Ferroelectricity in organic materials: from materials characteristics to de novo design
Abstract
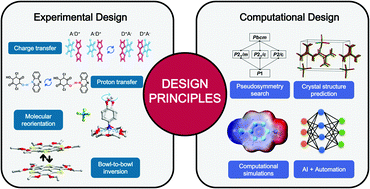
Supramolecular chemistry exploits weak, reversible interactions to form complex structures from simple components, which offers a solution for designing new ferroelectric materials. Organic ferroelectrics have been considered to be promising alternatives to conventional inorganic ferroelectrics due to their molecular mobility, flexibility and tunability of well-defined structures. However, ferroelectricity in organic solids remains underexplored, and the limited number of organic ferroelectrics with high performance hinders their practical applications. Therefore, the design of new organic ferroelectrics with promising ferroelectric properties is of great interest. In this perspective, we describe key material features and properties of organic ferroelectrics. Synthetic strategies and design principles are comprehensively reviewed. To further explore the possibility of organic ferroelectrics, we give our perspectives on the computational design and how to integrate computation and automation approaches for the accelerated discovery of organic ferroelectrics. Some of the challenges and opportunities in the field have been discussed. We hope this outlook can enable more research to accelerate the development of high-performance organic ferroelectrics.
- This article is part of the themed collection: Journal of Materials Chemistry C Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...