Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A novel low-cost plug-and-play multi-spectral LED based fluorometer, with application to chlorophyll detection
Sean M.
Power
 ,
Louis
Free
,
Adrian
Delgado
,
Louis
Free
,
Adrian
Delgado
 ,
Chloe
Richards
,
Elena
Alvarez-Gomez
,
Ciprian
Briciu-Burghina
,
Chloe
Richards
,
Elena
Alvarez-Gomez
,
Ciprian
Briciu-Burghina
 and
Fiona
Regan
and
Fiona
Regan
 *
*
DCU Water Institute, School of Chemical Sciences, Dublin City University, Dublin, Ireland. E-mail: fiona.regan@dcu.ie
First published on 11th October 2023
Abstract
In this paper a novel low-cost multi-spectral optical fluorometer is presented and evaluated. The device uses a range of LEDs in the blue and violet regions of the electromagnetic spectrum and a mini-spectrometer to detect the emitted fluorescence in the UV to IR spectrum region. Custom built electronics and software were designed to control the system and the components were housed in bespoke 3D printed parts. A number of known fluorophores were tested to determine the capabilities of the fluorometer. Application of the device is demonstrated for the detection of chlorophyll a (Chl a) from laboratory grown algae and from environmental samples while analytical performance is established using both in vivo and extracted Chl a fluorescence and by comparison with a benchtop fluorometer.
Introduction
With increasing environmental pressure due to global climate change, increases in global population and the need for sustainable obtained resources, water resources management is critical.1 Sensors and instrumentation play a key role in water management, and are becoming smaller, smarter and more economical.2–5 Optical based sensors are receiving great interest due recent advances in photonics and the ability to work in harsh environments.2,6 In aquatic environments, fluorescence based spectroscopy is a common technique used for detection and quantification of chlorophyll (Chl a),4,7,8 petroleum compounds,9 fDOM (fluorescent dissolved organic matter),10,11 tryptophan,12 algal populations13–15 and faecal indicator bacteria.16,17 Chlorophyll measurement in aquatic systems is an indicator of phytoplankton biomass and primary production in response to nutrients and light availability. Excessive enrichment of waters with nutrients causes eutrophication, harmful algal blooms and loss in species diversity.18,19 Analysis and monitoring of Chl a is critical in determining the trophic status of water bodies,20 time-series trends21 and where applicable reactive management. For example, the Marine Water Framework Directive (MWFD) aims to achieve ‘good environmental status of the EU's marine waters’ and the Water Framework Directive aims to achieve ‘good ecological status’.22 Chlorophyll fluorescence has been primarily measured in vitro after extraction in acetone and has led to the development of standard methods.23 Today, measurements are routinely carried in vivo using in situ submersible fluorometers7 and remotely using satellites and aircraft. Naturally, chlorophyll occurs in several distinct forms, with a and b being the major types found in higher plants and green algae. Combined, chlorophyll and other cellular components and pigments inside phytoplankton cells have a maximum absorption near 440 nm and maximum fluorescence at 685 nm.24,25 These excitation/emission pairs or similar have been used historically for chlorophyll measurements.26Fluorescent molecules absorb energy in the form of light, and after undergoing a non-radiative process, light of a longer wavelengths is emitted as fluorescence.27 This makes fluorescence a very sensitive and attractive techniques as the measurements are carried in the absence of incident light. For the detection of fluorescence, monochromators, such as a diffraction grating or optical filters are typically used to select the desired emission wavelength. Once filtered this light is typically directed towards a photo-multiplier tube (PMT) for detection. These systems for fluorescence detection are typically rather bulky and not suitable for in situ or continuous monitoring of local events. Thus the majority of environmental sensors, either for fluorescence or scatter measurements employ photodiodes as photodetectors and light emitting diodes (LED) as light sources.7 Selectivity to target analytes is thus achieved through the use of LEDs with narrow bandwidth emissions, laser diodes and optical filters which in general block or reduce the leaching of incident light into the photodiodes.16,28–33 In the race to miniaturisation and field application a range of photodetectors have been demonstrated in the literature for different applications and include: PMTs4,34,35 which although have excellent sensitivity require higher voltages to operate, camera sensors,36–38 PDs16,28,39 and CdS phototransistors.3,33
Due to the low cost, small size and improved performance these optical components have accelerated the development of in situ fluorometers. However, limitations still exist due to the complex composition of environmental waters and the overlapping of absorption or emission spectra associated with the presence of different optically active compounds.11,40 While PDs and PMTs provide poor spectral resolution, array detectors provide multi-spectral resolution which in turn captures multiple fluorescence emission bands associated with the presence of different constituents. Thus the higher resolution enables spectral deconvolution and the assigning of emission peaks to environmental constituents. Miniaturised spectrometers now exist to enable the fabrication of low-cost portable sensors with a recent demonstration for the use of the Hamamatsu C12880MA spectrometer chip for absorbance measurements.38
In this context, a novel plug-and-play LED board and miniaturised spectrometer system was designed and evaluated to detect and quantify in vivo fluorescence associated with Chl a. Initial performance was investigated with fluorescent chemical standards. System benchmarking was carried out against extracted Chl a reference measurements with laboratory cultured algae. Analysis of environmental samples was used to demonstrate system capabilities for in vivo Chl a detection and application to in situ monitoring.
Experimental
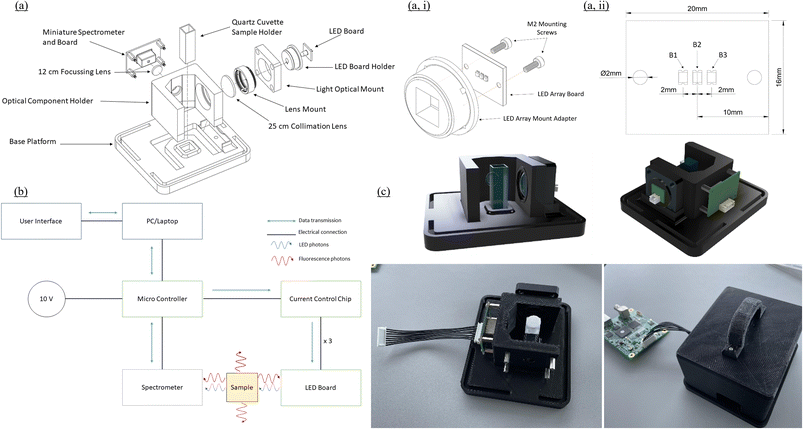
Fluorometer design and construction
| Component | Part number | Supplier | Quantity | Unit cost (€) | Total cost (€) | |
|---|---|---|---|---|---|---|
| a PCB-printed circuit board. | ||||||
| Optical | CMOS, photodetector | C12880MA | Hamamatsu Photonics, UK | 1 | 200 | 200 |
| Colimitation lens | 36689 | Edmund Optics, UK | 1 | 94.50 | 94.50 | |
| Plano-convex lens | 48668 | Edmund Optics, UK | 1 | 83.00 | 83.00 | |
| Lens tube | SM1L03 | ThorLabs, UK | 1 | 11.95 | 11.95 | |
| Threaded 30 mm cage plate | CP33/M | ThorLabs, UK | 1 | 17.35 | 17.35 | |
| 360 nm LED (B1) | ATS2012UV365 | Mouser Electronics, Ireland | 1 | 2.19 | 2.19 | |
| 380 nm LED (B2) | ATS2012UV385 | Mouser Electronics, Ireland | 1 | 1.56 | 1.56 | |
| 430 nm LED (B3) | KP-2012MBC | Farnell, Ireland | 1 | 0.86 | 0.86 | |
| Electronic | Teensy 3.2 microcontroller | DEV-13736 | Digikey, Ireland | 1 | 18.05 | 18.05 |
| 5 V LDO voltage regulator | L7805ACD2T-TR | Digikey, Ireland | 1 | 0.49 | 0.49 | |
| LED driver | TLC59116FIPWR | Digikey, Ireland | 1 | 1.92 | 1.92 | |
| Custom PCBs | Control board | — | Beta Layout, Ireland | 1 | 25.70 | 25.70 |
| LED mounting board | — | Beta layout, Ireland | 1 | 14.95 | 14.95 | |
| CMOS mounting board | — | Beta Layout, Ireland | 1 | 8.107 | 8.11 | |
| Total | 480.62 | |||||
Enclosure design iterations were carried out in SolidWorks (Fig. 1c) while the assembly parts were FDM (fused deposition modelling) 3D printed from PLA (polyacetic acid) filament using the Lulzbot Taz63D printer (Fig. 1c).
| LED | Dimensions (L × W × T) | Radiant flux (mW) | FWHM (nm) |
|---|---|---|---|
| B1 (λ = 360 nm) | 2 × 1.5 × 0.75 | 13 | 10 |
| B2 (λ = 380 nm) | 2 × 1.5 × 0.75 | 16 | 12 |
| B3 (λ = 430 nm) | 2 × 1.2 × 1.1 | 8 | 60 |
To detect the fluorescence emitted from the sample a 1.27 cm UV fused silica plano-convex lens with a 10 mm focal length was used to focus light onto the entrance slit of a Hamamatsu C12880MA mini-spectrometer. The mini-spectrometer has a spectral response range between 340 and 850 nm with a typical resolution of 12 nm (FWHM).
Light incident on the entrance slit of the spectrometer is passed to a reflective concave blazed grating. The light is reflected from the grating onto a high-sensitivity CMOS (complementary metal oxide semiconductor) linear image sensor with 288 pixels each corresponding to a particular wavelength, given by
| λ = A0 + B1n + B2n2 + B3n3 + B4n4 + B5n5 |
Proof of concept qualitative experiments
Initially, three high concentration solutions of quinine sulphate (Sigma-Aldrich, Ireland), extracted Chl a and cyan fluorescent protein (CFP) (Fisher Scientific, Ireland) were used for screening system application. Sample cells were 3.5 mL (10 × 10 mm) and UV quartz cuvettes, equipped with a Teflon stopper (Hellma Analytics). An appropriate integration time for each fluorophore was established by turning each LED on and adjusting the integration time until the peak of each fluorescence signal reached approximately 80–90% of the maximum signal. This ensured a high dynamic range without detector saturation.Analytical performance with laboratory standards
The analytical performance of the device was assessed with laboratory prepared standards of Basic Blue 3 dye (BB3), Sigma-Aldrich, Ireland and comparison with a Jasco FP-8300 benchtop fluorometer.Performance evaluation with algae cultures
Laboratory grown algae were used to determine the fluorometers capabilities to detect chlorophyll fluorescence. The diatom species Nitzschia ovalis (SAMS Limited, Scotland) was selected and cultured in filtered artificial seawater water (0.45 μm) supplemented with nutrients to make Guillard's F/2 + Si medium.42 The cultures were grown in batch growth system in 5 L Pyrex bottles (15–23 °C, 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 light
12 light![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dark, continuous light of 65 μmol m−2 s−1). The concentration of biomass was calculated by determining the concentration of Chl a in 25 mL aliquots that were filtered at low pressure (700 mb) through GF/F glass fibre filters (Whatman GF/F, pore size 0.7 μm) kept in the dark at −20 °C for 12 hours. Subsequently, 8 mL of 90% (v/v) acetone was added to each 25 mL filtered aliquot, followed by sonication in an ultrasound bath for 5 min. Extraction was carried out in the dark at 4 °C for at least three hours following centrifugation (5000 rpm, 10 minutes) and recovery of the supernatant. Absorbance was read at 665 nm and 750 nm in a Shimadzu UV-1800 UV-Vis double beam spectrophotometer allowing the calculation of Chl a concentration as outlined in ref. 43 and conversion to μg L−1 concentrations.
dark, continuous light of 65 μmol m−2 s−1). The concentration of biomass was calculated by determining the concentration of Chl a in 25 mL aliquots that were filtered at low pressure (700 mb) through GF/F glass fibre filters (Whatman GF/F, pore size 0.7 μm) kept in the dark at −20 °C for 12 hours. Subsequently, 8 mL of 90% (v/v) acetone was added to each 25 mL filtered aliquot, followed by sonication in an ultrasound bath for 5 min. Extraction was carried out in the dark at 4 °C for at least three hours following centrifugation (5000 rpm, 10 minutes) and recovery of the supernatant. Absorbance was read at 665 nm and 750 nm in a Shimadzu UV-1800 UV-Vis double beam spectrophotometer allowing the calculation of Chl a concentration as outlined in ref. 43 and conversion to μg L−1 concentrations.
Environmental case study
Environmental samples were used to determine if it was possible for the sensor to be used for in vivo fluorescence. Samples were collected from Dublin Harbour and can be classified as brackish. Sample were analysed untreated on the bench-top system, with fluorescence scans collected for all three LEDs.Results and discussion
LED evaluation
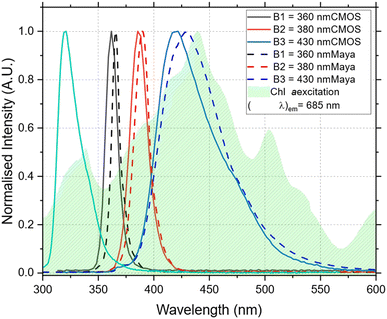
The normalized spectrum of each LED recorded by the mini-spectrometer and a commercial spectrometer, Maya 2000 PRO Series, Ocean Optics Inc., US is shown in Fig. 2. LEDs B1 and B2 have narrow band emissions with a FWHM (full width at half maximum) of 14 nm and 19 nm respectively as recorded on the mini spectrometer and 9 nm and 18 nm on the Maya spectrometer. B3 on the other hand has a much broader and asymmetrical spectrum than the other LEDs with a FWHM of 60 nm recorded on the mini-spectrometer and 58 nm on the Maya spectrometer. Overall, the LEDs cover a large portion of the blue and violet portions of the visible and near UV region of the spectrum making them good candidates for inducing fluorescence in fluorophores with excitation energies in this region. In particular, the LEDs were selected to excite Chl a and maximise light absorption but also to limit the leaching of scatter light into the photodetector at higher wavelengths (Fig. 2).Proof of concept qualitative experiments
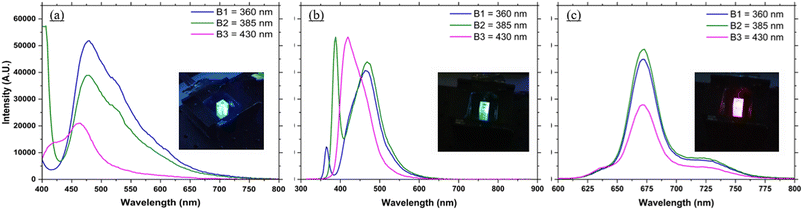
Fig. 3 shows the recorded spectrum of each of the proof of concept fluorophores, with an inlay of the visible fluorescence. It was possible using each LED to induce fluorescence in each of the fluorophores tested. Fig. 3a shows the signal recorded for each LED using quinine sulfate. The fluorescence from the fluorophore was visible to the eye, and it was also possible to record the fluorescence emitted from the fluorophore and spectrally resolve it using the mini-spectrometer. Quinine sulfate is a broadband fluorescence emitter, fluorescing between approximately 400–600 nm. As such there was some overlap between the LED signal and fluorescence signal for LEDs B1 and B2, while for LED B3 the LED and fluorescence signal combine together making it not possible to fully spectrally resolve the two signals, although it was still possible to see the fluorescence with the naked eye. Fig. 3b shows the fluorescence and spectrum recorded for each LED using CFP. Again in all cases fluorescence was visible seen using as three LEDs. For LED B3 it was once again not possible to spatially resolve the fluorescence signal from the LED signal, however using B1 and B2 there was a small overlap between the LED and fluorescence spectrum, there was sufficient resolution to spectrally resolve the two signals. CFP is a broadband fluorescent maker with a fluorescence spectrum between 410 and 500 nm (FWHM) peaking at 485 nm typically used as a biological marker for physiological processes, visualizing protein localization and detecting transgenic expression in vivo. Fig. 3c shows visually the fluorescence signal from the diatom algae solution and the fluorescence signal recorded for each LED. The chlorophyll sample is a broad emitter, with fluorescence from 625–725 nm. The signal has three peaks at 640 nm, 680 nm and 720 nm with the peak at 640 nm associated with Chl b and the peaks at 680 nm and 720 nm associated with Chl a. As the fluorescence was far from the LED signals it was possible to fully resolve the fluorescence signal from the LED signals. The signal detected was strongest for the blue LEDs (B1, B2 and B3) and much weaker for the UV LEDs (UV1 and UV2) due to limitations in how high the integration time could be set using the Hamamatsu software, set at 1 s. For later experiments, custom built software was written that allowed for longer integrations times up to typically 4 s, allowing weaker signals to be detected.Analytical performance with laboratory standards
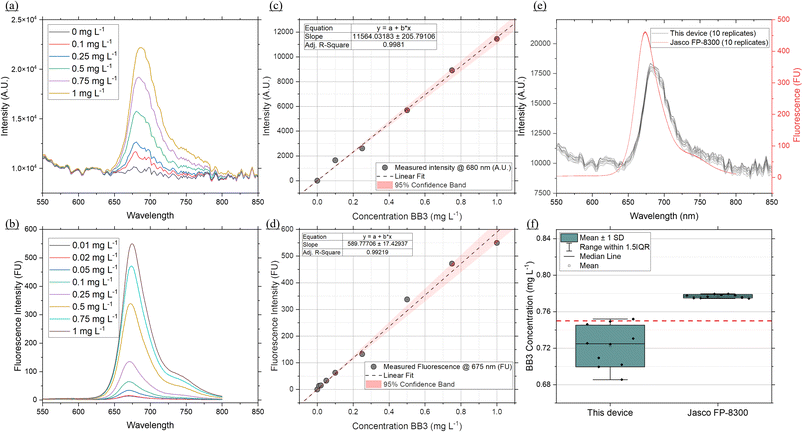
Fluorometers that measure in vivo fluorescence rely on analytical standards for calibration and quality control.44 This approach is used to ensure reproducibility by using a stable analytical grade standard. Once the devices are calibrated to a given standard, the response can be converted to equivalent standard concentration and conversion factors can be used to infer Chl a concentration from in vivo Chl a measurements.45 Fluorometers relying on in vivo measurements, target fluorescence emitted by Chl a molecules in photosystem II, for which the peak fluorescence emission is approx. 685 nm, although not all Chl a present is in photosystem II.45 Standard stability and excitation/emission overlap with Chl a is thus critical in selecting a suitable standard.44 For this reason and high solubility in water, Basic Blue 3 (BB3) was selected to benchmark the performance of the fluorometer developed here against a commercial benchtop fluorometer. Serial dilutions of BB3 in the 0–1 mg L−1 range were used to collect emission scans on both instruments (Fig. 4a and b) and develop calibration curves (Fig. 4c and d). This BB3 concentration range and the fluorescence emitted was determined previously, to correspond to the expected fluorescence of Chl a in the 0.01 to 10 μg L−1 range.45,46 The benchtop fluorometer was set to the highest sensitivity setting available while the fluorometer developed here used a 2 s integration time. This integration time was optimised to maximise the dynamic range of the instrument and reduce signal-to-noise ratio. While all the BB3 dilutions were successfully detected on the benchtop fluorometer, the CMOS based fluorometer could not detect the dilutions in the low range (i.e. 0.01, 0.02 and 0.05 mg L−1), Fig. 4, due to a higher signal-to-noise ratio. The calibration curve was thus constructed using the dilutions in the 0.1–1 mg L−1 range. To determine the precision and accuracy of the CMOS based fluorometer, a known concentration of BB3 (0.75 mg L−1) was run 10 times on both instruments (Fig. 4e). Using the developed calibration curves, each run was converted to BB3 concentration (mg L−1). The average concentration was found to be 0.72 ± 0.022 for this device and 0.78 ± 0.0018 for the Jasco fluorometer with a % RSD of 3.004 and 0.23 respectively (Fig. 4f). Although the Jasco fluorometer demonstrates a higher precision, the % RSD showed by the CMOS fluorometer is within the 5% margin of error acceptable for sensors with environmental applications.Application to extracted Chl a
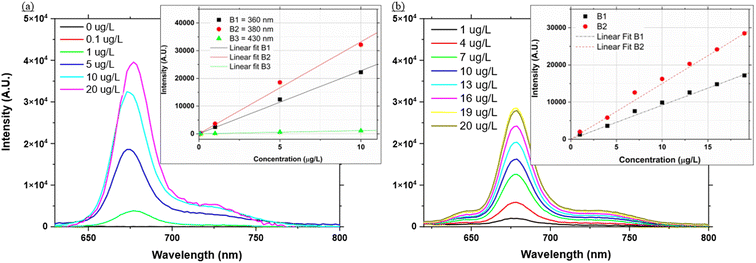
Fig. 5a shows a sample of the Chl a spectrum using different concentrations of Chl a extracted in acetone using LED B2 (λ = 380 nm). For each of the three LEDs used in this work it was possible to detect a fluorescence signal for Chl a concentrations between 1 and 20 μg L−1, with LED B2 achieving the highest signal for each concentration and LED B3 (λ = 430 nm) recording the lowest signal intensity.A calibration curve for each LED response was created by taking the peak of the fluorescence signal at 675 nm as recorded by the mini-spectrometer (Fig. 5a, Table 3). Due to the redshift in the fluorescence signal at 20 μg L−1 it was excluded from the calibration.
| LED | Extracted Chl a | In vivo Chl a | ||
|---|---|---|---|---|
| Slope | R 2 | Slope | R 2 | |
| a ND-not determined. | ||||
| B1 (λ = 360 nm) | 2248.1 | 0.997 | 878.39 | 0.991 |
| B2 (λ = 380 nm) | 3265.3 | 0.994 | 1498.4 | 0.995 |
| B3 (λ = 430 nm) | 115.03 | 0.991 | ND | ND |
In vivo application
Fig. 5b shows a sample of the spectra recorded using LED B2 of the diatom algae. Unlike the previous section these spectra were recorded in vivo. The measured response is widely used in environmental sensors as a proxy for Chl a concentration.45Fluorescence was successfully detected throughout the diatom algae concentrations with all the LEDs. LED B3 however showed the least change with increasing concentrations and was excluded from the calibration. Collected emission scans for LED B2 and calibration curves for LEDs B1 and B2 are presented in Fig. 5b while the calibration coefficients are presented in Table 3. Similarly to the extracted Chl a, LED B2 was the most sensitive to changes in concentration. Globally, Chl a concentrations range from 0.01 to 10 μg L−1.45,46 In terms of analytical performance, the system can target and successfully quantify Chl a at environmental concentrations for both in vivo measurements and combined acetone extraction.
Application to environmental samples
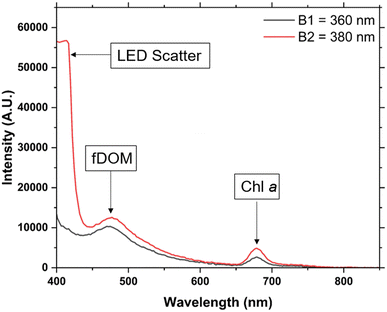
An example of the recorded fluorescence emission spectrum collected for environmental sample is shown in Fig. 6, between 400 and 850 nm for LEDs B1 and B2. No fluorescence signal was detected for B3, thus it is not shown. Using the calibration curves developed in the previous section it was possible to calculate the Chl a concentration by dividing the fluorescence signal peak by the slope of the calibration curve. Using the in vivo calibration curves developed with laboratory grown diatoms (Fig. 5b, Table 3) a concentration of 3.05 μg L−1 was calculated for LED B1 and 3.25 μg L−1 for LED B2 respectively. The Chl a concentration in the sample, following the standard method using filtration and acetone extraction was found to be 2.16 ± 0.24. Such discrepancy is expected as slope coefficients used are for in vivo Chl a fluorescence determined with laboratory cultures are expected to be different from environmental mixed populations. In general, the calibration of fluorimeters is carried out on site specific samples due to the large natural variability in the relationship between in situ fluorescence and extracted Chl a concentrations. Globally, the slope factor can range from 1 in the Arabian Sea region to greater than 6 in the Southern Ocean province, south of New Zeeland.45 With the two sources of uncertainty when measuring in vivo being the variability in the Chl a specific absorption47 and the variability in the fluorescence quantum yield.46The set-up while not as sophisticated as devices mentioned in the Introduction section was still consistently able to induce and detect fluorescence from all samples tested, and does however solve some issues presented such as costing, reliability and overall footprint, potentially allowing in situ real time monitoring of environmental events and changes over time. Algal species for example are a vital component of aquatic marine life, serving as the basis of the oceanic food chain. As a species, their environment is threatened by global warming and climate change, and therefore monitoring their levels in oceanic environments may be an important warning mechanism while tracking the impacts of climate change. Should a suitable housing environment be developed this technology may be deployable in marine environments to monitor changes in algal populations at a relatively low-cost.
The experimental work in this work showed that it was possible to detect fluorescence produced by Chl a samples at expected environmental concentrations and using the calibration curves quantify those values, making this technology a potentially vital tool for environmental monitoring. Detection capabilities within the μg L−1 are essential for real-world application, and similar devices have achieved this range using PMTs4,48 and PDs.28 The CMOS mini-spectrometer however has a spectral response range between 340 and 850 nm and a 12 nm spectral resolution. These key features enable spectral deconvolution of overlapping optical signatures and critically, detection of other optical targets within this range. Furthermore the compact, small design of the CMOS mini-spectrometer enables integration into submersible, in situ devices where size of electronic components is critical. The C12880MA chip employs a nano-printed reflective concave blazed grating which minimizes the light path and enables the realisation of small, compact detectors. By comparison, a longer optical path length is required when diffraction gratings are used in conjunction with CMOS based cameras.36 The use of LEDs means that the set-up can be flexible in determining the desired excitation wavelength for a particular fluorophore, by simply changing the LED to another wavelength in a plug-and-play manner or designing a multi-wavelength LED electronic board to hold a range of LEDs. The current optical design comprising collimating lenses facilitates the addition of multiple SMD LEDs on the same board thus enabling the detection and quantification of multiple target analytes. The optical components used were kept simple by limiting the optics to a collimating and focusing lens. More sophisticated and complicated components could be incorporated into the design in situations where narrow bands are required or the Stokes shift between the excitation and emission bands is too small.
Conclusions
Throughout this work a novel, modular fluorescence-based sensor has been developed. The sensor makes use of a range of LEDs to induce fluorescence in the sample under investigation and a miniature spectrometer to detect and spectrally resolve the incoming signal. A set of proof-of-concept experiments is presented to demonstrate the capabilities of the system in distinguishing between different fluorophores and optical signatures. Analytical performance of the device is established against analytical standards by comparison with a benchtop fluorometer. Application of the device is demonstrated for the detection and quantification of a Chl a extracts and Chl a in vivo from laboratory grown cultures and environmental samples. The simple design of the system enables a plug and play configuration, where the LEDs can be easily replaced or expanded to target other optical parameters of interest. In such a configuration specificity to target constituents can be achieved through the use of multi-LED arrays turned on sequentially, while the detector scans and records successive emission or scatter spectra. A key advantage of the device presented is the use the CMOS mini-spectrometer which provides the spectral resolution to resolve overlapping optical signatures by working in tandem with the sequentially operated LED board.Author contributions
Conceptualization: LF, SP, CBB, FR; data curation: LF, SP, CR, AD, EAG, CBB; funding acquisition: FR methodology: LF, SP, AD; project administration: CBB, FR; software: LF, SP; supervision: CBB, FR; writing original draft: LF; writing review & editing: SP, CBB, FR.Conflicts of interest
There are no conflicts to declare.Notes and references
- C. Whitt, J. Pearlman, B. Polagye, F. Caimi and F. Muller-Karger, et al., Future Vision for Autonomous Ocean Observations, Front. Mar. Sci., 2020, 7, 697 CrossRef.
- C. Briciu-Burghina, S. Power, A. Delgado and F. Regan, Sensors for Coastal and Ocean Monitoring, Annu. Rev. Anal. Chem., 2023, 16(1), 451–469 CrossRef PubMed.
- L. A. Porter, C. A. Chapman and J. A. Alaniz, Simple and Inexpensive 3D Printed Filter Fluorometer Designs: User-Friendly Instrument Models for Laboratory Learning and Outreach Activities, J. Chem. Educ., 2017, 94(1), 105–111 CrossRef CAS.
- Y. H. Shin, M. T. Gutierrez-Wing and J. W. Choi, A field-deployable and handheld fluorometer for environmental water quality monitoring, Micro Nano Syst. Lett., 2018, 6(1), 16 CrossRef.
- Z. Yang, T. Albrow-Owen, W. Cai and T. Hasan, Miniaturization of optical spectrometers, Science, 2021, 371(6528), eabe0722 CrossRef CAS PubMed.
- A. Delgado, C. Briciu-Burghina and F. Regan, Antifouling strategies for sensors used in water monitoring: review and future perspectives, Sensors, 2021, 21(2), 1–25 CrossRef PubMed.
- L. Zeng and D. Li, Development of in situ sensors for chlorophyll concentration measurement, J. Sens., 2015, 2015, 903509 CrossRef.
- A. M. F. Pinto, E. Von Sperling and R. M. Moreira, Chlorophyll-a determination via continuous measurement of plankton fluorescence: methodology development, Water Res., 2001, 35(16), 3977–3981 CrossRef CAS PubMed.
- R. N. Conmy, P. G. Coble, J. Farr, A. M. Wood and K. Lee, et al., Submersible optical sensors exposed to chemically dispersed crude oil: wave tank simulations for improved oil spill monitoring, Environ. Sci. Technol., 2014, 48(3), 1803–1810 CrossRef CAS PubMed.
- K. R. Murphy, K. D. Butler, R. G. M. Spencer, C. A. Stedmon, J. R. Boehme and G. R. Aiken, Measurement of dissolved organic matter fluorescence in aquatic environments: an interlaboratory comparison, Environ. Sci. Technol., 2010, 44(24), 9405–9412 CrossRef CAS PubMed.
- L. Jørgensen, C. A. Stedmon, T. Kragh, S. Markager, M. Middelboe and M. Søndergaard, Global trends in the fluorescence characteristics and distribution of marine dissolved organic matter, Mar. Chem., 2011, 126(1–4), 139–148 CrossRef.
- J. P. R. Sorensen, D. J. Lapworth, B. P. Marchant, D. C. W. Nkhuwa and S. Pedley, et al., In situ tryptophan-like fluorescence: a real-time indicator of faecal contamination in drinking water supplies, Water Res., 2015, 81, 38–46 CrossRef CAS PubMed.
- C. Bastien, R. Cardin, É. Veilleux, C. Deblois, A. Warren and I. Laurion, Performance evaluation of phycocyanin probes for the monitoring of cyanobacteria, J. Environ. Monit., 2011, 13(1), 110–118 RSC.
- E. Bertone, M. A. Burford and D. P. Hamilton, Fluorescence probes for real-time remote cyanobacteria monitoring: a review of challenges and opportunities, Water Res., 2018, 141, 152–162 CrossRef CAS PubMed.
- M. Beutler, K. H. Wiltshire, B. Meyer, C. Moldaenke and C. Lüring, et al., A fluorometric method for the differentiation of algal populations in vivo and in situ, Photosynth. Res., 2002, 72(1), 39–53 CrossRef CAS PubMed.
- B. Heery, C. Briciu-Burghina, D. Zhang, G. Duffy and D. Brabazon, et al., ColiSense, today's sample today: a rapid on-site detection of β-d-glucuronidase activity in surface water as a surrogate for E. coli, Talanta, 2016, 148, 75–83 CrossRef CAS PubMed.
- C. Briciu-Burghina, B. Heery, G. Duffy, D. Brabazon and F. Regan, Demonstration of an optical biosensor for the detection of faecal indicator bacteria in freshwater and coastal bathing areas, Anal. Bioanal. Chem., 2019, 411(29), 7637–7643 CrossRef CAS PubMed.
- V. H. Smith, Eutrophication of freshwater and coastal marine ecosystems: a global problem, Environ. Sci. Pollut. Res., 2003, 10(2), 126–139 CrossRef CAS PubMed.
- Y. Hautier, P. A. Niklaus and A. Hector, Competition for light causes plant biodiversity loss after eutrophication, Science, 2009, 324(5927), 636–638 CrossRef CAS PubMed.
- R. Beiras, Nonpersistent Inorganic Pollution, Mar. Pollut., 2018, pp. 31–39 Search PubMed.
- W. W. Gregg, N. W. Casey and C. R. McClain, Recent trends in global ocean chlorophyll, Geophys. Res. Lett., 2005, 32(3), 1–5 Search PubMed.
- European Commission, Directive 2000/60/EC of the European Parliament and of the council of 23 October 2000 establishing a framework for Community action in the field of water policy, https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32000L0060 Search PubMed.
- E. W. Rice, R. B. Baird, A. D. Eaton and L. S. Clesceri, Standard methods for the examination of water and wastewater, American public health association, Washington, DC, 2012, vol. 10 Search PubMed.
- M. Babin, C. S. Roesler and J. J. Cullen, Real-time coastal observing systems for marine ecosystem dynamics and harmful algal blooms: theory, instrumentation and modelling, UNESCO, 2008 Search PubMed.
- C. J. Lorenzen, A method for the continuous measurement of in vivo chlorophyll concentration, Deep-Sea Res. Oceanogr. Abstr., 1966, 13(2), 223–227 CrossRef.
- Y. Huot and M. Babin, Chlorophyll a Fluorescence in Aquatic Sciences: Methods and Applications, 2010, pp. 31–74 Search PubMed.
- J. R. Lakowicz, Principles of fluorescence spectroscopy, 2006, pp. 1–954 Search PubMed.
- J. J. Lamb, J. J. Eaton-Rye and M. F. Hohmann-Marriott, An LED-based fluorometer for chlorophyll quantification in the laboratory and in the field, Photosynth. Res., 2012, 114(1), 59–68 CrossRef CAS PubMed.
- M. Yoshida, T. Horiuchi and Y. Nagasawa, In situ multi-excitation chlorophyll fluorometer for phytoplankton measurements: Technologies and applications beyond conventional fluorometers, 2011 Search PubMed.
- T. Leeuw, E. S. Boss and D. L. Wright, In situ measurements of phytoplankton fluorescence using low cost electronics, Sensors, 2013, 13(6), 7872–7883 CrossRef CAS PubMed.
- Y. Pan and L. Qiu, A Submersible In Situ Highly Sensitive Chlorophyll Fluorescence Detection System, 2019 Search PubMed.
- R. I. Chowdhury, K. A. Wahid, K. Nugent and H. Baulch, Design and Development of Low-Cost, Portable, and Smart Chlorophyll-A Sensor, IEEE Sens. J., 2020, 20(13), 7362–7371 CAS.
- R. Bullis, J. Coker, J. Belding, A. De Groodt and D. W. Mitchell, et al., The Fluorino: A Low-Cost, Arduino-Controlled Fluorometer, J. Chem. Educ., 2021, 98(12), 3892–3897 CrossRef CAS.
- M. Brandl, T. Posnicek, R. Preuer and G. Weigelhofer, A Portable Sensor System for Measurement of Fluorescence Indices of Water Samples, IEEE Sens. J., 2020, 20(16), 1 Search PubMed.
- J. Bridgeman, A. Baker, D. Brown and J. B. Boxall, Portable LED fluorescence instrumentation for the rapid assessment of potable water quality, Sci. Total Environ., 2015, 524(525), 338–346 CrossRef PubMed.
- W. R. F. Silva, W. T. Suarez, C. Reis, V. B. Dos Santos and F. A. Carvalho, et al., Multifunctional Webcam Spectrophotometer for Performing Analytical Determination and Measurements of Emission, Absorption, and Fluorescence Spectra, J. Chem. Educ., 2021, 98(4), 1442–1447 CrossRef CAS.
- E. K. N. da Silva, V. B. dos Santos, I. S. Resque, C. A. Neves and S. G. C. Moreira, et al., A fluorescence digital image-based method using a 3D-printed platform and a UV-LED chamber made of polyacid lactic for quinine quantification in beverages, Microchem. J., 2020, 157(May), 104986 CrossRef CAS.
- K. Laganovska, A. Zolotarjovs, M. Vázquez, K. Mc Donnell and J. Liepins, et al., Portable low-cost open-source wireless spectrophotometer for fast and reliable measurements, HardwareX, 2020, 7, e00108 CrossRef PubMed.
- M. Tedetti, P. Joffre and M. Goutx, Development of a field-portable fluorometer based on deep ultraviolet LEDs for the detection of phenanthrene- and tryptophan-like compounds in natural waters, Sens. Actuators, B, 2013, 182, 416–423 CrossRef CAS.
- K. R. Murphy, C. A. Stedmon, T. D. Waite and G. M. Ruiz, Distinguishing between terrestrial and autochthonous organic matter sources in marine environments using fluorescence spectroscopy, Mar. Chem., 2008, 108(1–2), 40–58 CrossRef CAS.
- G. Schirripa Spagnolo and F. Leccese, LED Rail Signals: Full Hardware Realization of Apparatus with Independent Intensity by Temperature Changes, Electron, 2021, 10(11), 1291 CrossRef.
- M. D. Keller, R. C. Selvin, W. Claus and R. R. L. Guillard, Media for the Culture of Oceanic Ultraphytoplankton, J. Phycol., 2007, 23(4), 633–638 CrossRef.
- A. F. Marker, E. A. Nush and H. R. Rai, The measurement of photosynthetic pigments in freshwaters and standardization of methods: conclusions and recommendations, Arch. Hydrobiol., 1980, 14, 91–106 CAS.
- A. Earp, C. E. Hanson, P. J. Ralph, V. E. Brando and S. Allen, et al., Review of fluorescent standards for calibration of in situ fluorometers: recommendations applied in coastal and ocean observing programs, Opt. Express, 2011, 19(27), 26768 CrossRef PubMed.
- C. Roesler, J. Uitz, H. Claustre, E. Boss and X. Xing, et al., Recommendations for obtaining unbiased chlorophyll estimates from in situ chlorophyll fluorometers: a global analysis of WET Labs ECO sensors, Limnol. Oceanogr.: Methods, 2017, 15(6), 572–585 CrossRef CAS.
- T. L. Richardson, E. Lawrenz, J. L. Pinckney, R. C. Guajardo and E. A. Walker, et al., Spectral fluorometric characterization of phytoplankton community composition using the Algae Online Analyser®, Water Res., 2010, 44(8), 2461–2472 CrossRef CAS PubMed.
- A. Bricaud, A. A. Morel, L. Prieur and A. A. Morel, Factors of Some Phytoplankter, Limnol. Oceanogr., 1983, 28(5), 816–832 CrossRef.
- Z. Wang, W. Liu, Y. Zhang, W. Sima and J. Liu, A novel submersible phytoplankton fluorometer with multi-wavelength light emitting diode array as excitation source, Proc. SPIE-Int. Soc. Opt. Eng., 2007, 682817 CrossRef.
| This journal is © The Royal Society of Chemistry 2023 |