DOI:
10.1039/D2TA02914F
(Paper)
J. Mater. Chem. A, 2022,
10, 13492-13499
Achieving 15.81% and 15.29% efficiency of all-polymer solar cells based on layer-by-layer and bulk heterojunction structures†
Received
11th April 2022
, Accepted 6th June 2022
First published on 6th June 2022
Abstract
Wide bandgap polymer donor PM6 and narrow bandgap polymer acceptor PY-IT were selected to construct all-polymer solar cells (all-PSCs) with a layer-by-layer (LbL) or bulk heterojunction (BHJ) structure. The solvent additive 1-chloronaphthalene (CN) plays a vital role in improving the performance of all-PSCs. The power conversion efficiency (PCE) of LbL all-PSCs is improved to 15.81% from 13.67% by incorporating 1 vol% CN in PY-IT solution, benefiting from the simultaneously enhanced short circuit current density (JSC) of 22.61 mA cm−2 and fill factor (FF) of 73.62%. A similar phenomenon is also observed in BHJ all-PSCs whose PCE increased from 13.29% to 15.29% by incorporating 1 vol% CN in PM6:PY-IT blend solution, originating from the optimized phase separation for better exciton dissociation and charge transport in the BHJ active layers. Over 15% PCE improvement can be obtained in the BHJ and LbL all-PSCs by incorporating appropriate additive CN as a morphology regulator. It should be noticed that the PCEs of LbL all-PSCs with CN are higher than those of other all-PSCs, indicating that the LbL processing method combined with the additive should be a prospective strategy to fabricate highly efficient all-PSCs.
Introduction
All-polymer solar cells (all-PSCs) consisting of polymer donors and polymer acceptors have attracted extensive attention due to their superior features such as excellent mechanical durability and stability, as well as an outstanding film-forming property for large area roll-to-roll manufacturing. Benefiting from material innovation and device engineering, over 15% power conversion efficiency (PCE) of all-PSCs has been achieved in several studies.1–4 However, it should be noticed that the evolution of all-PSCs still lag behind that of small molecule acceptor based PSCs with PCEs over 19%, which is mainly due to the morphology regulation difficulty of active layers in commonly employed bulk heterojunction (BHJ) all-PSCs.5–8 The active layer morphology optimization is a huge challenge in BHJ all-PSCs because neighboring polymer chains are strongly intertwined and entangled during one step spin-coating of the polymer mixture solution. Very recently, the layer-by-layer (LbL) processing method has attracted extensive attention, in which the donor and acceptor layers are sequentially cast, which is conducive to maintaining the high purity of the donor/acceptor domain for efficient charge transport in LbL active layers.9–11 The key photovoltaic parameters of the typical studies of BHJ and LbL all-PSCs are listed in Table 1. The LbL all-PSCs exhibit a relatively large PCE in comparison with the corresponding BHJ all-PSCs prepared with the same donor and acceptor materials, suggesting that the LbL processing method may have great potential in achieving highly efficient all-PSCs. Recently, solvent additives have been separately introduced into donor or acceptor solutions to optimize the molecular arrangement and crystallites of each layer, which should be a simple and convenient strategy to further improve the performance of LbL-PSCs.12–15 An appropriate solvent additive can play a key role in optimizing phase separation for efficient exciton dissociation, as well as improving molecular crystallization and orientation for efficient hole and electron transport in active layers. In this work, an optimal PCE of 15.81% is achieved in LbL all-PSCs by adding CN to the acceptor solution.
Table 1 Key photovoltaic parameters of BHJ and LbL all-PSCs
| Active layer |
J
SC [mA cm−2] |
V
OC [V] |
FF [%] |
PCE [%] |
Ref. |
| PBDB-T:N2200 |
11.89 |
0.867 |
64.4 |
6.58 |
16
|
| PBDB-T/N2200 |
15.33 |
0.904 |
68.7 |
9.52 |
| PCE10:N2200 |
11.84 |
0.799 |
53.50 |
5.06 |
17
|
| PCE10/N2200 |
12.30 |
0.796 |
53.96 |
5.26 |
| PBDB-T:PYT |
22.64 |
0.887 |
70.02 |
14.06 |
| PBDB-T/PYT |
23.03 |
0.891 |
73.98 |
15.17 |
| PBQx-H-TF:PBTIC-γ-TSe |
23.17 |
0.91 |
65.85 |
13.91 |
18
|
| PBQx-H-TF/PBTIC-γ-TSe |
23.49 |
0.91 |
73.76 |
15.77 |
| PM6:PY-IT |
22.29 |
0.95 |
72.24 |
15.29 |
This work |
| PM6/PY-IT |
22.61 |
0.95 |
73.62 |
15.81 |
| PBDB-T/PYT |
23.07 |
0.91 |
77 |
16.05 |
19
|
| PM6:L15 |
22.51 |
0.95 |
71.31 |
15.19 |
20
|
| PM6/L15 |
23.58 |
0.94 |
73.17 |
16.15 |
Herein, a series of BHJ and LbL all-PSCs were composed of polymer PM6 as the donor, polymer PY-IT as the acceptor and CN as the solvent additive based on the normal structure of ITO/PEDOT:PSS/active layer/PNDIT-F3N/Al. The chemical structures, energy levels and absorption spectra of the used materials are exhibited in Fig. 1. The detailed HOMO and LUMO levels of the used materials are abstracted from ref. 21. The optimal LbL all-PSCs were achieved from PM6 chlorobenzene solution and PY-IT chloroform solution with 1 vol% CN, exhibiting a PCE of 15.81% along with an open circuit voltage (VOC) of 0.95 V, a short circuit current density (JSC) of 26.89 mA cm−2 and a fill factor (FF) of 75.79%. The optimal BHJ all-PSCs were achieved from the blend solutions with 1 vol% CN, exhibiting a PCE of 15.29% with an VOC of 0.95 V, a JSC of 22.29 mA cm−2 and an FF of 72.24%. The relatively large PCE (15.81% vs. 15.29%) of LbL all-PSCs vs. BHJ all-PSCs should benefit from the enhanced JSC and FF due to the optimized photon harvesting, suppressed charge recombination and improved charge collection. This work suggests that using the LbL processing method combined with a solvent additive can optimize photo harvesting, charge transport and molecular arrangement to fabricate highly efficient all-PSCs.
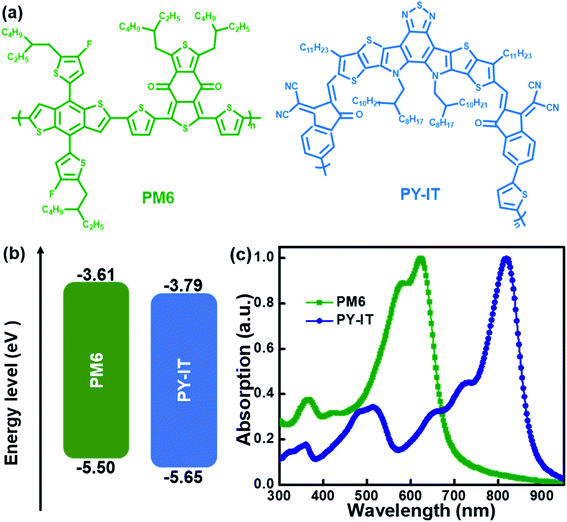 |
| | Fig. 1 (a) Chemical structures, (b) energy levels and (c) normalized absorption spectra of PM6 and PY-IT. | |
Results and discussion
The current density versus applied voltage (J–V) curves of the BHJ and LbL all-PSCs were measured under AM 1.5 G illumination with 100 mW cm−2 light intensity, as displayed in Fig. 2a. The PCE of BHJ or LbL all-PSCs can be improved from 13.29% to 15.29% or from 13.67% to 15.81% by incorporating 1 vol% CN into the PM6:PY-IT blend solution or PY-IT solution, respectively. Over 15% PCE improvement can be obtained from BHJ and LbL all-PSCs with CN compared to the corresponding all-PSCs without CN. The performance improvement of all-PSCs is mainly attributed to JSC enhancement from 20.76 mA cm−2 to 22.29 mA cm−2 for BHJ all-PSCs and from 21.12 mA cm−2 to 22.61 mA cm−2 for LbL all-PSCs, as well as FF increment from 67.36% to 72.24% for BHJ all-PSCs and from 68.14% to 73.62% for LbL all-PSCs. It should be noticed that the JSC and FF of LbL all-PSCs are larger than those of the corresponding BHJ all-PSCs, which should be mainly due to the weakened charge recombination and more efficient charge transport channels in LbL active layers. The increased JSC and FF of the BHJ and LbL all-PSCs with CN indicate that photon harvesting and morphology of active layers can be simultaneously improved by incorporating 1 vol% CN. The improved photon harvesting of BHJ and LbL all-PSCs with CN is well supported by the increased absorption spectra of the corresponding blend films incorporated with CN, as shown in Fig. S1.† To clarify the underlying reason for the effect of CN incorporation on the FF of all-PSCs, series resistance (RS) and shunt resistance (RSH) were calculated according to the J–V curves under light illumination. The RSH and RS are equal to differential resistance 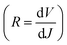 when VOC = 0 V and JSC = 0 V. The BHJ or LbL all-PSCs with CN show a smaller RS of 3.23 Ω cm2 or 2.84 Ω cm2 and a larger RSH of 883 Ω cm2 or 1031 Ω cm2 than the corresponding all-PSCs without CN, which can well support the relatively large FF of all-PSCs with CN. It is commonly reported that efficient PSCs also exhibit relatively small RS and relatively large RSH.22,23 The key photovoltaic parameters of all-PSCs and the calculated RS as well as RSH are summarized in Table 2.
when VOC = 0 V and JSC = 0 V. The BHJ or LbL all-PSCs with CN show a smaller RS of 3.23 Ω cm2 or 2.84 Ω cm2 and a larger RSH of 883 Ω cm2 or 1031 Ω cm2 than the corresponding all-PSCs without CN, which can well support the relatively large FF of all-PSCs with CN. It is commonly reported that efficient PSCs also exhibit relatively small RS and relatively large RSH.22,23 The key photovoltaic parameters of all-PSCs and the calculated RS as well as RSH are summarized in Table 2.
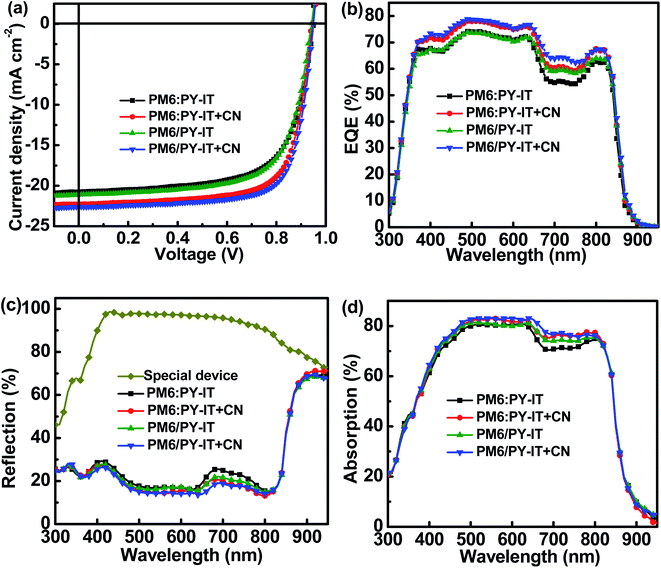 |
| | Fig. 2 (a) J–V curves of all-PSCs. (b) EQE spectra of all-PSCs. (c) Reflection spectra of all-PSCs and the special device. (d) Absorption spectra of active layers. | |
Table 2 Device parameters of the BHJ and LbL all-PSCsa
| Active layer |
J
SC [mA cm−2] |
Cal. JSC [mA cm−2] |
V
OC [V] |
FF [%] |
PCE (ave. ± dev.) [%] |
R
S [Ω cm2] |
R
SH [Ω cm2] |
|
The average and deviation (ave. ± dev.) of PCEs were calculated from 10 individual cells.
|
| PM6:PY-IT |
20.76 |
19.78 |
0.95 |
67.36 |
13.29 (13.13 ± 0.16) |
4.18 |
792 |
| PM6:PY-IT + CN |
22.29 |
21.21 |
0.95 |
72.24 |
15.29 (15.17 ± 0.12) |
3.23 |
883 |
| PM6/PY-IT |
21.12 |
20.24 |
0.95 |
68.14 |
13.67 (13.52 ± 0.15) |
3.77 |
806 |
| PM6/PY-IT + CN |
22.61 |
21.91 |
0.95 |
73.62 |
15.81 (15.69 ± 0.12) |
2.84 |
1031 |
The external quantum efficiency (EQE) spectra of all-PSCs were recorded and are exhibited in Fig. 2b. The calculated JSCs of all-PSCs with CN are higher than those of all-PSCs without CN, which agrees well with the tendency of the measured JSCs. The detailed calculated JSC values are listed in Table 2. The EQE values of all-PSCs with CN are larger than those of all-PSCs without CN in the wavelength range from 400 nm to 800 nm, which should be mainly caused by well-balanced photon harvesting, exciton dissociation, charge transport and collection in the corresponding all-PSCs with CN. To better understand the effect of employing CN on the EQE spectra of all-PSCs, the absorption spectra of the active layers in cells were investigated based on the reflection spectra of the relevant cells shown in Fig. 2c. A special cell ITO/PEDOT:PSS/poly(methyl methacrylate)PMMA/PNDIT-F3N/Al was constructed to investigate the influence of parasitic absorption in all-PSCs. The absorption spectra of the active layers in all-PSCs were evaluated using the difference between the reflection spectra of the specific device and those of all-PSCs, as exhibited in Fig. 2d.24,25 The shape of the absorption spectra of the active layers is very similar to the shape of the corresponding EQE spectra. Increased EQE values in the spectral range from 650 nm to 750 nm can be observed for BHJ all-PSCs with the CN additive, which should be attributed to the improved exciton utilization efficiency obtained by incorporating the appropriate CN additive. Meanwhile, the EQE of LbL all-PSCs are larger than that of BHJ all-PSCs, suggesting that the molecular arrangement of PY-IT can be well adjusted in neat films by incorporating CN for harvesting more photons, as confirmed from Fig. 2b.
To unveil the charge generation and exciton dissociation properties in active layers, photogenerated current density (Jph) versus effective voltage (Veff) of all-PSCs were recorded, as displayed in Fig. 3a. Here, the Jph is equal to the difference between the current density under AM 1.5G simulated solar illumination with 100 mW cm−2 light intensity (JL) and that under dark conditions (JD). The Veff is defined as:
| | | Veff = Vapplied − J × Rs − V0 | (1) |
Here
Vapplied and
V0 are the applied bias and the voltage under the
JL =
JD condition, respectively.
22 The
Jph of all-PSCs can be defined as
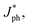 J&ph
J&ph and
Jsat under the short-circuit, maximal output-power and saturated state conditions, respectively.
26–28 The
Jsat values of both all-PSCs with the additive are higher than those of the corresponding all-PSCs without CN, indicating more efficient photon harvesting in the active layers with CN. The exciton dissociation efficiency (
ηD) and charge collection efficiency (
ηC) can be evaluated according to
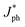
/
Jsat and
J&ph/
Jsat, respectively, as shown in Table S1
†.
29–32 The
ηD values of all-PSCs without or with CN are 92.92% or 94.61% for the BHJ structure, 92.66% or 94.43% for the LbL structure. The larger
ηD for the BHJ all-PSCs should be mainly attributed to more PM6:PY-IT interfaces in the BHJ active layers. Benefiting from the more donor enrichment at the bottom of the LbL active layer and more acceptor enrichment at the top of the LbL active layer, the
ηC values of LbL all-PSCs without or with CN are 81.51% or 82.11%, which are larger than the 80.92% or 82.01% of the corresponding BHJ all-PSCs.
33,34 It should be noticed that the
ηD and
ηC for all-PSCs with CN are higher than those of all-PSCs without CN, implying more effective exciton dissociation and charge collection in all-PSCs with CN. Furthermore, the photoluminescence (PL) spectra of pure PY-IT, PM6:PY-IT and PM6/PY-IT blend films without or with CN were investigated to further understand the exciton dissociation process in all-PSCs, as shown in
Fig. 3b.
35,36 It is apparent that PY-IT has a strong and broad PL emission with a PL peak at 845 nm. The emission intensity of PY-IT is markedly quenched in the PM6:PY-IT and PM6/PY-IT blend films. Meanwhile, the PL emission intensity of the blend films with CN is lower than that of the corresponding blend films without CN, suggesting an increased exciton dissociation efficiency in the blend films with CN.
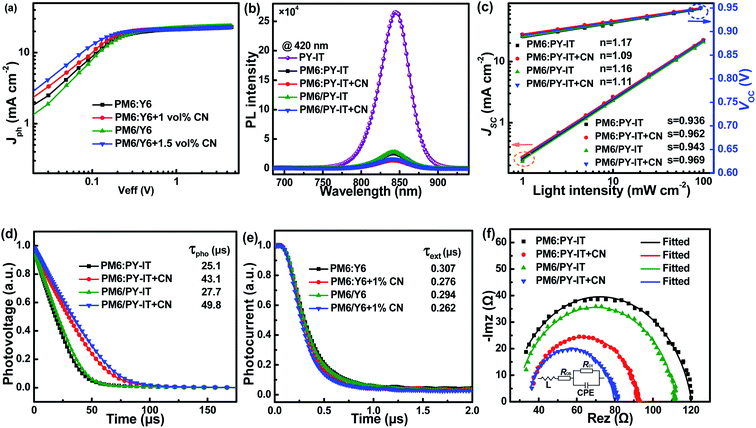 |
| | Fig. 3 (a) Jph–Veff curves of all-PSCs. (b) Photoluminescence spectra of the PY-IT film, and PM6:PY-IT and PM6/PY-IT blend films under 420 nm light excitation. (c) Jsc–Plight and Voc–Plight curves of all-PSCs. (d) Transient photovoltage curves of all-PSCs. (e) Transient photocurrent curves of all-PSCs. (f) Nyquist plots and the equivalent circuit of all-PSCs. | |
To better understand the impact of CN on the recombination dynamic process in the active layers, the J–V curves of all-PSCs were measured under different light intensities, as shown in Fig. S2.† Bimolecular recombination and space charge effects may result from the imbalanced hole and electron mobility, which are responsible for the dependence of JSC values on Plight expressed as:
as shown in
Fig. 3c.
37–39 The fitted coefficient
n represents the bimolecular recombination degree, and if the bimolecular recombination can be efficiently suppressed, it leads to an
s value close to 1.
40,41 The
n values of BHJ and LbL all-PSCs with CN are 0.962 and 0.969, which are larger than the 0.936 and 0.943 for all-PSCs without CN, respectively, indicating that bimolecular recombination can be restrained in all-PSCs by incorporating CN as the additive. The dependence of
VOC values on illumination
Plight can be expressed as:
| | VOC ∝ s(KT/q)ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Plight Plight | (3) |
In which
K,
T and
q represent the Boltzmann constant, absolute temperature and elementary charge, respectively. The fitted coefficient
s can give insight on the recombination process that dominates the devices. The
s values of all-PSCs are larger than 1, which evidences the presence of Shockley–Read–Hall recombination in all-PSCs.
42,43 The charge recombination characteristic can be further explored from the transient photovoltage (TPV) measurement, as exhibited in
Fig. 3d. The photocarrier lifetime (
τpho) can be extracted from TPV curves under open-circuit conditions.
44–46 The
τpho values of LbL or BHJ all-PSCs can be improved from 27.7 μs to 49.8 μs or from 25.1 μs to 43.1 μs by employing CN. The prolonged
τpho values indicate restrained charge recombination in BHJ and LbL all-PSCs with CN. The charge extraction time (
τext) can be fitted from the transient photocurrent (TPC) decay curves under short-circuit conditions.
47–49 As shown in
Fig. 3e, the fitted
τext values for BHJ and LbL all-PSCs with CN are 0.276 μs and 0.262 μs, which are shorter than the 0.307 μs and 0.294 μs for the BHJ and LbL all-PSCs without CN, respectively. The smaller
τext values reveal the efficient charge extraction in all-PSCs with CN. The longer photocarrier lifetime and the shorter charge extraction time can well support the enhanced JSC and FF in all-PSCs with CN.
To gain more insight into the charge transport and recombination process of all-PSCs, electrochemical impedance spectroscopy (EIS) measurements were carried out in the frequency range from 75 to 4.6 × 106 Hz under AM 1.5G illumination with 100 mW cm−2 light intensity. The Nyquist plots of all-PSCs measured at V = VOC are displayed in Fig. 3f and the inset shows the corresponding equivalent circuit model utilized to fit the Nyquist plot data. According to the equivalent circuit model, the fitting parameters of all-PSCs are listed in Table S2.† RCT represents charge transfer resistance, and ROS is associated with parasitic series resistance.50–52 The all-PSCs with CN present smaller RCT and ROS, which are conducive to the more efficient charge transport in the active layers with CN. The constant phase element (CPE) is introduced to describe the non-ideal behavior of capacitors. The CPE is defined using CPET and CPEP values according to the equation:53,54
Z is the impedance of a CPE,
w is the angular frequency, CPE
T is the capacitance value and CPE
P is the inhomogeneous constant varying between 0 and 1. The CPE
P values of BHJ or LbL all-PSCs are increased from 0.873 to 0.901 or from 0.886 to 0.912 by incorporating CN as the additive. The CPE
P values of BHJ and LbL all-PSCs with CN are much closer to 1 than those of the BHJ and LbL all-PSCs without CN, suggesting that there are fewer defects at the interface between PM6 and PY-IT as a result of employing CN. The time constant (
τ) of the active layer associated with charge transfer can be calculated from the equation:
55According to the
RCT and CPE
T values, the
τ values are 761.52 ns and 771.69 ns for LbL all-PSCs with and without CN, and 906.30 ns and 964.44 ns for BHJ all-PSCs with and without CN, respectively. The relatively small
τ values of all-PSCs with CN indicate inhibited charge recombination in the active layers with the CN additive.
Grazing incidence wide angle X-ray scattering (GIWAXS) characterization was employed to study the effect of introducing CN on molecular orientation properties. The 2D-GIWAXS patterns and the corresponding IP and OOP profiles of the neat films are exhibited in Fig. S3.† The neat PM6 film exhibits distinct (100) and (010) diffraction peaks in both OOP and IP directions, indicating the mixed face-on and edge-on molecular orientation of PM6. The intensity of the OOP (010) diffraction peak is enhanced in the PY-IT film with CN compared with that of the PY-IT film without CN, indicating that the face-on orientation of PY-IT is more ordered due to the incorporation of CN as the morphology regulator. The 2D-GIWAXS patterns and the corresponding OOP and IP profiles of the blend films are displayed in Fig. 4. The BHJ and LbL films exhibit the preferred face-on orientation, which is evidenced by the distinguishable lamellar stacking peak in the IP direction and the π–π stacking peak in the OOP direction. The positions of the diffraction peaks in the blend films are located between those of pure PM6 and PY-IT, signifying that the molecular arrangement in the blend films is codetermined by the donor and acceptor molecular arrangement.56 Enhanced scattering intensity of the OOP (010) and IP (100) diffraction peaks can be observed from the profiles of the blend films with CN, indicating that a more ordered face-on molecular arrangement should be formed in the BHJ and LbL blend films with CN for efficient charge transport along the normal direction of the substrate.57,58
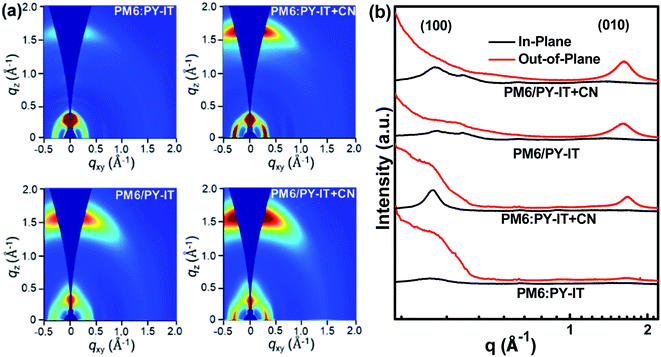 |
| | Fig. 4 (a) The 2D-GIWAXS patterns of PM6:PY-IT, PM6:PY-IT + CN, PM6/PY-IT and PM6/PY-IT + CN films. (b) The in-plane (IP, black line) and out-of-plane (OOP, red line) profiles abstracted from the 2D-GIWAXS patterns of the corresponding films. | |
Conclusion
In summary, the LbL and BHJ all-PSCs were fabricated based on the wide bandgap polymer PM6 as the donor and the narrow bandgap polymer PY-IT as the acceptor. The LbL or BHJ all-PSCs with CN exhibit PCEs of 15.81% or 15.29% due to the incorporation of 1 vol% CN in PY-IT solution or PM6:PY-IT blend solution, resulting from the simultaneously improved JSCs and FFs of LbL or BHJ all-PSCs with CN. Over 15% PCE improvement can be obtained in LbL or BHJ all-PSCs with CN, benefiting from the optimized photon harvesting, charge transport and molecular arrangement in LbL and BHJ active layers with CN. It should be highlighted that the PCEs of LbL all-PSCs are larger than those of the corresponding BHJ all-PSCs, which should be mainly due to the formation of more efficient charge transport channels by the LbL processing method. Overall, this work indicates that the LbL processing method combined with an additive should have great potential in preparing highly efficient all-PSCs.
Conflicts of interest
There are no conflicts of interest to declare.
Acknowledgements
This work was supported by the Fundamental Research Funds for the Central Universities (2022JBMC046) and the National Natural Science Foundation of China (62105017, 61975006, and 62175011).
References
- J. Du, K. Hu, J. Zhang, L. Meng, J. Yue, I. Angunawela, H. Yan, S. Qin, X. Kong, Z. Zhang, B. Guan, H. Ade and Y. Li, Nat. Commun., 2021, 12, 5264 CrossRef CAS PubMed.
- J. Zhang, C. Tan, K. Zhang, T. Jia, Y. Cui, W. Deng, X. Liao, H. Wu, Q. Xu, F. Huang and Y. Cao, Adv. Energy Mater., 2021, 11, 2102559 CrossRef CAS.
- Z. Genene, J. Lee, S. Lee, Q. Chen, Z. Tan, B. Abdulahi, D. Yu, T. Kim, B. Kim and E. Wang, Adv. Mater., 2022, 34, 2107361 CrossRef CAS PubMed.
- H. Fu, Y. Li, J. Yu, Z. Wu, Q. Fan, F. Lin, H. Woo, F. Gao, Z. Zhu and A. Jen, J. Am. Chem. Soc., 2021, 143, 2665–2670 CrossRef CAS PubMed.
- Y. Zhang, B. Wu, Y. He, W. Deng, J. Li, J. Li, N. Qiao, Y. Xing, X. Yuan, N. Li, C. Brabec, H. Wu, G. Lu, C. Duan, F. Huang and Y. Cao, Nano Energy, 2022, 93, 106858 CrossRef CAS.
- F. Cui, Z. Chen, J. Qiao, T. Wang, G. Lu, H. Yin and X. Hao, Adv. Funct. Mater., 2022, 32, 2200478 CrossRef.
- R. Sun, Y. Wu, X. Yang, Y. Gao, Z. Chen, K. Li, J. Qiao, T. Wang, J. Guo, C. Liu, X. Hao, H. Zhu and J. Min, Adv. Mater., 2022, 34, 2110147 CrossRef PubMed.
- C. He, Y. Pan, Y. Ouyang, Q. Shen, Y. Gao, K. Yan, J. Fang, Y. Chen, C. Ma, J. Min, C. Zhang, L. Zuo and H. Chen, Energy Environ. Sci., 2022 10.1039/d2ee00595f.
- X. Xu, L. Yu, H. Meng, L. Dai, H. Yan, R. Li and Q. Peng, Adv. Funct. Mater., 2021, 31, 2108797 Search PubMed.
- Y. Zheng, R. Sun, M. Zhang, Z. Chen, Z. Peng, Q. Wu, X. Yuan, Y. Yu, T. Wang, Y. Wu, X. Hao, G. Lu, H. Ade and J. Min, Adv. Energy Mater., 2021, 11, 2102135 CrossRef CAS.
- D. Li, C. Guo, X. Zhang, B. Du, C. Yu, P. Wang, S. Cheng, L. Wang, J. Cai, H. Wang, D. Liu, H. Yao, Y. Sun, J. Hou and T. Wang, Sci. China: Chem., 2022, 65, 373–381 CrossRef CAS.
- Y. Cui, S. Zhang, N. Liang, J. Kong, C. Yang, H. Yao, L. Ma and J. Hou, Adv. Mater., 2018, 30, 1802499 CrossRef PubMed.
- X. Wang, L. Zhang, L. Hu, Z. Xie, H. Mao, L. Tan, Y. Zhang and Y. Chen, Adv. Funct. Mater., 2021, 31, 2102291 CrossRef CAS.
- Q. Li, L. Wang, S. Liu, L. Guo, S. Dong, G. Ma, Z. Cao, X. Zhan, X. Gu, T. Zhu, Y. Cai and F. Huang, ACS Energy Lett., 2020, 5, 3637–3646 CrossRef CAS.
- W. Xu, X. Li, S. Jeong, J. Son, Z. Zhou, Q. Jiang, H. Woo, Q. Wu, X. Zhu, X. Ma and F. Zhang, J. Mater. Chem. C, 2022, 10, 5489–5496 RSC.
- Y. Xu, J. Yuan, S. Liang, J. Chen, Y. Xia, B. Larson, Y. Wang, G. Su, Y. Zhang, C. Cui, M. Wang, H. Zhao and W. Ma, ACS Energy Lett., 2019, 4, 2277–2286 CrossRef CAS.
- Q. Wu, W. Wang, Y. Wu, Z. Chen, J. Guo, R. Sun, J. Guo, Y. Yang and J. Min, Adv. Funct. Mater., 2021, 31, 2010411 CrossRef CAS.
- C. Cao, H. Wang, D. Qiu, T. Zhao, Y. Zhu, X. Lai, M. Pu, Y. Li, H. Li, H. Chen and F. He, Adv. Funct. Mater., 2022, 32, 2201828 CrossRef.
- Y. Zhang, B. Wu, Y. He, W. Deng, J. Li, J. Li, N. Qiao, Y. Xing, X. Yuan, N. Li, C. Brabec, H. Wu, G. Lu, C. Duan, F. Huang and Y. Cao, Nano Energy, 2022, 93, 106858 CrossRef CAS.
- B. Li, X. Zhang, Z. Wu, J. Yang, B. Liu, Q. Liao, J. Wang, K. Feng, R. Chen, H. Woo, F. Ye, L. Niu, X. Guo and H. Sun, Sci. China: Chem., 2022, 65, 1157–1163 CrossRef CAS.
- T. Liu, T. Yang, R. Ma, L. Zhan, Z. Luo, G. Zhang, Y. Li, K. Gao, Y. Xiao, J. Yu, X. Zou, H. Sun, M. Zhang, T. Dela Peña, Z. Xing, H. Liu, X. Li, G. Li, J. Huang, C. Duan, K. Wong, X. Lu, X. Guo, F. Gao, H. Chen, F. Huang, Y. Li, Y. Li, Y. Cao, B. Tang and H. Yan, Joule, 2021, 5, 914–930 CrossRef CAS.
- J. Vollbrecht, V. Brus, S. Ko, J. Lee, A. Karki, D. Cao, K. Cho, G. Bazan and T. Nguyen, Adv. Energy Mater., 2019, 9, 1901438 CrossRef.
- J. Vollbrecht and V. Brus, Org. Electron., 2020, 86, 105905 CrossRef CAS.
- X. Ma, A. Zeng, J. Gao, Z. Hu, C. Xu, J. Son, S. Jeong, C. Zhang, M. Li, K. Wang, H. Yan, Z. Ma, Y. Wang, H. Woo and F. Zhang, Natl. Sci. Rev., 2021, 8, nwaa305 CrossRef PubMed.
- X. Wang, Q. Sun, J. Gao, J. Wang, C. Xu, X. Ma and F. Zhang, Energies, 2021, 14, 4200 CrossRef CAS.
- M. Nam, J. Na, J. Shin, H. Lee, R. Chang and D. Ko, Nano Energy, 2020, 74, 104883 CrossRef CAS.
- K. Yang, J. Wang, Z. Zhao, Y. Sun, M. Liu, Z. Zhou, X. Zhang and F. Zhang, Chem. Eng. J., 2022, 435, 134973 CrossRef CAS.
- M. Jiang, H. Bai, H. Zhi, J. Sun, J. Wang, F. Zhang and Q. An, ACS Energy Lett., 2021, 6, 2898–2906 CrossRef CAS.
- C. Yan, H. Tang, R. Ma, M. Zhang, T. Liu, J. Lv, J. Huang, Y. Yang, T. Xu, Z. Kan, H. Yan, F. Liu, S. Lu and G. Li, Adv. Sci., 2020, 7, 2000149 CrossRef CAS PubMed.
- S. Zhang, X. Ma, C. Xu, W. Xu, S. Jeong, H. Woo, Z. Zhou, X. Zhang and F. Zhang, Macromol. Rapid Commun., 2022, 43, 2200345 CrossRef PubMed.
- X. Ma, Q. An, O. Ibraikulov, P. Lévêque, T. Heiser, N. Leclerc, X. Zhang and F. Zhang, J. Mater. Chem. A, 2020, 8, 1265–1272 RSC.
- R. Yu, G. Wu, Y. Cui, X. Wei, L. Hong, T. Zhang, C. Zou, S. Hu, J. Hou and Z. Tan, Small, 2021, 17, 2103497 CrossRef CAS PubMed.
- W. Xu, X. Ma, J. Son, S. Jeong, L. Niu, C. Xu, S. Zhang, Z. Zhou, J. Gao, H. Woo, J. Zhang, J. Wang and F. Zhang, Small, 2022, 18, 2104215 CrossRef CAS PubMed.
- M. Liu, J. Wang, K. Yang, Z. Zhao, Z. Zhou, Y. Ma, L. Shen, X. Ma and F. Zhang, J. Mater. Chem. C, 2021, 9, 6357–6364 RSC.
- H. Ning, Q. Jiang, P. Han, M. Lin, G. Zhang, J. Chen, H. Chen, S. Zeng, J. Gao, J. Liu, F. He and Q. Wu, Energy Environ. Sci., 2021, 14, 5919–5928 RSC.
- Z. Zhao, B. Liu, C. Xie, Y. Ma, J. Wang, M. Liu, K. Yang, Y. Xu, J. Zhang, W. Li, L. Shen and F. Zhang, Sci. China: Chem., 2021, 64, 1302–1309 CrossRef CAS.
- P. Heremans, L. Koster, M. Muccini, V. Mihailetchi, R. Ramaker, E. Meulenkamp, H. Xie and P. Blom, Appl. Phys. Lett., 2006, 86, 123509 Search PubMed.
- L. Koster, V. Mihailetchi, H. Xie and P. W. Blom, Appl. Phys. Lett., 2005, 87, 203502 CrossRef.
- J. Vollbrecht, J. Lee, S. Ko, V. Brus, A. Karki, W. Le, M. Seifrid, M. Ford, K. Cho, G. Bazan and T. Nguyen, J. Mater. Chem. C, 2020, 8, 15175–15182 RSC.
- H. Chen, T. Zhao, L. Li, P. Tan, H. Lai, Y. Zhu, X. Lai, L. Han, N. Zheng, L. Guo and F. He, Adv. Mater., 2021, 33, 2102778 CrossRef CAS PubMed.
- C. Xu, Z. Zhao, K. Yang, L. Niu, X. Ma, Z. Zhou, X. Zhang and F. Zhang, J. Mater. Chem. A, 2022, 10, 6291–6329 RSC.
- V. Brus, Org. Electron., 2016, 29, 1–6 CrossRef CAS.
- V. Brus, C. Proctor, N. Ran and T. Nguyen, Adv. Energy Mater., 2016, 6, 1502250 CrossRef.
- A. Chatri, S. Torabi, V. Corre and L. Koster, ACS Appl. Mater. Interfaces, 2018, 10, 12013–12020 CrossRef.
- Y. Lin, Y. Firdaus, F. Isikgor, M. Nugraha, E. Yengel, G. Harrison, R. Hallani, A. Labban, H. Faber, C. Ma, X. Zheng, A. Subbiah, C. Howells, O. Bakr, I. Culloch, S. Wolf, L. Tsetseris and T. Anthopoulos, ACS Energy Lett., 2020, 5, 2935–2944 CrossRef CAS.
- Z. Zhao, M. Liu, K. Yang, C. Xu, Y. Guan, X. Ma, J. Wang and F. Zhang, Adv. Funct. Mater., 2021, 31, 2106009 CrossRef CAS.
- V. Corre, A. Chatri, N. Doumon and L. Koster, Adv. Energy Mater., 2017, 7, 1701138 CrossRef.
- R. Wang, D. Zhang, S. Xie, J. Wang, Z. Zheng, D. Wei, X. Sun, H. Zhou and Y. Zhang, Nano Energy, 2018, 51, 736–744 CrossRef CAS.
- W. Gao, T. Liu, R. Sun, G. Zhang, Y. Xiao, R. Ma, C. Zhong, X. Lu, J. Min, H. Yan and C. Yang, Adv. Sci., 2020, 7, 1902657 CrossRef CAS PubMed.
- Z. Zhao, C. Xu, Y. Ma, K. Yang, M. Liu, X. Zhu, Z. Zhou, L. Shen, G. Yuan and F. Zhang, Adv. Funct. Mater., 2022, 32, 2203606 CrossRef.
- X. Ma, C. Tang, Y. Ma, X. Zhu, J. Wang, J. Gao, C. Xu, Y. Wang, J. Zhang, Q. Zheng and F. Zhang, ACS Appl. Mater. Interfaces, 2021, 13, 57684–57692 CrossRef CAS PubMed.
- T. Kumari, S. Lee, K. Lee, Y. Cho and C. Yang, Adv. Energy Mater., 2018, 8, 1800616 CrossRef.
- W. Li, J. Cai, F. Cai, Y. Yan, H. Yi, R. Gurney, D. Liu, A. Iraqi and T. Wang, Nano Energy, 2018, 44, 155–163 CrossRef CAS.
- C. Xu, X. Ma, Z. Zhao, M. Jiang, Z. Hu, J. Gao, Z. Deng, Z. Zhou, Q. An, J. Zhang and F. Zhang, Sol. RRL, 2021, 5, 2100175 CrossRef CAS.
- B. Xiao, M. Zhang, J. Yan, G. Luo, K. Gao, J. Liu, Q. You, H. Wang, C. Gao, B. Zhao, X. Zhao, H. Wu and F. Liu, Nano Energy, 2017, 39, 478–488 CrossRef CAS.
- K. Yang, Z. Zhao, M. Liu, Z. Zhou, K. Wang, X. Ma, J. Wang, Z. He and F. Zhang, Chem. Eng. J., 2022, 427, 131802 CrossRef CAS.
- K. Jiang, J. Zhang, Z. Peng, F. Lin, S. Wu, Z. Li, Y. Chen, H. Yan, H. Ade, Z. Zhu and A. Jen, Nat. Commun., 2021, 12, 468 CrossRef CAS.
- C. Xu, K. Jin, Z. Xiao, Z. Zhao, Y. Yan, X. Zhu, X. Li, Z. Zhou, S. Jeong, L. Ding, H. Woo, G. Yuan and F. Zhang, Sol. RRL, 2022, 6, 2200308 CrossRef.
|
| This journal is © The Royal Society of Chemistry 2022 |
Click here to see how this site uses Cookies. View our privacy policy here.  c,
Sang Young
Jeong
d,
Han Young
Woo
c,
Sang Young
Jeong
d,
Han Young
Woo
 d,
Zhengji
Zhou
d,
Zhengji
Zhou
 *e,
Qianqian
Sun
*e,
Qianqian
Sun
 *b and
Fujun
Zhang
*b and
Fujun
Zhang
 *a
*a

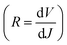 when VOC = 0 V and JSC = 0 V. The BHJ or LbL all-PSCs with CN show a smaller RS of 3.23 Ω cm2 or 2.84 Ω cm2 and a larger RSH of 883 Ω cm2 or 1031 Ω cm2 than the corresponding all-PSCs without CN, which can well support the relatively large FF of all-PSCs with CN. It is commonly reported that efficient PSCs also exhibit relatively small RS and relatively large RSH.22,23 The key photovoltaic parameters of all-PSCs and the calculated RS as well as RSH are summarized in Table 2.
when VOC = 0 V and JSC = 0 V. The BHJ or LbL all-PSCs with CN show a smaller RS of 3.23 Ω cm2 or 2.84 Ω cm2 and a larger RSH of 883 Ω cm2 or 1031 Ω cm2 than the corresponding all-PSCs without CN, which can well support the relatively large FF of all-PSCs with CN. It is commonly reported that efficient PSCs also exhibit relatively small RS and relatively large RSH.22,23 The key photovoltaic parameters of all-PSCs and the calculated RS as well as RSH are summarized in Table 2.

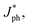 J&ph and Jsat under the short-circuit, maximal output-power and saturated state conditions, respectively.26–28 The Jsat values of both all-PSCs with the additive are higher than those of the corresponding all-PSCs without CN, indicating more efficient photon harvesting in the active layers with CN. The exciton dissociation efficiency (ηD) and charge collection efficiency (ηC) can be evaluated according to
J&ph and Jsat under the short-circuit, maximal output-power and saturated state conditions, respectively.26–28 The Jsat values of both all-PSCs with the additive are higher than those of the corresponding all-PSCs without CN, indicating more efficient photon harvesting in the active layers with CN. The exciton dissociation efficiency (ηD) and charge collection efficiency (ηC) can be evaluated according to 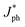 /Jsat and J&ph/Jsat, respectively, as shown in Table S1†.29–32 The ηD values of all-PSCs without or with CN are 92.92% or 94.61% for the BHJ structure, 92.66% or 94.43% for the LbL structure. The larger ηD for the BHJ all-PSCs should be mainly attributed to more PM6:PY-IT interfaces in the BHJ active layers. Benefiting from the more donor enrichment at the bottom of the LbL active layer and more acceptor enrichment at the top of the LbL active layer, the ηC values of LbL all-PSCs without or with CN are 81.51% or 82.11%, which are larger than the 80.92% or 82.01% of the corresponding BHJ all-PSCs.33,34 It should be noticed that the ηD and ηC for all-PSCs with CN are higher than those of all-PSCs without CN, implying more effective exciton dissociation and charge collection in all-PSCs with CN. Furthermore, the photoluminescence (PL) spectra of pure PY-IT, PM6:PY-IT and PM6/PY-IT blend films without or with CN were investigated to further understand the exciton dissociation process in all-PSCs, as shown in Fig. 3b.35,36 It is apparent that PY-IT has a strong and broad PL emission with a PL peak at 845 nm. The emission intensity of PY-IT is markedly quenched in the PM6:PY-IT and PM6/PY-IT blend films. Meanwhile, the PL emission intensity of the blend films with CN is lower than that of the corresponding blend films without CN, suggesting an increased exciton dissociation efficiency in the blend films with CN.
/Jsat and J&ph/Jsat, respectively, as shown in Table S1†.29–32 The ηD values of all-PSCs without or with CN are 92.92% or 94.61% for the BHJ structure, 92.66% or 94.43% for the LbL structure. The larger ηD for the BHJ all-PSCs should be mainly attributed to more PM6:PY-IT interfaces in the BHJ active layers. Benefiting from the more donor enrichment at the bottom of the LbL active layer and more acceptor enrichment at the top of the LbL active layer, the ηC values of LbL all-PSCs without or with CN are 81.51% or 82.11%, which are larger than the 80.92% or 82.01% of the corresponding BHJ all-PSCs.33,34 It should be noticed that the ηD and ηC for all-PSCs with CN are higher than those of all-PSCs without CN, implying more effective exciton dissociation and charge collection in all-PSCs with CN. Furthermore, the photoluminescence (PL) spectra of pure PY-IT, PM6:PY-IT and PM6/PY-IT blend films without or with CN were investigated to further understand the exciton dissociation process in all-PSCs, as shown in Fig. 3b.35,36 It is apparent that PY-IT has a strong and broad PL emission with a PL peak at 845 nm. The emission intensity of PY-IT is markedly quenched in the PM6:PY-IT and PM6/PY-IT blend films. Meanwhile, the PL emission intensity of the blend films with CN is lower than that of the corresponding blend films without CN, suggesting an increased exciton dissociation efficiency in the blend films with CN.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Plight
Plight

