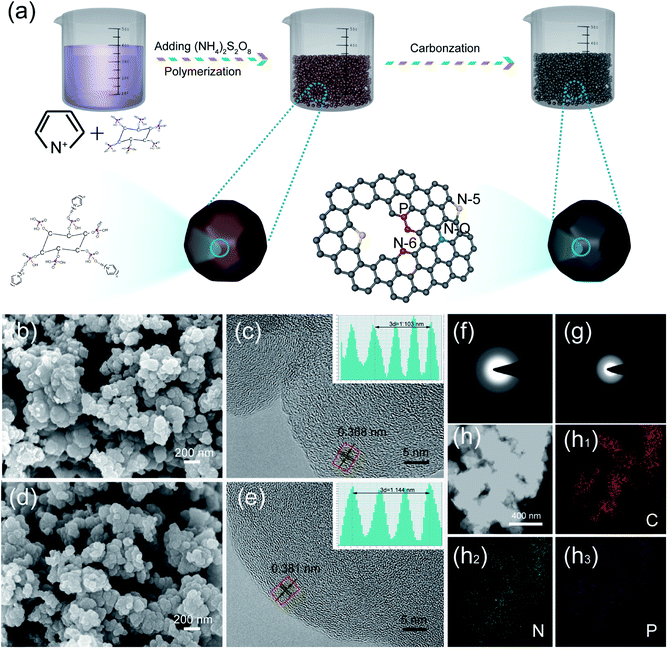
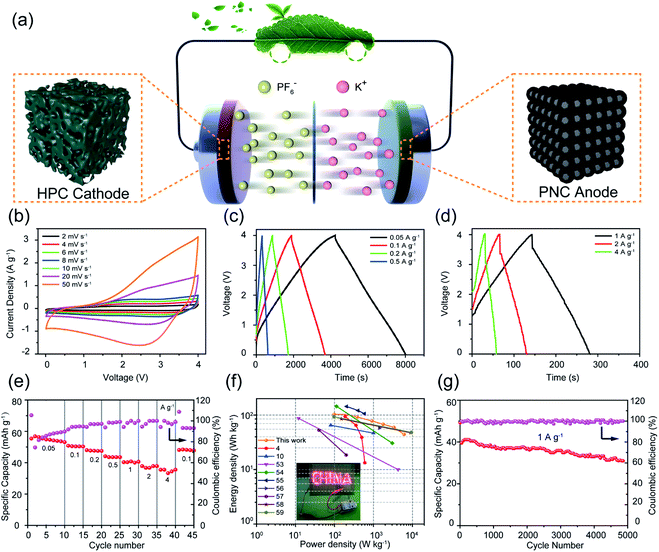
Rich-phosphorus/nitrogen co-doped carbon for boosting the kinetics of potassium-ion hybrid capacitors†
Zhenyu
Xie
a,
Jiannian
Xia
ab,
Daping
Qiu
a,
Jinying
Wei
ab,
Min
Li
a,
Feng
Wang
 *ac and
Ru
Yang
*ac and
Ru
Yang
 *ab
*ab
aState Key Laboratory of Chemical Resource Engineering, Beijing Key Laboratory of Electrochemical Process and Technology for Materials, Beijing University of Chemical Technology, Beijing 100029, P. R. China. E-mail: wangf@mail.buct.edu.cn; ruyang@mail.buct.edu.cn
bChangzhou Institute of Advanced Materials, Beijing University of Chemical Technology, Changzhou, Jiangsu 213000, P. R. China
cBeijing Advanced Innovation Center for Soft Matter Science and Engineering, Beijing University of Chemical Technology, Beijing 100029, China
First published on 13th November 2021
Abstract
Potassium-ion hybrid capacitors (PIHCs) meeting the high power density of capacitors and high energy density of batteries are seen as one of the most promising energy storage devices. However, this wonderful scene is undermined by the sluggish kinetics in the anode of PIHCs. Herein, a phosphorus and nitrogen co-doped carbon material (PNC) with a wide layer spacing (0.381 nm) and high content of phosphorus (1.27 at%) and nitrogen (6.37 at%) was synthesized with non-toxic pyrrole and phytic acid as the precursors by polymerization and carbonization. The wide layer spacing and rich heteroatoms served to accelerate the diffusion rate of potassium ions and increased the adsorption capacity of potassium ions. Benefiting from these characteristics, PNC delivered a remarkable specific capacity of 310 mA h g−1 at 0.025 A g−1, and a splendid rate capability of 149 mA h g−1 at 5 A g−1. As expected, the dual-carbon PIHC consisting of a PNC anode and a hierarchical porous carbon cathode delivered excellent energy density (103 W h kg−1), extraordinary power density (6106.2 W kg−1), and superior cycle lifespan. Our method can effectively solve the problems of the slow kinetics and low capacity of PIHCs, and provide a reference for further stimulation of their potential.
1. Introduction
Potassium-ion hybrid capacitors (PIHCs) with high power and energy have become a potential alternative to lithium-ion hybrid capacitors, due to the rich natural reserves of potassium and its low electrode potential similar to that of lithium.1,2 As one of the most important components of PIHCs, anode electrode materials are the key materials for high-performance PIHCs.3,4 Currently, various anode materials of PIHCs have been extensively studied and reported, such as alloyed materials,5 transition metal chalcogenides,6–9 phosphides,10 and carbon materials.11,12 Among these, carbon materials with low cost and high conductivity are one of a few anode electrode materials that can simultaneously maintain a high capacity, good rate performance, and durability.13,14 However, carbon materials are not perfect, because the large-sized potassium ions usually face a kinetic lag, leading to a violent volume expansion of carbon materials during the insertion process.15,16 Therefore, there is an urgent need to adopt certain strategies to accelerate the reaction kinetics and reduce the volume expansion.Kinetic hysteresis and volume expansion are two important factors that affect the rate performance, capacity, and life of carbon anodes.17,18 Considering their practical applications, an economical and effective strategy is to increase the active sites of potassium-ion adsorption, and widen the interlayer spacing of carbon materials through heteroatom doping.19,20 Simultaneously, heteroatom doping can also adjust the electronic structure of carbon materials and their surface properties, thereby enhancing their electrochemical properties further.21 Among the abundance of heteroatoms, nitrogen atoms can easily replace carbon atoms and enter the carbon skeleton to form nitrogen with different configurations (pyridine-N, pyrrole-N, and graphitized-N),3,22 because the size of nitrogen atoms is similar to that of carbon atoms. Studies have shown that pyridine-N and pyrrole-N are beneficial for the adsorption of potassium ions, and graphitized-N is helpful for the conductivity of carbon materials.23 Therefore, a large number of nitrogen-doped carbon materials have received extensive attention, such as nitrogen-doped hollow carbon spheres,19 nitrogen-doped porous carbon,24 and nitrogen-doped carbon nanosheets.25 Phosphorus atoms, which lie in the same main group as nitrogen atoms, are also commonly used to improve the properties of carbon atoms.26 Moreover, compared with nitrogen atoms, their larger atomic radius makes it easy for them to contribute electrons. Therefore, phosphorus-doped carbon materials generally exhibit better electron-donating ability, superior electron delocalization, and a higher defect center density than nitrogen-doped carbon materials.27,28 The insertion of phosphorus atoms, meanwhile, into the carbon lattice also brings about a larger covalent radius, which can significantly increase the distance between carbon layers.27,28 Although nitrogen and phosphorus doping offer many advantages, it is difficult to obtain a carbon material with high phosphorus and nitrogen content, due to the large differences in the sizes of phosphorus atoms and carbon or nitrogen atoms.
Herein, a P/N co-doped carbon material (PNC) with a wide layer spacing (0.381 nm) and abundant active sites was successfully prepared by a facile polymerization and carbonization. As a kind of PHIC anode, PNC delivered a high specific capacity, good rate performance (149 mA h g−1 even at 5 A g−1), and superior cycle performance. It was also found that P/N co-doping could not only expand the interlayer spacing of single N-doped carbon materials further, but also increase the proportion of active nitrogen in the total nitrogen content. The kinetic analysis showed that K+ energy storage in PNC was mainly dominated by the capacitive controlled process, reflecting the excellent reaction kinetics of PNC. Moreover, hierarchical porous carbon (HPC) with a narrow pore-size distribution and abundant nitrogen–oxygen functional groups was prepared with the same precursor as PNC by low-temperature carbonization and high-temperature activation. The HPC cathode exhibited excellent storage capacity. As expected, a dual-carbon PIHC consisting of the PNC anode and the HPC cathode delivered an excellent energy density (103 W h kg−1), awesome density (6106.2 W kg−1), and superior cycle life.
2. Experimental
2.1 Materials
Pyrrole (Py, 99%) was purchased from Ke Jing Star technology company limited. Ammonium persulfate ((NH4)2S2O8, ≥99.99%), phytic acid (PA, 60%), and potassium hydroxide (KOH, ≥99.999%) were purchased from Bei Jing Tong Guang Fine Chemicals company.2.2 Materials preparation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and dispersed evenly by ultrasonication for 10 min. Then, ammonium persulfate aqueous solution was quickly added into the above solution with vigorous stirring and a large number of black products could be observed after half a minute. The solution was continuously stirred for about 5 min and then polymerized for another 6 h at room temperature. Next, the as-precursors were washed with deionized water to remove the ammonium persulfate and then dried at 70 °C overnight. Finally, the precursors were finely ground and put into a tubular furnace for pyrolysis at 800 °C for 2 h under an argon atmosphere. The as-obtained samples were denoted as PNC. When only Py was included in the precursor, the associated products were named NC.
1 and dispersed evenly by ultrasonication for 10 min. Then, ammonium persulfate aqueous solution was quickly added into the above solution with vigorous stirring and a large number of black products could be observed after half a minute. The solution was continuously stirred for about 5 min and then polymerized for another 6 h at room temperature. Next, the as-precursors were washed with deionized water to remove the ammonium persulfate and then dried at 70 °C overnight. Finally, the precursors were finely ground and put into a tubular furnace for pyrolysis at 800 °C for 2 h under an argon atmosphere. The as-obtained samples were denoted as PNC. When only Py was included in the precursor, the associated products were named NC.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and then activated for 2 h at 900 °C under an argon atmosphere. After cooling to room temperature, the samples were washed with dilute hydrochloric acid and deionized water several times, and finally dried at 80 °C overnight.
4 and then activated for 2 h at 900 °C under an argon atmosphere. After cooling to room temperature, the samples were washed with dilute hydrochloric acid and deionized water several times, and finally dried at 80 °C overnight.
2.3 Material characterization
The morphology and microstructure of all the samples were measured by SEM (TESCAN), TEM (JEOL JSM-2100), XRD (Rigaku D/Max-2500VB), and Raman spectroscopy (Renishaw InVia Reflex, the wavelength of 532 nm). The surface elemental analysis was performed by XPS (Thermo Fisher Scientific ESCALAB250). The specific surface area and pore diameter were calculated by nitrogen adsorption–desorption measured by a Micrometrics ASAP 2020 system.2.4 Electrochemical evaluations
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DEC (1
DEC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) as the electrolyte in the cells (CR-2032). The PIHC device was composed of a PNC anode and an HPC cathode with mass ratios of 1
1, v/v) as the electrolyte in the cells (CR-2032). The PIHC device was composed of a PNC anode and an HPC cathode with mass ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 using the same electrolyte and separator like half-cells. All the cells were assembled in a glove box in an argon atmosphere.
3 using the same electrolyte and separator like half-cells. All the cells were assembled in a glove box in an argon atmosphere.
Galvanostatic charge/discharge (GCD) and the galvanostatic intermittent titration technique (GITT) were carried out on a battery testing system (LAND CT2001A, Wuhan). GITT was measured at a current density of 100 mA g−1 pulses for 30 min, followed by a relaxation time of 2 h. Cyclic voltammetry (CV) measurements were made by a CHI 760E workstation. The potential ranges of the anode half-cells, cathode half-cells, and PIHCs were 0.01–2.5 V (vs. K+/K), 2.0–4.0 V (vs. K+/K), and 0.01–4.0 V, respectively. The computational formulas of the energy density and power density of the PIHCs are as follows:29
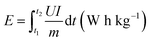 | (1) |
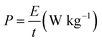 | (2) |
3. Results and discussion
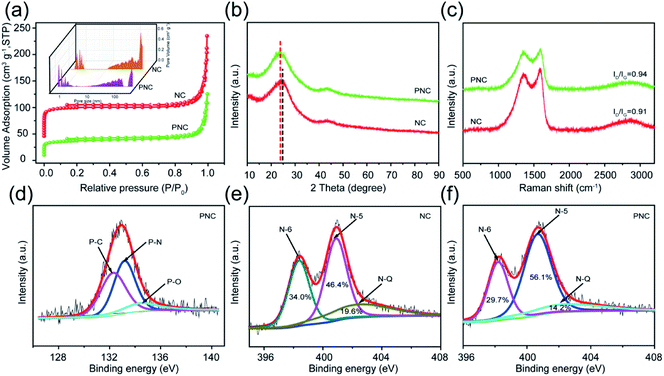
Phosphorus and nitrogen co-doped carbon materials (PNCs) were prepared with pyrrole (Py) and phytic acid (PA) as the precursors by polymerization and carbonization at 800 °C. The typical synthesis process is shown in Fig. 1a. For comparison, nitrogen-doped carbon materials (NC) were prepared by the precursor Py with the same synthesis procedure of PNC. The morphology and structure of the as-prepared samples were observed by SEM and HRTEM. It can be seen from Fig. 1b and d that the morphology of NC and PNC was almost the same, both of which showed irregular submicron particles. The disorder distribution of the lattice streaks in Fig. 1c and e indicated that each sample had a typical amorphous structure, while their corresponding hazy diffraction rings in the selected area electron diffraction (SEAD) images (Fig. 1f and g) also supported this. Although there were many apparent similarities between the two samples, their detailed microstructures showed obvious differences. As shown in Fig. 1c, a large number of short-domain graphitic layers for NC could be observed, and the interlayer spacing revealed in the inset image was 0.368 nm; while PNC displayed a wider interlayer spacing (0.381 nm), which is beneficial to the kinetics of the intercalation/deintercalation of potassium ions,29,30 than that of NC in the inset images of Fig. 1e. As shown in Fig. 1h1–h3, EDS elemental mapping was applied to the characterized elements in PNC. Obviously, the elements of C, N, and P were homogeneously distributed in PNC, which proved that P and N had been successfully doped in the samples.The pore parameters, which are the important microstructural properties, of NC and PNC were measured by nitrogen adsorption–desorption. As shown in Fig. 2a, the two samples both showed typical II and IV isotherms, indicating the existence of micropores, mesopores, and macropores.31,32 The pore structure parameters calculated from the corresponding adsorption–desorption isotherms are listed in Table S1.† It can be seen from the table that, compared with NC, the BET specific surface area of PNC was reduced from 301 m2 g−1 for NC to 114 m2 g−1; meanwhile, the pore volume calculated by the density functional theory also decreased from 0.26 cm3 g−1 for NC to 0.17 cm3 g−1. Although the addition of PA reduced the pore volume of PNC to some extent, the mesopores used to transport and store electrolyte ions were barely decreased. As shown in the pore-size distribution (inset graph in Fig. 2a), the pores of NC and PNC were both wedge-shaped pores composed of micropores, mesopores, and macropores. The crystallization characteristics and defect degree of NC and PNC were clarified by the XRD patterns and Raman spectra. As displayed in Fig. 2b, the XRD patterns of both samples showed broad (002) diffraction peaks and weak characteristic (100) peaks, indicating a disordered amorphous nature.33 Moreover, the (002) diffraction peak of PNC was shifted to lower angles than that of NC, suggesting PNC possessed a broader carbon layer spacing and more defects.34 The Raman spectra of the as-synthesized samples shown in Fig. 2c reveal the D band peak (≈1350 cm−1) belonging to the sp3 defect carbon and the G band peak (≈1560 cm−1), reflecting the crystallinity of sp2 carbon.35 Their intensity ratio (ID/IG) is considered to be the significant indicator for judging the defects of carbonaceous materials.36,37 The value of ID/IG of PNC (0.94) was higher than that of NC (0.91), which indicated richer defects, which is conducive to the exposure of more reaction sites.
The addition of PA not only introduced more defects but also inevitably caused changes in the composition and chemical state of the elements of the carbon materials. As shown in Fig. S1,† the XPS spectrum showed that the three elements of O, N, and C were all present in NC and PNC. In addition, P element at 1.27 at% could be detected in PNC (Fig. S1 and Table S2†). Notably, the P 2p high-resolution spectrum of PNC was composed of P–C (132.3 eV), P–N (133.2 eV), and P–O (134.5 eV) in Fig. 2d, suggesting that the phosphorus element had been successfully doped into the structure of the carbon materials. As reported, P–N and P–O are able to contribute to the pseudocapacitance of carbon materials, while P–C can offer additional capacity through the battery behavior.38 The N1s high-resolution spectra of PNC and NC (Fig. 2e and f) both contained three peaks: pyridinic-N (N–6, 398.2 eV), pyrrolic-N (N–5, 400.7 eV), and graphitic-N (N–Q, 402.2 eV).39,40 Interestingly, the total nitrogen content decreased to a certain extent after doping phosphorus, but the total percent of N–5 and N–6 increased from 80.4% of NC to 85.8% of PNC (Table S2†). On the contrary, the percent of N–Q in PNC lessened. The reason for this phenomenon is that P–C and P–N bonds were formed by replacing nitrogen or carbon atoms in N–6 and N–Q with phosphorus atoms.41 Moreover, the presence of some N/P functional groups and C–O (Fig. 2, S2, and Table S2†) is beneficial to the wettability of carbon materials.42,43 In short, PNC exhibited a wider interlayer spacing, higher defect degree, and higher active nitrogen content than NC. Simultaneously, it possessed adequate mesopores between 2 nm and 12 nm and additional phosphorus content.
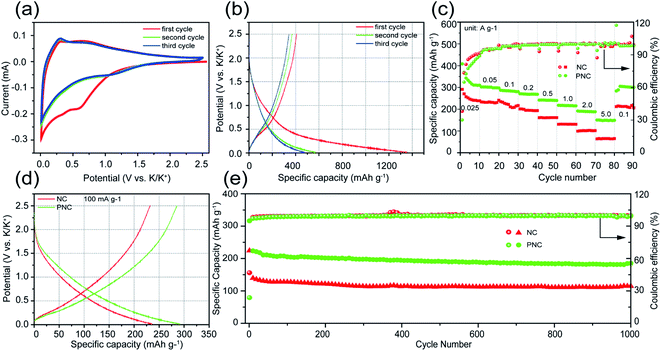
The electrochemical performance for potassium-ion storage of PNC and NC was evaluated in a potassium-ion half-cell. The CV curves of the first three cycles at a scan rate of 0.2 mV s−1 are shown in Fig. 3a. During the initial negative scan, the CV curve showed obvious broad valleys, which could be attributed to the reaction of potassium ions with surface functional groups, formation of a solid electrolyte interface (SEI), decomposition of the electrolyte, and the intercalation reaction of potassium ions, between 0 and 1.25 V.44,45 After the initial cycle, the almost coincident CV curves of the second and third cycles mean that the intercalation/deintercalation and adsorption/desorption processes of potassium ions had gradually stabilized, thus showing the excellent reversible performance of PNC. As displayed in Fig. 3b, the first three GCD curves of PNC were tested at a current density of 25 mA g−1. The first discharge capacity was higher than the other two, indicating that some irreversible side reactions occurred, which is consistent with the result from the first CV cycle, such as the formation of an SEI. In addition, the other two discharge curves hardly showed a discharge plateau, especially, below 0.1 V. This could effectively avoid the generation of dendrites.46 In Fig. 3c, the rate performanced of PNC and NC are compared in detail under the potential window between 0.01 and 2.5 V. PNC delivered excellent reversible specific capacities of 310, 297, 284, 268, 240, 215, 188, and 149 mA h g−1 when the current density was 0.025, 0.05, 0.1, 0.2, 0.5, 1, 2, and 5 A g−1, respectively. Simultaneously, there was a special phenomenon found where PNC with a low specific surface area exhibited a low initial coulombic efficiency (30.1% vs. 37.02% of NC), which could be attributed to PNC possessing more ultra-micropores with a pore diameter less than 0.8 nm (Table S1†) and richer heteroatoms, which could trigger more side reactions, than those of NC.47,48 Moreover, it was noteworthy that NC also revealed a good rate performance, but its specific capacity was lower than that of PNC. As reported, the potassium-ion storage process of heteroatom-doped carbon materials can be divided into the potassium-ion insertion process below 0.5 V and the adsorption process of potassium ions by the heteroatom functional groups and defect sites above 0.5 V.3,49Fig. 3d shows the GCD curves at the current density of 100 mA g−1. It can be seen that PNC (144.6 mA h g−1) delivered a higher potassium-ion adsorption capacity than that of NC (111.5 mA h g−1) above 0.5 V, which could be attributed to the additional pseudocapacitance from the functional group containing P. Similarly, PNC (141.1 mA h g−1) also possessed a higher potassium-ion intercalation capacity than that of NC (116.8 mA h g−1) below 0.5 V, due to the wider carbon layer spacing and more active sites in PNC. Cycle stability is one of the most important indicators to evaluate the performance of electrode materials. As shown in Fig. S3,† PNC exhibited a high reversible specific capacity (235.7 mA h g−1) and outstanding capacity retention (93.9%) at a current density of 0.2 A g−1 after 600 cycles. Notably, PNC could still output a specific capacity of 181 mA h g−1 at 1 A g−1 after 1000 cycles, which was better than that of NC (Fig. 3e), showing its outstanding stability. The above electrochemical measurement results (Fig. 3 and S3†) show that PNC possessed a better rate capability and lifespan than NC. Furthermore, compared with the reported carbon materials (Table S3†), PNC also presented excellent potassium-ion storage performance.
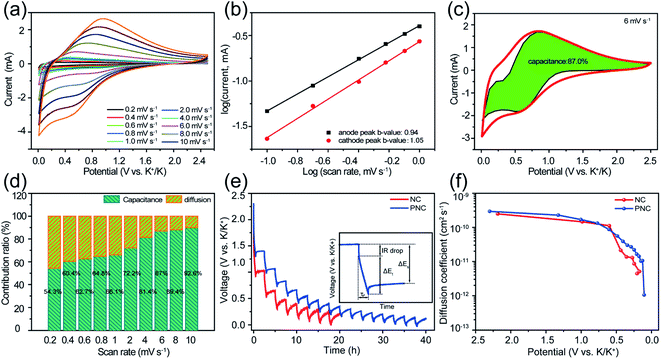
In order to in-depth dissect the potassium-ion storage mechanism of PNC, CV tests were performed at different scan rates in the potential window from 0.01 V to 2.5 V. With the increase in scan rate, the shape of the CV curves (Fig. 4a) maintained good similarity, suggesting an excellent rate performance and reversibility. The potassium-ion storage kinetics of PNC was evaluated through the functional relationship between the current density of the anode/cathode peak and the scan rate (eqn (S1)†). The log(v)–log(i) curves of PNC are presented in Fig. 4b, in which the slope b values of the anodic and cathodic peaks are 0.94 and 1.05, respectively, indicating that the process of potassium-ion storage was dominated by the surface-controlled behavior.50 Based on eqn (S2),† it could be calculated that 87% of the total capacity of PNC was contributed by the capacitive behavior at 6 mV s−1 (Fig. 4c). With the change in scan rate from 0.2 to 10 mV s−1, the contribution ratio of the capacitive behavior increased from 54% to 92.5% (Fig. 4d), which could be attributed to the high active N content and abundant P content. The K+ diffusion coefficients (Dk) of PNC and NC were calculated based on the GITT test results. As shown in Fig. 4e and f, the Dk values of PNC and NC both decreased with the continuous reduction of the discharge voltage, because the process of K+ storage changed from surface behavior to diffusion behavior. Furthermore, it was obvious that PNC showed higher Dk values than NC, which means PNC possessed a faster diffusion rate. This was attributed to the P/N co-doping introducing ample pseudocapacitance and significantly widening the carbon layer spacing.
As a significant constituent part of the PIHC device, a good cathode is vital. The concrete information on the structure, composition, and electrochemistry of the HPC cathode is shown in the ESI.† As shown in Fig. S4a–d,† the submicron precursors became a rich porous bulk after activation. The nitrogen adsorption–desorption tests (Table S4†) also exhibited that the activated samples contained rich micropores and mesopores. It is worth mentioning that the BET specific surface area of HPC was 3208 m2 g−1. Meanwhile, it can be seen from Fig. S5 and Table S4† that about 68% of the total volume was contributed by the efficient pore (d ≥ 0.8 nm) volume, which is critical for double-layer capacitance behaviors.51,52 In addition to the rich porous properties, HPC exhibited a high disorder degree (ID/IG = 1.15) according to the Raman spectrum in Fig. S5b.† As shown in the XPS spectrum (Fig. S5c†), 1.1 at% N and 8.76 at% O were found, which indicated an enhanced pseudocapacitance in HPC. The CV curves in Fig. S6a† displayed a quasi-rectangular shape at various scan rates, implying excellent capacitive behaviors and reversibility. As presented in Fig. S6b,† HPC possessed the highest capacity of 97 mA h g−1 at 0.1 A g−1 and a good rate performance. Furthermore, the capacitance retention was 71% at 2 A g−1 after 1000 cycles.
To appraise the practical application value of PNC and HPC electrode, dual-carbon PIHC devices composed of a PNC anode and an HPC cathode according to the mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 or 1
2 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (Fig. 5a) were tested in a high operating voltage window of 0.01–4.0 V. It was found that the PIHC with the mass ratio of 1
3 (Fig. 5a) were tested in a high operating voltage window of 0.01–4.0 V. It was found that the PIHC with the mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 exhibited a longer discharge time at the same condition, as seen in Fig. S7,† suggesting a better performance. Therefore, the electrochemical performance of PIHC with the mass ratio of 1
2 exhibited a longer discharge time at the same condition, as seen in Fig. S7,† suggesting a better performance. Therefore, the electrochemical performance of PIHC with the mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 was evaluated in Fig. 5. The CV curves of PHIC at different scan rates are shown in Fig. 5b. It can be seen that the response current of the CV curves at the low voltage region was smaller than that at the high voltage region, due to the existence of intercalation/deintercalation at low voltage. With the increase in scan rate, the shape of the CV curves was consistent, indicating the excellent rate capability of PIHC. As shown in Fig. 5c and d, the GCD curves of PHIC showed linear isosceles triangles at different current densities, indicating that the energy storage of PIHC was contributed by capacitance behavior. Based on the above GCD plots, the reversible specific capacities were 53.1, 50.3, 47.5, 43.5, 40.6, 37.8, and 35.5 mA h g−1 at the current densities of 0.05, 0.1, 0.2, 0.5, 1, 2, and 4 A g−1, respectively, exhibiting excellent rate capability (Fig. 5e). The Ragone plot in Fig. 5f presents the energy density–power densities relationship. The output energy density of the PIHC was 103, 103, 92.2, 78, 67.8, 59.5, and 44.1 W h kg−1 at the corresponding power density of 97.9, 206.5, 380.2, 914.7, 1768.7, 3346.9, and 6106.2 W kg−1, respectively. The energy density and power density are extremely competitive, even compared to the PIHCs reported currently.4,10,53–59 More significantly, the PIHC presented an ultralong lifespan with 64% capacity retention after 5000 cycles at 1 A g−1, showing the value of a potential device.
2 was evaluated in Fig. 5. The CV curves of PHIC at different scan rates are shown in Fig. 5b. It can be seen that the response current of the CV curves at the low voltage region was smaller than that at the high voltage region, due to the existence of intercalation/deintercalation at low voltage. With the increase in scan rate, the shape of the CV curves was consistent, indicating the excellent rate capability of PIHC. As shown in Fig. 5c and d, the GCD curves of PHIC showed linear isosceles triangles at different current densities, indicating that the energy storage of PIHC was contributed by capacitance behavior. Based on the above GCD plots, the reversible specific capacities were 53.1, 50.3, 47.5, 43.5, 40.6, 37.8, and 35.5 mA h g−1 at the current densities of 0.05, 0.1, 0.2, 0.5, 1, 2, and 4 A g−1, respectively, exhibiting excellent rate capability (Fig. 5e). The Ragone plot in Fig. 5f presents the energy density–power densities relationship. The output energy density of the PIHC was 103, 103, 92.2, 78, 67.8, 59.5, and 44.1 W h kg−1 at the corresponding power density of 97.9, 206.5, 380.2, 914.7, 1768.7, 3346.9, and 6106.2 W kg−1, respectively. The energy density and power density are extremely competitive, even compared to the PIHCs reported currently.4,10,53–59 More significantly, the PIHC presented an ultralong lifespan with 64% capacity retention after 5000 cycles at 1 A g−1, showing the value of a potential device.
4. Conclusion
In summary, an anode material based on P/N co-doping and wide interlayer spacing (0.381 nm) carbon was successfully fabricated by the polymerization reaction of pyrrole and phytic acid, and by carbonization. Benefiting from the abundant content of phosphorus (1.27 at%) and nitrogen (6.37 at%), endowing the rich adsorption capacity of potassium ions, and benefiting from the wide interlayer spacing accelerating the diffusion rate of potassium ions, the PNC electrode delivered a maximum specific capacity of 310 mA h g−1 at 0.025 A g−1, and 149 mA h g−1 even at a high current density of 5 A g−1, indicating its awesome rate performance. Moreover, kinetic analysis revealed that the mechanism of K+ storage in PNC was dominated by a capacitive controlled process. Encouragingly, a PIHC device constructed with the PNC anode and the HPC cathode exhibited a competitive energy density of 103 W h kg−1 and power density of 6106.2 W kg−1. Our method can effectively solve the current problems of the slow kinetics and low capacity of PIHC and provide a reference for further stimulation of their potential.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Thanks to the National Natural Science Foundation of China (grant no. 51772017, 51432003 and U20A20337) and Changzhou Sci &Tech Program (grant no. CJ20200015).References
- J. Chen, B. Yang, H. Hou, H. Li, L. Liu, L. Zhang and X. Yan, Adv. Energy Mater., 2019, 9, 1803894 CrossRef
.
- X. Zhou, L. Chen, W. Zhang, J. Wang, Z. Liu, S. Zeng, R. Xu, Y. Wu, S. Ye, Y. Feng, X. Cheng, Z. Peng, X. Li and Y. Yu, Nano Lett., 2019, 19, 4965–4973 CrossRef CAS PubMed
.
- D. Qiu, J. Guan, M. Li, C. Kang, J. Wei, Y. Li, Z. Xie, F. Wang and R. Yang, Adv. Funct. Mater., 2019, 29, 1903496 CrossRef
.
- B. Wang, E. H. Ang, Y. Yang, Y. Zhang, M. Ye, Q. Liu and C. Li, Chem. –Eur. J., 2021, 27, 512–536 CrossRef CAS
.
- K. Lei, C. Wang, L. Liu, Y. Luo, C. Mu, F. Li and J. Chen, Angew. Chem., Int. Ed., 2018, 130, 4777–4781 CrossRef
.
- H. Qiu, L. Zhao, M. Asif, X. Huang, T. Tang, W. Li, T. Zhang, T. Shen and Y. Hou, Energy Environ. Sci., 2020, 13, 571–578 RSC
.
- Y. Li, Y. Yang, P. Zhou, T. Gao, Z. Xu, S. Lin, H. Chen, J. Zhou and S. Guo, Matter, 2019, 1, 893–910 CrossRef
.
- J. Ge, B. Wang, J. Wang, Q. Zhang and B. Lu, Adv. Energy Mater., 2020, 10, 1903277 CrossRef CAS
.
- X. Mao, X. Gu, S. Wen, L. Zhang, P. Dai, L. Li, D. Liu, D. Li, Z. Li, K. Zhang and X. Zhao, Sustainable Energy Fuels, 2021, 5, 3240–3246 RSC
.
- Q. Tan, K. Han, W. Zhao, P. Li, Z. Liu, S. Li and X. Qu, Sustainable Energy Fuels, 2021, 5, 844–854 RSC
.
- Y. Sun, Y. Zhang, Z. Xing, D. Wei, Z. Ju and Q. Zhuang, Sustainable Energy Fuels, 2020, 4, 1216–1224 RSC
.
- J. Du, S. Gao, P. Shi, J. Fan, Q. J. Xu and Y. Min, J. Power Sources, 2020, 451, 227727 CrossRef CAS
.
- C. Li, X. Zhang, Z. Lv, K. Wang, X. Sun, X. Chen and Y. Ma, Chem. Eng. J., 2021, 414, 128781 CrossRef CAS
.
- C. Li, X. Zhang, K. Wang, F. Sun, C. M. Chen, F. Liu and Y. Ma, J. Energy Chem., 2021, 54, 352–367 CrossRef
.
- X. Hu, G. Zhong, J. Li, Y. Liu, J. Yuan, J. Chen, H. Zhan and Z. Wen, Energy Environ. Sci., 2020, 13, 2431–2440 RSC
.
- J. Ruana, Y. Zhao, S. Luo, T. Yuan, J. Yang, D. Sun and S. Zheng, Energy Storage Materials, 2019, 23, 46–54 CrossRef
.
- Z. Xie, D. Qiu, J. Xia, J. Wei, M. Li, F. Wang and R. Yang, ACS App. Mater. Inter., 2021, 13, 12006–12015 CrossRef CAS
.
- X. Hu, Y. Liu, J. Chen, L. Yi, H. Zhan and Z. Wen, Adv. Energy Mater., 2019, 9, 1970167 CrossRef CAS
.
- H. Li, Z. Cheng, Q. Zhang, A. Natan, Y. Yang, D. Cao and H. Zhu, Nano Lett., 2018, 18, 7407–7413 CrossRef CAS
.
- J. Yang, Z. Ju, Y. Jiang, Z. Xing, B. Xi, J. Feng and S. Xiong, Adv. Mater., 2018, 30, 1700104 CrossRef PubMed
.
- M. S. Ansari, T. N. J. I. Edison and Y. R. Lee, Sustainable Energy Fuels, 2020, 4, 5697–5708 RSC
.
- J. Ruan, X. Wu, Y. Wang, S. Zheng, D. Sun, Y. Song and M. Chen, J. Mater. Chem. A, 2019, 7, 19305–19315 RSC
.
- Y. Xie, Y. Chen, L. Liu, P. Tao, M. Fan, N. Xu, X. Shen and C. Yan, Adv. Mater., 2017, 29, 1702268 CrossRef
.
- Y. Zhou, Y. Zhu, B. Xu and X. Zhang, Sustainable Energy Fuels, 2021, 5, 396–400 RSC
.
- X. Chang, X. Zhou, X. Ou, C. S. Lee, J. Zhou and Y. Tang, Adv. Energy Mater., 2019, 9, 1902672 CrossRef CAS
.
- Y. Qian, S. Jiang, Y. Li, Z. Yi, J. Zhou, T. Li, Y. Han, Y. Wang, J. Tian, N. Lin and Y. Qian, Adv. Energy Mater., 2019, 9, 1901676 CrossRef
.
- J. P. Paraknowitsch and A. Thomas, Energy Environ. Sci., 2013, 6, 2839–2855 RSC
.
- C. H. Choi, S. H. Park and S. I. Woo, ACS Nano, 2012, 6, 7084–7091 CrossRef CAS
.
- D. Qiu, J. Guan, M. Li, C. Kang, J. Wei, F. Wang and R. Yang, Adv. Sci., 2020, 7, 2001681 CrossRef CAS
.
- R. C. Cui, B. Xu, H. J. Dong, C. C. Yang and Q. Jiang, Adv. Sci., 2020, 7, 1902547 CrossRef CAS
.
- N. Guo, M. Li, X. Sun, F. Wang and R. Yang, Green Chem., 2017, 19, 2595–2602 RSC
.
- J. Niu, J. Liang, R. Shao, M. Liu, M. Dou, Z. Li, Y. Huang and F. Wang, Nano Energy, 2017, 41, 285–292 CrossRef CAS
.
- J. G. Wang, H. Liu, X. Zhang, X. Li, X. Liu and F. Kang, Small, 2018, 14, 1703950 CrossRef
.
- H. Huang, R. Xu, Y. Feng, S. Zeng, Y. Jiang, H. Wang, W. Luo and Y. Yu, Adv. Mater., 2020, 32, 1904320 CrossRef CAS PubMed
.
- A. C. Ferrari and J. Robertson, Phys. Rev. B, 2000, 61, 14095–14107 CrossRef CAS
.
- W. Wei, Y. Zheng, M. Huang, J. Shi, L. Li, Z. Shi, S. Liu and H. Wang, Nanoscale, 2021, 13, 4911–4920 RSC
.
- Y. Sun, H. Wang, W. Wei, Y. Zheng, L. Tao, Y. Wang, M. Huang, J. Shi, Z. C. Shi and D. Mitlin, ACS Nano, 2021, 15, 1652–1665 CrossRef CAS
.
- Y. Qian, S. Jiang, Y. Li, Z. Yi, J. Zhou, T. Li, Y. Han, Y. Wang, J. Tian, N. Lin and Y. Qian, Adv. Energy Mater., 2019, 9, 1901676 CrossRef
.
- D. Ni, W. Sun, Z. Wang, Y. Bai, H. Lei, X. Lai and K. Sun, Adv. Energy Mater., 2019, 9, 1900036 CrossRef
.
- L. Hao, B. Luo, X. Li, M. Jin, Y. Fang, Z. Tang, Y. Jia, M. Liang, A. Thomas, J. Yang and L. Zhi, Energy Environ. Sci., 2012, 5, 9747–9751 RSC
.
- C. H. Choi, S. H. Park and S. I. Woo, ACS Nano, 2012, 6, 7084–7091 CrossRef CAS
.
- R. C. Cui, B. Xu, H. J. Dong, C. C. Yang and Q. Jiang, Adv. Sci., 2020, 7, 1902547 CrossRef CAS
.
- D. Wang, Z. Wang, Y. Li, S. Luo, K. Dong, Y. Liu and X. Qi, New J. Chem., 2018, 42, 14410–14416 RSC
.
- L. Fan, Q. Liu, S. Chen, K. Lin, Z. Xu and B. Lu, Nano Lett., 2015, 15, 7671–7677 CrossRef PubMed
.
- A. Mahmood, S. Li, Z. Ali, H. Tabassum, B. Zhu, Z. Liang, W. Meng, W. Aftab, W. Guo, H. Zhang, M. Yousaf, S. Gao, R. Zou and Y. Zhao, Adv. Mater., 2019, 31, 1805430 CrossRef PubMed
.
- X. Wu, Y. Chen, Z. Xing, C. W. K. Lam, S. S. Pang, W. Zhang and Z. Ju, Adv. Energy Mater., 2019, 9, 1900343 CrossRef
.
- B. Li, F. Dai, Q. Xiao, L. Yang, J. Shen, C. Zhang and M. Cai, Adv. Energy Mater., 2016, 6, 1600802 CrossRef
.
- M. Endo, T. Maeda, T. Takeda, Y. J. Kim, K. Koshiba, H. Hara and M. S. Dresselhaus, J. Electroche. Soc., 2001, 148, A910 CrossRef CAS
.
- X. Lin, J. Huang and B. Zhang, Carbon, 2019, 143, 138–146 CrossRef CAS
.
- J. Wang, J. Polleux, J. Lim and B. Dunn, J. Phys. Chem. C, 2007, 111, 14925–14931 CrossRef CAS
.
- L. Eliad, G. Salitra, A. Soffer and D. Aurbach, J. Phys. Chem. C, 2001, 105, 6880–6887 CrossRef CAS
.
- J. Zhou, Z. Li, W. Xing, H. Shen, X. Bi, T. Zhu, Z. Qiu and S. Zhuo, Adv. Funct. Mater., 2016, 26, 7955–7964 CrossRef CAS
.
- M. Moussa, S. A. Al-Bataineh, D. Losic and D. P. Dubal, Appl. Mater. Today, 2019, 16, 425–434 CrossRef
.
- F. Ming, H. Liang, W. Zhang, J. Ming, Y. Lei, A. H. Emwas and H. N. Alshareef, Nano Energy, 2019, 62, 853–860 CrossRef CAS
.
- Q. Shen, P. Jiang, H. He, C. Chen, Y. Liu and M. Zhang, Nanoscale, 2019, 11, 13511–13520 RSC
.
- X. Liu, G. A. Elia, B. Qin, H. Zhang, P. Ruschhaupt, S. Fang, A. Varzi and S. Passerini, ACS Energy Lett, 2019, 4, 2675–2682 CrossRef CAS
.
- F. Huang, W. Liu, Q. Wang, F. Wang, Q. Yao, D. Yan, H. Xu, B. Y. Xia and J. Deng, Electrochim. Acta, 2020, 354, 136701 CrossRef CAS
.
- C. Liu, Y. Xia, Y. Zhang, Q. Y. Zhou, H. B. He, F. D. Yu, Z. R. Wu, J. Liu, X. L. Sui, D. M. Gu and Z. B. Wang, ACS Appl. Mater. Inter., 2020, 12, 54773–54781 CrossRef CAS PubMed
.
- M. Xu, Y. Feng, B. Chen, R. Meng, M. Xia, F. Gu, D. Yang, C. Zhang and J. Yang, Nano-Micro Lett., 2021, 13, 42 CrossRef
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1se01627j |
| This journal is © The Royal Society of Chemistry 2022 |