Open Access Article
Open Access ArticleBiodegradable floating hydrogel baits as larvicide delivery systems against mosquitoes†
Marco
Piazzoni‡
a,
Agata
Negri
b,
Elisa
Brambilla
 c,
Laura
Giussani
a,
Simone
Pitton
c,
Laura
Giussani
a,
Simone
Pitton
 b,
Silvia
Caccia
b,
Sara
Epis
b,
Claudio
Bandi
b,
Silvia
Locarno
b,
Silvia
Caccia
b,
Sara
Epis
b,
Claudio
Bandi
b,
Silvia
Locarno
 *d and
Cristina
Lenardi
ad
*d and
Cristina
Lenardi
ad
aCIMAINA, Physics Department, Università degli Studi di Milano, Via Celoria 16, 20133, Milano, Italy
bDepartment of Biosciences and Pediatric CRC “Romeo ed Enrica Invernizzi”, University of Milan, Milan, ItalyVia Celoria 26, 20133, Milan, Italy
cDepartment of Pharmaceutical Sciences, Section of General and Organic Chemistry “A. Marchesisi”, Università degli Studi di Milano, Via Venezian 21, 20133, Milano, Italy
dPhysics Department, Università degli Studi di Milano, Via Celoria 16, 20133, Milano, Italy. E-mail: silvia.locarno@unimi.it
First published on 3rd August 2022
Abstract
Biological methods for mosquito larvae control are completely biodegradable and have null or limited effects on nontarget organisms. However, commercially available products have a low residual activity, with the consequent need for multiple applications that inevitably increase costs and the risk of resistance phenomena insurgence. Smart delivery systems made of hydrogels proved their efficacy in increasing the action duration of biolarvicides up to several months, but the lack of an efficient baiting mechanism to strongly attract the target pest remains a problem in practical applications. In this work, we investigated two novel hydrogel-based formulations of completely natural composition for baiting and killing larvae of Aedes albopictus mosquitos. The proposed materials consist of charged crosslinked polysaccharides (chitosan and cellulose) and are specifically manufactured to float in water, simulating organic matter usually present at breeding sites. Within the hydrogels’ matrix, yeast colonies of Saccharomyces cerevisiae were embedded as phagostimulants alongside a biolarvicide (Bacillus thuringiensis var. israelensis (Bti)). Despite the similar chemical nature and structure, chitosan-based hydrogels exhibited a markedly superior baiting potential compared to those made of cellulose and also succeeded in efficiently killing mosquito larvae just after a few hours from administration. We are confident that the proposed smart delivery hydrogel made of chitosan can be an enabling tool to attract mosquito larvae towards biopesticides of different nature without delocalizing active ingredients away from the breeding site and to simultaneously increase their residual activity, thus holding the potential of minimizing environmental pollution related to pest control and vector-borne disease prevention.
1. Introduction
Mosquitoes are one of the most widespread public health problems, in Europe and worldwide, because of their vector capacity for viruses, bacteria and protozoa which causes them to be among the deadliest animals for humans.1 Recent environmental changes accentuated the strong dynamism of such animal species and modulated, on a global scale, the spatio-temporal distribution of vectors, hosts and pathogens.2,3 It is indeed not uncommon to register epidemic outbreaks of mosquito-related diseases (e.g. dengue, yellow fever, West-Nile, chikungunya, zika, filariasis and malaria) in non-endemic areas.4Since mosquito adult forms are more difficult to control, due to the non-confined area where they live, it is essential to implement eradication strategies targeting immature stages (i.e. eggs, larvae, and pupae). Chemical larvicides have been dismissed in recent years because of water pollution, bioaccumulation and resistance insurgence related problems and are being substituted by those of biological origins.5–8 Biolarvicides can be derived from plants, bacteria, algae, lichens and fungi;9 some of the most extensively studied are oomycetes species (Leptolegnia chapmanii, Pythium sp. and Lagenidium giganteum),10 herbal species (Azadirachta indica)11 and bacterial species (Bacillus thuringiensis var. israelensis (Bti) and Bacillus sphaericus (Bs)).12,13 Their increasing use in integrated control programmes, where environmental management, personal protection and careful use of insecticides are crucial, is owed to specificity towards mosquito larvae with no side effects on mammals.14–16
Even if biolarvicides have the advantage of limited ecological impact, they have the drawback of a short duration of action due to high susceptibility to external atmospheric agents (e.g. sunlight and high temperature).17,18 The consequent need for multiple applications makes biolarvicides an expensive solution and increases the possibility of the emergence of resistance in mosquitoes.19 Therefore, to achieve an environmentally friendly formulation with a long shelf-life and efficacy, it is necessary to find an effective method to protect the active principle while simultaneously preventing its dispersion into the environment.
To overcome the above-listed challenges, a great deal of research is being focused on the development of new biolarvicide formulations and, in particular, smart delivery systems (i.e. engineered technologies for the targeted delivery and/or controlled release of substances) made of hydrogels showed very promising results.20
Hydrogels are crosslinked polymers capable of absorbing and retaining a large amount of water within their three-dimensional network. This is due to the presence of hydrophilic side groups on the polymer chains that compose the molecular backbone.21 A unique characteristic of hydrogel-based materials is the possibility of finely tuning their swelling and release properties by adjusting intrinsic parameters like crosslinking density, monomer types and network's charge.22 Therefore, if active ingredients (e.g. chemicals, cells, and enzymes) are encapsulated in the bulk architecture of the hydrogel, they will be released with a desired kinetic profile depending on their chemical nature, the hydrogel's matrix features and also the ionic composition of the external aqueous environment.23 Such technologies have been developed mainly for pharmaceutical applications but, in the last decades, they have also been engineered for biopesticide release.24–26
Only a few examples of smart delivery hydrogels employed specifically against mosquito larvae can be found in the literature. For instance, Borge and colleagues27 improved the release of the hydrophobic larvicidal extract of the plant Dendranthema grandiflora, by dispersing it in a block co-polymer of polybutylene succinate (PBS) and polyethylene glycol (PEG), causing mortality in larvae of Aedes aegypti. In another recent study, the residual activity of Bti against Aedes albopictus larvae was shown to increase up to several months when loaded into hydrogel capsules of PEG and hexadecanol.28
The fact that the hydrogels’ matrix can act as a shield for those active principles that can be inactivated by environmental agents is a potential enabling solution to diminish the number of biolarvicide administrations, therefore reducing both production costs and, most importantly, minimizing the risk of resistance insurgence in the target species.19,20 However, despite the undeniable efficiency upon increasing the residual activity of biolarvicides, the translation from laboratory to field conditions might not be that straightforward. As a matter of fact, it is not uncommon to attest a significant decrease in larvicide performance once placed in a real application environment (e.g. fountains, manholes, flowerpot dishes, and small ponds).29,30 Such incongruency can be attributed to the incapacity of the formulation to strongly attract towards itself the target species.31 A baiting mechanism is therefore paramount to ensure the specific insect to come in direct contact with the pesticide. Mosquito larvae are known to be attracted towards microorganisms that represent a feeding source and floating objects where they can find shelter from predators.32,33 To our knowledge, no hydrogel-based biolarvicide formulation has yet been reported of being both capable of floating and attracting like a phagostimulant.
Due to increased attention to environmental issues, many research interests are now moving towards the use of green and biodegradable substances. For this reason, in the context of control of pests and vectors, hydrogels made of natural polymers have become preferred over those of synthetic origins.31,34 Polysaccharides such as chitosan, alginate and cellulose have an attested renewability, biocompatibility, and nontoxicity and have already been successfully engineered to be used as smart delivery capsules against mosquito larvae.35–39
In this work, the potential of two novel smart delivery hydrogel-based formulations of completely natural origin is investigated in baiting and killing mosquito larvae. In more detail, the first one is a cationic hydrogel composed of crosslinked chitosan (ChitH) whereas the second one is an anionic hydrogel made of crosslinked cellulose (CellH). They were manufactured using an ad hoc liquid foam templating technique that allows generation of closed macro-pores entrapped in the matrix, so as to enable buoyancy in water. Within these hydrogels, colonies of the yeast Saccharomyces cerevisiae were embedded as phagostimulants and a commercially available Bti formulation as a biolarvicide. Physico-chemical analysis suggested that active ingredients (Bti and yeasts) were administered to the target species mainly by material erosion, framing the proposed hydrogels in the broad category of erodible smart delivery systems. However, only the ChitH formulation was proved to possess efficient biological activity towards Ae. albopictus larvae, both as a phagostimulant and as a larvicide. We strongly believe that the proposed smart delivery hydrogel made of chitosan can be a suitable ecofriendly tool against many different targets representing a public health concern or a challenge for the agriculture sector.
2. Experimental section
Chitosan (medium MW, 75–85% degree of deacetylation), sodium dodecyl sulphate (SDS) and citric acid were purchased from Sigma-Aldrich. Medium-viscosity hydroxyethylcellulose (HEC) was purchased from Farmlabor while carboxymethylcellulose sodium salt (CMCNa) and 1-ethyl-3(3-dimethylaminopropyl) carbodiimide (EDC) were purchased from Iris Biotech. Genipin was purchased from Wako Chemicals. All chemicals were used without further purification. Bti (VactoBac® 12 AS-Bti) was purchased from I.N.D.I.A (INDUPHARMA Srl). All solvents were of ACS grade or higher and were obtained from Sigma-Aldrich. Ultrapure water (resistivity 18.2 M cm) was obtained using a water purification system (MilliQ®Direct, EMD Millipore, Germany).Chitosan hydrogel formulation
One chitosan hydrogel (ChitH) tablet was prepared by slowly dissolving 10 mg of chitosan in 1 mL of a 1% v/v acetic acid solution and by adding 10 μL of 20 mM SDS solution. The mixture was stirred for 1 h at 300 rpm at room temperature (RT). Then, 100 μL of 44 mM genipin solution in EtOH 10% v/v were added, and the mixture was stirred at 600 rpm at RT for 30 min to blend everything together. For ChitH with Bti and yeasts (ChitH@Bti-Y), the active ingredients were added in the polymer mixture right after the genipin solution. The hydrogel preparation was performed from the microgram to multi-gram scale.Cellulose hydrogel formulation
One cellulose hydrogel (CellH) tablet was prepared according to a modified procedure described by Sannino et al.40 First, a mixture of HEC (8 mg) and CMCNa (24 mg) was dissolved in 1 mL of water by magnetic stirring at RT, until a clear and homogeneous solution was obtained. The final polymer content was 3 wt%, with a CMCNa/HEC weight ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. After the addition and mixing of 32 mg of EDC as a crosslinking agent, 50 μL aqueous solution containing 1 wt% of citric acid was added as a gelation catalyst. For CellH with Bti and yeasts (CellH@Bti-Y), the active ingredients were added in the polymer mixture before the citric acid solution. The hydrogel preparation was performed from a microgram to a multi-gram scale.
1. After the addition and mixing of 32 mg of EDC as a crosslinking agent, 50 μL aqueous solution containing 1 wt% of citric acid was added as a gelation catalyst. For CellH with Bti and yeasts (CellH@Bti-Y), the active ingredients were added in the polymer mixture before the citric acid solution. The hydrogel preparation was performed from a microgram to a multi-gram scale.
Liquid foam templating technique
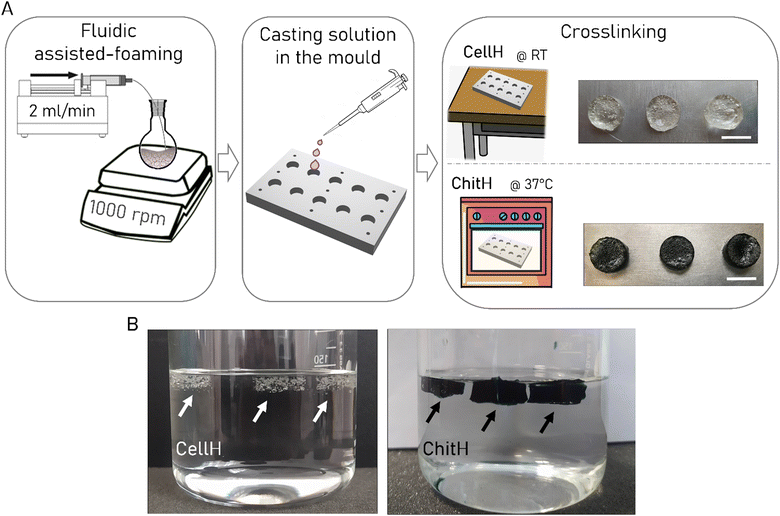
Before starting the liquid foam templating technique ChitH pre-polymer solution was cured at 42 °C for 35 min, whereas CellH pre-polymer solution was cured for 20 min at RT. Bubbles were generated inside the partially cured pre-polymer mixtures by injecting air at 10 μL min−1 with a syringe pump (KD Scientific, Thermo Fisher Scientific) in combination with vigorous stirring (1000 rpm). The procedure lasted 2 min, and then, the liquid foams were pipetted in the designated aluminium mould and were left to crosslink overnight. ChitH was cured at 37 °C whereas CellH was cured at RT. All hydrogels were moulded as small cylinders (∅ = 16 mm).Gel fraction
Gel samples were dried in an oven at 37 °C until a constant weight was reached, and then they were left to swell in MilliQ water for 4 days at room temperature. Swollen samples were again put in the oven at 37 °C until a constant weight was reached. The gel fraction degree (GF%) was obtained using formula (1):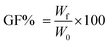 | (1) |
Swelling degree
Cured hydrogels were weighed before swelling in a large excess (150 mL) of NaCl aqueous solutions of increasing ionic strength (IS) (0 mM, 5 mM, 50 mM, and 500 mM). When the constant weight was reached, samples were removed from the aqueous solutions, gently tapped onto filter paper and weighed again. The swelling degree at equilibrium (Q%) is described using formula (2):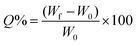 | (2) |
Weight loss
After reaching the maximum swelling degree in the NaCl swelling solutions (0 mM, 5 mM, 50 mM, and 500 mM), the weight loss of the gels was monitored over a period of 28 days. The weight loss (W%) was calculated using formula (3):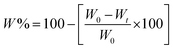 | (3) |
Uniaxial compression mechanical tests
Uniaxial compression tests were performed on hydrogel tablets with cylindrical shape (∅ = 16 mm) with a deformation rate of 5 mm min−1, using a traction machine (TVM-N, Sauter) coupled with a dynamometer FH-10 (Sauter) and the software AFH-FAST/FD (Sauter). Before testing, the samples were swelled in water for 24 h at RT. Three independent specimens were tested for every condition analysed. The stress (σ) was evaluated as the ratio between the forces measured by the load cell and the undeformed specimen cross-section. Strain (ε) was determined as the ratio between the crosshead displacement and the specimen height. Elastic moduli were obtained considering the elastic region of the stress–strain curve up to 10% of deformation. For CellH samples, the Young's modulus (E) was calculated whereas for ChitH samples the secant modulus (Es) was calculated.Mosquito breeding
The mosquitoes used in the present work are derived from the Rimini strain of the Asian tiger mosquito Aedes (Stegomyia) albopictus, established in 2004 from mosquito eggs collected in Rimini.41 The colony was maintained under standard conditions of humidity (70–80%) and temperature (28–30 °C) in the Insectary of the University of Milan. Adult mosquitoes were fed with animal blood to complete the reproductive cycle, while the larvae were reared with fish food (Tetra-fish, Melle).Fluorescent yeast culture for matrix inclusion
Saccharomyces cerevisiae cells that were GFP-labelled (SY2080 with integrative plasmid pRS306) were used to perform matrix inclusion and feeding experiments on Ae. albopictus mosquito larvae. Cells were grown in generic Yeast Extract–Peptone–Dextrose (YPD) medium enriched with 2% raffinose as a nutrient source and induced for 5 h with 30% galactose. Cells post-incubation were counted to reach a concentration of 107 cells mL−1 to be included in each hydrogel tablet.Ingestion test and fluorescence analysis
Two pools of five Ae. albopictus larvae were starved for 12 h in glass Petri dishes containing 50 mL of deionised water. One hydrogel tablet with yeast-GFP included (ChitH@Y or CellH@Y) was then added to the water. An empty matrix served as a control. After 24 h or 72 h larvae were dissected, separating the intestine from the carcass, and the midguts were observed using a SIM A1 confocal microscope (Nikon).Transmittance studies
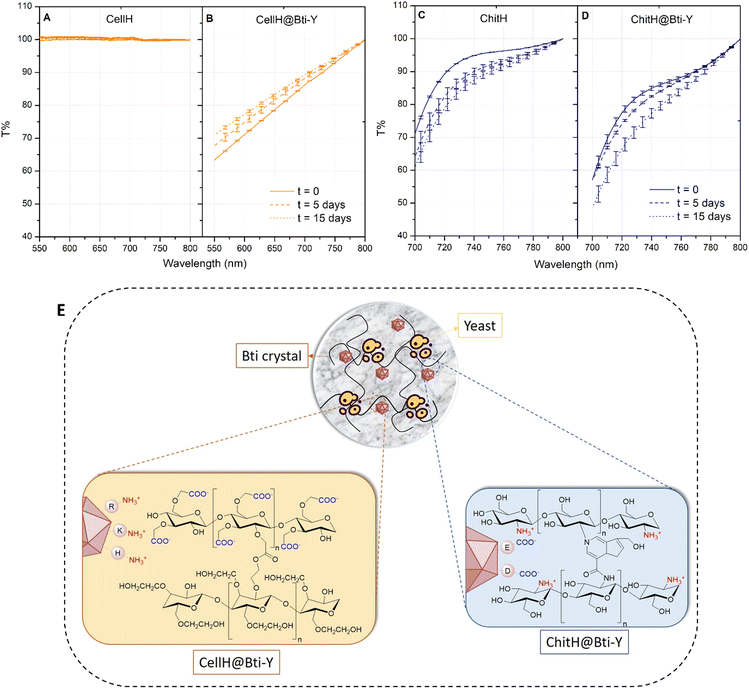
Hydrogel samples filled with Bti and yeasts (CellH@Bti-Y and ChitH@Bti-Y) were immersed in 150 mL of a 50 mM NaCl solution for 24 h and the transmittance (T%) spectra of the gels were recorded over a period of 15 days, using a UV-vis spectrophotometer (Cary 100 UV-vis, Agilent Technologies, Santa Clara, CA, USA). The wavelength range with steps of 1 nm was set between 550 and 800 nm for CellH@Bti-Y and between 700 and 800 nm for ChitH@Bti-Y. The T% values were averaged from three repeated measurements. Each measurement was performed on an independent gel tablet.Mortality bioassays
Pools of ten Ae. albopictus larvae were placed in Petri dishes containing deionised water. Larvae were exposed to a hydrogel tablet of different formulations (CellH@Bti-Y or ChitH@Bti-Y) placed onto the Petri dish; the empty matrices were used as a control for the test. The monitoring of live and dead larvae was carried out for a week.3. Results and discussion
Hydrogels’ design and manufacture
The rational design behind the proposed hydrogel formulations considered the need for a biodegradable floating system with baiting capability towards mosquito larvae that could be used as a delivery protective tool for the administration of biolarvicide molecules.For this purpose, the molecular backbone of both engineered hydrogels was completely made of natural polymers, in particular of polysaccharides, respectively, chitosan and cellulose. Polysaccharide-based hydrogels have indeed recently been attested to increase Bti residual activity up to several months.39 ChitH samples were crosslinked using genipin which is far known to have markedly lower cytotoxicity as compared with alternative crosslinkers such as glutaraldehyde.42 After crosslinking, they appeared as a dark-blue coloured tablet, a trend mark of oxygen radical-induced polymerization of genipin as well as its reaction with chitosan amino groups.43,44 This feature can both provide better Bti covering from UV radiation and more efficient larva baiting.33,45 CellH samples were instead obtained by crosslinking an aqueous mixture of HEC and CMCNa through the water-soluble carbodiimide EDC, under acidic conditions. This well-known chemical reaction induces the formation of ester bonds between the carboxyl groups provided by CMCNa and the hydroxyl groups provided by HEC. The crosslinking agent EDC is not incorporated into the gel structure, but it is changed into a urea derivative (EDU), which displays a very low degree of cytotoxicity and can be easily washed out from the polymeric network, increasing the biocompatibility of the final product.40
In both cases ionic hydrogels were obtained. While ChitH displayed positive charges, thanks to the presence of –NH3+ groups in the polymeric backbone, the negative fixed charges (–COO−) of the polyelectrolyte CMCNa induced the formation of anionic CellH.
The crosslinking efficacy was measured by gel fraction (GF%) experiments. The GF% of ChitH reached 79 ± 3% indicating a good degree of crosslinking formed in the polymer hydrogel network. On the other hand, the GF% value of CellH was found to be around 54 ± 1%, demonstrating and confirming that EDU molecules did not participate in the gel network, and they were free to spread out after swelling.
Afterwards, buoyancy was studied. In fact, floating objects have a natural baiting potential for mosquito larvae, since these insects normally exploit leaves or other organic materials to hide beneath and protect themself from natural surface- and bottom-predators or simply as an available microbial food resource while ensuring safer filter feeding.33 For such a reason small bubbles of air were entrapped in the hydrogel matrixes in order to allow them to float on water. This was achieved by developing an ad hoc liquid foam templating manufacturing procedure (Fig. 1A). A syringe pump was set to inflate air through a needle inside the pre-polymer solution, while a magnetic stirrer was used to break big bubbles into smaller ones (diameter in the millimetre range). When the liquid foam was generated, the solution was pipetted in the designated mould for crosslinking. A key aspect to ensure liquid foam stabilization before the onset of gelation is viscosity. Pre-polymer solutions possessing high viscosity can stabilize bubbles more efficiently and will prevent aging mechanisms (i.e. coalescence, coarsening and drainage) that inevitably disrupt the liquid foam.46,47 Both the ChitH and the CellH solutions proved to be not viscous enough to stabilise a foam. For this reason, we decide, on an empirical basis, to partially cure the ChitH pre-polymeric solution for 35 min and the CellH one for 20 min before starting the fluid-assisted foaming. Using this approach, we manage to successfully develop two floating Bti formulations (Fig. 1B). The versatility of liquid foam templating in converting virtually all kinds of pre-polymeric solutions into porous hydrogel materials makes this technique very appealing also in the context of preparing larvicide formulations. Moreover, the entire manufacturing procedure required mild conditions throughout the whole process, which not only ensure safety but also maintained low production costs, thus facilitating industrial scalability.
Hydrogels’ behaviour in aqueous environments
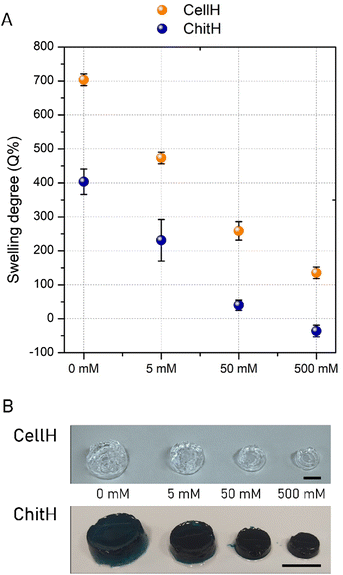
In hydrogels, the driving force for swelling arises from a difference between the water–polymer thermodynamic mixing force and the retractive force of the polymer chains.22 If charged species are present on the molecular backbone of the matrix's network and as free ions in the imbibing solution then the swelling process will be controlled by the previous two contributions as well as the ionic interactions between charges (i.e. Donnan's contribution).21Here, the swelling experiments were performed on both the investigated hydrogels in a solvent having a simple and well-defined composition. In particular, NaCl solutions with different concentrations (0 mM, 5 mM, 50 mM and 500 mM) were prepared in order to mimic the ionic strength of aqueous environments of practical interest (e.g. fountains, manholes, flowerpot dishes and small ponds). The hydrogels were immersed in a large reservoir of solvent and the variation in weight was recorded once the swelling equilibrium point is reached. The 0 mM NaCl solution (MilliQ water) represents the simplest solvent model tested, since the ion swelling pressure depends only on the ionic charges within the hydrogel. As expected, both the gels were able to absorb a large amount of water (Fig. 2A). However, the swelling capability is much higher in CellH (Q%0 = 703 ± 17%) than in ChitH (Q%0 = 404 ± 37%), suggesting that the negative charge of CellH imparted higher ionic strength with respect to the positive charge of ChitH. This result was further confirmed by varying the NaCl concentration. CellH samples showed a gradual decrease in swelling capability with the increase of salt concentration, due to reduction of the osmotic pressure between the hydrogel and the outer solution. ChitH samples resulted in a similar trend but with smaller swelling degree values. Also in this case, the maximum swelling degree was observed in ultrapure water and a gradual decrease and shrinkage were observed as the ionic strength of the outer solution increased up to 500 mM. When the salt concentration reached 50 mM, ChitH was near to be at osmotic equilibrium with the outer solution since the swelling degree value was close to 0% (Q%50 = 40 ± 15%). By further increasing the salt concentration, the ionic strength of the reservoir solution exceeded the ionic strength in the hydrogel's network, and this resulted in material shrinkage (Q%500 = −36 ± 17%).
When Bti and yeasts were included in the matrices (CellH@Bti-Y and ChitH@Bti-Y) the swelling degree trend of both hydrogels remained the same (Fig. S1, ESI†), indicating that active ingredients did not significantly impact water absorption dynamics. However, the overall values were slightly lower with respect to the empty matrices, suggesting a possible interaction between the charged amino acids of Bti and the charged side groups displayed in the hydrogels’ polymer backbones that decreased the osmotic pressure inside the gel networks.
The hydrogels’ weight variation was also monitored in the same range of ionic strength over a period of 28 days at RT. From the graph shown in Fig. 3A it can be observed that CellH samples maintained a steady weight in the 50 mM to 500 mM ionic strength range, whereas it underwent full degradation in 0 and 5 mM NaCl solutions after nearly a month. A hydrogel dimensional stability in a specific water solution mainly depends on its capacity of absorbing and retaining the solvent within the three-dimensional network. CellH massive swelling under 0 (Q%0 = 703 ± 17%) and 5 mM (Q%5 = 473 ± 17%) conditions exceeded the extensibility of crosslinked polymer segments to withhold together and eventually tore the material apart into many fragments.21 On the other hand, ChitH samples always maintained their weight above 70% of the initial value because the reduced amount of imbibed water did not compromise irreparably matrix integrity over the tested time window (Fig. 3B).
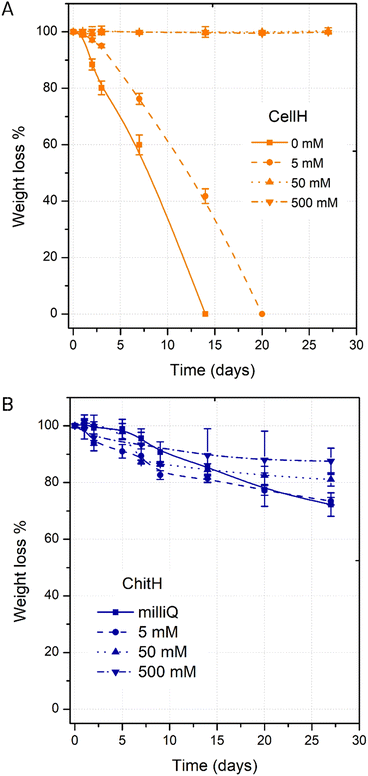 | ||
| Fig. 3 Weight loss (W%) of CellH (up) and ChitH (bottom) samples after 7, 14, 21, and 28 days in solutions of increasing ionic strength (0 mM, 50 mM, 50 mM and 500 mM). | ||
Hydrogels’ mechanical properties
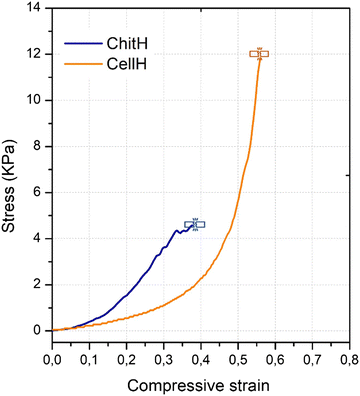
The hydrogels’ mechanical behaviour was assessed through uniaxial compressive mechanical tests. Fig. 4 shows the stress–strain relationship of the materials when compressed at a constant rate until fracture. CellH specimens showed a clear rubber-like elasticity behaviour. In fact, stress remained proportional to strain up until 30% of compression with respect to the initial height. After this value, yielding started to occur and deformation became plastic. On the other hand, ChitH specimens never quite manifested clear elastic deformation even at low strain values. Because of such differences in mechanical curves, we calculated the Young's modulus for the CellH samples (3.44 ± 0.4) and the secant modulus for ChitH (2.1 ± 1.8) (Table 1). Due to shorter crosslinks among polymer chains, ChitH also reported lower values of compressive strength (CS = 4.9 ± 0.4 kPa) and ultimate compression (UC = 0.4 ± 0.1) when compared to CellH (CS = 12.3 ± 2.9 kPa; UC = 0.6 ± 0.3). As a matter of fact, genipin is quite a short molecule and made the crosslinked network of the ChitH material extremely brittle.48 In contrast, the longer bridges holding together the cellulose polymer chains allowed the CellH material to experience sustained plastic deformation after the yield point.49 When matrices were filled with Bti and yeasts, mechanical properties did not change significantly (Fig. S2, ESI†).| Modulus (N m−2) | Compressive strength (kPa) | Ultimate compression (mm) | |
|---|---|---|---|
| CellH | 3.4 ± 0.4 | 12.3 ± 2.9 | 0.6 ± 0.3 |
| ChitH | 2.1 ± 1.8 | 4.9 ± 0.4 | 0.4 ± 0.1 |
Hydrogels’ biological activity
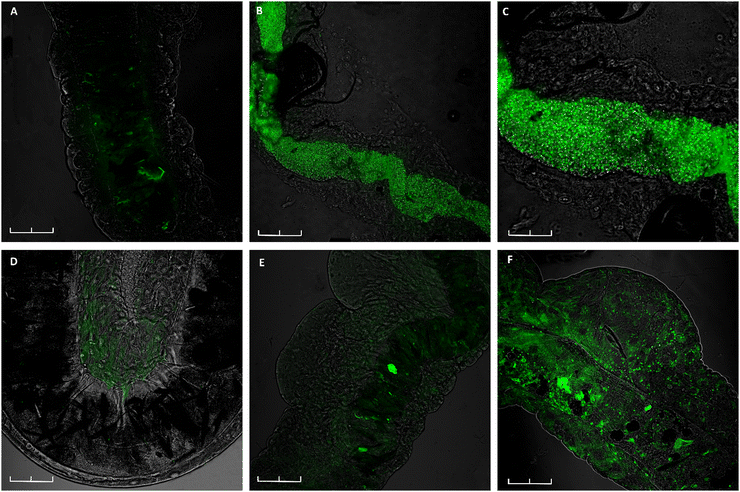
To address whether ChitH and CellH could represent an attractive feeding source for Ae. albopictus 3rd instar larvae, matrices only filled with fluorescent S. cerevisiae cells (yeast-GFP) were placed in water. Confocal microscopy images illustrate a clear green signal along the entire gastro-intestinal tract of the larvae when exposed to ChitH@Y already after 24 h (Fig. 5B and C). In contrast, from Fig. 5E and F it is visible that the intestine of larvae fed with CellH@Y did not present many cells inside the digestive tract within the same period of time and that a weak fluorescence signal could be observed only after 72 h of treatment.Chitosan-based hydrogels (ChitH@Bti-Y) proved also to be extremely efficient in killing Ae. albopictus larvae; in particular, the results obtained after mortality bioassays showed that, in the presence of these tablets, all the exposed larvae died after 12 h. In contrast, when the larvae were exposed to the CellH@Bti-Y hydrogels, they were still alive even after 72 h. Considering that Bti has an immediate effect once ingested, such a late mortality might suggest that larvae died upon starvation rather than the direct activity of the biolarvicide.
In order to elucidate the mechanisms of action of the hydrogel formulations, and in particular to what extent Bti and yeasts were retained/released from the matrices, transmittance analysis over a period of 15 days was performed. Hydrogel tablets filled with both Bti and yeasts were left to fully swell in a 50 mM NaCl solution and matrix optical transmittance (T%) was monitored over time. Such ionic strength was chosen because the hydrogel swelling ratio in larvae culture medium (i.e. mineral water) was similar to that registered in the 50 mM NaCl solution (Fig. S3, ESI†). A clear change in optical properties in the presence of active ingredients was particularly evident with cellulose-based hydrogels, since the empty matrix was completely transparent (T% = 100), while in the presence of Bti and yeasts, a significant quantity of light was absorbed and/or scattered by the system (Fig. 6A). T% remained quite stable over the period tested with a small decrease of the transmittance value in the range of 550–800 nm (Fig. 6B), suggesting that only a small portion of active ingredients were released from the matrix. On the other hand, the transmittance spectrum of empty chitosan-based hydrogels already showed significant optical absorbance, in particular below 700 nm, which is why a narrow wavelength range (700–800 nm) was considered for comparative analysis. Despite all, a difference between empty matrices and those with Bti and yeasts was still evident, since at time zero (t = 0) the T% function was shifted downwards (Fig. 6C). Most importantly, even for this formulation, T% remained quite unvaried after 15 days of immersion in the salty solution (Fig. 6D).
We hypothesized that active ingredient retention within the hydrogel matrices (Fig. S4, ESI†) can be influenced by two phenomena: electrostatic interactions and size exclusion. The Bti active principle consists of the sporulation products of B. thuringiensis which contains two types of toxins, crystal (Cry) and cytolytic (Cyt). These toxins are crystal proteins with a similar C-terminal hydrophilic region rich in basic and acidic amino acids such as lysine (K), arginine (R), histidine (H), aspartic acid (D) and glutamic acid (E).50 Therefore positively (R, K, and H) and negatively (E and D) charged amino acids can electrostatically interact with the anionic CellH network (–COO−) or with the cationic ChitH network (–NH3+) respectively, thus preventing Bti crystal diffusion in the outer solution (Fig. 6E). For what concern yeast cells instead, we believe that their diameter (∅ ≈ 30 μm) was way too large compared to the theoretical mesh size (ξ) of hydrogels (ξ ≈ 1–100 nm), giving a possible explanation of why they could not be released by diffusion.51,52
All together, these results point in the direction that active ingredients were mainly administered to larvae upon hydrogel matrix erosion and/or natural degradation and not because of diffusion through the meshes of crosslinked polymeric chains. The substantial superiority of the ChitH biological effect in terms of bating and killing the target species is therefore a reflection of its brittle nature and lower stability in aqueous environments. As a matter of fact, the immediate availability of yeast cells and Bti crystals contained in the chitosan-based matrix suggested that this material can be easily eroded by the filtering buccal apparatus of the animal right after immersion in the culture solution, enabling delivery of many yeast cells and of a lethal amount of Bti. In contrast, the marked rubber-like mechanics of the cellulose-based matrix most probably hindered its erosion, with the consequence of just a small number of yeast cells detected in the larvae intestinal tract as well as the complete absence of Bti residual activity.
Besides the larvae's inability to erode a substance with such a gelatinous consistency, attractiveness was further reduced due to CellH's transparent appearance. During biological assays it was observed that larvae preferred the blue-coloured ChitH and remained in its proximity for a longer time. Dark coloured objects can be indeed more easily confused with organic feeding sources as well as hiding places from predators, making a chitosan-based tablet a perfect bait for larvae.33
4. Conclusions
In this article, we reported a novel strategy for encapsulating microorganisms and biolarvicides in biocompatible floating materials acting as a shield against environmental agents and smart delivery systems. To validate our system, mosquito larvae of As. albopictus were proven to be drawn towards hydrogel matrices made of polysaccharides embedded with a commercially available Bti product and S. cerevisiae cells. The ChitH formulation proved to have more efficient phagostimulant activity compared to the CellH one and also to efficiently cause larvae mortality within just a few hours. These results are ascribable to the intrinsic brittleness of the crosslinked chitosan matrix in aqueous environments that made the material easily crumble by the larva brushes during feeding. Physico-chemical analysis suggested indeed that active ingredients (Bti and yeasts) were almost fully retained within the hydrogel network and could be delivered only by matrix erosion and/or degradation. We believe that such features, along with the natural composition of ChitH matrices that ensure environmental biocompatibility and nontoxicity, make the proposed engineered smart delivery hydrogel an appealing option for many applications. This might be considered as a proof-of-concept product to be possibly conjugated with other biolarvicides and/or microorganisms effective on different insect species that represent a problem for agriculture or public health.Author contributions
Conceptualization: C. Bandi, S. Caccia, S. Epis, C. Lenardi. Investigation and validation: E. Brambilla, L. Giussani, S. Locarno, A. Negri, M. Piazzoni, S. Pitton. Data curation: S. Locarno, M. Piazzoni. Writing – original draft: M. Piazzoni. Writing – review & editing: C. Bandi, S. Locarno, A. Negri, C. Lenardi, M. Piazzoni.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Italian Ministry of Education, University and Research, PRIN 2017 (2017J8JR57) to CB and SC. We would like to thank David Dallasega (Politecnico di Milano) and the UNITECH NOLIMITS platform (University of Milan), for the technical support in obtaining SEM images of the matrices and confocal microscopy images of larvae, respectively. The authors thank Prof. F. Lazzaro for the Saccharomyces cerevisiae strain.References
- “The 24 deadliest animals on Earth, ranked”, CNET, J. Learish, 2016, https://www.cnet.com/pictures/the-24-deadliest-animals-on-earth-ranked/, (accessed on March 2022).
- F. J. Colón-González, M. O. Sewe, A. M. Tompkins, H. Sjödin, G. A. Casallas, J. Rocklöv, C. Caminade and R. Lowe, Lancet Planet. Health, 2021, 5, 404–414 CrossRef.
- A. Negri, I. Arnoldi, M. Brilli, C. Bandi, P. Gabrieli and S. Epis, Parasites Vectors, 2021, 14, 534 CrossRef PubMed.
- “Vector-borne diseases”, WHO, 2020, https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases, (accessed March 2022).
- L. S. Tusting, J. Thwing, D. Sinclair, U. Fillinger, J. Gimnig, K. E. Bonner, C. Bottomley and S. W. Lindsay, The Cochrane Library, Wiley, United States, 2013 Search PubMed.
- M. Govindarajan and M. Rajeswary, J. Coastal Life Med., 2014, 2, 222–224 Search PubMed.
- M. A. Dar, G. Kaushik and J. F. Villarreal-Chiu, J. Environ. Manage., 2019, 239, 124–136 CrossRef CAS PubMed.
- S. A. Mapua, M. F. Finda, I. H. Nambunga, B. J. Msugupakulya, K. Ukio, P. P. Chaki, F. Tripet, A. H. Kelly, N. Christofides, J. Lezaun and F. O. Okumu, Malar. J., 2021, 20, 123 CrossRef.
- G. Benelli, C. L. Jeffries and T. Walker, Insects, 2016, 7, 52 CrossRef PubMed.
- E. J. Scholte, B. G. Knols, R. A. Samson and W. Takken, J. Insect Sci., 2004, 4, 19 CrossRef PubMed.
- R. Maheswaran and S. Ignacimuthu, A novel herbal formulation against dengue vector mosquitoes Aedes aegypti and Aedes albopictus, Parasitol. Res., 2012, 110, 1801–1813 CrossRef PubMed.
- P. K. Mittal, J. Vector Borne Dis., 2003, 40, 20–32 CAS.
- L. A. Lacey, J. Am. Mosq. Control Assoc., 2007, 23, 133–163 CrossRef CAS PubMed.
- B. M. Lee and G. I. Scott, Bull. Environ. Contam. Toxicol., 1989, 43(6), 827–832 CrossRef CAS PubMed.
- R. W. Merritt, E. D. Walker, M. A. Wilzbach, K. W. Cummins and W. T. Morgan, J. Am. Mosq. Control Assoc., 1989, 5, 397–415 CAS.
- L. A. Lacey and M. S. Mulla, Safety of Bacillus thuringiensis (H-14) and Bacillus sphaericus to non-target organisms in the aquatic environment, Safety of Microbial Insecticides, CRC Press, Boca Raton, 1990, pp. 169–188 Search PubMed.
- N. Becker, M. Zgomba, M. Ludwig, D. Petric and F. Rettich, J. Am. Mosq. Control Assoc., 1992, 8, 285–289 CAS.
- O. Aguilar-Meza, M. Ramírez-Suero, J. S. Bernal and M. Ramírez-Lepe, J. Econ. Entomol., 2010, 103, 570–576 CrossRef CAS.
- M. E. I. Badawy, N. E. M. Taktak, O. M. Awad, S. A. Elfiki and N. E. Abou El-Ela, Int. J. Mosq. Res., 2015, 45, 45–55 Search PubMed.
- W. E. Rudzinski, A. M. Dave, U. H. Vaishnav, S. G. Kumbar, A. R. Kulkarni and T. M. Aminabhavi, Des. Monomers Polym., 2002, 5, 39–65 CrossRef CAS.
- N. A. Peppas and A. R. Khare, Adv. Drug Delivery Rev., 1993, 11, 1–35 CrossRef CAS.
- N. A. Peppas, P. Bures, W. Leobandung and H. Ichikawa, Eur. J. Pharm. Biopharm., 2000, 6411, 27–46 CrossRef.
- Y. Samchenko, Z. Ulberg and O. Korotych, Adv. Colloid Interface Sci., 2011, 168, 247–262 CrossRef CAS PubMed.
- F. He, Q. Zhou, L. Wang, G. Yu, J. Li and Y. Feng, Appl. Clay Sci., 2019, 183, 105347 CrossRef CAS.
- A. Singh, et al. , Chem. Eng. J., 2022, 427, 131215 CrossRef CAS.
- N. A. Peppas, J. Z. Hilt, A. Khademhosseini and R. Langer, Adv. Mater., 2006, 18, 1345–1360 CrossRef CAS.
- G. R. Borges, M. G. Aboelkheir, F. G. de Souza Jr., K. C. Waldhelm and R. M. Kuster, Environ. Sci. Pollut. Res., 2020, 27, 23575–23585 CrossRef CAS.
- T. Liu, Y. Xie, F. Lin, L. Xie, W. Yang, X. Su, C. Ou, L. Luo, Q. Xiao, L. Gand and X. Chena, Pest Manage. Sci., 2021, 77, 741–748 CrossRef CAS.
- B. Huang, F. Chen, Y. Shen, K. Qian, Y. Wang, C. Sun, X. Zhao, B. Cui, F. Gao, Z. Zeng and H. Cui, Nanomaterials, 2018, 8, 102 CrossRef.
- D. Pimentel, J. Agric. Environ. Ethics, 1995, 8, 17–29 CrossRef.
- J. W. Tay, D. H. Choe, A. Mulchandani and M. K. Rust, J. Econ. Entomol., 2020, 113, 2061–2068 CrossRef CAS PubMed.
- N. Meriggi, M. Di Paola, D. Cavalieri and I. Stefanini, Front. Microbiol., 2020, 11, 1–8 CrossRef.
- D. Roberts, Bull. Entomol. Res., 2017, 107, 499–505 CrossRef CAS PubMed.
- B. K. Tyagi, P. Basu and S. Bhattacharya, Genetically modified and other innovative vector control technologies, Springer, Germany, 2021 Search PubMed.
- M. Anand, P. Sathyapriya, M. Maruthupandy and A. Hameedha Beevi, Front. Lab. Med., 2018, 2, 72–78 CrossRef.
- N. Taktak, M. Badawy, O. Awad, S. Elfiki and N. Abou El-Ela, Res. Rep. Trop. Med., 2016, 7, 23–38 Search PubMed.
- Z. Yang, H. Peng, W. Wang and T. Liu, J. Appl. Polym. Sci., 2010, 116, 2658–2667 CAS.
- J. Shin, S. M. Seo, I. K. Park and J. Hyun, Carbohydr. Polym., 2021, 254, 117381 CrossRef CAS.
- S. Nasser, M. P. M. da Costa, I. L. de Mellow Ferreira and J. B. P. Lima, Carbohydr. Polym. Technol. Appl., 2021, 2, 100125 CAS.
- A. Sannino, M. Madaghiele, M. G. Lionetto, T. Schettino and A. Maffezzoli, J. Appl. Polym. Sci., 2006, 102, 1524–1530 CrossRef CAS.
- R. Bellini, M. Calvitti, A. Medici, M. Carrieri, G. Celli and S. Maini, Area-Wide Control of Insect Pests, From Research to Field Implementation, ed. M. J. B. Vreysen, A. S. Robinson and J. Hendrichs, Springer, Germany, 2007, pp. 505–515 Search PubMed.
- M. F. Butler, Y. Ng and P. D. A. Pudney, J. Polym. Sci., Part A-1: Polym. Chem., 2003, 41, 3941–3953 CrossRef CAS.
- S. Dimida, C. Demitri, V. M. De Benedictis, F. Scalera, F. Gervaso and A. Sannino, J. Appl. Polym. Sci., 2015, 132, 1–8 CrossRef.
- R. A. A. Muzzarelli, Carbohydr. Polym., 2009, 77, 1–9 CrossRef CAS.
- N. L. Berry, E. P. Overholt, T. J. Fisher and C. E. Williamson, PLoS One, 2020, 15(12), e0244832 CrossRef PubMed.
- M. Piazzoni, E. Piccoli, L. Migliorini, E. Milana, F. Iberite, L. Vannozzi, L. Ricotti, I. Gerges, P. Milani, C. Marano, C. Lenardi and T. Santaniello, Soft Rob., 2021, 9, 224–232 CrossRef PubMed.
- S. Andrieux, A. Quell, C. Stubenrauch and W. Drenckhan, Adv. Colloid Interface Sci., 2018, 256, 276–290 CrossRef CAS PubMed.
- Y. W. Mak and W. W. F. Leung, J. Mater. Sci., 2019, 54, 10941–10962 CrossRef CAS.
- W. D. Callister and D. G. Rethwisch, Fundamentals of materials science and engineering: an integrated approach, John Wiley & Sons, United States, 2016 Search PubMed.
- H. Hwang and S. B. Gelvin, Plant Cell, 2004, 16, 3148–3167 CrossRef CAS PubMed.
- X. Zou, X. Zhao and L. Ye, Chem. Eng. J., 2015, 273, 92–100 CrossRef CAS.
- M. K. Matthew, S. Rehmann, K. M. Skeens, P. M. Kharkar, E. M. Ford, E. Maverakis and K. H. Lee, Physiol. Behav., 2011, 176, 139–148 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2sm00889k |
| ‡ Current address: Department of Biomedical, Surgical and Dental Sciences, University of Milan, Milan, Italy. |
| This journal is © The Royal Society of Chemistry 2022 |