Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Dual role of benzophenone enables a fast and scalable C-4 selective alkylation of pyridines in flow†
Jesús
Sanjosé-Orduna‡
 a,
Rodrigo C.
Silva‡
a,
Rodrigo C.
Silva‡
 ab,
Fabian
Raymenants
ab,
Fabian
Raymenants
 a,
Bente
Reus
a,
Jannik
Thaens
a,
Kleber T.
de Oliveira
a,
Bente
Reus
a,
Jannik
Thaens
a,
Kleber T.
de Oliveira
 b and
Timothy
Noël
b and
Timothy
Noël
 *a
*a
aFlow Chemistry Group, Van’t Hoff Institute for Molecular Sciences (HIMS), University of Amsterdam, Science Park 904, 1098 XH Amsterdam, The Netherlands. E-mail: t.noel@uva.nl; Web: https://www.noelresearchgroup.com/
bDepartamento de Química, Universidade Federal de São Carlos, SP 13565-905, Brazil
First published on 10th October 2022
Abstract
The efficient C-4 selective modification of pyridines is a major challenge for the synthetic community. Current strategies are plagued with at least one drawback regarding functional group-tolerant electronic activation of the heteroarene, mild generation of the required alkyl radicals, regioselectivity, safety and/or scalability. Herein, we describe a fast, safe and scalable flow process which allows preparation of said C-4 alkylated pyridines. The process involves a photochemical hydrogen atom transfer (HAT) event to generate the carbon-centered radicals needed to alkylate the C-2 blocked pyridine. In a two-step streamlined flow process, this light-mediated alkylation step is combined with a nearly instantaneous inline removal of the blocking group. Notably, cheap benzophenone plays a dual role in the pyridine alkylation mechanism by activating the hydrocarbon feedstock reagents via a HAT mechanism, and by acting as a benign, terminal oxidant. The key role of benzophenone in the operative reaction mechanism has also been revealed through a combination of experimental and computational studies.
Introduction
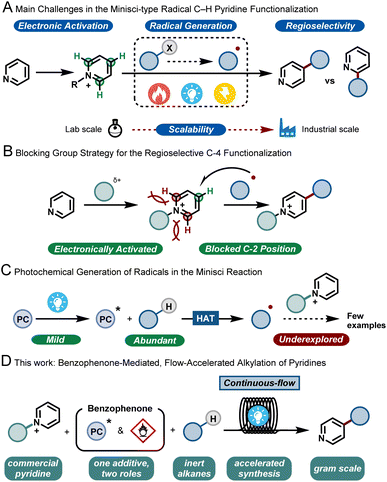
Pyridines are privileged scaffolds and their incorporation into biologically-active molecules can dramatically improve their potency.1 Hence, synthetic tools that can selectively edit this moiety are highly desired to enable the streamlined synthesis of various pharmaceuticals and agrochemicals.2In the 1970s, Minisci and co-workers reported upon the thermal generation of alkyl radicals via decarboxylation of carboxylic acids using silver salts. These nucleophilic radicals were subsequently exploited to alkylate various heteroarenes, including pyridines, under strongly acidic and oxidative conditions.3 More than 50 years later, this strategy, coined as the Minisci reaction, still stands and has evolved into a key method for the modification of heteroarenes.4 However, between academic discovery and practical use in the pharmaceutical and agrochemical industry, there remain many roadblocks for the widespread implementation of the Minisci reaction. These challenges include (i) a functional-group tolerant electrophilic activation of the basic heteroarene, (ii) a reliable and mild generation of the alkyl radicals; (iii) the poor regioselectivity of the radical addition to the aromatic ring5 leading to various byproducts and (iv) the safe and challenging scale up to relevant quantities for process chemists (Fig. 1A). In recent years, the use of bulky, C-2 blocking groups has proven to be very effective to enable both regioselectivity towards the C-4 position and efficient electrophilic activation of the pyridine moiety (Fig. 1B).6
With respect to the generation of radicals, since the initial thermal decarboxylation methodology developed by Minisci, several milder strategies have been discovered. As a prime example, photocatalysis has emerged in the past decade as a powerful tool for the efficient formation of open-shell reactive intermediates.7 Among the different radical precursors available, the direct activation of ubiquitous C–H bonds is perhaps one of the most desirable synthetic targets, as it bypasses the need for lengthy pre-functionalization steps. The homolytic cleavage of such inert C–H bonds via hydrogen atom transfer (HAT) has recently been shown to be a very powerful approach to enable both early and late stage functionalization of hydroalkanes.8
However, the coupling of the HAT-generated radical with activated pyridines is so far an underdeveloped strategy (Fig. 1C). Few examples are reported in the literature, usually requiring excess of hydrocarbons, inert conditions, transition metals and/or very extended reaction times, making such strategies less practical. Consequently, in order to make the transition from academic discovery to a scalable process, synthetic methodologies are urgently needed that use cheap and readily-available starting materials, involve few and non-toxic additives, require short reaction times and are scalable.
Given the experience of our group in developing multidisciplinary synthetic approaches,9 we wondered if the aforementioned issues could be simultaneously solved by developing a continuous-flow synthetic strategy. Flow technology has been shown to uniquely pair with photochemistry, by effectively reducing the reaction times, by improving reaction selectivity due to excellent mass and photon transfer characteristics, and by enabling facile scale up.10 Moreover, using such a flow strategy would also enable us to combine both the photochemical C-4 functionalization of the pyridine and the subsequent removal of the C-2 blocking group, ultimately delivering an operationally simple process that should ensure the preparation of large quantities of C-4 alkylated pyridines using hydrocarbons as cheap and abundantly available coupling partners (Fig. 1D).
Discussion
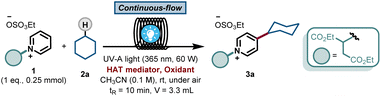
With this blueprint in mind, we commenced our investigations by testing a model system consisting of a commercially available C2-blocked pyridine 1,6h cyclohexane 2a, tetrabutylammonium decatungstate (TBADT) as the HAT photocatalyst11 and stoichiometric amounts of a terminal oxidant, i.e. (NH4)2S2O8. We subjected the initial reaction mixture under air in a continuous-flow microreactor (ID = 0.8 mm, 3.3 mL) and exposed it to UV-A light irradiation (λ = 365 nm, 60 W input power) (See ESI† for details). Under these conditions, we could observe the regioselective C-4 alkylation of 1 albeit with a low yield for 3a (Table 1, entry 1). In addition, precipitation of the photocatalyst and the oxidant in the reaction coil was observed, which would lead to clogging problems over time.12 We wondered if the highly-charged nature of TBADT could be an issue under these conditions, so we decided to switch to a simpler organic HAT mediator, such as benzophenone (BP1).13,14 Although similar low yields were obtained (Table 1, entry 2), a completely homogeneous solution was observed during the entire experiment.| Entry | 2a (eq.) | HAT mediator | Oxidant | 3a [%] |
|---|---|---|---|---|
a Yields determined by 1H NMR spectroscopy using 1,3,5-trimethoxybenzene as external standard.
b Ammonium persulfate required a CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture as solvent for solubility issues.
c In batch (See ESI for further details). 1) mixture as solvent for solubility issues.
c In batch (See ESI for further details).
|
||||
| 1 | 10 | TBADT (4 mol%) | (NH4)2S2O8 (2 eq.) | 24b |
| 2 | 10 | BP1 (20 mol%) | (NH4)2S2O8 (2 eq.) | 28b |
| 3 | 20 | BP1 (20 mol%) | Oxygen | 0 |
| 4 | 10 | BP1 (20 mol%) | None | 13 |
| 5 | 10 | BP1 (1 eq.) | 63 | |
| 6 | 10 | BP2 (1 eq.) | 36 | |
| 7 | 10 | BP3 (1 eq.) | 52 | |
| 8 | 10 | BP4 (1 eq.) | 55 | |
| 9 | 2.5 | BP1 (1.5 eq.) | 70 | |
| 10 | 2.5 | BP1 (1.5 eq.) | 27c | |
| 11 | 2.5 | None | 0 | |
|
||||
After selecting BP1 as the HAT mediator, different oxidants and solvents were screened (see ESI† for details). Unexpectedly, when using oxygen as oxidant, the reaction was completely suppressed (Table 1, entry 3). Notably, a blank experiment revealed another interesting observation: without the addition of any external oxidant, we observed the formation of 3a in 13% yield using 20 mol% of BP1 (Table 1, entry 4). This finding, in combination with entry 3, prompted us to question if benzophenone could also act as a terminal oxidant. When repeating the reaction with a stoichiometric amount of BP1, the product 3a was obtained in 63% yield, demonstrating the ability of BP1 to not only engage in the C(sp3)–H activation event, but also to serve as terminal oxidant (Table 1, entry 5). Next, we evaluated a diverse set of substituted benzophenones. Electron-rich (BP2, Table 1, entry 6), electron-poor (BP3, Table 1, entry 7) and a push–pull system (BP4, Table 1, entry 8) were subjected to the reaction conditions, but none of them outperformed the non-substituted and commercially available BP1 as mediator. Finally, increasing the amount of BP1 to 1.5 equivalents allowed us to decrease the equivalents of the hydroalkane 2a to 2.5 equivalents, delivering the target compound 3a in 70% yield (Table 1, entry 9).
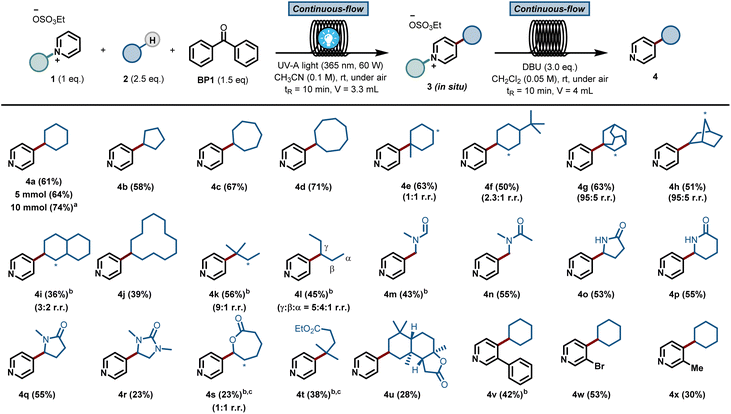
This result was obtained without any special precautions during the reaction preparation, including the use of wet reagents/solvents and working under air, making our reaction protocol particularly user-friendly. Interestingly, when repeating the reaction under conventional batch conditions, the yield for the benzophenone-mediated alkyl–pyridyl coupling dropped to 27% (Table 1, entry 10), highlighting the importance of an efficient irradiation of the reaction mixture under microfluidic conditions. No reactivity was observed when the reaction was performed without BP1 (Table 1, entry 11). Having found proper reaction conditions for the alkyl–pyridyl bond-forming reaction, we next aimed to remove the C2-blocking group attached to the pyridine moiety. To do so, we merged the reaction stream exiting the photochemical flow reactor with a dichloromethane solution containing the organic base 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU). In only 20 min overall residence time, we could perform both the photomediated alkylation and the removal of the blocking group without the need for intermediate isolation. In addition, this operationally simple protocol also allowed us to scale up the process without re-optimization of the reaction conditions, which often plagues conventional batch scale up procedures. Simply by continuously pumping starting materials into the reactor assembly, we could scale up the reaction to a gram-scale, with even slightly higher reaction yields (74% isolated yield for 4a, 91% purity, 210 mg hour−1).
The scope of pyridines that can be C-4 alkylated using our protocol is illustrated in Fig. 2. First, a diverse set of hydrocarbons bearing strong, non-activated C(sp3)–H bonds (4a–4l) were subjected to the photochemical reaction conditions. Benzophenone was able to cleave the C(sp3)–H bond in various cyclic alkane feedstocks, such as cyclopentane (4b), cycloheptane (4c), cyclooctane (4d), adamantane (4g), norbornane (4h), decalin (4i) and cyclododecane (4j) and the ensuing nucleophilic radical was subsequently coupled with the C2-blocked pyridine.
Also, linear alkanes, such as isopentane (4k) and n-pentane (4l), were selectively activated by benzophenone at their most hydridic C(sp3)–H bonds. Various amides, including dimethylformamide (DMF, 4m) and dimethylacetamide (DMA, 4n), could be activated α-to-N C(sp3)–H. Furthermore, 5 and 6-membered lactams (4o–4r), lactones (4s) and esters (4t) were compatible with our HAT-induced Minisci protocol. Notably, the sesquiterpene lactone natural product sclareolide (4u) could be regioselectively pyridinylated, demonstrating the potential of this protocol to act as a late-stage functionalization method. Finally, various C-3 substituted pyridines (4v–4x) were also compatible with the reaction conditions, giving rise to the corresponding C-4 alkylated compounds in good isolated yields. Unfortunately, alkanes bearing cyclic amines, cyclic ethers, aromatic rings or halogens gave rise to either low yields or complex reaction mixtures (see ESI† for details).
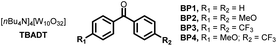
Next, we sought to investigate the mechanism of this process and, in particular, to elucidate the role of benzophenone. Using the initial rates method we could extract the experimental reaction rates at different concentrations (Fig. 3A).15 From these kinetic investigations, the reaction order of cyclohexane was determined to be 0.9, suggesting a first-order dependence regarding the hydrocarbon concentration under these conditions.
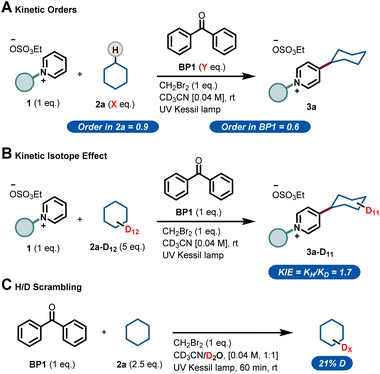 | ||
| Fig. 3 Experimental mechanistic investigations. (A) Kinetic orders. (B) Kinetic Isotope Effect (KIE). (C) H/D scrambling. | ||
Interestingly, the kinetic order of BP1 was measured to be 0.6. This fractional order suggests that the role of benzophenone is more complex, and implies that BP1 might be involved in different elementary steps through the entire mechanistic scenario. Similarly, to get further insights about the initial C(sp3)–H bond cleavage of cyclohexane, we examined the kinetic isotope effect (KIE) (Fig. 3B). To do that, we compared the initial rates of both a standard reaction and a reaction using deuterated cyclohexane (2a-d12). A KIE of 1.7 was determined, which suggests that the C(sp3)–H bond cleavage of the alkane might be involved in the rate-determining step.16 Further H/D scrambling experiments demonstrated the reversibility of the process under the described reaction conditions, as we observed partial deuteration of cyclohexane when repeating the reaction using D2O as cosolvent (Fig. 3C).
To further understand the experimentally-obtained mechanistic insights, we decided to simulate a plausible scenario that could explain this dual role of the benzophenone using density functional theory (DFT) calculations. The obtained qualitative reaction profile with the corresponding energies is depicted in Fig. 4 (See ESI† for further computational details). The reaction kicks off with UVA-light photoexcitation of the ground state benzophenone BP1 to its triplet state BP1*. This highly electrophilic species, regarded as the 0.0 in the energy profile, is responsible for the homolytic cleavage of a C(sp3)–H bond in the alkyl partner Cy1, through TS1 at 11.3 kcal mol−1. This low energetic barrier is in agreement with the absence of a primary KIE (Fig. 3B). The ensuing carbon-centered radical Cy2 resides in an endergonic position (−8.9 kcal mol−1) relative to BP1*. The reversibility of this step was observed experimentally (Fig. 3C), demonstrating that the reverse energetic barrier (20.2 kcal mol−1) can be overcome at room temperature under the described reaction conditions.
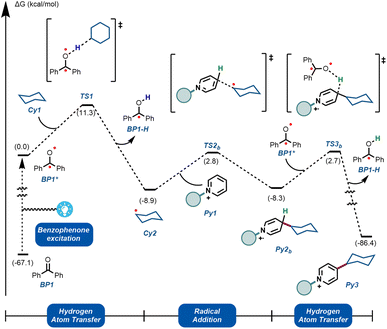 | ||
| Fig. 4 Computational mechanistic investigations. Calculations were performed at the ωB97X-D/6-31+G(d,p)/CPCM (acetonitrile) level of theory. See ESI† for computational details. | ||
Subsequently, species Cy2 can engage in a radical addition at the only electronically and sterically accessible position of Py1, through TS2b. Given that the reversibility of TS1 was demonstrated experimentally under these conditions, we can assume that the energetic barrier for TS2b (11.7 kcal mol−1) is also within reach. Finally, we simulated the re-aromatization of the reduced Py2b by a second molecule of BP1*via a second hydrogen atom transfer event in TS3b. The energetic barrier for this last step from Cy2 (11.0 kcal mol−1) is comparable to TS2b and to TS1, showcasing that all the elementary steps are feasible at room temperature. This demonstrates that BP1* can participate not only in the first HAT reaction, but also in the terminal oxidation of Py2b, thus explaining the fractional kinetic order observed experimentally (Fig. 3A).
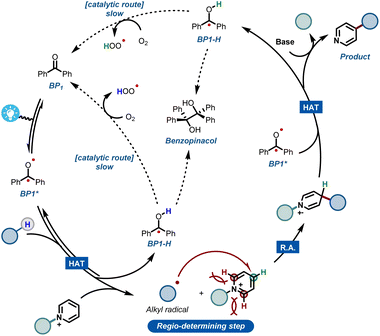
Combining all experimental and computational evidence, we can portray a plausible reaction mechanism for the benzophenone-enabled photomediated C4-alkylation of pyridines (Fig. 5). A photoexcited ketone in the triplet state BP1* is responsible for the cleavage of the strong C(sp3)–H bond via HAT, giving rise to a nucleophilic alkyl radical and concomitant formation of BP1-H. It should be noted that this protonated species can be re-oxidized to the original benzophenone under an oxygen atmosphere, leading to a catalytic pathway.14f However, this reoxidation pathway is particularly slow as shown by our experiments, making it less practical for scale up (see ESI†). The generated alkyl radical is subsequently added to the activated pyridine, establishing the targeted alkyl–pyridyl bond. Finally, the ensuing radical cation has to be rearomatized. Notably, this is the specific step that often requires harsh, external oxidants in many Minisci reaction protocols. However, under our set of reaction conditions, this step can be carried out mildly using a second molecule of BP1*.
It is worth noting that oxygen is also capable of engaging into this oxidation step, but again requiring quite long reaction times. The second molecule of reduced BP1-H can be re-oxidized in the same fashion by oxygen, closing what happens to be a rather slow, catalytic cycle.
Additionally, during our optimizations with BP1, we observed the formation of small quantities of the dimerized benzopinacol derivative. This evidence also corroborates with the proposed radical BP1-H and with the need for more than 1 eq. of BP1, thus suggesting a competition between the re-oxidation of BP1-H by O2 and its radical recombination (Fig. 5). Finally, the removal of the blocking group using a non-nucleophilic organic base gives rise to the desired C-4 alkylated pyridine.
Conclusions
In conclusion, we have developed an efficient, safe and scalable two-step flow protocol to enable the C-4 selective alkylation of pyridines. The uninterrupted process involves a benzophenone-induced HAT generation of radicals, a subsequent alkylation of a C-2 blocked pyridine and the ultimate removal of the blocking group, requiring only 20 min of reaction time in total. The scope of this transformation is broad, and the targeted pyridines can be alkylated with a variety of activated and non-activated hydrocarbon feedstocks. Finally, a combined experimental and computational mechanistic investigation was carried out to elucidate the reaction mechanism and to highlight the role of benzophenone as HAT reagent and mild oxidant.Data availability
Experimental details, used materials, sample preparation and analytical data (NMR) for the compounds 4a–4x. All the raw computational data is available on the ioChem-BD17 repository and can be freely accessed at https://doi.org/10.19061/iochem-bd-6-156.Author contributions
J. S.-O. conceived the idea for this work. J. S.-O. and J. T. carried out initial optimization studies. R. C. S. developed the refined reaction conditions and the substrate scope, with the help of B. R. and J. S.-O. carried out the experimental and computational mechanistic studies. F. R. and B. R. studied the effect of oxygen in the reaction. T. N. provided direction for the scientific strategy. J. S.-O. and T.N. wrote the manuscript with input from all the authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
R. C. S. scholarship was provided by the São Paulo Research Foundation – FAPESP – (grant numbers: 2021/09145-8), and thanks are also due to the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Financial Code 001. The authors would also like to acknowledge kind financial support from the European Union (FLIX, No. 862179, F. R. and T. N.).Notes and references
- (a) E. Vitaku, D. T. Smith and J. T. Njardarson, J. Med. Chem., 2014, 57, 10257–10274 CrossRef CAS PubMed; (b) Y. Ling, Z. Y. Hao, D. Liang, C. L. Zhang, Y. F. Liu and Y. Wang, Drug Des. Devel. Ther., 2021, 15, 4289–4338 CrossRef CAS PubMed; (c) P. Bhutani, G. Joshi, N. Raja, N. Bachhav, P. K. Rajanna, H. Bhutani, A. T. Paul and R. Kumar, J. Med. Chem., 2021, 64, 2339–2381 CrossRef CAS PubMed.
- (a) T. Cernak, K. D. Dykstra, S. Tyagarajan, P. Vachal and S. W. Krska, Chem. Soc. Rev., 2016, 45, 546–576 RSC; (b) J. Boström, D. G. Brown, R. J. Young and G. M. Keserü, Nat. Rev. Drug Discovery, 2018, 17, 709–727 CrossRef PubMed; (c) L. Zhang and T. Ritter, J. Am. Chem. Soc., 2022, 144, 2399–2414 CrossRef CAS PubMed.
- (a) F. Minisci, R. Galli, M. Cecere, V. Malatesta and T. Caronna, Tetrahedron Lett., 1968, 54, 5609–5612 CrossRef; (b) F. Minisci, R. Galli, V. Malatesta and T. Caronna, Tetrahedron, 1970, 26, 4083–4091 CrossRef CAS; (c) F. Minisci, R. Bernardi, F. Bertini, R. Galli and M. Perchinummo, Tetrahedron, 1971, 27, 3575–3579 CrossRef CAS.
- For selected reviews about the Minisci reaction: (a) M. A. J. Duncton, Med. Chem. Comm., 2011, 2, 1135–1161 RSC; (b) A. C. Sun, R. C. McAtee, E. J. McClain and C. R. J. Stephenson, Synthesis, 2019, 51, 1063–1072 CrossRef CAS PubMed; (c) R. S. J. Proctor and R. J. Phipps, Angew. Chem., Int. Ed., 2019, 58, 13666–13699 CrossRef CAS PubMed.
- (a) F. O'Hara, D. G. Blackmond and P. S. Baran, J. Am. Chem. Soc., 2013, 135, 12122–12134 CrossRef PubMed; (b) A. de A. Bartolomeu, R. C. Silva, T. J. Brocksom, T. Noël and K. T. de Oliveira, J. Org. Chem., 2019, 84, 10459–10471 CrossRef CAS PubMed.
- (a) Y. Nakao, Y. Yamada, N. Kashihara and T. Hiyama, J. Am. Chem. Soc., 2010, 132, 13666–13668 CrossRef CAS PubMed; (b) P. S. Fier, J. Am. Chem. Soc., 2017, 139, 9499–9502 CrossRef CAS PubMed; (c) Y. Moon, B. Park, I. Kim, G. Kang, S. Shin, D. Kang, M. H. Baik and S. Hong, Nat. Comm., 2019, 10, 4117 CrossRef PubMed; (d) I. Kim, S. Park and S. Hong, Org. Lett., 2020, 22, 8730–8734 CrossRef CAS PubMed; (e) W. Lee, S. Jung, M. Kim and S. Hong, J. Am. Chem. Soc., 2021, 143, 3003–3012 CrossRef CAS PubMed; (f) S. Shin, S. Lee, W. Choi, N. Kim and S. Hong, Angew. Chem., Int. Ed., 2021, 60, 7873–7879 CrossRef CAS PubMed; (g) B. Kweon, C. Kim, S. Kim and S. Hong, Angew. Chem., Int. Ed., 2021, 60, 26813–26821 CrossRef CAS PubMed; (h) J. Choi, G. Laudadio, E. Godineau and P. S. Baran, J. Am. Chem. Soc., 2021, 143, 11927–11933 CrossRef CAS PubMed; (i) H. Choi, G. R. Mathi, S. Hong and S. Hong, Nat. Comm., 2022, 13, 1776 CrossRef CAS PubMed; (j) Z. Zhang, Q. He, X. Zhang and C. Yang, Org. Biomol. Chem., 2022, 20, 1969–1973 RSC.
- (a) T. P. Yoon, M. A. Ischay and J. Du, Nat. Chem., 2010, 2, 527–532 CrossRef CAS PubMed; (b) D. Staveness, I. Bosque and C. R. J. Stephenson, Acc. Chem. Res., 2016, 49, 2295–2306 CrossRef CAS PubMed; (c) W.-M. Cheng, R. Shang, M.-C. Fu and Y. Fu, Chem. Eur. J., 2017, 23(11), 2537–2541 CrossRef CAS PubMed; (d) W.-M. Cheng, R. Shang and Y. Fu, ACS Catal., 2017, 7, 907–911 CrossRef CAS; (e) S. Crespi and M. Fagnoni, Chem. Rev., 2020, 120, 9790–9833 CrossRef CAS PubMed; (f) R. Cannalire, S. Pelliccia, L. Sancineto, E. Novellino, G. C. Tron and M. Giustiniano, Chem. Soc. Rev., 2021, 50, 866–897 RSC; (g) L. Candish, K. D. Collins, G. C. Cook, J. J. Douglas, A. Gómez-Suárez, A. Jolit and S. Keess, Chem. Rev., 2022, 122, 2907–2980 CrossRef CAS PubMed.
- For reviews about the use of hydrogen atom transfer for the generation of radicals see: (a) H. Cao, X. Tang, H. Tang, Y. Yuan and J. Wu, ChemCatChem, 2021, 1, 523–598 Search PubMed; (b) L. Capaldo, D. Ravelli and M. Fagnoni, Chem. Rev., 2022, 122, 1875–1924 CrossRef CAS PubMed.
- (a) G. Laudadio, S. Govaerts, Y. Wang, D. Ravelli, H. F. Koolman, M. Fagnoni, S. W. Djuric and T. Noël, Angew. Chem., Int. Ed., 2018, 57, 4078–4082 CrossRef CAS PubMed; (b) G. Laudadio, Y. Deng, K. van der Wal, D. Ravelli, M. Nuño, M. Fagnoni, D. Guthrie, Y. Sun and T. Noël, Science, 2020, 369, 92–95 CrossRef CAS PubMed; (c) T. Wan, L. Capaldo, G. Laudadio, A. V. Nyuchev, J. A. Rincón, P. García-Losada, C. Mateos, M. O. Frederick, M. Nuño and T. Noël, Angew. Chem., Int. Ed., 2021, 60, 17893–17897 CrossRef CAS PubMed; (d) D. Mazzarella, A. Pulcinella, L. Bovy, R. Broersma and T. Noël, Angew. Chem., Int. Ed., 2021, 60, 21277–21282 CrossRef CAS PubMed; (e) L. Capaldo, S. Bonciolini, A. Pulcinella, M. Nuño and T. Noël, Chem. Sci., 2022, 13, 7325–7331 RSC.
- (a) B. Gutmann, D. Cantillo and C. O. Kappe, Angew. Chem., Int. Ed., 2015, 54, 6688–6728 CrossRef CAS PubMed; (b) M. B. Plutschack, B. Pieber, K. Gilmore and P. H. Seeberger, Chem. Rev., 2017, 117, 11796–11893 CrossRef CAS PubMed; (c) S. Govaerts, A. Nyuchev and T. Noel, J. Flow. Chem., 2020, 10, 13–71 CrossRef CAS; (d) Z. Dong, Z. Wen, F. Zhao, S. Kuhn and T. Noël, Chem. Eng. Sci. X, 2021, 10, 100097 CAS; (e) L. Buglioni, F. Raymenants, A. Slattery, S. D. A. Zondag and T. Noël, Chem. Rev., 2022, 122, 2752–2906 CrossRef CAS PubMed.
- (a) M. C. Quattrini, S. Fujii, K. Yamada, T. Fukuyama, D. Ravelli, M. Fagnoni and I. Ryu, Chem. Commun., 2017, 53, 2335–2338 RSC; (b) D. Ravelli, M. Fagnoni, T. Fukuyama, T. Nishikawa and I. Ryu, ACS Catal., 2018, 8, 701–713 CrossRef CAS.
- (a) Z. Dong, S. D. A. Zondag, M. Schmid, Z. Wen and T. Noël, Chem. Eng. J., 2022, 428, 130968 CrossRef CAS; (b) A. Chaudhuri, S. D. A. Zondag, J. H. A. Schuurmans, J. van der Schaaf and T. Noël., Org. Proc. Res. Dev., 2022, 26, 1279–1288 CrossRef CAS PubMed.
- (a) J. Pérez-Prieto, R. E. Galian and M. A. Miranda, Mini Rev. Org. Chem., 2006, 3, 117–135 CrossRef; (b) G. Dormán, H. Nakamura, A. Pulsipher and G. D. Prestwich, Chem. Rev., 2016, 116, 15284–15398 CrossRef PubMed; (c) J. Mateos, S. Cuadros, A. Vega-Peñaloza and L. Dell’amico, Synlett, 2022, 33, 116–128 CrossRef CAS.
- For some selected examples of aromatic ketones acting as a hydrogen atom transfer mediator see: (a) C. B. Tripathi, T. Ohtani, M. T. Corbett and T. Ooi, Chem. Sci., 2017, 8, 5622–5627 RSC; (b) Y. Shen, Y. Gu and R. Martin, J. Am. Chem. Soc., 2018, 140, 12200–12209 CrossRef CAS PubMed; (c) Y. Li, M. Lei and L. Gong, Nat. Catal., 2019, 2, 1016–1026 CrossRef CAS; (d) C. Y. Huang, J. Li, W. Liu and C. J. Li, Chem. Sci., 2019, 10, 5018–5024 RSC; (e) Y. Gu, H. Yin, M. Wakeling, J. An and R. Martin, ACS Catal., 2022, 12, 1031–1036 CrossRef CAS; (f) A. Luridiana, D. Mazzarella, L. Capaldo, J. A. Rincon, P. Garcia-Losada, C. Mateos, M. O. Frederick, M. Nuno, W. J. Buma and T. Noël, ACS Catal., 2022, 12, 11216–11225 CrossRef CAS PubMed; (g) L. Zhang, B. Pfund, O. S. Wenger and X. Hu, Angew. Chem., Int. Ed., 2022, 61, e202202649 CAS.
- When measuring the kinetic orders of a photochemical reaction, the absorption of a photon by a molecule can be regarded as the first, bimolecular elementary reaction. Therefore, under controlled, identical light irradiation conditions, experimental apparent reaction rates can be compared. For further discussion see: (a) M. Hippler, J. Chem. Educ., 2003, 80, 1074–1077 CrossRef CAS; (b) S. Toby, J. Chem. Educ., 2005, 82, 37–38 CrossRef CAS.
- E. M. Simmons and J. F. Hartwig, Angew. Chem., Int. Ed., 2012, 51, 3066–3072 CrossRef CAS PubMed.
- M. Álvarez-Moreno, C. de Graaf, N. López, F. Maseras, J. M. Poblet and C. Bo, J. Chem. Inf. Model., 2015, 55, 95–1035 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: For the spectral data, experimental and computational experimental data. See DOI: https://doi.org/10.1039/d2sc04990b |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |