Multifluid electrospinning for multi-drug delivery systems: pros and cons, challenges, and future directions
Sahranur
Tabakoglu
 ,
Dorota
Kołbuk
,
Dorota
Kołbuk
 and
Paweł
Sajkiewicz
and
Paweł
Sajkiewicz
 *
*
Institute of Fundamental Technological Research, Polish Academy of Sciences, Pawinskiego 5B, 02-106 Warsaw, Poland. E-mail: psajk@ippt.pan.pl
First published on 19th October 2022
Abstract
The electrospinning method has been widely used to produce nano/micro fibers for various applications. As a drug delivery system, electrospun fibers display many advantages such as controlled drug delivery kinetics and the ability to deliver drugs locally. A drug delivery system improves delivery efficiency and reduces possible toxic effects. In particular, multiaxial fibers consisting of two or more fluid components have drawn attention for the simultaneous administration of multiple therapeutic agents for sustained delivery and effective treatment. This review discusses recently studied multi-compartment electrospun fibers, including side-by-side (Janus) and axially symmetric fibers – coaxial and triaxial – from the perspective of multi-drug incorporation. It begins with an overview of conventional uniaxial single-fluid electrospinning methods for drug delivery applications, then highlights the advantages of multi-compartment fibers for multi-substance loading/delivery and the advances in triaxial fibers that seem to be promising from the perspective of challenges for dressings and tissue regeneration. Furthermore, drug release mechanisms and kinetics are discussed in the controlled delivery of multiple therapeutics in fibers. In the conclusion, current biomedical applications of multi-drug delivery systems in selected applications and future perspectives are presented.
1. Introduction
Electrospinning is a nano- and submicron-scale material production method that has attracted much attention for drug delivery applications. This technique allows the fabrication of ultrafine nanofibers from various polymers with good structural properties, for example high surface-to-volume ratio, remarkable physicochemical properties, and flexibility, using a high-voltage electrical force.1–3 Nanofibers can provide effective drug-loading capacity and the desired release kinetics by using the right material and suitable production conditions.4 Contrary to traditional fiber production methods such as melt, dry, and wet spinning, the electrospinning technique forms fibers with diameters of less than 100 nm connected to porous membranes. Additionally, the parameters of the process determine electrospun membrane architecture – total porosity, pore size, fiber diameter, surface morphology – and make this method powerful and functional.5,6 To date, electrospun nanofibers features have been used for several kinds of application areas, including tissue engineering,7,8 drug delivery systems,9,10 cell culture studies,11 and many others.12–15 For effective and targeted drug delivery, electrospun nanofiber membranes are a challenging system. Furthermore, these fibers can mimic an extracellular matrix (ECM), which makes electrospinning an attractive method for producing functional materials for tissue engineering and drug delivery.16,17 There are many different specialized techniques of electrospinning, types of polymers, and process parameters, depending on the desired applications and related kinetics of drug release. One of the biggest challenges currently facing drug delivery system (DDS) design is achieving multiple drug delivery, with the combined delivery of two or more drugs and differing kinetics releases. Quick (burst) release might be followed by the remaining drugs’ slow (sustained) release over an extended period. Therapy combining two or more therapeutic agents is a cornerstone of modern cancer therapy, as it creates a synergistic and additive effect.18Besides the electrospun fibers, many kinds of DDS have been developed for multiple drug delivery; for instance, liposomes, polymer micelles,19 hydrogels,20 and various nanocarriers.21,22 For an effective DDS, some key points are taken into consideration: high drug-loading capacity and encapsulation efficiency, simultaneous delivery of therapeutics, drug release-time manipulation, cost, and operation friendliness. Compared with other DDS, the electrospinning strategy allows the selection of materials and therapeutics from a wide range. Furthermore, it allows the manipulation of the drug release rate by modification of the degradation rate of the fibers. Even more specifically, considering wound dressing, drug administration is easier than other methods because electrospun membranes can be easily placed in the defect area.23,24 Hydrogels are of special interest in DDS, but they may face problems related to the gelation process that result in washout of the loaded therapeutics. Electrospun fibers can work effectively against that problem.25 For these reasons and more, electrospinning is a competent strategy for DDS.
Conventional (uniaxial) electrospinning methods have already been reported in various drug delivery applications.26–28 Conventional electrospinning involves four primary constituents: a capillary tube, a spinneret (needle), a collector, and a high-voltage resource (Fig. 1). Many types of needle have been developed to allow uniaxial, coaxial, and triaxial formation. While the uniaxial needles allow the production of conventional single fibers, other fluid delivery geometries provide more complicated structures such as multilayer or core–shell fibers.29,30 One of the geometries currently of great interest in the drug delivery field is the core–shell structure produced by coaxial/triaxial electrospinning. The spinneret for coaxial electrospinning is composed of an inner needle connected to the core fluid reservoir, and an outer needle attached to the shell fluid one.
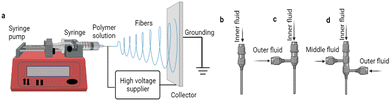 | ||
| Fig. 1 Conventional electrospinning process based on single fluid (a); uniaxial needle (b); coaxial needle (c); triaxial needle (d). | ||
Limitations for uniaxial fibers have been reported:
● limited compatibility of most drugs and biomolecules with the organic solvents used in the electrospinning process,31
● poor control of drug release profiles, with too fast release of drug, and being inadequate for the assumed treatment process,7
● limited delivery of poorly water-soluble drugs,32
● inadequate drug encapsulation efficiency,33
● lack of a common solvent for each polymer in single-blend electrospinning.34
To overcome these limitations, improvements in electrospinning are constantly proceeding, and bicomponent fibers, multijet electrospinning, needleless electrospinning in the case of scale-up, emulsion electrospinning, surface energy-free electrospinning, multifluid including coaxial, triaxial and side-by-side electrospinning have been explored.35–42 Coaxial electrospinning and the resulting core–shell nanofibers have many advantages as a drug delivery platform. These structures can protect the bioactivity of the molecules, e.g. proteins, drugs, and growth factors, and provide a more sustained and slower release than single nanofibers thanks to the shell structure.43 Furthermore, multiple drugs can be incorporated into core and shell layers, and their release mechanisms can be controlled to achieve two different release pathways.44 Coaxial fibers have higher drug loading efficiency than conventional (single blend) electrospinning. Notably, the initial burst release is relatively lower in coaxial fibers than in single ones consisting of the same polymer system.45 While this method has favorable attributes, it also has drawbacks from the application perspective. Incompatibility of the therapeutic agent and solvent used for polymer solution can still be a problem in this method, as in conventional uniaxial electrospinning.32 Additionally, initial burst release and controlled and sustained release of the agents are still issues that need improvement. Side-by-side electrospinning (Janus fibers) is another technique using two fluids for manipulation of multiple or sequential drug delivery. This method also has some drawbacks similar to coaxial. Therefore, multiaxial nanofiber production methods such as core–shell by triaxial electrospinning (Fig. 2), and also combinations of these, are being developed.38,39,46–49 All the techniques are discussed in detail in further sections considering multiple drug delivery.
 | ||
| Fig. 2 Representative scheme of different kinds of electrospun fibers; uniaxial (a), side-by-side (b); coaxial (c); triaxial (d). | ||
Triaxial electrospinning has become a meaningful subject in the field of modern drug delivery.50 In the triaxial method, the core material is surrounded by two different layers: intermediate and shell. All layers can be different materials, e.g. drugs, polymers, and solvents, as well as different or the same polymers.51–54 Furthermore, these polymers may be supplied/functionalized with multiple additives, for example growth factors, drugs, or nanoparticles.50 The triaxial method provides several advantages over coaxial, uniaxial electrospinning and Janus nanofibers, such as more complex structures for functional materials. Previous reports indicated that triaxial fibers exhibited better mechanical properties,55 a more sustained release profile,49 and enhanced biocompatibility52 over the coaxial ones. The principal aim of triaxial electrospinning is to solve the limitations of monolithic and core shell fibers: lack of sustained and controlled drug release, poor solubility of the drugs, the problem with loading multiple drugs, insufficient mechanical properties, biodegradation, and inadequate biocompatibility.
What is more, quad-axial electrospinning, using four fluids, is a quite new perspective in multifluid electrospinning for biomedical applications. Although it was first developed in 201456 it has been reported with controlled and sustained delivery only in 2021.57 This research reported that quad-axial fibers can provide more efficient and sequential release for drug delivery. Recently, a few review papers have mentioned it as a promising DDS for biomedical applications in the future.58–60 However, due to the lack of research studies, it is not possible to make an accurate comparison with other systems and say anything more.
This review aims to sum up the state-of-the-art in multiple drug treatments in the form of electrospun fibers in the last decade, emphasizing the advantages of triaxial electrospinning. Therefore, it compares different drug release systems based on electrospun membranes, highlighting their main advantages and disadvantages, such as uniaxial single-layer fibers, two-layer (coaxial) core–shell and side-by-side (Janus) fibers, as well as three-layer triaxial fibers. Finally, extended drug release mechanisms are reported from the perspective of selected applications and future development perspectives.
2. Multiple drug treatment using electrospun fibers: from the viewpoint of uniaxial electrospinning
In complicated pathophysiology and tissue regeneration processes, a single drug is mostly not sufficient to achieve the ideal therapeutic level.61,62 Due to the complexity of tissue regeneration, a combination of therapeutics including drugs,63,64 growth factors,65 genetic materials,66,67 and cells68 are needed for effective treatment. In the literature, it has already been reported that multiple therapeutic agent treatment has great advantage in wound healing,69,70 tissue regeneration,71–73 and cancer treatment.74–76 Furthermore, classical systemic treatment can cause undesired side effects through the application of a high dosage of drugs in a relatively short time (burst release). Additionally, such treatment exhibits inadequate therapeutic activity when the agent reaches the target area compared with targeted DDS using local vehicles like nanocarriers or micelles.77 Electrospun nano/microfibers have many advantages, i.e. minimizing adverse effects and achieving sufficient therapeutic level at the targeted area, and there have also been advances in loading multiple drugs.78,79In the case of uniaxial electrospinning there are a few strategies for realizing multiple drug delivery. In the simplest, drugs are loaded in a single polymeric solution resulting in a more or less homogeneous polymer solution (s.c. blend solution). The main drawbacks of this method are related to the adverse effect of the solvent on drug bioactivity, and the initial rapid release of the drugs, mostly due to the presence of the active molecules near the fiber surface and further diffusion from the surface/centre.80–82 For example, Zhang et al.74 studied dual anti-cancer drug delivery, 5-fluorouracil and oxaliplatin, incorporated into PLA fibers. The system resulted in a burst release of drugs of 30% through the diffusion of drugs near the fiber surface. In another study, fibers were electrospun from the blend of PCL–gelatin loaded with ciprofloxacin HCL (CIP) and quercetin.83 Approximately 65% of the drugs was released in the first 15 hours, followed by sustained release of the remainder over 4 days. The initial burst release was caused by solubilization and leaching of drugs entrapped near or directly on the surface during electrospinning. The extended release was due to the longer diffusion path of the rest of the medicines in the fiber. In another study, co-delivery of quercetin and a bioceramic cuprorivaite (Cup) resulted in the relatively sustained release of quercetin in quercetin/Cup-loaded fibers (Fig. 3a).84 An advantageous element in this strategy is related to the situation in which the used drugs differ in hydrophilicity. Considering the single-solvent system, the simultaneous incorporation of hydrophilic and hydrophobic drugs is also a big challenge in optimum conditions.85 Li et al.86 developed PLGA single fibers loaded with hydrophilic polyphenol compounds and hydrophobic drug DEX within acetone/DMF. It was reported that the fast release of hydrophilic agents accelerated the release of hydrophobic drugs and resulted in a similar release time profile for both molecules. In another study, the effect of hydrophobic drugs on the hydrophilic drug in the PVA polymer matrix was investigated.87 However, hydrophobic levonorgestrel (LNG) made the hydrophilic tenofovir (TVF) release more slowly compared with TVF-only loaded fibers, with both cumulative releases completed in 4 hours. Considering these drug characteristics and choosing one appropriate solvent is the tacit limitation of uniaxial application for multiple drug loading.
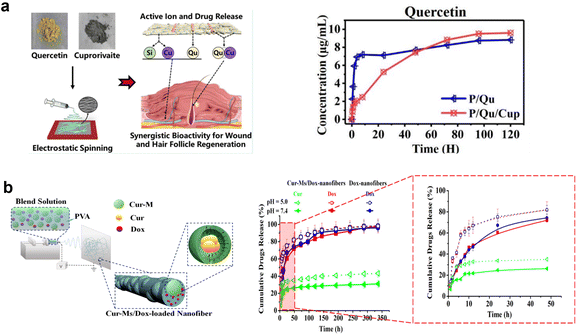 | ||
| Fig. 3 Schematic illustrations of drug-loaded electrospun fibers: (a) blend fiber. Adapted from ref. 84 with permission from American Chemical Society, Copyright 2020. (b) Second carrier in a fiber. Reprinted and adapted from ref. 91 with permission from John Wiley & Sons, Inc., Copyright 2014. | ||
To minimize the burst release of drugs from fibers electrospun from blends, the drugs are first encapsulated in various carriers such as nanoparticles or micelles.88,89 For example, a blend of PCL/gelatin was used as a matrix and mesoporous silica nanoparticles (MSNs) loaded with Alendronate sodium (ALN) were incorporated into the matrix.73 Compared with ALN in fibers without MSN, the release was much slower in the ALN–MSN-loaded fibers. Additionally, the surface coating was applied to MSNs to further prolong release in another study.90 Polymer micelles were loaded with hydrophilic and hydrophobic drugs simultaneously, and polymer micelles to improve the blend for electrospinning application were also developed. Yang et al.91 developed mono-methoxy poly(ethylene glycol)-block-poly(ε-caprolactone) (mPEG-PCL) polymer micelles to load curcumin, then loaded micelles incorporated into PVA polymeric matrix solution consisting of Doxorubicin (DOX) to be spun. The system resulted in a slower release of curcumin encapsulated in micelles regarding DOX in the first 12 hours (Fig. 3b). Table 1 shows some multi-drug-loaded uniaxial fiber studies from the literature, including a second carrier in single fiber. This table shows the polymers’ combinations affecting fiber diameter in the range of 80 nm–2 μm, with a release time of hours to days. Furthermore, the second-carrier advantage for prolonged release is seen obviously compared with single fibers. Research shows that PCL and PLGA were most favorable polyesters for these applications.
| Polymers | Drugs | Fiber diameter | Cumulative drug release time | Highlight | Reference |
|---|---|---|---|---|---|
| PCL with chitosan nanoparticles (nps) | Dexamethasone (DEX) in PCL | 370 ± 6 nm | 13 days | While DEX exhibited initial burst release of 30% in 12 h, AA exhibited smaller burst around 25% in 24 h. | Seddighian et al. (2021)111 |
| Ascorbic acid (AA) in chitosan nps | |||||
| PLLA | 6-Aminonicotinamide (6AN)/ibuprofen | 0.3 ± 0.1 μm | 9 days for 6AN, 5 days for ibuprofen | Release of 6AN is both drugs concentration dependent, release of ibuprofen is only 6AN concentration dependent | Schaub & Corey (2020)112 |
| PCL/gelatin | Quercetin/cuprorivaite (Cup) | N/A | 4 days | Cup plays an effective role in the sustained release of quercetin | Zhang et al. (2020)84 |
| PCL | Oxytetracycline (OTC)/zinc oxide (ZnO) | 350 ± 146 nm | 5 days | PCL-OTC/ZnO exhibited 120 h release with initial burst release of 31% in the first 10 h. On the contrary, PCL-OTC exhibited a 10 h release with initial burst release of 87% in the first 1 hour. | Dias et al. (2019)113 |
| PCL/gelatin with mesoporous silicate nanoparticle (MSN) | Alendronate (ALN) and silicate | 834 ± 33 nm | 35 days | MSN is effective in minimizing burst release of ALN. | Wang et al. (2019)73 |
| PVP | Amlodipine besylate/valsartan | 461 ± 72 nm at lowest drug concentration | 60 min | Both drugs showed rapid release profiles through the hydrophilic nature of PVP. | Bukhary et al. (2018)114 |
| 1270 ± 278 nm at highest drug concentration | |||||
| PCL | Acyclovir (ACY)/ciprofloxacin (CIP)/cyanocobalamin (B12) | 631 ± 344 nm | 150 hours for ACY, and 300 hours for B12, and CIP | The release times of CIP and B12 extended in multi-drug systems with respect to their single drug systems | Baskakova et al. (2016)115 |
| PLLA with MSNs | DOX and ibuprofen | 1.36 ± 0.32 μm | 90 days for DOX and 30 days for ibuprofen | MSNs-containing fibers showed steadier release than MSNs-free fibers. | Zhao et al. (2015)90 |
| PLGA | DEX/Green Tea Polyphenols (GTP) | ∼780 nm | 240 hours for GTP, 450 hours for DEX | The release rate of hydrophobic DEX gradually increased by increasing concentration of hydrophilic GTP | Li et al. (2014)86 |
| PVP | Paracetamol (PCM)/caffeine (CAF) | Varied in different drug amounts: 350–1900 nm | 175 h | Simultaneous sustained release for both substances. However, CAF release was slightly higher due to its higher solubility | Illangakoon et al. (2014)116 |
| Chitosan/PEO with PLGA nanoparticles | Vascular endothelial growth factor (VEGF)/platelet-derived growth factor-BB (PDGF-BB) | 116 ± 39 nm | 70 h for VEGF, 170 h for PGDF-BB | VEGF released 63% in burst in the first 1 h. PDGF-BB in PLGA nanoparticles released 28% in the first 2 hours. | Xie et al. (2013)65 |
Despite various more or less effective attempts to apply single-blend electrospinning techniques to multiple drug delivery, there are still serious problems that limit the practical use of this technique. First, sensitive molecules could lose their activity due to interaction with an organic solvent. Moreover, release time from the point of initial burst and uncontrolled release are still challenging points when considering the ideal therapeutic level. Although a second carrier relatively improved the release profile, distribution of the carriers is still limited. Therefore, novel DDSs such as multifluid electrospinning techniques need to be developed to prevent initial burst release, protect the activity of therapeutic agents, and achieve prolonged and well-controlled release profiles.
3. Mechanisms of drug release
In the nonwovens, the drug release profile is mainly related to the fiber composition (e.g., hydrophobicity/hydrophilicity of the polymer) and the arrangement of these different polymers into a single fiber in different architectures: monolithic, core–shell, and side-by-side fibers. Furthermore, specific polymer–drug interactions affect the mechanisms. In terms of the release profile, there are four main types: rapid, prolonged, stimulus-activated, and biphasic (two-stage manner) release.92 A rapid release profile is observed in single fibers consisting of a drug–polymer blend, because the drug molecules are located near the fiber surface. On the other hand, prolonged release can be achieved from more complicated nanofiber architectures, i.e. core–shell fiber structures with the shell layer as a barrier system, a blank middle layer between two layers loaded with bioactive molecules, or gradient distribution of the drug in each layer in multilayer fibers, such as triaxial systems (Fig. 4).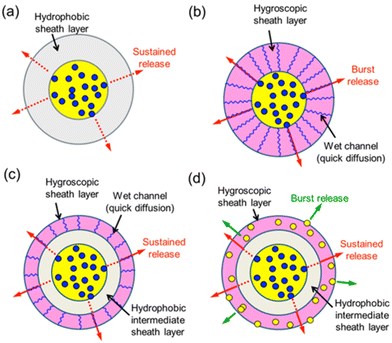 | ||
| Fig. 4 Schematic illustration of manipulation of release profiles by polymer characteristics and core–shell structure; (a) coaxial fiber with hydrophobic sheath; (b) coaxial fiber with hygroscopic sheath; (c) triaxial fiber with hygroscopic sheath; (d) triaxial fiber loaded with dual drugs. Reprinted from ref. 51 with permission from American Chemical Society, Copyright 2013. | ||
There are a few main mechanisms of drug release (Fig. 5a), but in the real-world situation of drug release, the overlap of various mechanisms described by different kinetic models and related equations is encountered. It is known that the real release profiles cannot be fitted by any single type of kinetic equation, since the release profile is a combination of different processes. The main mechanisms are related to drug diffusion, which can be divided into Fickian and non-Fickian type and erosion, and degradation of the polymer resulting in a higher rate of drug release.93 Considering the complexity of the situation, one should be aware of various specific situations related to the possibility, for instance, of polymer swelling, as well as to the more or less strong polymer–drug molecular interactions, affecting – sometimes seriously – the drug release kinetics. The first common situation is swelling in a highly hydrophilic polymer matrix, leading to the process in which diffusion of the drug through the swollen polymer, or erosion of the swollen polymer, should be taken into consideration. Whereas polymers with rapid/high swelling exhibit diffusion-controlled release, polymers with low swelling exhibit swelling-dependent release. Furthermore, in the case of non-swellable degradable polymers, drug release is controlled by erosion of the polymer surface.
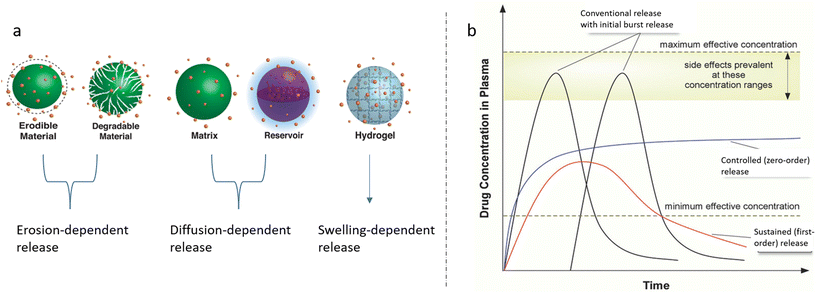 | ||
| Fig. 5 (a) Representative illustrations of release control mechanisms, i.e. erosion, diffusion, and swelling. Reprinted (adapted) from ref. 94 with permission from American Chemical Society, Copyright 2016. (b) Drug release profiles in terms of the drug concentration in plasma; conventional, sustained and controlled release. Reprinted (adapted) from ref. 97 with permission from John Wiley & Sons, Inc., Copyright 2018. | ||
Erosion-controlled release occurs by disruption of the surface of the material followed by polymer degradation, leading to open pores and related intense release.94 Additionally, drug release from non-biodegradable polymers is driven by the diffusion of the drugs through the matrix.95,96 For such diffusion-controlled DDS, the drug release rate is well correlated with the porosity of the polymer matrix, drug size and drug–polymer interaction, which imply the exudation of drug from the matrix. The overlapping mechanisms result in different types of release profiles such as conventional release by conventional treatment (tablet/injection), and controlled and sustained release by novel DDS (Fig. 5b).97 These mechanisms can be estimated by various kinetic models, including Fickian diffusion,98 which is based on Higuchi,99 Korsmeyer–Peppas,100 Hixon-Crowell, and first-order and zero-order reaction models.101 Since there are many studies in the literature on the kinetic models102–104 (Fig. 6a and b), this review focuses on the mechanisms related to multi-drug-loaded polymer systems.
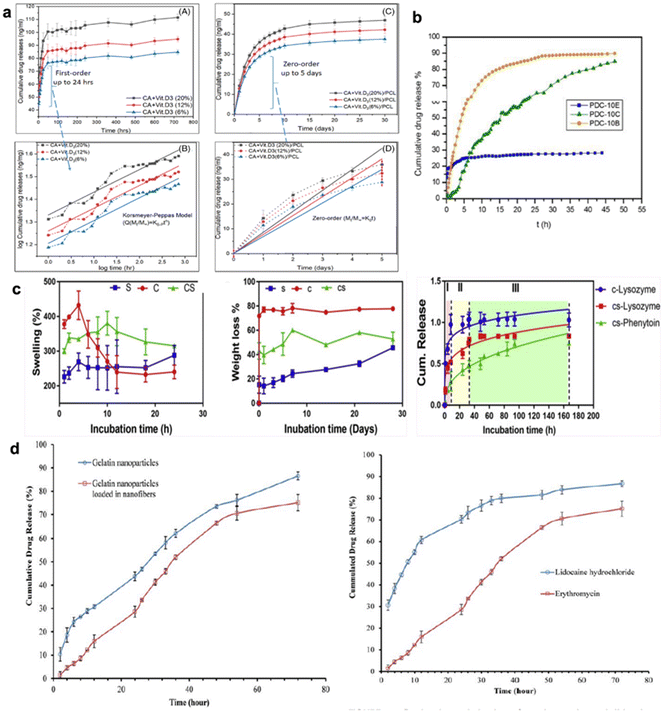 | ||
| Fig. 6 Schematic illustration of drug release profiles. (a) The release kinetics of vitamin D3 from CA and PCL fibers. Reprinted (adapted) from ref. 104 with permission from John Wiley & Sons, Inc., Copyright 2021; (b) the release profiles of Ciprofloxacin from PVA/dextran nanofibers developed by emulsion, blend, and coaxial electrospinning. Reprinted (adapted) from ref. 102 with permission from Elsevier, Copyright 2018; (c) drug release profiles core–shell (cs) and only core (c) in relation swelling and degradation of polymers. Reprinted (adapted) from ref. 118 with permission from Elsevier, Copyright 2020; (d) erythromycin release from particles and dual release of erythromycin and lidocaine from core–shell fibers. Reprinted (adapted) from ref. 120 with permission from John Wiley & Sons, Inc., Copyright 2015. | ||
Besides these main mechanisms, it is also worth noting that stimuli-responsive materials are considered in the development of smart delivery systems (modulated release) for effective drug release. Integrating stimuli-responsive materials into electrospun fibers is a challenging method to provide more controllable drug release and activity not only in vitro but also in the in vivo environment.105 Many kinds of system based on various stimuli can be developed, such as pH, temperature, light, ultrasound, magnetic field, electrical field, and combinations of these. The easiest way to develop such systems in electrospun fibers is surface modification.106 Another way is to use naturally environmentally responsive polymers such as chitosan, which is pH-sensitive; however, it is not easy to spin because of low solubility and stability.25,107 Furthermore, considering the thermal transition points of polymers, temperature-sensitive systems can be developed with suitable combinations, for example with P(NIPAAm) copolymers, PEG/PLGA block copolymers, and PEO/PPO block copolymers.108,109 Considering the human body and alongside some certain tissue and tumor areas, those systems sensitive to pH and temperature are the most important systems.110 On the other hand, internal stimulation is better way, rather than external triggers, because of some possible harmful effects on the body. Under certain circumstances, the polymer chain mobility increases, and links are cleaved resulting in the release of encapsulated therapeutics. Although this is an intelligent technology for effectively modulated release, there are some issues that need to be addressed such as which trigger method is the best for further clinical applications. Furthermore, its application is challenging from the viewpoint of multidrug delivery by multiaxial fibers, considering the complexity of the systems. Therefore, in multiaxial electrospun fiber systems, drug release is mostly currently modulated by the compositions of the fluids to be spun, drug–polymer interactions, and process parameters that influence the morphology of parameters. Here we discuss recent studies in the literature including multidrug release by multiaxial fibers.
Habibi Jouybari et al.117 worked on trilayer electrospun nanofibers including three different drugs – hydrophobic DOX, hydrophilic Paclitaxel (PTX), and 5-fluorouracil (5-FU) anti-cancer drugs – in each layer consisting of chitosan/polyvinyl alcohol (CS/PVA) as a core layer, and poly(lactic acid)/chitosan (PLA/CS) as a middle and shell layer. The release of 5-FU from the core occurs in a linear mode and is correlated with the sustained zero-order kinetic model, while the release of DOX and PTX, which are loaded into nanosheets in the shell layer, can be well described by the Korsemeyer–Peppas model, indicating that the role of the Fickian diffusion mechanism overlapped with other mechanisms such as erosion. The multilayer structure was compared with single fibres for each drug and the Fickian diffusion model was correlated with them. In another example, the poorly water-soluble drug ketoprofen (KET) inserted into the core and shell layer was analyzed.49 As in the study mentioned above related to the blank layer, a hydrophobic CA layer without drugs was placed between the drug-loaded CA core and PVP, which is a hydrophilic polymer shell layer. According to the pharmacokinetics results, KET release from the trilayer structure followed the Korsemeyer–Peppas model, indicating a zero-order kinetic model for sustained release, whereas the release from the core–shell without a blank middle layer was driven by Fickian diffusion. This shows that the release kinetics can be facilitated by loading the same active molecules into different layers in a multiaxial fiber. Furthermore, Zandi et al.118 studied a core–shell fiber consisting of gelatin (Gel) sheath and Gel/PVA core, loaded with phenytoin sodium as a drug molecule and lysozyme as a model protein, respectively. For each molecule, the release occurred in three stages including initial burst release, decreasing release rate, and finally steady-state conditions. The release mechanisms were driven by non-Fickian diffusion at the early stage and Fickian diffusion in later stages, through the dissolution of the PVA and swelling of the gelatin (Fig. 6c). Similar results were also reported by He et al.119 showing different release profiles of two drugs from one core–shell fiber drug reservoir. The core–shell structure was designed using PLGA loaded with hydrophobic metronidazole (MNA) as the shell layer and PVP loaded with hydrophilic naringin (NAR) as the core layer. MNA had a short-term quick release profile for one day because of the high biodegradability of PLA, whereas the NAR exhibited sustained and prolonged release for five days through the diffusion from the core layer. Some previous research for dual drug delivery using PLA single nanofibers loaded with 5-fluorouracil (5-Flu) and oxaliplatin was undertaken by Zhang et al.74 Both drugs exhibited a similar release profile, with initial burst release followed by constant release driven by the diffusion mechanism since the molecules are near the fiber surface. Another study using PCL/Gel single fiber for dual delivery was designed with a second carrier in the fiber structure.73 ALN, which is an osteoporosis agent, incorporated into MSNs can release silicate, showing angiogenic activity. In this system, the release kinetics of ALN for 40 days was driven by the slow degradation of PCL, while silicate release increasing gradually for 35 days was related to the hydrolysis of MSNs. In a different example using a second carrier, an antibiotic agent was loaded into gelatin nanoparticles (GNPs) and the GNPs were incorporated into a PVA/chitosan (CS)/lidocaine hydrochloride nanofibrous mat for dual delivery.120 Although the GNPs tended to rapidly swell and cause an initial burst release of loaded molecules, their incorporation into the core layer prevented the burst release of erythromycin (Fig. 6d). Moreover, the particle incorporation also decreased the swelling ratio of the PVA/CS/lidocaine nonwovens by increasing fiber rigidity. This was attributed to a decrement in the release rate of lidocaine in the dual system. It was reported that drug release from the shell is a closely fitted Fickian diffusion mechanism, while the release from the core is related to a non-Fickian mechanism and zero-order model based on a combination of diffusion and erosion mechanisms.
In summary, the release kinetics or mechanisms for multiple drug delivery cannot be generalized. They vary with regard to drug type, such as hydrophilicity, molecular size, and polymer characteristics; and biodegradability, hydrophobicity, and swellability. Furthermore, drug carrier architecture, which means core–shell, single fiber or second carrier, in a fiber is quite effective in determining which release mechanism or kinetics is the major one, or how the mechanisms appear sequentially. Note that instead of a single-fiber structure as a carrier, multiple-fiber designs such as core–shell, side-by-side or second carrier in the fiber, based on loading drugs into different carriers, could be more effective for two-stage controlled prolonged release of multiple drugs. According to the application's aim, the whole system could be designed considering the abovementioned criteria.
4. Current multifluid electrospinning methods for delivery
4.1 Janus fibers
Janus fibers represent side-by-side electrospinning technology, allowing the production of Janus nanoparticles and Janus nanofibers.121 In the beginning, a conventional side-by-side spinneret, which means two parallel capillaries, was used for production. Later, the special side-by-side spinneret, i.e., acentric spinneret, where the two sides combine into one full circle, allowing more robust and continuous production, was developed48,122–125 (Fig. 7A). Two-sided fibers improve functionalization compared with one monolithic fiber, because they have two physicochemically distinct surfaces responsive to hydrophilicity/hydrophobicity, magnetic properties, pH or thermal sensitivities.126–128 This allows the design of their own (different) functional features on both sides.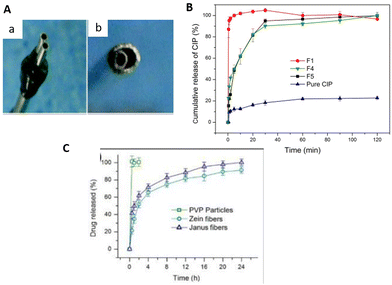 | ||
| Fig. 7 Janus fiber spinnerets and comparison of drug release profiles. (A) Conventional side-by-side spinneret (a); acentric spinneret (b). Reprinted (adapted) from ref. 123 with permission from the Royal Society of Chemistry. (B) CIP release from monolithic (F1); only CIP loaded Janus (F4); CIP and AgNPs loaded Janus (F5) fibers and pure drug particles. Reprinted (adapted) from ref. 48 with permission from Elsevier, Copyright 2020. (C) Cumulative FA release from PVP particles, single Zein and PVP–Zein Janus fibers. Reprinted (adapted) from ref. 131 with permission from Elsevier, Copyright 2020. | ||
For example, Yang et al.48 studied an anti-bacterial wound-dressing application using Janus nanofibers. The aim of their study was to improve the activity against bacteria by loading with two different agents. They used PVP and EC as polymer matrices for the sides of the Janus fiber loaded with ciprofloxacin (CIP) and silver nanoparticles (AgNPs), respectively. While >%90 of the CIP was released in the first 30 min, AgNPs extended the anti-bacterial activity for 72 h, which is quite effective in the first 24 h for infected wounds. As a result, they achieved a synergetic effect, providing initial fast action and long-lasting anti-bacterial activity using Janus fibers loaded with dual active agents (Fig. 7B). Another study was performed by Li et al.129 to investigate the double drug release behavior for combined therapy using Janus fibers in the form of a beads-on-a-string structure. They used KET and methylene blue (MB) as model drugs and incorporated them into the sides of PVP and EC as polymer matrices. According to the results of in vitro drug dissolution tests, the PVP–MB side exhibited rapid release for 0.5 h, while the EC–KET side showed a more sustained release for 24 h. In addition, another study using the same polymers was presented by Yu et al.130 for a different strategy in terms of drug release. In this study KET was incorporated into each side of the Janus structure, aiming to achieve a two-stage controlled release through the different hydrophobicity of each side. The PVP side dissolved quickly and exhibited rapid release for fast treatment, and EC provided a longer release time because of its higher hydrophobicity. This kind of strategy was also reported by Wang et al.131 with zein and PVP for Janus fiber sides which were both loaded with drug molecules. This group presented the controlled release of FA (ferulic acid), a poorly soluble drug molecule, in a two-stage manner by the difference in the hygroscopic structure of the polymer matrix (Fig. 7C). Table 2 summarizes some Janus fibers used for dual delivery from the literature. The table shows that the research is mostly related to drug models such as dyes and one drug inside two sides. In general, controlled release can be driven by wettability differences in polymers on each side for Janus systems. PVP, a hydrophilic matrix, is an extensively used pharmaceutical polymer among the developed systems. As a hydrophobic matrix, EC is also a commonly used pharmaceutical polymer, and both are biocompatible. This kind of system can provide tunable release profiles from the viewpoint of biphasic release to provide effective therapeutic activity according to the desired application. However, the release time for the systems is relatively short, of not more than 120 hours, as can be seen in the examples.
| Polymers | Drugs | Fiber diameter | Cumulative drug release time | Highlight | Reference |
|---|---|---|---|---|---|
| PVP | MB (-PVP) | 560 ± 30 nm | 0.5 h for MB | The PVP-MB side exhibited a typical erosion mechanism, while the EC-KET side was driven by a diffusion mechanism | Li et al. (2021)129 |
| EC | KET (-EC) | 24 h for KET | |||
| Sodium alginate (NA-Alg) | Minocycline hydrochloride (MH) | 3.5–15 mm | 120 h | Increment in fiber diameter led to decrease in drug release rate | Lai, Huang and Lui (2021)146 |
| Chitosan and carboxymethyl cellulose sodium (CMC-Na) | (NA-Alg) | ||||
| Malachite green (MG) | |||||
| (CMC-Na) | |||||
| PVP | Ciprofloxacin (CIP) | 840 ± 240 nm | 0.5 h for CIP | Immediate release of CIP provided strong anti-bacterial effect and AgNPs extended anti-bacterial action. | Yang et al. (2020)48 |
| EC | (-PVP) | 72 h anti-bacterial activity via AgNPs | |||
| Silver nanoparticles (AgNPs) (-EC) | |||||
| PVP | FA (both sides) | 570 ± 160 nm | 24 h | Biphasic release was obtained where the PVP side exhibited fast release for short term effect and Zein side provided further release. | Monglong Wang et al. (2020)131 |
| Zein | |||||
| PCL | Acriflavine (-PCL) | 17–22 μm | 4 days | Rapid release of Rh B was observed in first 6 h whereas the Acriflavine release extended over 4 days. | Yao et al. (2019)147 |
| PVP | Rh B (-PVP) | ||||
| PVP | Helicid (both sides) | 630 ± 110 nm | 30 min | It was achieved a fast release by improved hydrophilicity for rapid treatment | Wang et al. (2018)122 |
| PVP | DEX (both sides) | 250–400 nm | 14 h | Biphasic drug release, with an initial burst release followed by a sustained release due to the solubility differences of both polymers. | Geng et al. (2017)148 |
| PAN | |||||
| PVP | KET (both sides) | 600–630 nm | 24 h | It achieved two-stage controlled release due to the hydrophobicity difference in two sides. | Yu et al. (2016)130 |
| EC |
The two sides of the Janus fiber interact with the environment at the same time, and this could be useful for faster treatment and higher dosage release in a short time if needed. However, simultaneous interaction of each side with the environment is also a significant challenge from the viewpoint of sequential release and longer release time.132 Furthermore, regarding the side-by-side production process, the fluids need to be of similar characteristics in terms of conductivity, spinnability, and surface tension for the smooth production and perfect integration of the Janus fibers. These reasons restrict the material type, and thus the functional applications.48,122,131 At this point, the core–shell structure, allowing the use of wide-ranging fluids, including unspinnable ones, overcomes these limitations.
4.2 Coaxial electrospinning
To overcome the limitations of uniaxial fibers, core–shell fibers have been under development. These structures can be produced by electrospinning immiscible polymer blends using a coaxial nozzle or emulsion through a single nozzle. The first coaxial electrospinning was reported in 2002.133,134 Based on the conventional electrospinning setup, coaxial electrospinning has two spinnerets as inner and outer, which means two polymers’ solutions or liquids are pumped by two syringes, allowing the formation of core–shell fibers. In the beginning, the core layer was loaded with a drug to provide a longer release and protect the drug from the environment. The idea has been developed in particular for poorly water-soluble drugs which are encapsulated in fast-dissolving fibers. By using the coaxial technique these structures are covered with a shell layer to delay drug release. Among the recent studies using different modifications, there is drug-loaded hydrophilic PVP, a common polymer used, covered with hydrophobic poly(3-hydroxybutyric acidco-3-hydroxyvaleric acid) (PHBV), a natural component of blood, as shell layer.135 In another study, sucrolose, as a newer strategy, was used to modify the hydrophilicty of PVP on the shell layer.136 These strategies reported a longer release for coaxial fibers. Moving forward, this issue still is being researched from different perspectives using different material systems, such as electrospun nanohybrids including drug–crystalline nanoparticles,137 and drug–polymer composites.138Besides, coaxial electrospinning has been frequently used for multidrug delivery for the effective treatment of complex diseases. Drugs have been incorporated into the core and shell to simultaneously provide a synergistic drug76 effect and preserve bioactivity.139 This fiber architecture allows slower release from the core part and faster release from the shell layer.140 Usually, this type of nanofiber needs additional modifications to increase the stability of the structure/properties, such as reducing hydrophilicity/solubility in aqueous media. This is a case where natural polymers like collagen or gelatin, which are highly hydrophilic or soluble, result in a very rapid release.141–143 Many studies use coaxial electrospinning to load multiple bioactive agents and improve biocompatibility. Su et al.63 have developed poly(L-lactide-co-caprolactone) (PLLACL), PLLACL/collagen coaxial electrospun nanofibers loaded with two growth factors to promote bone tissue regeneration. They have compared blended and core–shell fibers in terms of the agent release, and reported that the core–shell structure showed more controlled release for 22 days over the blended ones (Fig. 8A). In addition to these, while the agent in the shell layer showed an initial burst release, the agent in the core layer exhibited steady long-term release. As a result, loading two growth factors provided sufficient activity for the whole period. In another study, PCL and collagen were used to fabricate core–shell nanofibers where the PCL is the core, providing mechanical support, and collagen is the shell, providing biocompatibility for a wound-dressing application.144 The shell layer was loaded with an anti-bacterial agent – Ag nanoparticles – and the core layer was loaded with Vitamin A (VA), a healing-promoting drug. The system resulted in the controlled release of VA within 72 h and the anti-bacterial activity of Ag nanoparticles. The synergistic effect of both agents provided an improvement in therapeutic effect. Another dressing strategy was developed by Chen et al.145 using HA/PCL nanofibrous membranes loaded with Ag nanoparticles. The core solution was prepared using Ag particles-embedded HA to provide anti-infection and anti-adhesion activity around the wound site, while the shell solution was PCL. Due to the hydrophobic shell, excessive initial release was prevented, and cumulative prolonged release was observed for 35 days.
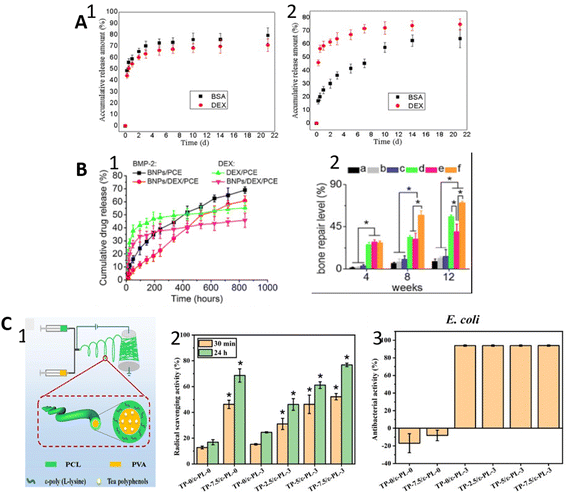 | ||
| Fig. 8 Schematic illustrations of the results of some studies. (A) The release profiles of BSA and DEX from uniaxial (1) and coaxial fibers (2). Reprinted (adapted) from ref. 63 with permission from Elsevier, Copyright 2012. (B) BMP-2 and DEX release profiles of their single delivery and dual delivery (1); bone repair level of for control group without material (a), PCE (b), NPs/PCE (c), BNPs/PCE (d), DEX/PCE (e), BNPs/DEX/PCE (f) (2). Reprinted (adapted) from ref. 149 with permission from Elsevier, Copyright 2015. (C) Production of coaxial fiber loaded with two therapeutic agents (1); antioxidant activity of coaxial fibers with different concentration of agents (2); anti-bacterial activity of coaxial fibers with different concentration of agents (3). Reprinted (adapted) from ref. 85 with permission from Elsevier, Copyright 2021. | ||
Nanofibers with the additive of nanoparticles for bone morphogenetic protein-2 (BMP-2) and DEX delivery were reported.149 BMP-2-loaded bovine serum albumin (BSA) nanoparticles stabilized with chitosan shell within DEX-loaded PCL/PEG co-electrospun fibers resulted in controlled two-stage release kinetics. Due to the multi-barrier structure protection, BMP-2 exhibited prolonged release similar to the zero-order release pattern for 35 days, whereas the DEX release was completed within 8 days. Therefore, DEX provided an immediate effect on bone repair while BMP-2 supported new bone formation for the long term. In comparison with only DEX- and only BMP-2-loaded fibers, the synergistic effects of the dual drug-loaded fibers displayed improvement on bone repair level (Fig. 8B). Based on these functional improvements, researchers have carried out various studies over the years.31,150–154 Herein, a quite new study was reported by Lan et al.85 for sequential delivery of natural active agents, which are an antioxidant tea polyphenol (TP) and anti-bacterial ε-poly(L-lysine) (ε-PL). The material system consisting of a PCL shell loaded with ε-PL and PVA core loaded with TP resulted in a two-stage controlled release for 96 h. They suggested that high initial release of ε-PL prevents bacterial growth in the early stage, followed by a longer antioxidant effect with sustained release of TP because of the shell layer. Compared with the single agent systems, co-delivery of both agents showed a greater effect in terms of the anti-bacterial and antioxidant activity (Fig. 8C). Among the recent new strategies, He et al.155 developed a spindles-on-a-string hybrid modifying coaxial electrospinning. Small-molecule solutions containing PEG and ibuprofen were used for treatment, along with core solutions containing EC and ibuprofen. They reported that the system provided a biphasic release profile in which there was a fast release through erosion of PEG and an extended release through diffusion from EC. Many combinations of multiple drug delivery in the literature use coaxial electrospinning (Table 3). While a few studies use the same material for core–shell, combinations of different materials are often used to manipulate the release profiles. The relocation of the drug in the layers has been investigated to achieve the target effect. Also analyzed is the effect of the second carrier such as MSN and BSA in the shell or core layer. Furthermore, various therapeutic agents such as drugs, natural antioxidants, growth factors, and organic/inorganic particles are reported. Cumulative drug release is achieved up to five weeks, and this is a considerably prolonged release time compared with Janus fibers.
| Polymer | Drug | Fiber diameter | Cumulative drug release time | Highlight | Reference |
|---|---|---|---|---|---|
| Core: c; shell: s. | |||||
| PCLs/PVAc | Tea polyphenol (TP)c/ε-poly (L-lysine) (ε-PL)s | 450–550 nm | 96 h | Sustained release was observed for both. However, ε-PL displayed faster release than TP due to the shorter diffusion path. | Lan et al. (2021)85 |
| PLGAs/PVPc | Pirfenidones/moxifloxacinc | 630 ± 300 nm | 1 h for Moxiflaxacin, 5 h for Pirfenidone | Mox showed rapid release of 60% in 30 min due to its and PVP'S hydrophilicity. Entrapment of Mox demonstrated sustained release is possible over a period of hours. | Tawfik et al. (2020)154 |
| PLAs,c | BMP-2c | 631 ± 158 nm | 72 h | TUDCA exhibited initial burst release 65% in fisrt 12 h, while BMP-2 showed sustained release without burst release. | Bhattarai et al. (2020)161 |
| Tauroursodeoxycholic acid (TUDCA)s | |||||
| PCLc | Curcumin (Cur)c | 328 ± 64 nm | 72 h | A rapid release of Lid and a sustained release of Cur were reported, while both pH responsive profile displayed in pH 5.4. | Guo et al. (2020)162 |
| Chitosan/polyethylene oxide (PEO)s | Lidocaine hydrochloride (Lid)s | ||||
| PLGAs | BSAc | 219 ± 107 nm | 18 days | Controlled release was achieved for both molecules. Core fluid composition and processing parameters are effective on controlling release behavior. | Wang and Windbergs (2019)31 |
| Acyclovirs | |||||
| PVPc | NARc | 1.73 ± 0.4 μm | 8 days | MTZ displayed initial short-term release, while NAR showed sustained long-term release. | He et al. (2019)163 |
| PLGA–PLLA–PDLLAs | MTZs | ||||
| PCLc | Icariin (ICA)c | 400–800 nm | 30 days | 50% MOX demonstrated a burst release in first 24 h, while only 20 ICA showed a burst release followed by slow sustained release. | Gong et al. (2019)164 |
| Gelatins | Moxifloxacins | ||||
| PLCL/Collogens | Salvianolic acid B (SAB) in MSNs | 821 ± 162 nm | 30 days | Both drug displayed sustained release where the SAB-MSN achieved a stable release without burst release | Kuang et al. (2018)165 |
| Heparinc | |||||
| PLLAc | BMP-2c | 335 ± 69 nm | 21 days | DEX showed early rapid release of 37.8% within 12 h, while BMP-2 exhibited a steadily increasing profile. | Li et al. (2018)166 |
| Zeins | DEXs | ||||
| PVA/gelatins | Bromelain (Br)s | 400–500 nm | 120 hours | Both drugs demonstrated sustained release for 120 hours where the Br of 75% released within 1 day while SAB of 50% released within 2 days. | Shoba et al. (2017)167 |
| PCLc | SABc | ||||
| PCLs | Silvers | 80–120 μm | 5 weeks | Sustained release which was started with initial burst release was observed for both drugs | Chen et al. (2017)168 |
| Pluronic-F 127c | Gentamicinc | ||||
| PCLc | Resveratrol (RSV)c | 240 ± 50 nm | 120 hours | RSV showed slower release than FA due to its incorporation into the core in which a sustained release pattern was observed for both molecules. | Poornima and Korrapati, (2016)169 |
| Chitosans | Ferulic acid (FA)s | ||||
| PLGAs | BMP-2c | 201 ± 76 nm | 35 days | All bioactive molecules exhibited a similar sustained release profile. | Hsu et al. (2016)170 |
| Vancomycin and ceftazidimes | |||||
| PCL-co-poly(ethylene glycol) (PCE) copolymers | BMP-2 in BSA npsc | 350–400 nm | 8 days for DEX, 35 days for BMP-2 | Due to the protection by the multi barrier structure, BMP-2 exhibited a gentler release pattern similar to a zero-order release pattern. | Li et al. (2015)149 |
| Chitosanc | DEXs | ||||
| PCLs | Ag npss | 344 ± 92 nm | 4 days for Ag, 21 days for HA | Hydrophobic PCL sheath prevented the initial burst release of highly hydrophilic HA. | Chen et al. (2015)145 |
| PEOc | Hyaluronic acid (HA)c | ||||
| PLGA/Collogens | Fibronectin (FN)/cadherin 11 (CDH)c | 465 ± 138 nm | 15 days | The release rate of drugs was concentration independent in which the drugs of 25% released in first 24 h followed by a sustained release. | Wang et al. (2014)151 |
| PVAc | |||||
| Gelatin/PCL-co-poly-(ε-caprolactone) (PLLCL)s | Multiple epidermal induction factors (EIF) embedded in BSAc | 366 ± 125 nm | 15 days | No initial burst release was observed for core–shell structure while the single blends exhibited burst release within 3 days. | Jin et al. (2013)171 |
| Poly(L-lactide-co-caprolactone) (PLLACL)/collagens | BMP-2c | 337–365 nm | 22 days | The DEX exhibited a more dramatic initial burst release than BMP-2 within 2 days. | Su et al. (2012)63 |
| DEXs | |||||
Despite great progress, coaxial electrospinning also has some restrictions. Reports have already demonstrated that coaxial fibers play an essential role in preserving the bioactivity and contribute to the sustained release of the loaded molecules as compared with conventional spinning and Janus fibers. However, some matters still need to be improved to achieve superior results in drug delivery applications: enhancing the solubility of insufficiently water-soluble drugs, further protecting the molecules, and promoting the sustained and controlled release. Therefore, research groups are exploring and developing alternative techniques, e.g., triaxial electrospinning techniques integrating the middle layer between the core and shell layers for multiple drug treatments.
4.3 Triaxial electrospinning
In the triaxial electrospinning process, triaxial needles made of three concentric standard needles are used to simultaneously infuse up to three different fluids pumped by three syringe pumps. This system allows the use of different solvents, polymers, or only bioactive molecule solutions in the core. The first study about triaxial electrospinning aiming at core–shell-manner fiber manufacturing was reported in 2007 by Lallave et al.156 The shell layer was pure ethanol as a lubricating agent to avoid solidification of concentrated solutions on the spinneret by compensating for solvent evaporation during the spinning process. Furthermore, not all the solutions used in triaxial electrospinning need to be electro-spinnable. Material choice and system parameters vary in accordance with the aim of the application. Since the system is complicated and not yet investigated completely, it still needs to be researched more deeply. On the other hand, as core–shell nanofibers produced by triaxial electrospinning have more material fractions than conventional ones, this may be a more technically powerful approach to providing functionality to materials.157,158 In the literature, there are reports highlighting the advantages of the triaxial method over the coaxial one. Many studies have preferred natural polymers such as gelatin and collagen as a sheath layer due to their biocompatibility, but this situation leads to burst release due to the high hydrophilicity of these materials. A hydrophobic intermediate layer is placed between the core and outer layer to solve this problem.51 Similarly, in a more recent study, Yang et al.49 have stated that a blank (without drug) polymer layer inserted as an intermediate layer between two polymer layers loaded with drug molecules resulted in sustained and longer release time with linear release kinetics. These authors worked with coaxial and triaxial fibers as drug vehicles. Both coaxial and triaxial fibers were composed of the same materials, but a blank cellulose acetate (CA) layer was used as a middle layer in the triaxial fibers (Fig. 9A). A great advantage of CA as a blank layer is related to its biodegradability and the abundant availability of cellulose.53,159,160 According to the results of the release kinetics of the drug molecule, the triaxial structure exhibited longer sustained release and linear release kinetics over the coaxial fibers. It has been suggested that the blank polymer layer could play a vital role in evolving the discrete drug distribution within the nanofiber. The general idea is that when the natural and hygroscopic layer is used for the shell to obtain appropriate biocompatibility, using a hydrophobic intermediate layer forces the diffusion of active molecules from the core through the intermediate layer and results in longer sustained release instead of rapid release. This kind of developed structure and polymer type are just one of the ideas that have encouraged the development of the triaxial production method in terms of drug release.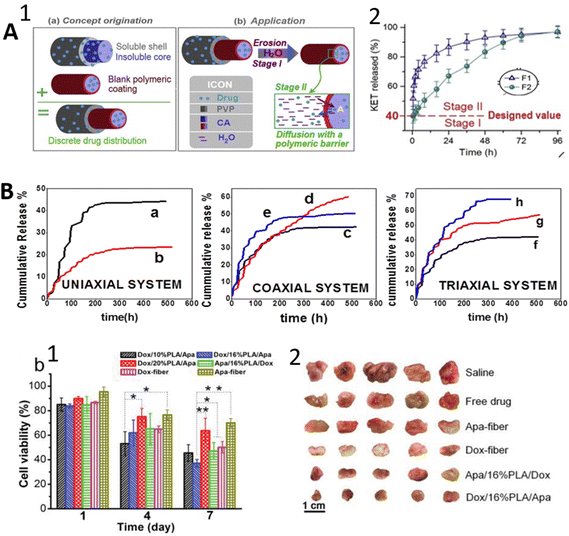 | ||
| Fig. 9 Schematic illustrations of the results of some studies. (A) The structure of trilayer fiber with discrete drug distribution and release mechanisms (1); release profile of KET from coaxial (F1) and triaxial (F2) (2). Reprinted (adapted) from ref. 49 with permission from Elsevier, Copyright 2020. (B) Comparison of three systems where the (a) BSA-FITC release from uniaxial PLGA electrospun fibers; (b) RhB release from uniaxial PLGA electrospun fibers; (c) BSA-FITC release from coaxial PLGA and gelatin electrospun fibers; (d) BSA-FITC release coaxial PLGA with RhB and gelatin with BSA-FITC electrospun fibers; (e) RhB release from Coaxial PLGA with RhB and gelatin with BSA-FITC electrospun fibers; (f) BSA-FITC release from triaxial PLGA, gelatin with BSA-FITC and PCL electrospun fibers; (g) RhB release from triaxial PLGA with RhB, gelatin with BSA-FITC and PCL electrospun fibers; (h) BSA-FITC release from triaxial PLGA with RhB, gelatin with BSA-FITC and PCL electrospun fibers. Reprinted (adapted) from ref. 158. (C) (1) In vitro cell cytotoxicity test on cancer cell line of single and triaxial fibers with different drug locations and middle layer concentration; (2) ex vivo tumors treated by single and triaxial fibers with different drug locations and middle layer concentration. Reprinted (adapted) from ref. 174 with permission from John Wiley & Sons, Inc., Copyright 2019. | ||
Another advantage of the triaxial method is that it provides highly controlled release of multiple drugs by arranging different drugs into individual layers.52 In particular, complex regenerative processes need to combine multifarious therapies involving drugs, proteins, growth factors, genetic materials, and cells.64–68 It is possible to place a wide variety of drugs in the core and the shell to form an intermediate layer providing a barrier structure. Such a scheme would prohibit drug–drug interaction and each molecule would release at a different time. Therefore, they would exhibit synergistic effects at relevant times properly. Besides, the incorporation of drugs into layers could be decided according to the functional aim of the nanofibers’ application.
The broader possibility for multiple drug loading also makes this method very attractive, considering that multiple-drug treatment can be more effective in targeting an ideal therapeutic effect than conventional single-drug treatment.77 Among different kinds of drug delivery systems-nanoparticles, hydrogels, micelles, conventional electrospun fibers- novel triaxial electrospun fibers have been of great interest for simultaneous multiple drug loading due to the characteristics mentioned above.
It has been shown that such a three-layer structure contributes to the mechanical properties of the developed fibers. It has been reported that triaxial nanofibers exhibited a huge increment in mechanical properties compared with coaxial nanofibers.55 Among recent studies, Nagiah et al.158 worked on triaxial nanofibers using a strong core to improve the mechanical properties while loading drugs into the sheath and intermediate layer for multiple simultaneous drug releases. They used PCL for a mechanically strong core, and for incorporating different drugs PLGA and gelatin were used as shell and the intermediate layer, respectively. Rhodamine B was incorporated into the sheath layer for the model drug molecules, and fluorescein isothiocyanate (FITC) Bovine Serum Albumin (BSA) was loaded into the intermediate layer. Their results showed that triaxial fibers exhibited enhanced mechanical properties over coaxial ones with a PLGA shell and gelatin core. Multiple drugs in two coaxial and triaxial fibers improved the cumulative release for 24 days, with the small molecule Rhodamine B in the sheath layer and larger molecule FITC-BSA in the intermediate layer (Fig. 9B). Additionally, it was observed that the initial burst release was relatively decreased in triaxial fibers. In another study, a triaxial methodology was used to provide sustained release by using a drug-loaded core and thin-layer coating for the intermediate layer.53 In this study, coaxial and triaxial fiber samples were compared in terms of drug release, and it was reported that triaxial fibers prevented the initial burst release over the coaxial fibers regardless of the thickness of the coating layer. Besides, unspinnable fluids as a mixture of solutions of acetic acid and acetone as outer and blank cellulose acetate layer as middle were used. For the core layer, a poorly soluble antioxidant molecule FA-loaded protein (gliadin) solution, which is spinnable, was used to produce high-quality triaxial fibers. Compared with the coaxial system with the same materials, the new system provided prolonged release, depending on shell thickness, for 48 hours and eliminated initial burst release. Thus, a new modified triaxial structure has been suggested in this study. From the previous studies, Han and Steckl (2013)51 worked on dual drug loading into the shell and core layer of triaxial fiber by using the intermediate layer as a barrier. They used two different dyes – keyacid blue and uranine (KAB and KAU) – to simulate the drug release profiles and worked with the combination of PCL and PVP (polyvinylpyrrolidone) as fiber materials. It was observed that triaxial fibers exhibited 24 times slower release over the coaxial fibers loaded with the same molecules. Additionally, it was suggested that this developed structure provides quick treatment by rapid release from the sheath layer and long-term treatment by sustained release from the core layer. In a quite new study, Xu et al.172 reported a comparison of coaxial and triaxial loaded fibers with the same active molecule, model drug metformin hydrochloride (MET), from the point of sustained release and initial burst release. In contrast with the study mentioned above, electrospinnable solutions of CA for the inner and middle and non-electrospinnable solvent for an outer layer were used to manufacture non-defective fibers. According to the results, the triaxial structure eliminated the initial burst release and provided a longer sustained release of around 24 hours, while the coaxial ones provided a release of around 12 hours. Unlike these, as a different kind of strategy, Yu et al.173 studied drug gradient distribution using the same drug, KET, with different concentrations in each layer of triaxial fiber produced from ethyl cellulose (EC) as a fiber-forming matrix for three layers. The drug content in each layer gradually decreased from the inner to the outer layer to provide a release gradient. These authors compared triaxial and monolithic fibers in terms of sustained drug release (zero-order kinetics) and initial burst release. There was no observed initial burst release for the triaxial fibers, while the monolithic fibers exhibited rapid release then continued to slow. The triaxial fibers had a constant slow rate release for almost one day. A different study combining three different drugs was presented by Habibi Jouybari et al.117 To provide simultaneous controlled release of different drug molecules, Doxorubicin (DOX), Paclitaxel (PTX), and 5-fluorouracil (5-FU) anti-cancer drugs were incorporated into triaxial fibers composed of PLA, PVA (polyvinyl alcohol), and chitosan in combination. In this combination, the intermediate layer was a blank layer without a drug, the core layer was loaded with one drug, and the shell layer was loaded with nanosheets carrying two drugs. The strategy resulted in controlled release for around 600 hours for three drugs without initial burst release in comparison with the single-blend ones. Among recent studies, chemotherapeutic drug DOX in the inner cavities within the fiber and the tumor vascular inhibitor apatinib (Apa) in the outer fiber matrix were combined in a trilayer fiber architecture consisting of glycerol–PLA–PCL layers.174 The actual aim was to compare the effect of drug release sequence with the triaxial fiber-loaded Apa inner and Dox outer. In vitro and in vivo results showed that Dox/PLA/Apa, with the Apa in the outer layer, is more effective for tumor inhibition for a longer time (Fig. 9C). Furthermore, the synergetic effect of the drugs was achieved through time-programmed sequential delivery due to the position of the drugs in the fiber. On the other hand, co-delivery of two therapeutic agents resulted in higher anti-tumor activity than their single-drug-loaded fibers.
Another study was performed by Wang et al.,175 who used a fiber-forming polymer matrix, CA, for the intermediate layer and used shell and core layers as a drug reservoir for Acyclovir, which is an antiviral drug. However, they used only drug–solvent mixing for the core, whereas the shell was a drug–polymer composite. They reported that the triaxial system eliminated the initial burst release and prolonged the sustained release over the coaxial system loaded with the same active ingredients. A similar study for multiple drug release was performed by Huang et al.176 They used a drug–polymer composite as a middle layer and only drug in a solvent as the core layer, where the shell layer was pure solvent in which EC was used as a filament-forming matrix and KET was used as a model drug. They achieved a constant drug release rate without burst release when compared with monolithic fibers loaded with the same drug molecule. It has been suggested that this research showed that the drug-loaded shell section provided better-sustained release in terms of zero-order release kinetics over the drug-free shell section studied previously by Yang et al.159 Besides, due to the complexity of the method and the variety of parameters, it is hard to determine one precise manufacturing strategy, and studies of diverse strategies are ongoing. In contrast with the aforementioned method, Hou et al.177 used a drug-free solvent-based shell layer, drug–polymer composite middle layer, and drug–liquid inner layer to produce high drug-loading triaxial fibers, using CA and ferulic acid (FA) for fiber-forming matrix and drug model, respectively. They achieved a drug loading of around 72% and clear sustained drug release for 48 hours. Considering all these, triaxial fiber production and drug incorporation into the fibers have resulted in many suggestions, such as polymer characteristics, active ingredient type, production conditions, and drug release strategies. As shown in Table 4, systems gradient/discrete distributions have been tried in which a single drug is placed in more than one layer in triaxial fibers or blank layer placement has been studied for prolonged release.
| Polymer | Drug | Fiber diameter | Cumulative drug release time | Highlight | Reference |
|---|---|---|---|---|---|
| Core: c; middle: m; shell: s. | |||||
| CAc,m | METc,m | 560 ± 75 nm | 24 h | Sustained release with almost no initial burst release was observed. | Xu et al. (2021)172 |
| Solvent mixture of acetone, N,N-dimethyl acetamide (DMAc), and ethanols | |||||
| PCLc | Rh Bs (FITC-BSA)m,c | 1.16 ± 0.23 μm | 24 days | The addition of hydrophilic RhB in the sheath led to a higher release of BSA-FITC from core/intermediate layers whereas the sustained release was observed in the whole system. | Nagiah et al. (2020)158 |
| PLGAs | |||||
| Gelm | |||||
| Glycerolc | DOXc | ∼1.31 μm | 12 days for DOX | Rapid release of DOX is achieved by rupturing of internal cavities of fiber, sustained release of Apa is obtained by slow degradation of shell polymer | Li et al. (2020)174 |
| PLAm | Apas | 36 days for Apa | |||
| PCLs | |||||
| Ethyl cellulose (EC)m | Ketoprofen (KET)m,c | 640 ± 130 nm | 32 h | In the core-shell system, using a drug loaded-shell was able to provide better zero-order release than drug free-shell. | Huang et al. (2020)176 |
| Pure solvents | |||||
| CAm,s | Acyclovir (ACY)m,c | 670 ± 70 nm | 48 h | A sustained release without burst release was achieved | Wang et al. (2020)175 |
| CAm | FAm,c | 760 nm ± 130 nm | 72 h | The initial burst release effect was completely eliminated in which zero-order kinetic release was observed. | Hou et al. (2020)177 |
| Solvent mixtures | |||||
| Chitosan/polyvinyl alcohol (CS/PVA)c | DOX/PTXs | 200–500 nm | 600 h for DOX and PTX | DOX and PTX release decreased with the increment in shell thickness where the 5-FU release profile followed by zero-order release behavior. | Habibi Jouybari et al. (2019)117 |
| PLA/CSm,s | 5-FUc | 700 h for 5-FU | |||
| CAm | Ibuprofenc | 700–900 nm | 60 h | When the CA layer thickness increased, release time extended. Minimal initial burst release was observed independent of the thickness. | Yang et al. (2019)160 |
| Pure solvents | |||||
| PCL/Gels,c | Doxc | 30.0 ± 17.0 μm | 24 h | Release behavior can be controlled by permeability of material where the combining hydrophilic–hydrophobic layers in fiber. | Khalf and Madihally (2017)30 |
| Gelm | |||||
| ECc,s,m | KETc,s,m | 740 ± 60 nm | 20 h | A linear release profile without burst release was achieved. | Yu et al. (2015)173 |
| PVPc | KABc | 648 ± 259 nm | 25 h | A rapid release from the shell and a sustained release from the core were observed. | Han and Steckl (2013)51 |
| PCLm | KAUs | ||||
| PCLs | |||||
Moreover, spinnable or non-spinnable materials and their effects in different layers have been investigated. Although the studies using different drugs are relatively few at the moment, since the material and composition are studied in detail, there are also cases where two or three different drugs are studied together. Elimination of initial burst release is one of the significant results. While the system has great promise, it is quite complicated. Therefore, more detailed research and testing of different strategies are still needed.
5. Applications of multidrug-loaded fibers
Electrospun fibers have been used as a drug delivery system for the treatment of various diseases. These systems have already been proposed for many kinds of biomedical applications, e.g. regenerative medicine, wound dressing, implants, and cancer treatment.28,178–180 To date, many kinds of therapeutic agents including proteins, DNA/RNA, anti-bacterial agents including inorganic nanoparticles, antiviral, and anti-cancer drugs have been loaded into fibers for appropriate applications.181–183 This section summarizes some key applications of multidrug-loaded electrospun fibers.5.1 Regenerative medicine
Since multi-drug-loaded fibers have many advantages, as discussed before, they are used in various fields of regenerative medicine such as bone regeneration, periodontal regeneration, and nerve tissue engineering.In the case of bone tissue engineering the main aim is to promote osteogenesis, leading to natural bone growth/repair.184 In addition to the fact that electrospun fibers can mimic natural extra cellular matrix (ECM), they can efficiently deliver the therapeutics to improve the osteogenesis, e.g. growth factors/proteins, and drugs.185–187 BMP-2, which is the most used growth factor, and drugs, for instance DEX, as promoting agents for osteogenesis have been extensively studied in the literature.188–190 For example, Wang et al.191 aimed for sequential release of VEGF and BMP-2 from PLA-PDLLA core–shell fibers to extend osteogenic activity. After 4 weeks, more new bone formation was observed with respect to the corresponding single fibers (Fig. 10A). Core–shell silk fibroin (SF)/PCL/PVA consisting of BMP-2 in the core and CTGF in the shell was fabricated to promote different stages of bone regeneration.47 The results demonstrated that CTGF played a role in short-term bone healing, while BMP-2 provided further bone formation by sustained release.
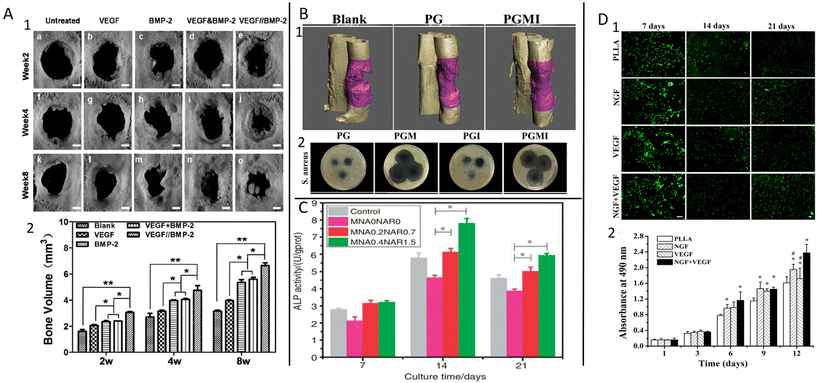 | ||
| Fig. 10 Examples of some regenerative medicine applications. (A) Bone repair level comparison (1); new bone formation volume (2) where the VEGF&BMP-2 is blended and VEGF//BMP-2 is core–shell formation. Reprinted (adapted) from ref. 191 with permission from Elsevier, Copyright 2015. (B) Representative 3D micro-CT images of the regenerated bone at 3 months post-surgery treated by the fibrous membranes where the without drug (PCL-gelatin (PG)); dual drug-loaded MOX-ICA (PGMI) (1); images of the inhibition zone around the fibrous membranes against Gram-positive S. aureus where the PG, MOX-loaded (PGM), ICA-loaded (PGI), and PGM membranes (2). Reprinted (adapted) from ref. 164 with permission from Elsevier, Copyright 2019. (C) Effect of membranes on osteogenic marker alkaline phosphatase (ALP) activity with different concentrations of drugs. Reprinted (adapted) from ref. 163 with permission from SAGE Publications, Copyright 2020. (D) Live/dead staining of induced pluripotent stem cells (iPSCs)-derived human neural crest stem cells (NCSCs) (iPSCs-NCSCs) seeded on different nanofibrous where the green represents live cells and red represents dead cells (1); cell viability of iPSCs-NCSCs seeded on different nanofibrous scaffolds (2). Reprinted (adapted) from ref. 200 with permission from Elsevier, Copyright 2018. | ||
Drugs, including synthetic and natural drugs, are also used for enhancing osteogenic differentiation. DEX is one of the synthetic drugs widely utilized as a bone formation-promoting factor.190 Scientists reported that a combination of DEX and BMP-2 in fiber could be an optimal material for a controlled and improved regeneration process due to the rapid release of DEX and sustained release of BMP-2.166 It achieved an improvement in osteogenic differentiation due to the synergetic effects of different release profiles.149 In addition to these, icariin, a natural medicine, has been suggested as a promoting agent for cell osteogenicity.187,192 Furthermore, adding anti-bacterial agents to fibers is essential in the early stage of the bone regeneration process. For example, Gong et al.164 incorporated icariin and anti-bacterial drug MOX into core–shell fiber, where the icariin is in the core and MOX is in the shell layer. It was reported that the anti-bacterial drug release prevented bacterial growth in the early stage and contributed to enhancing osteogenic markers through the slow release of icariin (Fig. 10B).
Periodontal tissue engineering aims to reduce bacterial infection using anti-bacterial agents for effective tissue regeneration to treat inflammatory periodontal diseases causing systemic side effects.193,194 To overcome the adverse effects of systemic antibiotics, local delivery systems such as electrospun fibers are used to deliver the therapeutic agents to the target site directly. Furthermore, osteogenic agents could also be incorporated into the same system to improve tissue regeneration. For example, an anti-bacterial agent MTZ and osteogenesis-promoting agent NAR were incorporated into the PLGA/PVP core–shell system to provide a synergistic effect.163 The desired therapeutic effect was obtained by the rapid release of MTZ (in the shell) inhibiting bacterial growth and prolonged release of NAR (in the core) promoting cell differentiation (Fig. 10C). Oxytetracycline hydrochloride (OTC) and ZnO were used to investigate the synergetic effect on bacterial infection using a PCL polymer fiber-forming matrix.113 However, ZnO was not effective as an anti-bacterial agent; it decreased the release rate of OTC by reducing the wettability of fibers. Thus, a prolonged anti-bacterial effect was provided by OTC. Many more studies have been reported to investigate such dual effects in this field.195–197
Another hot topic in regenerative medicine is nerve tissue regeneration, considering the limited ability of natural regeneration of this tissue. Therefore, incorporating multiple growth factors into fibers to promote nerve tissue has become a challenge for scientists.198,199 Xia and Lv (2018)200 developed PLLA fibers including VEGF on the surface and NGF in the core to provide angiogenesis first and nerve regeneration later for a better recovery mechanism. Rapid release of VEGF promoted neovascularization, while the prolonged release of NGF improved cell proliferation (Fig. 10D). It has been suggested that dual delivery of both factors enhanced regeneration functionality. Additionally, the synergetic effect of NGF and ciliary neurotrophic factor (CNTF) was reported as being greater in inducing glial cell and neuron growth together with respect to their single ones.201 Furthermore, during nerve regeneration to enhance functional recovery by controlling the immune response, anti-inflammatory agents, e.g. ibuprofen, curcumin, and anti-inflammatory peptides, can also be loaded into the fibers.199,202,203 There is much research on this subject, but it still needs to be deeply investigated because of the unpredictable structure.
5.2 Wound dressing
Wound dressing is a field where much research has been conducted in biomedical technologies. The dressing aims to reduce inflammation and promote cell proliferation during the wound-healing process for more effective recovery.204 A perfect wound dressing material should provide broad-spectrum anti-bacterial activity while considering the warm, moist, and nutrient-rich skin conditions that elevate bacterial growth.205 In the literature, several anti-bacterial agents such as drugs, peptides, and nanoparticles have been incorporated into electrospun fibers for appropriate wound healing.206–208 Multiple therapeutic agents can be incorporated into the fibers to facilitate the multifunctional dressing and improve the process. For example, Vitamin A (VA) and Vitamin E (VE) were loaded in gelatin nanofibers to obtain a functional wound-dressing material.209 The system exhibited sustained release of both agents over 60 h and inhibited bacterial growth after incubation for 2 days. However, it has been reported that VE is more effective for bacterial inhibition while VA is a greater inducer of cell proliferation. VE was combined with antioxidant selenium nanoparticles (Se NPs) in PCL/Gel nanofibers by Doostmohammadi et al.210 They achieved a synergetic effect through the sustained release profile of both and complete wound closure and re-epithelization with respect to the study mentioned above (Fig. 11A). J. Yang et al.48 incorporated ciprofloxacin and Ag NPs into Janus PVP/EC fibers. Rapid release of 90% of ciprofloxacin from the PVP side provided strong early anti-bacterial activity, while the sustained release of Ag NPs from the EC side inhibited bacterial growth for a longer time. Furthermore, growth factors that induce generation of new skin also play a vital role in wound healing. For example, it has been reported that controlled dual delivery of recombinant human epidermal growth factor (rhEGF) and recombinant human basic fibroblast growth factor (rhbFGF) exhibits a synergetic effect on accelerating epidermal and mesenchymal regeneration by promoting cell differentiation.211 Vascular endothelial growth factor (VEGF) and transforming growth factor beta 3 (TGF-β3) were delivered by PLGA fibers.212In vivo results showed that co-delivery of the growth factors provided much better composed skin and higher blood vessel formation on the defect site with respect to single-factor-loaded PLGA fibers (Fig. 11B). Considering these advantages, a combination of the growth factors epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF) and an anti-bacterial agent silver sulfadiazine (SSD) was incorporated into a trilayer fiber mat including PCL bottom, Cs/PEO middle, and PCL/collagen top layers, with the growth factors in the middle and SSD in the bottom layer.213 Evaluation of the healing process for 14 days found better epithelization with fibers including growth factors compared with fibers without growth factors. In another example, Ponericin G1 (PonG1), an anti-bacterial peptide, and bFGF were applied for the surface modification of PLGA fibers.214In vitro results proved that while accelerating cell proliferation, PonG1 decreases bacterial growth. In vivo results showed that their co-delivery made wound-healing time shorter. In addition to these, anti-inflammatory pharmaceutics including flurbiprofen, DEX, and diclofenac sodium can also be incorporated into the material to promote the wound-healing process.182,215,216 | ||
| Fig. 11 Schematic illustrations of wound dressing and cancer treatment applications. (A) Visual appearance of wound excised on rat: I; day 0, 13th day of; negative control (II); PCL/GEL (III); traditional wound-healing medicine (IV); PCL/GEL/Se NPs scaffold (V); and PCL/GEL/Se NPs/VE scaffold (VI). Reprinted (adapted) from ref. 210 with permission from SAGE Publications, Copyright 2021; (B) visual appearance of wound excised on a rat: treated by nanofiber only; with single factor (V-free: without VEGF; T-free: without TGF-β3); codelivery of two factors (coa-dual). Reprinted (adapted) from ref. 212 with permission from Elsevier, Copyright 2017; (C) bioluminescence signal intensity (tumor progression) images show anti-tumor efficacy in in mice from sham (no treatment), drug implant and drug/gene dual-implant groups where the tumor cells are displayer in the brain (red color indicating the highest bioluminescence intensity). Reprinted (adapted) from ref. 222 with permission from Elsevier, Copyright 2013. (D) Fluorescence photomicrograph indicating cell density of cancer cells: control (untreated) (1); fiber membrane-1 wt% methotrexate (2); fiber membrane-1 wt% methotrexate, 2 wt% triterpenoids (3); fiber membrane-1 wt% methotrexate, 4 wt% triterpenoids (4); fiber membrane-1 wt% methotrexate, 6 wt% triterpenoids (5). Reprinted (adapted) from ref. 228 with permission from Elsevier, Copyright 2020. (E) Confocal images indicating cell density of cancer cells treated with blank triaxial fibers (a); single-layer fiber containing DOX, PTX and 5-FU (b); tri-layer nanofibers containing DOX, PTX and 5-FU with core (c). Reprinted (adapted) from ref. 117 with permission from Elsevier, Copyright 2019. | ||
5.3 Cancer treatment
Due to the complex physiological structure and drug resistance arising from ongoing treatments of tumors, conventional single-drug therapy might not be a sufficient route for cancer treatment. Combining chemotherapy using multiple drugs has been proposed to enhance the therapeutic effect through the synergetic effect of multiple drugs.217–219 Therefore, electrospun fibers are a promising drug delivery system because their high drug-loading capacity, tunable release properties, ability to mimic ECM, and potential for a combination of therapeutics.220Wei et al.221 have developed hydrophilic drug (DOX) embedded in ZnO nanoparticles and hydrophobic drug camptothecin (CPT)-loaded ZnO/PLA/Gel fibers to treat tissue defects after tumor resection. In vitro results demonstrated that co-delivery of the drugs exhibited intense anti-tumor effects through the fast release of CPT and sustained release of DOX over 120 hours. In this dual-delivery system, distinct mechanisms of action of both drugs were applied synergistically while the initial rapid release of CPT played a vital role in suppressing cancer cells. A gene/drug (matrix metalloproteinase-2 (MMP-2)/PAX) dual-delivery system has been proposed to block cell proliferation, tumor invasion, and angiogenesis.222 Gene-embedded polyethyleneimine (PEI) carriers were encapsulated in PLGA fibers for sustained release for both bioactive molecules. In vivo tests proved the dual-delivery system's synergetic therapeutic effect, showing remarkable tumor regression with respect to single-drug delivery fibers (Fig. 11C). A similar strategy was applied to develop combined therapy by DOX and a drug resistance-related gene inhibitor protein apatinib (AP) in a trilayer structured fiber, leading to increased DOX accumulation in the tumor site.223 This method can also be modified with siRNA delivery to knock down the expression of related genes.224 Furthermore, drugs have also been combined with organic/inorganic particles, such as iron-oxide and CaCO3, for triggered cancer therapies, i.e. magnetic, thermal, and pH responsive.225–227 In a different study, Ganoderma lucidum triterpenoids (GLT), which is a fungus-derived natural medicine possessing anti-cancer effects such as anti-proliferation and anti-inflammation, was used in combination with the anti-cancer drug methotrexate (MTX).228 GLT was incorporated in the PCL core and MTX was incorporated in the PEO shell layer of a core–shell fiber structure for controlled delivery. MTX displayed a biphasic release profile with an initial burst release in the early stage, while GLT was released more slowly in a sustained manner over 180 hours. It was reported that a higher GLT concentration provided a higher anti-cancer effect and synergetic effect of both drugs in comparison with single MTX-loaded fibers (Fig. 11D). Moreover, Habibi Jouybari et al.117 developed three anti-cancer drugs including 5-FU, DOX, and PTX-loaded triaxial fibers to provide high activity against cancer cells. The combination of DOX and PTX results in blocking cells in the G2 phase (rapid cell growth phase) in the cell cycle, and 5-FU pushs the cellsto kill them and promote tumor cell apoptosis. To apply this mechanism for a prolonged time a trilayer structure was developed, resulting in cumulative drug release of over 700 hours. In vitro results showed that drug-loaded trilayer fibers exhibited higher cytotoxicity for cancer cells than drug-loaded single-layer fibers (Fig. 11E).
Although there are many different strategies and much research for cancer treatment, it is still a big challenge due to the unique and complex tumor microenvironment. There are more detailed reviews based on electrospun fibers by Abid et al.,229 Khodadadi et al.,230 and Zhao and Cui.231
6. Conclusions and future perspectives
From the perspective of multi-drug loading, nanofibers formed using multifluid electrospinning, i.e., Janus fibers and co- and tri-axial fibers, have shown significant advantages over uniaxial (single fluid) fibers. Different bioactive molecules such as drugs, growth factors/proteins, genomic molecules, and natural medicine products can be simultaneously incorporated into multiaxial fibers. Multi-drug therapy reduces the risk of developing cell resistance to treatment and increases functionality. More effective control of multi-drug release by manipulating diffusion/erosion mechanisms using hygroscopic characteristics of different polymers and adjusting diffusion pathways by using polymer layers in a rapid, sustained, and/or sequential manner makes these methods promising. The antibiotic resistance of some bacteria to drugs is becoming more and more severe, and the need to use precise multi-drug therapies will increase in the future.Furthermore, varying the polymer relocation of therapeutic agents in layers in core–shell fibers makes it possible to precisely modulate drug-release mechanisms and kinetics. This is why triaxial fibers are making significant advances in sustained release with zero-order kinetics compared with core–shell fibers by coaxial electrospinning. Core–shell fibers have been keenly investigated. In the future, more attention will be given to triaxial fibers, giving the broader possibility of combining drugs and modulating release kinetics.
From the viewpoint of modulation of drug-release profile, the implementation of smart delivery systems into multifluid electrospinning for multidrug delivery is also expected to gain more attention. Intelligent drug delivery could provide ideal therapeutic effects through the drug release at specific time intervals to fulfill the needs of the body. These systems have great potential in biomedicine for more advanced control and efficient release of drugs. However, several parameters must be considered, such as stimulus method, i.e., external or internal stimulation, selection of appropriate material, biocompatibility, FDA approval, patient convenience, efficiency, cost, etc. Therefore, further investigations are necessary for practical applications in the future.
Multi-drug-loaded nonwovens, made of, for example Janus core–shell using coaxial/triaxial fibers, are promising in regenerative medicine, wound dressing, and cancer treatment. Their complexity enhances the synergetic effects of drugs, lower drug dosage, functional healing process, prolonged anti-tumor/bacterial/antioxidant and cell proliferation/inhibition activities, enabling sequential release leading to an optimal treatment regimen. However, there are still challenges and a lack of studies from the clinical application perspective, and at least in vivo studies on animals. Furthermore, most in vivo studies on healthy animals do not consider comorbidities like diabetes, cardiovascular problems, and hypertension. Although using several therapeutic agents in one fiber will require additional clinical trials in animals and humans, these studies are becoming necessary, considering the shortcomings of current therapies. Meanwhile, there has not been a clinical trial for Janus or core–shell fibers; the keywords ‘Janus, coaxial, triaxial, multiaxial’ were not matched with any study on clinicaltrials.gov.
It is also worth noting that biosensing employing multilevel structured nanofibers is another main scientific field of interest for researchers, to understand the human being at the cellular level through biological reactions. Electrospun fibers have not only ability to use a wide range of materials, but also to have complex structure owing to spinneret and collector design. Thanks to these unique characteristics of electrospinning, embedding of various combinations of active agents including carbon materials, ceramics, metals, biological enzymes, and metal NPs as well as drugs is possible. Implementation of the detection agents into nanofibers to develop smart sensing devices might be a novel approach for biomedical applications in the future.
Green manufacturing has emerged across industries, propelled by a growing awareness of the negative environmental and health impacts associated with traditional manufacturing. There is a significant need to implement large-scale green manufacturing of biomaterials in the medical device field.232 A new perspective is developing of electrospun biomaterials according sustainable rules. In the literature several authors have already focused on green polymers,233–235 natural polymers,144,158,159 and green solvents for fabrication of electrospun scaffolds.232,236
The next change in future decades is to save energy. Considering all types of electrospinning, one of the important issues that need to be improved is energy saving for cost reduction and technological feasibility. Researchers have started to implement new strategies such as a solid Teflon-core needle instead of traditional metal-based needle for lower energy consumption. Since the electrospinning technique has a wide range of possibilities regarding selection of raw materials and the ability for ease of operation in production of functional materials, the development of cost-friendly nanofibers might be expected to draw more attention for future applications. Furthermore, the combination of different polymeric film production techniques with electrospinning could be a trending topic to take advantage of nanofibers in biomedical applications.
Sustainable manufacturing in electrospinning has the potential to transform the production of materials for various medical applications. This is the future perspective which will strongly reduce the negative environmental aspects of the process.
To conclude, multiaxial fibers are an innovative way to develop complex structures for effective treatments. Currently, there is interest in multidrug delivery systems to provide particular desired drug-release profiles. However, there are still limitations that need to be overcome, and we should focus on overcoming the deficiencies by applying clinical trials to make dreams about reliable therapy come true.
Author contributions
Conceptualization, S. T.; validation, S. T., D. K., and P. S.; writing—original draft preparation, S. T.; writing, review and editing, S. T., D. K., and P. S., visualization, S. T., D. K., and P. S.; supervision, P. S. and D. K. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The first author would like to acknowledge support from the Project no. POWR.03.02.00-00-IO28/17-01 implemented under the Operational Programme Knowledge Education Development 2014–2020, co-financed by the European Social Fund.References
- K. Ghosal, C. Agatemor, Z. Špitálsky, S. Thomas and E. Kny, Chem. Eng. J., 2019, 358, 1262–1278 CrossRef CAS
.
- B. Niemczyk-Soczynska, J. Dulnik, O. Jeznach, D. Kolbuk and P. Sajkiewicz, Micron, 2021, 145, 103066 CrossRef CAS
.
- O. Jeznach, D. Kołbuk, M. Marzec, A. Bernasik and P. Sajkiewicz, RSC Adv., 2022, 12, 11303–11317 RSC
.
- Ş. M. Eskitoros-Togay, Y. E. Bulbul, S. Tort, F. Demirtaş Korkmaz, F. Acartürk and N. Dilsiz, Int. J. Pharm., 2019, 565, 83–94 CrossRef
.
- A. Khalf and S. V. Madihally, Eur. J. Pharm. Biopharm., 2017, 112, 1–17 CrossRef CAS PubMed
.
- Y. Zhuang, K. Lin and H. Yu, Front. Chem., 2019, 7, 495–495 CrossRef CAS
.
- J. Xue, M. He, Y. Niu, H. Liu, A. Crawford, P. Coates, D. Chen, R. Shi and L. Zhang, Int. J. Pharm., 2014, 475, 566–577 CrossRef CAS PubMed
.
- H. Yoshimoto, Y. M. Shin, H. Terai and J. P. Vacanti, Biomaterials, 2003, 24, 2077–2082 CrossRef CAS PubMed
.
- A. Yin, R. Luo, J. Li, X. Mo, Y. Wang and X. Zhang, Colloids Surf., B, 2017, 152, 432–439 CrossRef CAS
.
- J. Zeng, X. Xu, X. Chen, Q. Liang, X. Bian, L. Yang and X. Jing, J. Controlled Release, 2003, 92, 227–231 CrossRef CAS PubMed
.
- T. Sun, D. Norton, R. J. McKean, J. W. Haycock, A. J. Ryan and S. MacNeil, Biotechnol. Bioeng., 2007, 97, 1318–1328 CrossRef CAS PubMed
.
- K. M. Yun, C. J. Hogan, Y. Matsubayashi, M. Kawabe, F. Iskandar and K. Okuyama, Chem. Eng. Sci., 2007, 62, 4751–4759 CrossRef CAS
.
- P. Akangah, S. Lingaiah and K. Shivakumar, Compos. Struct., 2010, 92, 1432–1439 CrossRef
.
- M. Alcoutlabi, H. Lee, J. V. Watson and X. Zhang, J. Mater. Sci., 2013, 48, 2690–2700 CrossRef CAS
.
- S. Jiang, G. Duan, H. Hou, A. Greiner and S. Agarwal, ACS Appl. Mater. Interfaces, 2012, 4, 4366–4372 CrossRef CAS
.
- W. Huang, Y. Xiao and X. Shi, Adv. Fiber Mater., 2019, 1, 32–45 CrossRef
.
- B. Gong, X. Zhang, A. A. Zahrani, W. Gao, G. Ma, L. Zhang and J. Xue, Exploration, 2022, 2, 20210035 CrossRef
.
- R. Bayat Mokhtari, T. S. Homayouni, N. Baluch, E. Morgatskaya, S. Kumar, B. Das and H. Yeger, Oncotarget, 2017, 8, 38022–38043 CrossRef PubMed
.
- A. Dadwal, A. Baldi and R. Kumar Narang, Artif. Cells, Nanomed., Biotechnol., 2018, 46, 295–305 CrossRef CAS
.
- Z. Sun, C. Song, C. Wang, Y. Hu and J. Wu, Mol. Pharm., 2020, 17, 373–391 CAS
.
- H.-W. An, M. Mamuti, X. Wang, H. Yao, M.-D. Wang, L. Zhao and L.-L. Li, Exploration, 2021, 1, 20210153 CrossRef
.
- Y. Liu, F. Wu, Y. Ding, B. Zhu, Y. Su and X. Zhu, Adv. Fiber Mater., 2019, 1, 152–162 CrossRef
.
- E. J. Torres-Martinez, J. M. Cornejo Bravo, A. Serrano Medina, L. Pérez González and L. J. Villarreal Gómez, Curr. Drug Delivery, 2018, 15, 1360–1374 CrossRef CAS
.
-
R. Di Gesù, A. Merlettini, C. Gualandi and M. L. Focarete, in Core-Shell Nanostructures for Drug Delivery and Theranostics, 2018, pp. 405–430, DOI:10.1016/b978-0-08-102198-9.00014-4
.
- S. Gao, G. Tang, D. Hua, R. Xiong, J. Han, S. Jiang, Q. Zhang and C. Huang, J. Mater. Chem. B, 2019, 7, 709–729 RSC
.
- A. J. Meinel, O. Germershaus, T. Luhmann, H. P. Merkle and L. Meinel, Eur. J. Pharm. Biopharm., 2012, 81, 1–13 CrossRef CAS
.
- Y. Ding, W. Li, F. Zhang, Z. Liu, N. Zanjanizadeh Ezazi, D. Liu and H. A. Santos, Adv. Funct. Mater., 2019, 29, 1802852 CrossRef
.
- S. M. S. Shahriar, J. Mondal, M. N. Hasan, V. Revuri, D. Y. Lee and Y.-K. Lee, Nanomaterials, 2019, 9(4), 532 CrossRef CAS PubMed
.
- S. Ramakrishna, K. Fujihara, W.-E. Teo, T. Yong, Z. Ma and R. Ramaseshan, Mater. Today, 2006, 9, 40–50 CrossRef CAS
.
- A. Khalf and S. V. Madihally, Mater. Sci. Eng., C, 2017, 76, 161–170 CrossRef CAS PubMed
.
- J. Wang and M. Windbergs, Int. J. Pharm., 2019, 556, 363–371 CrossRef CAS PubMed
.
- R. Augustine, A. A. Zahid, A. Hasan, M. Wang and T. J. Webster, Int. J. Nanomed., 2019, 14, 8573–8588 CrossRef CAS
.
- T. Hai, X. Wan, D.-G. Yu, K. Wang, Y. Yang and Z.-P. Liu, Mater. Des., 2019, 162, 70–79 CrossRef CAS
.
- L. D. Tijing, W. Choi, Z. Jiang, A. Amarjargal, C.-H. Park, H. R. Pant, I.-T. Im and C. S. Kim, Curr. Appl. Phys., 2013, 13, 1247–1255 CrossRef
.
- N. Eslahi, A. Mahmoodi, N. Mahmoudi, N. Zandi and A. Simchi, Polym. Rev., 2020, 60, 144–170 CrossRef CAS
.
- M. A. Taemeh, A. Shiravandi, M. A. Korayem and H. Daemi, Carbohydr. Polym., 2020, 228, 115419 CrossRef CAS PubMed
.
- Y. Du, X. Zhang, P. Liu, D. G. Yu and R. Ge, Front. Chem., 2022, 10, 944428 CrossRef CAS PubMed
.
- W. Jiang, P. Zhao, W. Song, M. Wang and D.-G. Yu, Biomolecules, 2022, 12, 1110 CrossRef CAS
.
- M. Wang, D.-G. Yu, G. R. Williams and S. W. A. Bligh, Pharmaceutics, 2022, 14, 1208 CrossRef CAS PubMed
.
- Y. Liu, H. Lv, Y. Liu, Y. Gao, H. Y. Kim, Y. Ouyang and D.-G. Yu, Mater. Today Chem., 2022, 25, 100974 CrossRef CAS
.
- H. Liu, H. Wang, X. Lu, V. Murugadoss, M. Huang, H. Yang, F. Wan, D.-G. Yu and Z. Guo, Adv. Compos. Hybrid Mater., 2022, 5, 1017–1029 CrossRef CAS
.
- D.-G. Yu, M. Wang and R. Ge, WIREs Nanomed. Nanobiotechnol., 2022, 14(3), e1772 CAS
.
- L. E. Sperling, K. P. Reis, P. Pranke and J. H. Wendorff, Drug Discovery Today, 2016, 21, 1243–1256 CrossRef CAS
.
- Y. Lu, J. Huang, G. Yu, R. Cardenas, S. Wei, E. K. Wujcik and Z. Guo, WIREs Nanomed. Nanobiotechnol., 2016, 8, 654–677 CrossRef CAS
.
- B. Pant, M. Park and S. J. Park, Pharmaceutics, 2019, 11(7), 305 CrossRef CAS PubMed
.
- K. J. Lee, T.-H. Park, S. Hwang, J. Yoon and J. Lahann, Langmuir, 2013, 29, 6181–6186 CrossRef CAS
.
- G. Cheng, C. Yin, H. Tu, S. Jiang, Q. Wang, X. Zhou, X. Xing, C. Xie, X. Shi, Y. Du, H. Deng and Z. Li, ACS Nano, 2019, 13, 6372–6382 CrossRef CAS
.
- J. Yang, K. Wang, D.-G. Yu, Y. Yang, S. W. A. Bligh and G. R. Williams, Mater. Sci. Eng., C, 2020, 111, 110805 CrossRef CAS PubMed
.
- Y. Yang, S. Chang, Y. Bai, Y. Du and D.-G. Yu, Carbohydr. Polym., 2020, 243, 116477 CrossRef CAS
.
- K. Ghosal, R. Augustine, A. Zaszczynska, M. Barman, A. Jain, A. Hasan, N. Kalarikkal, P. Sajkiewicz and S. Thomas, React. Funct. Polym., 2021, 163, 104895 CrossRef CAS
.
- D. Han and A. J. Steckl, ACS Appl. Mater. Interfaces, 2013, 5, 8241–8245 CrossRef CAS PubMed
.
- W. Liu, C. Ni, D. B. Chase and J. F. Rabolt, ACS Macro Lett., 2013, 2, 466–468 CrossRef CAS PubMed
.
- X. Liu, Y. Yang, D.-G. Yu, M.-J. Zhu, M. Zhao and G. R. Williams, Chem. Eng. J., 2019, 356, 886–894 CrossRef CAS
.
- J. Zhu, H. Ye, D. Deng, J. Li and Y. Wu, J. Biomater. Appl., 2020, 34, 1282–1293 CrossRef CAS
.
- S. Jiang, G. Duan, E. Zussman, A. Greiner and S. Agarwal, ACS Appl. Mater. Interfaces, 2014, 6, 5918–5923 CrossRef CAS
.
- S. Labbaf, H. Ghanbar, E. Stride and M. Edirisinghe, Macromol. Rapid Commun., 2014, 35, 618–623 CrossRef CAS PubMed
.
- X. Zhang, C. Chi, J. Chen, X. Zhang, M. Gong, X. Wang, J. Yan, R. Shi, L. Zhang and J. Xue, Mater. Des., 2021, 206, 109732 CrossRef CAS
.
- M. Zare, K. Dziemidowicz, G. R. Williams and S. Ramakrishna, Nanomaterials, 2021, 11(8), 1968 CrossRef CAS
.
- D. G. Yu, M. Wang, X. Li, X. Liu, L. M. Zhu and S. W. Annie Bligh, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2020, 12, e1601 Search PubMed
.
- X. Liu, H. Xu, M. Zhang and D.-G. Yu, Membranes, 2021, 11(10), 770 CrossRef CAS PubMed
.
- Y.-H. Kim and Y. Tabata, Adv. Drug Delivery Rev., 2015, 94, 28–40 CrossRef CAS
.
- H. S. Kim, X. Sun, J. H. Lee, H. W. Kim, X. Fu and K. W. Leong, Adv. Drug Delivery Rev., 2019, 146, 209–239 CrossRef CAS PubMed
.
- Y. Su, Q. Su, W. Liu, M. Lim, J. R. Venugopal, X. Mo, S. Ramakrishna, S. S. Al-Deyab and M. El-Newehy, Acta Biomater., 2012, 8, 763–771 CrossRef CAS PubMed
.
- E. K. Tsekoura, A. L. Helling, J. G. Wall, Y. Bayon and D. I. Zeugolis, Biomed. Mater., 2017, 12, 035013 CrossRef CAS
.
- Z. Xie, C. B. Paras, H. Weng, P. Punnakitikashem, L. C. Su, K. Vu, L. Tang, J. Yang and K. T. Nguyen, Acta Biomater., 2013, 9, 9351–9359 CrossRef CAS
.
- S. A. Abbah, D. Thomas, S. Browne, T. O'Brien, A. Pandit and D. I. Zeugolis, Sci. Rep., 2016, 6, 20922 CrossRef CAS
.
- I. Fernandez-Piñeiro, I. Badiola and A. Sanchez, Biotechnol. Adv., 2017, 35, 350–360 CrossRef
.
- M. Isakson, C. de Blacam, D. Whelan, A. McArdle and A. J. P. Clover, Stem Cells Int., 2015, 2015, 831095–831095 CAS
.
- L. Zhao, L. Niu, H. Liang, H. Tan, C. Liu and F. Zhu, ACS Appl. Mater. Interfaces, 2017, 9, 37563–37574 CrossRef CAS
.
- R. Ahmed, M. Tariq, I. Ali, R. Asghar, P. Noorunnisa Khanam, R. Augustine and A. Hasan, Int. J. Biol. Macromol., 2018, 120, 385–393 CrossRef CAS
.
- M. S. Kang, J.-H. Kim, R. K. Singh, J.-H. Jang and H.-W. Kim, Acta Biomater., 2015, 16, 103–116 CrossRef CAS PubMed
.
- M. Farokhi, F. Mottaghitalab, M. A. Shokrgozar, K. L. Ou, C. Mao and H. Hosseinkhani, J. Controlled Release, 2016, 225, 152–169 CrossRef CAS PubMed
.
- Y. Wang, W. Cui, X. Zhao, S. Wen, Y. Sun, J. Han and H. Zhang, Nanoscale, 2019, 11, 60–71 RSC
.
- J. Zhang, X. Wang, T. Liu, S. Liu and X. Jing, Drug Delivery, 2016, 23, 784–790 CrossRef CAS
.
- R. Pushpalatha, S. Selvamuthukumar and D. Kilimozhi, J. Drug Delivery Sci. Technol., 2017, 39, 362–371 CrossRef CAS
.
- F. Wang, L. Zhang, X. Bai, X. Cao, X. Jiao, Y. Huang, Y. Li, Y. Qin and Y. Wen, ACS Appl. Mater. Interfaces, 2018, 10, 22767–22775 CrossRef CAS PubMed
.
- S. Ni, X. Fan, J. Wang, H. Qi and X. Li, Ann. Biomed. Eng., 2014, 42, 214–221 CrossRef PubMed
.
- S. T. Yohe, V. L. Herrera, Y. L. Colson and M. W. Grinstaff, J. Controlled Release, 2012, 162, 92–101 CrossRef CAS
.
- X. Feng, J. Li, X. Zhang, T. Liu, J. Ding and X. Chen, J. Controlled Release, 2019, 302, 19–41 CrossRef CAS PubMed
.
- D. Painuly, U. Nisha, S. Arya and J. B. Sangeeth Krishnan, J. Drug Delivery Sci. Technol., 2019, 51, 454–463 CrossRef CAS
.
- H. Yu, X. Chen, J. Cai, D. Ye, Y. Wu and P. Liu, J. Biomater. Sci., Polym. Ed., 2019, 30, 64–76 CrossRef CAS PubMed
.
- M. Liu, Y. Zhang, S. Sun, A. R. Khan, J. Ji, M. Yang and G. Zhai, J. Drug Targeting, 2019, 27, 270–282 CrossRef CAS PubMed
.
- G. Ajmal, G. V. Bonde, P. Mittal, G. Khan, V. K. Pandey, B. V. Bakade and B. Mishra, Int. J. Pharm., 2019, 567, 118480 CrossRef CAS PubMed
.
- Z. Zhang, Q. Dai, Y. Zhang, H. Zhuang, E. Wang, Q. Xu, L. Ma, C. Wu, Z. Huan, F. Guo and J. Chang, ACS Appl. Mater. Interfaces, 2020, 12, 12489–12499 CrossRef CAS
.
- X. Lan, Y. Liu, Y. Wang, F. Tian, X. Miao, H. Wang and Y. Tang, Int. J. Pharm., 2021, 601, 120525 CrossRef CAS PubMed
.
- J. Li, R. Fu, L. Li, G. Yang, S. Ding, Z. Zhong and S. Zhou, Pharm. Res., 2014, 31, 1632–1643 CrossRef CAS PubMed
.
- A. K. Blakney, E. A. Krogstad, Y. H. Jiang and K. A. Woodrow, Int. J. Nanomed., 2014, 9, 2967–2978 CrossRef
.
- X. Ren, Y. Han, J. Wang, Y. Jiang, Z. Yi, H. Xu and Q. Ke, Acta Biomater., 2018, 70, 140–153 CrossRef CAS
.
- X. Liu, W. Zhang, Y. Wang, Y. Chen, J. Xie, J. Su and C. Huang, J. Controlled Release, 2020, 320, 201–213 CrossRef CAS
.
- X. Zhao, J. Zhao, Z. Y. Lin, G. Pan, Y. Zhu, Y. Cheng and W. Cui, Colloids Surf., B, 2015, 130, 1–9 CrossRef CAS PubMed
.
- G. Yang, J. Wang, L. Li, S. Ding and S. Zhou, Macromol. Biosci., 2014, 14, 965–976 CrossRef CAS PubMed
.
- S. Kajdič, O. Planinšek, M. Gašperlin and P. Kocbek, J. Drug Delivery Sci. Technol., 2019, 51, 672–681 CrossRef
.
- M. Sadri, A. Mohammadi and H. Hosseini, Nanomed. Res. J., 2016, 1, 112–121 Search PubMed
.
- M. W. Tibbitt, J. E. Dahlman and R. Langer, J. Am. Chem. Soc., 2016, 138, 704–717 CrossRef CAS PubMed
.
- Y. Fu and W. J. Kao, Expert Opin. Drug Delivery, 2010, 7, 429–444 CrossRef CAS PubMed
.
-
R. A. Siegel and M. J. Rathbone, in Fundamentals and Applications of Controlled Release Drug Delivery, ed. J. Siepmann, R. A. Siegel and M. J. Rathbone, Springer US, Boston, MA, 2012, pp. 19–43, DOI:10.1007/978-1-4614-0881-9_2
.
- O. S. Fenton, K. N. Olafson, P. S. Pillai, M. J. Mitchell and R. Langer, Adv. Mater., 2018, 30, 1705328 CrossRef PubMed
.
- P. L. Ritger and N. A. Peppas, J. Controlled Release, 1987, 5, 37–42 CrossRef CAS
.
- T. Higuchi, J. Pharm. Sci., 1963, 52, 1145–1149 CrossRef CAS
.
- R. W. Korsmeyer, R. Gurny, E. Doelker, P. Buri and N. A. Peppas, Int. J. Pharm., 1983, 15, 25–35 CrossRef CAS
.
- P. Costa and J. M. Sousa Lobo, Eur. J. Pharm. Sci., 2001, 13, 123–133 CrossRef CAS PubMed
.
- A. M. Moydeen, M. S. Ali Padusha, E. F. Aboelfetoh, S. S. Al-Deyab and M. H. El-Newehy, Int. J. Biol. Macromol., 2018, 116, 1250–1259 CrossRef CAS PubMed
.
-
S. Nagarajan, M. Bechelany, N. S. Kalkura, P. Miele, C. P. Bohatier and S. Balme, in Applications of Targeted Nano Drugs and Delivery Systems, ed. S. S. Mohapatra, S. Ranjan, N. Dasgupta, R. K. Mishra and S. Thomas, Elsevier, 2019, pp. 595–625, DOI:10.1016/B978-0-12-814029-1.00020-X
.
- M. Ahmad Wsoo, S. Izwan Abd Razak, S. Shahir, H. Ahmed Abdullah Al-Moalemi, M. Rafiq Abdul Kadir and N. Hasraf Mat Nayan, Polym. Adv. Technol., 2021, 32, 3664–3678 CrossRef CAS
.
- S. Chen, S. K. Boda, S. K. Batra, X. Li and J. Xie, Adv. Healthc. Mater., 2018, 7, e1701024 CrossRef PubMed
.
- J. Jiang, J. Xie, B. Ma, D. E. Bartlett, A. Xu and C. H. Wang, Acta Biomater., 2014, 10, 1324–1332 CrossRef CAS PubMed
.
- B. Ardeshirzadeh, N. A. Anaraki, M. Irani, L. R. Rad and S. Shamshiri, Mater. Sci. Eng., C, 2015, 48, 384–390 CrossRef CAS PubMed
.
- J. Xue, C. Zhu, J. Li, H. Li and Y. Xia, Adv. Funct. Mater., 2018, 28, 1705563 CrossRef PubMed
.
- R. P. Shaikh, V. Pillay, Y. E. Choonara, L. C. du Toit, V. M. Ndesendo, P. Bawa and S. Cooppan, AAPS PharmSciTech, 2010, 11, 441–459 CrossRef CAS
.
- A. K. Bajpai, S. K. Shukla, S. Bhanu and S. Kankane, Prog. Polym. Sci., 2008, 33, 1088–1118 CrossRef CAS
.
- A. Seddighian, F. Ganji, M. Baghaban-Eslaminejad and F. Bagheri, Prog. Biomater., 2021, 10, 65–76 CrossRef CAS PubMed
.
- N. J. Schaub and J. M. Corey, Int. J. Pharm., 2020, 574, 118871 CrossRef CAS PubMed
.
- A. M. Dias, F. G. da Silva, A. P. D. F. Monteiro, A. D. Pinzón-García, R. D. Sinisterra and M. E. Cortés, Mater. Sci. Eng., C, 2019, 103, 109798 CrossRef CAS PubMed
.
- H. Bukhary, G. R. Williams and M. Orlu, Int. J. Pharm., 2018, 549, 446–455 CrossRef CAS PubMed
.
- A. Baskakova, S. Awwad, J. Q. Jiménez, H. Gill, O. Novikov, P. T. Khaw, S. Brocchini, E. Zhilyakova and G. R. Williams, Int. J. Pharm., 2016, 502, 208–218 CrossRef CAS PubMed
.
- U. E. Illangakoon, H. Gill, G. C. Shearman, M. Parhizkar, S. Mahalingam, N. P. Chatterton and G. R. Williams, Int. J. Pharm., 2014, 477, 369–379 CrossRef CAS PubMed
.
- M. Habibi Jouybari, S. Hosseini, K. Mahboobnia, L. A. Boloursaz, M. Moradi and M. Irani, Colloids Surf., B, 2019, 179, 495–504 CrossRef CAS PubMed
.
- N. Zandi, R. Lotfi, E. Tamjid, M. A. Shokrgozar and A. Simchi, Mater. Sci. Eng., C, 2020, 108, 110432 CrossRef CAS
.
- P. He, Q. Zhong, Y. Ge, Z. Guo, J. Tian, Y. Zhou, S. Ding, H. Li and C. Zhou, Mater. Sci. Eng., C, 2018, 90, 549–556 CrossRef CAS PubMed
.
- S. Fathollahipour, A. Abouei Mehrizi, A. Ghaee and M. Koosha, J. Biomed. Mater. Res., Part A, 2015, 103, 3852–3862 CrossRef CAS PubMed
.
- S. Madhugiri, A. Dalton, J. Gutierrez, J. P. Ferraris and K. J. Balkus, J. Am. Chem. Soc., 2003, 125, 14531–14538 CrossRef CAS PubMed
.
- K. Wang, X. K. Liu, X. H. Chen, D. G. Yu, Y. Y. Yang and P. Liu, ACS Appl. Mater. Interfaces, 2018, 10, 2859–2867 CrossRef CAS PubMed
.
- D.-G. Yu, J.-J. Li, M. Zhang and G. R. Williams, Chem. Commun., 2017, 53, 4542–4545 RSC
.
- H. Liu, H. Wang, X. Lu, V. Murugadoss, M. Huang, H. Yang, F. Wan, D.-G. Yu and Z. Guo, Adv. Compos. Hybrid Mater., 2022, 5, 1017–1029 CrossRef CAS
.
- M. Wang, D.-G. Yu, G. R. Williams and S. W. Bligh, Pharmaceutics, 2022, 14(6), 1208 CrossRef CAS PubMed
.
- L. D. Tijing, M. T. G. Ruelo, A. Amarjargal, H. R. Pant, C.-H. Park and C. S. Kim, Mater. Chem. Phys., 2012, 134, 557–561 CrossRef CAS
.
- Z. Jiang, L. D. Tijing, A. Amarjargal, C. H. Park, K.-J. An, H. K. Shon and C. S. Kim, Composites, Part B, 2015, 77, 311–318 CrossRef CAS
.
-
S. Khoee and A. Nouri, in Design and Development of New Nanocarriers, ed. A. M. Grumezescu, William Andrew Publishing, 2018, pp. 145–180, DOI:10.1016/B978-0-12-813627-0.00004-1
.
- D. Li, M. Wang, W.-L. Song, D.-G. Yu and S. W. A. Bligh, Biomolecules, 2021, 11, 635 CrossRef CAS PubMed
.
- D.-G. Yu, C. Yang, M. Jin, G. R. Williams, H. Zou, X. Wang and S. W. Annie Bligh, Colloids Surf., B, 2016, 138, 110–116 CrossRef CAS PubMed
.
- M. Wang, D. Li, J. Li, S. Li, Z. Chen, D.-G. Yu, Z. Liu and J. Z. Guo, Mater. Des., 2020, 196, 109075 CrossRef CAS
.
- L. He, Y. Deng-Guang, W. Menglong and N. Tingbao, Mater. Highlights, 2021, 2, 18–22 CrossRef
.
- I. G. Loscertales, A. Barrero, I. Guerrero, R. Cortijo, M. Marquez and A. M. Gañán-Calvo, Science, 2002, 295, 1695–1698 CrossRef CAS PubMed
.
- Z. Sun, E. Zussman, A. L. Yarin, J. H. Wendorff and A. Greiner, Adv. Mater., 2003, 15, 1929–1932 CrossRef CAS
.
- Y. Liu, X. Chen, Y. Liu, Y. Gao and P. Liu, Polymers, 2022, 14(3), 469 CrossRef CAS PubMed
.
- T. Ning, Y. Zhou, H. Xu, S. Guo, K. Wang and D. G. Yu, Membranes, 2021, 11(11), 802 CrossRef CAS PubMed
.
- H. Lv, S. Guo, G. Zhang, W. He, Y. Wu and D. G. Yu, Polymers, 2021, 13, 4286 CrossRef CAS PubMed
.
- X. Liu, M. Zhang, W. Song, Y. Zhang, D. G. Yu and Y. Liu, Gels, 2022, 8(6), 357 CrossRef CAS
.
- E. Pugliese, J. Q. Coentro and D. I. Zeugolis, Adv. Mater., 2018, 30, 1704324 CrossRef
.
- R. A. Perez and H.-W. Kim, Acta Biomater., 2015, 21, 2–19 CrossRef CAS PubMed
.
- K. Kim, Y. K. Luu, C. Chang, D. Fang, B. S. Hsiao, B. Chu and M. Hadjiargyrou, J. Controlled Release, 2004, 98, 47–56 CrossRef CAS PubMed
.
- V. M. Merkle, L. Zeng, M. J. Slepian and X. Wu, Biopolymers, 2014, 101, 336–346 CrossRef CAS PubMed
.
- T. Wang, X. Ji, L. Jin, Z. Feng, J. Wu, J. Zheng, H. Wang, Z.-W. Xu, L. Guo and N. He, ACS Appl. Mater. Interfaces, 2013, 5, 3757–3763 CrossRef CAS
.
- Q. Wei, F. Xu, X. Xu, X. Geng, L. Ye, A. Zhang and Z. Feng, Front. Mater. Sci., 2016, 10, 113–121 CrossRef
.
- C.-H. Chen, S.-H. Chen, K. T. Shalumon and J.-P. Chen, Acta Biomater., 2015, 26, 225–235 CrossRef CAS PubMed
.
- W.-F. Lai, E. Huang and K.-H. Lui, Asian J. Pharm. Sci., 2021, 16, 77–85 CrossRef PubMed
.
- Z.-C. Yao, J.-C. Wang, B. Wang, Z. Ahmad, J.-S. Li and M.-W. Chang, J. Drug Delivery Sci. Technol., 2019, 50, 372–379 CrossRef CAS
.
- Y. Geng, P. Zhang, Q. Wang, Y. Liu and K. Pan, J. Mater. Chem. B, 2017, 5, 5390–5396 RSC
.
- L. Li, G. Zhou, Y. Wang, G. Yang, S. Ding and S. Zhou, Biomaterials, 2015, 37, 218–229 CrossRef CAS PubMed
.
- H. Yu, P. Yang, Y. Jia, Y. Zhang, Q. Ye and S. Zeng, Colloids Surf., B, 2016, 146, 63–69 CrossRef CAS PubMed
.
- J. Wang, X. Cui, Y. Zhou and Q. Xiang, Connect. Tissue Res., 2014, 55, 292–298 CrossRef CAS PubMed
.
- J. Wang and M. Windbergs, Eur. J. Pharm. Sci., 2018, 124, 71–79 CrossRef CAS PubMed
.
- J. Yoon, H.-S. Yang, B.-S. Lee and W.-R. Yu, Adv. Mater., 2018, 30, 1704765 CrossRef PubMed
.
- E. A. Tawfik, D. Q. M. Craig and S. A. Barker, Int. J. Pharm., 2020, 581, 119296 CrossRef CAS PubMed
.
- H. He, M. Wu, J. Zhu, Y. Yang, R. Ge and D.-G. Yu, Adv. Fiber Mater., 2021, 4, 305–317 CrossRef
.
- M. Lallave, J. Bedia, R. Ruiz-Rosas, J. Rodríguez-Mirasol, T. Cordero, J. C. Otero, M. Marquez, A. Barrero and I. G. Loscertales, Adv. Mater., 2007, 19, 4292–4296 CrossRef CAS
.
- K. Zhao, W. Wang, Y. Yang, K. Wang and D.-G. Yu, Results Phys., 2019, 15, 102770 CrossRef
.
- N. Nagiah, C. J. Murdock, M. Bhattacharjee, L. Nair and C. T. Laurencin, Sci. Rep., 2020, 10, 609 CrossRef CAS
.
- G.-Z. Yang, J.-J. Li, D.-G. Yu, M.-F. He, J.-H. Yang and G. R. Williams, Acta Biomater., 2017, 53, 233–241 CrossRef CAS
.
- Y. Yang, W. Li, D.-G. Yu, G. Wang, G. R. Williams and Z. Zhang, Carbohydr. Polym., 2019, 203, 228–237 CrossRef CAS PubMed
.
- D. P. Bhattarai, M. H. Kim, H. Park, W. H. Park, B. S. Kim and C. S. Kim, Chem. Eng. J., 2020, 389, 123470 CrossRef CAS
.
- H. Guo, S. Tan, J. Gao and L. Wang, J. Mater. Chem. B, 2020, 8, 1759–1770 RSC
.
- P. He, Y. Li, Z. Huang, Z.-Z. Guo, B. Luo, C.-R. Zhou and H. Li, J. Biomater. Appl., 2019, 34, 1041–1051 CrossRef
.
- M. Gong, C. Huang, Y. Huang, G. Li, C. Chi, J. Ye, W. Xie, R. Shi and L. Zhang, Nanomedicine, 2019, 17, 124–136 CrossRef CAS PubMed
.
- H. Kuang, Y. Wang, J. Hu, C. Wang, S. Lu and X. Mo, ACS Appl. Mater. Interfaces, 2018, 10, 19365–19372 CrossRef CAS PubMed
.
- R. Li, Y. Ma, Y. Zhang, M. Zhang and D. Sun, Colloids Surf., B, 2018, 169, 384–394 CrossRef CAS PubMed
.
- E. Shoba, R. Lakra, M. Syamala Kiran and P. S. Korrapati, Biomed. Mater., 2017, 12, 035005 CrossRef PubMed
.
- S. Chen, L. Ge, A. Mueller, M. A. Carlson, M. J. Teusink, F. D. Shuler and J. Xie, Nanomedicine, 2017, 13, 1435–1445 CrossRef CAS PubMed
.
- B. Poornima and P. S. Korrapati, Carbohydr. Polym., 2017, 157, 1741–1749 CrossRef CAS PubMed
.
- Y.-H. Hsu, C.-T. Lin, Y.-H. Yu, Y.-C. Chou, S.-J. Liu and E.-C. Chan, Int. J. Nanomed., 2016, 11, 3927–3937 CrossRef CAS PubMed
.
- G. Jin, M. P. Prabhakaran, D. Kai and S. Ramakrishna, Eur. J. Pharm. Biopharm., 2013, 85, 689–698 CrossRef CAS PubMed
.
- H. Xu, X. Xu, S. Li, W.-L. Song, D.-G. Yu and S. W. Annie Bligh, Biomolecules, 2021, 11(9), 1330 CrossRef CAS PubMed
.
- D.-G. Yu, X.-Y. Li, X. Wang, J.-H. Yang, S. W. A. Bligh and G. R. Williams, ACS Appl. Mater. Interfaces, 2015, 7, 18891–18897 CrossRef CAS PubMed
.
- X. Li, Y. He, J. Hou, G. Yang and S. Zhou, Small, 2020, 16, 1902262 CrossRef CAS PubMed
.
- M. Wang, J. Hou, D.-G. Yu, S. Li, J. Zhu and Z. Chen, J. Alloys Compd., 2020, 846, 156471 CrossRef CAS
.
- C.-K. Huang, K. Zhang, Q. Gong, D.-G. Yu, J. Wang, X. Tan and H. Quan, Int. J. Biol. Macromol., 2020, 152, 68–76 CrossRef CAS PubMed
.
- J. Hou, J. Yang, X. Zheng, M. Wang, Y. Liu and D.-G. Yu, Int. J. Pharm., 2020, 583, 119397 CrossRef CAS PubMed
.
- L. J. Villarreal-Gómez, J. M. Cornejo-Bravo, R. Vera-Graziano and D. Grande, J. Biomater. Sci., Polym. Ed., 2016, 27, 157–176 CrossRef PubMed
.
- P. Kamble, B. Sadarani, A. Majumdar and S. Bhullar, J. Drug Delivery Sci. Technol., 2017, 41, 124–133 CrossRef CAS
.
- R. Contreras-Cáceres, L. Cabeza, G. Perazzoli, A. Díaz, J. M. López-Romero, C. Melguizo and J. Prados, Nanomaterials, 2019, 9(4), 656 CrossRef PubMed
.
- M. Jin, D.-G. Yu, X. Wang, C. F. G. C. Geraldes, G. R. Williams and S. W. A. Bligh, Adv. Healthcare Mater., 2016, 5, 977–985 CrossRef CAS PubMed
.
- S. Wen, Y. Hu, Y. Zhang, S. Huang, Y. Zuo and Y. Min, Mater. Sci. Eng., C, 2019, 100, 514–522 CrossRef CAS PubMed
.
- C. Liu, C. Wang, Q. Zhao, X. Li, F. Xu, X. Yao and M. Wang, Biomed. Mater., 2018, 13, 044107 CrossRef PubMed
.
- J. K. Bar, T. Kowalczyk, P. G. Grelewski, S. Stamnitz, M. Paprocka, J. Lis, A. Lis-Nawara, S. An and A. Klimczak, Materials, 2022, 15(5), 1900 CrossRef CAS PubMed
.
- C. Li, C. Vepari, H. J. Jin, H. J. Kim and D. L. Kaplan, Biomaterials, 2006, 27, 3115–3124 CrossRef CAS PubMed
.
- A. Martins, A. R. C. Duarte, S. Faria, A. P. Marques, R. L. Reis and N. M. Neves, Biomaterials, 2010, 31, 5875–5885 CrossRef CAS PubMed
.
- M. Gong, C. Chi, J. Ye, M. Liao, W. Xie, C. Wu, R. Shi and L. Zhang, Colloids Surf., B, 2018, 170, 201–209 CrossRef CAS PubMed
.
- J. K. Park, J.-H. Shim, K. S. Kang, J. Yeom, H. S. Jung, J. Y. Kim, K. H. Lee, T.-H. Kim, S.-Y. Kim, D.-W. Cho and S. K. Hahn, Adv. Funct. Mater., 2011, 21, 2906–2912 CrossRef CAS
.
- L. Yin, S. Yang, M. He, Y. Chang, K. Wang, Y. Zhu, Y. Liu, Y. Chang and Z. Yu, J. Mater. Sci.: Mater. Med., 2017, 28, 94 CrossRef PubMed
.
- A. S. Timin, A. R. Muslimov, M. V. Zyuzin, O. O. Peltek, T. E. Karpov, I. S. Sergeev, A. I. Dotsenko, A. A. Goncharenko, N. D. Yolshin, A. Sinelnik, B. Krause, T. Baumbach, M. A. Surmeneva, R. V. Chernozem, G. B. Sukhorukov and R. A. Surmenev, ACS Appl. Mater. Interfaces, 2018, 10, 34849–34868 CrossRef CAS PubMed
.
- Y. Wang, Y. Wei, X. Zhang, M. Xu, F. Liu, Q. Ma, Q. Cai and X. Deng, Chem. Eng. J., 2015, 273, 490–501 CrossRef CAS
.
- H. Liu, W. Li, B. Luo, X. Chen, W. Wen and C. Zhou, Mater. Sci. Eng., C, 2017, 79, 399–409 CrossRef CAS
.
- Š. Zupančič, S. Baumgartner, Z. Lavrič, M. Petelin and J. Kristl, J. Drug Delivery Sci. Technol., 2015, 30, 408–416 CrossRef
.
- Š. Zupančič, L. Casula, T. Rijavec, A. Lapanje, M. Luštrik, A. M. Fadda, P. Kocbek and J. Kristl, J. Controlled Release, 2019, 316, 223–235 CrossRef
.
- S. Chen, Y. Hao, W. Cui, J. Chang and Y. Zhou, J. Mater. Sci., 2013, 48, 6567–6577 CrossRef CAS
.
- M. Ranjbar-Mohammadi, M. Zamani, M. P. Prabhakaran, S. H. Bahrami and S. Ramakrishna, Mater. Sci. Eng., C, 2016, 58, 521–531 CrossRef CAS PubMed
.
- Y. Wang, H. Li, Y. Feng, P. Jiang, J. Su and C. Huang, Int. J. Nanomed., 2019, 14, 963–976 CrossRef CAS PubMed
.
- M. Heidari, S. H. Bahrami, M. Ranjbar-Mohammadi and P. B. Milan, Mater. Sci. Eng., C, 2019, 103, 109768 CrossRef CAS PubMed
.
- J. Qian, Z. Lin, Y. Liu, Z. Wang, Y. Lin, C. Gong, R. Ruan, J. Zhang and H. Yang, Smart Mater. Med., 2021, 2, 260–279 CrossRef
.
- B. Xia and Y. Lv, Mater. Sci. Eng., C, 2018, 82, 253–264 CrossRef CAS PubMed
.
- T. M. Dinis, G. Vidal, R. R. Jose, P. Vigneron, D. Bresson, V. Fitzpatrick, F. Marin, D. L. Kaplan and C. Egles, PLoS One, 2014, 9, e109770 CrossRef PubMed
.
- L. R. Pires, V. Guarino, M. J. Oliveira, C. C. Ribeiro, M. A. Barbosa, L. Ambrosio and A. P. Pêgo, J. Tissue Eng. Regener. Med., 2016, 10, E154–E166 CrossRef CAS PubMed
.
- Z. He, H. Zang, L. Zhu, K. Huang, T. Yi, S. Zhang and S. Cheng, Int. J. Nanomed., 2019, 14, 721–732 CrossRef PubMed
.
- S. Selvaraj and N. N. Fathima, ACS Appl. Mater. Interfaces, 2017, 9, 5916–5926 CrossRef CAS
.
- J. He, Y. Liang, M. Shi and B. Guo, Chem. Eng. J., 2020, 385, 123464 CrossRef CAS
.
- P.-O. Rujitanaroj, N. Pimpha and P. Supaphol, Polymer, 2008, 49, 4723–4732 CrossRef CAS
.
- Y. Xi, J. Ge, Y. Guo, B. Lei and P. X. Ma, ACS Nano, 2018, 12, 10772–10784 CrossRef CAS PubMed
.
- G. Rivero, M. Meuter, A. Pepe, M. G. Guevara, A. R. Boccaccini and G. A. Abraham, Colloids Surf., A, 2020, 587, 124313 CrossRef CAS
.
- H. Li, M. Wang, G. R. Williams, J. Wu, X. Sun, Y. Lv and L.-M. Zhu, RSC Adv., 2016, 6, 50267–50277 RSC
.
- M. Doostmohammadi, H. Forootanfar, M. Shakibaie, M. Torkzadeh-Mahani, H.-R. Rahimi, E. Jafari, A. Ameri and B. Amirheidari, J. Biomater. Appl., 2021, 36, 193–209 CrossRef CAS
.
- O. Mirdailami, M. Soleimani, R. Dinarvand, M. R. Khoshayand, M. Norouzi, A. Hajarizadeh, M. Dodel and F. Atyabi, J. Biomed. Mater. Res., Part A, 2015, 103, 3374–3385 CrossRef CAS PubMed
.
- M. S. Lee, T. Ahmad, J. Lee, H. K. Awada, Y. Wang, K. Kim, H. Shin and H. S. Yang, Biomaterials, 2017, 124, 65–77 CrossRef CAS PubMed
.
- F. Nejaddehbashi, M. Hashemitabar, V. Bayati, M. Abbaspour, E. Moghimipour and M. Orazizadeh, Artif. Organs, 2019, 43, 413–423 CrossRef CAS
.
- J. Zhan, H. Xu, Y. Zhong, Q. Wu and Z. Liu, Mater. Des., 2020, 188, 108432 CrossRef CAS
.
- X. Zhang, R. Guo, J. Xu, Y. Lan, Y. Jiao, C. Zhou and Y. Zhao, J. Biomater. Appl., 2015, 30, 512–525 CrossRef CAS
.
- D. Kharaghani, P. Gitigard, H. Ohtani, K. O. Kim, S. Ullah, Y. Saito, M. Q. Khan and I. S. Kim, Sci. Rep., 2019, 9, 12640 CrossRef
.
- C. M. Hu and L. Zhang, Biochem. Pharmacol., 2012, 83, 1104–1111 CrossRef CAS
.
- J. J. Grob, M. M. Amonkar, B. Karaszewska, J. Schachter, R. Dummer, A. Mackiewicz, D. Stroyakovskiy, K. Drucis, F. Grange, V. Chiarion-Sileni, P. Rutkowski, M. Lichinitser, E. Levchenko, P. Wolter, A. Hauschild, G. V. Long, P. Nathan, A. Ribas, K. Flaherty, P. Sun, J. J. Legos, D. O. McDowell, B. Mookerjee, D. Schadendorf and C. Robert, Lancet Oncol., 2015, 16, 1389–1398 CrossRef CAS
.
- J. Larkin, V. Chiarion-Sileni, R. Gonzalez, J. J. Grob, C. L. Cowey, C. D. Lao, D. Schadendorf, R. Dummer, M. Smylie, P. Rutkowski, P. F. Ferrucci, A. Hill, J. Wagstaff, M. S. Carlino, J. B. Haanen, M. Maio, I. Marquez-Rodas, G. A. McArthur, P. A. Ascierto, G. V. Long, M. K. Callahan, M. A. Postow, K. Grossmann, M. Sznol, B. Dreno, L. Bastholt, A. Yang, L. M. Rollin, C. Horak, F. S. Hodi and J. D. Wolchok, N. Engl. J. Med., 2015, 373, 23–34 CrossRef
.
-
A. R. K. Sasikala, A. R. Unnithan, C. H. Park and C. S. Kim, in Biomimetic Nanoengineered Materials for Advanced Drug Delivery, ed. A. R. Unnithan, A. R. K. Sasikala, C. H. Park and C. S. Kim, Elsevier, 2019, pp. 11–36, DOI:10.1016/B978-0-12-814944-7.00002-3
.
- J. Wei, J. Hu, M. Li, Y. Chen and Y. Chen, RSC Adv., 2014, 4, 28011–28019 RSC
.
- C. Lei, Y. Cui, L. Zheng, P. K. Chow and C. H. Wang, Biomaterials, 2013, 34, 7483–7494 CrossRef CAS PubMed
.
- Y. He, X. Li, J. Ma, G. Ni, G. Yang and S. Zhou, Small, 2019, 15, 1804397 CrossRef PubMed
.
- T. Al-Attar and S. V. Madihally, Int. J. Pharm., 2019, 569, 118599 CrossRef CAS PubMed
.
- X. Zhao, Z. Yuan, L. Yildirimer, J. Zhao, Z. Y. Lin, Z. Cao, G. Pan and W. Cui, Small, 2015, 11, 4284–4291 CrossRef CAS PubMed
.
- A. R. K. Sasikala, A. R. Unnithan, Y.-H. Yun, C. H. Park and C. S. Kim, Acta Biomater., 2016, 31, 122–133 CrossRef CAS PubMed
.
- F. Serio, N. Silvestri, S. Kumar Avugadda, G. E. P. Nucci, S. Nitti, V. Onesto, F. Catalano, E. D'Amone, G. Gigli, L. L. del Mercato and T. Pellegrino, J. Colloid Interface Sci., 2022, 607, 34–44 CrossRef CAS PubMed
.
- S.-F. Shen, L.-F. Zhu, J. Liu, A. Ali, A. Zaman, Z. Ahmad, X. Chen and M.-W. Chang, J. Drug Delivery Sci. Technol., 2020, 59, 101865 CrossRef CAS
.
- S. Abid, T. Hussain, Z. A. Raza and A. Nazir, Mater. Sci. Eng., C, 2019, 97, 966–977 CrossRef CAS PubMed
.
- M. Khodadadi, S. Alijani, M. Montazeri, N. Esmaeilizadeh, S. Sadeghi-Soureh and Y. Pilehvar-Soltanahmadi, J. Biomed. Mater. Res., Part A, 2020, 108, 1444–1458 CrossRef CAS PubMed
.
- J. Zhao and W. Cui, Adv. Fiber Mater., 2020, 2, 229–245 CrossRef CAS
.
- C. Z. Mosher, P. A. P. Brudnicki, Z. Gong, H. R. Childs, S. W. Lee, R. M. Antrobus, E. C. Fang, T. N. Schiros and H. H. Lu, Biofabrication, 2021, 13, 035049 CrossRef CAS PubMed
.
- T. Zhang, B. A. Howell and P. B. Smith, Ind. Eng. Chem. Res., 2017, 56, 1661–1670 CrossRef CAS
.
- D. Kolbuk, O. Jeznach, M. Wrzecionek and A. Gadomska-Gajadhur, Polymers, 2020, 12, 1731 CrossRef CAS PubMed
.
- M. Wrzecionek, A. Bandzerewicz, E. Dutkowska, J. Dulnik, P. Denis and A. Gadomska-Gajadhur, Polym. Adv. Technol., 2021, 32, 3955–3966 CrossRef CAS
.
- J. Dulnik, D. Kołbuk, P. Denis and P. Sajkiewicz, Eur. Polym. J., 2018, 104, 147–156 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2023 |



