Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Improving the thermoelectric performance of ZrNi(In,Sb)-based double half-Heusler compounds†
Shiyang
He‡
ab,
Amin
Bahrami‡
*a,
Pingjun
Ying
a,
Lars
Giebeler
 a,
Xiang
Zhang
a,
Xiang
Zhang
 c,
Kornelius
Nielsch
ab and
Ran
He
c,
Kornelius
Nielsch
ab and
Ran
He
 *a
*a
aLeibniz Institute of Solid State and Materials Science, 01069 Dresden, Germany. E-mail: a.bahrami@ifw-dresden.de; r.he@ifw-dresden.de
bInstitute of Materials Science, Technische Universität Dresden, 01062 Dresden, Germany
cNational Center for International Joint Research of Micro-Nano Molding Technology, School of Mechanics and Safety Engineering, Zhengzhou University, 450001 Zhengzhou, China
First published on 31st May 2022
Abstract
The complexity of crystal structures plays an intriguing role in manipulating properties in thermoelectrics, spintronics, and batteries. In comparison to the widely studied ternary half-Heusler thermoelectric compounds, quaternary double half-Heusler compounds are promising due to their intrinsically low lattice thermal conductivities (κL). However, they have been much less investigated due to the limited material availability. In this study, we report a new double half-Heusler compound based on ZrNi(In,Sb). Upon tuning the ratio of In/Sb from 0.5/0.5 to 0.4/0.6 and reducing the nominal concentrations of Zr and Ni by 10%, we greatly reduce the intensities of the impurity-phase peaks in the diffraction patterns. An even better phase purity, in combination with an optimized power factor, is realized by substituting Co at the Ni sites. Further alloying Hf at the Zr sites enhances the point defect scattering of phonons, which yielded a minimum κL of ∼1.8 W m−1 K−1 and a maximum zT of ∼0.5 for Zr0.7Hf0.2Ni0.65Co0.25In0.4Sb0.6 at 973 K. Our work thus confirms the intrinsically low κL of ZrNi(In,Sb) double half-Heusler compounds and indicates their promising applications upon further improving their electrical transport properties.
1 Introduction
Exotic or even anomalous physical phenomena are often observed in materials by designing complex crystal structures, such as enhancing the mechanical properties with multiple elements alloyed in high-entropy alloys1 and facilitating the diffusion of lithium ions with the desired atom site occupancies in battery materials.2 Therefore, it is imaginable that similarly amazing electrical and thermal transport properties can be achieved in robust materials with the designed complex crystal structures to enable a tailored manipulation of the transport mechanism of carriers.3Thermoelectric (TE) technology is a promising sustainable solution to alleviate the energy crisis and environmental pollution.4 The performance of TE materials is determined by the coupled electrical and thermal transport, as manifested in the expression of the figure-of-merit, zT, given by zT = S2ρ−1(κele + κL)−1T, where S, ρ, κele, κL, and T are the Seebeck coefficient, the resistivity, the electrical thermal conductivity, the lattice thermal conductivity and the absolute temperature, respectively.5 Thus, good TE materials require high power factors (PF = S2ρ−1) as well as low total thermal conductivity to realize high zT.6 However, optimizing the zT is challenging due to the intercorrelation of the transport parameters (S, ρ, and κele), such as via the carrier concentration.7 To realize effective decoupling, tremendous efforts are devoted, which can generally be categorized into two approaches.8 One is to maximize the PF by optimizing the carrier concentration via doping or band engineering,9 while another one targets seeking materials or processes to minimize the thermal conductivity.10
As a prominent TE material candidate in the moderate temperature region, half-Heusler (HH) compounds have attracted significant attention due to their low cost, high thermal stability, and mechanical robustness, as well as their excellent electrical properties.11 The bandgap of HH compounds varies from 0.1 to 3.7 eV based on the composition.11 The valence electron count (VEC) per unit cell has been confirmed to play a critical role in the transport properties.12 A slight variation from the 18 VEC (i.e., through doping) will optimize the PF, and the high PF is marked as the unique advantage of HH compounds.13 For instance, an ultrahigh PF value of ∼10.6 mW m−1 K−2 has been achieved in Ti-doped NbFeSb, and a record output power density of ∼22 W cm−2 is obtained in a single-leg TE device.14 In another example, Zhu et al. reported an outstanding zT of ∼1.5 at 923 K for p-type HH in a Sn-doped ZrCoBi alloy with a high PF (3.7 mW m−1 K−2 at 823 K).15 Despite the excellent electronic performance, in comparison with other classic thermoelectric materials (e.g. Bi2Te3-, PbTe- and Cu2Se-based TE materials),13,16,17 the intrinsically large κL of HH compounds impedes their further zT improvement. Because of their innately low thermal conductivity, the nominal 19-electron half-Heusler compounds,18 or defective half-Heusler compounds,19 have recently been identified as attractive TE material options for HH compounds. As a result, the primary objective of discovery in the TE discipline is to create novel HH alloys with low κL yet strong electrical characteristics.
The complexity of a primitive unit cell is a key factor to reduce the κL of HH compounds. Anand et al.20 compared the phonon frequency dependence of calculated cumulative κL of ternary (TiCoSb) and quaternary (Ti2FeNiSb2) HH compounds. A κL reduction of ∼50% as found for Ti2FeNiSb2 occurred in the high energy range of the phonons. In comparison to TiCoSb, a lower group velocity and a higher scattering rate of Ti2FeNiSb2 have been found, which indicates that quaternary HH compounds with more complex crystal structures would in general have lower κL than the traditional ternary HH compounds. Hence, HH systems are promising candidates for designing complex crystal structures to suppress the κL. Anand et al. predicted a total number of 713 possible ternary, defect-free HH compounds, of which 487 have been fully explored.20 However, quaternary double HH compounds, based on the same calculations, have been rarely investigated, with only TiFe1−xNixSb being successfully synthesized.21 The lowest κL of 2.5 W m−1 K−1 at 973 K has been achieved in TiFe0.6Ni0.4Sb while still reaching a high PF (∼1.5 mW m−1 K2), indicating that enhancing the complexity of a primitive unit cell for HH alloys can dramatically suppress the κL.21 Thus, quaternary HH compounds have huge potential to enable the discovery of further new highly performing TE materials due to the huge unexplored phase space of ∼7719 quaternary HH compounds.20
In this study, a new n-type double HH compound based on ZrNi(In,Sb) is realized (Fig. 1(a)). Starting from ZrNiIn0.5Sb0.5, we find obvious impurity peaks besides the main HH phase by X-ray powder diffraction (XRD). The intensity of the impurity peaks is substantially reduced after tuning the composition by changing the nominal In/Sb ratio to 0.4/0.6. Lowering the nominal amount of Zr and Ni by 10% improves this impurity phase reduction. Further substituting Co at the Ni sites not only tunes the carrier concentrations to realize an almost doubled peak PF from 1.08 to 2.03 mW m−1 K2 at 973 K in Zr0.9Ni0.65Co0.25In0.4Sb0.6 but also further removes the impurity phase completely according to the detection limit of the diffractometer. Additionally, we further suppressed the κL from 2.10 to 1.81 W m−1 K−1 at 973 K by alloying a small amount of Hf to partially substitute Zr on the respective sites, which is comparable with other HH materials that have shown minimized κL (Fig. 1(b)). As a result of simultaneously optimizing the electrical properties and suppressing the κL, the final zT values were enhanced by 336% from 0.11 to 0.48 at 973 K in a compound with the composition of Zr0.7Hf0.2Ni0.65Co0.25In0.4Sb0.6 (Fig. 1(c)), demonstrating the great potential of ZrNi(In,Sb) HH compounds for intermediate- and high-temperature energy conversion.
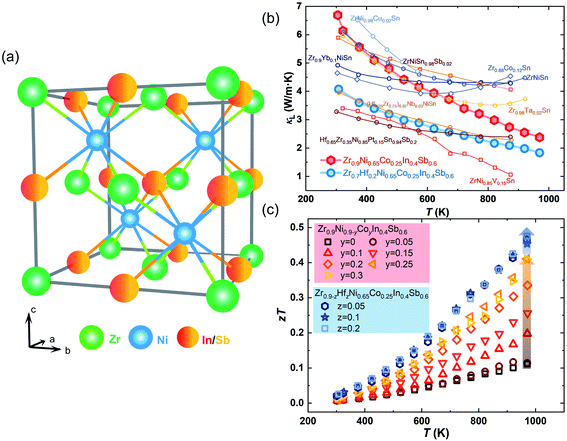 | ||
Fig. 1 (a) Unit cell of the crystal structure of ZrNi(In,Sb) double HH compounds (space group F![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3m). (b) Comparison of temperature-dependent κL among other low κL HH systems. (c) Temperature-dependent zT values of ZrNi(In,Sb) double HH compounds.2,38–41,42 3m). (b) Comparison of temperature-dependent κL among other low κL HH systems. (c) Temperature-dependent zT values of ZrNi(In,Sb) double HH compounds.2,38–41,42 | ||
2 Experimental
2.1 Materials synthesis
The samples with nominal compositions of ZrNiInxSb1−x (x = 0.4–0.6), Zr0.9Ni0.9−yCoyIn0.4Sb0.6 (y = 0–0.3) and Zr0.9−zHfzNi0.65Co0.25In0.4Sb0.6 (z = 0.05–0.2) are prepared by arc melting, ball milling, and hot-pressing processes. The stoichiometrically weighed raw materials (Zr sponges, 99.2%; Ni granules, 99.98%; In granules, 99.9%; Sb shots, 99.8%; Hf sponges, 99.9%; Co powder, 99.9%) were melted three times in an Ar-protected chamber to form homogeneous ingots. Subsequently, the ingots were loaded into an Ar-filled stainless steel jar and ball milled for 4 h in a SPEX 8000D machine. The ball-milled powders were pressed into pellets by spark plasma sintering (AGUS-PECS SPS-210Gx) at 1173 K for 5 minutes under vacuum with a uniaxial pressure of 50 MPa.2.2 Phase identification and microstructure characterization
X-ray powder diffraction (XRD) measurements for bulk samples were performed using a Bruker D8 with a Co source in the Bragg–Brentano mode to examine the phase purity and crystal structure of the samples. For the Rietveld analysis, the powders were prepared as flat samples, where powder less than the tip of a spatula was glued on acetate foil. The sample was inserted into a Stoe Stadi P powder diffractometer equipped with a Mo source and a Ge(111) monochromator that yield a single wavelength of 0.7093 Å. The position sensitive detector was a Mythen 1K from Dectris. The samples were measured in the range of 10° ≤ 2θ ≤ 60° in the transmission mode. The recorded data were analyzed according to the Rietveld method22 with the program package Fullprof/WinPlotR.23 With the profile function nPr = 7 for the structure model refinement, the isotropic temperature factors (Biso) and the asymmetry parameters Asy1 and Asy2 were refined. An instrumental resolution function was deduced from the NIST 640d silicon standard. All data were used as measured. For the main HH phase, the ZrCoSb structure model with the space group F![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3m of Romaka et al.24 was adopted to carry out investigation on the compounds. The fractured surface of the samples was analyzed by field emission scanning electron microscopy (Sigma300-ZEISS FESEM). Inductively coupled plasma-optical emission spectrometry (ICP-OES) measurements were conducted with an iCAP 6500 Duo View (Thermo Fisher Scientific) after aqueous acidic digestion.
3m of Romaka et al.24 was adopted to carry out investigation on the compounds. The fractured surface of the samples was analyzed by field emission scanning electron microscopy (Sigma300-ZEISS FESEM). Inductively coupled plasma-optical emission spectrometry (ICP-OES) measurements were conducted with an iCAP 6500 Duo View (Thermo Fisher Scientific) after aqueous acidic digestion.
2.3 Thermoelectric measurement
Both sides of the obtained pellets were polished after spark plasma sintering (AGUS-PECS SPS-210Gx), and the thermoelectric properties were directly quantified afterward. The temperature-dependent Seebeck coefficient (S) and electrical resistivity (ρ) were measured by the standard four-probe method (LSR-3, Linseis). The temperature-dependent thermal diffusivity (λ) was evaluated by a laser flash method in a helium atmosphere (LFA 1000, Linseis). The density (ρ) of the samples was measured by the Archimedes method, and the heat capacity (Cp) was calculated using the Dulong–Petit law. The thermal conductivity (κtot) was calculated according to the relationship κtot = λρCp. The carrier concentration and Hall mobility were determined from 10 K to 300 K with Hall measurements in the van-der-Pauw geometry with a physical property measurement system (9 T LOT Dynacool system).3 Results and discussion
3.1 Phase identification and purification
The bulk XRD patterns of all the samples of ZrNi(In,Sb) double HH are shown in Fig. S1.† Compared with the crystal structure of ZrCoSb,24 the main reflections of all the samples correspond well with the MgAgAs-type with the space group F![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3m (No. 216). In the starting compound, ZrNiIn0.5Sb0.5, we also find multiple impurities with the main reflections indexed as Zr2Ni2In and ZrNi2In. The impurities vary in concentration upon changing the ratio of In/Sb. For example, with an In/Sb ratio of 0.6/0.4, the main reflection intensity of Zr2Ni2In (at 2θ ∼ 49° with Co Kα radiation) is significantly enhanced. In contrast, the ZrNi2In reflection almost vanishes with an In/Sb ratio of 0.4/0.6. Furthermore, since other impurities are intermetallics of Zr–Ni in ZrNiIn0.4Sb0.6, we modified the composition by reducing the nominal concentrations of Zr and Ni by 10%. This way, only a small fraction of an In-based impurity remains in the sample with the nominal composition Zr0.9Ni0.9In0.4Sb0.6. Moreover, we substituted Co for Ni, forming Zr0.9Ni0.9−yCoyIn0.4Sb0.6 (y = 0–0.3). Interestingly, we found that the remaining In-based impurity almost disappears with higher concentrations of Co (y = 0.25 and 0.3). Nevertheless, by substituting Hf at Zr sites to form Zr0.9−zHfzNi0.65Co0.25In0.4Sb0.6, the slight impurity reflection of In-based impurity reappears with low intensity, and the second phase of impurity becomes more prominent with increased Hf content (Fig. S1(c)†). All of these experimental observations suggest that ZrNiInxSb1−x-based compounds exhibit the HH crystal structure, although most end up with slight impurities. The phase-evolvement dynamics of the studied system remain unclear and could be rather complex due to its multi-element nature. It may originate from the composition-dependent state of equilibrium, or simply from the multi-step processing under partially harsh conditions. For example, In and Sb have much higher vapor pressure compared to Zr and Ni, so the reduced nominal concentration of Zr and Ni in improving the phase purity may simply be because it compensates for the losses of In and Sb during the arc melting process. A more detailed analysis demands further study in the future.
3m (No. 216). In the starting compound, ZrNiIn0.5Sb0.5, we also find multiple impurities with the main reflections indexed as Zr2Ni2In and ZrNi2In. The impurities vary in concentration upon changing the ratio of In/Sb. For example, with an In/Sb ratio of 0.6/0.4, the main reflection intensity of Zr2Ni2In (at 2θ ∼ 49° with Co Kα radiation) is significantly enhanced. In contrast, the ZrNi2In reflection almost vanishes with an In/Sb ratio of 0.4/0.6. Furthermore, since other impurities are intermetallics of Zr–Ni in ZrNiIn0.4Sb0.6, we modified the composition by reducing the nominal concentrations of Zr and Ni by 10%. This way, only a small fraction of an In-based impurity remains in the sample with the nominal composition Zr0.9Ni0.9In0.4Sb0.6. Moreover, we substituted Co for Ni, forming Zr0.9Ni0.9−yCoyIn0.4Sb0.6 (y = 0–0.3). Interestingly, we found that the remaining In-based impurity almost disappears with higher concentrations of Co (y = 0.25 and 0.3). Nevertheless, by substituting Hf at Zr sites to form Zr0.9−zHfzNi0.65Co0.25In0.4Sb0.6, the slight impurity reflection of In-based impurity reappears with low intensity, and the second phase of impurity becomes more prominent with increased Hf content (Fig. S1(c)†). All of these experimental observations suggest that ZrNiInxSb1−x-based compounds exhibit the HH crystal structure, although most end up with slight impurities. The phase-evolvement dynamics of the studied system remain unclear and could be rather complex due to its multi-element nature. It may originate from the composition-dependent state of equilibrium, or simply from the multi-step processing under partially harsh conditions. For example, In and Sb have much higher vapor pressure compared to Zr and Ni, so the reduced nominal concentration of Zr and Ni in improving the phase purity may simply be because it compensates for the losses of In and Sb during the arc melting process. A more detailed analysis demands further study in the future.
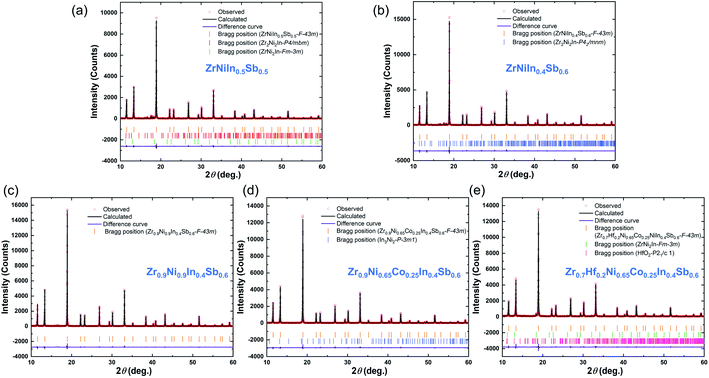
Subsequently, to evaluate the lattice parameters and phase contents, Rietveld analyses based on the XRD results were performed for four specimens with the nominal compositions of ZrNiIn0.5Sb0.5, ZrNiIn0.4Sb0.6, Zr0.9Ni0.9In0.4Sb0.6, Zr0.9Ni0.65Co0.25In0.4Sb0.6, and Zr0.7Hf0.2Ni0.65Co0.25In0.4Sb0.6 as shown in Fig. 2, and the detailed information can be found in Table S1.†
For the Rietveld analyses, the structural model of Romaka et al.24 was adopted for the previously discussed elements. The resulting structural model fits the X-ray powder diffraction patterns to a great extent and with a phase content > 93 wt% to almost 99 wt% depending on the total amount of minor phases. Here, the specimen with the nominal composition ZrNiIn0.5Sb0.5 has the highest percentage of ∼7 wt% and ∼1 to 2 wt% for all other compounds. The evaluated sizes of the lattice parameter a result in the following order of the compounds: ZrNiIn0.4Sb0.6 > ZrNiIn0.5Sb0.5 > Zr0.9Ni0.9In0.4Sb0.6 > Zr0.9Ni0.65Co0.25In0.4Sb0.6 > Zr0.7Hf0.2Ni0.65Co0.25In0.4Sb0.6 reflecting the different concentrations of substituent elements and their respective radii. As Sb has a smaller radius than Zr, Ni, and Co, reduced lattice parameters are expected, and the lattice parameters of the individual compounds therewith follow their composition obtained by the ICP-OES analyses shown in Table S2.† We find that while the concentrations of other elements agree well with the nominal one, In always displays certain deficiencies that may originate from the losses during the arc melting. Additionally, increased Sb and Ni/Co concentrations are found, whereas the In concentration decreased. Both may lead to the resulting lattice parameter order. The occupancy has not been included in this consideration as these results need further investigation to explain them plausibly. Fig. 3 illustrates the fracture surface obtained by SEM (scanning electron microscopy) for the compounds ZrNiInxSb1−x (x = 0.4–0.6). The grain boundaries on this scale become less defined with decreasing In content, as displayed in Fig. 3(b) and (c). Additionally, the average grain size values of ZrNiInxSb1−x are similar based on statistics but still show a slightly increasing trend with lower In content. This behavior may be attributed to the distinct response to the current-assisted sintering process, as will be discussed soon since the Sb-rich compound is more conductive than the In-rich one. It is worth noting that the increasing trend of grain size is more visible, while the electrical resistivities increased in Co-doped samples. As shown in Fig. S2,† the clear grain boundaries can be observed, and the grain size significantly increased with increased Co doping content (Fig. S2(h)†). Additionally, grain sizes above 1 μm appeared and were distributed uniformly – marked with the red circles – when the Co-doped content reached 0.3 (Fig. S2†).
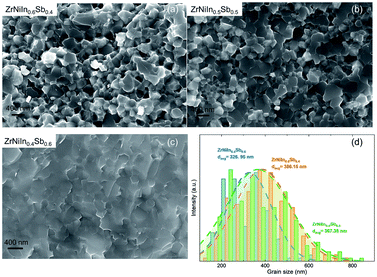 | ||
| Fig. 3 The fracture surfaces obtained by SEM for (a) ZrNiIn0.6Sb0.4, (b) ZrNiIn0.5Sb0.5 and (c) ZrNiIn0.4Sb0.6, and the distribution of the (d) grain size for the above composition. | ||
3.2 Electrical transport properties
As is known, HH compounds involve p-type/n-type transport with a valence electron count (VEC) less and more than 18, respectively. As displayed in Fig. 4(a), the Seebeck coefficients exhibit an increasing trend upon heating. All samples show a strong n-type conductor behavior even with x = 0.6 that nominally has a VEC of 17.8. This finding is related to the loss of In, as documented by the ICP-OES results. The temperature-dependent ρ of ZrNiInxSb1−x (x = 0.4–0.6) are given in Fig. 4(b). All ρ of the investigated samples increase for the measured temperature range pointing to degenerated or metal-like behaviors. A lower Sb content increases the value of ρ from 1.27 μΩ m to 2.33 μΩ m at 300 K. The nominal deficiency of Zr/Ni has little influence on ρ, suggesting that the effect of a second phase on the electrical properties is negligible for such a heavily doped conductor. Finally, a maximum PF of ∼1.8 mW m−1 K2 is obtained for ZrNiSb0.5In0.5. However, Zr0.9Ni0.9Sb0.6In0.4 exhibits relatively low impurities, which is the reason it was selected for subsequent optimizations. | ||
| Fig. 4 Temperature dependence of S (a and d), ρ (b and e) and PF (c and f) for ZrNi(In,Sb) double HH compounds. | ||
Considering the low electrical resistivity and the Seebeck coefficient, it is desirable to reduce the carrier concentration to optimize the power factor, which is realized by substituting Co for Ni. As illustrated in Fig. 4(d) and (e), both the electrical resistivity and the Seebeck coefficient of Zr0.9Ni0.9−yCoyIn0.4Sb0.6 (y = 0–0.3) are linearly dependent on the temperature in the degenerated nature. The value of ρ increases monotonically from 2.35 to 12.57 μΩ m with the doping level of Co at 973 K (Fig. 4(e)). Correspondingly, the S also increases three times from −51.3 to −151.1 μV K−1 at 973 K (Fig. 4(d)), implying that the Co doping could effectively tune the electrical properties. Benefiting from the huge increase of S, the PF displays ∼100% enhancement from 1.07 to 2.04 mW m−1 K2 for the composition of y = 0.25 (Fig. 4(f)).
The variation in the electrical properties can be understood from the results of the carrier concentration (n) and the Hall mobility (μ). Fig. 5(a) and (b) show the Hall measurement results in the range of 10–300 K. The n values of all the samples are found in the range of 1021 cm−3 and are weakly dependent on the temperature. As shown in Fig. 5(a), the ZrNiIn0.5Sb0.5 sample has the lowest Hall concentration (nH) among the ZrNiIn1−xSbx samples in the temperature range of 10–300 K. The Hall concentration was found to increase by either increasing the In/Sb ratio to 0.6/0.4 or decreasing it to 0.4/0.6. Whereas the increased nH stems from an increased VEC in the Sb-rich composition (nominally the VECs are 18 for ZrNiIn0.5Sb0.5, and 18.2 for ZrNiIn0.4Sb0.6), it has more complex origins in the In-rich composition because of the much higher concentration of impurities (Fig. S1(a)†). Interestingly, the carrier concentration changes little by decreasing the nominal concentrations of both Zr and Ni by 10% from ZrNiIn0.4Sb0.6 to Zr0.9Ni0.9In0.4Sb0.6. The Co-substituted composition, Zr0.9Ni0.65Co0.25In0.4Sb0.6, displays a further reduced n to ∼2 × 1021 cm−3, thus explaining its higher power factor in Fig. 4(f). Additionally, Fig. 5(a) shows that the substitution of Hf on the Zr site doesn't influence the nH significantly due to the isovalent nature of Zr and Hf, as well as the lanthanide-contraction of Hf as has been discussed by Liu et al.25 Accordingly, the electrical properties do not change significantly upon Hf substitution, as shown in Fig. S3.†
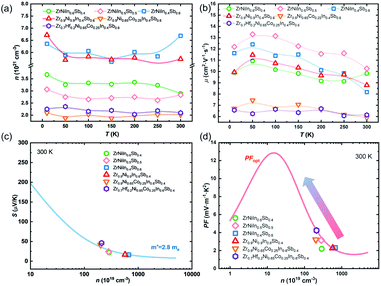 | ||
| Fig. 5 (a) Temperature dependence of n; (b) μ and (c) n dependence of |S| at 300 K ; (d) optimum PF at 300 K for the ZrNi(In,Sb) double HH compounds. | ||
On the other hand, the μ values for all the Co-free samples are similar, typically ranging from 14 to 10 cm2 V−1 s−1. The substitution of Co in Zr0.9Ni0.65Co0.25In0.4Sb0.6 yields a reduction in the mobility by nearly ∼40%, probably due to the enhanced electron scattering through alloying. Based on the Hall measurement and electrical measurement results, we plotted the carrier-concentration as a function of the absolute Seebeck coefficient in Fig. 5(c) together with the calculations to evaluate the DOS effective mass m*, using the single parabolic band (SPB) model,26 which is estimated to be 2.8me and similar to the ZrNiSn-based compounds.27–29 This approach is reasonable since the band edges mainly constitute the d-orbitals from the transition metals (Zr and Ni), so the modification at the most electronegative sites (In/Sb or Sn) is not expected to change the effective mass drastically. Fig. 5(d) shows the n-dependent optimum PF of ZrNi(In,Sb) samples at 300 K following an SPB model,26 together with our experimental results. Clearly, there is great potential to further improve the power factor by further reducing the carrier concentration. However, this idea is not realized by increasing the Co concentration to y = 0.3, as shown in Fig. 4, which might originate from an enhanced alloy scattering when the Co amount exceeds a certain limit. Therefore, other approaches are sought to further enhance the thermoelectric performance, which will be introduced later.
3.3 Thermal transport properties
Fig. 6(a) plots the temperature-dependent total thermal conductivity κtot of the ZrNiInxSb1−x-based HH compounds. The κtot of the ZrNiInxSb1−x compound decreases weakly with increased temperature and In/Sb ratio. For example, the κtot decreases from 9.90 W m−1 K−1 in ZrNiIn0.4Sb0.6 to 7.65 W m−1 K−1 at 973 K in ZrNiIn0.6Sb0.4. Fig. 6(b) shows the κL, as obtained by subtracting the electronic component κe using the Wiedemann–Franz law, κe = LσT, where L is the Lorenz number determined by the formula L = 1.5 + exp(−|S|/116) (Fig. S4(a)†).30κL shows a decrease from 2.22 W m−1 K−1 to 0.98 W m−1 K−1 at 973 K with a shrinking In/Sb ratio. Apparently, κe dominates the heat conduction, suggesting the importance of reducing the carrier concentration for a lower κtot. Additionally, the introduced deficiencies of Zr and Ni further suppress the κL to ∼0.4 W m−1 K−1 at 973 K, which is even lower than the theoretical Cahill minimum κL (∼0.7 W m−1 K−1 for ZrNiSn). Admittedly, such an extremely low κL may originate from an overestimated κe due to the inaccurate evaluation of the Lorenz number for such a complex system. Nevertheless, these results do confirm the low-κL nature of the double HH compounds ZrNi(In,Sb), which has been rarely realized in ternary half-Heusler compounds. Glassbrenner and Slack suggested that the κL follows the T−1 relationship if only the Umklapp scattering process above the Debye temperature is considered.31 The curves in Fig. 6(b) indicate that such a T−1 relationship is roughly followed except for ZrNiIn0.6Sb0.4, implying that the phonon scattering is dominated by the phonon–phonon interaction. The κL ∼ T−0.67 relationship of ZrNiIn0.6Sb0.4 implies other dominant phonon scattering mechanisms such as point defects or impurities.32 Furthermore, we compare in Fig. 6(c) our κL with other double half-Heusler compounds based on theoretical calculations and experimentally discovered TiFe1−xNixSb at 300 K. The κL of ZrNi(In,Sb) is found to be comparable with TiFe1−xNixSb, and much lower than the values as predicted by theoretical calculations (e.g., 13.60 W m−1 K−1 for ZrNiIn0.5Sb0.5).20 We thus demonstrate the great potential of ZrNi(In,Sb) as a low-κL platform to realize subsequent improvement of zT.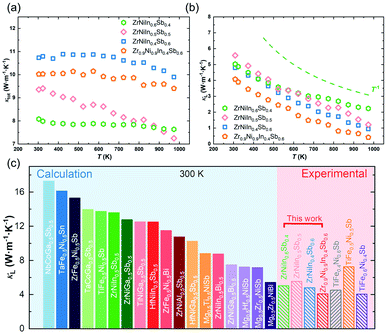 | ||
| Fig. 6 Temperature dependence of (a) κtot and κL (b) for ZrNiInxSb1−x, and (c) the comparison of κL of the calculated/experimental double HH alloys at 300 K.20 | ||
Subsequently, we investigated the dependence of the thermal transport properties on the Co and Hf substitutions. In parallel, the temperature-dependent κtot and κL of Zr0.9Ni0.9−yCoyIn0.4Sb0.6 and Zr0.9−zHfzNi0.65Co0.25In0.4Sb0.6 compounds are presented in Fig. 7. For Zr0.9Ni0.9−yCoyIn0.4Sb0.6, while κtot is suppressed by increasing the Co content (Fig. 7(a)), κL displays an inverse trend (Fig. 7(b)). Thus, the reduction of κe is very important to overcompensate the rebound of κL. Furthermore, the increased κL with respect to the increased Co content is counter-intuitive, since heavier alloying usually scatters phonons more strongly. The Rietveld analysis (Table S1†) suggested that the half-Heusler phase has a phase content of ∼98.6 mass% for the Zr0.9Ni0.65Co0.25In0.4Sb0.6 sample; therefore, the impurity phases should contribute negligibly to the varied lattice thermal conductivity. Furthermore, we observe a trend of grain enlargement with more Co following the SEM results (Fig. S2†), which could be partially accountable for the enhanced κL. The mechanism of such a grain-enlargement is not well understood, but it has been widely reported for half-Heusler materials that, under the same preparation conditions, a lower electrical resistivity usually yields a small grain size.33,34 This is probably due to less Joule heating produced during sintering. In addition, our group recently revealed that a higher doping level will induce, spontaneously, a stronger charge-compensation effect in the half-Heusler compounds by producing charged point defects to counteract the extrinsic doping.34 This effect is widely appreciated in tuning the electronic transport, but it can also significantly intensify the phononic scattering. The substitution of Co at the Ni sites reduces the carrier concentration/doping level, thus reducing the formation of charged point defects that are against the extrinsic doping. Admittedly, considering the complex multiple-elemental nature and the formation of impurities, it is very challenging to quantify the point defect through refinement based on a Mo anode. Diffraction patterns with higher quality are necessary such as those obtained by synchrotron or even neutron radiation. These will be subject to future studies.
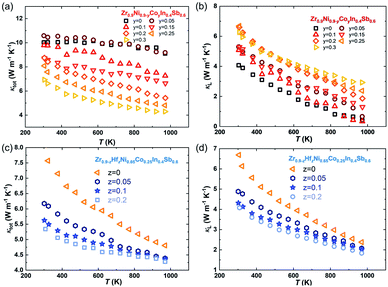 | ||
| Fig. 7 Temperature dependence of κtot (a and c) and κL (b and d) for the Co-doped and Hf-doped ZrNiInxSb1−x based HH alloy samples. | ||
For Zr0.9−zHfzNi0.65Co0.25In0.4Sb0.6 samples, the κtot gradually decreases as the Hf content increases, while there is only a negligible difference of κe (Fig. S4(c)†). Hence, the decrease of κtot is related to a stronger mass fluctuation induced by higher Hf contents. In particular, the lowest κL of Zr0.7Hf0.2Ni0.65Co0.25In0.4Sb0.6 is ∼4.1 W m−1 K−1 at room temperature and ∼1.81 W m−1 K−1 at 973 K as given in Fig. 7(d). Finally, the zT values increase from ∼0.1 to ∼0.4 at 973 K with a tuned carrier concentration by Co-doping and are further improved to ∼0.5 at 973 K with suppressed lattice thermal conductivity realized in the compound with the nominal stoichiometry Zr0.7Hf0.2Ni0.65Co0.25In0.4Sb0.6 as shown in Fig. 1(c). Additionally, the optimum zT of the newly discovered ZrNi(In,Sb) compounds is comparable with that of other double HH systems21,35–37 (Fig. S5†), demonstrating that ZrNi(In,Sb) compounds enrich the HH families and can potentially be applied in electricity generation.
4 Conclusion
In this work, we proposed and examined the thermoelectric properties of new double HH ZrNi(In,Sb) compounds. We also demonstrated how the phase purity evolves by controlling the In/Sb ratio and the nominal content of Zr and Ni, doping the Ni sites with Co, and substituting Hf for Zr. The interrelated TE parameters are partially decoupled, through which simultaneously optimized electronic and phononic properties could be realized. A reduced κL ∼ 1.8 W m−1 K−1 and a peak zT ∼ 0.5 are obtained for the compound with a nominal stoichiometry of Zr0.7Hf0.2Ni0.65Co0.25In0.4Sb0.6 at 973 K. These findings reveal the role of Co doping in improving the PF via tuning the charge carrier concentration and the role of Hf in suppressing κL by enhancing the point defect-related phonon scattering. The enhanced TE performance indicated the effectiveness of this strategy and suggests its potential for further enhancement, which may help enrich HH families and discover novel materials.Author contributions
S. H., A. B., and R. H. designed the research; A. B., P. Y., and S. H. prepared the samples; S. H. and A. B. performed the microstructure characterization studies; S. H., A. B., and R. H. measured the thermoelectrical properties; S. H. conducted the Hall measurement, and X. Z. analyzed the data; L. G. conducted the powder XRD and Rietveld analysis; S. H., A. B., R. H., and K. N. prepared the manuscript, and all the authors commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by Deutsche Forschungsgemeinschaft (DFG), Project Number 453261231. A. B. acknowledges the Alexander von Humboldt Foundation for the Postdoctoral Research Fellowship funding. P. Y. acknowledges the financial support from the strategic project at IFW Dresden on “Wireless sensor devices for high temperature applications”.Notes and references
- S. Zhou, T. Mei, X. Wang and Y. Qian, Nanoscale, 2018, 10, 17435–17455 RSC
.
- D. B. Miracle and O. N. Senkov, Acta Mater., 2017, 122, 448–511 CrossRef CAS
.
- C. A. Cox, E. S. Toberer, A. A. Levchenko, S. R. Brown, G. J. Snyder, A. Navrotsky and S. M. Kauzlarich, Chem. Mater., 2009, 21, 1354–1360 CrossRef CAS
.
- A. Bahrami, G. Schierning and K. Nielsch, Adv. Energy Mater., 2020, 10(19), 1904159 CrossRef CAS
.
- S. He, Y. Li, L. Liu, Y. Jiang, J. Feng, W. Zhu, J. Zhang, Z. Dong, Y. Deng, J. Luo, W. Zhang and G. Chen, Sci. Adv., 2020, 6, eaaz8423 CrossRef CAS PubMed
.
- S. He, S. Lehmann, A. Bahrami and K. Nielsch, Adv. Energy Mater., 2021, 11, 2101877 CrossRef CAS
.
- X. Qi, J. Chen, K. Guo, S. He, J. Yang, Z. Li, J. Xing, J. Hu, H. Luo, W. Zhang and J. Luo, Chem. Eng. J., 2019, 374, 494–501 CrossRef CAS
.
- S. He, J. Yang, A. Bahrami, X. Zhang, R. He, M. Hantusch, S. Lehmann and K. Nielsch, ACS Appl. Energy Mater., 2022, 5, 4041–4046 CrossRef CAS
.
- L. You, Z. Li, Q. Ma, S. He, Q. Zhang, F. Wang, G. Wu, Q. Li, P. Luo, J. Zhang and J. Luo, Research, 2020, 2020, 1–8 CrossRef PubMed
.
- S. He, Y. Yang, Z. Li, J. Zhang, C. Wang, W. Zhang and J. Luo, Sci. China Mater., 2021, 64, 1751–1760 CrossRef CAS
.
- S. Chen and Z. Ren, Mater. Today, 2013, 16, 387–395 CrossRef CAS
.
- T. Fang, K. Xia, P. Nan, B. Ge, X. Zhao and T. Zhu, Mater. Today Phys., 2020, 13, 100200 CrossRef
.
- C. Fu, T. Zhu, Y. Pei, H. Xie, H. Wang, G. J. Snyder, Y. Liu, Y. Liu and X. Zhao, Adv. Energy Mater., 2014, 4, 1400600 CrossRef
.
- R. He, D. Kraemer, J. Mao, L. Zeng, Q. Jie, Y. Lan, C. Li, J. Shuai, H. S. Kim, Y. Liu, D. Broido, C.-W. Chu, G. Chen and Z. Ren, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 13576–13581 CrossRef CAS PubMed
.
- H. Zhu, R. He, J. Mao, Q. Zhu, C. Li, J. Sun, W. Ren, Y. Wang, Z. Liu, Z. Tang, A. Sotnikov, Z. Wang, D. Broido, D. J. Singh, G. Chen, K. Nielsch and Z. Ren, Nat. Commun., 2018, 9, 1–9 CrossRef PubMed
.
- J.-F. Li and J. Liu, Phys. Status Solidi, 2006, 203, 3768–3773 CrossRef CAS
.
- F. Kong, J. Bai, Y. Zhao, Y. Liu, J. Shi, Z. Wang and R. Xiong, Appl. Phys. Lett., 2019, 115, 203901 CrossRef
.
- K. Xia, P. Nan, S. Tan, Y. Wang, B. Ge, W. Zhang, S. Anand, X. Zhao, G. J. Snyder and T. Zhu, Energy Environ. Sci., 2019, 12, 1568–1574 RSC
.
- K. Xia, Y. Liu, S. Anand, G. J. Snyder, J. Xin, J. Yu, X. Zhao and T. Zhu, Adv. Funct. Mater., 2018, 28, 1705845 CrossRef
.
- S. Anand, M. Wood, Y. Xia, C. Wolverton and G. J. Snyder, Joule, 2019, 3, 1226–1238 CrossRef CAS
.
- Z. Liu, S. Guo, Y. Wu, J. Mao, Q. Zhu, H. Zhu, Y. Pei, J. Sui, Y. Zhang and Z. Ren, Adv. Funct. Mater., 2019, 29, 1905044 CrossRef CAS
.
- H. M. Rietveld, J. Appl. Crystallogr., 1969, 2, 65–71 CrossRef CAS
.
- T. Roisnel and J. Rodríquez-Carvajal, Mater. Sci. Forum, 2001, 378–381, 118–123 CAS
.
- V. V. Romaka, L. Romaka, P. Rogl, Y. Stadnyk, N. Melnychenko, R. Korzh, Z. Duriagina and A. Horyn, J. Alloys Compd., 2014, 585, 448–454 CrossRef CAS
.
- Y. Liu, C. Fu, K. Xia, J. Yu, X. Zhao, H. Pan, C. Felser and T. Zhu, Adv. Mater., 2018, 30, 1800881 CrossRef PubMed
.
- J. Zhu, X. Zhang, M. Guo, J. Li, J. Hu, S. Cai, W. Cai, Y. Zhang and J. Sui, npj Comput. Mater., 2021, 7, 116 CrossRef CAS
.
- A. Bahrami, P. Ying, U. Wolff, N. P. Rodríguez, G. Schierning, K. Nielsch and R. He, ACS Appl. Mater. Interfaces, 2021, 13, 38561–38568 CrossRef CAS PubMed
.
- C. Fu, M. Yao, X. Chen, L. Z. Maulana, X. Li, J. Yang, K. Imasato, F. Zhu, G. Li, G. Auffermann, U. Burkhardt, W. Schnelle, J. Zhou, T. Zhu, X. Zhao, M. Shi, M. Dressel, A. V. Pronin, G. J. Snyder and C. Felser, Adv. Sci., 2020, 7, 1902409 CrossRef CAS PubMed
.
- C. Yu, H. Xie, C. Fu, T. Zhu and X. Zhao, J. Mater. Res., 2012, 27, 2457–2465 CrossRef CAS
.
- S. He, A. Bahrami, X. Zhang, I. G. Martínez, S. Lehmann and K. Nielsch, Adv. Mater. Technol., 2021, 2100953 Search PubMed
.
- C. J. Glassbrenner and G. A. Slack, Phys. Rev., 1964, 134, A1058–A1069 CrossRef
.
- W. Ren, H. Zhu, Q. Zhu, U. Saparamadu, R. He, Z. Liu, J. Mao, C. Wang, K. Nielsch, Z. Wang and Z. Ren, Adv. Sci., 2018, 5, 1800278 CrossRef PubMed
.
- H. Zhu, R. He, J. Mao, Q. Zhu, C. Li, J. Sun, W. Ren, Y. Wang, Z. Liu, Z. Tang, A. Sotnikov, Z. Wang, D. Broido, D. J. Singh, G. Chen, K. Nielsch and Z. Ren, Nat. Commun., 2018, 9, 2497 CrossRef PubMed
.
- R. He, T. Zhu, Y. Wang, U. Wolff, J.-C. Jaud, A. Sotnikov, P. Potapov, D. Wolf, P. Ying, M. Wood, Z. Liu, L. Feng, N. P. Rodriguez, G. J. Snyder, J. C. Grossman, K. Nielsch and G. Schierning, Energy Environ. Sci., 2020, 13, 5165–5176 RSC
.
- A. Karati, S. Ghosh, R. C. Mallik, R. Shabadi, B. S. Murty and U. V. Varadaraju, J. Mater. Eng. Perform., 2022, 31, 305–317 CrossRef CAS
.
- R. Hasan, T. Park, S. Kim, H.-S. Kim, S. Jo and K. H. Lee, Advanced Energy and Sustainability Research, 2022, 2100206 CrossRef
.
- Q. Wang, X. Li, C. Chen, W. Xue, X. Xie, F. Cao, J. Sui, Y. Wang, X. Liu and Q. Zhang, Phys. Status Solidi, 2020, 217, 2000096 CrossRef CAS
.
- H. Xie, H. Wang, C. Fu, Y. Liu, G. J. Snyder, X. Zhao and T. Zhu, Sci. Rep., 2014, 4, 1–6 Search PubMed
.
- B. Gong, Y. Li, F. Liu, J. Zhu, X. Wang, W. Ao, C. Zhang, J. Li, H. Xie and T. Zhu, ACS Appl. Mater. Interfaces, 2019, 11, 13397–13404 CrossRef CAS PubMed
.
- N. S. Chauhan, S. Bathula, A. Vishwakarma, R. Bhardwaj, B. Gahtori, A. Kumar and A. Dhar, ACS Appl. Energy Mater., 2018, 1, 757–764 CrossRef CAS
.
- X. Yang, Z. Jiang, J. Li, H. Kang, D. Liu, F. Yang, Z. Chen, E. Guo, X. Jiang and T. Wang, Nano Energy, 2020, 78, 105372 CrossRef CAS
.
- S. Katsuyama, H. Matsushima and M. Ito, J. Alloys Compd., 2004, 385, 232–237 CrossRef CAS
.
Footnotes |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ta02413f |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |