Multichannel charge transfer enhanced radiative decay and RISC in TADF materials containing multiple donors and acceptors†
Zhaolong
He
a,
Jiuyan
Li
 *a,
Di
Liu
*a,
Di
Liu
 *ab,
Huihui
Wan
c,
Yongqiang
Mei
a and
Chunlong
Shi
a
*ab,
Huihui
Wan
c,
Yongqiang
Mei
a and
Chunlong
Shi
a
aFrontiers Science Center for Smart Materials, College of Chemical Engineering, Dalian University of Technology, 2 Linggong Road, Dalian, 116024, China. E-mail: jiuyanli@dlut.edu.cn; liudi@dlut.edu.cn
bState Key Laboratory of Luminescent Materials and Devices, South China University of Technology, Guangzhou, 510640, China
cInstrumental Analysis Center, Dalian University of Technology, Dalian, 116024, China
First published on 17th November 2022
Abstract
Coexistence of through-bond charge transfer (TBCT) and through-space charge transfer (TSCT) is observed to strongly enhance the performance of thermally activated delayed fluorescence (TADF) materials that contain multiple donors and acceptors. A group of TADF emitters were developed with carbazole as the donor, benzophenone as the acceptor, and phenylene as the linking bridge. TBCT was responsible for the TADF feature of the para-linked analogue p-tCz-BP, while both TBCT and TSCT were observed in the ortho-linked isomer o-tCz-BP. The multiple-donor–acceptor analogue D-tCz-D-BP was proved to exhibit multi-channel TBCT and TSCT processes with more near-degenerate excited states and more TSCT (81.0%) contribution than o-tCz-BP (66.3%). Thus D-tCz-D-BP combines the merits of both the high oscillator strength of p-tCz-BP and the tiny energy splitting (ΔEST) between the lowest singlet and triplet excited states caused by the twisted conformation of o-tCz-BP, leading to a high rate constant of radiation (kr) of 1.01 × 107 s−1, of reverse intersystem crossing (krisc) of 0.56 × 106 s−1 and a high photoluminescence quantum yield of 96.8%. The sky-blue organic light-emitting diode (OLED) of D-tCz-D-BP exhibited an external quantum efficiency (EQE) of 24.9%, much higher than those of p-tCz-BP (6.3%) and o-tCz-BP (11.1%). D-tCz-D-BP was also capable of hosting an orange-red iridium phosphor to form a two-emitting-component white OLED that realized an EQE of 18.8% and a high CRI of 80.
Introduction
Recently, thermally activated delayed fluorescence (TADF) materials with high electroluminescent (EL) efficiency and low cost have attracted considerable attention as third-generation organic light-emitting diode (OLED) materials.1,2 Since the spins of the injected charges are uncorrelated, the ratio of singlet excitons to triplet excitons generated by electronic excitation is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.3,4 For TADF emitters, small energy splitting between the lowest singlet and triplet states (ΔEST) is indispensable to promote triplet to singlet spin conversion via an effective reverse intersystem crossing (RISC) process and full utilization of both singlet and triplet excitons for light emission, finally achieving an internal quantum efficiency (IQE) of 100%.2,5,6 A typical strategy to minimize the ΔEST is adopting large steric-hindrance induced twisted intramolecular through-bond charge transfer (TBCT) or through-space charge transfer (TSCT) to separate the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) on the individual electron donor (D) and acceptor (A) unit, which results in small HOMO–LUMO overlaps.4,7,8 According to Fermi's golden rule, a small ΔEST favors facilitation of efficient RISC processes and thus increases the reverse intersystem crossing rate (krisc).9,10 However, small overlap of the HOMO and LUMO usually generates a small oscillator strength (f), radiative decay rate (kr) and low photoluminescence quantum yield (PLQY).11,12 Hence, generating a balance between a small ΔEST and high krisc, kr and PLQY is essential for excellent TADF materials.
3.3,4 For TADF emitters, small energy splitting between the lowest singlet and triplet states (ΔEST) is indispensable to promote triplet to singlet spin conversion via an effective reverse intersystem crossing (RISC) process and full utilization of both singlet and triplet excitons for light emission, finally achieving an internal quantum efficiency (IQE) of 100%.2,5,6 A typical strategy to minimize the ΔEST is adopting large steric-hindrance induced twisted intramolecular through-bond charge transfer (TBCT) or through-space charge transfer (TSCT) to separate the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) on the individual electron donor (D) and acceptor (A) unit, which results in small HOMO–LUMO overlaps.4,7,8 According to Fermi's golden rule, a small ΔEST favors facilitation of efficient RISC processes and thus increases the reverse intersystem crossing rate (krisc).9,10 However, small overlap of the HOMO and LUMO usually generates a small oscillator strength (f), radiative decay rate (kr) and low photoluminescence quantum yield (PLQY).11,12 Hence, generating a balance between a small ΔEST and high krisc, kr and PLQY is essential for excellent TADF materials.
Recently, researchers have reported the combinational effects of TBCT and TSCT in one single molecule, in which the ortho-linkage of the D and A units on the phenylene bridge endows large D–A dihedral angles and cofacial interactions with a small D–A spatial distance, leading to small ΔEST and high kr, krisc and PLQY. For instance, Chi et al. adopted a sterically hindered asymmetric D–A–D′ configuration to suppress non-radiative decay and accelerate radiative decay, gaining a high PLQY of 91.9%. As a result, the non-doped emitter (2Cz-DPS) exhibited a high external quantum efficiency (EQE) of 28.7% in OLEDs.2 Yang et al. reported the combinational effect of TBCT and TSCT in one single TADF molecule, which could provide multiple charge transfer pathways, increase the PLQY and reduce the ΔEST simultaneously, achieving high-efficiency TADF emitters (one of the EQEs had a high value up to 28.1%).6,13,14 Duan et al. developed TADF molecules with ortho-linked multiple donor-acceptor (ortho-Dn–A) motifs to create near-degenerate excited states for the reinforcement of spin–orbit coupling (SOC). The incorporation of TBCT and TSCT enlarges the oscillator strength (f), and the optimal ortho-D3–A compound achieved a high PLQY (100%), krisc (106 s−1) and kr (107 s−1) and generated a high EQE over 30% when it acted as the sensitizer for a doped emitter in OLEDs.15 As mentioned above, the positions or numbers of D and A can affect not only the charge transfer format but also the performance of the emitter.16–21 Furthermore, some TBCT-TSCT type TADF emitters, especially blue emitting ones, have been used as hosts and/or host emitters to fabricate phosphorescent OLEDs (PhOLEDs) and/or white OLEDs (WOLEDs) at very low doping concentrations.22,23
A group of TADF molecules, namely p-tCz-BP, o-tCz-BP and D-tCz-D-BP (Scheme 1), was designed using tert-butylcarbazole as the donor, benzophenone as the acceptor and phenylene as the linking bridge. The linking style between the donor and acceptor was varied as para- or ortho- in isomers p-tCz-BP and o-tCz-BP to investigate the influence of D–A charge transfer to the electronic and TADF properties. Through-bond charge transfer (TBCT) was observed in the para-linked p-tCz-BP, where significant HOMO–LUMO overlapping results in a large f of 0.258 and a high kr of 2.25 × 107 s−1 but a relatively large ΔEST (0.23 eV). While both TBCT and through-space charge transfer (TSCT) were proved in the ortho-linked o-tCz-BP, which has a more twisted conformation and small spatial distance of ortho-substituted donor–acceptor moieties accompanied by a small HOMO–LUMO overlap, resulting in a tiny ΔEST of near zero and a high krisc of 2.85 × 106 s−1. By increasing the donor and acceptor contents in D-tCz-D-BP, i.e. with double ortho-linked donor–acceptor pairs on the phenylene bridge, multi-channel charge transfer was confirmed including para-TBCT, ortho-TBCT, and TSCT. D-tCz-D-BP shows more near-degenerate excited states and a higher intramolecular TSCT (81.0%) contribution than o-tCz-BP (66.3%). By inheriting the advantages of both p-tCz-BP and o-tCz-BP, D-tCz-D-BP exhibits a good balance between large f and small ΔEST, thus achieving a high kr of 1.01 × 107 s−1, a high krisc of 0.56 × 106 s−1 and a high PLQY of 96.8% simultaneously. The sky-blue OLED with D-tCz-D-BP as the doped emitter gained a maximum EQE of 24.9%, much higher than those of p-tCz-BP (6.3%) and o-tCz-BP (11.1%). D-tCz-D-BP also acted as an excellent host or a host emitter for a red iridium (III) complex (Ir2) to fabricate a red phosphorescence OLED and a two-emitting-component white OLED with an acceptable performance.
Results and discussion
Synthesis and characterization
The chemical structures of p-tCz-BP, o-tCz-BP and D-tCz-D-BP are shown in Scheme 1, and their synthesis is provided in Scheme S1 in the ESI.† The important intermediates 1, 2, and 3, i.e. the brominated phenylene intermediates, were first synthesized according to the literature methods.24–26 Then the nucleophilic addition of these brominated intermediates with 4-methylbenzaldehyde in the presence of n-BuLi followed by further oxidation with pyridinium chlorochromate generated the final products p-tCz-BP, o-tCz-BP and D-tCz-D-BP at moderate yields. These compounds have good solubilities in common organic solvents and could be purified by column chromatography and repeated recrystallization. Details of the syntheses and structure characterization are provided in the ESI.†Theoretical calculations
Density functional theory (DFT) calculation at the B3LYP-D3BJ/def2-SVP level and time-dependent DFT (TD-DFT) calculation at the PBE0/def2-SVP level were performed to gain insights into the electronic and structural properties of these compounds. As shown in Fig. 1a, in the optimized ground-state geometries of p-tCz-BP, o-tCz-BP and D-tCz-D-BP, the dihedral angles between the linking phenylene and donor moieties are 51.3°, 68.9° and 62.6°, respectively. Evidently, the carbazole donor ring was more twisted due to the steric hindrance effect when the donor and acceptor moieties are ortho-linked on the phenylene bridge. Meanwhile, in o-tCz-BP and D-tCz-D-BP molecules, the donor moieties show a tilted face-to-face alignment with the ortho-substituted acceptor moieties at a minimum spatial distance of 2.94 Å and 2.96 Å (Fig. 1a) between the non-hydrogen atoms, respectively, suggesting a more effective intramolecular π–π interaction between donor and acceptor and indicating the possible existence of TSCT. In contrast, TBCT seems exclusive in the p-tCz-BP molecule as the para-substitution increases the spatial distance between the donor and acceptor. The oscillator strengths (f) of these molecules were calculated to be 0.258, 0.0025 and 0.0528, respectively. It was found that D-tCz-D-BP inherits the twisted configuration and small donor–acceptor spatial distance from o-tCz-BP, but further gains a balance in the f values between p-tCz-BP and o-tCz-BP and finally reveals a moderate f of 0.0528. The frontier molecular orbitals (FMOs) distribution is shown in Fig. 1b, and the HOMOs of these molecules are mainly located on the tCz units while the LUMOs are located on the BP units. Clearly, large orbital overlap on the phenylene bridge is observed for p-tCz-BP, leading to a large theoretical ΔEST of 0.54 eV (Fig. 1c). In contrast, small orbital overlaps were observed for o-tCz-BP and D-tCz-D-BP, and thus achieving small theoretical ΔESTs of 0.09 and 0.13 eV, respectively. The suitable HOMO and LUMO overlap on the phenylene bridge suggests that charge can also be transferred through the phenylene moiety directly, confirming the possibility of TBCT in these molecules. It is interesting that the HOMO and HOMO−1 of D-tCz-D-BP are nearly degenerated (Table S1, ESI†), and may both contribute to the CT states. Fig. 1c shows the TD-DFT calculated excited states of these molecules. For D-tCz-D-BP, there are many near-degenerate singlet (S1–S2) and triplet excited states (T2–T6), which are close to the T1 state with small energy gaps and thus definitely promote an efficient multichannel RISC process. The spin–orbit coupling matrix elements (SOCME) between these states were also calculated and are illustrated in Table S2 (ESI†). It was reported that within an empirical energy range of 0.37 eV and a high SOC value (>0.3 cm−1),6,27,28 the numbers of valid RISC channels (blue-grey background) found in p-tCz-BP, o-tCz-BP and D-tCz-D-BP are 0, 4, and 10, respectively. According to Fermi's golden rule, krisc is proportional to 〈S|Ĥsoc|T/ΔEST〉, in which 〈S|Ĥsoc|T〉 is the spin orbit coupling (SOC) matrix element between the excited singlet (S) and triplet (T) states.9,10 Hence, o-tCz-BP and D-tCz-D-BP favor possessing a faster RISC pathway. More importantly, D-tCz-D-BP possesses much more valid RISC channels along with an increased oscillator strength (f = 0.0528), indicating the multi-donor–acceptor molecular architecture may be favorable for the light-emitting performance theoretically. Of course, the energy of 0.37 eV is within an empirical range that only represents a rough trend. This empirical energy range should be considered along with the experimentally determined energy values when they are used to interpret the RISC and TADF mechanisms. | ||
| Fig. 1 (a) Optimized ground geometries, (b) HOMO/LUMO distributions, and (c) energy level diagram of the PBE0/def2-SVP calculated excited states of p-tCz-BP, o-tCz-BP and D-tCz-D-BP. | ||
To explore the existence of weak intramolecular interactions between donor and acceptor, the independent gradient model based on the Hirshfeld partition of molecular density (IGMH) analyses in Multiwfn was studied,29–31 and the sign (λ2)ρ colored IGMH isosurface maps are shown in Fig. 2. The green regions of the IGMH isosurface maps show the presence of obvious intramolecular interactions, indicating that intramolecular through-space charge transfer can occur between donor and acceptor fragments in o-tCz-BP and D-tCz-D-BP. By integrating the transition density that was localized on/not on the phenylene linker, the proportions of TBCT/TSCT contribution to the S1 states were calculated to be 33.7%/66.3% and 19.0%/81.0% for o-tCz-BP and D-tCz-D-BP, respectively. The natural transition orbital (NTO) and triplet state spin density distribution (TSDD) were calculated to analyze the transition characteristics (Fig. S1, ESI†). Apparently, three molecules reveal a typical charge-transfer (CT) feature in their S1 states; p-tCz-BP shows a combined local-excited triplet state (3LE) and charge-transfer triplet states (3CT), while o-tCz-BP and D-tCz-D-BP exhibit a strong 3CT feature instead of the 3LE feature. Accordingly, different linking styles of donor and acceptor and multiple donor–acceptor pairs in one single molecule will generate different TBCT/TSCT proportions and subtly determine the excited states.19,21
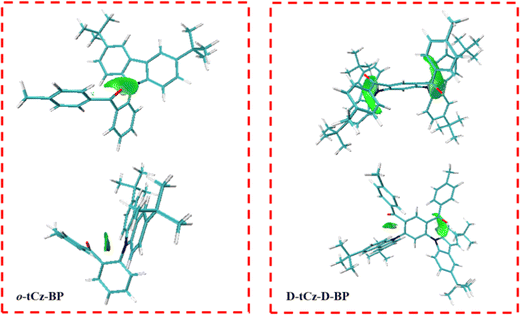 | ||
| Fig. 2 IGMH isosurface maps of o-tCz-BP and D-tCz-D-BP from different perspectives (isosurfaces of δginter = 0.004 a.u.). | ||
According to the optimized ground state geometries of these molecules, TD-DFT calculations were conducted to reveal the excited state energy-levels of the corresponding fragment molecules (Fig. S2, ESI† and Fig. 1c). The characters of low-lying singlet and triplet states other than S1 and T1 were also obtained by analyzing the hole and particle distributions in NTO analyses (Fig. S1, ESI†). Combined with fragment molecular calculations and NTO analyses of these molecules, it was found that the 3LEA and 3LED were located energetically close to the 3CT (TBCT and TSCT) states or mixed with the 3CT states. According to the El-Sayed rule, spin–orbital coupling between the 1CT and 3CT states is forbidden due to identical spatial orbital angular momentums, while that between the 1CT and 3LE states is permitted.32,33 Meanwhile, if 1CT and 3CT states stem from different charge transfer excited states, e.g. TBCT and TSCT, the SOC between 1CT and 3CT states should also be allowed. The 3LEA, 3LED and mixed 3LE and 3CT states can therefore serve as intermediate excited states between the 3CT and 1CT states to mediate spin–orbital coupling and promote the RISC process.17,33,34 According to Fermi's golden rule, krisc is proportional to 〈S|Ĥsoc|T〉/ΔEST. Hence, both large SOC and small ΔEST are optimum for a faster RISC process.
Based on the excited state alignment and the calculated SOC, the corresponding charge transfer channels and RISC mechanisms are proposed for these three emitters in Fig. 3. p-tCz-BP is characterized by an exclusive para-TBCT pathway and a relatively large ΔEST, which are not favorable for an efficient RISC process. However, due to the existence of intermediate excited states and considerable SOC between S1 and T3 states with different spatial orbital angular momentums, the T1 state can still be pumped to T2 and T3 states via vibrational coupling (VC) and thus transformed into the S1 state via RISC. Additionally, a large ΔEST and oscillator strength (f) normally refer to inferior krisc and superior kr, respectively. As for o-tCz-BP that has an ortho-linked and face-to-face donor and acceptor, there are two intramolecular charge transfer channels, i.e. ortho-TBCT (33.7%) and TSCT (66.3%), which lead to an extremely small ΔEST. At the same time, due to small energy differences between these triplet excited states, T1 (3CT) and T6 (3CT) states can be transformed into the intermediate excited states of T2 (3LEA), T3 (3LED) and T4 (3LEA) via vibrational coupling (VC) and internal conversion (IC), and then converted to S2 (1CT) or S1 (1CT) states. Based on the small energy gaps between these intermediate triplet excited states and S1 state and large SOC values, a multichannel RISC process is reasonable for o-tCz-BP and rather high krisc values can be expected. With increasing the contents of both donor and acceptor to form D-tCz-D-BP, the rich position relationships between the donor and acceptor generated three different types of charge transfer pathways, including para-TBCT, ortho-TBCT and TSCT. As calculated in Fig. 2, the content ratios are TBCT (19%, para-TBCT + ortho-TBCT) and TSCT (81%). Within an empirical energy range of 0.37 eV, there are much more intermediate triplet excited states that can be transformed into S1 or S2 states, most of which have a high SOC value (>0.3 cm−1), predicting the feasibility of a multichannel RISC process. Based on high SOC values, small ΔEST and considerable oscillator strength (f), superior krisc and kr can be safely anticipated simultaneously for D-tCz-D-BP.
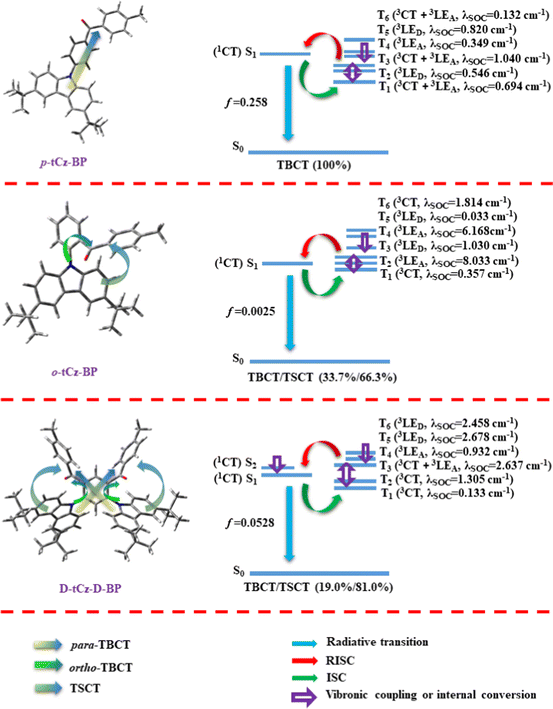 | ||
| Fig. 3 Charge transfer models (left) and the excited state alignment and supposed RISC mechanism (right) for p-tCz-BP, o-tCz-BP, and D-tCz-D-BP. | ||
Crystal structure and electrochemical properties
X-ray single crystal diffraction analysis for D-tCz-D-BP was performed. The details for the single crystal are listed in Table S3 in the ESI.† As shown in Fig. 4a, the dihedral angles between donors and the phenylene bridge are 61.95° and 61.57°, and the minimum π–π spatial distance between the non-hydrogen atoms in the acceptor and donor planes is 2.910 Å, confirming the coexistence of ortho-TBCT and TSCT effects. These results are in agreement with the theoretical calculations. It can be observed that there are multiple intermolecular and intramolecular interactions in its crystal packing (Fig. 4b), which are expected to restrict intramolecular motions and enhance the molecular rigidify, thus alleviating non-radiative decay and improving emission efficiency.The electrochemical properties of these compounds were examined by cyclic voltammetry (CV) in DCM (anodic scan) and DMF solutions (cathodic scan), as shown in Fig. S3 (ESI†). Using the onset potentials of the first oxidation and reduction wave (Eonsetox and Eonsetred), the HOMO and LUMO energies for all these compounds were estimated according to the empirical equations EHOMO = −e(Eonsetox + 4.4) and EHOMO = −e(Eonsetred + 4.4). The HOMO and LUMO levels were −5.55/−2.79, −5.52/−2.67 and −5.58/2.89 eV for p-tCz-BP, o-tCz-BP and D-tCz-D-BP, respectively. The different LUMOs for regioisomers p-tCz-BP and o-tCz-BP might be ascribed to different substitution positions, which finally impact the acceptor strength.18 The electrochemical data are listed in Table S4 (ESI†).
Photophysical properties
The ultraviolet-visible (UV-vis) absorption in dilute toluene (10−5 M) and photoluminescence (PL) spectra in different solvents at room temperature (RT) of p-tCz-BP, o-tCz-BP and D-tCz-D-BP are shown in Fig. 5a and the relevant photophysical data are summarized in Table S4 (ESI†). The high-energy absorption bands in the range of 290–350 nm should be assigned to local transitions of tCz and BP fragments, and the corresponding weak and broad bands above 355 nm are attributed to intramolecular charge transfer (ICT) from the donor to the acceptor. The PL spectra of p-tCz-BP and o-tCz-BP in solvents with different polarities exhibit broad and structureless bands, which could be assigned to the typical ICT emission, and the bathochromic shift displays a distinct positive solvatochromism, confirming their PL all originate from the CT excited states. The PL spectra of D-tCz-D-BP exhibit a dual emission feature in low polar solvents like hexane or toluene, in which the short-wavelength and long-wavelength bands should be assigned to the LE state and CT state emission, respectively, since the LE state emission at around 450 nm is almost independent of the solvent polarity in position but gradually gave place to the CT emission band with increasing solvent polarity while the CT emission band exhibited a distinct positive solvatochromism.According to the onsets of fluorescence spectra at room temperature and phosphorescence spectra at 77 K measured in 8 wt% doped PPF films (Fig. 5b), the S1 and T1 energies of p-tCz-BP, o-tCz-BP and D-tCz-D-BP were experimentally determined to be 3.10/2.87, 3.00/2.92 and 2.86/27.1 eV, and the corresponding ΔEST were calculated to be 0.23, 0.08 and 0.15 eV, respectively (Table 1). The experimental ΔEST values of o-tCz-BP and D-tCz-D-BP were in good agreement with the TD-DFT calculated values. The S1 energy level of o-tCz-BP was smaller than that of p-tCz-BP, which could be ascribed to the enhanced charge transfer (two charge transfer channels) in the o-tCz-BP molecule. The smaller ΔEST (0.08 eV) of o-tCz-BP could also be ascribed to the enhanced charge transfer (especially TSCT) and greatly reduced S1 energy due to reduced HOMO–LUMO overlaps caused by the twisted structure. For a similar reason, both greatly reduced S1 and T1 energy levels were observed for D-tCz-D-BP owing to the multi-donor–acceptor interactions,19 resulting in small ΔEST values of 0.15 eV. The relevant photophysical data are summarized in Table 1 and Table S4 (ESI†).
| Compound | ΔEST [eV] | Φ [%] | Φ PF/ΦDF [%] | Φ p/Φd [%] | τ PF [ns] | τ DF [μs] | k r/knr [106 s−1] | k isc [106 s−1] | k risc [106 s−1] |
|---|---|---|---|---|---|---|---|---|---|
| p-tCz-BP | 0.23 | 28.8 | 6.7/22.1 | 23.2/76.8 | 3.0 | 528.8 | 22.5/55.7 | 259.1 | 0.0082 |
| o-tCz-BP | 0.08 | 57.8 | 1.3/56.5 | 2.2/97.8 | 5.5 | 15.9 | 2.33/1.70 | 177.9 | 2.85 |
| D-tCz-D-BP | 0.15 | 96.8 | 12.1/84.7 | 12.5/87.5 | 12.0 | 14.4 | 10.1/0.33 | 73.2 | 0.56 |
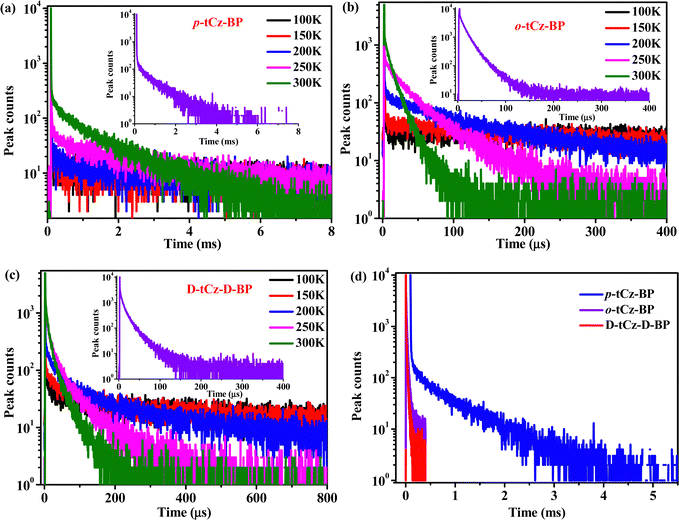
To confirm the TADF properties, the transient PL decay of these emitters doped in PPF (2,8-bis(diphenylphosphoryl)dibenzo[b,d]furan) film was measured at RT (Fig. 6 inset). Each transient curve consists of a prompt fluorescence (PF) and the delayed emission components. The emission at different delay times was detected with spectra identical to the PF (Fig. S6, ESI†), suggesting that the delayed emission and the PF originated from the same singlet excited state, i.e. the delayed fluorescence (DF). The PF have lifetimes (τPF) of 3.0, 5.5, and 12.0 ns (Fig. S7, ESI†), and the DF with lifetimes (τDF) of 528.8, 15.9 and 14.0 μs for p-tCz-BP, o-tCz-BP and D-tCz-D-BP were obtained, and the corresponding absolute PLQYs (Φ) were measured as 28.8%, 57.8% and 96.8%, respectively. The long DF lifetime and low PLQY of p-tCz-BP can be ascribed to large ΔEST. Although the τDF of o-tCz-BP and D-tCz-D-BP are almost equally short, the much higher PLQY of D-tCz-D-BP definitely favors gaining a good TADF performance. The positive temperature dependence of the delayed emission intensity (Fig. 6) along with the small ΔEST values confirmed the TADF nature for these three emitters.35 Taking into account the contributions of the prompt (Φp) and delayed (Φd) components to the total PLQYs (Φ), the ΦDF gradually increases from 22.1%, via 56.5% to 84.7% for p-tCz-BP, o-tCz-BP and D-tCz-D-BP, respectively, happening to be consistent with the trend of the TSCT proportions contributed to the S1 state (0, 66.3% and 81.0%, sequentially). According to the transient PL data and PLQY values, all relevant rate constants of different kinetic processes were calculated following the reported equations.36,37 The rate constants of radiation (kr) were calculated to be 2.25 × 107 s−1, 0.23 × 107 s−1 and 1.01 × 107 s−1, the rate constants of RISC (krisc) were calculated to be 0.0082 × 106 s−1, 2.85 × 106 s−1 and 0.56 × 106 s−1, and the rate constants of non-radiation (knr) were calculated to be 55.7 × 106 s−1, 1.70 × 106 s−1 and 0.33 × 106 s−1 for p-tCz-BP, o-tCz-BP and D-tCz-D-BP, respectively. The high kr of p-tCz-BP and D-tCz-D-BP should be ascribed to large f values, the high krisc of o-tCz-BP and D-tCz-D-BP should be attributed to small ΔEST, favorable SOC, and much more valid RISC channels. Due to the restricted intramolecular motions and enhanced molecular rigidity in the crystal packing, D-tCz-D-BP gained the lowest knr, favoring alleviation of the non-radiative decay and improving the emission efficiency. Obviously, D-tCz-D-BP with multi-donor–acceptor para/ortho-linked architecture not only possesses a high PLQY, low knr and high rate of TSCT contribution, but it also combines the merits of p-tCz-BP and o-tCz-BP. The photophysical data of the investigated molecules are listed in Table 1.
Electroluminescent devices
To further investigate the electroluminescence (EL) performances of p-tCz-BP, o-tCz-BP and D-tCz-D-BP, multilayer OLEDs were fabricated with the device configuration of indium tin oxide (ITO)/poly-(3,4ethylenedioxythiophene):poly(styrene sulfonic acid) (PEDOT:PSS, 40 nm)/1,1-bis[(di-4tolylamino)phenyl]cyclohexane (TAPC, 20 nm)/4,4′,4′′-tri(Ncarbazolyl) triphenylamine (TCTA, 5nm)/(1,5-bis(9-carbazolyl)benzene) (mCP, 5 nm)/PPF:dopant (8 wt%, 20 nm)/(2,8-bis(diphenylphosphoryl)dibenzo[b,d]furan) (PPF, 5 nm)/(3,3′-(5′-(3-(pyridine-3-yl)phenyl)[1,1′:3′,1′′-terphenyl]-3,3′′-diyl)dipyridine (TmPyPB, 40 nm)/LiF (1 nm)/Al (200 nm). The energy level diagram of the OLEDs and the chemical structures of these materials are depicted in Fig. 7a and b. In these devices, PEDOT:PSS and TAPC served as the hole-injection layer (HIL) and the hole-transporting layer (HTL), PPF as the host material, TCTA, mCP and PPF as exciton-blocking materials, and TmPyPB and LiF as the electron-transporting layer (ETL) and electron-injection layer (EIL), respectively. The EL characteristics of devices A, B and C based on p-tCz-BP, o-tCz-BP and D-tCz-D-BP were depicted in Fig. 7c and d and the corresponding device performances were summarized in Table 2. As shown in Fig. 7c and d, emission peaks at 450, 466, and 490 nm for p-tCz-BP, o-tCz-BP and D-tCz-D-BP were obtained, respectively, corresponding to CIE coordinates of (0.16, 0.11), (0.17, 0.23) and (0.22, 0.40). Spectral bathochromic shifts should be ascribed to the progressively enhanced intramolecular charge transfer (ICT) strength. According to the EL spectra and current density–voltage–brightness (J–V–B) characteristics, the maximum current efficiency (CE), maximum power efficiency (PE) and EQE were calculated to be 6.7 cd A−1, 3.5 lm W−1 and 6.3% for device A, 18.5 cd A−1, 17.0 lm W−1 and 11.1% for device B and 54.8 cd A−1, 52.2 lm W−1 and 24.9% for device C, respectively. The inferior EL performances of device A could be ascribed to the lower PLQY, larger ΔEST, large knr and longer τDF of p-tCz-BP, and the inferior EL performances of device B could be ascribed to the small f, moderate PLQY and relatively large knr of o-tCz-BP. On the contrary, the superior EL performance of device C should be ascribed to the absolutely high PLQY, large kr and krisc, and small ΔEST and knr of D-tCz-D-BP. Evidently, thanks to the multichannel charge transfer feature gained in D-tCz-D-BP, both the radiation transition and RISC were efficiently enhanced and balanced with an extremely high PLQY (96.8%), high krisc (0.56 × 106 s−1) and low knr (0.33 × 106 s−1), which finally resulted in the excellent performance of the D-tCz-D-BP-based TADF-OLED.| Device | V on [V] | η c [cd A−1] | η p [lm W−1] | η ext [%] | λ EL [nm] | FWHMd [nm] | CIE(x,y)e |
|---|---|---|---|---|---|---|---|
| a V on, turn-on voltage at 1 cd m−2. b η c, ηp, ηext: the current efficiency, power efficiency, and external quantum efficiency for devices A, B, C, R and W, and the values are in the order of maximum/at 100 cd m−2/at 500 cd m−2. c λ EL, EL peak wavelength at 8 V. d FWHM, full width at half maximum. e CIE(x,y), Commission International de I’Eclairage coordinates at 8 V. | |||||||
| A | 5.8 | 6.7/1.4/0.4 | 3.5/0.5/0.1 | 6.3/1.4/0.4 | 450 | 62 | 0.16,0.12 |
| B | 3.4 | 18.5/16.0/13.5 | 17.0/9.8/6.3 | 11.1/9.6/8.2 | 468 | 74 | 0.17,0.23 |
| C | 3.3 | 54.8/40.3/34.6 | 52.2/28.2/19.7 | 24.9/18.5/15.7 | 490 | 90 | 0.22,0.40 |
| R | 3.5 | 26.3/25.2/22.8 | 20.2/12.8/9.4 | 21.0/20.2/18.3 | 618 | 69 | 0.62,0.36 |
| W | 3.2 | 39.6/29.3/26.2 | 38.8/21.1/15.2 | 18.8/13.5/12.1 | 500,606 | — | 0.41,0.42 |
Inspired by the excellent performance of D-tCz-D-BP in its TADF-OLEDs, it was also explored D-tCz-D-BPas a host to sensitize iridium phosphors to fabricate phosphorescence OLEDs with a configuration of ITO/PEDOT:PSS (40 nm)/TAPC (20 nm)/mCP (5 nm)/D-tCz-D-BP:Ir2 (3 wt%, 20 nm)/PPF (5 nm)/TmPyPB (40 nm)/LiF (1 nm)/Al (200nm) (device R). The home-made Ir2 was used as the red emitting dopant and its structure is shown in Fig. S8 in the ESI.†![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38Fig. 8a–c illustrate the J–V–B characteristics, efficiency curves and the EL spectra of device R, and the related EL data are summarized in Table 2. At an optimized doping concentration of 3 wt%, device R displayed red emission with a peak at 618 nm and CIE coordinates of (0.62, 0.36). The single emission from the phosphorescent dopant indicated the sufficient energy transfer from the host to dopant. The device R displayed a maximum CE of 26.3 cd A−1 and EQE of 21.0%. The pure red emission from the iridium dopant with the acceptable efficiency of device R manifests that D-tCz-D-BP is a promising host material for a phosphorescent emitter. With further reducing the doping concentration of Ir2 in D-tCz-D-BP to 0.2 wt% (device W, Fig. 8a–c), both the sky-blue emission from the D-tCz-D-BP host and the red phosphorescence from the Ir2 dopant were obtained simultaneously, generating warm white emission (Fig. 8c). Device W exhibited a maximum ηc of 39.6 cd A−1, ηp of 38.8 lm W−1, and ηext of 18.8%. With the driving voltage going from 6 to 9 V, the CIE coordinates showed a slight change from (0.43,0.42) to (0.40, 0.42), and a CRI from 79 to 80, respectively. The good performance of the WOLED including acceptable efficiency and the high CRI value should be ascribed to the intrinsically excellent TADF feature and good charge transporting ability of D-tCz-D-BP when it acted as the host emitter in a white OLED. As shown in Fig. 8d, at such a low doping concentration of 0.2 wt%, the Förster energy transfer from the singlet excitons of D-tCz-D-BP to the iridium dopant molecules followed by the easy and efficient intersystem crossing of the iridium phosphor should dominate the excitation of phosphorescent dopant molecules.
38Fig. 8a–c illustrate the J–V–B characteristics, efficiency curves and the EL spectra of device R, and the related EL data are summarized in Table 2. At an optimized doping concentration of 3 wt%, device R displayed red emission with a peak at 618 nm and CIE coordinates of (0.62, 0.36). The single emission from the phosphorescent dopant indicated the sufficient energy transfer from the host to dopant. The device R displayed a maximum CE of 26.3 cd A−1 and EQE of 21.0%. The pure red emission from the iridium dopant with the acceptable efficiency of device R manifests that D-tCz-D-BP is a promising host material for a phosphorescent emitter. With further reducing the doping concentration of Ir2 in D-tCz-D-BP to 0.2 wt% (device W, Fig. 8a–c), both the sky-blue emission from the D-tCz-D-BP host and the red phosphorescence from the Ir2 dopant were obtained simultaneously, generating warm white emission (Fig. 8c). Device W exhibited a maximum ηc of 39.6 cd A−1, ηp of 38.8 lm W−1, and ηext of 18.8%. With the driving voltage going from 6 to 9 V, the CIE coordinates showed a slight change from (0.43,0.42) to (0.40, 0.42), and a CRI from 79 to 80, respectively. The good performance of the WOLED including acceptable efficiency and the high CRI value should be ascribed to the intrinsically excellent TADF feature and good charge transporting ability of D-tCz-D-BP when it acted as the host emitter in a white OLED. As shown in Fig. 8d, at such a low doping concentration of 0.2 wt%, the Förster energy transfer from the singlet excitons of D-tCz-D-BP to the iridium dopant molecules followed by the easy and efficient intersystem crossing of the iridium phosphor should dominate the excitation of phosphorescent dopant molecules.
Conclusions
By grafting two pairs of ortho-linked carbazole donor-benzophenone acceptors on the phenylene bridge, a novel TADF emitter D-tCz-D-BP was developed. Multichannel charge transfer, including para-TBCT (through-bond charge transfer), ortho-TBCT and TSCT (through-space charge transfer), was proved by single crystal analysis and theoretical calculations in D-tCz-D-BP, which essentially enhanced both the radiation and RISC processes and thus resulted in a high kr of 1.01 × 107 s−1, a high PLQY of 96.8%, and a high krisc of 0.56 × 106 s−1. The sky-blue TADF-OLED with D-tCz-D-BP as the doped emitter exhibited a maximum EQE of 24.9%, much higher than those of the reference analogues p-tCz-BP (6.3%) and o-tCz-BP (11.1%) that contain either a para- or ortho-linked donor–acceptor. D-tCz-D-BP also exhibited good performance with a high EQE of 18.8% and a high CRI of 80 in the two-emitting-component white OLED when it acted as the host emitter for a red iridium phosphor. It was demonstrated that multichannel charge transfer is a practical strategy to design high-performance TADF emitters for OLED applications.Author contributions
Zhaolong He: conceptualization, investigation, writing – original draft. Jiuyan Li: funding acquisition, investigation, supervision, writing – review and editing. Di Liu: investigation, project administration, resource, supervision. Huihui Wan: spectra acquisition and structure characterization. Yongqiang Mei: data curation, visualization. Chunlong Shi: formula analysis, software.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We thank the National Natural Science Foundation of China (U1801258 and 22078051), the Fundamental Research Funds for the Central Universities (DUT22LAB610) and the Open Fund of the State Key Laboratory of Luminescent Materials and Devices (South China University of Technology, 2021-skllmd-03) for financial support of this work.References
- X. Q. Wang, S. Y. Yang, Q. S. Tian, C. Zhong, Y. K. Qu, Y. J. Yu, Z. Q. Jiang and L. S. Liao, Angew. Chem., Int. Ed., 2021, 60, 5213–5219 CrossRef CAS PubMed.
- Z. Yang, Z. Mao, C. Xu, X. Chen, J. Zhao, Z. Yang, Y. Zhang, W. Wu, S. Jiao, Y. Liu, M. P. Aldred and Z. Chi, Chem. Sci., 2019, 10, 8129–8134 RSC.
- P. K. Samanta, D. Kim, V. Coropceanu and J. L. Bredas, J. Am. Chem. Soc., 2017, 139, 4042–4051 CrossRef CAS PubMed.
- X. Li, J. Li, D. Liu, D. Li and R. Dong, New J. Chem., 2020, 44, 9743–9753 RSC.
- H.-Z. Li, F.-M. Xie, K. Zhang, Y. Shen, W. Zhou, Y.-Q. Li, W.-J. Wang and J.-X. Tang, Chem. Eng. J., 2022, 436, 135234 CrossRef CAS.
- X. Zheng, R. Huang, C. Zhong, G. Xie, W. Ning, M. Huang, F. Ni, F. B. Dias and C. Yang, Adv. Sci., 2020, 7, 1902087 CrossRef CAS PubMed.
- X. Liang, Z. L. Tu and Y. X. Zheng, Chem. – Eur. J., 2019, 25, 5623–5642 CrossRef CAS PubMed.
- Z. G. Wu, H. B. Han, Z. P. Yan, X. F. Luo, Y. Wang, Y. X. Zheng, J. L. Zuo and Y. Pan, Adv. Mater., 2019, 31, 1900524 CrossRef PubMed.
- Z. Lin, R. Kabe, K. Wang and C. Adachi, Nat. Commun., 2020, 11, 191 CrossRef CAS PubMed.
- J. U. Kim, I. S. Park, C. Y. Chan, M. Tanaka, Y. Tsuchiya, H. Nakanotani and C. Adachi, Nat. Commun., 2020, 11, 1765 CrossRef CAS PubMed.
- J. Xue, Q. Liang, R. Wang, J. Hou, W. Li, Q. Peng, Z. Shuai and J. Qiao, Adv. Mater., 2019, 31, 1808242 CrossRef PubMed.
- W. Li, X. Cai, B. Li, L. Gan, Y. He, K. Liu, D. Chen, Y. C. Wu and S. J. Su, Angew. Chem., Int. Ed., 2019, 58, 582–586 CrossRef CAS PubMed.
- X. Lv, Y. Wang, N. Li, X. Cao, G. Xie, H. Huang, C. Zhong, L. Wang and C. Yang, Chem. Eng. J., 2020, 402, 126173 CrossRef CAS.
- M. Ouyang, L. Xing, Q. Chen, H. Huang, M. Zhu, K. Hu, Y. Liu, W.-C. Chen, Y. Huo and C. Yang, J. Mater. Chem. C, 2021, 9, 1678–1684 RSC.
- T. Huang, Q. Wang, S. Xiao, D. Zhang, Y. Zhang, C. Yin, D. Yang, D. Ma, Z. Wang and L. Duan, Angew. Chem., Int. Ed., 2021, 60, 23771–23776 CrossRef CAS PubMed.
- D. Zhang, X. Song, A. J. Gillett, B. H. Drummond, S. T. E. Jones, G. Li, H. He, M. Cai, D. Credgington and L. Duan, Adv. Mater., 2020, 32, 1908355 CrossRef CAS PubMed.
- H. Noda, X. K. Chen, H. Nakanotani, T. Hosokai, M. Miyajima, N. Notsuka, Y. Kashima, J. L. Bredas and C. Adachi, Nat. Mater., 2019, 18, 1084–1090 CrossRef CAS PubMed.
- N. A. Kukhta, H. F. Higginbotham, T. Matulaitis, A. Danos, A. N. Bismillah, N. Haase, M. K. Etherington, D. S. Yufit, P. R. McGonigal, J. V. Gražulevičius and A. P. Monkman, J. Mater. Chem. C, 2019, 7, 9184–9194 RSC.
- A. Danos, D. Gudeika, N. A. Kukhta, R. Lygaitis, M. Colella, H. F. Higginbotham, A. N. Bismillah, P. R. McGonigal, J. V. Grazulevicius and A. P. Monkman, J. Mater. Chem. C, 2022, 10, 4737–4747 RSC.
- G. Haykir, M. Aydemir, A. Danos, S. Gumus, G. Hizal, A. P. Monkman and F. Turksoy, Dyes Pigm., 2021, 194, 109579 CrossRef CAS.
- F.-M. Xie, J.-X. Zhou, Y.-Q. Li and J.-X. Tang, J. Mater. Chem. C, 2020, 8, 9476–9494 RSC.
- J. Zeng, J. Guo, H. Liu, Z. Zhao and B. Z. Tang, Adv. Funct. Mater., 2020, 30, 2000019 CrossRef CAS.
- H. Chen, H. Liu, P. Shen, J. Zeng, R. Jiang, Y. Fu, Z. Zhao and B. Z. Tang, Adv. Opt. Mater., 2021, 9, 2002019 CrossRef CAS.
- T. Sudyoadsuk, S. Petdee, P. Chasing, P. Therdkatanyuphong, C. Kaiyasuan, W. Waengdongbung, S. Namuangruk and V. Promarak, Dyes Pigm., 2021, 195, 109721 CrossRef.
- Y. H. Lee, S. Park, J. Oh, S.-J. Woo, A. Kumar, J.-J. Kim, J. Jung, S. Yoo and M. H. Lee, Adv. Opt. Mater., 2018, 6, 1800385 CrossRef.
- H. L. Lee, W. J. Chung and J. Y. Lee, Small, 2020, 16, 1907569 CrossRef CAS PubMed.
- R. Chen, Y. Tang, Y. Wan, T. Chen, C. Zheng, Y. Qi, Y. Cheng and W. Huang, Sci. Rep., 2017, 7, 6225 CrossRef PubMed.
- Y. Tao, R. Chen, H. Li, J. Yuan, Y. Wan, H. Jiang, C. Chen, Y. Si, C. Zheng, B. Yang, G. Xing and W. Huang, Adv. Mater., 2018, 30, 1803856 CrossRef PubMed.
- T. Lu and F. Chen, J. Comput. Chem., 2012, 33, 580–592 CrossRef CAS PubMed.
- C. Lefebvre, G. Rubez, H. Khartabil, J. C. Boisson, J. Contreras-Garcia and E. Henon, Phys. Chem. Chem. Phys., 2017, 19, 17928–17936 RSC.
- T. Lu and Q. Chen, J. Comput. Chem., 2022, 43, 539–555 CrossRef CAS PubMed.
- M. A. El-Sayed, J. Chem. Phys., 1963, 38, 2834–2838 CrossRef CAS.
- Y. Song, M. Tian, R. Yu and L. He, ACS Appl. Mater. Interfaces, 2021, 13, 60269–60278 CrossRef CAS PubMed.
- M. K. Etherington, J. Gibson, H. F. Higginbotham, T. J. Penfold and A. P. Monkman, Nat. Commun., 2016, 7, 13680 CrossRef CAS PubMed.
- L. Gan, Z. Xu, Z. Wang, B. Li, W. Li, X. Cai, K. Liu, Q. Liang and S. J. Su, Adv. Funct. Mater., 2019, 29, 1808088 CrossRef.
- W. Li, B. Li, X. Cai, L. Gan, Z. Xu, W. Li, K. Liu, D. Chen and S. J. Su, Angew. Chem., Int. Ed., 2019, 58, 11301–11305 CrossRef CAS PubMed.
- R. Dong, J. Li, D. Liu, D. Li, Y. Mei, M. Ma and J. Jiang, Adv. Opt. Mater., 2021, 9, 2100970 CrossRef CAS.
- H. Tian, D. Liu, J. Li, M. Ma, Y. Lan, W. Wei, R. Niu and K. Song, New J. Chem., 2021, 45, 11253–11260 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2174202. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2tc03875g |
| This journal is © The Royal Society of Chemistry 2022 |