Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Study on drug substance degradation under headspace GC conditions of residual solvent analysis
Hua
Zhao
 *a,
Doug
Phillipson
b,
Jiangwei
Li
*a,
Doug
Phillipson
b,
Jiangwei
Li
 a and
Frank
Wu
a and
Frank
Wu
 a
a
aNeurocrine Biosciences Inc, 12780 El Camino Real, San Diego, CA 92130, USA. E-mail: lzhao@neurocrine.com
b14148 Bahama Cove, Del Mar, CA 92014, USA
First published on 25th January 2023
Abstract
Isobutyraldehyde (IBA) was detected in drug substance (DS) containing an amino acid group using a headspace-gas chromatography (HS-GC) method. High-performance liquid chromatography-mass-spectrometry (HPLC-MS) data from an HS vial confirmed that IBA was a degradant. The HS-GC method was modified to minimize IBA by keeping the HS oven temperature lower than 80 °C.
According to ICH Q3C guidelines, residual solvents of drug substances are analyzed as one of many tests run to ensure patient safety. The HS-GC methodology is a preferred methodology for residual solvent analysis.1,2
During residual solvent analysis by HS-GC, where DS is dissolved in a high boiling solvent and heated for a period of time before the gases above the liquid are equilibrated and injected into the GC system, an unexpected peak was observed when the HS oven temperature was set at 120 °C. The artifact peak was identified as isobutyraldehyde (IBA) by GC-MS.
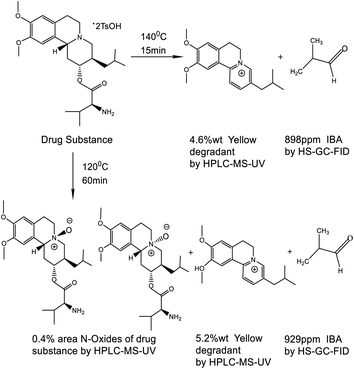
When the drug substance was subjected to the residual solvent method conditions, IBA was routinely observed at a low level of approximately 50 ppm. A much higher level of IBA (1092 ppm) was observed in an in process control (IPC) test sample. A comprehensive study was conducted on the headspace method using both HS-GC-flame ionization detector (FID)/MS and HPLC-ultraviolet (UV)/MS. The drug substance (see Scheme 1 for structure), contains an L-valine ester and it was hypothesized that the IBA observed was a result of a “Strecker Degradation”3,4 of the α-amino acid valine to yield the one carbon shorter degradation product, IBA. The main purpose of this study is to understand the mechanism and kinetics of IBA formation when the drug substance that contains an amino acid group is under the headspace oven temperature during residual solvent testing.
Four experiments were designed to explore the effect of headspace parameters and find out the critical parameters for IBA formation. Both HS-GC-FID/MS and HPLC-UV/MS have been utilized to study the mechanism by analyzing both gas and liquid phases of the sample after heating in a headspace oven. The four experiments are summarized in Table 1.
| Exp. | Design | Objective |
|---|---|---|
| I | 20 mg mL−1 drug substance at 80 °C headspace oven temperature shaken for 15 min with spiked 479 ppm and 974 ppm of IBA | To confirm that IBA is not present in the drug substance at 80 °C |
| II | 20 mg mL−1 drug substance at headspace oven temperatures of 80, 100, 120, 130, 140 °C shaken for 15 min | To study the impact of different parameters on the residual solvent method (120 °C, 15 min) |
| III | 20 mg mL−1 drug substance at a headspace oven temperature of 120 °C shaken for 30 and 60 min | |
| IV | 0.02 mg mL−1L-valine at headspace oven temperatures of 80, 100, 120, 130, 140 °C shaken for 15 min | To confirm that IBA is a degradant from L-valine |
The chemicals and reagents are listed below:
The drug substance is provided by Neurocrine Bioscience Inc.
L-Valine: MP Biomedicals LLC. Catalog#19476905 5 GM.
Isobutyraldehyde (IBA): Aldrich Product#240788, >99%.
The HS-GC method is listed in Tables 2 and 3.
| System | Agilent GC7890A with FID&MS 5977B detectors with a column splitter and a Headspace autosampler Agilent 7697A |
| Column | HP5, length = 30 m, internal diameter = 0.32 mm, film thickness = 1.8 μm or equivalent |
| Injector temperature | 180 °C |
| Detector temperature | 260 °C |
| Column temperature | Isotherm at 40 °C for 4 min; from 40 °C to 50 °C at 4 °C min; from 50 °C to 240 °C at 50 °C min−1 |
| Injector | Split ratio 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| Carrier gas | Helium, constant flow 2.5 mL min−1 |
| Makeup gas | Nitrogen flow 30 mL min−1 |
| Detector | FID (air 400 mL min−1, hydrogen 40 mL min−1) |
| Detector | Agilent 5977B: MS (50–200 m/z) |
| Run time | 10.3 min |
| GC diluent | N,N-Dimethylacetamide (DMA) with 40 μg mL−1 of butanol as internal standard |
| Oven temperature | 120 °C |
| Loop temperature | 130 °C |
| Transfer line temperature | 140 °C |
| Vial equilibration time | 15 min |
| Pressurization time | 1.0 min |
| Vial pressurization | 15 psi |
| Loop filling time | 0.10 min |
| Loop equilibration time | 0.05 min |
| Injection time | 1.00 min |
| Loop volume | 1.0 mL |
| Shaking speed | High |
| Vial | 20 mL headspace vial filled with 5 mL of solution |
HPLC method is listed in Tables 4 and 5.
| System | Agilent 1260 LCMS system |
| Column | Phenomenex kinetex C18, 3 × 100 mm, 1.7 μm |
| Temperature | 50 °C |
| Mobile phase A | 50 mM ammonium formate in water |
| Mobile phase B | Acetonitrile |
| Flow rate | 0.48 mL min−1 |
| Injection volume | 4 μL |
| Wavelength | 280 nm, bandwidth: 4 nm |
| MS | Agilent 6120: ESI, positive scan mode (100–1000 m/z) |
| HPLC diluent | 0.1% formic acid in water: acetonitrile 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
| Time | %A | %B |
|---|---|---|
| 0.0 | 95 | 5 |
| 4.0 | 84 | 16 |
| 12.0 | 75 | 25 |
| 15.0 | 5 | 95 |
| 15.1 | 95 | 5 |
| 18.0 | 95 | 5 |
GC-HS sample preparation: working standard solutions (WSS) were prepared by dissolving appropriate amounts of IBA in GC diluent to make 0.02 mg mL−1.
Sample solutions were prepared by appropriately dissolving 100 mg of the drug substance sample or L-valine into 5 mL of GC diluent.
Spiked sample solutions were prepared by spiking 200 μL and 400 μL of 0.25 mg mL−1 IBA in GC diluent standard solutions into sample solutions, respectively. The spiked IBA concentrations were approximately 500 and 1000 ppm, respectively.
HPLC sample preparation: after the headspace experiment was completed, 1 mL of the headspace sample solution was diluted to 19 mL of HPLC diluent to make an HPLC sample solution.
IBA is quantified using the WSS solution and run at the same temperature.
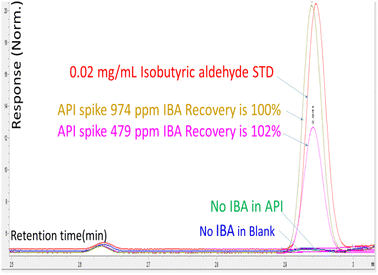
Experiment I provided assurance that IBA could be quantified in the headspace solution if it were present as an impurity in the DS. There was no IBA detected when the drug substance was analyzed with the headspace oven temperature set to 80 °C. When the drug substance was spiked with 479 and 974 ppm of IBA at 80 °C for 15 min, the IBA recoveries were 102% and 100%, respectively. The spiking experiment demonstrated that IBA was not present as an impurity in the drug substance (shown in Fig. 1).
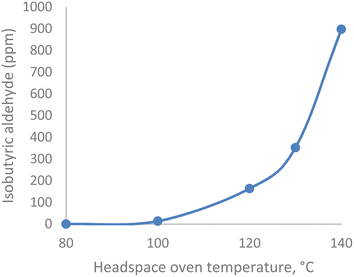
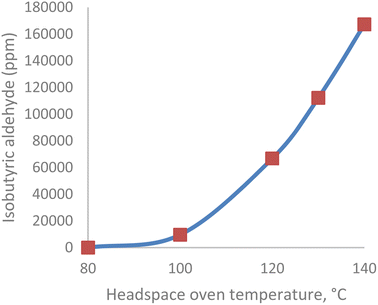
Experiment II examined the IBA content of head space vials subjected to increasing equilibration temperatures. The IBA content of the DS sample increased exponentially from 14 ppm to 898 ppm when the headspace oven temperature was increased from 100 °C to 140 °C. A graph of IBA content as a function of temperature is shown in Fig. 2. The valine portion of the drug substance degraded to IBA when the oven temperature was >100 °C.
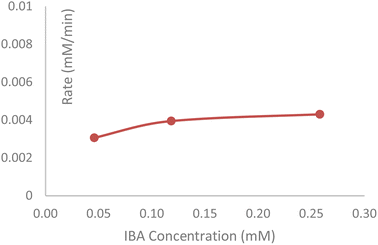
In Experiment III, the IBA content of the headspace vial was studied as a function of equilibration time at 120 °C. IBA was shown to increase linearly from 164, 429, and 929 ppm with headspace equilibration times at 15, 30, and 60 min. The IBA degradation with equilibration time is a zero-order reaction (shown in the red line in Fig. 3). When the drug substance was shaken in the HS oven at 120 °C for 60 min, the liquid content of the HS vial was analyzed by HPLC-UV-MS, and the data showed the formation of three related degradation products, one of which no longer contained the valine amino acid of the DS (see yellow degradant structure in Scheme 1), confirming that IBA was a degradant, formed during the HS-GC analysis. A total of 0.4% area of two N-oxides of the drug substance was observed by HPLC and confirmed by mass spectrometry. This oxidation reaction (see Scheme 1) observed in the HS oven was in alignment with the oxidation of the drug substance with an oxidant such as hydrogen peroxide (H2O2). During the forced degradation study of the drug substance with 3% H2O2 at 25 °C for 24 h, a total of 1.9% area of two N-oxides was observed.
If the IBA behaved like a residual solvent pre-existing in the drug substance sample, such as acetonitrile, a small linear increase in concentration with temperature and no increase in concentration over equilibration time would be expected. Both experiments II and III demonstrate that IBA is a degradant of the drug substance and not a pre-existing residual solvent in the drug substance.
In addition, after the headspace experiment II was completed, the liquid contents of the headspace vials were evaluated using HPLC-UV and MS, and one major HPLC-UV peak in the chromatogram was the same yellow degradation product found in experiment III. This degradation product was previously identified as a known drug substance impurity, with the valine functional group acting as a leaving group during aromatization (see Scheme 1). The major yellow degradant increased from 0.9 to 4.6 wt% proportionally to oven temperature, as shown in Fig. 4. The pictures of a colorless initial sample to a yellow sample at 140 °C are an indication of yellow degradant formation.
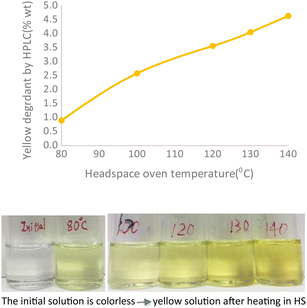 | ||
| Fig. 4 The yellow degradant in the HS sample solution increased proportionally with the HS oven temperature. | ||
The IBA degradation product of L-valine under the HS-GC conditions was also confirmed in experiment IV, and the amount of IBA increased exponentially as a function of headspace oven temperature from 100–140 °C. The HS-GC trend of L-valine to IBA is similar to that seen with the drug substance degrading to IBA when the headspace oven temperature increased over the same interval. This result was taken as further confirmation that the presence of IBA under our residual solvent headspace conditions was due to the degradation of the L-valine portion, as shown in Fig. 5.
All the evidence indicates that the observed IBA resulted from a “Strecker Degradation”3,4 of the α-amino acid valine to yield the one carbon shorter IBA. The degradation pathways and some of the degradants observed using HS-GC and LC-MS are shown in Scheme 1.
The IBA is not present in the drug substance as a process solvent or impurity because it is not detected at a headspace oven temperature of 80 °C. Both the drug substance and L-valine, under the headspace conditions (120 °C), show an artifact peak corresponding to IBA. The Strecker degradation of amino acids to their corresponding aldehydic decomposition products under GC-HS conditions is a possibility where an amino acid is a functional unit of a compound on test for residual solvents.
The critical HS-GC system parameter controlling IBA formation is the headspace oven temperature. The results above suggest that to avoid a drug substance containing an amino acid that might degrade in the HS oven, the HS oven temperature should be set at 80 °C or lower to minimize the formation of the degradation product of IBA. The result described above may be applicable to related chemicals with amino acid esters when subjected to HS-GC or other high-temperature thermal degradation.
Author contributions
Hua Zhao performed the experiments, analyzed the data, and wrote the original draft. Doug Phillipson is responsible for supervision, review, and editing. Jiangwei Li and Frank Wu contributed to the review and editing.Conflicts of interest
All authors declare that they have no conflicts of interest.References
- H. Lee, Control of chloroethane in raw materials and drug substances using headspace/gas chromatography analysis, Pharmaceutical Industry Practices on Genotoxic Impurities. 2014, p. 14 Search PubMed.
- F. R. Cafferata and C. J. Manzione, Kinetics and Mechanism of Thermolysis using Headspace-Gas Chromatographic Analysis Lázaro, J. Chromatogr. Sci., 2001, 39(2), 45–48 Search PubMed.
- G. P. Rizzi, The Strecker Degradation of Amino Acids: Newer Avenues for flavor Formation, Food Rev. Int., 2008, 24(4), 416–435 CrossRef CAS.
- D. R. Cremer and K. Eichner, The reaction kinetics for the formation of Strecker aldehydes in low moisture model systems and in plant powders, Food Chem., 2000, 71, 37–43 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |