Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
High wavenumber Raman spectroscopy for intraoperative assessment of breast tumour margins†
Jennifer
Haskell
 ab,
Thomas
Hubbard
ab,
Thomas
Hubbard
 ab,
Claire
Murray
b,
Benjamin
Gardner
ab,
Claire
Murray
b,
Benjamin
Gardner
 ab,
Charlotte
Ives
ab,
Douglas
Ferguson
ab,
Charlotte
Ives
ab,
Douglas
Ferguson
 ab and
Nick
Stone
ab and
Nick
Stone
 *ab
*ab
aPhysics and Astronomy, Faculty of Environment, Science and Economy, University of Exeter, Exeter, Devon, UK. E-mail: n.stone@exeter.ac.uk
bRoyal Devon University Healthcare NHS Foundation Trust, Exeter, Devon, UK
First published on 28th July 2023
Abstract
Optimal oncological results and patient outcomes are achieved in surgery for early breast cancer with breast conserving surgery (BCS) where this is appropriate. A limitation of BCS occurs when cancer is present at, or close, to the resection margin – termed a ‘positive’ margin – and re-excision is recommended to reduce recurrence rate. This is occurs in 17% of BCS in the UK and there is therefore a critical need for a way to assess margin status intraoperatively to ensure complete excision with adequate margins at the first operation. This study presents the potential of high wavenumber (HWN) Raman spectroscopy to address this. Freshly excised specimens from thirty patients undergoing surgery for breast cancer were measured using a surface Raman probe, and a multivariate classification model to predict normal versus tumour was developed from the data. This model achieved 77.1% sensitivity and 90.8% specificity following leave one patient out cross validation, with the defining features being differences in water content and lipid versus protein content. This demonstrates the feasibility of HWN Raman spectroscopy to facilitate future intraoperative margin assessment at specific locations. Clinical utility of the approach will require further research.
Introduction
Primary surgery and tumour excision remains the standard first line of treatment for patients with early breast cancer. Breast conserving surgery, (BCS), combined with radiotherapy, where the tumour is removed by wide local excision (lumpectomy) with a margin of surrounding healthy tissue, is oncologically equivalent to mastectomy (removal of the whole breast)1 and is the preferred option where possible. Unlike mastectomy, this allows the patient to retain the majority of their breast tissue for much improved aesthetic and psychological outcomes.2,3 However, with breast conserving surgery comes the problem of positive tumour margins, where the tumour is very close to the edge of the removed tissue, increasing the potential for abnormal tissue to remain behind. The current UK protocol requires a clear margin of 1 mm of healthy tissue to be removed around the entirety of the tumour.4According to a meta-analysis of 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 162 women undergoing breast conserving surgery, local recurrence is twice as likely when margins are positive.5 The current protocol6,7 for locating the tumour on a macroscopic level involves palpation by the surgeon, and specimen radiograph for non-palpable tumours, but the only way to conclusively assess margins is through histopathological assessment, the results of which are not available until days or weeks after the operation.8 Therefore, patients often need follow up surgery to re-excise any remaining tumour. In the UK, on average 17.2% of patients undergo re-excision operations, but this can be as high as 40% in some individual hospital units.7 This comes at significant financial cost, as well as all the risks associated with additional surgery for the patient including, morbidity, anxiety, prolonged wound healing, infection and worse cosmetic outcome.7,9 Therefore, a method which can assess tumour margins intraoperatively is needed.
162 women undergoing breast conserving surgery, local recurrence is twice as likely when margins are positive.5 The current protocol6,7 for locating the tumour on a macroscopic level involves palpation by the surgeon, and specimen radiograph for non-palpable tumours, but the only way to conclusively assess margins is through histopathological assessment, the results of which are not available until days or weeks after the operation.8 Therefore, patients often need follow up surgery to re-excise any remaining tumour. In the UK, on average 17.2% of patients undergo re-excision operations, but this can be as high as 40% in some individual hospital units.7 This comes at significant financial cost, as well as all the risks associated with additional surgery for the patient including, morbidity, anxiety, prolonged wound healing, infection and worse cosmetic outcome.7,9 Therefore, a method which can assess tumour margins intraoperatively is needed.
There are currently many different approaches being researched within the field to try to achieve this. Frozen section analysis (FSA) and imprint cytology (IC) allow intraoperative assessment of the resected specimen by pathologists, and both methods have been proven to reduce re-excision rates.10,11 However FSA tends add about 30 minutes on average to the operation time,10,12 and breast tissue is difficult to cryosection due to its adiposity.10,13 IC by contrast only takes about 15 minutes,12 but at the expense of sensitivity,10 with only the very surface layer of cells being assessed.10 And with this, both methods require pathologists’ time and are subject to sampling errors.10,13
Alternatives include imaging modalities such as ultrasound (US) and micro-CT. A review by Colakovic et al. of 16 studies showed that on average US guided resection achieved negative margins 86% of the time, with an average re-excision rate of 9%; compared to 24% for wire guided localisations (of those studies reporting this value).14 Yet, a large proportion of patients may be unsuitable for this; ductal carcinoma in situ (DCIS) is often identified by calcifications, and due to their acoustic properties, US cannot reliably detect them.15–17 This is not a problem with micro-CT, using and this method specimens can be assessed within 15 minutes, with a spatial resolution reported as <1 μm.15,18 Yet there are only limited studies assessing this method's capability for assessing margins; Qiu et al. suggest in one of their studies that were the method used to directly inform the necessity of re-excision, rates would have been reduced from 32% to 14%, despite the same study only achieving 56% sensitivity.18 It is also acknowledged that the diagnostic performance of X-rays is reduced with higher density tissue.15
Photoacoustic imaging is a newer technique that has been applied to breast cancer. This technique works through the generation and detection of acoustic waves from tissue when it undergoes themoelastic expansion following the application of a pulsed laser beam.19 Through haemoglobin targeting, this technique has allowed the detection of malignant breast lesions through cancer induced angiogenesis.20 More recently, lipids and collagen have been mapped using optoacoustic tomography in ex vivo breast tissue, which could provide new ways to detect and localise breast tumours.21 Specifically, Kosik et al. developed a lipid-weighted Intraoperative Photoacoustic Screening scanner, which was used to measure breast lumpectomy specimens. The tumour volumes determined by the system showed a significant positive correlation and were statistically similar to that determined by dynamic contrast enhanced magnetic resonance imaging.20 The specimen scans were completed in a clinically feasible time of only 6 minutes,20 with depths greater than 2 cm achievable, helped by the fact that acoustic waves are less scattered in tissues than optical photons.19,20 However, pressure was required to enable measurement of the lumpectomy samples to maximise laser fluence at increased depths; this may be the reason for the 5.1 cm average overestimate in maximum tumour diameter.20
Exploration in this field has yielded several commercially available products using different methods to address the problem. These include the iKnife, MarginProbe and ClearEdge, where the former utilises mass spectrometry, while the latter two systems make use of bio-impedance spectroscopy through slightly varied approaches.22,23 The iKnife has the benefit that it integrates easily into current surgical practice, and it achieves high sensitivity and specificity (94.9% and 93.4% respectively),22 rivalling if not improving upon the more traditional methods. Its resolution is limited, however, to 4 mm which is the width of the blade used.22 Furthermore, it entirely destroys the tissue it samples, making validation of the result difficult. The MarginProbe® on the other hand is the only intraoperative margin assessment device currently approved by the Food and Drug Administration (FDA).15 While it is frequently reported to significantly reduce re-excision rates,24–26 and only takes 5 minutes to assess a specimen,27 the sensitivity and specificity levels achieved tend to be reduced compared to other methods discussed. For example, in a study of 596 patients, Schnabel et al. demonstrate a sensitivity and specificity of margin assessment as 75% and 46% respectively, with deliberate tuning of the classification model to sacrifice specificity for enhanced sensitivity.25 In comparison, the ClearEdge system reportedly achieves better sensitivity and specificity (87.3% and 75.6%) for assessing margins, and has the added advantage that it can be tuned to probe a particular depth within the specimen, e.g. 2 mm.23 Dixon et al. found that re-excision rates were reduced from 37% to 17% using ClearEdge,23 but there is currently little other literature to corroborate this, and the re-excision rate was reduced to the UK average from a comparatively high baseline.
Raman spectroscopy provides another different approach to the problem. Chemical information is obtained through the change of energy, or frequency shift, of light photons when they interact inelastically with molecular bonds. This shift is chemically specific, producing a spectrum of peaks corresponding to the bonds present in a tissue sample, i.e. a ‘spectral fingerprint’. The technique has been used in several forms to successfully discriminate various forms of cancer.28–31 Measuring fresh frozen ex vivo breast samples with a Raman microscope, Haka et al. characterised normal tissue, invasive carcinoma and benign fibrocystic change with a sensitivity of 94% and specificity of 96%,28 but the protocol used here would take too long with necessary sampling to be feasible for margin assessment. The group then used this database to characterise new measurements of breast tissue margins taken with a Raman probe intraoperatively.32 While this achieved perfect sensitivity and specificity values, the sample size is very small – 30 spectra from nine patients – and only one measurement turned out to be positive for cancer.32
Raman has also been demonstrated to be feasible for intraoperative identification of lymph node involvement in both breast cancer surgery33,34 and for identifying primary and secondary cancers in head and neck surgery.35 All of these studies utilise the fingerprint region of the spectrum, complex but rich in molecular information on the phenotypic cellular molecular composition.
There are a number of studies that have demonstrated that water concentration is increased in cancerous tissues. The first study to identify this was conducted by Damadian in 1971. Using nuclear magnetic resonance (NMR) on rat models with sarcoma and hepatoma, prolonged relaxation times of water protons in malignant tissue compared to normal tissue were observed.36 Further NMR studies have confirmed this finding, again in rat and mouse models,37 and a small number of human samples,38 with relaxation times correlating with an increase in the hydration of the malignant tissue.37 This has been demonstrated specifically in breast cancer, with tumour tissue measured to contain a higher water-fat ratio than normal tissue.39,40 Corroborating this further are studies utilising diffuse optical spectroscopy (DOS), which when used in vivo indicated that malignant breast tissue contained 20% reduced lipid content and about 50% increased water.41
The Raman fingerprint (FP) region is unsuitable for water analysis with low signals dominated by other molecular species, but the high wavenumber (HWN) region is very sensitive to water. In fact, some studies have demonstrated dual illumination methods to measure both the HWN region and the FP region for the extra information that lies there. Qi et al. demonstrated the use of a Raman probe with simultaneous laser excitation at both 681 nm and 785 nm for HWN and FP region measurement respectively, with the collected signal being decoupled by an algorithm the group devised. From the HWN spectra, they could ascertain water content of in vivo skin.42 Similarly, Masson et al. used a Raman probe with dual laser excitation which could switch between 680 nm and 785 nm. From the HWN water band they were able to quantify the water content of tissue phantoms as well as from ex vivo mouse cervix tissue in different conformations validated by the wet and dry weights of the samples.43 Assessing the HWN region only, a Raman probe was applied intraoperatively for margin analysis in oral cancer by Barroso et al.44 While their work was not used to influence resection rates, it achieved a sensitivity of 99% and specificity of 92% based on the difference in water content of normal versus cancerous tissue, which was stark (p < 0.0001).44 Using this principle, the group later demonstrated how water levels decrease with distance across margins, starting high within the tumour and decreasing across an inadequate margin, and decreasing further towards an adequate margin.45 Several studies utilising the HWN region have also found water content to be higher in cancerous versus non-cancerous breast tissue. This includes samples from rat models46 and snap frozen – defrosted human samples.47 The latter, undertaken by Hubbard et al., was important in establishing that fluorescence from surgical dye used routinely in breast surgery can be minimised by measuring the HWN region at a longer illumination wavelength of 785 nm.47 But due to the reduced quantum efficiency of CCDs in the HWN region at this wavelength, it was necessary to use an InGaAs camera instead, unlike in the previously mentioned studies by Qi, Masson, and Barroso et al. Abramczyk et al. successfully discerned normal from tumour in fresh breast samples using HWN Raman spectroscopy, but their protocol involved taking thousands of spectra using a Raman microscope, which would take too long to implement intraoperatively.48 To improve on the preliminary work by Hubbard et al., fresh samples should be measured. Yet, along with the work by Barroso et al. and Hubbard et al., the study convincingly proves the potential of this approach for assessing breast margins. The HWN spectrum has also been used as a screening tool, by Liao et al., to identify suspicious areas followed by definitive assessment with the FP region,49 however, in this study the water peak between 3035–3680 cm−1 was not included in the analysis, and the diagnostic ability of HWN RS for breast cancer remains under investigated. Therefore, the following study aims to establish whether HWN Raman spectroscopy using a handheld Raman probe can discern tumour versus non-tumour status in ex vivo fresh breast tissue with measurements taken in a clinically relevant setting and timeframe.
Materials and methods
Tissue samples
Tissue specimens resected as part of standard oncological resection from 30 patients were measured at the Royal Devon and Exeter Hospital (Royal Devon University Healthcare NHS Foundation Trust). Only patients undergoing mastectomy were approached, as these specimens could be sliced open to allow access to the tumour without affecting subsequent histopathological assessment.Live subject statement
All experiments were performed in accordance with NHS Health Research Authority (HRA) guidelines, and approved by the North West – Greater Manchester Central Research Ethics Committee (REC). Written informed consents were obtained from human participants of this study.Following ethical approval (IRAS ID: 210732, study title: Raman spectroscopy for rapid analysis of pathology of the breast) eligible patients received information about the study at least 24 hours before their operation. Informed consent was then obtained in the morning of their surgery.
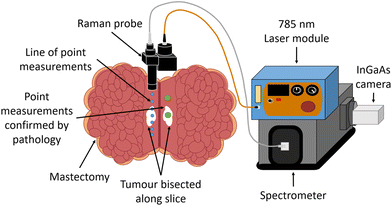
Once the specimen was removed, the surgeon located the tumour and sliced the specimen to bisect it, allowing access to the tumour surface for measurement, as seen in Fig. 1. The sample was relocated to a nearby room in the theatre block for Raman measurements. The specimen was only kept for measurement for a maximum of 20 minutes before being returned to the theatre and re-entered into the standard sample pathway to pathology assessment.
Raman instrumentation
A diagram of the experimental setup is shown in Fig. 1. Raman measurements were taken using a homebuilt handheld surface probe, connected to a 785 nm laser (Innovative Photonic Solutions, USA) and a Kaiser Holospec spectrometer with a 785 nm edge (Semrock 50 mm Edgebasic® Long wave pass) filter (Kaiser optical systems inc, Ann Arbour, USA) with an InGaAs camera (iDus InGaAs 1.7 μm, Andor, Belfast, UK). The use of the combination of laser and InGaAs camera enabled sensitive detection of the high wavenumber region, not possible with silicon-based CCDs at this wavelength. The probe design contains a 105 μm core fibre, a collimating lens and then a 785 nm laser bandpass filter (Semrock Maxline®), with collimated light then passing through a 785 nm dichroic mirror (Thorlabs, NJ, USA). A 12.5 mm diameter plano-convex f = 20 mm lens with anti-reflective coating (Thorlabs, NJ, USA) was used along with a 20 mm spacer tube, producing a 325 mW beam at the sample with spot size at the focus measured with a beam profiler to be 1.6 mm in diameter. The end of the spacer was placed in contact with the sample when measurements were taken, maintaining the optical working distance required by the f = 20 mm lens that collected the scattered light. This ensured optimum collection at all times when in contact with the tissue. As the spacer was a tube, any pressure applied to the periphery of the region of interest had no effect on the central region measured. Elastically scattered light was rejected by the dichroic filter (Semrock 785 nm) and a 785 nm edge filter (Semrock Razoredge® longpass) before the remaining inelastically scattered light was focused into a multimodal low OH fibre bundle 7 × 105 μm core (Thorlabs BFL105LS02) with round array at one end translating into a linear array, enabling the array to be efficiently launched into the spectrometer mounted with a 100 μm slit. Calculations based on the optical configuration showed the optical collection area to be from a 0.64 mm spot on the surface of the sample. Note the mismatch in the illumination and collection areas, resulting in a range of spatially offset signals (∼0.5 mm to 1.1 mm offsets50), likely to result in a range of signals collected from the surface and tissue depths of up to ∼1 mm. We have tested the instrument with a number of scenarios using lard/protein rich tissue phantoms and demonstrated that protein rich tissues can only be probed when lightly buried by lipid rich materials. From this, we have ascertained that the maximum depth for detection of epithelial tissue beneath adipose tissues to be ∼1 mm with the current set up. Furthermore, phantom tumours, within a lipid rich volume, with a surface area between 0.5 and 1 mm2 were detectable with this set up.51 Spectra were collected across the region from 0 to 4000 cm−1, but the HWN region from 2500 to 4000 cm−1 was focused on in data analysis.Raman measurement protocol
Approved laser local rules were followed at all times. Before specimen measurements took place, paracetamol was measured as a calibration standard, with 1 s exposure time, and the wavenumber axis was corrected using a polynomial fit. A line of point measurements was taken along the specimen slice, with step sizes of either 0.5 or 1 cm depending on specimen size. Additional single point measurements were taken on ‘tumour’ and ‘normal’ tissue respectively, and were indicated with black and blue ink respectively for confirmation with pathology. For each point measurement on the specimen, 3 spectra were taken, each with 5 s exposure time. The number of measurements taken for each specimen varied, with the aim being to maximise the number of measurements possible within the allocated timeframe.Histopathological examination
Each of the inked measurement locations were assessed by histopathology through routine haematoxylin and eosin (H&E) stained sections taken from larger tissue blocks of the measured locations. The pathologist's assessment of the status of the examined tissue – tumour or normal – and the type of tissue in the surrounding area (fat, stroma, etc.) was collated.Data analysis
Data analysis was carried out using MATLAB (Mathworks, 2018a).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N) levels (S
N) levels (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N < 10), were removed. All remaining spectra were pre-processed before analysis, starting with the subtraction of a spectrum taken with the laser switched off to remove fixed pattern noise. A median filter was then used to remove cosmic rays, then data was baselined to remove background signal, and then normalised; for visualisation and ratio calculations, data were normalised to the CH peak at 2935 cm−1, and for multivariate analysis data were vector normalised.
N < 10), were removed. All remaining spectra were pre-processed before analysis, starting with the subtraction of a spectrum taken with the laser switched off to remove fixed pattern noise. A median filter was then used to remove cosmic rays, then data was baselined to remove background signal, and then normalised; for visualisation and ratio calculations, data were normalised to the CH peak at 2935 cm−1, and for multivariate analysis data were vector normalised.
• The data validated by histopathology only (194 spectra)
• All data from all measurements (1620 spectra)
• A reduced dataset including data assessed by pathology and data from the line spectra originating from only the most extreme tumour and normal locations (770 spectra).
It is important to clarify that only the first data group contained spectra from tissue that had all been fully validated by histopathology. The second group containing all spectra consists of all pathology validated spectra as well as the rest of the spectra for which the ground truth could only be estimated by the surgeon at the time of measurement. The final, reduced dataset was included to minimise some potential experimental errors, described as follows. In some cases, the tumour was difficult to access based on its location within the specimen, so it is unclear whether the tumour had been directly measured by Raman without precise histopathological validation, and so these measurements were removed from this dataset. Also, the surgeon's indication of the tumour was an estimate, and the exact location was difficult to ascertain in the moment of measurement. Therefore, in the reduced dataset, measurements were retained when there was confidence that they were centred on tumour or benign tissue and the data from the less certain boundary regions was removed to minimise incorrect assignments.
Results and discussion
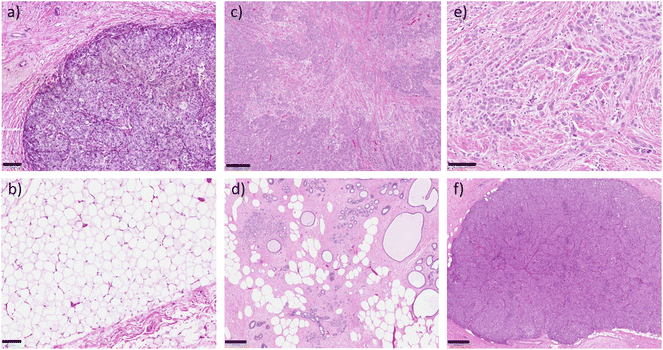
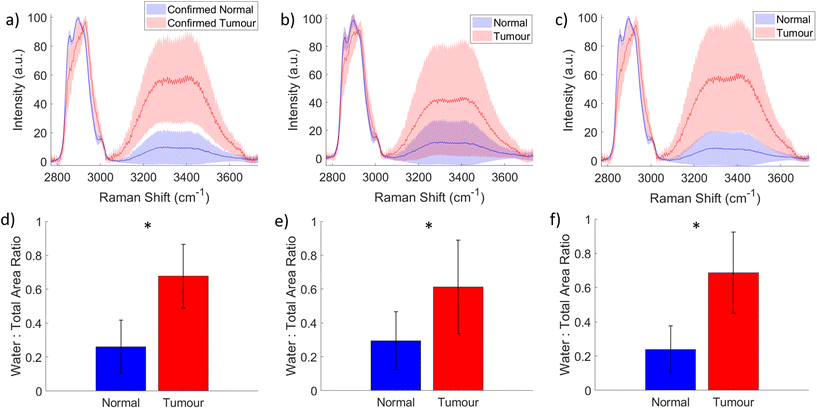
Fig. 2 shows several images of areas of histological haematoxylin and eosin (H&E) stained sections. These sections were taken from the positions that were inked on the specimen following Raman measurement for the purpose of matching the Raman spectra with the correct pathology of the measured area. Figures (a) and (b), and figures (c) and (d) respectively, show tumour and benign tissue from the same specimen. (a) Depicts invasive ductal carcinoma while (b) shows the normal fibroadipose tissue from another area on the specimen. (c) Is also an image of invasive ductal carcinoma, this time with sclerotic stroma, and (d) is an example of benign fibrocystic change. Invasive ductal carcinoma was the most commonly pathology measured in this study, with a number of cases of invasive lobular carcinoma, and some with mixed types, and small numbers of rarer types. These include one case of pleomorphic lobular carcinoma, shown in (e), and one case of solid papillary carcinoma, shown in (f).In total, 1620 spectra were analysed – 1051 normal, and 569 tumour. Fig. 3 shows the mean and standard deviation of the normal versus tumour HWN spectra for each of the dataset groupings described in the methods; (a) accounts for the histopathology confirmed spectra, (194 spectra), (b) accounts for all spectra, including those confirmed by histopathology (1620), and (c) the reduced dataset, (770 spectra). The band between 3000–3700 cm−1 forms as a convolution of several peaks, largely attributed to water.52 There was a significant difference in the WTAR between normal vs. tumour in the histopathology confirmed data (normal vs. tumour 0.26 vs. 0.68; p < 0.001), full dataset (normal vs. tumour; 0.29 vs. 0.62; p < 0.001) and reduced dataset (normal vs. tumour 0.24 vs. 0.68; p < 0.001), as per Fig. 3). This indicates that tumour tissue has increased water content compared to normal tissue, as expected based on the literature. Although not universal, normal breast tissue is more likely to have a higher proportion of adipose tissue to fibrous tissue whilst tumour tissue is more likely to have a higher proportion of protein rich fibrous stroma, which is more associated with a high water content.53 Tumours in general also have a higher density of cells than normal tissue and this tends to mean an increased water content. This is because diffusion is impeded in tissues with high cellularity, meaning water is trapped within cells, as evidenced by diffusion-weighted MRI studies.54 There are two visible maximum points across the water band, at around 3299 cm−1 and 3391 cm−1 on average between the three datasets, which are associated with differences in the binding status of water.52,55 In this region, water peaks at lower wavenumbers indicate a higher degree of intramolecular hydrogen bonding, whereas higher wavenumbers indicate more free water.52,55 That said the picture is complicated by the fact that there is an N–H stretch contribution to the signals at around 3300 cm−1 originating from proteins found in the tissues, so those areas with higher protein density will have a stronger peak in a similar location to that originating from OH stretch affected by higher intramolecular hydrogen bonding.56 In the normal spectra in this figure, the two maxima are similar in intensity, if very slightly higher at the lower wavenumber peak, unlike in the tumour spectra, where there is a slight increase in the higher wavenumber peak. This could potentially indicate a slightly higher concentration of free water, or only partially bound water in the tumour tissue compared to the normal (although some of this will be driven by the NH stretch contribution). In support of this, a study by Chung et al. using broadband DOS found that malignant breast tissue contains significantly higher free water compared to normal tissue.57 A possible explanation for this, as well as for the higher water content of tumour tissue in general is the influence of angiogenesis. Vessels formed through this process are inefficient and leak plasma, amongst other substances, into the surrounding tissue.58
The other important difference between the two spectra is the position of the peak between 2800 cm−1 and 3040 cm−1. The normal spectra have prominent peaks at 2859 cm−1 and 2900 cm−1, which account for CH2 lipid stretching, and 3002 cm−1, CH lipid stretching, with a maximum at the 2900 cm−1 peak. By contrast, in this region the tumour spectrum has a peak located at 2931 cm−1, attributable to protein CH2 stretching.59 The reduction of lipids and increase in protein in the tumour tissue is supported by the literature,41,46 although for measurements that did not have the histopathological confirmation obtained, it is possible that normal fibro-glandular tissue could have been measured in some cases. However, importantly the trend for separation based on both water and protein content is evident specifically in Fig. 3(a), for which the Raman measurements were coupled with histopathological assessment. Furthermore, this shows that the Raman spectra of normal tissue is significantly different from the tumour, confirming the ability of HWN Raman spectroscopy to detect cancer in freshly excised breast tissue.
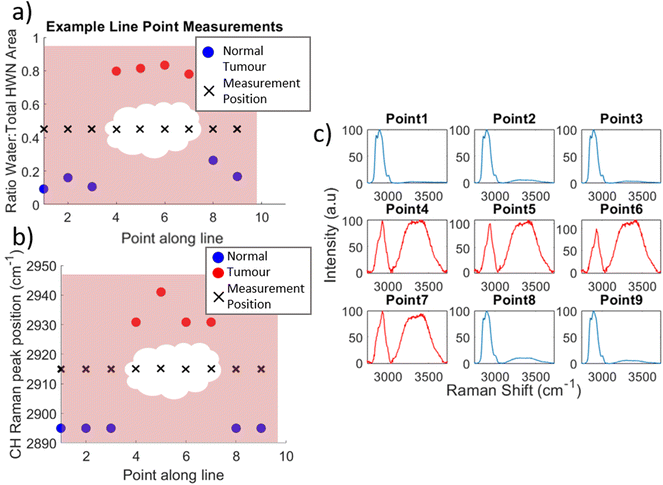
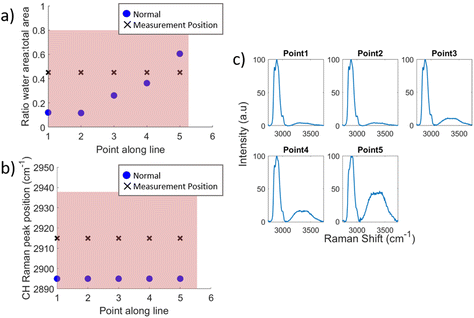
The differences seen between the mean spectra can be used to indicate the location of a tumour within a specimen as demonstrated in Fig. 4. This figure demonstrates the change in water content and fat/protein content across a line of measurements taken along a single specimen slice and indicates the position of the tumour as estimated by the surgeon. Fig. 5 by contrast shows the data from another line of measurements, line 2, taken from the same specimen but at a distance from the tumour. At each measurement position, for both Fig. 4 and 5, the WTAR and wavenumber at which the maximum of the CH peak is located is plotted in (a) and (b) respectively, with the corresponding mean spectrum for each point shown alongside in (c). In Fig. 4, the tumour position can be clarified by the locations where the WTAR and CH peak position are highest. For this particular specimen, the WTAR for measurements of the tumour are consistently around 0.8, with the average at 0.81. By contrast, the normal measurements for this line have an average WTAR of 0.12. In terms of CH peak position, this is located at a higher wavenumber for the tumour measurements compared to the normal; 2933.5 cm−1 compared to 2895 cm−1 respectively. In Fig. 5, the CH position of line 2, away from the tumour, also remains stable at 2895 cm−1. The average WTAR for this line, 0.29, is higher than that of the normal points in Fig. 4 but is still much reduced compared to the values obtained from tumour measurements. However, the values do increase towards the end of the line, but are still reduced compared to the tumour measurements, indicating some levels of variation in the WTAR amongst normal tissue.
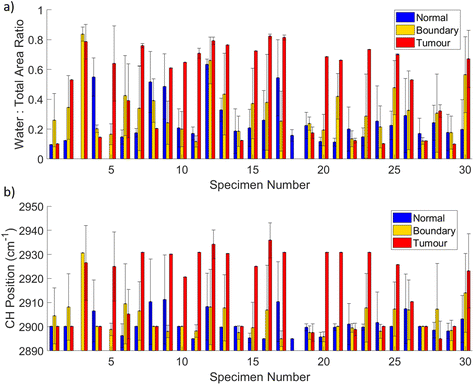
While this figure shows a very clear distinction between normal and tumour measurements across this particular specimen, this was not necessarily the case for all. That said, it should be noted that these measurements were not assessed by histopathology and the tumour location was an estimate by the surgeon based on palpation and visual assessment, and there is also dependence on the depth of the slice into the tumour. The reduced dataset accounts for this somewhat in that spectra from boundary regions were removed to minimise incorrect assignment, but this does not explain all of the variation. Fig. 6 shows bar graphs indicating the mean WTAR (Fig. 6(a)) and CH peak position (Fig. 6(b)) for normal (blue), boundary (yellow) and tumour (red) positions from the line measurements taken on each specimen, with the standard deviation as the error bars. Looking at the mean values, for the majority of specimens both the WTAR and CH peak wavenumber of tumour measurements is higher than that of normal measurements. This is true for 19/30 specimens in terms of the WTAR and 17/30 in terms of CH peak position. This increases to 20/30 specimens when considering those specimens whose boundary measurements are increased compared to normal. Importantly, there are three specimens where there was either no normal tissue or no tumour tissue measured, and thus not allowing this comparison. In the remaining 27, almost half of the specimens demonstrate a trend where the WTAR or CH position of normal measurements < boundary < tumour. While this is not always the case, this indicates an incremental increase in WTAR and CH position between normal tissue and within the threshold of the tumour. This has been demonstrated by Barroso et al. on oral tissue.45 On average across all specimens, the WTAR was 0.26 for normal, 0.32 for boundary and 0.50 for tumour. The CH position was on average 2901 cm−1 for normal, 2903 cm−1 for boundary and 2916 cm−1 for tumour. It is important to note that for both metrics there can be large variation across individual specimens, as well as variation between patients, as base levels of tissue water content is different for each individual.60
Yet, there are a small number of specimens that demonstrate a higher WTAR or CH peak position for normal tissue rather than tumour or boundary tissue. A possible explanation for this is that there may be contributions from dense glandular tissue which, has higher protein and water content than fibroadipose tissue.53,60 This could also be a factor in the increase in WTAR and broadening of the CH peak seen in the measurements at the end of the line in Fig. 5. While the maximum CH peak position did not change, the increase in intensity at 2921 cm−1 is indicative of protein.59 In addition, fat is much more highly scattering than protein, so if there were a small amount of protein-rich tissue in amongst fatty tissue in these measurement locations the lipid signal would still dominate. Also, tumour cells can exhibit discrete single cell invasion resulting in a very low tumour cellularity within tissue predominantly composed of fat.61
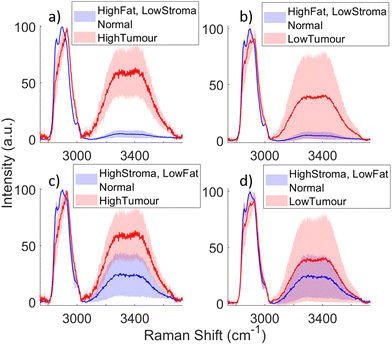
As seen in Fig. 3(b) and (c) there is some degree of overlap in the standard deviations of both the water band and the CH peak between normal and tumour spectra. This overlap is explored in Fig. 7, which shows normal vs. tumour HWN spectra with varying concentrations of fat and stroma in the former, and tumour cells in the latter, as estimated by the pathologist from the histology-assessed dataset. In this figure levels of a specific tissue type are described as ‘high’ or ‘low’; ‘high’ refers to an estimate of greater than or equal to 50% concentration of that tissue type in the section examined by the pathologist in the vicinity of the Raman point measurement, and ‘low’ refers to less than 50% of that tissue type. The spectra shown are the mean of the spectra designated as high/low of the particular tissue type, and the standard deviation is shown in the shaded area. As it was not possible to control the levels of fat or stroma in the human samples, spectra taken from areas of tissue determined to be ‘high’ in one and simultaneously ‘low’ in the other were used. Across all four plots, it is clear that spectra with high levels of tumour have the highest mean water content, as well a more consistent shift towards 2931 cm−1 in the CH peak. Fig. 7(a) shows the opposite is true for normal spectra which are highly fatty; having little to no water content and a very well defined lipid CH peak at 2900 cm−1, these spectra are easily separated from spectra with high tumour levels. The same can be said for the means of the spectra in Fig. 7(b), but the spectra with low tumour levels have a much larger standard deviation, likely owed to contributions from other normal tissue types amongst tumour cells in the area measured. Also, the shifted CH peak is not as well defined and has higher variation. Therefore, while highly fatty normal spectra will be largely distinguished from material with low tumour cell concentration, it is possible that some spectra will be misclassified. A similar conclusion can be drawn from the comparison between high tumour and high stroma normal spectra in Fig. 7(c). Although the mean spectra are sufficiently separate in terms of both the water content and the CH peak position, there is some overlap in the standard deviations, meaning some spectra could be misclassified. To note, the CH peak in the normal spectrum with high stroma is still indicative of the presence of lipids, but shows no or minimal indication of the presence of protein. But, as seen in Fig. 5, this is somewhat expected due to the signal from lipids being much more intense than that from proteins (lipids generate more Raman scattering than a similar concentration of protein molecules). Furthermore, Fig. 7(d) indicates where the biggest classification problem arises, in comparing high stroma normal spectra with low tumour spectra. While the latter still has higher water content on average, the water content of the mean normal spectrum is very close to this, and their standard deviations completely overlap, indicating that this situation is the most likely for incorrect classification to occur. Therefore, if the concentration of tumour cells reduces towards the boundary of the tumour with normal tissue, this could be problematic when it comes to detecting tumour margins. Likewise, with tumours whose cells are particularly infiltrative in nature, this may again limit the detection of these cells.
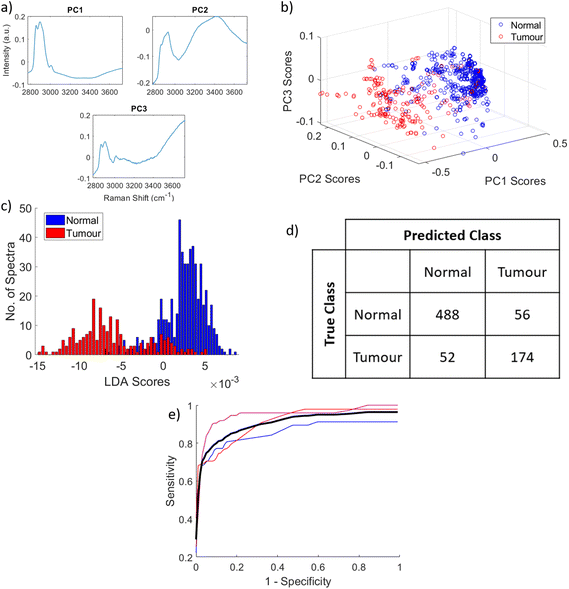
Fig. 8 demonstrates the classification of the spectra into ‘tumour’ or ‘normal’ through the PCA–LDA model. The model uses the reduced dataset, where tumour-normal boundary measurements were excluded, to minimise errors in training the model. Fig. 8(a) Shows the loadings of PC1, PC2 and PC3, which were the most significant PCs based on ANOVA testing (p < 0.001). From the statistical testing PC1 is clearly the most significant, demonstrating that the highest variation within the data was a strong lipid CH peak with reduced contributions from the water band. PC2 illustrates the significance of a shift in the CH peak towards higher wavenumbers along with a pronounced increase in signal from water. PC3 relates a reduction in lipid contributions with an increase in the water contribution above 3400 wavenumbers. It should be noted that the region of the spectrum defined here as that relating to the OH stretch of the water also includes some signal form NH stretch at 3300 cm−1. The scores of PCs 1, 2 and 3 are plotted against each other in a scatter graph in Fig. 8(b) and this shows good separation between normal and tumour. This is evident again in Fig. 8(c) – a histogram of the subsequent LDA scores. Above zero is a large concentration of normal spectra, with tumour spectra distributed mostly below zero. The tumour spectra are spread much further across the axis indicating the increased heterogeneity of these spectra compared to normal. The specific numbers of correctly and incorrectly classified spectra based on this model are shown in the confusion matrix in Fig. 8(d). Following leave-one-patient-out cross validation, of 544 normal spectra, 488 were correctly classified as normal, and only 56 were misclassified as tumour. Of 226 tumour spectra, 174 were correctly classified as tumour, while 52 were incorrectly classified as normal. This equates to a sensitivity of 77.1%, specificity of 90.8% and overall accuracy of 86.0%, and area under the mean ROC curve following 3-fold cross validation of 0.8993. For completeness a model using data from just the CH region (2800–3000 cm−1) of the spectra, where the difference in lipid and protein rich tissues are most apparent, was also used. This resulted in sensitivity and specificity of 76.1% and 91.7% respectively following leave-one-patient-out cross validation.
Conclusion
This study demonstrates the ability of HWN Raman spectroscopy to distinguish between cancerous and normal breast tissue from freshly excised human samples. The key factors enabling this are the difference in water content, including a potential difference in the behaviour of bound and free water, and fat/protein within normal versus tumour, which this technique is able to detect and utilise for classification via multivariate modelling. To advance this line of research towards use for intraoperative margin assessment, it is important that the precision of this method is interrogated further. This could be achieved by having a pathologist present for the Raman measurements, and measuring smaller samples, perhaps in the form of a micro-tissue array. Overall, this work highlights that this method can achieve the fundamental classification of tumour versus normal on clinically relevant breast samples, and that it has great potential for future development towards intraoperative margin assessment.Author contributions
Jennifer Haskell – data curation, formal analysis, investigation, methodology, project administration, resources, software, validation, visualization, writing – original draft, review and editing. Thomas Hubbard – investigation, methodology, project administration, resources, software, writing – review and editing. Claire Murray – investigation, methodology, resources, writing – review and editing. Benjamin Gardner – software, writing – review and editing. Charlotte Ives – resources. Douglas Ferguson – resources, supervision, editing. Nick Stone – conceptualization, formal analysis, funding acquisition, methodology, project administration, resources, software, supervision, validation, writing – review and editing.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was funded by the EPSRC, grant number EP/P012442/1 and the University of Exeter funded the PhD studentship of J. Haskell who is first author. Thanks go to all the staff in the surgical department of the Royal Devon and Exeter hospital, especially breast surgeons Rachel Tillett, Sisse Olsen and Hannah Knight, as well as Tanwen Wright in the pathology department, and research nurses Linda Park and Tania Nightingale. Thanks also to Pavel Matousek at Rutherford Appleton Laboratory for the valuable discussions.References
- B. Fisher, M. Bauer, R. Margolese, R. Poisson, Y. Pilch and C. Redmond, et al., Five-Year Results of a Randomized Clinical Trial Comparing Total Mastectomy and Segmental Mastectomy with or without Radiation in the Treatment of Breast Cancer, N. Engl. J. Med., 1985, 312(11), 665–673, DOI:10.1056/NEJM198503143121101.
- W. S. Schain, M. E. Dunn, A. S. Lichter and L. I. Pierce, Mastectomy versus Conservative Surgery and Radiation Therapy Psychosocial Consequences, Cancer, 1994, 73(4), 1221–1228 CrossRef CAS PubMed https://acsjournals.onlinelibrary.wiley.com/doi/10.1002/1097-0142 .
- S. K. Al-Ghazal, L. Fallowfield and R. W. Blamey, Comparison of psychological aspects and patient satisfaction following breast conserving surgery, simple mastectomy and breast reconstruction, Eur. J. Cancer, 2000, 36(15), 1938–1943 CrossRef CAS PubMed.
- Association of Breast Surgery (ABS), Association of Breast Surgery Consensus Statement Margin Width in Breast Conservation Surgery, 2015. Available from: https://www.associationofbreastsurgery.org.uk Search PubMed.
- N. Houssami, P. Macaskill, M. Luke Marinovich and M. Morrow, The association of surgical margins and local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy: A meta-analysis, Ann. Surg. Oncol., 2014, 21(3), 717–730 CrossRef PubMed.
- S. Downey, L. Chagla, V. Chandran, A. Gandhi, G. Layer and A. Sahu, et al., Best Practise Guidelines For Surgeons In Breast Cancer Screening, 2018. https://associationofbreastsurgery.org.uk/media/64276/final-screening-guidelines-2018.pdf Search PubMed.
- S. S. K. Tang, S. Kaptanis, J. B. Haddow, G. Mondani, B. Elsberger and M. K. Tasoulis, et al., Current margin practice and effect on re-excision rates following the publication of the SSO-ASTRO consensus and ABS consensus guidelines: a national prospective study of 2858 women undergoing breast-conserving therapy in the UK and Ireland, Eur. J. Cancer, 2017, 84, 315–324 CrossRef PubMed.
- KEY PERFORMANCE INDICATORS IN PATHOLOGY Recommendations from the Royal College of Pathologists [Internet], 2013. Available from: https://www.rcpath.org/static/e7b7b680-a957-4f48-aa78e601e42816de/Key-Performance-Indicators-in-Pathology-Recommendations-from-the-Royal-College-of-Pathologists.pdf.
- T. S. Menes, P. I. Tartter, I. Bleiweiss, J. H. Godbold, A. Estabrook and S. R. Smith, The consequence of multiple re-excisions to obtain clear lumpectomy margins in breast cancer patients, Ann. Surg. Oncol., 2005, 12(11), 881–885 CrossRef PubMed.
- K. Esbona, Z. Li and L. G. Wilke, Intraoperative imprint cytology and frozen section pathology for margin assessment in breast conservation surgery: A systematic review, Ann. Surg. Oncol., 2012, 19(10), 3236–3245 CrossRef PubMed.
- T. P. Olson, J. Harter, A. Muñoz, D. M. Mahvi and T. M. Breslin, Frozen section analysis for intraoperative margin assessment during breast-conserving surgery results in low rates of re-excision and local recurrence, Ann. Surg. Oncol., 2007, 14(10), 2953–2960 CrossRef CAS PubMed.
- J. J. Keating, C. Fisher, R. Batiste and S. Singhal, Advances in intraoperative margin assessment for breast cancer, Curr. Surg. Rep., 2016, 4(4), 1–8 Search PubMed.
- R. Emmadi and E. L. Wiley, Evaluation of resection margins in breast conservation therapy: The pathology perspective-past, present, and future, Int. J. Surg. Oncol., 2012, 2012, 180259, DOI:10.1155/2012/180259.
- N. Colakovic, D. Zdravkovic, Z. Skuric, D. Mrda, J. Gacic and N. Ivanovic, Intraoperative ultrasound in breast cancer surgery-from localization of non-palpable tumors to objectively measurable excision, World J. Surg. Oncol., 2018, 16(1), 1–7 CrossRef PubMed.
- J. Schwarz and H. Schmidt, Technology for Intraoperative Margin Assessment in Breast Cancer, Ann. Surg. Oncol., 2020, 27(7), 2278–2287, DOI:10.1245/s10434-020-08483-w.
- L. N. F. Smith, I. T. Rubio, R. Henry-Tillman, S. Korourian and V. S. Klimberg, Intraoperative ultrasound-guided breast biopsy, Am. J. Surg., 2000, 180(6), 419–423 CrossRef CAS PubMed.
- M. Ramos, J. C. Díaz, T. Ramos, R. Ruano, M. Aparicio and M. Sancho, et al., Ultrasound-guided excision combined with intraoperative assessment of gross macroscopic margins decreases the rate of reoperations for non-palpable invasive breast cancer, Breast, 2013, 22(4), 520–524, DOI:10.1016/j.breast.2012.10.006.
- S. Q. Qiu, M. D. Dorrius, S. J. de Jongh, L. Jansen, J. de Vries and C. P. Schröder, et al., Micro-computed tomography (micro-CT) for intraoperative surgical margin assessment of breast cancer: A feasibility study in breast conserving surgery, Eur. J. Surg. Oncol., 2018, 44(11), 1708–1713 CrossRef PubMed.
- A. B. E. Attia, G. Balasundaram, M. Moothanchery, U. S. Dinish, R. Bi and V. Ntziachristos, et al., A review of clinical photoacoustic imaging: Current and future trends, Photoacoustics, 2019, 16, 100144, DOI:10.1016/j.pacs.2019.100144.
- I. Kosik, M. Brackstone, A. Kornecki, A. Chamson-Reig, P. Wong and J. J. L. Carson, Lipid-weighted intraoperative photoacoustic tomography of breast tumors: Volumetric comparison to preoperative MRI, Photoacoustics, 2020, 18, 100165, DOI:10.1016/j.pacs.2020.100165.
- Y. Goh, G. Balasundaram, H. M. Tan, T. C. Putti, S. W. Tang and C. W. Q. Ng, et al., Biochemical “decoding” of breast ultrasound images with optoacoustic tomography fusion: First-in-human display of lipid and collagen signals on breast ultrasound, Photoacoustics, 2022, 27, 100377, DOI:10.1016/j.pacs.2022.100377.
- E. R. St John, J. Balog, J. S. McKenzie, M. Rossi, A. Covington and L. Muirhead, et al., Rapid evaporative ionisation mass spectrometry of electrosurgical vapours for the identification of breast pathology: Towards an intelligent knife for breast cancer surgery, Breast Cancer Res., 2017, 19(1), 1–14 CrossRef PubMed.
- J. M. Dixon, L. Renshaw, O. Young, D. Kulkarni, T. Saleem and M. Sarfaty, et al., Intra-operative assessment of excised breast tumour margins using ClearEdge imaging device, Eur. J. Surg. Oncol., 2016, 42(12), 1834–1840, DOI:10.1016/j.ejso.2016.07.141.
- M. Thill, C. Dittmer, K. Baumann, K. Friedrichs and J. U. Blohmer, MarginProbe® - Final results of the German post-market study in breast conserving surgery of ductal carcinoma in situ, Breast, 2014, 23(1), 94–96, DOI:10.1016/j.breast.2013.11.002.
- F. Schnabel, S. K. Boolbol, M. Gittleman, T. Karni, L. Tafra and S. Feldman, et al., A randomized prospective study of lumpectomy margin assessment with use of marginprobe in patients with nonpalpable breast malignancies, Ann. Surg. Oncol., 2014, 21(5), 1589–1595 CrossRef PubMed.
- T. M. Allweis, Z. Kaufman, S. Lelcuk, I. Pappo, T. Karni and S. Schneebaum, et al., A prospective, randomized, controlled, multicenter study of a real-time, intraoperative probe for positive margin detection in breast-conserving surgery, Am. J. Surg., 2008, 196(4), 483–489 CrossRef PubMed.
- M. Thill, MarginProbe®: Intraoperative margin assessment during breast conserving surgery by using radiofrequency spectroscopy, Expert Rev. Med. Devices, 2013, 10(3), 301–315 CrossRef CAS PubMed.
- A. S. Haka, K. E. Shafer-Peltier, M. Fitzmaurice, J. Crowe, R. R. Dasari and M. S. Feld, Diagnosing breast cancer by using Raman spectroscopy, Proc. Natl. Acad. Sci. U. S. A., 2005, 102(35), 12371–12376 CrossRef CAS PubMed https://www.pnas.org/doi/abs/10.1073/pnas.0501390102 .
- N. Stone, C. Kendall, J. Smith, P. Crow and H. Barr, Raman spectroscopy for identification of epithelial cancers, Faraday Discuss., 2004, 126, 141–157 RSC https://pubs.rsc.org/en/content/articlehtml/2004/fd/b304992b .
- Z. Huang, A. McWilliams, H. Lui, D. I. McLean, S. Lam and H. Zeng, Near-infrared Raman spectroscopy for optical diagnosis of lung cancer, Int. J. Cancer, 2003, 107(6), 1047–1052 CrossRef CAS PubMed.
- P. Crow, A. Molckovsky, N. Stone, J. Uff, B. Wilson and L. M. Wongkeesong, Assessment of fiberoptic near-infrared raman spectroscopy for diagnosis of bladder and prostate cancer, Urology, 2005, 65(6), 1126–1130 CrossRef CAS PubMed.
- A. S. Haka, Z. Volynskaya, J. A. Gardecki, J. Nazemi, J. Lyons and D. Hicks, et al., In vivo Margin Assessment during Partial Mastectomy Breast Surgery Using Raman Spectroscopy, Cancer Res., 2006, 66(6), 3317–3322 CrossRef CAS PubMed https://aacrjournals.org/cancerres/article/66/6/3317/527477/In-vivo-Margin-Assessment-during-Partial .
- M. Sattlecker, C. Bessant, J. Smith and N. Stone, Investigation of support vector machines and Raman spectroscopy for lymph node diagnostics, Analyst, 2010, 135(5), 895–901 RSC https://pubs.rsc.org/en/content/articlehtml/2010/an/b920229c .
- J. Horsnell, P. Stonelake, J. Christie-Brown, G. Shetty, J. Hutchings and C. Kendall, et al., Raman spectroscopy—A new method for the intra-operative assessment of axillary lymph nodes, Analyst, 2010, 135(12), 3042–3047 RSC https://pubs.rsc.org/en/content/articlehtml/2010/an/c0an00527d .
- G. R. Lloyd, L. E. Orr, J. Christie-Brown, K. McCarthy, S. Rose and M. Thomas, et al., Discrimination between benign, primary and secondary malignancies in lymph nodes from the head and neck utilising Raman spectroscopy and multivariate analysis, Analyst, 2013, 138(14), 3900–3908 RSC https://pubs.rsc.org/en/content/articlehtml/2013/an/c2an36579k .
- R. Damadian, Tumor detection by nuclear magnetic resonance, Science, 1971, 171(3976), 1151–1153 CrossRef CAS PubMed.
- C. Kiricuta and V. Simplăceanu, Tissue Water Content and Nuclear Magnetic Resonance in Normal and Tumor Tissues, Cancer Res., 1975, 35, 1164–1167 Search PubMed https://aacrjournals.org/cancerres/article-pdf/35/5/1164/2394055/cr0350051164.pdf .
- D. P. Hollis, J. S. Economou, L. C. Parks, J. C. Eggleston, L. A. Saryan and J. L. Czeisler, Nuclear Magnetic Resonance Studies of Several Experimental and Human Malignant Tumors, Cancer Res., 1973, 33(9), 2156–2160 CAS.
- P. E. Sijens, H. K. Wijrdeman, M. A. Moerland, C. J. G. Bakker, J. W. A. H. Vermeulen and P. R. Luyten, Human breast cancer in vivo: H-1 and P-31 MR spectroscopy at 1.5 T, Radiology, 1988, 169(3), 615–620 CrossRef CAS PubMed.
- N. R. Jagannathan, M. Singh, V. Govindaraju, P. Raghunathan, O. Coshic and P. K. Julka, et al., Volume localized in vivo proton MR spectroscopy of breast carcinoma: variation of water±fat ratio in patients receiving chemotherapy, NMR Biomed., 1998, 11, 414–422 CrossRef CAS PubMed https://analyticalsciencejournals.onlinelibrary.wiley.com/doi/10.1002/ .
- A. E. Cerussi, N. S. Shah, D. Hsiang, A. Durkin, J. A. Butler and B. J. Tromberg, In vivo absorption, scattering, and physiologic properties of 58 malignant breast tumors determined by broadband diffuse optical spectroscopy, J. Biomed. Opt., 2006, 11(4), 044005, DOI:10.1117/12337546 https://www.spiedigitallibrary.org/journals/journal-of-biomedical-optics/volume-11/issue-4/044005/In-vivo-absorption-scattering-and-physiologic-properties-of-58-malignant/10.1117/1.2337546.full .
- Y. Qi, R. Zhang, P. Rajarahm, S. Zhang, A. B. Ebrahim Attia, R. Bi and M. Olivo, Simultaneous Dual-Wavelength Source Raman Spectroscopy with a Handheld Confocal Probe for Analysis of the Chemical Composition of In Vivo Human Skin, Anal. Chem., 2023, 95(12), 5240–5247, DOI:10.1021/acs.analchem.2c05065.
- L. E. Masson, C. M. O'Brien, I. J. Pence, J. L. Herington, J. Reese and T. G. Van Leeuwen, et al., Dual excitation wavelength system for combined fingerprint and high wavenumber Raman spectroscopy, Analyst, 2018, 143(24), 6049–6060 RSC https://pubs.rsc.org/en/content/articlehtml/2018/an/c8an01989d .
- E. M. Barroso, R. W. H. Smits, T. C. B. Schut, I. Ten Hove, J. A. Hardillo and E. B. Wolvius, et al., Discrimination between Oral Cancer and Healthy Tissue Based on Water Content Determined by Raman Spectroscopy, Anal. Chem., 2015, 87(4), 2419–2426 CrossRef CAS PubMed.
- E. M. Barroso, R. W. H. Smits, C. G. F. Van Lanschot, P. J. Caspers, I. Ten Hove and H. Mast, et al., Water concentration analysis by Raman spectroscopy to determine the location of the tumor border in oral cancer surgery, Cancer Res., 2016, 76(20), 5945–5953 CrossRef CAS PubMed.
- A. F. García-Flores, L. Raniero, R. A. Canevari, K. J. Jalkanen, R. A. Bitar and H. S. Martinho, et al., High-wavenumber FT-Raman spectroscopy for in vivo and ex vivo measurements of breast cancer, Theor. Chem. Acc., 2011, 130(4–6), 1231–1238 Search PubMed https://link.springer.com/article/10.1007/s00214-011-0925-9 .
- T. J. E. Hubbard, A. P. Dudgeon, D. J. Ferguson, A. C. Shore and N. Stone, Utilization of Raman spectroscopy to identify breast cancer from the water content in surgical samples containing blue dye, Transl. Biophotonics, 2021, 3(2), e202000023 Search PubMed https://onlinelibrary.wiley.com/doi/full/10.1002/tbio.202000023 .
- H. Abramczyk, B. Brozek-Pluska, J. Surmacki, J. Jablonska-Gajewicz and R. Kordek, Hydrogen bonds of interfacial water in human breast cancer tissue compared to lipid and DNA interfaces, J. Biophys. Chem., 2011, 02(02), 159–170 Search PubMed.
- Z. Liao, M. G. Lizio, C. Corden, H. Khout, E. Rakha and I. Notingher, Feasibility of integrated high-wavenumber Raman imaging and fingerprint Raman spectroscopy for fast margin assessment in breast cancer surgery, J. Raman Spectrosc., 2020, 51(10), 1986–1995 CrossRef CAS.
- S. Mosca, C. Conti, N. Stone and P. Matousek, Spatially offset Raman spectroscopy, Nat. Rev. Methods Primers, 2021, 1(1), 21, DOI:10.1038/s43586-021-00019-0.
- T. Hubbard, Using Raman Spectroscopy for Intraoperative Margin Analysis in Breast Conserving Surgery, University of Exeter, 2020 Search PubMed.
- C. Choe, J. Lademann and M. E. Darvin, Depth profiles of hydrogen bound water molecule types and their relation to lipid and protein interaction in the human stratum corneum in vivo, Analyst, 2016, 141(22), 6329–6337 RSC https://pubs.rsc.org/en/content/articlehtml/2016/an/c6an01717g .
- S. J. Graham, S. Ness, B. S. Hamilton and M. J. Bronskill, Magnetic resonance properties of ex vivo breast tissue at 1.5 T, Magn. Reson. Med., 1997, 38(4), 669–677 CrossRef CAS PubMed.
- Y. Guo, Y. Q. Cai, Z. L. Cai, Y. G. Gao, N. Y. An and L. Ma, et al., Differentiation of clinically benign and malignant breast lesions using diffusion-weighted imaging, J. Magn. Reson. Imaging, 2002, 16(2), 172–178 CrossRef PubMed.
- R. Vyumvuhore, A. Tfayli, H. Duplan, A. Delalleau, M. Manfait and A. Baiilet-Guffroy, Effects of atmospheric relative humidity on Stratum Corneum structure at the molecular level: Ex vivo Raman spectroscopy analysis, Analyst, 2013, 138(14), 4103–4111 RSC.
- P. J. Caspers, G. W. Lucassen, E. A. Carter, H. A. Bruining and G. J. Puppels, In vivo confocal raman microspectroscopy of the skin: Noninvasive determination of molecular concentration profiles, J. Invest. Dermatol., 2001, 116(3), 434–442, DOI:10.1046/j.1523-1747.2001.01258.x.
- S. H. Chung, A. E. Cerussi, C. Klifa, H. M. Baek, O. Birgul and G. Gulsen, et al., In vivo water state measurements in breast cancer using broadband diffuse optical spectroscopy, Phys. Med. Biol., 2008, 53(23), 6713–6727 CrossRef CAS PubMed.
- S. Ziyad and M. L. Iruela-Arispe, Molecular Mechanisms of Tumor Angiogenesis, Genes Cancer, 2011, 2(12), 1085–1096 CrossRef PubMed https://journals.sagepub.com/doi/full/10.1177/1947601911432334 .
- Z. Movasaghi, S. Rehman and I. U. Rehman, Raman Spectroscopy of Biological Tissues, Appl. Spectrosc. Rev., 2007, 42(5), 493–541 CrossRef CAS https://www.tandfonline.com/doi/abs/10.1080/05704920701551530 .
- N. Boyd, L. Martin, S. Chavez, A. Gunasekara, A. Salleh and O. Melnichouk, et al., Breast-tissue composition and other risk factors for breast cancer in young women: a cross-sectional study, Lancet Oncol., 2009, 10(6), 569–580 CrossRef PubMed.
- P. Friedl and K. Wolf, Tumour-cell invasion and migration: diversity and escape mechanisms, Nat. Rev. Cancer, 2003, 3(5), 362–374 CrossRef CAS PubMed https://www.nature.com/articles/nrc1075 .
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3an00574g |
| This journal is © The Royal Society of Chemistry 2023 |