Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Dynamic kinetic resolution of γ,γ-disubstituted indole 2-carboxaldehydes via NHC-Lewis acid cooperative catalysis for the synthesis of tetracyclic ε-lactones†
Kuruva
Balanna
a,
Soumen
Barik
a,
Sayan
Shee
a,
Rajesh G.
Gonnade
 b and
Akkattu T.
Biju
b and
Akkattu T.
Biju
 *a
*a
aDepartment of Organic Chemistry, Indian Institute of Science, Bangalore-560012, India. E-mail: atbiju@iisc.ac.in; Web: https://orgchem.iisc.ac.in/atbiju/
bCentre for Materials Characterization, CSIR-National Chemical Laboratory, Dr Homi Bhabha Road, Pune-411008, India
First published on 29th August 2022
Abstract
The ubiquity of ε-lactones in various biologically active compounds inspired the development of efficient and enantioselective routes to these target compounds. Described herein is the enantioselective synthesis of indole-fused ε-lactones by the N-heterocyclic carbene (NHC)-Lewis acid cooperative catalyzed dynamic kinetic resolution (DKR) of in situ generated γ,γ-disubstituted indole 2-carboxaldehydes. The Bi(OTf)3-catalyzed Friedel–Crafts reaction of indole-2-carboxaldehyde with 2-hydroxy phenyl p-quinone methides generates γ,γ-disubstituted indole 2-carboxaldehydes, which in the presence of NHC and Bi(OTf)3 afforded the desired tetracyclic ε-lactones in up to 93% yield and >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 er. Moreover, preliminary studies on the mechanism of this formal [4 + 3] annulation are also provided.
1 er. Moreover, preliminary studies on the mechanism of this formal [4 + 3] annulation are also provided.
Introduction
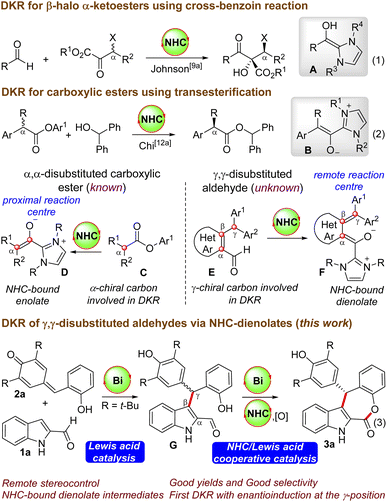
Functionalized ε-lactones are important structural motifs present in various biologically active compounds and this core is responsible for the flavor and aroma in many natural products.1 For instance, the natural products rubellins A and B have the benzo-fused ε-lactone moiety connected to the anthraquinone unit, and they exhibit photodynamic activity.2 Moreover, 9-dehydroxyeurotinone and 2-O-methyl-9-dehydoxyeurotinone have a dibenzo-fused ε-lactone core, and they are useful due to their antimicrobial and cytotoxic activity.3 Given the potential applications of ε-lactone-containing compounds, the development of rapid and facile routes for the enantioselective synthesis of ε-lactone derivatives have received remarkable attention. The Baeyer-Villiger oxidation of cyclohexanones constitutes one of the traditional approaches to access ε-lactones.4 Moreover, transition metal-catalyzed ring-expansion reactions and carbonylation processes could also provide straightforward access to ε-lactones.5 Herein, we report the enantioselective synthesis of tetracyclic indole-fused ε-lactones by the N-heterocyclic carbene (NHC)-Lewis acid catalyzed dynamic kinetic resolution (DKR) of in situ generated γ,γ-disubstituted indole 2-carboxaldehydes.6,7NHC-catalyzed DKR strategies are employed for the conversion of racemic substrates to enantiomerically pure products.8 Generally, carbene-catalyzed DKR approaches are applicable to racemic carbonyl compounds, where the enantioinduction takes place at the α-carbon centre. For instance, Goodman and Johnson reported the DKR of β-halo α-ketoesters by utilizing the NHC-catalyzed cross-benzoin reaction, where the reaction proceeds via the generation of the nucleophilic Breslow intermediate A (Scheme 1, eqn (1)).9–11 Moreover, Chi and co-workers demonstrated the NHC-catalyzed DKR of α-alkyl α-aryl carboxylic esters via the transesterification strategy, and the NHC- enolate B is the key intermediate (eqn (2)).12 In all these cases, the α-carbon center is involved in the DKR process, where the generated chiral center is proximal to the reacting center (generation of D from C), and intriguingly, the synthesis of enantioenriched γ-substituted carboxylic esters from racemic starting materials via the DKR process is not known.13 This will be interesting as the γ-carbon center will be remote from the reacting carbonyl center and enantioinduction will be challenging (conversion of E to F). In this context, we envisioned the NHC-catalyzed DKR of the γ,γ-disubstituted aldehyde G derived from the unprotected indole-2-carboxaldehyde,14 which can be generated in situ by the Lewis acid-catalyzed Friedel–Crafts reaction of indole 2-aldehyde 1a with the o-hydroxyphenyl-substituted p-quinone methide 2a. This formal [4 + 3] annulation reaction afforded indole-fused ε-lactone 3a in good yields and selectivities. The optimal Lewis acid was Bi(OTf)3, which plays dual roles: (a) in catalyzing the initial Friedel–Crafts reaction generating G, and (b) then the involvement in the DKR process for the esterification reaction in cooperation with NHCs.15 Intriguingly, although NHC-catalyzed DKR strategies are known for the enantioselective synthesis of β-lactones, γ-lactones and δ-lactones, the related DKR strategies for ε-lactones are unknown. It may be noted in this context that NHC-catalyzed synthesis of fused ε-lactones by the [4 + 3] annulation of o-quinone methides with enal-derived homoenolates was uncovered independently by Ye’s16 and Scheidt’s groups.17 Moreover, a related NHC-homoenolate route for the synthesis of spirooxindole ε-lactones (without involving the DKR process) is demonstrated by Li’s18a and Enders’ groups.18b
Results and discussion
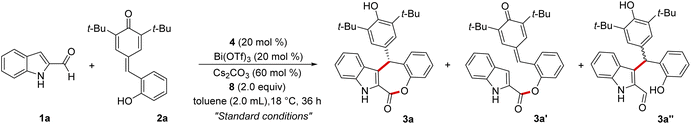
Driven by the idea of inducing stereocontrol at a remote position using the DKR strategy, the present study was initiated by treating indole 2-carboxaldehyde 1a with the p-quinone methide 2a in the presence of NHC generated from the chiral triazolium salt 4 using Cs2CO3 as the base under oxidative conditions using the bisquinone 8. Interestingly, under these conditions, the desired indole-fused ε-lactone 3a was formed in 68% yield and a 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
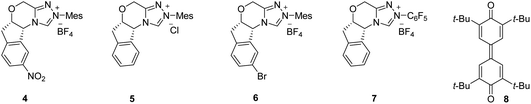
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 enantiomeric ratio (er) (Table 1, entry 1). The product 3a was formed by the initial Friedel–Crafts reaction of 1a with 2a catalyzed by Bi(OTf)3 (generating in situ3a''), followed by the NHC/Lewis acid-catalyzed DKR via a stereoselective esterification reaction. Notably, the ester 3a′ (formed by the esterification of 1a with the phenol moiety of 2a),19 and the Friedel–Crafts product 3a′′ were not isolated under these conditions. Moreover, compared to the carbene formed from 4, other chiral triazolium salts 5–7 provided less yield and selectivity of 3a (entries 2–4). The screening of other bases and solvents revealed that Cs2CO3 is the optimal base and toluene is the best solvent for this transformation (entries 5–10). The use of Sc(OTf)3 as the Lewis acid and CF3SO3H as the Brønsted acid for initiating the Friedel–Crafts reaction was also not efficient (entries 11 and 12). In addition, performing the reaction with 10 mol% of 4 or using 1.0 equiv. of 8 resulted in an incomplete reaction with the isolation of the Friedel–Crafts adduct 3a′′ maintaining high selectivity (entries 13,14). Hence, entry 1 was selected as the best condition for the substrate scope analysis.20
5 enantiomeric ratio (er) (Table 1, entry 1). The product 3a was formed by the initial Friedel–Crafts reaction of 1a with 2a catalyzed by Bi(OTf)3 (generating in situ3a''), followed by the NHC/Lewis acid-catalyzed DKR via a stereoselective esterification reaction. Notably, the ester 3a′ (formed by the esterification of 1a with the phenol moiety of 2a),19 and the Friedel–Crafts product 3a′′ were not isolated under these conditions. Moreover, compared to the carbene formed from 4, other chiral triazolium salts 5–7 provided less yield and selectivity of 3a (entries 2–4). The screening of other bases and solvents revealed that Cs2CO3 is the optimal base and toluene is the best solvent for this transformation (entries 5–10). The use of Sc(OTf)3 as the Lewis acid and CF3SO3H as the Brønsted acid for initiating the Friedel–Crafts reaction was also not efficient (entries 11 and 12). In addition, performing the reaction with 10 mol% of 4 or using 1.0 equiv. of 8 resulted in an incomplete reaction with the isolation of the Friedel–Crafts adduct 3a′′ maintaining high selectivity (entries 13,14). Hence, entry 1 was selected as the best condition for the substrate scope analysis.20
| entry | Variation of the standard conditionsa | Yield of 3ab (%) | er of 3ac | Yield of 3a′b (%) | Yield of 3a′′b (%) |
|---|---|---|---|---|---|
| a Standard conditions: 1a (0.12 mmol), 2a (0.168 mmol), 4 (20 mol%), Bi(OTf)3 (20 mol%), Cs2CO3 (60 mol%), 8 (2.0 equiv.), toluene (2.0 mL), 18 °C and 36 h. b Yields of the column chromatography purified products are provided. c The er was established by HPLC analysis on a chiral stationary phase. | |||||
| 1 | None | 68 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
<5 | <5 |
| 2 | 5 Instead of 4 | 62 | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
<5 | <5 |
| 3 | 6 Instead of 4 | 11 | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
<5 | 66 |
| 4 | 7 Instead of 4 | 19 | 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19 |
<5 | <5 |
| 5 | K2CO3 instead of Cs2CO3 | 36 | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
<5 | 25 |
| 6 | KOt-Bu instead of Cs2CO3 | <5 | -Nd- | <5 | <5 |
| 7 | DABCO instead of Cs2CO3 | <5 | -Nd- | <5 | <5 |
| 8 | THF instead of toluene | <5 | -Nd- | 71 | <5 |
| 9 | DME instead of toluene | <5 | -Nd- | 67 | <5 |
| 10 | Mesitylene instead of toluene | 22 | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
<5 | 18 |
| 11 | Sc(OTf)3 instead of Bi(OTf)3 | 52 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
<5 | <5 |
| 12 | CF3SO3H instead of Bi(OTf)3 | 60 | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
<5 | <5 |
| 13 | 10 mol% of 4 instead of 20 mol% | 31 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
<5 | 48 |
| 14 | 1.0 equiv. of 8 instead of 2.0 equiv. | 39 | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
<5 | 28 |
|
|||||
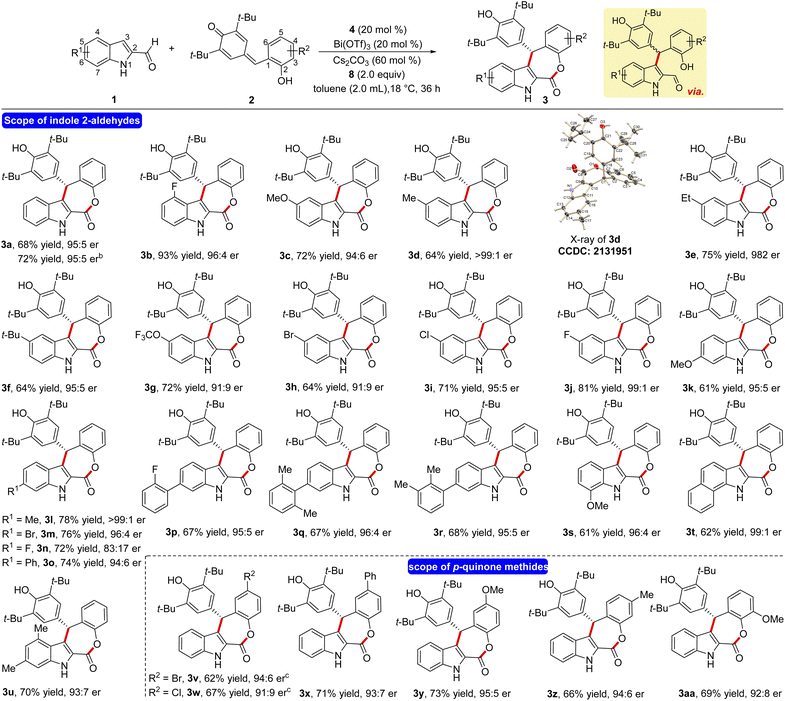
Having the optimized reaction conditions in hand, the scope and limitations of the present NHC-catalyzed DKR has been examined. First, the variation of the indole 2-carboxaldehyde has been studied. The unsubstituted parent aldehyde worked well and 4-fluoro substituted aldehyde furnished the tetracyclic ε-lactone 3b in 93% yield and 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 er (Scheme 2). The formation of 3a in 72% yield and 95
4 er (Scheme 2). The formation of 3a in 72% yield and 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 er on a 1.0 mmol scale indicates that the present DKR process is scalable and practical. A variety of electronically different substituents at the 5-position of indole 2-carboxaldehyde was well tolerated under the optimized conditions and the corresponding ε-lactones were formed in good yields and selectivities (3c–3j). In the case of the methyl derivative 3d, the structure and the absolute stereochemistry of the chiral center were confirmed using X-ray analysis of the crystals.21 Moreover, substrates bearing different groups at the 6-position of indole 2-carboxaldehye underwent a smooth NHC-catalyzed annulation reaction to afford the desired products in good yields and er values (3k–3r). In addition, the reaction using 7-methoxy indole 2-carboxaldehyde furnished the product 3s in 61% yield and 96
5 er on a 1.0 mmol scale indicates that the present DKR process is scalable and practical. A variety of electronically different substituents at the 5-position of indole 2-carboxaldehyde was well tolerated under the optimized conditions and the corresponding ε-lactones were formed in good yields and selectivities (3c–3j). In the case of the methyl derivative 3d, the structure and the absolute stereochemistry of the chiral center were confirmed using X-ray analysis of the crystals.21 Moreover, substrates bearing different groups at the 6-position of indole 2-carboxaldehye underwent a smooth NHC-catalyzed annulation reaction to afford the desired products in good yields and er values (3k–3r). In addition, the reaction using 7-methoxy indole 2-carboxaldehyde furnished the product 3s in 61% yield and 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 er. Furthermore, disubstituted indole -aldehydes also provided good yield of the target product thus expanding the scope of this annulation (3t and 3u).
4 er. Furthermore, disubstituted indole -aldehydes also provided good yield of the target product thus expanding the scope of this annulation (3t and 3u).
Next, the variation in the o-hydroxyphenyl-substituted p-quinone methide moiety was studied. The p-quinone methides having –Br, –Cl, Ph and –OMe groups at the 5-position are well tolerated under the present conditions and the desired annulated products are formed in reasonable yields and selectivities (3v–3y). Moreover, –Me and –OMe groups at the 4- and 3-position of 2 did not affect the reaction outcome and the target ε-lactones are formed in good yields and er values (3z and 3aa).
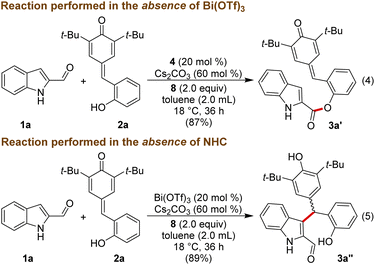
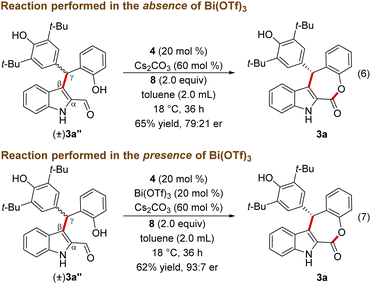
To get insight into the mechanism of the reaction, a few mechanistic experiments were performed. When the reaction of 1a was performed with 2a in the absence of Bi(OTf)3, the reaction furnished the ester product 3a′ in 87% yield, and 3a was not formed under these conditions (Scheme 3, eqn (4)). Notably, related esterification reactions catalyzed by NHCs are reported by Studer and co-workers.19 Moreover, treatment of 1a with 2a in the absence of NHC resulted in the formation of the Friedel–Crafts adduct 3a′′ in 89% yield (eqn (5)).
The lack of the desired product 3a formation in the absence of either Bi(OTf)3 or NHC indicates the role of these two catalysts for the direct and enantioselective synthesis of the ε-lactone 3a. To get further insight into the role of Bi(OTf)3 in the DKR process, the Friedel–Crafts alkylation product 3a′′ was treated with NHC generated from 4 under oxidative conditions in the absence of Bi(OTf)3. This reaction afforded 3a in 65% yield and 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 er (Scheme 4, eqn (6)). Interestingly, when the same reaction was conducted in the presence of Bi(OTf)3 the product 3a was formed in 62% yield and an improved er of 93
21 er (Scheme 4, eqn (6)). Interestingly, when the same reaction was conducted in the presence of Bi(OTf)3 the product 3a was formed in 62% yield and an improved er of 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 shedding light on the role of a Lewis acid in the DKR process (eqn (7)).22 It is reasonable to assume that the Bi(III) Lewis acid is involved in coordination with the NHC-bound dienolate and the phenolic –OH moiety for the facile dienolate protonation and intramolecular acylation.23,24
7 shedding light on the role of a Lewis acid in the DKR process (eqn (7)).22 It is reasonable to assume that the Bi(III) Lewis acid is involved in coordination with the NHC-bound dienolate and the phenolic –OH moiety for the facile dienolate protonation and intramolecular acylation.23,24
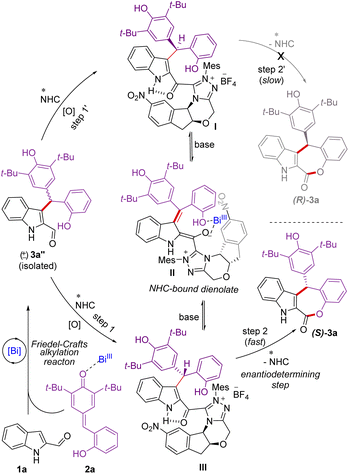
Mechanistically, in the presence of Lewis acidic Bi(OTf)3, indole 2-carboxaldehyde 1a25 adds to the p-quinone methide 2a generating in situ the racemic γ,γ-disubstituted indole 2-carboxaldehyde 3a′′ through an intermolecular Friedel–Crafts alkylation reaction (Scheme 5). Under oxidative conditions, the addition of NHC to the aldehyde 3a′′ could generate the diastereomeric NHC-bound acylazoliums I and III.26 It is reasonable to assume that the NHC acylazolium I could not undergo intramolecular acylation due to the presence of a bulky chiral indanone core of the catalyst. Hence, the formation of (R)-3a is not feasible. On the other hand, the NHC acylazolium III undergoes facile intramolecular acylation to afford the desired product (S)-3a as the aminoindanol and the bulky 2,6-di-tert-butyl phenolic moieties are on the opposite side. The acylazolium I under basic conditions could form the NHC-dienolate intermediate II,6j,27 which could undergo enantioselective protonation to generate the intermediate III, which can further undergo acylation to form the product (S)-3a. During the re-protonation of NHC-bound dienolate intermediate II, Bi(OTf)3 is likely involved in the coordination with the dienolate and –OH moieties to facilitate protonation and then esterification.
In conclusion, we have presented the NHC-Lewis acid cooperative catalyzed DKR for the enantioselective synthesis of tetracyclic indole-fused ε-lactones, a formal [4 + 3] annulation. The transiently generated γ,γ-disubstituted indole 2-carboxaldehydes from indole-2-carboxaldehyde and 2-hydroxy phenyl p-quinone methides using Bi(OTf)3 catalysis underwent an efficient DKR process, where the NHC-bound dienolates are the key intermediates. In the presence of NHC and Bi(OTf)3, facile ε-lactonization takes place with enantioinduction at the γ-position. The tetracyclic ε-lactones are formed in up to 93% yield and >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 er. The stereoinduction at the remote γ-carbon, mild reaction conditions, and in situ generation of the racemic substrate for DKR are the notable features of the present annulation reaction.
1 er. The stereoinduction at the remote γ-carbon, mild reaction conditions, and in situ generation of the racemic substrate for DKR are the notable features of the present annulation reaction.
Data availability
Details of the experimental procedures, mechanistic experiments, characterization data of all the tetracyclic indole-fused ε-lactones, and X-ray data of 3d.Author contributions
K. B. and A. T. B. conceived and designed the project. K. B. performed the optimization studies, substrate scope analysis and mechanistic studies. S. B. and S. S. helped in the substrate scope studies. R. G. G. performed the X-ray crystallographic analysis. K. B. and A. T. B. wrote the manuscript. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Generous financial support by the Science and Engineering Research Board (SERB), Government of India (File Number: DIA/2018/000018) is gratefully acknowledged. K. B. is thankful to UGC and S. B. is thankful to IISc, respectively, for a fellowship and S. S. is thankful to the Ministry of Education for the PMRF. We also thank Ms. Malini George for the experimental support, and Dr Trinadh Kaicharla for the useful discussion.Notes and references
- For selected examples, see: (a) L. Wang, S. Xie, L. Ma, Y. Chen and W. Lu, Bioorg. Med. Chem., 2015, 23, 1950 CrossRef CAS PubMed; (b) Y. Luo, S. Yu, L. Tong, Q. Huang, W. Lu and Y. Chen, Eur. J. Med. Chem., 2012, 54, 281 CrossRef CAS PubMed; (c) S.-P. Yang, H.-D. Chen, S.-G. Liao, B.-J. Xie, Z.-H. Miao and J.-M. Yue, Org. Lett., 2011, 13, 150 CrossRef CAS PubMed; (d) K. C. Nicolaou and H. Xu, Chem. Commun., 2006, 6, 600 RSC ; See also:; (e) S. Schulz and S. Hötling, Nat. Prod. Rep., 2015, 32, 1042 RSC; (f) T. Janecki, Natural Lactones and Lactams: Synthesis, Occurrence and Biological Activity, John Wiley & Sons. 2013 CrossRef.
- A. Arnone, L. Camarda, G. Nasini and G. Assante, J. Chem. Soc., Perkin Trans. 1, 1986, 255 RSC.
- H.-J. Yan, X.-M. Li, C.-S. Li and B.-G. Wang, Helv. Chim. Acta, 2012, 95, 163 CrossRef CAS.
- For selected reviews, see: (a) G.-J. ten Brink, I. W. C. E. Arends and R. A. Sheldon, Chem. Rev., 2004, 104, 4105 CrossRef PubMed; (b) G. Strukul, Angew. Chem., Int. Ed., 1998, 37, 1198 CrossRef.
- (a) R. Chanthateyanonth and H. Alper, Adv. Synth. Catal., 2004, 346, 1375 CrossRef CAS; (b) M. Murakami, T. Tsuruta and Y. Ito, Angew. Chem., Int. Ed., 2000, 39, 2484 CrossRef CAS; (c) B. El Ali, K. Okuro, G. Vasapollo and H. Alper, J. Am. Chem. Soc., 1996, 118, 4264 CrossRef CAS.
- For selected reviews on NHC organocatalysis, see: (a) R. Song, Y. Xie, Z. Jin and Y. R. Chi, Angew. Chem., Int. Ed., 2021, 60, 26026 CrossRef CAS PubMed; (b) J. Wang, C. Zhao and J. Wang, ACS Catal., 2021, 11, 12520 CrossRef CAS; (c) H. Ohmiya, ACS Catal., 2020, 10, 6862 CrossRef CAS; (d) S. Barik and A. T. Biju, Chem. Commun., 2020, 56, 15484 RSC; (e) T. K. Das and A. T. Biju, Chem. Commun., 2020, 56, 8537 RSC; (f) T. Ishii, K. Nagao and H. Ohmiya, Chem. Sci., 2020, 11, 5630 RSC; (g) X.-Y. Chen, Z.-H. Gao and S. Ye, Acc. Chem. Res., 2020, 53, 690 CrossRef CAS PubMed; (h) S. Mondal, S. R. Yetra, S. Mukherjee and A. T. Biju, Acc. Chem. Res., 2019, 52, 425 CrossRef CAS; (i) K. J. R. Murauski, A. A. Jaworski and K. A. Scheidt, Chem. Soc. Rev., 2018, 47, 1773 RSC; (j) X.-Y. Chen, Q. Liu, P. Chauhan and D. Enders, Angew. Chem., Int. Ed., 2018, 57, 3862 CrossRef CAS; (k) D. M. Flanigan, F. Romanov-Michailidis, N. A. White and T. Rovis, Chem. Rev., 2015, 115, 9307 CrossRef CAS PubMed; (l) R. S. Menon, A. T. Biju and V. Nair, Beilstein J. Org. Chem., 2016, 12, 444 CrossRef CAS PubMed; (m) M. N. Hopkinson, C. Richter, M. Schedler and F. Glorius, Nature, 2014, 510, 485 CrossRef CAS; (n) R. S. Menon, A. T. Biju and V. Nair, Chem. Soc. Rev., 2015, 44, 5040 RSC.
- A. T. Biju, Wiley-VCH Verlag GmbH & Co. KGa A, Boschstr.12, 69469.
- (a) C. De Risi, O. Bortolini, G. Di Carmine, D. Ragno and A. Massi, Synthesis, 2019, 51, 1871 CrossRef CAS; (b) S. Chen, Y.-H. Shi and M. Wang, Chem.–Asian J., 2018, 13, 2184 CrossRef CAS PubMed; (c) Z. Wang, D. Pan, T. Li and Z. Jin, Chem.–Asian J., 2018, 13, 2149 CrossRef CAS.
- (a) C. G. Goodman and J. S. Johnson, J. Am. Chem. Soc., 2014, 136, 14698 CrossRef CAS PubMed ; See also:; (b) C. G. Goodman, M. M. Walker and J. S. Johnson, J. Am. Chem. Soc., 2015, 137, 122 CrossRef CAS PubMed.
- For pioneering reports on NHC-catalyzed DKR, see: (a) D. T. Cohen, C. C. Eichman, E. M. Phillips, E. R. Zarefsky and K. A. Scheidt, Angew. Chem., Int. Ed., 2012, 51, 7309 CrossRef CAS PubMed ; See also:; (b) R. C. Johnston, D. T. Cohen, C. C. Eichman, K. A. Scheidt and P. H.-Y. Cheong, Chem. Sci., 2014, 5, 1974 RSC.
- R. Breslow, J. Am. Chem. Soc., 1958, 80, 3719 CrossRef CAS.
- (a) X. Chen, J. Z. M. Fong, J. Xu, C. Mou, Y. Lu, S. Yang, B.-A. Song and Y. R. Chi, J. Am. Chem. Soc., 2016, 138, 7212 CrossRef CAS PubMed ; See also:; (b) B. Liu, R. Song, J. Xu, P. K. Majhi, X. Yang, S. Yang, Z. Jin and Y. R. Chi, Org. Lett., 2020, 22, 3335 CrossRef CAS PubMed.
- For selected NHC-catalyzed reactions via DKR strategies, see: (a) Y.-Y. Gao, C.-L. Zhang, L. Dai, Y.-F. Han and S. Ye, Org. Lett., 2021, 23, 1361 CrossRef CAS PubMed; (b) D. Guo, Q. Peng, B. Zhang and J. Wang, Org. Lett., 2021, 23, 7765 CrossRef CAS PubMed; (c) Y. Liu, P. K. Majhi, R. Song, C. Mou, L. Hao, H. Chai, Z. Jin and Y. R. Chi, Angew. Chem., Int. Ed., 2020, 59, 3859 CrossRef CAS PubMed; (d) K.-Q. Chen, Z.-H. Gao and S. Ye, Angew. Chem., Int. Ed., 2019, 58, 1183 CrossRef CAS PubMed; (e) S. Mondal, S. Mukherjee, T. K. Das, R. Gonnade and A. T. Biju, ACS Catal., 2017, 7, 3995 CrossRef CAS; (f) X. Yang, P. K. Majhi, H. Chai, B. Liu, J. Sun, T. Liu, Y. Liu, L. Zhou, J. Xu, J. Liu, D. Wang, Y. Zhao, Z. Jin and Y. R. Chi, Angew. Chem., Int. Ed., 2021, 60, 159 CrossRef CAS PubMed.
- For the NHC-catalyzed activation of N-H free indoles and subsequent reaction with electrophiles, see: (a) K. Balanna, K. Madica, S. Mukherjee, A. Ghosh, T. Poisson, T. Besset, G. Jindal and A. T Biju, Chem. - Eur. J., 2020, 26, 818 CrossRef CAS; (b) Y. Liu, G. Luo, X. Yang, S. Jiang, W. Xue, Y. R. Chi and Z. Jin, Angew. Chem., Int. Ed., 2020, 132, 450 CrossRef; (c) Q.-P. Peng, S.-J. Li, B. Zhang, D.-H. Guo, Y. Lan and J. Wang, Commun. Chem., 2020, 3, 177 CrossRef CAS; (d) C. Wang, Z. Li, J. Zhang and X.-P. Hui, Org. Chem. Front., 2020, 7, 1647 RSC.
- For a DKR strategy under NHC-Lewis acid cooperative catalysis, see: (a) Z. Wu, F. Li and J. Wang, Angew. Chem., Int. Ed., 2015, 54, 1629 CrossRef CAS PubMed ; For related reports on NHC-Lewis acid cooperative catalysis, see:; (b) S. Bera, R. C. Samanta, C. G. Daniliuc and A. Studer, Angew. Chem., Int. Ed., 2014, 53, 9622 CrossRef CAS; (c) S. Lu, S. B. Poh, W.-Y. Siau and Y. Zhao, Angew. Chem., Int. Ed., 2013, 52, 1731 CrossRef CAS PubMed; (d) B. Cardinal-David, D. E. A. Raup and K. A. Scheidt, J. Am. Chem. Soc., 2010, 132, 5345 CrossRef CAS PubMed; (e) G. Yang, D. Guo, D. Meng and J. Wang, Nat. Commun., 2019, 10, 3062 CrossRef PubMed; (f) Y. Wu, M. Li, J. Sun, G. Zheng and Q. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202117340 CAS.
- H. Lv, W.-Q. Jia, L.-H. Sun and S. Ye, Angew. Chem., Int. Ed., 2013, 52, 8607 CrossRef CAS.
- J. Izquierdo, A. Orue and K. A. Scheidt, J. Am. Chem. Soc., 2013, 135, 10634 CrossRef CAS PubMed.
- (a) W. Li, H. Yuan, Z. Liu, Z. Zhang, Y. Cheng and P. Li, Adv. Synth. Catal., 2018, 360, 2460 CrossRef CAS; (b) Q. Liu, S. Li, X.-Y. Chen, K. Rissanen and D. Enders, Org. Lett., 2018, 20, 3622 CrossRef CAS PubMed.
- For the NHC-catalyzed esterification of aldehydes, see: S. De Sarkar, S. Grimme and A. Studer, J. Am. Chem. Soc., 2011, 132, 1190 CrossRef.
- For details, see the Supporting Information†.
- CCDC 2131951 (3d) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from the Data Centre via https://www.ccdc.cam.ac.uk/data_request/cif.
- J. Mo, X. Chen and Y. R. Chi, J. Am. Chem. Soc., 2012, 134, 8810 CrossRef CAS PubMed.
- Performing the reaction of 3a’ under the optimized conditions to check the feasibility of intramolecular cyclization leading to 3a did not work indicating that Friedel-Crafts alkylation proceeds prior to annulation.
- To get insight into the DKR process, the reaction of 3a′′ was performed under the optimized conditions for 12 h and the ee of the recovered 3a′′ was determined. Detection of 3a′′ in the racemic form is an indication that the present reaction is proceeding via DKR.
- Presently, this annulation works with indole 2-carboxaldehydes as nucleophiles, and the attempted reactions with other aldehydes such as pyrrole 2-carboxaldehyde, benzofuran 2-carboxaldehyde and 3,4,5-trimethoxy benzaldehyde as nucleophiles did not afford the desired annulation products under the optimized conditions.
- The reaction of N-methyl indole-2-carbaldehyde instead of 1a under the optimized reaction conditions afforded N-methyl ε-lactones in 93% yield and 50:50 er. This result indicates that free N-H required for getting selectivity, and the N-H group is possibly involved in H-bonding with the acylazolium moiety.
- X. Chen, S. Yang, B. A. Song and Y. R. Chi, Angew. Chem., Int. Ed., 2013, 52, 11134 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. Details on experimental procedures, characterization data of all compounds. CCDC 2131951. For ESI and crystallographic data in CIF or other electronic format see https://doi.org/10.1039/d2sc03745a |
| This journal is © The Royal Society of Chemistry 2022 |