofunctional dressings is avoided.
Finally, interactivity, understood as the communication between humans and digital devices, is the key concept for the next-generation of biomaterials. In fact, the collection of recently published works presented herein do evidence the turn this research area is taking.
An important advantage of HA is that it allows for its manufacturing in a wide variety of forms, which include gels, after proper chemical functionalization, sheets of solid materials or lightly woven meshes, among others. This easiness of fabrication adapts the HA-based platform to a specific biological setting, where mechanical parameters, such as robustness, flexibility, and stability are of major importance and, hence, should not be overlooked. In that regard, 3D manufacturing methods, or even 4D methods, already used with other biopolymers, are anticipated to be applied to HA-based hydrogels in the short term. Similarly, HA-hydrogels could be combined with electrospun mats for improved performance and more morphologically complex substrates.
Although the biological activity of HA-based hydrogel wound dressings is already significantly better than that of other materials in terms of healing, it could be further improved by adding other biomolecules or stem cells. Indeed, new avenues are being explored that include gene therapy, addition of hormones/enzymes, and the controlled effect of growth factors.
Large-scale manufacturing with adequate production rates and reproducibility represents another challenge in this line of research, and it would ensure successful commercialization. Besides, as we are moving towards an era of personalized medicine, efforts need to be also focused on developing portable low-cost ES equipment, with accessible components and low maintenance, as well as a wide catalogue of dressings that could be adjusted in situ to better match the type of wound that requires treatment.
To conclude, the wound closure process can become a difficult one when a challenging environment is faced with numerous factors perpetuating non-healing conditions, such as poor circulation, prolonged inflammation, nerve damage, infection, or slow rate of blood vessel formation, etc. To reverse that, clinically relevant actions need to be performed in combination with currently available therapies and future treatment options, as presented herein. Most importantly, the transition from lab to clinic needs to be ensured and, therefore, any significant outcome should be shared with as many clinicians and general practitioners as possible. As always, the finish line will be crossed when a functional and cheap clinical alternative is available for all.
Author contributions
V. C. summarized the literature studies. M. M. P.-M. conceived, supervised, and wrote the manuscript with the assistance of V. C. C. A. revised the manuscript. M. M. P.-M. and C. A. secured funding. All authors read and approved the final manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This publication is part of the I + D + i project PID2021-125767OB-I00 funded by MCIN/AEI/10.13039/501100011033 and, as appropriate, by “ERDF A way of making Europe”, by the European Union. M. M. P.-M. thanks the Ministerio de Educación y Formación Profesional for the Junior Beatriz Galindo Award (BG20/00216).References
- K. W. Cho, S.-H. Sunwoo, Y. J. Hong, J. H. Koo, J. H. Kim, S. Baik, T. Hyeon and D.-H. Kim, Chem. Rev., 2022, 122, 5068–5143 CrossRef CAS PubMed.
- Y. Liu, M. Pharr and G. A. Salvatore, ACS Nano, 2017, 11, 9614–9635 CrossRef CAS PubMed.
- Y. Zhou, C. Wan, Y. Yang, H. Yang, S. Wang, Z. Dai, K. Ji, H. Jiang, X. Chen and Y. Long, Adv. Funct. Mater., 2019, 29, 1806220 CrossRef.
- L. Dong, M. Wang, J. Wu, C. Zhu, J. Shi and H. Morikawa, ACS Appl. Mater. Interfaces, 2022, 14, 9126–9137 CrossRef CAS PubMed.
- W. Zhang, L. Xu, M. Zhao, Y. Ma, T. Zheng and L. Shi, Soft Matter, 2022, 18, 1644–1652 RSC.
- C. Li, RSC Adv., 2021, 11, 33835–33848 RSC.
- F. Fu, J. Wang, H. Zeng and J. Yu, ACS Mater. Lett., 2020, 2, 1287–1301 CrossRef CAS.
- L. P. da Silva, S. C. Kundu, R. L. Reis and V. M. Correlo, Trends Biotechnol., 2020, 38, 24–49 CrossRef CAS PubMed.
- A. J. Hayes and J. Melrose, Adv. Ther., 2020, 3, 2000151 CrossRef CAS.
- V. Falanga, in Principles of Tissue Engineering, ed. R. Lanza, R. Langer, J. P. Vacanti and A. Atala, Academic Press, 2020, 5th edn, pp. 1331–1352 Search PubMed.
- B. K. Sun, Z. Siprashvili and P. A. Khavari, Science, 2014, 346, 941–945 CrossRef CAS PubMed.
- R. Laurano, M. Boffito, G. Ciardelli and V. Chiono, Eng. Regen., 2022, 3, 182–200 Search PubMed.
- J. R. Bardill, M. R. Laughter, M. Stager, K. W. Liechty, M. D. Krebs and C. Zgheib, Acta Biomater., 2022, 138, 73–91 CrossRef CAS PubMed.
- G. Thakral, J. LaFontaine, B. Najafi, T. K. Talal, P. Kim and L. A. Lavery, Diabet. Foot Ankle, 2013, 4, 22081 CrossRef PubMed.
- S. B. Rajendran, K. Challen, K. L. Wright and J. G. Hardy, J. Funct. Biomater., 2021, 12(40), 1–17 Search PubMed.
- M. Zhao, Semin. Cell Dev. Biol., 2009, 20, 674–682 CrossRef CAS PubMed.
- C. Korupalli, H. Li, N. Nguyen, F.-L. Mi, Y. Chang, Y.-J. Lin and H.-W. Sung, Adv. Healthcare Mater., 2021, 10, 2001384 CrossRef CAS PubMed.
- J. G. Powers, C. Higham, K. Broussard and T. J. Phillips, J. Am. Acad. Dermatol., 2016, 74, 607–625 CrossRef PubMed.
- Y. Lu, Y. Wang, J. Zhang, X. Hu, Z. Yang, Y. Guo and Y. Wang, Acta Biomater., 2019, 89, 217–226 CrossRef CAS PubMed.
- C. Wu, L. Shen, Y. Lu, C. Hu, Z. Liang, L. Long, N. Ning, J. Chen, Y. Guo, Z. Yang, X. Hu, J. Zhang and Y. Wang, ACS Appl. Mater. Interfaces, 2021, 13, 52308–52320 CrossRef CAS PubMed.
- C. Wang, X. Jiang, H.-J. Kim, S. Zhang, X. Zhou, Y. Chen, H. Ling, Y. Xue, Z. Chen, M. Qu, L. Ren, J. Zhu, A. Libanori, Y. Zhu, H. Kang, S. Ahadian, M. R. Dokmeci, P. Servati, X. He, Z. Gu, W. Sun and A. Khademhosseini, Biomaterials, 2022, 285, 121479 CrossRef CAS PubMed.
- X. Wang, W. Zhang, Q. Zhou and F. Ran, Chem. Eng. J., 2023, 452, 139491 CrossRef CAS.
- T. Distler, C. Polley, F. Shi, D. Schneidereit, M. D. Ashton, O. Friedrich, J. F. Kolb, J. G. Hardy, R. Detsch, H. Seitz and A. R. Boccaccini, Adv. Healthcare Mater., 2021, 10, 2001876 CrossRef CAS PubMed.
- J. Li, S. Guan, J. Su, J. Liang, L. Cui and K. Zhang, Curr. Drug Delivery, 2021, 18, 836–846 CAS.
- S. Li, Q. Dong, X. Peng, Y. Chen, H. Yang, W. Xu, Y. Zhao, P. Xiao and Y. Zhou, ACS Nano, 2022, 16, 11346–11359 CrossRef CAS PubMed.
- G. Abatangelo, V. Vindigni, G. Avruscio, L. Pandis and P. Brun, Cells, 2020, 9, 1743 CrossRef CAS PubMed.
- M. Litwiniuk, A. Krejner and T. Grzela, Wounds, 2016, 28, 78–88 Search PubMed.
- R. D. Price, S. Myers, I. M. Leigh and H. A. Navsaria, Am. J. Clin. Dermatol., 2005, 6, 393–402 CrossRef PubMed.
- J. Voigt and V. R. Driver, Wound Repair Regener., 2012, 20, 317–331 CrossRef PubMed.
- T. Murakami, S. Otsuki, Y. Okamoto, K. Nakagawa, H. Wakama, N. Okuno and M. Neo, Connect. Tissue Res., 2019, 60, 117–127 CrossRef CAS PubMed.
- Y.-W. Ding, Z.-Y. Wang, Z.-W. Ren, X.-W. Zhang and D.-X. Wei, Biomater. Sci., 2022, 10, 3393–3409 RSC.
- M. S. Birajdar, H. Joo, W.-G. Koh and H. Park, Biomater. Res., 2021, 25, 8 CrossRef CAS PubMed.
- M. F. P. Graça, S. P. Miguel, C. S. D. Cabral and I. J. Correia, Carbohydr. Polym., 2020, 241, 116364 CrossRef PubMed.
- S. Wang, S. Tavakoli, R. P. Parvathaneni, G. N. Nawale, O. P. Oommen, J. Hilborn and O. P. Varghese, Biomater. Sci., 2022, 10, 6399–6412 RSC.
- Y.-W. Ding, X.-W. Zhang, C.-H. Mi, X.-Y. Qi, J. Zhou and D.-X. Wei, Smart Mater. Struct., 2023, 4, 59–68 Search PubMed.
- I. Carayon, A. Gaubert, Y. Mousli and B. Philippe, Biomater. Sci., 2020, 8, 5589–5600 RSC.
- R. Yu, H. Zhang and B. Guo, Nano-Micro Lett., 2021, 14, 1 Search PubMed.
- J. H. Min, M. Patel and W.-G. Koh, Polymers, 2018, 10, 1078 CrossRef PubMed.
- M. Shin, K. H. Song, J. C. Burrell, D. K. Cullen and J. A. Burdick, Adv. Sci., 2019, 6, 1901229 CrossRef CAS PubMed.
- X. Chen, H. Zhang, X. Yang, W. Zhang, M. Jiang, T. Wen, J. Wang, R. Guo and H. Liu, Molecules, 2021, 26, 4037 CrossRef CAS PubMed.
- G. Ferreres, S. Pérez-Rafael, J. Torrent-Burgués and T. Tzanov, Int. J. Mol. Sci., 2021, 22, 13428 CrossRef CAS PubMed.
- W. Li, X. Zhao, T. Huang, Y. Ren, W. Gong, Y. Guo, J. Wang and Q. Tu, Int. J. Biol. Macromol., 2021, 191, 60–70 CrossRef CAS PubMed.
- S. Pérez-Rafael, K. Ivanova, I. Stefanov, J. Puiggalí, L. J. del Valle, K. Todorova, P. Dimitrov, D. Hinojosa-Caballero and T. Tzanov, Acta Biomater., 2021, 134, 131–143 CrossRef PubMed.
- Y. Xiong, Y. Xu, F. Zhou, Y. Hu, J. Zhao, Z. Liu, Q. Zhai, S. Qi, Z. Zhang and L. Chen, Bioeng. Transl. Med., 2022, e10373 Search PubMed.
- J. Yu, L. Cheng, Z. Jia, X. Han, H. Xu and J. Jiang, J. Polym. Environ., 2022, 30, 1330–1343 CrossRef CAS.
- W. Shi, Y. Kong, Y. Su, M. A. Kuss, X. Jiang, X. Li, J. Xie and B. Duan, J. Mater. Chem. B, 2021, 9, 7182–7195 RSC.
- X. Qi, Y. Huang, S. You, Y. Xiang, E. Cai, R. Mao, W. Pan, X. Tong, W. Dong, F. Ye and J. Shen, Adv. Sci., 2022, 9, 2106015 CrossRef CAS PubMed.
- B. Zhang, Y. Lv, C. Yu, W. Zhang, S. Song, Y. Li, Y. Chong, J. Huang and Z. Zhang, Biomater. Adv., 2022, 137, 212869 CrossRef CAS PubMed.
- M. Deng, Y. Wu, Y. Ren, H. Song, L. Zheng, G. Lin, X. Wen, Y. Tao, Q. Kong and Y. Wang, J. Controlled Release, 2022, 350, 613–629 CrossRef CAS PubMed.
- A. Mohandas, S. Kumar PT, B. Raja, V.-K. Lakshmanan and R. Jayakumar, Int. J. Nanomed., 2015, 10, 53–66 CAS.
- M. R. El-Aassar, N. G. El-Beheri, M. M. Agwa, H. M. Eltaher, M. Alseqely, W. S. Sadik and L. El-Khordagui, Int. J. Biol. Macromol., 2021, 167, 1552–1563 CrossRef CAS PubMed.
- S. Yao, J. Chi, Y. Wang, Y. Zhao, Y. Luo and Y. Wang, Adv. Healthcare Mater., 2021, 10, 2100056 CrossRef CAS PubMed.
- Y. Liang, X. Zhao, T. Hu, B. Chen, Z. Yin, P. X. Ma and B. Guo, Small, 2019, 15, 1900046 CrossRef PubMed.
- Y. Ren, S. Ma, D. Zhang, S. Guo, R. Chang, Y. He, M. Yao and F. Guan, Int. J. Biol. Macromol., 2022, 210, 218–232 CrossRef CAS PubMed.
- J. L. Patarroyo, J. Cifuentes, L. N. Muñoz, J. C. Cruz and L. H. Reyes, Heliyon, 2022, 8, e09145 CrossRef CAS PubMed.
- X. Ou, L. Guan, W. Guo, X. Zhang, S. Wu, D. Guo, R. Li, A. V. Zvyagin, Q. Lin and W. Qu, Mater. Des., 2022, 224, 111284 CrossRef CAS.
- Y. Wang, Y. Lu, J. Zhang, X. Hu, Z. Yang, Y. Guo and Y. Wang, J. Mater. Chem. B, 2019, 7, 538–547 RSC.
- F. Azadmanesh, M. Pourmadadi, J. Zavar Reza, F. Yazdian, M. Omidi and B. F. Haghirosadat, Biotechnol. Prog., 2021, 37, e3132 CAS.
- C. H. Lee, S. Y. Song, Y. J. Chung, E. K. Choi, J. Jang, D. H. Lee, H. D. Kim, D.-U. Kim and C. B. Park, ACS Appl. Bio Mater., 2022, 5, 761–770 CrossRef CAS PubMed.
- H. Rastin, B. Zhang, A. Mazinani, K. Hassan, J. Bi, T. T. Tung and D. Losic, Nanoscale, 2020, 12, 16069–16080 RSC.
- M. Talikowska, X. Fu and G. Lisak, Biosens. Bioelectron., 2019, 135, 50–63 CrossRef CAS PubMed.
- J. Yang, G. Choe, S. Yang, H. Jo and J. Y. Lee, Biomater. Res., 2016, 20, 31 CrossRef PubMed.
- Y. Yang, H. Xu, M. Li, Z. Li, H. Zhang, B. Guo and J. Zhang, ACS Appl. Mater. Interfaces, 2022, 14, 41726–41741 CrossRef CAS PubMed.
- M. Björninen, A. Siljander, J. Pelto, J. Hyttinen, M. Kellomäki, S. Miettinen, R. Seppänen and S. Haimi, Ann. Biomed. Eng., 2014, 42, 1889–1900 CrossRef PubMed.
- L. Fan, C. Xiao, P. Guan, Y. Zou, H. Wen, C. Liu, Y. Luo, G. Tan, Q. Wang, Y. Li, P. Yu, L. Zhou and C. Ning, Adv. Healthcare Mater., 2022, 11, 2101556 CrossRef CAS PubMed.
- J. Qu, X. Zhao, Y. Liang, Y. Xu, P. X. Ma and B. Guo, Chem. Eng. J., 2019, 362, 548–560 CrossRef CAS.
- X. Jin, Y. Shang, Y. Zou, M. Xiao, H. Huang, S. Zhu, N. Liu, J. Li, W. Wang and P. Zhu, ACS Appl. Mater. Interfaces, 2020, 12, 56681–56691 CrossRef CAS PubMed.
- C. Wu, L. Long, Y. Zhang, Y. Xu, Y. Lu, Z. Yang, Y. Guo, J. Zhang, X. Hu and Y. Wang, J. Controlled Release, 2022, 344, 249–260 CrossRef CAS PubMed.
- D. Aycan, N. Dolapçı, Ö. G. Karaca and N. Alemdar, J. Appl. Polym. Sci., 2022, 139, e52761 CrossRef CAS.
- Y. Xu, P. A. Patsis, S. Hauser, D. Voigt, R. Rothe, M. Günther, M. Cui, X. Yang, R. Wieduwild, K. Eckert, C. Neinhuis, T. F. Akbar, I. R. Minev, J. Pietzsch and Y. Zhang, Adv. Sci., 2019, 6, 1802077 CrossRef PubMed.
- P. Liu, K. Jin, Y. Zong, M. He, C. Lu, H. Li, Y. Wang and C. Li, Biomater. Sci., 2022, 10, 1795–1802 RSC.
- Y. Liang, J. He and B. Guo, ACS Nano, 2021, 15, 12687–12722 CrossRef CAS PubMed.
- N. Pandey, L. Soto-Garcia, S. Yaman, A. Kuriakose, A. U. Rivera, V. Jones, J. Liao, P. Zimmern, K. T. Nguyen and Y. Hong, Biomater. Adv., 2022, 134, 112589 CrossRef PubMed.
- J. Hu, M. Tao, F. Sun, C. Chen, G. Chen and G. Wang, Int. J. Biol. Macromol., 2022, 222, 55–64 CrossRef CAS PubMed.
- M. Gong, F. Yan, L. Yu and F. Li, Cell Death Dis., 2022, 13, 1–11 Search PubMed.
- J. Zhu, H. Zhou, E. M. Gerhard, S. Zhang, F. I. Parra Rodríguez, T. Pan, H. Yang, Y. Lin, J. Yang and H. Cheng, Bioact. Mater., 2023, 19, 360–375 CrossRef PubMed.
- R. Lv, Z. Bei, Y. Huang, Y. Chen, Z. Zheng, Q. You, C. Zhu and Y. Cao, Macromol. Rapid Commun., 2020, 41, 1900450 CrossRef CAS PubMed.
- L. Fan, J. Xie, Y. Zheng, D. Wei, D. Yao, J. Zhang and T. Zhang, ACS Appl. Mater. Interfaces, 2020, 12, 22225–22236 CrossRef CAS PubMed.
- Z. Cao, Y. Luo, Z. Li, L. Tan, X. Liu, C. Li, Y. Zheng, Z. Cui, K. W. K. Yeung, Y. Liang, S. Zhu and S. Wu, Macromol. Biosci., 2021, 21, 2000252 CrossRef CAS PubMed.
- R. Yu, M. Li, Z. Li, G. Pan, Y. Liang and B. Guo, Adv. Healthcare Mater., 2022, 11, 2102749 CrossRef CAS PubMed.
- Z. Xu, G. Liu, J. Huang and J. Wu, ACS Appl. Mater. Interfaces, 2022, 14, 7680–7689 CrossRef CAS PubMed.
- Z. Xu, G. Liu, P. Liu, Y. Hu, Y. Chen, Y. Fang, G. Sun, H. Huang and J. Wu, Acta Biomater., 2022, 147, 147–157 CrossRef CAS PubMed.
- L. Migliaccio, P. Manini, D. Altamura, C. Giannini, P. Tassini, M. G. Maglione, C. Minarini and A. Pezzella, Front. Chem., 2019, 7, 162 CrossRef CAS PubMed.
- X. Zhang, Y. Peng, X. Wang and R. Ran, ACS Appl. Polym. Mater., 2021, 3, 1899–1911 CrossRef CAS.
- M. Li, Y. Liang, Y. Liang, G. Pan and B. Guo, Chem. Eng. J., 2022, 427, 132039 CrossRef CAS.
- A. Guo, B. Song, B. Reid, Y. Gu, J. V. Forrester, C. A. B. Jahoda and M. Zhao, J. Invest. Dermatol., 2010, 130, 2320–2327 CrossRef CAS PubMed.
- P. Lavrador, M. R. Esteves, V. M. Gaspar and J. F. Mano, Adv. Funct. Mater., 2021, 31, 2005941 CrossRef CAS.
- T. Wang, J. Song, R. Liu, S. Y. Chan, K. Wang, Y. Su, P. Li and W. Huang, ACS Appl. Mater. Interfaces, 2022, 14, 14596–14606 CrossRef CAS PubMed.
- L. Fan, L. Hu, J. Xie, Z. He, Y. Zheng, D. Wei, D. Yao and F. Su, Biomater. Sci., 2021, 9, 5884–5896 RSC.
- L. Fan, Z. He, X. Peng, J. Xie, F. Su, D.-X. Wei, Y. Zheng and D. Yao, ACS Appl. Mater. Interfaces, 2021, 13, 53541–53552 CrossRef CAS PubMed.
- Y. Zhao, Z. Li, S. Song, K. Yang, H. Liu, Z. Yang, J. Wang, B. Yang and Q. Lin, Adv. Funct. Mater., 2019, 29, 1901474 CrossRef.
- S. Tian, M. Wang, X. Wang, L. Wang, D. Yang, J. Nie and G. Ma, ACS Biomater. Sci. Eng., 2022, 8, 1867–1877 CrossRef CAS PubMed.
- B. G. Molina, L. J. del Valle, P. Turon, E. Armelin and C. Alemán, J. Phys. Chem. C, 2019, 123, 22181–22190 CrossRef CAS.
- B. G. Molina, L. J. del Valle, J. Casanovas, S. Lanzalaco, M. M. Pérez-Madrigal, P. Turon, E. Armelin and C. Alemán, Adv. Healthcare Mater., 2021, 10, 2100425 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |
