Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A MOF-based electronic nose for carbon dioxide sensing with enhanced affinity and selectivity by ionic-liquid embedment†
Peng
Qin
,
Salih
Okur
,
Yunzhe
Jiang
and
Lars
Heinke
 *
*
Karlsruhe Institute of Technology (KIT), Institute of Functional Interfaces (IFG), Hermann-von-Helmholtz-Platz 1, 76344 Eggenstein-Leopoldshafen, Germany. E-mail: Lars.Heinke@kit.edu
First published on 28th November 2022
Abstract
The unequivocal detection of CO2 is important in many situations, like in the living environment, plant cultivation and the conservation of cultural relics and archives. Due to their large specific surface areas and highly ordered and tunable structures, metal–organic frameworks (MOFs) have the potential to improve CO2 sensing, however, they often suffer from low CO2 affinity and selectivity. Ionic liquids (ILs) have high CO2 affinity, but their performance in sensors is hampered by their nonporous, liquid form. Here, we present a low-cost and portable CO2 sensor system based on an array of gravimetric sensors made of MOF films with embedded ILs in the pores. The array is composed of MOF films of two different structures, which are HKUST-1 and UiO-66, filled with 3 different types of ILs and 2 different pore-filling levels, resulting in an array of up to 14 different sensors. We show that the different combinations of IL and MOF result in different affinities for CO2 and other analytes. With the help of machine learning using a neural network, the sensor array was used to quantify the CO2 concentration as well as to distinguish CO2 from other gases and vapors, like nitrogen, ethanol, methanol and water, and to distinguish certain binary mixtures. While the MOF-sensor array without IL achieves only a small accuracy for determining the CO2 concentration, the IL@MOF sensor array can accurately classify the gas types (98% accuracy) in mixed gas atmospheres of unknown composition and concentration as well as can determine the CO2 gas concentration with an average error of only 2.7%. Using only MOFs with a pronounced chemical stability (like UiO-66) in the sensor array also allows the detection and identification of CO2 in a humid atmosphere. Moreover, the presented sensor system has very high sensitivity with a CO2 limit of detection below 100 ppm, which is four times smaller than the CO2 concentration in air. This work shows the unprecedented performance of the sensor arrays of MOFs with embedded ILs, referred to as IL@MOF-electronic nose (IL@MOF-e-nose), for sensing the composition and concentration of CO2 gas mixtures.
Introduction
Carbon dioxide is an essential component of the atmosphere, a frequent product of life activities and industrial processes as well as the major culprit of the global greenhouse effect.1 Apart from the climate damage, high CO2 levels can have a variety of effects and can be harmful to human health. When the concentration remains at 0.3% or higher for a long time, people may experience significant headaches, and people will feel dizzy at CO2 concentrations of 4–5%.2 CO2 concentrations of at least 9% for more than 5 minutes may cause the death of humans.3 In indoor situations, the main CO2 sources are exhaled breath and open fires, e.g. from fireplaces, candles and gas cookers. Especially in relatively sealed rooms (e.g. air-conditioned rooms without ventilation and air exchange), the CO2 concentration gradually increases and can quickly reach critical values. There, the CO2 detection is vital. In addition to the indoor applications, carbon dioxide sensing is critical for many different industrial applications, including landfills,4 horticulture,5 controlled atmosphere storage and packaging6 and metal treatment.7Conventional CO2 sensors generally use Non-Dispersive Infrared (NDIR) technology.8 Once there is moisture in the analyte gas or the CO2 concentration is high, the absorption bands overlap and the CO2 quantification is inaccurate. Photoacoustic CO2 sensing is a mature technology,9–11 but so far it has only been used in expensive laboratory-scale applications. Gravimetric sensors, like quartz crystal microbalance (QCM) sensors, have the advantages of simple device structure, high sensitivity, low cost, short analysis time and reusability.12,13 The resonance frequency, which is proportional to the mass of the adsorbed molecules, is used as the sensor signal.14 The affinity of the sensor can be tuned by modifying the QCM sensor surface. For example, QCM-based sensors modified with nanoporous materials with large specific surface areas have been used for the detection of various gases and vapors.15–18 QCM-based CO2 sensors have been presented but, while the detection of large CO2 concentrations is straightforward, the detection limit and sensitivity are, to date, too poor for practical applications.19 The main challenge for gravimetric CO2 sensors is the active sensing material which should have a high affinity towards CO2, but a low affinity towards other gases, and CO2 should adsorb reversibly allowing for repeatable sensor usage.
MOFs are a class of nanoporous materials, composed of metal nodes connected by organic ligand molecules.20,21 The structure and properties of MOFs can be designed to a wide extent. In this way, the chemical building blocks, the pore size and shape, the surface functionality and even orderliness have been modified to optimize the CO2 adsorption capacity.22–26 However, for practical applications, the CO2 affinity has to be significantly increased and the cross-sensitivity to other gases has to be generally decreased. Ionic liquids (ILs) are a class of unique liquids with high CO2 affinities.27,28 ILs are composed of organic cationic and anionic molecules and have a wide structural and functional diversity.29–31 Based on their CO2 affinity, ILs are used as active sensing materials for sensing CO2.32–34 However, their liquid and non-porous nature hamper their application in sensors.35,36 Nanoporous MOF materials can serve as host materials for the embedment of ILs, and the combination of the two can enhance the CO2 sensing performance.37,38 IL@MOF composites were also used in various applications, for example, they show a strong performance in CO2 adsorption39–42 and separation,43–46 I2 capture,47 heterogeneous catalysis48–51 or battery applications.52–54 For sensor applications, the cross-sensitivity, which means that the CO2-concentration signal is interfered by the presence of humidity and other gas or vapor molecules, is often a severe issue, but unexplored to date. A powerful method to minimize the cross-sensitivity is to use arrays of sensors with different affinities. Such a sensor system is also referred to as electronic nose, e-nose.55–57
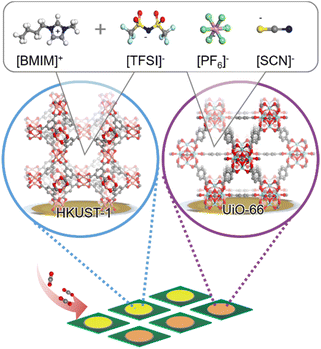
Here, we present a sensing system capable of detecting CO2 and its mixtures with common vapor molecules. It is based on an electronic nose (e-nose), which is an array of QCM sensors coated with 2 different nanoporous MOF films, which are MOFs of type HKUST-1 and UiO-66, Fig. 1. (HKUST-1 stands for Hong Kong University of Science and Technology 1, also known as Cu3(BTC)2 with BTC = benzene-1,3,5-tricarboxylate), and UiO stands for Universitetet i Oslo.) To enhance the CO2 affinity and selectivity, six sensors of each MOF structure were loaded with three different ionic liquids: [BMIM][SCN], [BMIM][TFSI], [BMIM][PF6] with pore fillings of 20% and 50%. ([BMIM] stands for 1-butyl-3-methylimidazolium, [SCN] for thiocyanate, [TFSI] for bis(trifluoromethylsulfonyl)imide and [PF6] for hexafluorophosphate). Higher IL-pore-fillings are avoided, due to the decreased free pore volume resulting in slow analyte diffusion and uptake. MOF films of type HKUST-1 and UiO-66 were chosen, because of: (1) their appropriate pore size, which is large enough to host ILs; (2) their pronounced stability, also when loaded with IL. Moreover, UiO-66 is also stable under humid conditions. (3) The syntheses of both MOFs were optimized allowing the preparation of homogeneous and highly crystalline films. The responses of the e-nose to different gases or vapors of CO2, N2, ethanol, methanol and water as well as some mixtures were tested. As expected, the sensor signal (or uptake) of most analyte molecules was reduced by the IL embedment in the MOF pores, due to the reduced free pore volume. On the other hand, the IL embedment results in an increased uptake of CO2 and water upon IL-embedment, despite the reduced free pore volume. The ratio of the IL-embedment-caused signal increase for CO2 varies with the MOF structure and the type of IL, resulting in different sensitivities of the different IL@MOF-sensors. With a neural network-based machine learning algorithm,58 the data of the sensor array were analyzed. As a result, the e-nose can quantify the CO2 concentration down to a limit of detection below the CO2 level in air and can distinguish between the different gases and mixtures with 98% accuracy. This study shows that the ionic-liquid-loading in nanoporous materials can enhance the CO2 sensitivity and selectivity and that such IL@MOF-based sensor arrays show superior performance for CO2 sensing.
Experimental section
MOF film syntheses
All MOF films are prepared on silver-coated QCM sensors. The HKUST-1 films were prepared using a layer-by-layer (lbl) method by sequentially exposing the substrate surface to the solutions of the metal nodes and of the linker molecules, resulting in surface-mounted MOFs (SURMOFs).59,60 The MOF films with an HKUST-1 structure61 were prepared from an ethanolic solution of 1 mM copper acetate and 0.2 mM BTC. The lbl SURMOF synthesis was performed by the spray method62 with 100 synthesis cycles. Prior to the SURMOF syntheses, all QCM substrates were functionalized with a 11-mercapto-1-undecanol (MUD) self-assembled monolayer (SAM).UiO-66 films were prepared by the vapor-assisted conversion (VAC) method.63 ZrOCl2, terephthalic acid (BDC) and acetic acid were dissolved in DMF as the precursor solution. DMF and acetic acid mixture solution were filled in the glass vessel as a vapor source. One drop (50 μL) of the precursor solution was deposited uniformly on the sensor surface. The vessel was then sealed and heated to 120 °C for 3 hours. Finally, the samples were dried in vacuum at room temperature.
Embedment of IL in MOF films
The loading of the MOF films with IL was performed by immersing the samples in an IL/acetonitrile solution, as optimized in previous publications.64–66 The immersion was carried out at room temperature for about 20 minutes. Afterwards, the sample was rinsed for about 2 seconds using acetonitrile and then dried in a stream of pure nitrogen. The IL concentration in acetonitrile was 20% and 50%, respectively, resulting in roughly 20% and 50% IL pore filling, respectively.64–66 The sensor names with the MOF structures and the IL-loadings are given in Table 1.| Sensor name | IL and MOF | IL loading in IL pairs per unit cell | Sensitivity in Hz/%vol | LOD in %vol | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CO2 | N2 | Ethanol | Methanol | Water | CO2 | N2 | Ethanol | Methanol | Water | |||
| S1 | HKUST-1 (empty) | 0 | 47.041 | 17.077 | 141.2 | 190.64 | 95.941 | 0.0102 | 0.0281 | 0.0034 | 0.0025 | 0.005 |
| S2 | [BMIM][TFSI]20% @HKUST-1 | 5.3 ± 1.4 | 47.657 | 13.109 | 118.4 | 164.61 | 136.72 | 0.0101 | 0.0336 | 0.0041 | 0.0029 | 0.0035 |
| S3 | [BMIM][TFSI]50% @HKUST-1 | 9.8 ± 0.3 | 53.307 | 10.016 | 110.13 | 154.4 | 188.6 | 0.009 | 0.0479 | 0.0044 | 0.0031 | 0.0025 |
| S4 | [BMIM][SCN]20% @HKUST-1 | 6.1 ± 1.1 | 51.116 | 14.213 | 128.42 | 169.45 | 170.8 | 0.0094 | 0.0338 | 0.0037 | 0.0028 | 0.0028 |
| S5 | [BMIM][SCN]50% @HKUST-1 | 11.9 ± 0.4 | 56.174 | 10.937 | 110.74 | 161.2 | 212.68 | 0.0085 | 0.0439 | 0.0043 | 0.003 | 0.0023 |
| S6 | [BMIM][PF6]20% @HKUST-1 | 5.1 ± 1.6 | 47.026 | 13.276 | 117.98 | 164.15 | 149.4 | 0.0102 | 0.0362 | 0.0041 | 0.0029 | 0.0032 |
| S7 | [BMIM][PF6]20% @HKUST-1 | 9.7 ± 1.1 | 52.995 | 10.002 | 109.45 | 154.35 | 196.2 | 0.0091 | 0.048 | 0.0044 | 0.0031 | 0.0024 |
| S8 | UiO-66 (empty) | 0 | 37.477 | 11.268 | 104.25 | 146.56 | 94.85 | 0.0128 | 0.0426 | 0.0046 | 0.0033 | 0.0051 |
| S9 | [BMIM][TFSI]20% @UiO-66 | 1.7 ± 0.6 | 39.144 | 8.656 | 88.084 | 129.57 | 127.75 | 0.0123 | 0.0555 | 0.0054 | 0.0037 | 0.0038 |
| S10 | [BMIM][TFSI]50% @UiO-66 | 5.5 ± 0.6 | 41.397 | 7.075 | 81.506 | 123.98 | 151.57 | 0.0116 | 0.0678 | 0.0059 | 0.0039 | 0.0032 |
| S11 | [BMIM][SCN]20% @UiO-66 | 2.3 ± 0.6 | 40.108 | 9.664 | 93.637 | 133.48 | 136.25 | 0.012 | 0.0497 | 0.0051 | 0.0036 | 0.0035 |
| S12 | [BMIM][SCN]50% @UiO-66 | 6.2 ± 0.9 | 44.157 | 7.67 | 83.631 | 126.62 | 192.68 | 0.0109 | 0.0626 | 0.0057 | 0.0038 | 0.0025 |
| S13 | [BMIM][PF6]20% @UiO-66 | 2.2 ± 0.9 | 38.983 | 8.63 | 87.545 | 129.12 | 141.99 | 0.0123 | 0.0556 | 0.0055 | 0.0037 | 0.0034 |
| S14 | [BMIM][PF6]50% @UiO-66 | 4.9 ± 0.6 | 41.127 | 7.058 | 81.062 | 123.72 | 155.17 | 0.0117 | 0.068 | 0.0059 | 0.0039 | 0.0031 |
Characterization of samples
The gas flow system used to generate a specific content of the target analyte is shown in Fig. S1.† Argon is used as the carrier gas, while CO2 and nitrogen are the analyte gases. Their flow rates are controlled by mass flow controllers. Different concentrations of CO2 (or nitrogen) are realized by mixing the pure argon and CO2 gas streams (or nitrogen stream). The mixing ratio of the two gas streams is controlled by mass flow controllers. For example, the flow rate in the argon channel is 196.06 ml min−1 and the CO2 stream is 3.94 ml min−1, resulting in a gas mixture with a CO2 concentration of 1.97%vol (or 2 kPa). Methanol, ethanol and water enriched gas flows were prepared like in our previous publications.67,68 One stream passes through the gas wash bottle and bubbles through the liquid VOC to produce VOC-enriched vapor. The other stream is pure argon. The two streams are then combined and the VOC concentration can be controlled by adjusting the flow rates.
Each sensing experiment consists of 3 phases. First, the sensor is in a pure argon gas stream and the MOF pores are emptied. This is the baseline. Then, the gas stream is instantaneously switched to the analyte-enriched argon gas with a constant analyte concentration. The adsorption of analyte molecules in the MOF film leads to an increase in mass and, thus, in a change in the frequency of the QCM sensor, which is the sensor signal. The third step is the desorption of the adsorbed molecules in pure argon gas. The adsorption step is 30 minutes long. The desorption step is typically 60 minutes long to ensure that all gas molecules are desorbed. In the experiments, 35 different analyte concentrations, see Table S1,† were used. All experiments were performed at room temperature (295 K).
 | ||
| Fig. 2 E-nose data as a function of time. The signals are shown for the exposure to CO2 with a concentration of 1.97%vol (a), 1.48%vol (b), 0.98%vol (c) and 0.19%vol (d) as well as 0.19% of CO2 with 10% humidity (e) and 20% humidity (f), respectively. The gas concentrations are labelled in the panels. The color code for the MOF sensors is shown in the legend, the sample names are given in Table S1.† The red arrows indicate the data taken for the sensor performance analysis (Fig. 3–5). | ||
Results and discussion
The entire e-nose, i.e., the sensor array, is composed of 14 QCM sensors coated with HKUST-1 MOF films (7 times) and with UiO-66 MOF films (7 times). While one MOF film each remained empty (without IL-loading), 6 HKUST-1 films and 6 UiO-66 films were loaded with three different ionic liquids and 20% and 50% pore filling, respectively. The X-ray diffractograms of the samples (Fig. S2†) show that the MOF films are crystalline with the target HKUST-1 and UiO-66 structures. The diffractograms of the samples are very similar, indicating a very similar crystallinity of the samples. In addition, the diffraction patterns show that the crystal structures of all samples remain unchanged upon IL loading. The samples were further characterized by infrared and energy-dispersive X-ray (EDX) spectroscopy, Fig. S4 and S5,† verifying the targeted MOF structure and IL embedment. The amount of the IL content per MOF unit cell is quantified by EDX spectroscopy. The determined content is shown in Table 1 and S2†.To explore the performance of the sensor array, gas sensing experiments were conducted for different concentrations of CO2, N2, ethanol, methanol and H2O. Argon was chosen as the carrier gas because of the small interaction between the noble gas and the nanoporous host, resulting in a very small argon uptake at room temperature. When the gas flow through the e-nose was switched from initially pure argon to the analyte-enriched argon flow, the frequency shifts of all QCM sensors changed, which are proportional to the mass of the adsorbed analyte molecules in the MOF films. The frequency shifts were recorded as sensing signals. In this way, the response of the sensor array was explored for different compositions and different concentrations of gas mixtures, i.e., CO2 (0.09–1.97%vol), N2 (0.09–1.97%vol), ethanol (0.09–1.97%vol), methanol (0.09–1.97%vol) and water vapor (0.09%vol and 0.19%vol). In addition, the response of the sensor array to CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N2 = 1
N2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 gas mixtures (2%vol and 0.09%vol in argon) and to 0.19%vol CO2 with different humidity levels (10%, 20% and 30%, which correspond to concentrations of 0.23%vol, 0.46%vol and 0.68%vol) was also explored. In total, 35 experiments with different concentrations and compositions were performed, see Table S1.†Fig. 2 shows the frequency shift of the HKUST-1 QCM sensor array when exposed to CO2 at different concentrations. Further adsorption data are shown in Fig. S6–S18.† All transient uptake (i.e., analyte exposure) and release curves are qualitatively similar.
1 gas mixtures (2%vol and 0.09%vol in argon) and to 0.19%vol CO2 with different humidity levels (10%, 20% and 30%, which correspond to concentrations of 0.23%vol, 0.46%vol and 0.68%vol) was also explored. In total, 35 experiments with different concentrations and compositions were performed, see Table S1.†Fig. 2 shows the frequency shift of the HKUST-1 QCM sensor array when exposed to CO2 at different concentrations. Further adsorption data are shown in Fig. S6–S18.† All transient uptake (i.e., analyte exposure) and release curves are qualitatively similar.
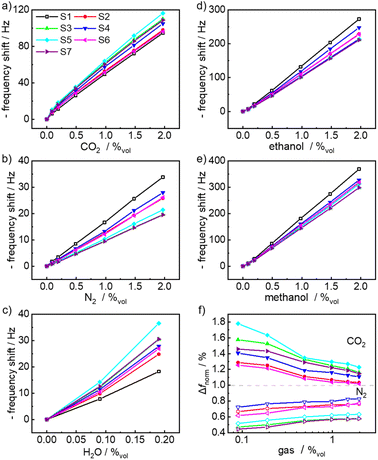
All sensor signals as a function of different concentrations of CO2, N2, ethanol, methanol and water are shown in Fig. 3 for the HKUST-1 sensors (sensors S1–S7) and in Fig. S20 and S21† for the UiO-66 sensors (sensors S8–S14).
The data for all sensors and gas molecules are essentially linear and can be described with the Henry's region of a Langmuir isotherm. Fig. 3f shows the ratios of the CO2 and N2 uptake of an individual IL-loaded sensor relative to the CO2 and N2 uptake by the empty HKUST-1 sensor. The relative values of ethanol, methanol and water uptake are shown in Fig. S19.† As shown, there is a significant difference in the adsorption of CO2 and water versus N2, ethanol and methanol by the IL-loaded sensors. The IL-embedment results in a smaller uptake of nitrogen, ethanol and methanol. This is caused by the IL in the pores, reducing the available pore volume of the MOF. On the other hand, the uptakes of water and CO2 increase for the IL-loaded sensors compared to the empty-MOF sensors. For example, the uptake of CO2 by HKUST1 loaded with 50% pore filling of [BMIM][SCN] increased by 69%, compared to the empty MOF sensor. This is due to the stronger solubilization ability of the ionic liquid for CO2 (and water).74 Although CO2 is a non-polar molecule, it has a large quadruple dipole moment and polarizability, which account for its strong interaction with the IL molecules. The interaction between the CO2 molecules and the IL molecules in the pore is based on the electrostatic interaction between the ionic liquid and CO2.75,76 In addition, weak dispersive interaction may contribute to the energy.76 It was found that the interaction between CO2 and the IL, and thus the solubility, is dominated by the anions, rather than the cations.75 In line with solubility studies of different (bulk) ILs based on BMIM,75 the interaction of CO2 with TFSI is larger than that with PF6, see Fig. 3f or Table 1.
The slopes of the linear range of the isotherm, which correspond to the sensitivity, are shown in Table 1. It is worth noting that each sensor has a different slope for the same gas molecule. This is caused by the fact that the MOF material is loaded with different concentrations and different types of ILs. For CO2, the [BMIM][SCN]50%@HKUST-1 sensor showed the largest sensitivity, followed by the [BMIM][TFSI]50% @HKUST-1. More importantly, the same sensor showed different sensitivities for different analytes. For the HKUST-1 sensors, the CO2 sensitivity ranges between 47.0 Hz/%vol for the empty MOF (S1) and 56.2 Hz/%vol for the MOF with 50% pore filling of [BMIM][SCN] (S5). The sensitivities of the HKUST-1 sensors for N2, ethanol, methanol and water are in the ranges of 10–17 Hz/%vol, 109–141 Hz/%vol, 154–191 Hz/%vol and 95–213 Hz/%vol, respectively. For UiO-66, the CO2 sensitivity ranges between 37.5 Hz/%vol for the empty MOF (S8) and 44.2 Hz/%vol for the MOF with 50% pore filling of [BMIM][SCN] (S12). The sensitivities of the UiO-66 sensors for N2, ethanol, methanol and water are in the ranges of 7.0–11.3 Hz/%vol, 81–104 Hz/%vol, 124–147 Hz/%vol and 95–193 Hz/%vol, respectively. Most importantly, the different combinations of IL and MOF structures result in different sensitivities for each analyte. This feature is important for distinguishing the analytes via machine learning, see below (and see also Fig. 4 below).
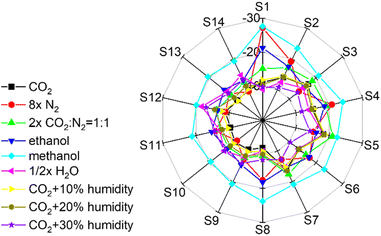 | ||
Fig. 4 Radar plot of the frequency shift Δf for exposure to gases of 0.19%vol concentration. The gases are CO2, N2, CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N2 = 1 N2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, ethanol, methanol, H2O, CO2 + 10%, 20% and 30% humidity, respectively, see labels. Each axis (with a scale from 0 to 30 Hz) represents a different sensor, see Table 1. For better visibility, the data of nitrogen have been multiplied by 8, of CO2 1, ethanol, methanol, H2O, CO2 + 10%, 20% and 30% humidity, respectively, see labels. Each axis (with a scale from 0 to 30 Hz) represents a different sensor, see Table 1. For better visibility, the data of nitrogen have been multiplied by 8, of CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N2 = 1 N2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture by 2 and the H2O data have been divided by 2. 1 mixture by 2 and the H2O data have been divided by 2. | ||
The limits of detection (LODs, Table 1) of individual sensors are calculated as 3 times the standard deviation divided by the sensitivity. As standard deviation, we used the average value of the standard deviation of each individual sensor, determined from the baselines in Fig. 2 and S6–S9† before the analyte exposure begins (at about 1000 s). The average value of the standard deviation is 0.16 Hz. For the HKUST-1, while the empty sample (without IL) has a CO2 LOD of 0.0102%vol, the LODs of the IL@MOF sensors are significantly smaller, with the smallest LOD of only 0.0085%vol (i.e., 85 ppm). This is lower than the present atmospheric CO2 concentration of about 350 ppm (i.e. 0.035%vol). The sensor arrays also have very low detection limits for N2, ethanol and methanol, ranging from 0.0025 to 0.068%vol.
In addition to exploring the sensor response to different concentrations of CO2 and different concentrations of N2, the sensor performance for different concentrations of CO2–N2 and CO2–water mixtures (in argon) was also experimentally explored. In Fig. S10 and S11,† the sensor array signal data versus time for different concentrations of CO2 and N2 gas mixtures are shown. The application of the sensor array in humid environments was also investigated. The response of the sensor array to 0.19%vol CO2 with 10%vol, 20%vol and 30%vol humidity was tested, Fig. S17 and S18.† Due to the high stability of UiO-MOFs77 and due to the reproducibility of the uptake by UiO-66 in a moist atmosphere,78 the UiO-66 sensor performance is not hampered by the humidity and the sensor can operate normally at 30%vol humidity. On the other hand, the HKUST-1 sensor exhibits a decrease in sensor signal at 30%vol humidity (compare Fig. 2e and f and S17†), which is attributed to the water-induced formation of defects in HKUST-1, decreasing the analyte uptake.79 This means, while the IL@UiO-sensors are suitable for practical applications, also in a humid environment, the application of the IL@HKUST-1 is limited to a dry atmosphere.
Fig. 4 shows the radar plots of the frequency shift of the sensor array for a gas concentration of 0.19%vol. Radar plots for different gas concentrations are shown in Fig. S23.† The radar plots show that each analyte has a unique response pattern that can be distinguished visually. This means that the sensor array can qualitatively distinguish between the gas molecules.
To quantitatively analyze the sensor performance for the different gas molecules, the sensor array data were analyzed with a machine learning algorithm, here a neural network was used. The confusion matrix visually reflects the classification performance of the algorithm, where the correct classification is on the diagonal. For a given analyte concentration, the confusion matrixes for CO2, N2, ethanol, methanol and CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N2 = 1
N2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 are shown in Fig. S24.† It shows that the sensor array can accurately discriminate the analytes.
1 are shown in Fig. S24.† It shows that the sensor array can accurately discriminate the analytes.
Typically, the components, composition and concentration of the analytes are unknown in a real-life sensing problem. Fig. S25† shows the confusion matrix for all the sensor data explored for different compositions and different concentrations of gases, from 0.09%vol content to 1.97%vol content. Their average classification accuracy is 93.8%.
The results of the sensor performance with the neural-network algorithm using only the data from some sensors (i.e. only a part of the sensor array) as input data are shown in Fig. 5. There, the input data are either only from the two empty MOF sensors, two IL-loaded-MOF sensors, two water-stable IL-loaded-UiO sensors and the entire sensor array. All analytes regardless of the concentration are individual classes. It shows that the full sensor array applied to all data without assigned concentrations (i.e. classification of CO2, N2, ethanol, methanol and CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N2 = 1
N2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 without concentration information markers) achieves an outstanding classification accuracy of 98.0% (Fig. 5j). It shows that the IL@MOF-e-nose is fully capable of analyzing the gases (and mixtures) with unknown concentrations. For comparison, the data from only two sensors (S1 and S8) of plain MOF, i.e., HKUST-1 and UiO-66 without IL loading, cannot clearly distinguish CO2 from the other analytes, (Fig. 5a). The average accuracy is 84.5%, but only 66.3% for CO2, which is unacceptable for practical applications.
1 without concentration information markers) achieves an outstanding classification accuracy of 98.0% (Fig. 5j). It shows that the IL@MOF-e-nose is fully capable of analyzing the gases (and mixtures) with unknown concentrations. For comparison, the data from only two sensors (S1 and S8) of plain MOF, i.e., HKUST-1 and UiO-66 without IL loading, cannot clearly distinguish CO2 from the other analytes, (Fig. 5a). The average accuracy is 84.5%, but only 66.3% for CO2, which is unacceptable for practical applications.
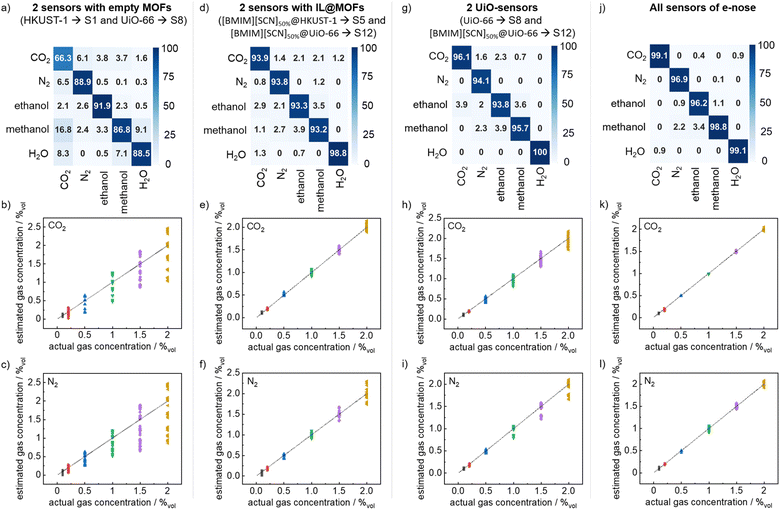 | ||
Fig. 5 The confusion matrixes and the comparison of actual gas concentrations with the estimated gas concentrations, as a result of the sensor data analysis with the neural-network algorithm. (a–c) The input data are from sensors S1 and S8, i.e. HKUST-1 and UiO-66 without IL-loading. (d–f) The input data are from sensors S5 and S12, i.e. [BMIM][SCN]50%@HKUST-1 and [BMIM][SCN]50%@UiO-66. (g–i) The input data are from two UiO-sensors (S8 and S12), i.e. UiO-66 and [BMIM][SCN]50%@UiO-66. (j–l) The input data comprise the data from all sensors (S1–S14). All concentrations of all CO2, N2, ethanol, methanol and CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N2 = 1 N2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 are classified as one analyte each. (It corresponds to Fig. S25,† however, ignoring the gas concentration). 1 are classified as one analyte each. (It corresponds to Fig. S25,† however, ignoring the gas concentration). | ||
On the other hand, two sensors of MOF (one HKUST-1 and one UiO-66) with embedded IL (i.e. [BMIM][SCN]50% @HKUST-1, S5 and [BMIM][SCN]50% @UiO-66, S12) can distinguish all analytes with an average accuracy of 94.6%, and can identify CO2 with 93.9% accuracy (Fig. 5d).
For using only two water-stable UiO-MOFs, empty (S8) and filled with [BMIM][SCN]50% (S12), the average classification accuracy is even slightly higher, 95.9% (Fig. 5g). This shows that 2 UiO-MOF-sensors filled with IL can be an excellent e-nose.
Based on the neural network, the quantitative analysis of different gas concentrations can be achieved by regressing a mixture of gases of different compositions using the sensor data not used in the training network, see Fig. 5 b, c, e, f, h, g, k and l. Using the data of the entire sensor array, the estimated concentration essentially agrees with the real CO2 concentration with an average error of 2.7%.
The average error in estimating the N2 concentration is 3.2%. The IL@MOF-e-nose not only has superb sensitivity and selectivity for a single target gas, but also can accurately predict the type and concentration of the gases. However, the regression task using only two sensors (S1 and S8) without IL-loading does not allow the quantitative analysis of the different gas concentrations, see Fig. 5b and c. The average error in the predicted CO2 concentration is 37.5% and the average error in the predicted N2 concentration reaches 39%.
For two IL-loaded sensors with high sensitivities (S5 and S12, Fig. 5e and f), the average errors for predicting the CO2 and N2 concentrations are 9.1% and 10.7%, respectively. For using only 2 water-stable UiO-MOFs (S8 and S12,†Fig. 5h and i), the average error for predicting the CO2 and N2 concentrations are 9.3% and 10.4%, respectively. The error in predicting the gas concentration is relatively small, so the sensor array enables effective prediction of the type and concentration of the target gas, especially CO2.
The effect of ambient humidity on the sensor operation is explored in Fig. S26.† The entire sensor arrays can operate normally at humidity levels below 30%. When the humidity is 10% and 20%, the average error of the estimated CO2 concentration is 5.2%. The average error in the estimated CO2 concentration is 9.8% when the humidity is 30%. Using only the data of the UiO-sensors as the input (S8–S14), which are stable in humid environments, the average error in the estimated CO2 concentration is 5.5%. For using only the data from 2 UiO-sensors, the average error in the estimated CO2 concentration is still rather small, only 6.2%. This demonstrates the great performance of the e-nose under humid conditions.
The transient signal of the sensor array can be described with a mono-exponential decay function with time constants of 0.6 to 2.0 min for the CO2 uptake and roughly 6 min for the CO2 release, see Table S3.† Based on the time constants, the sensor response time and recovery time can be calculated. On the other hand, although the signals have not yet reached its equilibrium values in the analyte atmosphere, the (non-equilibrium) data of the e-nose can be used for discriminating the analytes. The accuracy of discrimination of the sensor data as a function of time is shown in Fig. 6. The data show that the sensor array reaches its final classification accuracy about 2 minutes after start of the analyte exposure. This means, although the sensor response time has not yet passed, the sensor array is able to classify the analytes precisely.
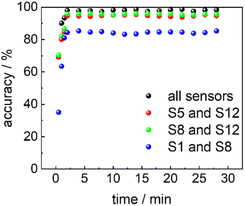 | ||
| Fig. 6 Discrimination accuracy of 5 analytes (see Fig. 5a, d, g and j) at different time intervals. Each value is determined from 100 consecutive data points, corresponding to approximately 2 minute time intervals, where the final data points end at the values on the x-axis. The input data comprise data from the entire sensor array (black), the data for sensors S5 and S12 (red) and the data from sensors S1 and S8 (green). The x-axis is the time after the start of exposure to the analytes. | ||
The reproducibility of the data was explored by repeating the same analyte exposure experiments 4 times, see Fig. S27 and S28.† The data show that the signal is reproducible with only very small deviations between the different experiments. The average standard deviations between the signal were approximately 0.5 Hz, which is much smaller than the signal magnitude (of about 100 Hz).
While the experiments here were performed at room temperature, the QCM transducer also allows the sensing at other temperatures. With the knowledge of the adsorption enthalpy of the analytes in the IL@MOF material, the sensor response at different temperatures can be predicted. While the small changes of the resonance frequency occur when strongly changing the gas flow rate through the sensor cell, the sensor response is not affected by changes of the flow rate. The QCM sensors can be reused for preparing new IL@MOF films, after removing the MOF films from the surface (e.g., by ultrasonication in water or aqueous acidic acid).
Conclusions
A gravimetric sensor array based on 14 QCM sensors coated with HKUST-1 and UiO-66 MOF films loaded with three different ionic liquids, [BMIM][SCN], [BMIM][TFSI] and [BMIM][PF6], at 20% and 50% concentrations is presented. The investigation of sensor response to different gases and vapor analytes, including CO2, N2, ethanol, methanol, water and some mixtures as well as simultaneous classification and regression based on neural-network machine learning analysis for different concentrations were performed. The data show that, in addition to an increase in sensitivity, the embedment of IL in the pores of MOFs enhances the selectivity significantly, especially with respect to CO2. The presented IL@MOF-e-nose has a very low limit of detection of 0.0085%vol for CO2 and excellent selectivity. Moreover, the e-nose was also able to predict the concentrations of the analytes with a very small error rate in the range of a few percent. By using only robust MOFs (i.e. UiO-66) as the IL-host, the sensor array also shows excellent performance in a humid environment, showing its potential for practical real-life applications.Considering the huge variety of ionic liquids and nanoporous materials like MOFs, there exist endless possibilities of combining them and optimizing the selectivities. Thus, we believe there is large potential for sensors with nanoporous materials with enhanced sensitivities obtained by ionic-liquid embedment. We like to stress that the performance of the e-nose strongly depends on the used sensor, and picking the best sensors from a large selection is not a trivial task, see for instance (ref. 80 and 81). Therefore, we assume that the e-nose performance can be further enhanced by optimizing the sensor selection as well by optimizing the combinations of the MOF and IL.
Author contributions
PQ performed all experiments, supported by SO. YJ recorded the SEM images. PQ and LH wrote the manuscript, approved by all authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge funding through the China Scholarship Council (CSC) and the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation, DFG HE 7036/5).Notes and references
- J. Rogelj, A. Popp, K. V. Calvin, G. Luderer, J. Emmerling, D. Gernaat, S. Fujimori, J. Strefler, T. Hasegawa, G. Marangoni, V. Krey, E. Kriegler, K. Riahi, D. P. van Vuuren, J. Doelman, L. Drouet, J. Edmonds, O. Fricko, M. Harmsen, P. Havlík, F. Humpenöder, E. Stehfest and M. Tavoni, Nat. Clim. Change, 2018, 8, 325–332 CrossRef CAS.
- K. Permentier, S. Vercammen, S. Soetaert and C. Schellemans, Int J Emerg Med., 2017, 10, 14 CrossRef PubMed.
- H. R. Ludwig, S. G. Carirelli and J. J. Whalen, Documentation for Immediately Dangerous to Life or Health Concentrations (IDLHs), National Institute for Occupational Safety and Health (NIOSH), 1994, p. 82 Search PubMed.
- A. Lohila, T. Laurila, J.-P. Tuovinen, J. H. Mika Aurela, M. P. Tea Thum, J. Rinne and T. Vesala, Environ. Sci. Technol., 2007, 41, 2717–2722 CrossRef CAS PubMed.
- S. A. Prior, G. B. Runion, S. C. Marble, H. H. Rogers, C. H. Gilliam and H. A. Torbert, HortScience, 2011, 46, 158–162 CAS.
- S. Neethirajan, D. S. Jayas and S. Sadistap, Food Bioprocess Technol., 2008, 2, 115–121 CrossRef.
- M. Ahila, J. Dhanalakshmi, J. C. Selvakumari and D. P. Padiyan, Mater. Res. Express, 2016, 3, 105025 CrossRef.
- X. Tan, H. Zhang, J. Li, H. Wan, Q. Guo, H. Zhu, H. Liu and F. Yi, Nat. Commun., 2020, 11, 5245 CrossRef CAS PubMed.
- H. S. M. d. Vries, M. A. J. Wasono, F. J. M. Harren, E. J. Woltering, H. C. P. M. v. d. Valk and J. Reuss, Postharvest Biol. Technol., 1996, 8, 1–10 CrossRef.
- C. G. Teodoro, D. U. Schramm, M. S. Sthel, G. R. Lima, M. V. Rocha, J. R. Tavares and H. Vargas, Infrared Phys. Technol., 2010, 53, 151–155 CrossRef CAS.
- T. Yang, W. Chen and P. Wang, Appl. Spectrosc. Rev., 2020, 56, 143–170 CrossRef.
- S. K. Vashist and P. Vashist, J. Sens., 2011, 2011, 1–13 CrossRef.
- H.-H. Lu, Y. K. Rao, T.-Z. Wu and Y.-M. Tzeng, Sens. Actuators, B, 2009, 137, 741–746 CrossRef CAS.
- G. Sauerbrey, Zeitschrift fiir Physik, 1959, 155, 206–222 CrossRef CAS.
- N. L. Torad, S. Zhang, W. A. Amer, M. M. Ayad, M. Kim, J. Kim, B. Ding, X. Zhang, T. Kimura and Y. Yamauchi, Adv. Mater. Interfaces, 2019, 6, 1900849 CrossRef.
- E. Haghighi and S. Zeinali, Microporous Mesoporous Mater., 2020, 300, 110065 CrossRef CAS.
- L. Wang, Sens. Actuators, A, 2020, 307, 111984 CrossRef CAS.
- S. Okur, P. Qin, A. Chandresh, C. Li, Z. Zhang, U. Lemmer and L. Heinke, Angew. Chem., Int. Ed. Engl., 2021, 60, 3566–3571 CrossRef CAS PubMed.
- J. Devkota, K. J. Kim, P. R. Ohodnicki, J. T. Culp, D. W. Greve and J. W. Lekse, Nanoscale, 2018, 10, 8075–8087 RSC.
- O. M. Yaghi, M. J. Kalmutzki and C. S. Diercks, Introduction to Reticular Chemistry: Metal-Organic Frameworks and Covalent Organic Frameworks, Wiley, 2019, p. 552, ISBN: 978-3-527-34502-1 Search PubMed.
- S. Kaskel, The Chemistry of Metal-Organic Frameworks: Synthesis, Characterization, and Applications, Wiley, 2016, P. 904, ISBN: 978-3-527-33874-0 Search PubMed.
- J. An and N. L. Rosi, J. Am. Chem. Soc., 2010, 132, 5578–5579 CrossRef CAS PubMed.
- L. Valenzano, B. Civalleri, S. Chavan, G. T. Palomino, C. O. Areán and S. Bordiga, J. Phys. Chem. C, 2010, 114, 11185–11191 CrossRef CAS.
- H.-Y. Cho, D.-A. Yang, J. Kim, S.-Y. Jeong and W.-S. Ahn, Catal. Today, 2012, 185, 35–40 CrossRef CAS.
- S. Choi, T. Watanabe, T. H. Bae, D. S. Sholl and C. W. Jones, J. Phys. Chem. Lett., 2012, 3, 1136–1141 CrossRef CAS PubMed.
- T. Ghanbari, F. Abnisa and W. M. A. Wan Daud, Sci. Total Environ., 2020, 707, 135090 CrossRef CAS PubMed.
- J. L. Anderson, J. K. Dixon and J. F. Brennecke, Acc. Chem. Res., 2007, 40, 1208–1216 CrossRef CAS PubMed.
- M. J. Muldoon, S. N. V. K. Aki, J. L. Anderson, J. K. Dixon and J. F. Brennecke, J. Phys. Chem. B, 2007, 9001–9009 CrossRef CAS PubMed.
- H. Weingartner, Angew. Chem., Int. Ed. Engl., 2008, 47, 654–670 CrossRef PubMed.
- J. D. Holbrey and K. R. Seddon, Clean Prod. Process., 1999, 1, 223–236 Search PubMed.
- R. D. Rogers and K. R. Seddon, Science, 2003, 302, 792–793 CrossRef PubMed.
- D. S. Silvester, Analyst, 2011, 136, 4871–4882 RSC.
- D. Wei and A. Ivaska, Anal. Chim. Acta, 2008, 607, 126–135 CrossRef CAS PubMed.
- K. P. S. Hussan, H. H. Moidu, M. S. Thayyil, T. V. Jinitha, A. Antony and G. Govindaraj, J. Mater. Sci.: Mater. Electron., 2021, 32, 25164–25174 CrossRef CAS.
- R. V. Barrulas, M. Zanatta, T. Casimiro and M. C. Corvo, Chem. Eng. J., 2021, 411, 128528 CrossRef CAS.
- S. Zhang, K. Dokko and M. Watanabe, Mater. Horiz., 2015, 2, 168–197 RSC.
- M. Mohamedali, A. Henni and H. Ibrahim, Microporous Mesoporous Mater., 2019, 284, 98–110 CrossRef CAS.
- W. Chen, Z. Zhang, C. Yang, J. Liu, H. Shen, K. Yang and Z. Wang, J. Membr. Sci., 2021, 636, 119581 CrossRef CAS.
- A. Aijaz, T. Akita, H. Yang and Q. Xu, Chem. Commun., 2014, 50, 6498–6501 RSC.
- Z. Bian, X. Zhu, T. Jin, J. Gao, J. Hu and H. Liu, Microporous Mesoporous Mater., 2014, 200, 159–164 CrossRef CAS.
- C. Chen, N. Feng, Q. Guo, Z. Li, X. Li, J. Ding, L. Wang, H. Wan and G. Guan, J. Colloid Interface Sci., 2018, 521, 91–101 CrossRef CAS PubMed.
- J. M. Vicent-Luna, J. J. Gutierrez-Sevillano, S. Hamad, J. Anta and S. Calero, ACS Appl. Mater. Interfaces, 2018, 10, 29694–29704 CrossRef CAS PubMed.
- J. Ma, Y. Ying, X. Guo, H. Huang, D. Liu and C. Zhong, J. Mater. Chem. A, 2016, 4, 7281–7288 RSC.
- M. Zeeshan, V. Nozari, M. B. Yagci, T. Isik, U. Unal, V. Ortalan, S. Keskin and A. Uzun, J. Am. Chem. Soc., 2018, 140, 10113–10116 CrossRef CAS PubMed.
- H. M. Polat, M. Zeeshan, A. Uzun and S. Keskin, Chem. Eng. J., 2019, 373, 1179–1189 CrossRef CAS.
- W. Chen, Z. Zhang, C. Yang, J. Liu, H. Shen, K. Yang and Z. Wang, J. Membr. Sci., 2021, 636, 119581 CrossRef CAS.
- Y. Tang, H. Huang, J. Li, W. Xue and C. Zhong, J. Mater. Chem. A, 2019, 7, 18324–18329 RSC.
- Q.-x. Luo, X.-d. Song, M. Ji, S.-E. Park, C. Hao and Y.-q. Li, Appl. Catal., A, 2014, 478, 81–90 CrossRef CAS.
- S. Abednatanzi, A. Abbasi and M. Masteri-Farahani, Catal. Commun., 2017, 96, 6–10 CrossRef CAS.
- S. Abednatanzi, K. Leus, P. G. Derakhshandeh, F. Nahra, K. De Keukeleere, K. Van Hecke, I. Van Driessche, A. Abbasi, S. P. Nolan and P. V. Der Voort, Catal. Sci. Technol., 2017, 7, 1478–1487 RSC.
- H. M. A. Hassan, M. A. Betiha, S. K. Mohamed, E. A. El-Sharkawy and E. A. Ahmed, J. Mol. Liq., 2017, 236, 385–394 CrossRef CAS.
- Z. Wang, R. Tan, H. Wang, L. Yang, J. Hu, H. Chen and F. Pan, Adv. Mater., 2018, 30 Search PubMed.
- T. Chen, S. Chen, Y. Chen, M. Zhao, D. Losic and S. Zhang, Mater. Chem. Front., 2021, 5, 1771–1794 RSC.
- Z.-J. Zheng, H. Ye and Z.-P. Guo, Energy Environ. Sci., 2021, 14, 1835–1853 RSC.
- C. Gonzalez Viejo, S. Fuentes, A. Godbole, B. Widdicombe and R. R. Unnithan, Sens. Actuators, B, 2020, 308, 127688 CrossRef CAS.
- M. Taştan and H. Gökozan, Appl. Sci., 2019, 9, 3435 CrossRef.
- S. Esfahani, A. Tiele, S. O. Agbroko and J. A. Covington, Sensors (Basel), 2020, 20, 6875 CrossRef CAS PubMed.
- H. A. Rowley, S. Member, S. Baluja and T. Kanade, IEEE Trans. Pattern Anal. Mach. Intell., 1998, 20, 23–28 CrossRef.
- O. Shekhah, H. Wang, D. Zacher, R. A. Fischer and C. Wöll, Angew. Chem., Int. Ed. Engl., 2009, 48, 5038–5041 CrossRef CAS PubMed.
- L. Heinke and C. Wöll, Adv. Mater., 2019, 31, e1806324 CrossRef PubMed.
- S. S.-Y. Chui, S. M.-F. Lo, J. P. H. Charmant, A. G. Orpen and I. D. Williams, Science, 1999, 283, 1148–1150 CrossRef CAS PubMed.
- S. Hurrle, S. Friebe, J. Wohlgemuth, C. Wöll, J. Caro and L. Heinke, Chem.–Eur. J., 2017, 23, 2294–2298 CrossRef CAS PubMed.
- E. Virmani, J. M. Rotter, A. Mahringer, T. von Zons, A. Godt, T. Bein, S. Wuttke and D. D. Medina, J. Am. Chem. Soc., 2018, 140, 4812–4819 CrossRef CAS PubMed.
- A. B. Kanj, R. Verma, M. Liu, J. Helfferich, W. Wenzel and L. Heinke, Nano Lett., 2019, 19, 2114–2120 CrossRef CAS PubMed.
- M. Vazquez, M. Liu, Z. Zhang, A. Chandresh, A. B. Kanj, W. Wenzel and L. Heinke, ACS Appl. Mater. Interfaces, 2021, 13, 21166–21174 CrossRef CAS PubMed.
- Z. Zhang, C. Li, A. Chandresh and L. Heinke, Ionics, 2021, 487–494, DOI:10.1007/s11581-021-04249-w.
- P. Qin, B. A. Day, S. Okur, C. Li, A. Chandresh, C. E. Wilmer and L. Heinke, ACS Sens., 2022, 7, 1666–1675 CrossRef CAS PubMed.
- P. Qin, S. Okur, C. Li, A. Chandresh, D. Mutruc, S. Hecht and L. Heinke, Chem. Sci., 2021, 12, 15700–15709 RSC.
- M. Barriault, I. Alexander, N. Tasnim, A. O'Brien, H. Najjaran and M. Hoorfar, Sens. Actuators, B, 2021, 326, 129012 CrossRef CAS.
- S. C. van de Leemput, J. Teuwen, B. van Ginneken and R. Manniesing, J. Open Res. Softw., 2019, 4, 1576 CrossRef.
- A. L. Maas, A. Y. Hannun and A. Y. Ng, Proc. Int. Conf. Mach. Learn., 2013, 30, 3 Search PubMed.
- A. A. Lydia and F. S. Francis, Int. J. Inf. Comput. Sci, 2019, 6, 566–568 Search PubMed.
- D. Harvey, S. Leybourne and P. Newbold, Int. J. Forecast., 1997, 13, 281–291 CrossRef.
- C. Cadena, J. L. Anthony, J. K. Shah, T. I. Morrow, J. F. Brennecke and E. J. Maginn, J. Am. Chem. Soc., 2004, 126, 5300–5308 CrossRef CAS PubMed.
- S. J. Zeng, X. Zhang, L. P. Bai, X. C. Zhang, H. Wang, J. J. Wang, D. Bao, M. D. Li, X. Y. Liu and S. J. Zhang, Chem. Rev., 2017, 117, 9625–9673 CrossRef CAS PubMed.
- H. Weingaertner, Angew. Chem., Int. Ed., 2008, 47, 654–670 CrossRef CAS PubMed.
- N. C. Burtch, H. Jasuja and K. S. Walton, Chem. Rev., 2014, 114, 10575–10612 CrossRef CAS PubMed.
- C. Li, A. Chandresh, Z. Zhang, S. Moulai and L. Heinke, Adv. Mater. Interfaces, 2022, 9, 2101947 CrossRef CAS.
- L. Heinke, Z. Gu and C. Wöll, Nat. Commun., 2014, 5, 4562 CrossRef CAS PubMed.
- J. A. Gustafson and C. E. Wilmer, J. Phys. Chem. C, 2017, 121, 6033–6038 CrossRef CAS.
- J. A. Gustafson and C. E. Wilmer, ACS Sens., 2019, 4, 1586–1593 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ta06324g |
| This journal is © The Royal Society of Chemistry 2022 |