Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Nanomaterial-based surface-enhanced Raman scattering spectroscopy for sensing and diagnostics of gas molecules in environment and healthcare
Jai
Prakash
 *ac,
Paulo Roberto
de Oliveira
b,
H. C.
Swart
*ac,
Paulo Roberto
de Oliveira
b,
H. C.
Swart
 c,
Marina
Rumyantseva
c,
Marina
Rumyantseva
 d,
M.
Packirisamy
d,
M.
Packirisamy
 e,
Bruno C.
Janegitz
b and
Xiaogan
Li
e,
Bruno C.
Janegitz
b and
Xiaogan
Li
 f
f
aDepartment of Chemistry, National Institute of Technology Hamirpur, Hamirpur-177005, Himachal Pradesh, India. E-mail: jaip@nith.ac.in
bLaboratory of Sensors, Nanomedicine and Nanostructured Materials, Federal University of São Carlos, Araras, São Paulo 13600-970, Brazil
cDepartment of Physics, University of the Free State (UFS), Bloemfontein, ZA 9300, South Africa
dChemistry Department, Moscow State University, Moscow, 119991, Russian Federation
eOptical-Bio Microsystems Laboratory, Department of Mechanical, Industrial and Aerospace Engineering, Concordia University, Montreal, QC H3G1M8, Canada
fSchool of Microelectronics, Faculty of Electronic Information and Electrical Engineering, Key Lab. of Liaoning for Integrated Circuits Technology, Dalian University of Technology, Dalian, China
First published on 13th September 2022
Abstract
The detection and identification of molecular traces in the environment as well as in the human breath are very significant for the development of a healthy society. This is extremely important from the environmental control and medical diagnostics point of view. A great deal of research is being conducted in the field of gas sensing using a variety of sensing techniques. Even though several sensing materials with low cost have been developed for sensing toxic gases with relatively high sensitivity, however, sensors suffer from certain drawbacks such as poor sensitivity at room temperature. Another fact is that traditional sensors fail to discriminate the signals of different target molecules in the case of multiplex gases. Therefore, developing a sensing tool with high sensitivity, good selectivity, and simple operation for gas detection is of great significance to environment monitoring/protection and human healthcare. In this context, the surface-enhanced Raman spectroscopy (SERS) technique has emerged as one of the promising surface sensitive techniques for the ultra-detection of various kinds of species, such as chemical and biological molecules, including environmental gaseous pollutants. SERS not only provides ultrasensitive detection of molecules but also provides fingerprints of individual gases out of the mixtures, which are very complicated in the case of traditional methods. Recent advances in the SERS technique in sensing toxic and harmful environmental gases are highlighted. Additionally, this technique has shown promising application as a gas biomarker in the human breath to detect related diseases in the human body, which has also been discussed along with its possible application in the detection of SARS-CoV-2 in the breath of COVID-19 patients. Furthermore, challenges in the application of SERS technology for gas detection in the environment and their practical implementations in air pollution control, as well as human healthcare, are addressed.
1. Introduction
The detection and identification of some species at molecular levels in the air are important from environmental safety and control point of view, along with other technological processes such as food safety, warfare, and medical diagnosis.1–5 Particularly, in the case of very toxic and harmful gases present in the surroundings affecting human health, it becomes necessary to detect and identify the molecular traces with extreme sensitivity for fast control and cure of the problem that can occur.6 Sometimes, even very low concentrations of toxic gases, i.e., as low as part-per-billion (ppb), can be critical if inhaled for a long time.2,7,8With fast-growing modern industries and agricultural waste, there is a huge production of toxic and harmful gases such as NH3, NO2, NO, SO2, and volatile organic compounds (VOCs) among others, which have adverse effects not only on the environment but also on the health of human beings.7,9,10 Some of the gases play a major role in damaging the natural resources in the open environment producing acid rain, photochemical smog, etc. Similarly, some gases, including smog, are toxic to the human body, creating a lot of problems, particularly related to respiratory diseases, and also affect many organs of the human body.11,12 Hence, these poisonous and flammable gaseous chemicals in the environment cause several diseases in the human body when exceeding a certain level. For example, NO2 causes respiratory and cardiovascular diseases at a level higher than 1 part-per-million (ppm), with symptoms similar to those of asthma.13,14 NH3 has an adverse effect on the body, skin, and also on eyes.15 On the other hand, H2S is another very notoriously toxic gas that can cause sudden death if a person has exposure to >700 ppm.16 It also has adverse effects on cardiovascular and neurological systems.17 Therefore, monitoring very low concentration levels of these toxic and harmful gases in the environment is essential for people to avoid health issues. In addition, this ultra-sensitivity detection of these toxic and harmful gases could also be exploited as a biomarker for the diagnosis of various diseases by monitoring human breath.3 This is possible by examining the exhaled gases of the patients. Breath analysis could provide a promising way to diagnose diseases by detecting traces of various gases in exhaled breath.3,18 For example, the presence of an abnormal level of NH3 in exhaled air could be a sign of kidney failure. The detection of H2 and CH4 in the exhaled air could be an indication of intestinal diseases. Similarly, the presence of nitrogen-based oxide (NOx) gases in the human breath provides information about respiratory diseases such as bronchial asthma, chronic cough, chronic obstructive pulmonary disease, etc.18–22
The above discussion indicates that ultra-detection of these toxic and harmful gases in the environment as well as in human breath could be a promising way to control and improve the health issues in society. Furthermore, it could be possible by the development of high-performance gas sensors. In the past few years, there has been a growing interest in developing compact and reliable sensors for gas detection in applications for human health and environmental control.2,7,13–15,18,23–27 These include gas chromatography/mass spectroscopy, chemiluminescence, electrochemical, chemoresistance, laser spectroscopy, etc.10,11,14,27–30 Even though several sensing materials with a low cost have been developed for sensing toxic gases with relatively high sensitivity,7,9,14,15,18,31–36 sensors can present some drawbacks, such as poor sensitivity at room temperature.14 Another fact is that typical sensors detect the electrical transfer resistance upon the adsorption of analytes onto the surface of the sensing nanomaterial, and it remains challenging to discriminate the signals of target molecules from those of other gas molecules.14,23
In addition to these techniques, the surface-enhanced Raman spectroscopy (SERS) technique has shown very promising application as an ultrasensitive molecular sensing tool and has the potential to increase gas sensitivity by several orders of magnitude.23,24,34,37–41 SERS is a promising technique for ultrasensitive detection of chemical, biological, and environmental pollutants, with the technique capable of detecting single molecules.42 The gas sensing response of SERS-based sensors is rapid and more reliable than that of conventional sensors, which lag behind due to limitations of low selectivity, high operation temperature, etc. This technique involves the application of nanomaterial-based SERS active substrates composed of noble metals43–45 or metal oxide semiconductors37,46–48 or their nanocomposites.40,49–51 Particularly, plasmonic noble metal nanostructures (Ag/Au) are known to be the best SERS substrates because of their unique localized surface plasmon resonance (LSPR) properties,39,52–55 which provide a high SERS enhancement factor (EF). Furthermore, recent advancement in synthesis and tailoring properties of plasmonic nanostructures (Au/Ag, alloys, etc.) has improved the molecular level sensing using the SERS technique with sensitivity limit down to sub-ppm level.41 For example, Kim et al.56 developed a plasmonic-SERS-based high-performance NO2 gas sensor with a limit of detection of 0.1 ppm that could be operated under ambient conditions. Similarly, a highly sensitive 3D Au–Ag bimetallic nanowire-based SERS sensing substrate with a high density of hot spots and strong binding between NO2 along with a large interconnected 3D Au surface was fabricated that facilitated the adsorption of gas molecules enhancing the Raman signals. On the other hand, metal oxide semiconductors such as TiO2, ZnO, CuO, etc.46,48,57–60 are also used but are not so efficient as SERS substrates for ultrasensitive detection due to low SERS EF. However, these semiconductors, including 2D nanomaterials (i.e., GO),36,61–63 in combination with plasmonic nanostructures provide excellent results in SERS sensing applications.64–66 For example, Myoung et al.67 demonstrated that Ag NPs on SiO2 substrates could be used as a real-time vapor phase SERS detector with a detectable range of 0.6 to 800 ppm. Moreover, the SERS technique has also been used to detect different kinds of toxic gases and VOCs in the breath analysis of human beings as a gas biomarker for the diagnosis of various diseases.24,68–71 For example, recently, Chen et al.24 developed SERS based approach to detect VOCs biomarkers using Au NPs and reduced graphene oxide (GO) nanocomposites in both simulated and real breath samples with ultra-sensitivity and multiplexing. This experiment provides a way to distinguish early gastric cancer (EGC) and advanced gastric cancer (AGC) patients from healthy persons by monitoring the exhaled breath samples from patients. Similarly, Fu et al.72 demonstrated that the detection of VOCs using the SERS technique could be helpful in the early diagnosis of lung cancer. The MIL-100(Fe) platform with Au NPs was employed to detect toluene and other VOCs. The limit of detection for toluene gas as a biomarker for lung cancer was found to be 0.48 ppb level with an EF of 1010.
Although researched extensively, SERS technology using functional nanomaterials with short and long-term multifunctional activities in the field of environment monitoring/control and healthcare as gas sensors has rarely been reviewed. This review provides fundamental details of SERS technology in brief, using conventional and modern portable SERS handheld spectrophotometers for point-of-care testing, which needs attention to explore for real practical applications. The emerging application of SERS technology as a gas sensing tool for the detection of toxic and harmful gases and gas biomarkers has been discussed with an emphasis on its simple and cost effective use for the benefit of society. Furthermore, its applications in environmental control and healthcare have been explored. It is to be noted that a few reviews seem to be similar but these are entirely different because all are focused on a particular theme, either on healthcare (mainly on a particular disease or materials) or environment related (on a particular problem).73–78 The proposed article is focused on environmental and healthcare applications, including the pandemic COVID-19 problem and scientific solutions with a potential for commercialization because of its portability and simple handling as compared to the traditional sensor/biosensors/environmental sensors. This is an emerging research area where various plasmonic based nanomaterials and SERS technique could be potentially applied for the analysis of the human breath to monitor human health as well as for the detection of such gases in environmental monitoring, as shown schematically in Fig. 1. This review article deals with such progress in the field of SERS technique as an ultrasensitive gas sensor in environmental and multiplex gas biomarker in human health.
2. Surface-enhanced Raman scattering
As discussed above, traditional methods have been potentially used to perform qualitative analysis and quantitative detection of chemical and biological analytes; however, these are found to be somehow limited by lower detection sensitivity of the analytes and response time. Although there has been a lot of progress in the past decade to improve the detection sensitivity and response time, some traditional techniques are not appropriately designed to carry and are not easy to operate in the field for practical applications.79 Therefore, research on sensing tools, which can provide high sensitivity and selectivity of the analytes along with short detection time, would be of great significance and it is the need of the day. Furthermore, the detection tool should be portable so that it can be carried anywhere for practical applications, especially when it is being used for environmental and diagnosis purposes, especially to fight a situation like the COVID-19 pandemic. In this context, SERS technology, which uses a Raman spectrophotometer, is being used as an ultrasensitive detection tool with a new spectrum detection technology. It has the potential not only to identify the fingerprints of different analytes but also to detect a lower concentration as a single molecular detection tool. Due to modern technological development in the last few years, various portable SERS tools have been invented with high sensitivity, selectivity, and rapid response, which could replace traditional sensing tools.As discussed above, in SERS technology, SERS active substrates are used, which are generally tailored nanostructures of noble metals, particularly Ag or Ag nanostructures or hybrid nanostructures composed of noble metals with other functional nanomaterials. The unique LSPR property of the noble metals is tailored to achieve high sensitivity in the SERS technique. It is based on the enhancement of Raman scattering signals of the molecules adsorbed on the surface of nanostructured SERS active substrates when the plasmon frequency is in resonance with that of the probe radiation used in the Raman spectrophotometer.8,38,40,48,80 It could be explained by electromagnetic and chemical mechanisms, whereas electromagnetic SERS enhancement has been the most effective and highly acceptable.38,40,42,48,80
The electromagnetic SERS mechanism is mainly due to the LSPR of noble metals, which shows a high EF due to their ease of fabrication and biocompatibility; however, there are some drawbacks when used as sole nanostructured materials in the form of powder or solutions.79,81 Recent developments demonstrate that surface nanoengineering of noble metal-based SERS active substrates and their composites with semiconductors provide better morphology with ‘hot spot’ nano-architectures that are suitable for higher Raman signals due to the synergetic effect of electromagnetic and chemical enhancement.34,48,79 It has been reported to be capable of detecting a single molecule at low concentrations of analyte molecules.82 SERS technique has shown its capability to detect small molecules, i.e., organic pollutants, environmental gaseous molecules, ions, proteins, antibodies, nucleic acids, etc.39,47,83 along with large and complex molecules, i.e., viruses, bacteria, etc.82,84–86 In the present review, the application of SERS has been described in the field of environment and healthcare as a gas sensing tool for the detection of toxic/harmful environmental gases as well as gas biomarkers in human breath. For that purpose, a set is generally designed for gas sensing using the SERS technique, as shown schematically in Fig. 2. The gaseous specimen is either used as a gas flow or collected in the chamber containing the SERS active substrates. After the analyte molecules are adsorbed on the surface of the SERS active substrate, it is kept under the focus of the laser beam from the Raman spectrophotometer, and the Raman scattering signals are collected through the detector. Similarly, it is possible to collect human breath in the analyzing chamber, and gas biomarkers can be detected. On the other hand, in the case of solid or liquid specimen sensing, no such set is required, as shown in Fig. 2. The SERS substrate adsorbed with the analyte molecules (after drying if it is in liquid) can be directly kept under the focus of the laser beam from the Raman spectrophotometer, and the SERS signals are collected. In all such cases, the SERS active substrates and their morphologies, including shape/size, play an important role in the SERS signal enhancement.
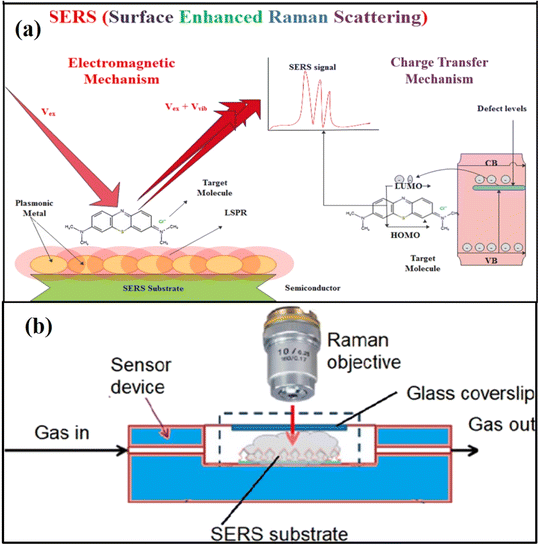 | ||
| Fig. 2 Schematics of (a) SERS mechanism of electromagnetic enhancement and chemical enhancements80 (b) experimental setup for SERS measurements in the gas phase. Reprinted with permission from ref. 2. | ||
A lot of progress has been reported in the last few years in designing various 0–3D SERS active substrates using noble metals and semiconductors to enhance the EF and detect lower concentrations of analyte molecules, as discussed in the next sections, particularly in the case of detection of toxic gases and biomarkers in the environment and human breath, respectively. In addition, portable SERS devices have been developed, which are capable of several types of molecular detection in a convenient manner demonstrating as a promising candidate for point-of-care testing87 as also discussed in the next section.
3. Application of SERS in environmental gas sensing
Due to the growing industries, agriculture, and population, the environment is full of many toxic and harmful gases, i.e., CO2, CO, NH3, NO2, NO, SO2, VOCs, etc.,14,72,88–90 and these gases affect the healthy environment as well as the health of human beings. Some of the gases affect human health badly, even when present in trace amounts. As discussed above, SERS is a promising technique to detect and identify various molecular species, and several studies have reported the synthesis of tailored SERS active substrates for environmental gas sensing.3.1 SERS detection of C, N, and S based environmental gases
As discussed in section 1, several toxic and harmful gases could be present in the environment affecting human health. The most available gases belong to C, N, and S, which have been extensively investigated. Recently, Huang et al.91 reported a very effective SERS substrate based on ultrathin ZIF-8 wrapped on Au-dotted Ag-nanowires (NWs) for CO2 detection. Bontempi et al.88 recently designed SiO2/TiO2 core/shell beads for Raman detection of environmental CO2 under real working conditions during the solar to fuel transforming reaction. The morphology of the designed SERS substrate was tailored to trap the light for SERS resonance for enhanced sensitivity and low concentration detection of CO2. Similarly, Yao et al.92 developed a novel SERS and resonance Rayleigh scattering (RRS) coupled di-mode method for the detection of CO using Au-based nano-enzyme catalysis as well as Au as nanoprobe. It has been demonstrated that asthma symptoms become worse when a patient is exposed to NO2 gas between 0.10 and 0.53 ppm.56 Kim et al.56 developed a 3D nanoarchitecture-based SERS substrate composed of Au coated 1D Ag (NWs) for the detection of NO2 gas. As shown in the scanning electron microscopy (SEM) images of Fig. 3a–c, 3D multi-layered Ag NWs of diameter 45.8 ± 6.2 nm were fabricated via vacuum filtration. This method produced interconnected 3D structures forming a high density of crosspoints. At the crosspoints, two NWs were closely stacked and parallel non-touching Ag NWs formed hot spots, which contributed to the SERS detection by enhancing the local electromagnetic field in the gaps due to the strong plasmonic coupling effect. Furthermore, the thick Au film was deposited onto the 3D stacked Ag NWs by sputtering the produced 3D Au–Ag bimetallic nanostructures (Fig. 3b). The average diameter of these nanostructures was estimated to be 62.6 ± 9.7 nm after depositing the 15 nm thick Au layer onto the 3D Ag NWs. The proposed SERS sensor exhibited LOD with 0.1 ppm of NO2 along with on-site and rapid detection. It was also demonstrated that the SERS NO2 gas sensing could be performed under ambient conditions using a handheld Raman spectrometer, as shown in Fig. 3d–f. The ultra-detection NO2 SERS sensing was performed in vehicle exhaust tests, and high sensitivity was observed, attributed to the stable complex formation of NO2 molecules on Au surface of than with Ag in 3D Au–Ag bimetallic nanoarchitecture. It was also proposed that this SERS substrate and a handheld Raman spectrophotometer set-up could be promising for many environmental and medical applications, including in-house air quality tests, gas sensing, and health care monitoring.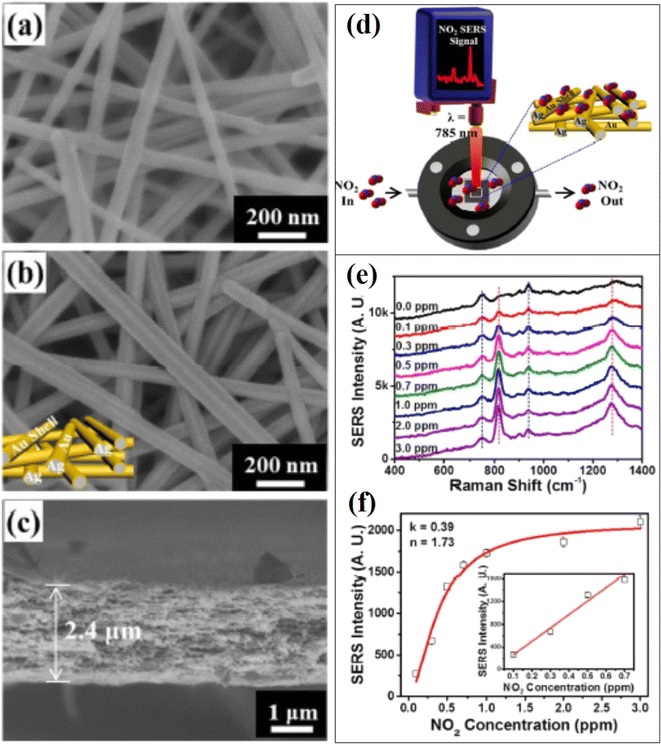 | ||
| Fig. 3 SEM micrographs of 3D stacked (a) Ag nanowires (NWs) and (b) Au-coated Ag NWs. (c) X-SEM micrograph of the NWs. (d) Schematic representation of in situ NO2 gas detection process under ambient conditions. (e) SERS spectra of NO2 gas at different concentrations from 0.1 ppm to 3 ppm. (f) Calibration curve based on the SERS intensity at 810 cm−1 as a function of the NO2 gas concentration.56 | ||
In another work, Kim et al.93 demonstrated that similar SERS-active plasmonic chips with a 3D nanoporous architecture composed of Ag NWs deposited with Au hemispheres on their surfaces were extremely sensitive with fast NO2 gas sensitivity at room temperature. Recently, Li et al.94 studied the interaction of oxides of S and N (i.e., NO2, NO3, SO3, and SO4) on plasmonic metal Ag surfaces. It was demonstrated with the help of spectroscopic evidence and density functional theory (DFT) calculations during the NO and SO2 plasma-based removal and formation of intermediates by in situ SERS. Moreover, this study provided novel identification of such surface species with their vibrational modes, molecular adsorption, and orientation while adsorbed on Ag NPs through experimental as well as DFT studies. Similarly, different SERS substrates were designed to detect SO2 and NO2 in different kinds of samples.89,95–99
Kumar et al. and Sharma et al.42,90 developed a SERS-based NH3 sensor for the detection of NH3 gas using a substrate composed of Au NPs decorated onto partial embedded 2D polystyrene colloidal crystals (PSCCs), partially embedded into flexible polydimethylsiloxane (PDMS) elastomer. SERS signals of NH3 molecules were found to be enhanced with an increase in NH3 concentration from 100 to 1000 ppm. Zhang et al.100 recently demonstrated that low concentration detection of NH3 could be possible by in situ SERS detection. Rae et al.23 investigated CO and N2O gas sensing using AgPd SERS substrates, where the maximum enhancement factor that could be achieved was 105. Sharma et al.42 designed an effective SERS substrate composed of Au NPs decorated on a controlled morphology of 2D PSCCs (200–500 nm diameter), partially embedded into flexible PDMS elastomer for SERS sensing of various gaseous molecules NH3, H2O2, N2O, and H2S. Design of such nanostructure with Au NPs (of size 8 nm) deposited by radio frequency (RF) magnetron sputtering on PSCCs creating a hot spot with large electromagnetic enhancement provides excellent SERS sensitivity and reliability. It occurred due to the self-assembled flexible hexagonally close-packed PSCCs producing 75 and 25 nm voids in the 500 and 200 nm PSCCs monolayers acting as an effective plasmonic propagator for SERS sensors signal.90
3.2 SERS detection of VOCs and other gases
As discussed in section 1, several VOCs could be present in the environment and have been investigated using SERS sensing applications. For example, Wong et al.101 proposed multiplex SERS-based detection of VOCs using leaning pillar substrates (i.e., Si nanopillars coated with Ag). The Si nanopillars were 50–80 nm wide, and their heights were found to be 600–1600 nm. These specially designed leaning pillar substrates with Ag tips on the top exhibited better adsorption of the VOCs analyte molecules resulting in enhanced Raman signal attributed to the LSP ‘hot spots’ between adjacent leaning nanopillars. Using these Ag–Si SERS active substrate systems, a multiplex detection for mixed acetone and ethanol VOCs was investigated, and these VOCs in different ratios were successfully detected in a multiplex format. The limits of detection were found to be 0.0017 and 0.0037 ng for ethanol and acetone vapor molecules, respectively. These designed nanopillar substrates exhibited a state-of-the-art Raman enhancement over large areas, as shown in Fig. 4(a). The novelty of leaned pillars with Ag tips was their large aspect ratio, which provided flexibility for leaning towards nearby pillars, creating self-assembled electromagnetic hot spots when illuminated by the laser. A SERS ultrasensitive device to detect ethanol vapor composed of Ag NPs of size 60 nm modified polyvinylpyrrolidone (PVP) was proposed by Wang et al.102 Chen et al.103 demonstrated that Au@ZIF-8 core–shell nanostructures with 55 nm Au NPs and a shell thickness of 3 nm exhibited better SERS response for the detection of various VOC gas molecules, such as toluene, ethylbenzene, and chlorobenzene. Similarly, Xia et al. proposed PDMS-coated 1-propanethiol-modification of Au@Ag NPs for enhanced sensitivity of various aromatic vapors in the atmospheric environment.104 50 nm Au@Ag NPs were prepared by first synthesizing Au NPs of 30 nm through epitaxial growth, followed by treatment with AgNO3 solution and PDMS. It was found that due to greater adsorption by PDMS and strong LSPR properties of Au@Ag NPs, there was SERS enhancement of about two orders of magnitude compared to only NPs.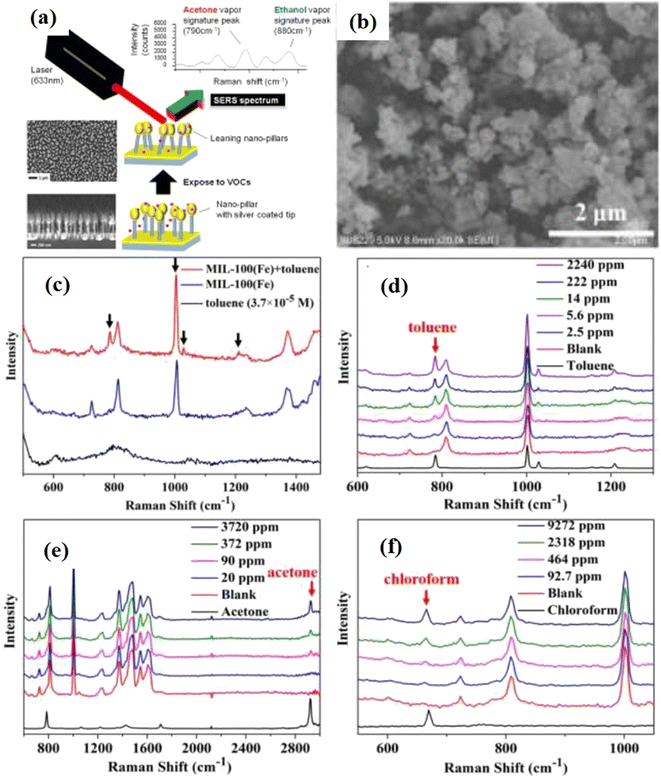 | ||
| Fig. 4 (a) SEM micrographs of the nanopillar with a schematic of SERS detection. Reprinted with permission form ref. 101 (b) SEM micrograph of MIL-100(Fe) (c) SERS spectra of the toluene solution, its vapour and SERS substrate, and (d)–(f) are the SERS spectra of toluene, acetone, chloroform vapors, respectively, on SERS substrate. Reprinted with permission from ref. 72. | ||
Recently, Snitka et al.2 developed a hybrid SERS platform of Si membrane and Ag NPs with a high density of ‘hot spots’ for molecular trace detection of various VOCs in the environment. First, 3D Ag/Si SERS substrates were prepared by using a Si wafer immersed in AgNO3 and HF solution, followed by the formation of micro-pores through a photoelectrochemical etching process. The LOD of hydrazine was found to be 10−12 mol L−1 from the solution and 0.1 ppm from the vapor phase. Whereas 0.5 ppb LOD was recorded for anisole vapors in the air using Si–Ag hybrid SERS membrane platform. It was concluded that such hybrid systems could be designed for sensing in space applications. Similarly, Nemciauskas et al.105 fabricated novel 3D printed SERS-based molecular sensors composed of nanoplasmonic Si membrane integrated with a gas micropump. The membrane-based sensing platform exhibited high sensitivity of 4.6 × 10−9 M towards anisole vapors. Fu et al.72 studied the role of MOFs as SERS substrates for the ultrasensitive multiplex detection of toluene and other VOCs. Au NPs and MIL-100 (Fe) MOFs system, as shown in Fig. 4(b), was used as an ideal SERS substrate, and a multiplex sensing of VOCs such as toluene, acetone, chloroform, etc. was demonstrated, as shown in Fig. 4(c–f). A high order EF was achieved for toluene, and it was also demonstrated that it could be useful for diagnostic lung cancer with a ppm detection limit in the human body. It was also demonstrated that the process of adsorption and desorption of gases could be studied using real-time SERS technology and applied to the specific identification of intermediates in catalytic reactions. Several VOCs such as benzenethiol, 2,4-dinitrotoluene, pyridine, 4-nitrophenol, toluene, benzene, nitrobenzene, and aromatic vapors have been detected using SERS as a probe for different purposes.104,106–109 Heleg-Shabtai et al.110 reported ultrasensitive detection of gas-phase VX and HD, which are toxic chemical warfare agents (CWA) and nerve agents (NAs).
The above gas sensing experiments and their fruitful results exhibit the applicability of Raman technology to the environment as well as homeland security. Some recent reviews could be referred to for the detailed study of VOCs detection using SERS.111,112 The above discussion reveals that the synthesis of nanomaterials with better morphology and surface properties is an important aspect for enhanced SERS detection of gases/vapours. Some important findings in this regard are summarized in Table 1. The nanostructures with ‘hot spot’ creating higher electromagnetic enhancement along with higher adsorption capability are promising SERS substrates in the case of gaseous molecular detection.
| S. No. | Nanostructures as SERS substrate/morphology | Method of fabrication | Detection of gases and VOCs | Important results/observation | Ref. |
|---|---|---|---|---|---|
| 1. | ZIF-8 wrapping on Au-dotted Ag-NWs | Solvothermal polyol method | CO2 | High selectivity for CO2 molecules with good stability and repeatability | 91 |
| 2. | Crystals SiO2/TiO2 core/shell beads | Atomic layer deposition of TiO2 on SiO2 spheres | CO2 | Real time detection of CO2 as a function of different temperature | 88 |
| 3. | 3D Au coated 1D Ag NWs | Vacuum filtration of an Ag NW solution through a porous microfiber filter followed by Au sputtering | NO2 | On-site detection of LOD 0.1 ppm NO2 due to the high density of hot spots | 56 |
| 4. | 3D nanoporous architecture composed of Ag NWs deposited with Au hemispheres | Vacuum filtration of an Ag NW solution through a porous microfiber filter followed by Au sputtering and thermal annealing | NO2 | Extremely sensitive and rapid detection of NO2 gas to 10 ppb using a simple handheld Raman spectrometer | 93 |
| 5. | AgPd NPs | Simple colloidal chemistry method | CO and N2O | SERS EF of 4 × 105 for CO and 1 × 105 for N2O | 23 |
| 6. | Au NPs decorated 2D PSCCs partially embedded in the flexible PDMS substrate | Self assembled 2D PSCCSs deposited with Au NPs by RF magnetron sputtering | NH3, H2O2, N2O, and H2S | Sensing response, strongly dependent on the crystal size | 42, 90 |
| 7. | Si nano-pillars coated with Ag | Si nanopillars were fabricated by reactive ion etching process followed by Ag coating using electron beam evaporation | Acetone and ethanol | Higher LSPR response due to hot spots between adjacent leaning nanopillars leading to the LOD 0.0017 and 0.0037 ng for acetone and ethanol, respectively. EF ~1011 | 101 |
| 8. | Au@ZIF-8 core–shell NPs | Citrate reduction method for Au NPs followed by controlling the ZIF-8 shell thickness through quenching terminated the reaction process | Toluene, ethylbenzene, and chlorobenzene | Owing to the excellent gas adsorption of the ZIF-8 shell and the LSPR effect of the Au core, Au@ZIF-8 NPs with a shell thickness of 3 nm exhibited better SERS enhancement | 103 |
| 10. | Ag NPs modified PVP | Chemical reduction method for Ag NPs | Ethanol | Act as a potential SERS substrate for VOCs | 102 |
| 11. | PDMS-coated Au@Ag NPs | Au@Ag NPs prepared by a seeds growth method followed by AgNO3 solution treatment and PDMS | Aromatic VOCs | Greater adsorption by PDMS and strong LSPR of plasmonic NPs resulted in about two orders of magnitude SERS enhancement as compared to only NPs | 104 |
| 12. | 3D Ag/Si membranes | Reduction of Ag on Si wafer followed by photoelectrochemical etching | Hydrazine | LOD of hydrazine was found to be 0.1 ppm from the vapor phase. EF of 104 was achieved | 2 |
| 13. | Au NPs and MIL-100 (Fe) MOFs | Au NP colloid solution was prepared and dropped on MIL-100(Fe) MOFs | VOCs such as toluene, acetone, chloroform, etc. | The LOD of MIL-100(Fe) for toluene was found to be 2.5 ppm, which was better with Ag-MOFs and decreased to 0.48 ppb due to “hot spots” in between Au NPs, resulting in EF of 1010 | 72 |
| 14. | 3D Ag/Si membranes | Reduction of Ag on Si wafer followed by photoelectrochemical etching using 3D printing | Anisole vapor | Synthesized SERS substrates achieved ppb sensitivity and identification in gas detection | 105 |
| 15. | Au NPs (variable sizes) | Salt reduction followed by UV-assisted photo-chemical reduction approach | Dinitrotoluene vapor | EF of 5.6 × 106 (for 117 nm Au-NPs) | 106 |
| 16. | Au NPs | Salt reduction followed by UV-assisted photo-chemical reduction approach | 4-Nitrophenol vapor | A detection limit of 0.5 ppb was achieved | 107 |
| 17. | PDMS coated Au NPs monolayer film | Seed growth method of Au NPs followed by PDMS coating | Toluene, nitrobenzene, benzene | LOD of 0.5 ppm, 0.6 ppm, and 78 ppm for toluene, nitrobenzene, and benzene, respectively | 108 |
| 18. | Ag film over a nanosphere (AgFON) | Ag films (200 nm thick) were deposited over silica nanosphere using the thermal vapor deposition system | Benzenethiol | LOD 6 ppm | 109 |
| 19. | Silver nanofilm (AgNF) with NPs of ∼42 nm diameter | — | SO2 | Label-free assay, but with higher sensitivity with LOD of 10−6 M | 89 |
| 20. | Au NPs modified onto quartz fibres | Seeds growth method of Au NPs | Gas-phase VX and HD | (LOD) of 0.008 μg L−1 and 0.054 μg L−1 were achieved for VX and HD, respectively | 110 |
4. Emerging application of SERS for breath diagnosis in human health
There is great progress in the past few years in ultrasensitive gas detection in various fields of medical and environmental monitoring using the SERS technique, which has provided excellent results with high selectivity, sensitivity, and multiplex gas detection capability. In this section, the use of the SERS technique in breath diagnosis for healthcare applications is discussed.4.1 For multiplex potential gas biomarker in the human breath of patients
Several toxic gases and VOCs can be produced by different biochemical and physiological processes in various parts of the human body, which are exhaled in human breath, for example, NH3, NOx, H2S, alcohols, aldehydes, acids, ketones, etc. In case of any abnormal metabolic state or abnormal biochemical/physiological processes within the body, the level of these gaseous components would not be normal in the exhaled air of human breath. Importantly, detection of abnormally high concentrations of these gases in exhaled breath could be promising for identifying various diseases because these gases are biomarkers of some diseases.The vibrational fingerprints of molecular structures provide specific Raman peaks for different gaseous molecules. Wong et al.101 first demonstrated the multiplex SERS detection of VOCs, which was found to be very useful for the detection of multiplexity gas biomarkers in human breath analysis for the diagnosis of various diseases. Recently, Chen et al.24 developed SERS based approach to detect VOCs biomarkers using Au NPs and reduced graphene oxide (GO) nanocomposites for both simulated and real breath samples with ultra sensitivity and multiplexing. These Au NPs-reduced GO SERS samples were fabricated by depositing Au NPs on the reduced GO, which provides excellent LSPR properties and adsorption properties for the VOC biomarkers to be adsorbed on the surface, resulting in the enhancement of SERS signals (Fig. 5I). As shown in Fig. 5II (A and B), the simulation provided well-defined clusters for the simulated breath of healthy persons (a), early gastric cancer (EGC) (b), and advanced gastric cancer (AGC) (c) patients. The relation between 14 Raman bands corresponding to the VOC biomarkers was studied and summarized. The 14 Raman bands in the Raman spectra were taken as the fingerprints of biomarker patterns to distinguish EGC and AGC patients from healthy persons and were used for breath analysis based on the Au-GO SERS sensor. Fig. 5II (C and D) show the SERS spectra of simulated and real breath samples of healthy persons along with EGC and AGC patients. This experiment provides a way to distinguish EGC and AGC patients from healthy persons by monitoring the exhaled breath samples from the patients.
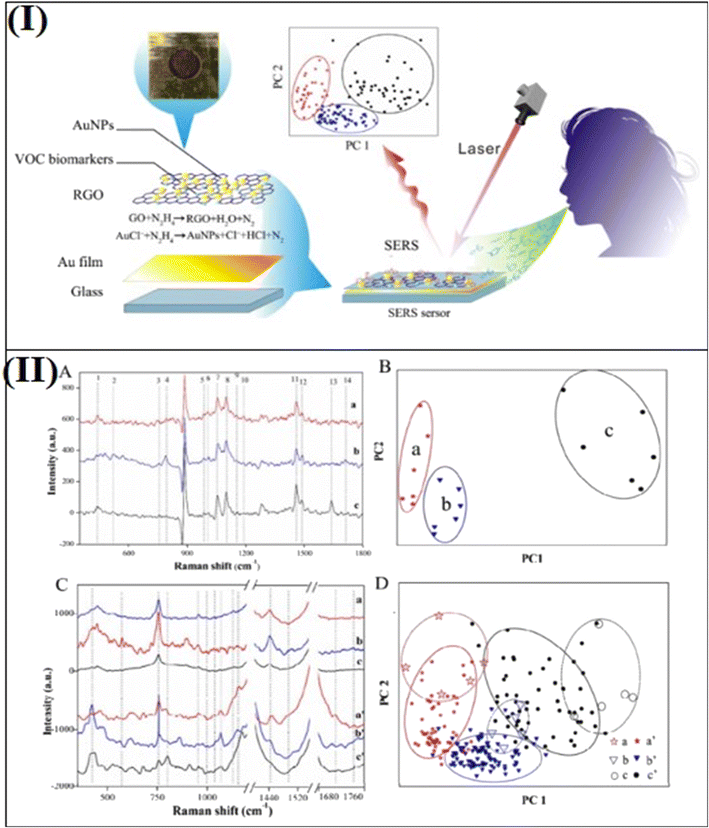 | ||
| Fig. 5 (I) Schematic of SERS sensing tool and involved breath analysis. (II) (A) SERS spectra of VOC biomarker patterns. (B) PCA of the data set of biomarker patterns of healthy persons and EGC and AGC patients. Each data point in the PCA corresponds to the area of 14 bands in the processed SERS spectra. (C) SERS spectra of simulated and real breath samples of healthy persons and EGC and AGC patients. (D) PCA of the data set of simulated and real breath samples of healthy persons and EGC and AGC patients. Each data point in the PCA corresponds to the area of 14 bands in the SERS spectra. Reprinted with permission from ref. 24. | ||
Similarly, Fu et al.72 demonstrated the detection of VOCs using the SERS technique, which could be helpful in the early diagnosis of lung cancer. The MIL-100(Fe) platform with Au NPs onto this was employed to detect toluene and other VOCs. The limit of detection for toluene gas as a biomarker for lung cancer was 0.48 ppb with an EF of 1010. This is an emerging area in which the SERS technique could be potentially applied for the analysis of human breath to monitor human health. Lauridsen et al.113,114 developed SERS based breath nanosensor composed of Au-coated Si nanopillars and studied the SERS detection of hydrogen cyanide (HCN) at ppb level for human breath analysis (Fig. 6a and b). These Au-coated Si nano-pillar SERS substrates were fabricated by reactive ion etching of Si wafer followed by sputtered Au deposition. It formed 225 nm Au caps on Si nanopillars producing hot spots with an enhanced electromagnetic field for SERS detection. The detection of HCN is known to be a Pseudomonas aeruginosa (PA) biomarker for lung infections in cystic fibrosis (CF) patients. In HCN, a Raman band at ∼2133 cm−1 shows the characteristic peak due to the presence of a triple bond between C and N in cyanide. This is the novel characteristic of HCN detection using SERS.
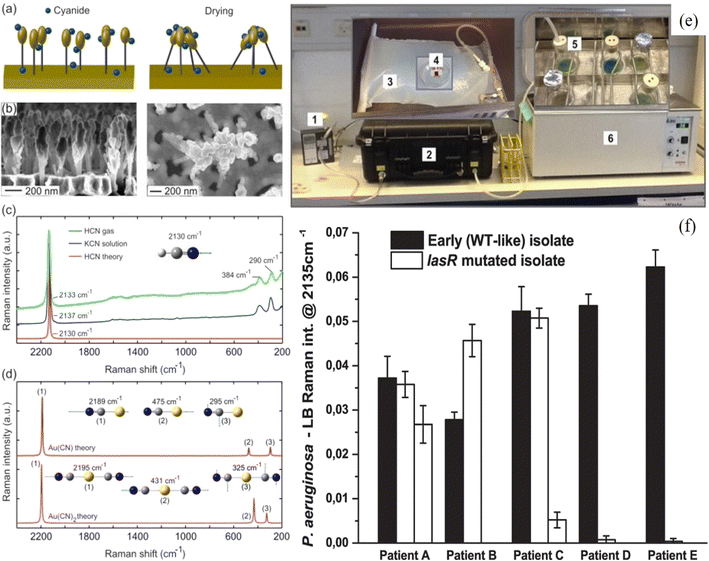 | ||
| Fig. 6 Schematic of leaning of nanopillars (a) and SEM images (b) before and after leaning (courtesy of Kaiyu Wu). (c) SERS spectra of HCN(g) and KCN(aq) and theoretical Raman spectrum of HCN. (d) Theoretical Raman spectrum of Au(CN) and Au(CN)2.114 (e) Demonstration of experimental set-up and detection of HCN using SERS substrate and (f) cyanide production of clinical P. aeruginosa overnight cultures from 5 CF patients. SERS detection of the biomarker hydrogen cyanide from Pseudomonas aeruginosa cultures isolated from cystic fibrosis patients.113 | ||
A direct HCN SERS detection was performed first in the gas phase by exposing 5 ppm HCN on Au coated Si nanopillar substrate and correlated with the theoretical SERS spectrum of HCN on Au. Fig. 6(c) shows the SERS spectra in both gas (HCN) and liquid (KCN) with Raman band at 2133 cm−1. Experimentally observed Raman bands at 290 and 384 cm−1 were explained by theoretical calculations, which showed the formation of Au–cyanide complexes (Fig. 6(d)). Fig. 6(e) shows the experimental setup of HCN detection using Au–Si nanopillar SERS substrates. Further, 12 clinical PA strains isolated from 5 CF patients and PA reference strains were studied for real practical application using SERS detection of HCN biomarker. As shown in Fig. 6(f), the intensity of the Raman band at 2135 cm−1 exhibited by all early (WT-like) strains and also some of the lasR mutated strains isolated from pediatric CF patients corresponded to the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N stretching band. That indicated the emission of HCN from the CF patients, and it was concluded that PA infections could be detected at an earlier stage because daily breath sampling with an immediate output could be possible with a point-of-care SERS device.113 Similarly, Qiao et al.115 fabricated a ZIF-8 layer coated with self-assembled Au superparticles (GSPs) based SERS gas biomarker detector. The SERS substrate was designed to slow the flow rate of gaseous biomarkers and depress the exponential decay of the electromagnetic field around the GSP surfaces. The GSPs were functionalized by Schiff base reaction with 4-aminothiophenol for capturing the gaseous biomarkers. They studied the detection of gaseous aldehydes released as a result of tumor-specific tissue composition and metabolism, thereby acting as indicators of lung cancer bombarding onto SERS-active GSPs substrates through a ZIF-8 channel. With the help of such an arrangement, gaseous aldehydes could be detected with a 10 ppb LOD. This experiment demonstrated the application of SERS for in vitro diagnosis of early-stage lung cancer. Interestingly, Park et al.116 demonstrated that SERS based substrates can also be used as a gas biomarker for living or dried plants through the detection of VOCs emitting from plants. Through SERS detection, it was possible to detect different VOCs and differentiate between healthy and caterpillar-infested plants.
N stretching band. That indicated the emission of HCN from the CF patients, and it was concluded that PA infections could be detected at an earlier stage because daily breath sampling with an immediate output could be possible with a point-of-care SERS device.113 Similarly, Qiao et al.115 fabricated a ZIF-8 layer coated with self-assembled Au superparticles (GSPs) based SERS gas biomarker detector. The SERS substrate was designed to slow the flow rate of gaseous biomarkers and depress the exponential decay of the electromagnetic field around the GSP surfaces. The GSPs were functionalized by Schiff base reaction with 4-aminothiophenol for capturing the gaseous biomarkers. They studied the detection of gaseous aldehydes released as a result of tumor-specific tissue composition and metabolism, thereby acting as indicators of lung cancer bombarding onto SERS-active GSPs substrates through a ZIF-8 channel. With the help of such an arrangement, gaseous aldehydes could be detected with a 10 ppb LOD. This experiment demonstrated the application of SERS for in vitro diagnosis of early-stage lung cancer. Interestingly, Park et al.116 demonstrated that SERS based substrates can also be used as a gas biomarker for living or dried plants through the detection of VOCs emitting from plants. Through SERS detection, it was possible to detect different VOCs and differentiate between healthy and caterpillar-infested plants.
As discussed above, biomarker detection using the SERS technique in human breath is a unique way to diagnose several diseases in the human body. Again, it is important to mention that designing SERS substrates through tailoring of shape or nano-architecturing is an important factor in enhancing the sensitivity for the gas biomarker at lower concentrations in the human breath. Porous nanomaterials embedded with plasmonic nanostructures could be better SERS substates with higher adsorption capability of the gas species, resulting in higher sensitivity. In addition, 3D nano-architecturing with plasmonic hot spots with tunable gaps could be implemented for better results in the detection of gas biomarkers in human breath and diagnostics. Some important results have been summarized in Table 2, including nanostructured SERS substrates used for the detection of gas biomarkers in human breath indicating certain diseases in the human body.
| S. No. | Nanostructures as SERS substrate/morphology | Method of fabrication | Gas biomarkers in human breath | Indication of related diseases | Ref. |
|---|---|---|---|---|---|
| 1. | Au NPs-reduced GO nanocomposites | GO prepared by Hummers method followed by deposition of a 300 nm thick Au film by sputtering | 14 VOC biomarkers in human breath | EGC and AGC | 24 |
| 2. | Au NPs and MIL-100 (Fe) MOFs | Au NPs colloid solution was prepared and dropped on MIL-100(Fe) MOFs | 4-Ethylbenzaldehyde, acetone, and isopropanol | Lung cancer | 72 |
| 3. | Au coated Si nanopillars | Reactive ion etching of Si wafer followed by Au sputtered deposition | HCN is known to be Pseudomonas aeruginosa (PA) biomarker | Lung infections in CF | 113, 114 |
| 4. | ZIF-8 layer coated with Au superparticles | Au superparticles synthesized by microemulsion assembly method coated by A ≈ 150 nm thick shell of ZIF-8 | Gaseous aldehydes | Early-stage lung cancer | 115 |
| 5. | Tenax-TA deposited on a layer of Ag-nanosphere | Ag nanosphere from AgNO3 reduction followed by a coating of Tenax-TA polymer solution | Cotton VOCs | Caterpillar infestation in plants | 116 |
4.2 Promising detection of SARS-CoV-2 in the breath of COVID-19 patients
Since 2020, the whole world has been fighting the most damaging coronavirus pandemic, and even after the development of many effective vaccines, provided little relax from the pandemic situation. There is still a chance of spreading more dangerous variants of the coronavirus. It shows the urgent need to develop novel technologies, medical innovations, or innovative materials for controlling SARS-CoV-2 infection. The mode of infection of SARS-CoV-2 could be through the surface, air, or water.117 However, the problem of the coronavirus is still a question as 2nd and 3rd waves in 2021 and 2022, respectively, could not be controlled and more are expected in the future with a possibility of new corona variants. It has been reported in many surveys and recent articles that the spreading of coronavirus takes place via airborne transmission of the SARS-CoV-2 virus through aerosols.117,118 Efficient detection of SARS-CoV-2 from exhaled breath is required because it can provide a more accurate and direct way to detect the virus and minimize community transmissions.119,120 However, detection of SARS-CoV-2 in aerosols/breath and studying the risk of the same is not so easy and is challenging. Shan et al.121 demonstrated that a nanomaterial-based sensor array with multiplexed capabilities for the detection and monitoring of COVID-19 from exhaled breath could be possible. They fabricated a sensor composed of different Au NPs linked to organic ligands. The system developed a sensing layer that could be swelled or shrunk upon exposure to VOCs, leading to a change in the electric signals. Recently, Subali et al.122 presented a review on the potential of VOCs based breath analysis for COVID-19 screening using gas chromatography and mass spectrometry methods. It was concluded that COVID-19 patients showed a distinct pattern of VOCs in their breath analysis.Li et al.118 fabricated a swirling aerosol collection device for collecting viral particles in exhaled breath emitted by a patient and subsequently detected SARS-CoV-2 using reverse transcription-polymerase chain reaction (RT-PCR). Recently, Viklund et al.123 demonstrated that SARS-CoV-2 can be detected in exhaled aerosol/breath sampled during a few minutes of breathing or coughing. This is one of the most reliable diagnostic techniques for viral/bacterial infection along with some other techniques82,86 (Fig. 7). However, the RT-PCR method takes a long time to diagnose SARS-CoV-2 because of the required thermocycling steps.124 Wu et al. recently demonstrated that SERS-based PCR detection method using Au NPs could be useful in shortening the diagnosis time.124 SERS is a paradigmatic example of a nanomaterial-based highly sensitive diagnostic method as it has shown its potential with great capability for very low-level specific identification of various viruses, bacteria, and other pathogens.125,126 The use of SERS in the detection of viruses/bacteria is promising because of its feasibility in biosensing. As discussed above, it is a simple technique that utilizes SERS-active nanomaterials as substrate and a Raman spectrometer for the detection of biochemical structures and can be used in the fast detection of viruses like SARS-CoV-2 virus.125,126 It is better than a biochemical-based diagnosis, which needs many chemicals and other complicated processes.
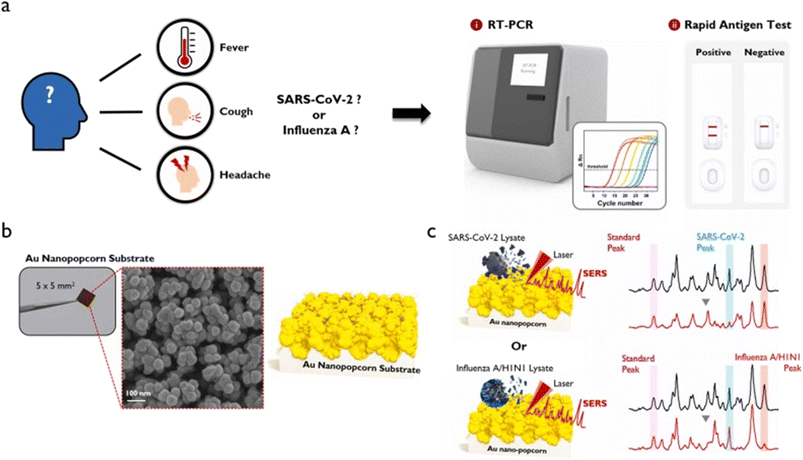 | ||
| Fig. 7 (a) Symptoms that appear when infected with SARS-CoV-2 or influenza A, and RT-PCR and rapid antigen kit for their diagnosis. (b) Photograph and SEM image of Au nanopopcorn substrate. (c) Working principle of dual aptamer-immobilized Au nanopopcorn substrate for virus assays. Reprinted with permission from ref. 86. | ||
Recently, Chen et al.86 developed SERS-based dual functional sensor for rapid classification of SARS-CoV-2 and influenza A/H1N1 infection (Fig. 7). Au nanopopcorn based SERS substrate was fabricated and DNA aptamers that selectively bind to SARS-CoV-2 and influenza A/H1N1 were immobilized together on Au nanopopcorn substrate. Raman reporters (Cy3 and RRX), attached to the terminal of DNA aptamers, could generate strong SERS signals in the nanogap of the Au nanopopcorn substrate. When SARS-CoV-2 or influenza A virus approached the Au nanopopcorn substrate, the corresponding DNA aptamer selectively detached from the substrate due to the significant binding affinity between the corresponding DNA aptamer and the virus resulting in the SERS intensity change with increasing target virus concentration. In this way, it was concluded that it could be possible to estimate whether a suspected patient is infected with SARS-CoV-2 or influenza A using the SERS-based DNA aptasensor as shown in Fig. 7.86
There is limited use of SERS-based diagnosis of SARS-CoV-2 virus, which shows its great potential to be used for fast screening of SARS-CoV-2 virus and suitable for such pandemics.87 In a recent review, Liu et al.87 discussed an overview of fundamental knowledge about the SERS mechanism, including chemical and electromagnetic field enhancement mechanisms, the design and fabrication of SERS substrates based on materials, progress in using SERS for point-of-care testing in biochemical sensing, including SARS-CoV-2 virus and its clinical applications. SERS technique has been used to identify a Raman fingerprint of SARS-CoV-2 in the saliva of patients affected by COVID-19.85,128 As an immune response to COVID-19 infection, generally, patients produce SARS-CoV-2-specific antibodies, which are directed against specific proteins.82,127 SERS has been used for the detection of SARS-CoV-2-specific antibodies IgM/IgG in sera of COVID-19 patients using conventional lateral flow assay.129–131 Daoudi et al.127 demonstrated SERS sensing of SARS-CoV-2 S1 spike protein of the SARS-CoV-2 virus family by fabricating AgNPs/SiNWs nanohybrid based sensors, as shown in Fig. 8(step 1–3). The Si NWs have an important impact on SERS, not only by increasing the specific surface area but also by enhancing the local electromagnetic field.132,133 Furthermore, the flexible SERS substrates are more conducive to gas detection.134 These were prepared by reactive ion etching and Ag NPs deposition. These SiNWs/AgNPs SERS-based sensors were used to detect the spike protein at a concentration down to 9.3 × 10−12 M. Strong and dominant Raman peaks at 1280, 1404, 1495, 1541, and 1609 cm−1 were observed at a fraction of a minute corresponding to various CH2 wagging more of several amino acids of spike proteins present in the virus (Fig. 8a–g).127
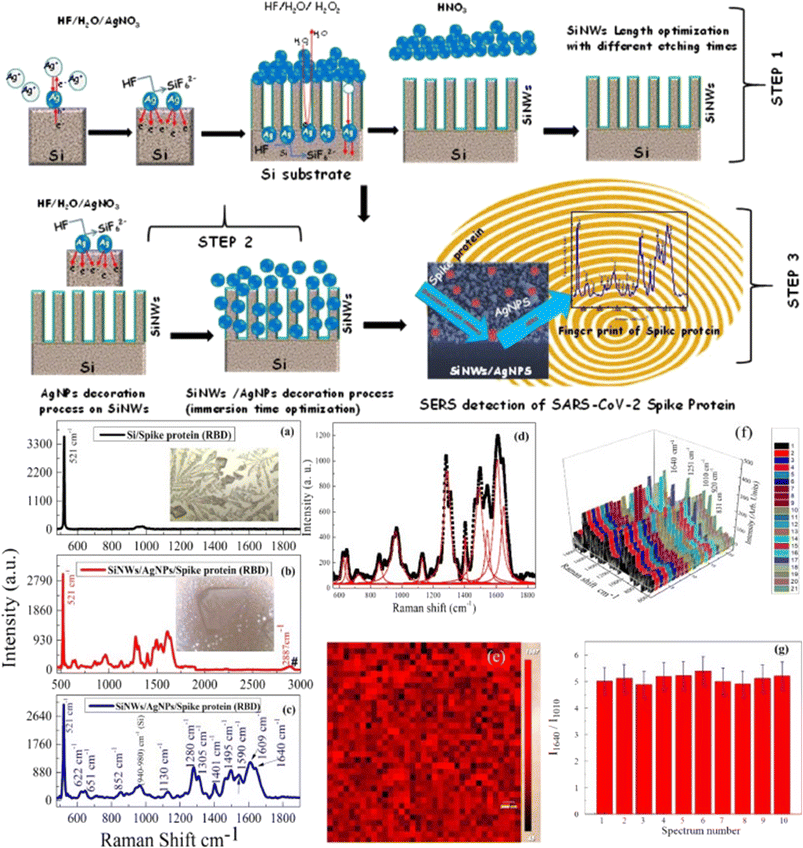 | ||
| Fig. 8 Step 1–3: Schematic for fabrication and fast optical detection of the spike protein of the SARS-COV-2 using Si–Ag based SERS substrates. Raman spectra of SARS-CoV-2 spike protein on (a) flat Si and Si–Ag based SERS substrate between (b) 500 cm−1 to 3000 cm−1 and (c) 500 cm−1 to 1800 cm−1. Insets are the optical microscopy images of spike proteins. (d) Lorentz fit for the experimental data (e) mapping of spike protein detection on SERS substrate. (f) Various scans taken randomly at many points and (g) the corresponding statistical analysis of Raman peak intensity ratios (I1640/I1010) for 10 different positions. Reprinted with permission from ref. 127. | ||
Zhang et al.135 demonstrated that using portable Raman spectrometers and Ag-nanorod (of diameter and length of 211 ± 45 and 737 ± 52 nm, respectively)-based SERS substrates, ultra-fast and onsite interrogation of SARS-CoV-2 in water could be carried out within 5 min. These Ag nanorod based SERS substrates were prepared by oblique angle deposition on Si substrate by electron evaporation/sputtering method. More than 23 water samples were tested with accuracy onsite, which proved that it has the potential to be used as an easy and portable measurement for spot detection of SARS-CoV-2 to evaluate disinfection performance and explore viral survival in environmental media. Similarly, Leong et al.120 designed a noninvasive and point-of-care handheld portable SERS sensor-based breathalyzer containing a chip with Ag nanocubes (of length 120 ± 5 nm) as SERS active substrates. It was explained that the exhaled breath could be filled into the breathalyzer of the SERS device for a few seconds, where the breath components would interact with the SERS active substrates. Once the breathalyzer was loaded into a portable Raman spectrometer, potential COVID-19 breath biomarkers could be detected by analyzing the SERS spectra. Breath VOC biomarkers have been detected for COVID-19,120,136 such as the work demonstrated by Leong et al.,120 that SERS spectra of the breath of COVID-positive and -negative people were different in regions responsive to ketones, alcohols, and aldehydes. The collected data were used to develop a statistical model for COVID diagnosis, in which over 500 people were tested using the developed breathalyzer and compared with their RT-PCR tests. Results were found to be very close in both the tests; however, the SERS-based test could be performed very fast as compared to RT-PCR and was carried out on-site. It was concluded that developing this type of breathalyzer could be a potential tool to detect COVID-19 or any other viruses directly and would open a way to stop the community transmissions.
It is evident from the above discussed literature that SERS based detection of SARS-CoV-2 in the breath of COVID-19 patients is promising as compared to the traditional methods and the advancement of portable Raman spectrophotometer has made it more convenient. It provides a facility to do the detection on site without visiting the laboratory, which is generally not advised for COVID-19 patients. More efforts are needed to design and tailor the nanomaterials for tuning surface area and flexibility. It will help in tuning their SERS activity for better sensitivity to SARS-CoV-2 or other dangerous viruses.
5. Summary, challenges, and future prospects
The ultrasensitive detection of toxic and harmful gases is required for industrial process monitoring and environmental control as well as for medical diagnostics in human health. Nanomaterials are at the heart of nanotechnology and are being utilized as promising and primary materials for modern techniques in the field of energy, environment, and biomedical. In this context, nanomaterials based on the SERS technique is one of such techniques being used as a multidisciplinary tool in almost all field of science. This technique uses novel functional nanomaterials based on noble metals or semiconductors, providing higher sensitivity and selectivity for the detection and sensing of chemical or biological molecules. SERS provides a rapid, highly sensitive/selective tool and direct evidence of analyte molecules as compared to the traditional gas sensing techniques with a special property of discriminating several molecules based on their unique vibrational fingerprints with a high spectral resolution.In this review, the emerging application of SERS as a gas sensing tool for the detection of toxic and harmful gases has been discussed. Particularly, the use of SERS has been explored because of its emerging and potential applications for gas sensing in the environment and human health, with emphasis on the recent advances in these fields. In recent years, SERS has shown promising application in the detection of gas biomarkers in the human breath to detect related diseases in the human body, which has been discussed in detail. It is simpler and more favorable for sensing environmental gases or diagnostic human health using handheld portable Raman spectrometers. The detection of breath gases/VOCs using such a tool provides an excellent way to detect SARS-CoV-2 or similar viruses directly from the breath of the patient, which is at present limited by bulky instrumentation and inflexible analysis protocol. Recent advancements in detecting SARS-CoV-2 in the breath of COVID-19 patients have been discussed with emphasis on the use of portable SERS for direct and fast detection. There are only a few reports available on this important research in the literature.
However, the use of SERS nanotechnology has not been explored much yet in the field of environmental gas sensing or for the detection of gas biomarker/SARS-CoV-2/other viruses in human breath and need more attention for practical applications in society. Therefore, there is a big scope for developing nanomaterial based SERS techniques in the field of environment and human health. This has led to several open platforms to work further and explore the research in several directions – (i) poor affinity of gaseous molecules on the surface of the metal NPs is a big challenge for ultrasensitive detection using SERS.2 This interaction needs more experimental and theoretical work to provide a better protocol for surface modification or to develop efficient nanomaterials to better enrich molecules on the metallic surfaces for highly selective SERS gas sensors.91,93 (ii) In the case of environmental gas sensing, sometimes cooling of the substrate for better adsorption of the gas molecules on the surface of the SERS active substrate is required, which limits the practical applications and also stability of SERS measurement that need to be explored in the future research.101 (iii) Portable SERS technology could emerge as a promising candidate for point-of-care testing using SERS detection technology, which needs attention to explore.87 (iv) For practical applications of the SERS technique, proper integration of SERS active nanomaterials with handheld portable Raman spectrometers is required for higher sensitivity and gas/virus selectivity capabilities.110 (v) There is an urgent need to explore SERS based research for virus detection, such as SARS-CoV-2, for direct and fast detection, which is limited by the traditional approaches.84 (vi) Looking at the current COVID-19 pandemic, the development of a highly sensitive, portable, and cost effective technique for direct breath analysis of COVID-19 patients or any viral infection would be a game-changer in this field of sensors and diagnostics.137
In summary, the nanomaterial-based SERS technique is a promising sensing tool for various applications in the field of environmental control to human health for diagnosis. It has the potential to compete with other traditional methods with high sensitivity and selectivity. Eventually, it can play a vital role in the direct detection of SARS-CoV-2 in the exhaled breath of COVID-19 patients with portable applicability for fast detection, which is the need of the hour and has a lot of future research possibilities to work in this direction.
Conflicts of interest
The authors declare that they don't have any known competing financial interests or personal relationships that could have appeared to influence the work reported in this review article.Acknowledgements
The author (JP) thanks the Department of Science and Technology (DST), India, for the prestigious INSPIRE Faculty award [INSPIRE/04/2015/002452(IFA15-MS-57)] along with research grant.References
- L. Zhang and M. Fang, Nanomaterials in pollution trace detection and environmental improvement, Nano Today, 2010, 5, 128–142 CrossRef CAS.
- V. Snitka, D. Batiuskaite, I. Bruzaite, U. Lafont, Y. Butenko and C. Semprimoschnig, Surface-enhanced Raman scattering sensors for biomedical and molecular detection applications in space, CEAS Space J., 2021, 13, 509–520 CrossRef PubMed.
- L. Huang, Y. Zhu, C. Xu, Y. Cai, Y. Yi and K. Li, et al., Noninvasive Diagnosis of Gastric Cancer Based on Breath Analysis with a Tubular Surface-Enhanced Raman Scattering Sensor, ACS Sens., 2022, 7, 1439–1450 CrossRef CAS PubMed.
- W.-L. Wang, J.-L. Lu, J.-L. Gu, L.-F. Xie, J. Chang and B. Zou, et al., Rapid qualitative and quantitative analysis of trace aconitum phytotoxin by SERS, Food Chem., 2022, 391, 133234 CrossRef CAS PubMed.
- J. Sun, Z. Zhang, H. Li, H. Yin, P. Hao and X. Dai, et al., Ultrasensitive SERS Analysis of Liquid and Gaseous Putrescine and Cadaverine by a 3D-Rosettelike Nanostructure-Decorated Flexible Porous Substrate, Anal. Chem., 2022, 94, 5273–5283 CrossRef CAS PubMed.
- J. Prakash, Samriti, A. Kumar, H. Dai, B. C. Janegitz and V. Krishnan, et al., Novel rare earth metal–doped one-dimensional TiO2 nanostructures: Fundamentals and multifunctional applications, Mater. Today Sustain., 2021, 13, 100066 CrossRef.
- Q. Feng, B. Huang and X. Li, Graphene-Based Heterostructure Composite Sensing Materials for Detection of Nitrogen-Containing Harmful Gases, Adv. Funct. Mater., 2021, 31, 2104058 CrossRef CAS.
- J. Prakash, S. Sun, H. C. Swart and R. K. Gupta, Noble metals-TiO2 nanocomposites: From fundamental mechanisms to photocatalysis, surface enhanced Raman scattering and antibacterial applications, Appl. Mater. Today, 2018, 11, 82–135 CrossRef.
- Y. Zeng, S. Lin, D. Gu and X. Li, Two-Dimensional Nanomaterials for Gas Sensing Applications: The Role of Theoretical Calculations, Nanomaterials, 2018, 8, 851 CrossRef PubMed.
- Q. Feng, Y. Zeng, P. Xu, S. Lin, C. Feng and X. Li, et al., Tuning the electrical conductivity of amorphous carbon/reduced graphene oxide wrapped-Co3O4 ternary nanofibers for highly sensitive chemical sensors, J. Mater. Chem. A, 2019, 7, 27522–27534 RSC.
- B. C. Janegitz, T. A. Silva, A. Wong, L. Ribovski, F. C. Vicentini and M. P. Taboada Sotomayor, et al., The application of graphene for in vitro and in vivo electrochemical biosensing, Biosens. Bioelectron., 2017, 89, 224–233 CrossRef CAS.
- X. Wang, X. Li, Y. Zhao, Y. Chen, J. Yu and J. Wang, The influence of oxygen functional groups on gas-sensing properties of reduced graphene oxide (rGO) at room temperature, RSC Adv., 2016, 6, 52339–52346 RSC.
- J. Wu, Z. Wu, H. Ding, Y. Wei, W. Huang and X. Yang, et al., Flexible, 3D SnS2/Reduced graphene oxide heterostructured NO2 sensor, Sens. Actuators, B, 2020, 305, 127445 CrossRef CAS.
- H. Bai, H. Guo, J. Wang, Y. Dong, B. Liu and Z. Xie, et al., A room-temperature NO2 gas sensor based on CuO nanoflakes modified with rGO nanosheets, Sens. Actuators, B, 2021, 337, 129783 CrossRef CAS.
- A. G. Bannov, M. V. Popov, A. E. Brester and P. B. Kurmashov, Recent Advances in Ammonia Gas Sensors Based on Carbon Nanomaterials, Micromachines, 2021, 12, 186 CrossRef.
- S. B. Singh and H. C. Lin, Hydrogen Sulfide in Physiology and Diseases of the Digestive Tract, Microorganisms, 2015, 3, 866–889 CrossRef CAS PubMed.
- C. H. K. Wu, The role of hydrogen sulphide in lung diseases, Biosci. Horiz., 2013, 6, hzt009 Search PubMed.
- X. Zhou, Z. Xue, X. Chen, C. Huang, W. Bai and Z. Lu, et al., Nanomaterial-based gas sensors used for breath diagnosis, J. Mater. Chem. B, 2020, 8, 3231–3248 RSC.
- P. Španěl and D. Smith, What is the real utility of breath ammonia concentration measurements in medicine and physiology?, J. Breath Res., 2018, 12, 027102 CrossRef PubMed.
- P. Trefz, M. Schmidt, P. Oertel, J. Obermeier, B. Brock and S. Kamysek, et al., Continuous Real Time Breath Gas Monitoring in the Clinical Environment by Proton-Transfer-Reaction-Time-of-Flight-Mass Spectrometry, Anal. Chem., 2013, 85, 10321–10329 CrossRef CAS PubMed.
- B. Henderson, G. Lopes Batista, C. G. Bertinetto, J. Meurs, D. Materić and C. C. W. G. Bongers, et al., Exhaled Breath Reflects Prolonged Exercise and Statin Use during a Field Campaign, Metabolites, 2021, 11, 192 CrossRef CAS PubMed.
- H. Li, J. Wu, A. Wang, X. Li, S. Chen and T. Wang, et al., Effects of ambient carbon monoxide on daily hospitalizations for cardiovascular disease: a time-stratified case-crossover study of 460,938 cases in Beijing, China from 2013 to 2017, Environ. Health, 2018, 17, 82 CrossRef CAS PubMed.
- S. I. Rae and I. Khan, Surface enhanced Raman spectroscopy (SERS) sensors for gas analysis, Analyst, 2010, 135, 1365–1369 RSC.
- Y. Chen, Y. Zhang, F. Pan, J. Liu, K. Wang and C. Zhang, et al., Breath Analysis Based on Surface-Enhanced Raman Scattering Sensors Distinguishes Early and Advanced Gastric Cancer Patients from Healthy Persons, ACS Nano, 2016, 10, 8169–8179 CrossRef CAS PubMed.
- A. K. Davey, X. Gao, Y. Xia, Z. Li, M. N. Dods and S. Delacruz, et al., Amine-functionalized metal-organic framework ZIF-8 toward colorimetric CO2 sensing in indoor air environment, Sens. Actuators, B, 2021, 344, 130313 CrossRef CAS.
- X. Yang, A. S. P. Chang, B. Chen, C. Gu and T. C. Bond, High sensitivity gas sensing by Raman spectroscopy in photonic crystal fiber, Sens. Actuators, B, 2013, 176, 64–68 CrossRef CAS.
- J. Krämer, R. Kang, L. M. Grimm, L. De Cola, P. Picchetti and F. Biedermann, Molecular Probes, Chemosensors, and Nanosensors for Optical Detection of Biorelevant Molecules and Ions in Aqueous Media and Biofluids, Chem. Rev., 2022, 122(3), 3459–3636 CrossRef PubMed.
- L. V. Jodar, F. A. Santos, V. Zucolotto and B. C. Janegitz, Electrochemical sensor for estriol hormone detection in biological and environmental samples, J. Solid State Electrochem., 2018, 22, 1431–1438 CrossRef CAS.
- G. C. M. de Oliveira, J. R. Camargo, N. C. S. Vieira and B. C. Janegitz, A new disposable electrochemical sensor on medical adhesive tape, J. Solid State Electrochem., 2020, 24, 2271–2278 CrossRef CAS.
- W. S. Fernandes-Junior, L. F. Zaccarin, G. G. Oliveira, P. R. de Oliveira, C. Kalinke and J. A. Bonacin, et al., Electrochemical Sensor Based on Nanodiamonds and Manioc Starch for Detection of Tetracycline, J. Sens., 2021, 2021, 6622612 Search PubMed.
- S.-B. Wang, Y.-F. Huang, S. Chattopadhyay, S. Jinn Chang, R.-S. Chen and C.-W. Chong, et al., Surface plasmon-enhanced gas sensing in single gold-peapodded silica nanowires, NPG Asia Mater., 2013, 5, e49 CrossRef CAS.
- T. K. Pathak, V. Kumar, J. Prakash, L. P. Purohit, H. C. Swart and R. E. Kroon, Fabrication and characterization of nitrogen doped p-ZnO on n-Si heterojunctions, Sens. Actuators, A, 2016, 247, 475–481 CrossRef CAS.
- J. Prakash, J. C. Pivin and H. C. Swart, Noble metal nanoparticles embedding into polymeric materials: From fundamentals to applications, Adv. Colloid Interface Sci., 2015, 226, 187–202 CrossRef CAS PubMed.
- J. Prakash, H. C. Swart, G. Zhang and S. Sun, Emerging applications of atomic layer deposition for the rational design of novel nanostructures for surface-enhanced Raman scattering, J. Mater. Chem. C, 2019, 7, 1447–1471 RSC.
- N. Singh, J. Prakash and R. K. Gupta, Design and engineering of high-performance photocatalytic systems based on metal oxide–graphene–noble metal nanocomposites, Mol. Syst. Des. Eng., 2017, 2, 422–439 RSC.
- B. Balasubramaniam, N. Singh, P. Kar, A. Tyagi, J. Prakash and R. K. Gupta, Engineering of transition metal dichalcogenide-based 2D nanomaterials through doping for environmental applications, Mol. Syst. Des. Eng., 2019, 4, 804–827 RSC.
- N. Singh, J. Prakash, M. Misra, A. Sharma and R. K. Gupta, Dual Functional Ta-Doped Electrospun TiO2 Nanofibers with Enhanced Photocatalysis and SERS Detection for Organic Compounds, ACS Appl. Mater. Interfaces, 2017, 9, 28495–28507 CrossRef CAS PubMed.
- J. Prakash, R. A. Harris and H. C. Swart, Embedded plasmonic nanostructures: synthesis, fundamental aspects and their surface enhanced Raman scattering applications, Int. Rev. Phys. Chem., 2016, 35, 353–398 Search PubMed.
- J. Prakash, V. Kumar, R. E. Kroon, K. Asokan, V. Rigato and K. H. Chae, et al., Optical and surface enhanced Raman scattering properties of Au nanoparticles embedded in and located on a carbonaceous matrix, Phys. Chem. Chem. Phys., 2016, 18, 2468–2480 RSC.
- J. Prakash, Fundamentals and applications of recyclable SERS substrates, Int. Rev. Phys. Chem., 2019, 38, 201–242 Search PubMed.
- G. C. Phan-Quang, H. K. Lee, H. W. Teng, C. S. L. Koh, B. Q. Yim and E. K. M. Tan, et al., Plasmonic Hotspots in Air: An Omnidirectional Three-Dimensional Platform for Stand-Off In-Air SERS Sensing of Airborne Species, Angew. Chem., Int. Ed., 2018, 57, 5792–5796 CrossRef CAS.
- S. K. Sharma, P. Kumar, S. Barthwal, S. Sharma and A. Sharma, Highly Sensitive Surface-Enhanced Raman Scattering (SERS)- Based Multi Gas Sensor : Au Nanoparticles Decorated on Partially Embedded 2D Colloidal Crystals into Elastomer, ChemistrySelect, 2017, 2, 6961–6969 CrossRef CAS.
- P. Kumar, M. C. Mathpal, A. K. Tripathi, J. Prakash, A. Agarwal and M. M. Ahmad, et al., Plasmonic resonance of Ag nanoclusters diffused in soda-lime glasses, Phys. Chem. Chem. Phys., 2015, 17, 8596–8603 RSC.
- M. Fan, F.-J. Lai, H.-L. Chou, W.-T. Lu, B.-J. Hwang and A. G. Brolo, Surface-enhanced Raman scattering (SERS) from Au:Ag bimetallic nanoparticles: the effect of the molecular probe, Chem. Sci., 2013, 4, 509–515 RSC.
- G. M. Das, R. V. William, V. R. Dantham and R. Laha, Study on SERS activity of Au-Ag bimetallic nanostructures synthesized using different reducing agents, Phys. E, 2021, 129, 114656 CrossRef CAS.
- P. Kumar, M. Chandra Mathpal, J. Prakash, B. C. Viljoen, W. D. Roos and H. C. Swart, Band gap tailoring of cauliflower-shaped CuO nanostructures by Zn doping for antibacterial applications, J. Alloys Compd., 2020, 832, 154968 CrossRef CAS.
- Samriti, Prateek, M. C. Joshi, R. K. Gupta and J. Prakash, Hydrothermal synthesis and Ta doping of TiO2 nanorods: Effect of soaking time and doping on optical and charge transfer properties for enhanced SERS activity, Mater. Chem. Phys., 2022, 278, 125642 CrossRef CAS.
- Samriti, V. Rajput, R. K. Gupta and J. Prakash, Engineering metal oxide semiconductor nanostructures for enhanced charge transfer: fundamentals and emerging SERS applications, J. Mater. Chem. C, 2022, 10, 73–95 RSC.
- J. Prakash, P. Kumar, R. A. Harris, C. Swart, J. H. Neethling and A. J. van Vuuren, et al., Synthesis, characterization and multifunctional properties of plasmonic Ag–TiO<sub>2</sub>nanocomposites, Nanotechnology, 2016, 27, 355707 CrossRef PubMed.
- M. C. Mathpal, P. Kumar, S. Kumar, A. K. Tripathi, M. K. Singh and J. Prakash, et al., Opacity and plasmonic properties of Ag embedded glass based metamaterials, RSC Adv., 2015, 5, 12555–12562 RSC.
- E. Ashok Kumar, N. Riswana Barveen, T.-J. Wang, T. Kokulnathan and Y.-H. Chang, Development of SERS platform based on ZnO multipods decorated with Ag nanospheres for detection of 4-nitrophenol and rhodamine 6G in real samples, Microchem. J., 2021, 170, 106660 CrossRef CAS.
- J. Prakash, A. Tripathi, V. Rigato, J. C. Pivin, J. Tripathi and K. H. Chae,
et al., Synthesis of Au nanoparticles at the surface and embedded in carbonaceous matrix by 150
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) keV Ar ion irradiation, J. Phys. D: Appl. Phys., 2011, 44, 125302 CrossRef.
keV Ar ion irradiation, J. Phys. D: Appl. Phys., 2011, 44, 125302 CrossRef. - J. Prakash, A. Tripathi, S. Gautam, K. H. Chae, J. Song and V. Rigato, et al., Phenomenological understanding of dewetting and embedding of noble metal nanoparticles in thin films induced by ion irradiation, Mater. Chem. Phys., 2014, 147, 920–924 CrossRef CAS.
- P. Kumar, M. C. Mathpal, J. Prakash, S. Hamad, S. V. Rao and B. C. Viljoen, et al., Study of Tunable Plasmonic, Photoluminscence, and Nonlinear Optical Behavior of Ag Nanoclusters Embedded in a Glass Matrix for Multifunctional Applications, Phys. Status Solidi A, 2019, 216, 1800768 CrossRef.
- P. Kumar, M. Chandra Mathpal, J. Prakash, G. Jagannath, W. D. Roos and H. C. Swart, Plasmonic and nonlinear optical behavior of nanostructures in glass matrix for photonics application, Mater. Res. Bull., 2020, 125, 110799 CrossRef CAS.
- S. Kim, D.-H. Kim and S.-G. Park, Highly sensitive and on-site NO2 SERS sensors operated under ambient conditions, Analyst, 2018, 143, 3006–3010 RSC.
- T. Gupta, Samriti, J. Cho and J. Prakash, Hydrothermal synthesis of TiO2 nanorods: formation chemistry, growth mechanism, and tailoring of surface properties for photocatalytic activities, Mater. Today Chem., 2021, 20, 100428 CrossRef CAS.
- V. Kumar, J. Prakash, J. P. Singh, K. H. Chae, C. Swart and O. M. Ntwaeaborwa, et al., Role of silver doping on the defects related photoluminescence and antibacterial behaviour of zinc oxide nanoparticles, Colloids Surf., B, 2017, 159, 191–199 CrossRef CAS.
- J. Prakash, V. Kumar, L. J. B. Erasmus, M. M. Duvenhage, G. Sathiyan and S. Bellucci, et al., Phosphor Polymer Nanocomposite: ZnO:Tb3+ Embedded Polystyrene Nanocomposite Thin Films for Solid-State Lighting Applications, ACS Appl. Nano Mater., 2018, 1, 977–988 CrossRef CAS.
- P. Liang, Y. Cao, Q. Dong, D. Wang, D. Zhang and S. Jin, et al., A balsam pear-shaped CuO SERS substrate with highly chemical enhancement for pesticide residue detection, Microchim. Acta, 2020, 187, 335 CrossRef CAS.
- X. Liang, N. Li, R. Zhang, P. Yin, C. Zhang and N. Yang, et al., Carbon-based SERS biosensor: from substrate design to sensing and bioapplication, NPG Asia Mater., 2021, 13, 8 CrossRef CAS.
- Samriti, Manisha, Z. Chen, S. Sun and J. Prakash, Design and engineering of graphene nanostructures as independent solar-driven photocatalysts for emerging applications in the field of energy and environment, Mol. Syst. Des. Eng., 2022, 7, 213–238 RSC.
- J. Prakash, Mechanistic Insights into Graphene Oxide Driven Photocatalysis as Co-Catalyst and Sole Catalyst in Degradation of Organic Dye Pollutants, Photochem, 2022, 2, 651–671 CrossRef.
- D. Mathivanan, K. S. Shalini Devi, G. Sathiyan, A. Tyagi, V. A. O. P. da Silva and B. C. Janegitz, et al., Novel polypyrrole-graphene oxide-gold nanocomposite for high performance hydrogen peroxide sensing application, Sens. Actuators, A, 2021, 328, 112769 CrossRef CAS.
- S. Verma, D. S. Mal, P. R. de Oliveira, B. C. Janegitz, J. Prakash and R. K. Gupta, A facile synthesis of novel polyaniline/graphene nanocomposite thin films for enzyme-free electrochemical sensing of hydrogen peroxide, Mol. Syst. Des. Eng., 2022, 7, 158–170 RSC.
- Z. Li, S. C. Xu, C. Zhang, X. Y. Liu, S. S. Gao and L. T. Hu, et al., High-performance SERS substrate based on hybrid structure of graphene oxide/AgNPs/Cu film@pyramid Si, Sci. Rep., 2016, 6, 38539 CrossRef CAS PubMed.
- N. Myoung, H. K. Yoo and I.-W. Hwang, Raman gas sensing of modified Ag nanoparticle SERS, SPIE, 2014 Search PubMed.
- M. Zhang, Y. Liu, P. Jia, Y. Feng, S. Fu and J. Yang, et al., Ag Nanoparticle-Decorated Mesoporous Silica as a Dual-Mode Raman Sensing Platform for Detection of Volatile Organic Compounds, ACS Appl. Nano Mater., 2021, 4, 1019–1028 CrossRef CAS.
- W. Zhu, J. A. Hutchison, M. Dong and M. Li, Frequency Shift Surface-Enhanced Raman Spectroscopy Sensing: An Ultrasensitive Multiplex Assay for Biomarkers in Human Health, ACS Sens., 2021, 6, 1704–1716 CrossRef CAS PubMed.
- J. Plou, P. S. Valera, I. García, C. D. L. de Albuquerque, A. Carracedo and L. M. Liz-Marzán, Prospects of Surface-Enhanced Raman Spectroscopy for Biomarker Monitoring toward Precision Medicine, ACS Photonics, 2022, 9, 333–350 CrossRef CAS PubMed.
- C. Song, Y. Wang, Y. Lei and J. Zhao, SERS-Enabled Sensitive Detection of Plant Volatile Biomarker Methyl Salicylate, J. Phys. Chem. C, 2022, 126, 772–778 CrossRef CAS.
- J.-H. Fu, Z. Zhong, D. Xie, Y.-J. Guo, D.-X. Kong and Z.-X. Zhao, et al., SERS-Active MIL-100(Fe) Sensory Array for Ultrasensitive and Multiplex Detection of VOCs, Angew. Chem., Int. Ed., 2020, 59, 20489–20498 CrossRef CAS PubMed.
- Y. Hang, J. Boryczka and N. Wu, Visible-light and near-infrared fluorescence and surface-enhanced Raman scattering point-of-care sensing and bio-imaging: a review, Chem. Soc. Rev., 2022, 51, 329–375 RSC.
- S. Fornasaro, D. Cialla-May, V. Sergo and A. Bonifacio, The Role of Surface Enhanced Raman Scattering for Therapeutic Drug Monitoring of Antimicrobial Agents, Chemosensors, 2022, 10, 128 CrossRef CAS.
- L. Song, J. Chen, B. B. Xu and Y. Huang, Flexible Plasmonic Biosensors for Healthcare Monitoring: Progress and Prospects, ACS Nano, 2021, 15, 18822–18847 CrossRef CAS PubMed.
- M. Haroon, M. Tahir, H. Nawaz, M. I. Majeed and A. A. Al-Saadi, Surface-enhanced Raman scattering (SERS) spectroscopy for prostate cancer diagnosis: A review, Photodiagn. Photodyn. Ther., 2022, 37, 102690 CrossRef CAS.
- A. Kozik, M. Pavlova, I. Petrov, V. Bychkov, L. Kim and E. Dorozhko, et al., A review of surface-enhanced Raman spectroscopy in pathological processes, Anal. Chim. Acta, 2021, 1187, 338978 CrossRef CAS PubMed.
- V. Cupil-Garcia, P. Strobbia, B. M. Crawford, H.-N. Wang, H. Ngo and Y. Liu, et al., Plasmonic nanoplatforms: From surface-enhanced Raman scattering sensing to biomedical applications, J. Raman Spectrosc., 2021, 52, 541–553 CrossRef CAS.
- T. Wang, P. Dong, C. Zhu, W. Gao, P. Sha and Y. Wu, et al., Fabrication of 2D titanium carbide MXene/Au nanorods as a nanosensor platform for sensitive SERS detection, Ceram. Int., 2021, 47, 30082–30090 CrossRef CAS.
- J. Prakash, Samriti, D. N. Wijesundera, I. Rajapaksa and W.-K. Chu, Ion beam nanoengineering of surfaces for molecular detection using surface enhanced Raman scattering, Mol. Syst. Des. Eng., 2022, 7, 411–421 RSC.
- M. Wu, M. He, Q. Hu, Q. Wu, G. Sun and L. Xie, et al., Ti3C2 MXene-Based Sensors with High Selectivity for NH3 Detection at Room Temperature, ACS Sens., 2019, 4, 2763–2770 CrossRef CAS PubMed.
- J. Sitjar, J.-D. Liao, H. Lee, H.-P. Tsai, J.-R. Wang and P.-Y. Liu, Challenges of SERS technology as a non-nucleic acid or -antigen detection method for SARS-CoV-2 virus and its variants, Biosens. Bioelectron., 2021, 181, 113153 CrossRef CAS PubMed.
- N. Feliu, M. Hassan, E. Garcia Rico, D. Cui, W. Parak and R. Alvarez-Puebla, SERS Quantification and Characterization of Proteins and Other Biomolecules, Langmuir, 2017, 33, 9711–9730 CrossRef CAS PubMed.
- I. N. Kurochkin, A. V. Eremenko, E. G. Evtushenko, N. L. Nechaeva, N. N. Durmanov and R. R. Guliev, et al., SERS for Bacteria, Viruses, and Protein Biosensing, in Macro, Micro, and Nano-Biosensors: Potential Applications and Possible Limitations, ed. M. Rai, A. Reshetilov, Y. Plekhanova and A. P. Ingle, Springer International Publishing, Cham, 2021, pp. 75–94 Search PubMed.
- M. Zhang, X. Li, J. Pan, Y. Zhang, L. Zhang and C. Wang, et al., Ultrasensitive detection of SARS-CoV-2 spike protein in untreated saliva using SERS-based biosensor, Biosens. Bioelectron., 2021, 190, 113421 CrossRef CAS PubMed.
- H. Chen, S.-K. Park, Y. Joung, T. Kang, M.-K. Lee and J. Choo, SERS-based dual-mode DNA aptasensors for rapid classification of SARS-CoV-2 and influenza A/H1N1 infection, Sens. Actuators, B, 2022, 355, 131324 CrossRef CAS PubMed.
- X. Liu, J. Guo, Y. Li, B. Wang, S. Yang and W. Chen, et al., SERS substrate fabrication for biochemical sensing: towards point-of-care diagnostics, J. Mater. Chem. B, 2021, 9, 8378–8388 RSC.
- N. Bontempi, L. Carletti, C. De Angelis and I. Alessandri, Plasmon-free SERS detection of environmental CO2 on TiO2 surfaces, Nanoscale, 2016, 8, 3226–3231 RSC.
- D. Kong, W. Zhu and M. Li, A facile and sensitive SERS-based platform for sulfite residues / SO2 detection in food, Microchem. J., 2021, 165, 106174 CrossRef CAS.
- P. Kumar, M. Soni, T. Arora and S. K. Sharma, 2D Colloidal Crystals Based SERS Sensors for NH3 Detection, 2015 IEEE International Symposium on Nanoelectronic and Information Systems, 2015, pp. 277–280 Search PubMed.
- K. Huang, S. Gong, L. Zhang, H. Zhang, S. Li and G. Ye, et al., Ultrathin ZIF-8 wrapping on Au-dotted Ag-nanowires for highly selective SERS-based CO2 gas detection, Chem. Commun., 2021, 57, 2144–2147 RSC.
- D. Yao, G. Wen, L. Gong, C. Li, A. Liang and Z. Jiang, A Highly Sensitive SERS and RRS Coupled Di-Mode Method for CO Detection Using Nanogolds as Catalysts and Bifunctional Probes, Nanomaterials, 2020, 10, 450 CrossRef CAS PubMed.
- S. Kim, H. S. Jung, D.-H. Kim, S.-H. Kim and S.-G. Park, 3D nanoporous plasmonic chips for extremely sensitive NO2 detection, Analyst, 2019, 144, 7162–7167 RSC.
- S. Li, B. Zhao, A. Aguirre, Y. Wang, R. Li and S. Yang, et al., Monitoring Reaction Intermediates in Plasma-Driven SO2, NO, and NO2 Remediation Chemistry Using In Situ SERS Spectroscopy, Anal. Chem., 2021, 93, 6421–6427 CrossRef CAS.
- B. Fan, Y. Wang, Z. Li, D. Xun, J. Dong and X. Zhao, et al., Si@Ag@PEI substrate-based SERS sensor for rapid detection of illegally adulterated sulfur dioxide in traditional Chinese medicine, Talanta, 2022, 238, 122988 CrossRef CAS PubMed.
- H. Matsuta and K. Hirokawa, SERS observation of the adsorption behavior of SO2 on silver powder surfaces at nearly real environmental conditions, Appl. Surf. Sci., 1987, 27, 482–486 CrossRef CAS.
- L. Mandrile, I. Cagnasso, L. Berta, A. M. Giovannozzi, M. Petrozziello and F. Pellegrino, et al., Direct quantification of sulfur dioxide in wine by Surface Enhanced Raman Spectroscopy, Food Chem., 2020, 326, 127009 CrossRef CAS PubMed.
- D. Li, Y. Ma, H. Duan, W. Deng and D. Li, Griess reaction-based paper strip for colorimetric/fluorescent/SERS triple sensing of nitrite, Biosens. Bioelectron., 2018, 99, 389–398 CrossRef CAS.
- J. Chen, S. Pang, L. He and S. R. Nugen, Highly sensitive and selective detection of nitrite ions using Fe3O4@SiO2/Au magnetic nanoparticles by surface-enhanced Raman spectroscopy, Biosens. Bioelectron., 2016, 85, 726–733 CrossRef CAS PubMed.
- S. Zhang and T. Zhang, In Situ Detection of Low Amounts of Ammonia, Trends Chem., 2021, 3, 339–341 CrossRef CAS.
- C. L. Wong, U. S. Dinish, M. S. Schmidt and M. Olivo, Non-labeling multiplex surface enhanced Raman scattering (SERS) detection of volatile organic compounds (VOCs), Anal. Chim. Acta, 2014, 844, 54–60 CrossRef CAS PubMed.
- X.-M. Wang, X. Li, W.-H. Liu, C.-Y. Han and X.-L. Wang, Gas Sensor Based on Surface Enhanced Raman Scattering, Materials, 2021, 14, 388 CrossRef CAS PubMed.
- Q.-Q. Chen, R.-N. Hou, Y.-Z. Zhu, X.-T. Wang, H. Zhang and Y.-J. Zhang, et al., Au@ZIF-8 Core–Shell Nanoparticles as a SERS Substrate for Volatile Organic Compound Gas Detection, Anal. Chem., 2021, 93, 7188–7195 CrossRef CAS PubMed.
- D. Xia, Q. Guo, M. Ge, Y. Yuan, M. Xu and J. Yao, On-line sensitive detection of aromatic vapor through PDMS/C3H7S-assisted SERS amplification, RSC Adv., 2016, 6, 53289–53295 RSC.
- K. Nemciauskas, L. Traksele, A. Salaseviciene and V. Snitka, A silicon membrane-silver nanoparticles SERS chip for trace molecules detection, Microelectron. Eng., 2020, 225, 111282 CrossRef CAS.
- M. K. Khaing Oo, C.-F. Chang, Y. Sun and X. Fan, Rapid, sensitive DNT vapor detection with UV-assisted photo-chemically synthesized gold nanoparticle SERS substrates, Analyst, 2011, 136, 2811–2817 RSC.
- M. K. Khaing Oo, Y. Guo, K. Reddy, J. Liu and X. Fan, Ultrasensitive Vapor Detection with Surface-Enhanced Raman Scattering-Active Gold Nanoparticle Immobilized Flow-Through Multihole Capillaries, Anal. Chem., 2012, 84, 3376–3381 CrossRef CAS.
- C. Qian, Q. Guo, M. Xu, Y. Yuan and J. Yao, Improving the SERS detection sensitivity of aromatic molecules by a PDMS-coated Au nanoparticle monolayer film, RSC Adv., 2015, 5, 53306–53312 RSC.
- K. B. Biggs, J. P. Camden, J. N. Anker and R. P. V. Duyne, Surface-Enhanced Raman Spectroscopy of Benzenethiol Adsorbed from the Gas Phase onto Silver Film over Nanosphere Surfaces: Determination of the Sticking Probability and Detection Limit Time, J. Phys. Chem. A, 2009, 113, 4581–4586 CrossRef CAS PubMed.
- V. Heleg-Shabtai, H. Sharabi, A. Zaltsman, I. Ron and A. Pevzner, Surface-enhanced Raman spectroscopy (SERS) for detection of VX and HD in the gas phase using a hand-held Raman spectrometer, Analyst, 2020, 145, 6334–6341 RSC.
- N. H. Ly, S. J. Son, S. Jang, C. Lee, J. I. Lee and S.-W. Joo, Surface-Enhanced Raman Sensing of Semi-Volatile Organic Compounds by Plasmonic Nanostructures, Nanomaterials, 2021, 11, 2619 CrossRef CAS PubMed.
- H.-X. Wang, Y.-W. Zhao, Z. Li, B.-S. Liu and D. Zhang, Development and Application of Aptamer-Based Surface-Enhanced Raman Spectroscopy Sensors in Quantitative Analysis and Biotherapy, Sensors, 2019, 19, 3806 CrossRef CAS PubMed.
- R. K. Lauridsen, L. M. Sommer, H. K. Johansen, T. Rindzevicius, S. Molin and L. Jelsbak, et al., SERS detection of the biomarker hydrogen cyanide from Pseudomonas aeruginosa cultures isolated from cystic fibrosis patients, Sci. Rep., 2017, 7, 45264 CrossRef CAS PubMed.
- R. K. Lauridsen, T. Rindzevicius, S. Molin, H. K. Johansen, R. W. Berg and T. S. Alstrøm, et al., Towards quantitative SERS detection of hydrogen cyanide at ppb level for human breath analysis, Sens. Bio-Sens. Res., 2015, 5, 84–89 CrossRef.
- X. Qiao, B. Su, C. Liu, Q. Song, D. Luo and G. Mo, et al., Selective Surface Enhanced Raman Scattering for Quantitative Detection of Lung Cancer Biomarkers in Superparticle@MOF Structure, Adv. Mater., 2018, 30, 1702275 CrossRef PubMed.
- J. Park, J. A. Thomasson, C. C. Gale, G. A. Sword, K.-M. Lee and T. J. Herrman, et al., Adsorbent-SERS Technique for Determination of Plant VOCs from Live Cotton Plants and Dried Teas, ACS Omega, 2020, 5, 2779–2790 CrossRef CAS PubMed.
- J. Prakash, J. Cho and Y. K. Mishra, Photocatalytic TiO2 nanomaterials as potential antimicrobial and antiviral agents: Scope against blocking the SARS-COV-2 spread, Micro Nano Eng., 2022, 14, 100100 CrossRef CAS.
- X. Li, J. Li, Q. Ge, Y. Du, G. Li and W. Li, et al., Detecting SARS-CoV-2 in the Breath of COVID-19 Patients, Front. Med., 2021, 8 DOI:10.3389/fmed.2021.604392.
- C. Duan, L. Buerer, J. Wang, S. Kaplan, G. Sabalewski and G. D. Jay, et al., Efficient Detection of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) from Exhaled Breath, J. Mol. Diagn., 2021, 23, 1661–1670 CrossRef CAS PubMed.
- S. X. Leong, Y. X. Leong, E. X. Tan, H. Y. F. Sim, C. S. L. Koh and Y. H. Lee, et al., Noninvasive and Point-of-Care Surface-Enhanced Raman Scattering (SERS)-Based Breathalyzer for Mass Screening of Coronavirus Disease 2019 (COVID-19) under 5 min, ACS Nano, 2022, 16, 2629–2639 CrossRef CAS PubMed.
- B. Shan, Y. Y. Broza, W. Li, Y. Wang, S. Wu and Z. Liu, et al., Multiplexed Nanomaterial-Based Sensor Array for Detection of COVID-19 in Exhaled Breath, ACS Nano, 2020, 14, 12125–12132 CrossRef CAS PubMed.
- A. D. Subali, L. Wiyono, M. Yusuf and M. F. A. Zaky, The potential of volatile organic compounds-based breath analysis for COVID-19 screening: a systematic review & meta-analysis, Diagn. Microbiol. Infect. Dis., 2022, 102, 115589 CrossRef CAS PubMed.
- E. Viklund, S. Kokelj, P. Larsson, R. Nordén, M. Andersson and O. Beck, et al., Severe acute respiratory syndrome coronavirus 2 can be detected in exhaled aerosol sampled during a few minutes of breathing or coughing, Influenza Other Respir. Viruses, 2022, 16, 402–410 CrossRef CAS PubMed.
- Y. Wu, H. Dang, S.-G. Park, L. Chen and J. Choo, SERS-PCR assays of SARS-CoV-2 target genes using Au nanoparticles-internalized Au nanodimple substrates, Biosens. Bioelectron., 2022, 197, 113736 CrossRef CAS.
- A. Pramanik, Y. Gao, S. Patibandla, K. Gates and P. C. Ray, Bioconjugated Nanomaterial for Targeted Diagnosis of SARS-CoV-2, Acc. Mater. Res., 2022, 3(2), 134–148 CrossRef CAS.
- S. S. Sinha, S. Jones, A. Pramanik and P. C. Ray, Nanoarchitecture Based SERS for Biomolecular Fingerprinting and Label-Free Disease Markers Diagnosis, Acc. Chem. Res., 2016, 49, 2725–2735 CrossRef CAS.
- K. Daoudi, K. Ramachandran, H. Alawadhi, R. Boukherroub, E. Dogheche and M. A. E. Khakani, et al., Ultra-sensitive and fast optical detection of the spike protein of the SARS-CoV-2 using AgNPs/SiNWs nanohybrid based sensors, Surf. Interfaces, 2021, 27, 101454 CrossRef CAS PubMed.
- C. Carlomagno, D. Bertazioli, A. Gualerzi, S. Picciolini, P. I. Banfi and A. Lax, et al., COVID-19 salivary Raman fingerprint: innovative approach for the detection of current and past SARS-CoV-2 infections, Sci. Rep., 2021, 11, 4943 CrossRef CAS.
- S. Srivastav, A. Dankov, M. Adanalic, R. Grzeschik, V. Tran and S. Pagel-Wieder, et al., Rapid and Sensitive SERS-Based Lateral Flow Test for SARS-CoV2-Specific IgM/IgG Antibodies, Anal. Chem., 2021, 93, 12391–12399 CrossRef CAS PubMed.
- K. V. Serebrennikova, N. A. Byzova, A. V. Zherdev, N. G. Khlebtsov, B. N. Khlebtsov and S. F. Biketov, et al., Lateral Flow Immunoassay of SARS-CoV-2 Antigen with SERS-Based Registration: Development and Comparison with Traditional Immunoassays, Biosensors, 2021, 11, 510 CrossRef CAS PubMed.
- H. Liu, E. Dai, R. Xiao, Z. Zhou, M. Zhang and Z. Bai, et al., Development of a SERS-based lateral flow immunoassay for rapid and ultra-sensitive detection of anti-SARS-CoV-2 IgM/IgG in clinical samples, Sens. Actuators, B, 2021, 329, 129196 CrossRef CAS.
- W. K. Choi, T. H. Liew, M. K. Dawood, H. I. Smith, C. V. Thompson and M. H. Hong, Synthesis of Silicon Nanowires and Nanofin Arrays Using Interference Lithography and Catalytic Etching, Nano Lett., 2008, 8, 3799–3802 CrossRef CAS PubMed.
- J. Yang, F. Luo, T. S. Kao, X. Li, G. W. Ho and J. Teng, et al., Design and fabrication of broadband ultralow reflectivity black Si surfaces by laser micro/nanoprocessing, Light: Sci. Appl., 2014, 3, e185 CrossRef CAS.
- M. S. S. Bharati and V. R. Soma, Flexible SERS substrates for hazardous materials detection: recent advances, Opto-Electron. Adv., 2021, 4, 210048 CAS.
- D. Zhang, X. Zhang, R. Ma, S. Deng, X. Wang and X. Wang, et al., Ultra-fast and onsite interrogation of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in waters via surface enhanced Raman scattering (SERS), Water Res., 2021, 200, 117243 CrossRef CAS.
- H. Chen, X. Qi, J. Ma, C. Zhang, H. Feng and M. Yao, Breath-borne VOC Biomarkers for COVID-19, medRxiv, 2020, preprint, 2020.06.21.20136523, DOI:10.1101/2020.06.21.20136523.
- G. Giovannini, H. Haick and D. Garoli, Detecting COVID-19 from Breath: A Game Changer for a Big Challenge, ACS Sens., 2021, 6, 1408–1417 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |