Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
An aqueous photo-controlled polymerization under NIR wavelengths: synthesis of polymeric nanoparticles through thick barriers†
Zilong
Wu
 a,
Wenbo
Fang
a,
Chenyu
Wu
a,
Wenbo
Fang
a,
Chenyu
Wu
 b,
Nathaniel
Corrigan
b,
Nathaniel
Corrigan
 a,
Tong
Zhang
a,
Sihao
Xu
a and
Cyrille
Boyer
a,
Tong
Zhang
a,
Sihao
Xu
a and
Cyrille
Boyer
 *a
*a
aCluster for Advanced Macromolecular Design and Australian Centre for NanoMedicine, School of Chemical Engineering, The University of New South Wales, Sydney, NSW 2052, Australia. E-mail: cboyer@unsw.edu.au
bQingdao Institute for Theoretical and Computational Sciences, Institute of Frontier and Interdisciplinary Science, Shandong University, Qingdao 266237, Shandong, P. R. China
First published on 1st September 2022
Abstract
We report an aqueous and near-infrared (NIR) light mediated photoinduced reversible addition–fragmentation chain transfer (photo-RAFT) polymerization system using tetrasulfonated zinc phthalocyanine (ZnPcS4−) as a photocatalyst. Owing to the high catalytic efficiency and excellent oxygen tolerance of this system, well-controlled polyacrylamides, polyacrylates, and polymethacrylates were synthesized at fast rates without requiring deoxygenation. Notably, NIR wavelengths possess enhanced light penetration through non-transparent barriers compared to UV and visible light, allowing high polymerization rates through barriers. Using 6.0 mm pig skin as a barrier, the polymerization rate was only reduced from 0.36 to 0.21 h−1, indicating potential for biomedical applications. Furthermore, longer wavelengths (higher λ) can be considered an ideal light source for dispersion photopolymerization, especially for the synthesis of large diameter (d) nanoparticles, as light scattering is proportional to d6/λ4. Therefore, this aqueous photo-RAFT system was applied to photoinduced polymerization-induced self-assembly (photo-PISA), enabling the synthesis of polymeric nanoparticles with various morphologies, including spheres, worms, and vesicles. Taking advantage of high penetration and reduced light scattering of NIR wavelengths, we demonstrate the first syntheses of polymeric nanoparticles with consistent morphologies through thick barriers.
Introduction
Photoinduced reversible deactivation radical polymerization (photo-RDRP)1–58 techniques enable the production of polymers with low dispersity and defined architectures and provide a high degree of spatio–temporal reaction control. As a useful photo-RDRP variant, photoinduced reversible addition–fragmentation chain transfer polymerization (photo-RAFT) polymerization process26–58 often uses ppm range photocatalysts (PCs) to activate RAFT agents under visible light without the need for deoxygenation.48–52 Although photo-RDRP systems that use visible light (λ = 400–700 nm) are well-established, systems regulated by near-infrared light (NIR, λ = 700–2500 nm) are still rare.20–26,43–47 This is mainly due to the difficulty in using long wavelength, low energy (E = h/λ) irradiation to drive photochemical reactions.59 While PCs with NIR light absorption exist, they commonly possess lower redox potentials of excited state (S1 and T1), resulting in limited catalytic performance and inability to mediate photoinduced electron/energy transfer (PET) processes.43 However, compared to shorter wavelengths, NIR light has enhanced penetration through non-transparent barriers,60 which can be beneficial for materials engineering and potential biomedical applications. For instance, transdermal photopolymerization has been employed for injectable hydrogel systems, minimizing operative wounds and conferring spatiotemporal control towards gelation.61–63Meanwhile, using aqueous media instead of organic solvents for polymerizations provides economic and environmental benefits, further increasing these systems for bio-applications.50,61–64 However, long-wavelength-mediated aqueous photo-RDRP systems have seldom been reported,17,20,51 due to the typically low solubility of NIR absorbing catalysts in aqueous media. In 2020, Qiao and co-workers developed an aqueous visible- and NIR-mediated photoinduced electron/energy transfer reversible addition–fragmentation chain transfer (PET-RAFT) polymerization process catalyzed by a self-assembled carboxylated porphyrin photocatalyst.51 However, this system suffers from low polymerization rates under NIR irradiation and provided only low monomer conversions without deoxygenation. Recently, the groups of Pan and Pang reported the successful utilization of upconversion nanoparticles as heterogeneous photocatalysts to induce photoinduced atom-transfer radical polymerizations (photo-ATRP) under NIR laser light in aqueous media.17,20 Nevertheless, prior deoxygenation and high-intensity light sources were required in these systems to achieve appreciable monomer conversions.
In this study, aqueous photo-RAFT systems catalyzed by tetrasulfonated zinc phthalocyanine (ZnPcS4−) under NIR light irradiation (λmax = 730 nm) were successfully developed. ZnPcS4− has been used widely as a photosensitizer in photodynamic therapy (PDT) owing to its absorption of extended wavelengths and excellent solubility in water,65–70 however, this is the first example of ZnPcS4− as a PC to mediate photopolymerization. These aqueous systems exhibited excellent oxygen tolerance, providing high polymerization rates and polymers with narrow molecular weight distributions (MWDs). Taking advantage of the enhanced penetration of NIR light, photopolymerizations were performed with non-transparent barriers between the light source and the reaction vessel, resulting in high polymerization rates (0.21–0.34 hour−1) and well-defined polymers (dispersity (Đ) < 1.15). Although photopolymerization through barriers has been demonstrated in previous NIR light mediated RDRP systems,21–23,44–46,51 this is the first aqueous system which displays fast polymerization in the presence of thick barriers and does not require deoxygenation. Aqueous media and excellent oxygen tolerance facilitate the potential application of this NIR system in materials engineering and biomedicine.50,61–64
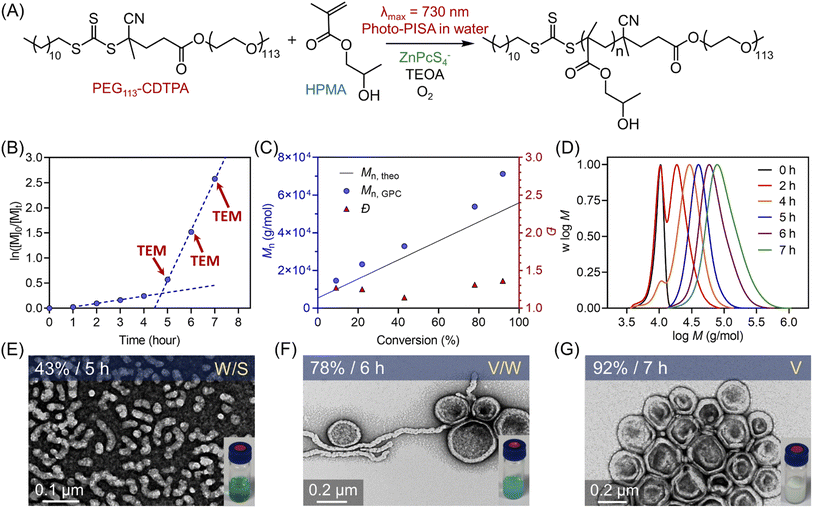
In addition, the developed polymerization systems are highly suited for aqueous polymerization-induced self-assembly (PISA) as they do not require deoxygenation, which simplifies the synthetic procedure. More importantly, long wavelength (λ) NIR light is an ideal energy source to perform photoinitiated polymerization-induced self-assembly (photo-PISA),71–82 especially for synthesizing large (d) nanoparticles, as light scattering is directly proportional to d6/λ4.83–87 Reduced scattering diminishes light intensity gradients in the reaction media, promoting light penetration in colloidal dispersions, which can favor the production of polymeric nanoparticles with more well-defined morphologies. Photo-RAFT mediated aqueous dispersion polymerization of 2-hydroxypropyl methacrylate (HPMA) was conducted using a poly(ethylene glycol) (PEG) functionalized RAFT agent as the first stabilizing block. As a result, nanoparticles with various morphologies, including spheres, worms, and vesicles, were successfully synthesized by simply changing the targeted degree of polymerization (DP). Furthermore, polymeric nanoparticles with consistent morphologies were synthesized via photo-PISA through a biological barrier (6.0 mm thick pig skin). To our knowledge, this is the first example of polymeric nanoparticle synthesis through thick barriers using NIR light. Like the barrier-free photo-PISA, we observed a similar evolution of nanoparticle morphologies, i.e., from spheres to vesicles, when a biological barrier was introduced. The successful synthesis of large vesicles (∼200 nm diameter) through thick barriers indicated the high efficiency of this system.
Results and discussion
Aqueous photo-RAFT polymerization catalyzed by ZnPcS4− under NIR light without deoxygenation
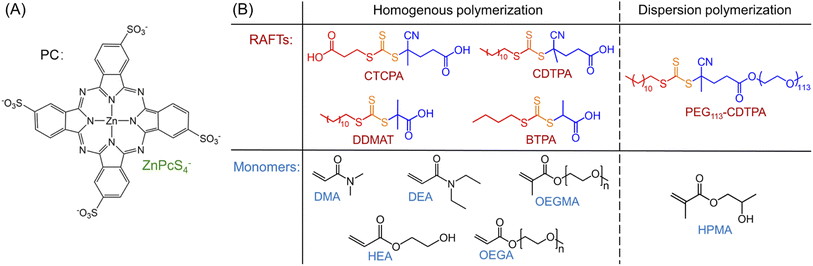
Under NIR light (λmax = 730 nm) irradiation, tetrasulfonated zinc phthalocyanine (ZnPcS4−, Scheme 1A and ESI, Fig. S1†) was utilized as a water-soluble photocatalyst (PC) to mediate photo-RAFT polymerization. N,N-Dimethylacrylamide (DMA) and 4-((((2-carboxyethyl)thio)carbonothioyl)thio)-4-cyanopentanoic acid (CTCPA) were selected as the model monomer and RAFT agent, respectively, owing to their solubility in water (Scheme 1B).PET-RAFT systems were first tested in the presence of ZnPcS4− as PC. As PET-RAFT polymerization can be carried out under two different quenching pathways, namely an oxidative quenching pathway (OQP, Scheme 2A)43,46,48 and a reductive quenching pathway (RQP, Scheme 2B).39,40 We decided to investigate the efficiency of each pathway in the presence of ZnPcS4− under inert atmosphere. Under the OQP, a modest monomer conversion was noted (9% after 10 hours; Table 1, #1), whereas, under the RQP in the presence of triethanolamine (TEOA), a higher monomer conversion was observed (20% after 3 hours; Table 1, #2). In the absence of ZnPcS4−, both OQP and RQP systems exhibited no monomer conversion (Table 1, #3 and 4). As expected, PET-RAFT polymerization via OQP was completely inert without deoxygenation (Table 1, #5), as radical species were quenched by dissolved oxygen. However, surprisingly in the case of RQP in the presence of air, the monomer conversion reached 65% in 3 hours (Table 1, #6). To confirm this surprising result in the presence of TEOA and air, we performed polymerization kinetics with and without deoxygenation. A much higher apparent propagation rate (kpapp) (0.36 hour−1versus 0.08; ESI, Fig. S2†) was determined when the polymerization was conducted without deoxygenation, which suggests a new reaction pathway in the presence of TEOA and oxygen. Control experiments without deoxygenation demonstrated the need for the presence of ZnPcS4− (Table 1, #7), TEOA (Table 1, #5), and light (Table 1, #8), as negligible monomer conversions were observed in the absence of either species or light irradiation. Furthermore, in the presence of ZnPcS4−, TEOA, and oxygen, the control experiment without RAFT agent displayed a modest monomer conversion (18% after 10 hours), suggesting that initiating species can be formed in the absence of RAFT agent (Table 1, #9). This is in contrast to PET-RAFT polymerization via RQP (i.e., in the presence of ZnPcS4− and TEOA after deoxygenation) where we did not observe monomer conversion in the absence of RAFT agent (Table 1, #10). In the presence of oxygen, photopolymerization displays a different pathway (Table 1, #6 and 9) from PET-RAFT process (Table 1, #2 and 10). Regardless of the conditions employed, PDMA was successfully synthesized in a controlled manner (Table 1, #1, 2, and 6; ESI, Table S1 and Fig. S3†), as indicated by the excellent agreement between theoretical (Mn,theo) and experimental (Mn,GPC) molecular weights, and low dispersity (Đ) determined by gel permeation chromatography (GPC).
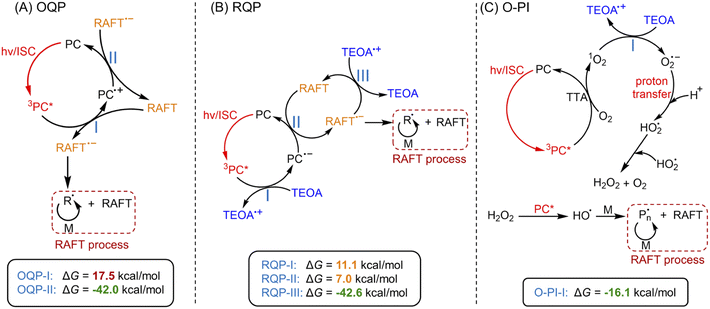 | ||
| Scheme 2 Proposed mechanism of photo-RAFT polymerization catalyzed by ZnPcS4−via three pathways.a Note: a(A) PET-RAFT polymerization via an oxidative quenching pathway (OQP) and (B) a reductive quenching pathway (RQP). (C) Photo-RAFT polymerization via an oxygen-mediated photoinitiation (O-PI) system. Values indicate the Gibbs free energy change (ΔG) of electron transfer reactions in three pathways calculated by DFT (coordinates and energies of PC, RAFT agent, TEOA, and O2 were shown in ESI†). | ||
| # | RAFT agent | PC | Deoxygenation | Reducing agent | Time (hour) | α (%) | k p app (hour−1) | M n,theo (g mol−1) | M n,GPC (g mol−1) | Đ |
|---|---|---|---|---|---|---|---|---|---|---|
a Notes: Reactions were performed at room temperature under NIR light (λmax = 730 nm; I = 60 mW cm−2) in water at [DMA]/[water] = 50 (v/v). A fixed reaction stoichiometry of [DMA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CTCPA] [CTCPA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [TEOA] [TEOA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [ZnPcS4−] = 200 [ZnPcS4−] = 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01 (50 ppm ZnPcS4− relative to monomer) was used.
b Theoretical molecular weight was calculated using the following equation: Mn,theo = [M]0/[RAFT agent]0 × MWM × α + MWRAFT agent, where [M]0, [RAFT agent]0, MWM, α, and MWRAFT agent correspond to initial monomer concentration, initial RAFT agent concentration, molar mass of monomer, monomer conversion determined by 1H NMR, and molar mass of RAFT agent, respectively.
c Molecular weight and dispersity (Đ) were determined by GPC analysis (DMAC as eluent) calibrated using PMMA standards.
d Reaction was placed in the dark. 0.01 (50 ppm ZnPcS4− relative to monomer) was used.
b Theoretical molecular weight was calculated using the following equation: Mn,theo = [M]0/[RAFT agent]0 × MWM × α + MWRAFT agent, where [M]0, [RAFT agent]0, MWM, α, and MWRAFT agent correspond to initial monomer concentration, initial RAFT agent concentration, molar mass of monomer, monomer conversion determined by 1H NMR, and molar mass of RAFT agent, respectively.
c Molecular weight and dispersity (Đ) were determined by GPC analysis (DMAC as eluent) calibrated using PMMA standards.
d Reaction was placed in the dark.
|
||||||||||
| 1 | CTCPA | ZnPcS4− | Yes | — | 10 | 9 | 0.01 | 2100 | 2600 | 1.16 |
| 2 | CTCPA | ZnPcS4− | Yes | TEOA | 3 | 20 | 0.08 | 4300 | 4900 | 1.12 |
| 3 | CTCPA | — | Yes | — | 10 | 0 | — | — | — | — |
| 4 | CTCPA | — | Yes | TEOA | 10 | 0 | — | — | — | — |
| 5 | CTCPA | ZnPcS4− | No | — | 0 | 0 | — | — | — | — |
| 6 | CTCPA | ZnPcS4− | No | TEOA | 3 | 65 | 0.36 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.09 |
| 7 | CTCPA | — | No | TEOA | 10 | 0 | — | — | — | — |
| 8d | CTCPA | ZnPcS4− | No | TEOA | 10 | 0 | — | — | — | — |
| 9 | — | ZnPcS4− | No | TEOA | 10 | 18 | — | — | 260![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
2.62 |
| 10 | — | ZnPcS4− | Yes | TEOA | 10 | 0 | — | — | — | — |
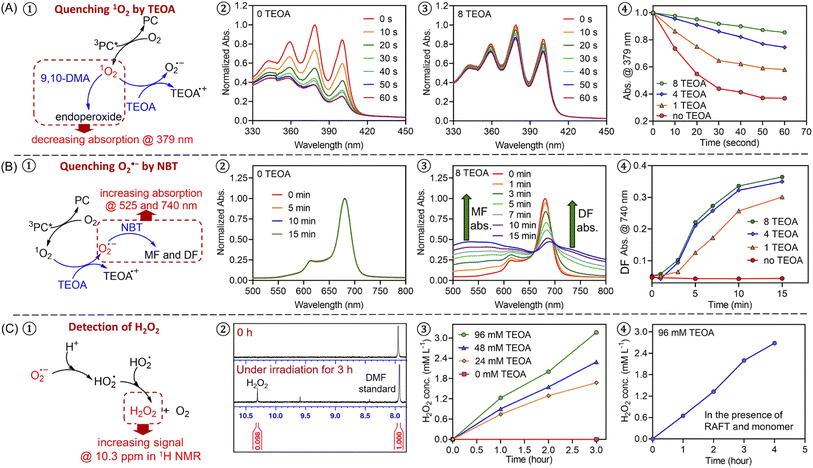
To further investigate the photopolymerization mechanism in the presence of TEOA without deoxygenation, we performed a model reaction in water containing ZnPcS4−, TEOA, and air under light irradiation. 1H NMR spectroscopy was employed to monitor the formation of new species. Interestingly, with increasing irradiation time, we observed the emergence of a new signal at ∼10.3 ppm via1H NMR (Fig. 1C, #2; ESI, Fig. S4B†), which was attributed to the formation of hydrogen peroxide (H2O2). The addition of commercial H2O2 in the reaction medium was carried out resulting in an increased intensity of this signal (ESI, Fig. S4C†), confirming the formation of H2O2. Notably, a faster generation of H2O2 was observed in the presence of a higher concentration of TEOA (Fig. 1C, #3; ESI, Fig. S5†), indicating the crucial role of TEOA in H2O2 formation. In a following experiment, RAFT agent and monomer were added, the signal at 10.3 ppm was also detected (Fig. 1C, #4; ESI, Fig. S6†), showing that RAFT agent or monomer does not interfere with the formation of H2O2. Having established the formation of H2O2, we turned our attention to its role in the initiation of this polymerization. Previous publications examining zinc phthalocyanines for photodynamic therapy demonstrated their capability to photosensitize (hydro)peroxides to generate initiating species under light irradiation (ESI, Fig. S7A†).88,89 To determine if H2O2 can be activated by ZnPcS4−, a small quantity of commercial H2O2 (24.3 mM) was added to the reaction mixture consisting of ZnPcS4−, DMA, and CTCPA in water. Under NIR light exposure, we observed 68% monomer conversion after 2 hours (ESI, Fig. S7B,† green line), which indicated that H2O2 was successfully activated by this system. In the absence of light or ZnPcS4−, no polymerization was observed (ESI, Fig. S7B,† red points).
Intrigued by the formation of H2O2, we decided to investigate the mechanism in detail. First, a series of control experiments were carried out to confirm the role of ZnPcS4−, oxygen and light in the production of H2O2. After deoxygenation, no formation of H2O2 was detected after 3 hours in the presence of ZnPcS4− and TEOA under NIR light irradiation (ESI, Fig. S4E†). Without deoxygenation, similar results were obtained in the absence of ZnPcS4− or light (ESI, Fig. S4D and F†). As reported in previous publications,90,91 H2O2 can be generated from reactive oxygen species (ROS), such as singlet oxygen (1O2) or superoxide (O2˙−). Under light irradiation and in the presence of excited ZnPcS4− (3PC*), oxygen (O2) can be transformed into singlet oxygen (1O2) by triplet–triplet annihilation (TTA; Scheme 2C).92,93 To confirm the formation of 1O2, we performed model experiments in the absence of TEOA and RAFT agents but in the presence of ZnPcS4− and 9,10-dimethylanthracene (9,10-DMA) as 1O2 quencher94 (Fig. 1A, #1; ESI, Scheme S1A†) using water and DMF as the solvent. DMF was used to increase the solubility of 9,10-DMA and replace the monomer in our system. Under irradiation, we observed a decrease of 9,10-DMA signals between 330 and 420 nm (Fig. 1A, #2) due to the formation of endoperoxide (ESI, Scheme S1A†), confirming the generation of 1O2. According to previous reports,95–97 we hypothesized that 1O2 can react with TEOA to yield superoxide (O2˙−) (Scheme 2C, O-PI-I). To confirm this assumption, we performed model reactions where different concentrations of TEOA were gradually added, and we measured the consumption of 9,10-DMA. Interestingly, with an increasing concentration of TEOA, we noted a significantly slower disappearance of the characteristic peaks of 9,10-DMA (at ∼379 nm), suggesting that 1O2 was quenched by TEOA (Fig. 1A, #3 and 4; ESI, Fig. S8†). To confirm that O2˙− was generated from the electron transfer between TEOA and 1O2 (Scheme 2C, O-PI-I), quenching experiments of O2˙− in the presence of nitrotetrazolium blue chloride (NBT) were performed (Fig. 1B, #1). Indeed, NBT reacts with O2˙− (ESI, Scheme S1B†) to yield monoformazan (MF; λmax at ∼525 nm) and diformazan (DF; λmax at ∼740 nm).98 In the absence of TEOA, no absorptions of characteristic MF and DF signals were observed, indicating the absence of O2˙− formation (Fig. 1B, #2). In contrast, two characteristic signals of MF and DF appeared when TEOA was added to the mixture, suggesting O2˙− generation (Fig. 1B, #3). Additionally, a faster increase in absorption of MF and DF was observed when a higher ratio of TEOA was added (Fig. 1B #4; ESI, Fig. S9†), indicating a more efficient generation of O2˙− in this condition which is in accord with the literature.98 Subsequently, the protonation of O2˙− (Scheme 2C, proton transfer) results in the formation of hydroperoxyl radical (HO2˙),99,100 which is regarded as a precursor of H2O2.90,91 The accumulation of H2O2 was confirmed by the presence of a signal at 10.3 ppm determined by 1H NMR analysis (Fig. 1C; ESI, Fig. S5 and S6†).
In addition to experimental evidence, a series of density-functional theory (DFT) calculations were performed to calculate the Gibbs free energy change (ΔG) for the three different reaction pathways (Scheme 2). The reaction step possessing a lower value of ΔG indicates a more favorable electron transfer. ΔG values of each reaction involved with electron transfer are listed in Scheme 2. In this work, PET-RAFT polymerization via RQP is more favorable than OQP, showing lower values of ΔG (11.1 kcal mol−1 for RQP-I and 7.0 kcal mol−1 for RQP-II versus OQP-I = 17.5 kcal mol−1). This is in agreement with the faster polymerization rate measured in PET-RAFT polymerization via RQP than OQP (0.08 hour−1versus 0.01 hour−1 in ESI, Fig. S2†). Furthermore, ΔG of the electron transfer from TEOA to 1O2 in O-PI pathway was calculated as −16.1 kcal mol−1, which is significantly more favorable than the activation steps both in OQP and RQP. This computational result confirms our experimental observation that O-PI displays significantly faster polymerization (ESI, Fig. S2†) than OQP and RQP.
Optimization experiments of ZnPcS4− mediated photo-RAFT system
After investigating the activation mechanism in the presence of O2 and TEOA, we turned our attention to the optimization of polymerization kinetics by first varying the TEOA concentrations. Kinetics studies of photopolymerization were investigated with four different TEOA ratios related to the RAFT agent, specifically 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (24 mM), 2
1 (24 mM), 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (48 mM), 4
1 (48 mM), 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (96 mM), and 8
1 (96 mM), and 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (192 mM) (ESI, Fig. S10 and Table S2†). As expected, photopolymerization with the lowest concentration of TEOA, i.e., 1
1 (192 mM) (ESI, Fig. S10 and Table S2†). As expected, photopolymerization with the lowest concentration of TEOA, i.e., 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (ESI, Fig. S10, red line; Table S2,† #1), resulted in the slowest kpapp (0.06 hour−1) and the longest inhibition period (∼90 min). As TEOA acts as an electron donor for 1O2 to generate O2˙− according to our proposed mechanism (Scheme 2C), the formation and protonation of O2˙− are restricted at a low concentration of TEOA, leading to the sluggish formation of H2O2 (Fig. 1C, #3). As H2O2 plays a crucial role in this photo-RAFT polymerization system, the slow polymerization rate and long induction period observed in the low concentration of TEOA can be attributed to the slow generation of H2O2. By increasing the TEOA ratio from 1
1 (ESI, Fig. S10, red line; Table S2,† #1), resulted in the slowest kpapp (0.06 hour−1) and the longest inhibition period (∼90 min). As TEOA acts as an electron donor for 1O2 to generate O2˙− according to our proposed mechanism (Scheme 2C), the formation and protonation of O2˙− are restricted at a low concentration of TEOA, leading to the sluggish formation of H2O2 (Fig. 1C, #3). As H2O2 plays a crucial role in this photo-RAFT polymerization system, the slow polymerization rate and long induction period observed in the low concentration of TEOA can be attributed to the slow generation of H2O2. By increasing the TEOA ratio from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 2
1 to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the inhibition time was significantly reduced from 90 min to 30 min, and kpapp increased significantly from 0.06 to 0.27 hour−1 (ESI, Fig. S10, orange line; Table S2,† #2). When [TEOA]
1, the inhibition time was significantly reduced from 90 min to 30 min, and kpapp increased significantly from 0.06 to 0.27 hour−1 (ESI, Fig. S10, orange line; Table S2,† #2). When [TEOA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [RAFT agent] increased to 4
[RAFT agent] increased to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the performance of polymerization was further improved, exhibiting a shorter induction period of around 15 min and a faster reaction rate of 0.36 hour−1 (ESI, Fig. S10, green line; Table S2,† #3). However, as the TEOA ratio was increased to 8
1, the performance of polymerization was further improved, exhibiting a shorter induction period of around 15 min and a faster reaction rate of 0.36 hour−1 (ESI, Fig. S10, green line; Table S2,† #3). However, as the TEOA ratio was increased to 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, only a very slight increase in kpapp was observed from 0.36 to 0.38 hour−1 (ESI, Fig. S10, blue line; Table S2,† #4), indicating that 4
1, only a very slight increase in kpapp was observed from 0.36 to 0.38 hour−1 (ESI, Fig. S10, blue line; Table S2,† #4), indicating that 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 can be regarded as the optimized condition.
1 can be regarded as the optimized condition.
Subsequently, the concentration of PC in this system was optimized (ESI, Fig. S11 and Table S3†). As expected, photopolymerization with 50 ppm ZnPcS4− showed a faster apparent propagation rate (0.36 hour−1; ESI, Fig. S11, green line; Table S3,† #2) than 20 ppm (0.24 hour−1; ESI, Fig. S11, red line; Table S3,† #1). This result can be explained by the faster production of 1O2 at a higher concentration of ZnPcS4−, which favors H2O2 generation and photoinitiation. However, as ZnPcS4− concentration increased further to 100 ppm, the kpapp slightly decreased from 0.36 to 0.33 hour−1 (ESI, Fig. S11, blue line; Table S3,† #3), which can be attributed to limiting light penetration in the system due to the absorption of ZnPcS4−.
To demonstrate the versatility of this PC to activate different RAFT agents, three other RAFT agents, including 4-cyano-4-[(dodecylsulfanylthiocarbonyl)sulfanyl]pentanoic acid (CDTPA), 2-(dodecylthiocarbonothioylthio)-2-methylpropionic acid (DDMAT), and 2-(butylthiocarbonothioylthio)propanoic acid (BTPA) (Scheme 1B), were tested in this aqueous system using a relatively high monomer concentration (50 v% (49 wt%)) in water, to enable the solubilization of these RAFT agents. Compared to BTPA-mediated photopolymerization (ESI, Fig. S12, red line; Table S4,† #4), CDTPA and DDMAT (ESI, Fig. S12, orange and blue lines; Table S4,† #2 and 3) displayed faster polymerization rates and better control. This can be attributed to favorable activation of the tertiary R group of CDTPA and DDMAT than the secondary R group of BTPA.43,101,102 Moreover, owing to the better water-solubility of CTCPA than CDTPA and DDMAT, a faster polymerization rate and narrower MWD of synthesized PDMA were also observed in the presence of CTCPA (ESI, Fig. S12A, green line; Table S4,† #1). In summary, the optimal polymerization condition was established using CTCPA as the RAFT agent with [TEOA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CTCPA] = 4
[CTCPA] = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in the presence of 50 ppm ZnPcS4−.
1 in the presence of 50 ppm ZnPcS4−.
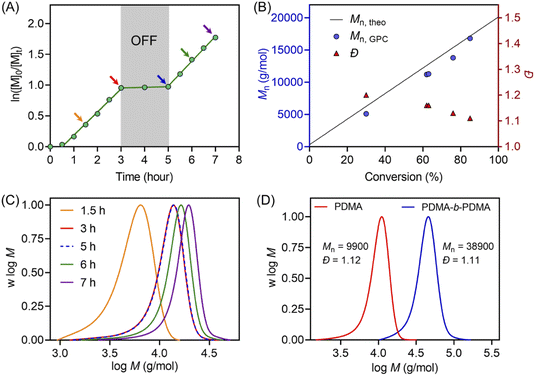
Temporal control of this aqueous photo-RAFT polymerization was demonstrated (Fig. 2A) by no monomer conversion when the light was turned OFF. Polymerization resumed when the reaction was placed under light irradiation with little change in kpapp (kpappbefore = 0.38 hour−1versus kpappafter = 0.40 hour−1). In the dark condition, H2O2 was not activated by ZnPcS4−, and as a consequence, did not form radicals to initiate the photopolymerization. An excellent agreement between Mn,theo and Mn,GPC and a decreasing Đ from 1.2 to 1.1 were observed during the polymerization (Fig. 2B). Moreover, the MWDs of synthesized PDMA shifted toward higher molecular weight with increasing irradiation time (Fig. 2C), confirming the controlled character of this aqueous photo-RAFT polymerization. To confirm the maintenance of trithiocarbonate at the end of the polymerization, PDMA was successfully chain extended by adding fresh DMA, leading to clear shifts of MWDs of PDMA-b-PDMA (Fig. 2D). Similar results were obtained when poly(oligo(ethylene glycol)methyl ether methacrylate) (POEGMA) was chain extended in the presence of DMA to yield POEGMA-b-PDMA block copolymers (ESI, Fig. S13†). Similar to photopolymerizations performed after deoxygenation (ESI, Fig. S14B†), the high end group fidelity of polymers synthesized in the presence of oxygen was also confirmed by 1H NMR spectroscopy (ESI, Fig. S14A†). Signals at 5.1 ppm attributed to the CH adjacent to the trithiocarbonate on the R-group side remained in a similar ratio to the CH3 protons from the n-butyl Z-group under both deoxygenated and non-deoxygenated conditions. Subsequently, the degrees of polymerization (DPs) varied from 100 to 800 (ESI, Table S5 and Fig. S15†), resulting in the preparation of PDMA with narrow and symmetric MWDs (Đ < 1.2). To investigate the versatility of this system toward various monomers, we decided to expand the family and type of water-soluble monomers (ESI, Table S6 and Fig. S16†). In the presence of ZnPcS4−, TEOA and CTCPA, N,N-diethylacrylamide (DEA), 2-hydroxyethyl acrylate (HEA), oligo(ethylene glycol)methyl ether acrylate (OEGA, Mn = 300 g mol−1), and oligo(ethylene glycol)methyl ether methacrylate (OEGMA, Mn = 480 g mol−1) were successfully polymerized in a controlled manner (Đ < 1.35) under NIR light irradiation without requiring prior deoxygenation.
Aqueous photo-RAFT polymerizations through various barriers under NIR light
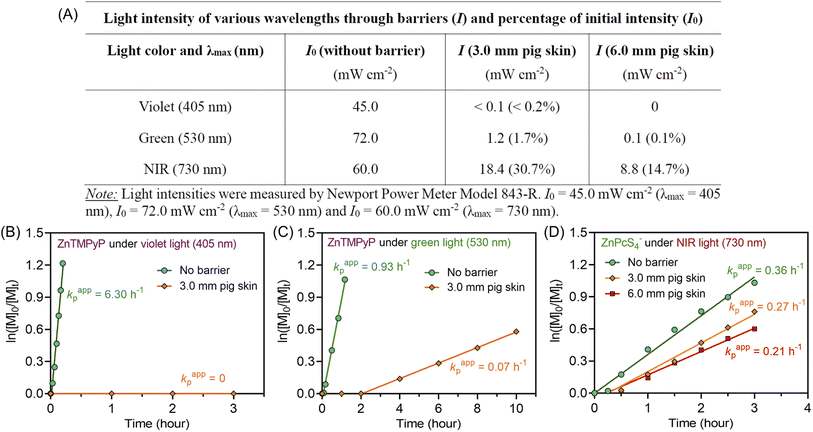
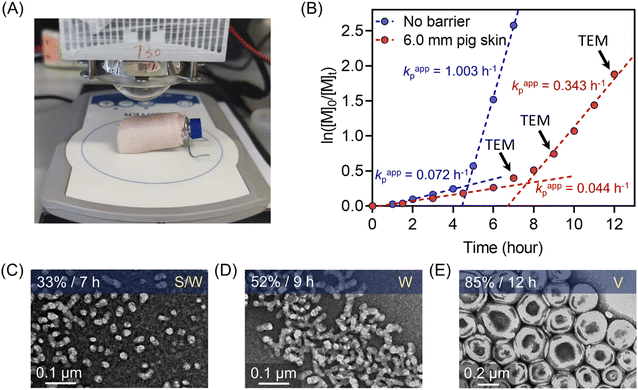
Notably, compared with UV and visible light, longer wavelengths, such as NIR light, have enhanced penetration through non-transparent materials, making photopolymerization feasible to conduct through thick barriers (ESI, Fig. S17†). A series of photopolymerizations catalyzed by ZnPcS4− was performed through various barriers without prior deoxygenation. In the presence of 0.1 and 0.2 mm print paper, although kpapp decreased from 0.36 to 0.26 and 0.21 hour−1 (ESI, Fig. S18A†), polymers were successfully synthesized in a controlled manner (ESI, Table S7,† #2 and 3). In addition, biological barriers, such as pig skin, were tested in this system. Kpapp declined slightly from 0.36 to 0.34 hour−1 (ESI, Fig. S18B,† blue line) when 1.5 mm pig skin was used as the barrier between the NIR LED light and the reaction mixture. When the thickness of pig skin increased further to 3.0 mm, a decrease in kpapp was noted as 0.27 hour−1 (Fig. 3D, orange line). Surprisingly, through the 6.0 mm thickness of pig skin, a relatively fast kpapp of 0.21 hour−1 was still achieved in this long wavelengths mediated system (Fig. 3D, red line). As a result, polymers were synthesized successfully through barriers with different thicknesses in the presence of oxygen, showing good agreement between Mn,theo and Mn,GPC (ESI, Table S7†), narrow MWDs with low dispersity (Đ < 1.15; ESI, Fig. S18C and D), and high end group fidelity (ESI, Fig. S19†).To emphasize the enhanced penetration of NIR light through non-transparent barriers compared to visible light (400–700 nm), the light intensity of various wavelengths (violet light, λmax = 405 nm; green light, λmax = 530 nm; NIR light λmax = 730 nm) were measured before and after passing through barriers with different thicknesses (Fig. 3A). Through 3.0 mm pig skin, the light intensity (I) of violet light decreased to 0.2% of the initial value (I0). While green light was less attenuated than violet light, the light intensity still dropped to only 1.7% of the original I0 value after passing through 3.0 mm pig skin. In contrast to the limited light penetration of these shorter wavelengths, 730 nm light was able to penetrate through the barriers in appreciable quantities, with 30.7% and 14.7% of the initial light passing through 3.0 and 6.0 mm pig skin, respectively.
To compare the photopolymerization performance between the visible light mediated system and the NIR light mediated system through barriers, zinc meso-tetra(N-methyl-4-pyridyl) porphine tetrachloride (ZnTMPyP; Fig. S20†) was employed as a model PC owing to its water solubility and broad absorption in visible regions50 (ESI, Table S8 and Fig. S21†). Under violet (λmax = 405 nm) and green (λmax = 530 nm) light irradiation and in the absence of barriers, photo-RAFT polymerization catalyzed by ZnTMPyP displayed significantly fast polymerization rates (Fig. 3B and C, 6.30 h−1 under violet light and 0.93 h−1 under green light), which is much faster than NIR light system (0.36 h−1) under similar light intensities (Iviolet = 45 mW cm−2; Igreen = 72 mW cm−2; INIR = 60 mW cm−2). However, in the presence of 3.0 mm pig skin as the barrier, polymerization under violet light showed no monomer conversion after 3 hours (Fig. 3B). Meanwhile, a very long induction period (2 hours) and a slow polymerization rate (0.07 h−1) were noted when green light was employed (Fig. 3C). In contrast to violet and green light systems, photo-RAFT mediated by NIR light under NIR showed a slight decrease in kpapp from 0.36 to 0.27 h−1 (Fig. 3D) when a 3 mm pig skin was introduced.
Aqueous photo-PISA catalyzed by ZnPcS4− under NIR light in the presence of oxygen
Inspired by the monomer versatility and oxygen tolerance, this aqueous photo-RAFT polymerization system was applied to photoinitiated polymerization-induced self-assembly (photo-PISA) to synthesize polymeric nanoparticles. Notably, light scattering limits the light penetration through a dispersion polymerization reaction according to the Rayleigh light scattering equation (proportional to d6/λ4), where d is the diameter of the particles and λ is the wavelength of incident light. Therefore, longer wavelengths of irradiation are more suitable to mediate photoinduced dispersion polymerization, especially for synthesizing large nanoparticles.In this work, PEG113-CDTPA macromolecular RAFT agent was used as the first stabilizing block, and the chains were extended in the presence of hydroxypropyl methacrylate (HPMA) as the core-forming monomer in this aqueous photo-RAFT dispersion polymerization (Scheme 1B and Fig. 4A). Optimization experiments with various ratios of TEOA (ESI, Table S9†) were conducted using a 20 wt% solids content of HPMA in water with 1 mL reaction volume in a 2 mL glass vial under NIR light irradiation without prior deoxygenation. Interestingly, in contrast to homogenous polymerization showing good efficiency at [TEOA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CTCPA] = 2 (ESI, Table S2,† #2: 53% monomer conversion in 3 hours), a limited monomer conversion (8% monomer conversion in 24 hours) was observed in dispersion photopolymerization using this ratio (ESI, Table S9† #2). This was attributed to the lower monomer content used in dispersion polymerization in contrast to homogenous polymerization (20 wt% versus 50 v% (49 wt%)). As [TEOA]
[CTCPA] = 2 (ESI, Table S2,† #2: 53% monomer conversion in 3 hours), a limited monomer conversion (8% monomer conversion in 24 hours) was observed in dispersion photopolymerization using this ratio (ESI, Table S9† #2). This was attributed to the lower monomer content used in dispersion polymerization in contrast to homogenous polymerization (20 wt% versus 50 v% (49 wt%)). As [TEOA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [PEG113-CDTPA] increased further to 4
[PEG113-CDTPA] increased further to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, monomer conversion reached 99% in 24 hours as indicated by 1H NMR spectroscopy (ESI, Fig. S22†). GPC analysis confirmed successful chain extension of PEG113-CDTPA and the preparation of narrow molecular weight diblock copolymers (ESI, Fig. S23†).
1, monomer conversion reached 99% in 24 hours as indicated by 1H NMR spectroscopy (ESI, Fig. S22†). GPC analysis confirmed successful chain extension of PEG113-CDTPA and the preparation of narrow molecular weight diblock copolymers (ESI, Fig. S23†).
Subsequently, the kinetics study of ZnPcS4− mediated photo-RAFT dispersion polymerization of HPMA were investigated at a reaction stoichiometry of [HPMA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [PEG113-CDTPA]
[PEG113-CDTPA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [TEOA]
[TEOA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [ZnPcS4−] = 350
[ZnPcS4−] = 350![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.02 at 20 wt% solids content. As expected, photo-RAFT dispersion polymerization was successfully catalyzed by ZnPcS4− without requiring prior deoxygenation under 730 nm light irradiation, although a short inhibition period of 1 hour was observed (Fig. 4A). As reported previously,49,50,64 we noted two different regimes: a low kpapp (0.072 hour−1) at the starting of the dispersion polymerization (Fig. 4B, 0–4 h), followed by an acceleration of kpapp (1.003 hour−1) which was attributed to the formation of nanoparticles (micellization). To confirm this assumption, transmission electron microscopy (TEM) was performed at different time points (t = 5, 6, and 7 hours). At t = 5 hours, the early stage of the micellization was confirmed by the presence of spherical nanoparticles with ∼20 nm diameter and short worms (Fig. 4E). As the photopolymerization continued, the morphologies of nanoparticles evolved from a mixture of spheres and worms to worms and vesicles (Fig. 4F). At the final point of the kinetics (92% monomer conversion at t = 7 hours), pure vesicles with ∼250 nm diameter were observed (Fig. 4G). This evolution in morphology was supported by the apparent change from a transparent solution to a cloudy (milky) solution (Fig. 4E–G, photos at right bottom). In addition, the size distribution of these particles increased with higher monomer conversions, as indicated by dynamic light scattering (DLS) analyses (ESI, Table S10 and Fig. S24†). Moreover, this aqueous dispersion polymerization was performed in a controlled manner, as indicated by the analysis of the diblock copolymers by GPC (Fig. 4C and D). We observed a good agreement between Mn,GPC and Mn,theo and relatively low MWD (Đ < 1.4). Concerning Đ, a decrease was observed first from 1.27 to 1.14 before the micellization (Fig. 4C), which can be attributed to the photoactivation of PEG113-CDTPA macroRAFT agent to PEG113-b-PHPMA with increasing irradiation time as indicated by GPC analyses (Fig. 4D: 0, 2, 4, and 5 hours). After that, Đ increased gradually from 1.14 to 1.36 in the phase of dispersion polymerization (Fig. 4C, last three red triangle points). In addition, a clear shift of MWDs towards higher molecular weight with increasing reaction time was observed (Fig. 4D) in this photo-PISA system, indicating the living characters of synthesized polymers.
0.02 at 20 wt% solids content. As expected, photo-RAFT dispersion polymerization was successfully catalyzed by ZnPcS4− without requiring prior deoxygenation under 730 nm light irradiation, although a short inhibition period of 1 hour was observed (Fig. 4A). As reported previously,49,50,64 we noted two different regimes: a low kpapp (0.072 hour−1) at the starting of the dispersion polymerization (Fig. 4B, 0–4 h), followed by an acceleration of kpapp (1.003 hour−1) which was attributed to the formation of nanoparticles (micellization). To confirm this assumption, transmission electron microscopy (TEM) was performed at different time points (t = 5, 6, and 7 hours). At t = 5 hours, the early stage of the micellization was confirmed by the presence of spherical nanoparticles with ∼20 nm diameter and short worms (Fig. 4E). As the photopolymerization continued, the morphologies of nanoparticles evolved from a mixture of spheres and worms to worms and vesicles (Fig. 4F). At the final point of the kinetics (92% monomer conversion at t = 7 hours), pure vesicles with ∼250 nm diameter were observed (Fig. 4G). This evolution in morphology was supported by the apparent change from a transparent solution to a cloudy (milky) solution (Fig. 4E–G, photos at right bottom). In addition, the size distribution of these particles increased with higher monomer conversions, as indicated by dynamic light scattering (DLS) analyses (ESI, Table S10 and Fig. S24†). Moreover, this aqueous dispersion polymerization was performed in a controlled manner, as indicated by the analysis of the diblock copolymers by GPC (Fig. 4C and D). We observed a good agreement between Mn,GPC and Mn,theo and relatively low MWD (Đ < 1.4). Concerning Đ, a decrease was observed first from 1.27 to 1.14 before the micellization (Fig. 4C), which can be attributed to the photoactivation of PEG113-CDTPA macroRAFT agent to PEG113-b-PHPMA with increasing irradiation time as indicated by GPC analyses (Fig. 4D: 0, 2, 4, and 5 hours). After that, Đ increased gradually from 1.14 to 1.36 in the phase of dispersion polymerization (Fig. 4C, last three red triangle points). In addition, a clear shift of MWDs towards higher molecular weight with increasing reaction time was observed (Fig. 4D) in this photo-PISA system, indicating the living characters of synthesized polymers.
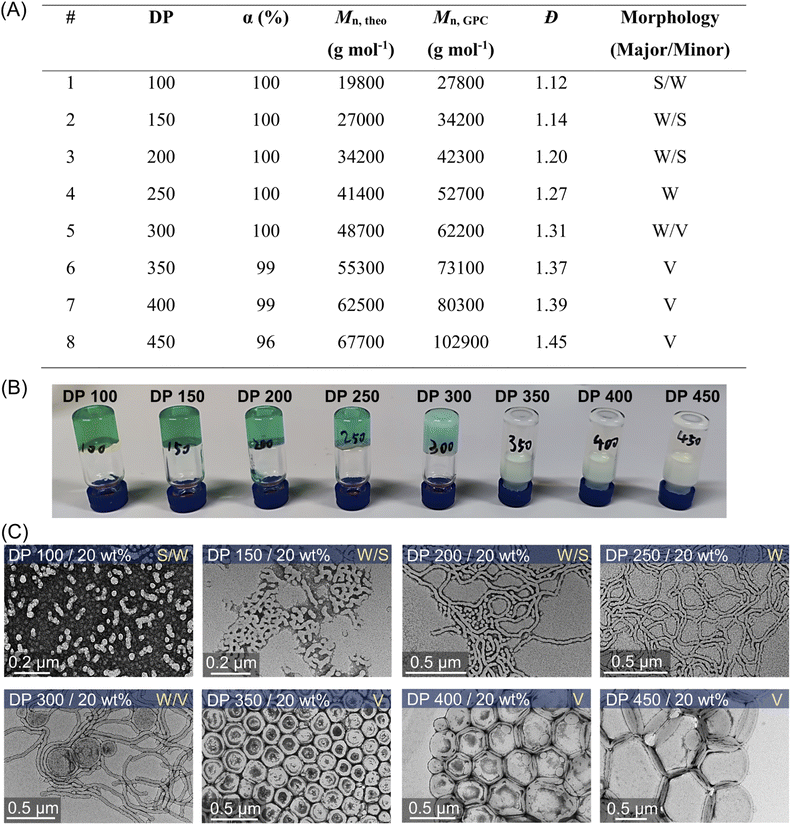
Morphological evolution of synthesized nanoparticles with different targeted DPs and solids content
This aqueous and oxygen tolerant photo-PISA system was investigated further with various targeted DPs at 20 wt% solids content after reaching high monomer conversions (>95%). Overall, dispersion polymerization targeting lower DPs exhibited closer values between Mn,GPC and Mn,theo (Fig. 5A), resulting in the synthesis of PEG113-b-PHPMA with narrower MWDs than polymerization with higher DPs (ESI, Fig. S25†). The reactions ranging from 100 to 300 DPs were significantly viscous, as shown by the digital photos of vials (Fig. 5B), which is likely to correspond to the formation of worms morphologies. Meanwhile, appearances of increasingly cloudy reactions were observed in the reactions with higher targeted DPs, especially for 250, 300, and 350 DPs (Fig. 5B). This indicated that larger-size nanoparticles were prepared with higher DPs, which was also confirmed by DLS measurements (ESI, Table S13, #9–12; Fig. S28C†). Furthermore, TEM was carried out to observe the morphologies for these nanoparticles with different DPs (Fig. 5C). First, a mixture of the majority of spheres and the minority of short worms (S/W) was observed with 100 DP (Fig. 5C, top first). With increasing DPs to 150 and 200, the population of nanoparticles evolved into worms (major) and spheres (minor) (W/S; Fig. 5C, top second and third). Among these two DPs, much longer worms were observed in 200 DP (Fig. 5C, top third) than in 150 DP. Moreover, the morphology of pure worms (W) was observed in the dispersion polymerization with 250 DP (Fig. 5C, top fourth). As the targeted DP increased to 300, a mixed morphology appeared again, consisting of worms and some vesicles (W/V; Fig. 5C, bottom first). As DPs increased further from 350 to 450, we observed pure vesicles with increasing sizes from ∼200 to ∼500 nm (Fig. 5C, bottom last three).The evolution of the morphology was also studied at 10 and 15 wt% solids concentration by varying DPs from 100 to 400. A phase diagram was plotted, reflecting the morphologies of these nanoparticles versus various DPs and solids content (Fig. 6A). At 10 wt% solids content, although the morphologies of synthesized PEG113-b-PHPMA did not show any transformation with increasing DPs, we observed an apparent increase of spherical nanoparticle sizes from ∼25 nm at 100 DP to ∼56 nm at 400 DP (Fig. 6B, bottom row). In contrast to 10 wt%, the change in morphology at 15 wt% solids concentration was similar to 20 wt%, evolving from S/W to V with increasing DPs. On the other hand, there are size differences of synthesized nanoparticles between 15 wt% (Fig. 6B, top row) and 20 wt% (Fig. 5C), which can be easily observed in TEM and DLS analyses (ESI, Table S13 and Fig. S28†). For an example of 400 DP, the diameter of synthesized vesicles at 15 wt% was ∼200 nm, while at 20 wt% solids concentration, the size was ∼400 nm. Overall, a phase diagram was successfully plotted in this photo-PISA system (Fig. 6A), facilitating the nanoparticle synthesis with specific morphologies and sizes under NIR light irradiation. Furthermore, PEG113-b-PHPMA copolymers forming the nanoparticles displayed narrow MWDs (ESI, Fig. S26 and S27†) and a good correlation between Mn,GPC and Mn,theo (ESI, Tables S11 and S12†).
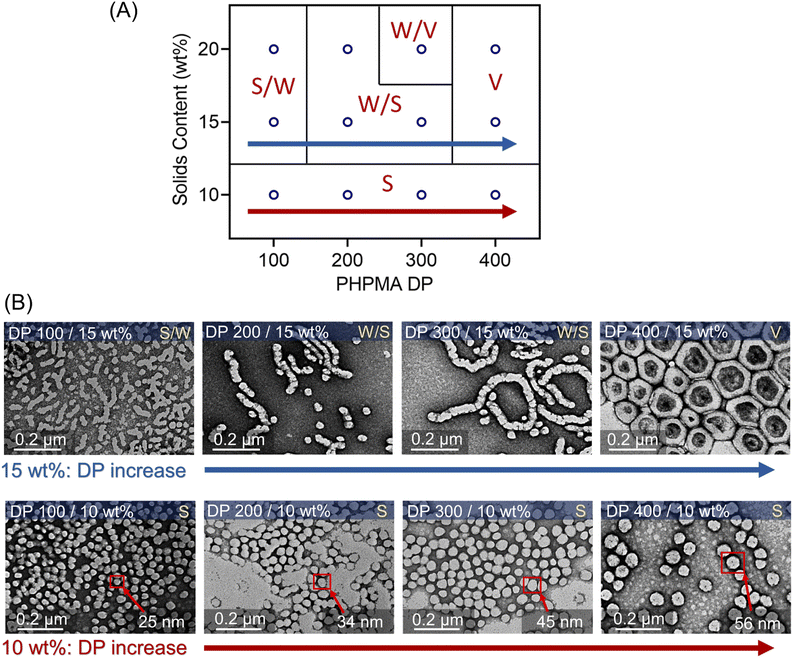 | ||
Fig. 6 (A) Phase diagram of photo-PISA synthesized by ZnPcS4− mediated dispersion photopolymerization of HPMA. Polymerizations were conducted under the irradiation of 730 nm light (I = 60 mW cm−2) for 24 hours without prior deoxygenation in water at fixed reaction stoichiometries of [PEG113-CDTPA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [TEOA] [TEOA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [ZnPcS4−] 1 [ZnPcS4−] 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.02. (B) TEM images of synthesized polymeric nanoparticles at 10 and 15 wt% solids content (note: TEM images at 20 wt% were presented in Fig. 5C). 0.02. (B) TEM images of synthesized polymeric nanoparticles at 10 and 15 wt% solids content (note: TEM images at 20 wt% were presented in Fig. 5C). | ||
Synthesis of polymeric nanoparticles through thick barriers under NIR wavelengths
As demonstrated previously in homogenous photopolymerizations, longer wavelengths possess enhanced penetration through non-transparent materials, enabling the synthesis of polymers through thick barriers. Photopolymerization through barriers can be considered more challenging in dispersion polymerization than in the homogenous solution, due to the light scattering of particles impeding the light penetration through the reaction media. As the homogenous photo-RAFT process exhibited high efficiency using 6.0 mm pig skin as the barrier (Fig. 3), this thickness of pig skin was selected in the photo-PISA system (Fig. 7A). Compared to barrier-free photopolymerization, kpapp declined slightly from 0.072 to 0.044 hour−1 before micellization (0–7 hours) in the presence of the pig skin barrier (Fig. 7B). After micellization (7–12 hours), kpapp decreased more significantly from 1.003 (without barrier) to 0.343 hour−1 (with barrier), which was attributed to the presence of light scattering due to the formation of the nanoparticles. However, the evolution of nanoparticle morphologies was not affected by the inclusion of this barrier. At t = 7 hours (33% monomer conversion), PEG113-b-PHPMA exhibited a mixed morphology consisting of spheres and worms (Fig. 7C), which evolved to pure worms (Fig. 7D) under light irradiation for additional two hours. At 12 hours, TEM indicated the formation of vesicles with ∼200 nm diameter (Fig. 7E). Moreover, this photoinduced dispersion polymerization system enabled the synthesis of well-defined polymers through thick barriers, showing a linear relationship between Mn,GPC and monomer conversion (ESI, Fig. S29†).Conclusion
ZnPcS4− was demonstrated as an efficient photocatalyst to mediate aqueous photo-RAFT polymerization under NIR light irradiation, enabling the synthesis of various polymers (polyacrylamide, polyacrylate, and polymethacrylate) in a controlled manner without the need for prior deoxygenation. Owing to the enhanced penetration capabilities afforded by NIR wavelengths, photopolymerizations were successfully conducted through thick barriers at fast polymerization rates in the presence of air. As longer wavelengths display less light scattering, this aqueous and NIR light mediated system was applied in the photo-PISA system, enabling the successful preparation of polymeric nanoparticles with consistent morphologies in water. Moreover, an evolution from spheres to vesicles was observed with increasing monomer conversion in the kinetics study. By plotting a phase diagram of this photo-PISA system, the targeted synthesis of polymeric nanoparticles with specific morphologies and sizes was accessible. Notably, polymeric nanoparticles with precise morphologies were successfully synthesized through 6.0 mm pig skin under NIR light irradiation, owing to enhanced light penetration and reduced light scattering afforded by long wavelengths.Data availability
All data associated with this article have been included in the main text and ESI.†Author contributions
Z. W. and C. B. conceived the project and designed the experiments. Z. W. carried out the photopolymerizations and characterization. Z. W. and W. F. carried out the synthesis of polymeric nanoparticles. Z. W. carried out the DFT calculations with C. W.’s help. Z. W. carried out the detection of ROS species with T. Z.’s help. Z. W. carried out the TEM with S. X.’s help. Z. W. wrote the original draft with further revisions by N. C. and C. B.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors acknowledge the facilities and technical assistance provided by the Mark Wainwright Analytical Centre at UNSW Sydney for support in TEM imaging.References
- A. Anastasaki, V. Nikolaou, A. Simula, J. Godfrey, M. Li, G. Nurumbetov, P. Wilson and D. M. Haddleton, Macromolecules, 2014, 47, 3852–3859 CrossRef CAS.
- A. Anastasaki, V. Nikolaou, Q. Zhang, J. Burns, S. R. Samanta, C. Waldron, A. J. Haddleton, R. McHale, D. Fox, V. Percec, P. Wilson and D. M. Haddleton, J. Am. Chem. Soc., 2014, 136, 1141–1149 CrossRef CAS PubMed.
- B. P. Fors and C. J. Hawker, Angew. Chem., Int. Ed., 2012, 51, 8850–8853 CrossRef CAS PubMed.
- B. P. Fors, J. E. Poelma, M. S. Menyo, M. J. Robb, D. M. Spokoyny, J. W. Kramer, J. H. Waite and C. J. Hawker, J. Am. Chem. Soc., 2013, 135, 14106–14109 CrossRef CAS PubMed.
- J. C. Theriot, C.-H. Lim, H. Yang, M. D. Ryan, C. B. Musgrave and G. M. Miyake, Science, 2016, 352, 1082–1086 CrossRef CAS PubMed.
- N. J. Treat, H. Sprafke, J. W. Kramer, P. G. Clark, B. E. Barton, J. Read de Alaniz, B. P. Fors and C. J. Hawker, J. Am. Chem. Soc., 2014, 136, 16096–16101 CrossRef CAS.
- M. A. Tasdelen, M. Uygun and Y. Yagci, Macromol. Chem. Phys., 2010, 211, 2271–2275 CrossRef CAS.
- M. A. Tasdelen, M. Uygun and Y. Yagci, Macromol. Rapid Commun., 2011, 32, 58–62 CrossRef CAS.
- D. Konkolewicz, K. Schröder, J. Buback, S. Bernhard and K. Matyjaszewski, ACS Macro Lett., 2012, 1, 1219–1223 CrossRef CAS PubMed.
- T. G. Ribelli, D. Konkolewicz, S. Bernhard and K. Matyjaszewski, J. Am. Chem. Soc., 2014, 136, 13303–13312 CrossRef CAS.
- T. G. Ribelli, D. Konkolewicz, X. Pan and K. Matyjaszewski, Macromolecules, 2014, 47, 6316–6321 CrossRef CAS.
- B. Wenn, M. Conradi, A. D. Carreiras, D. M. Haddleton and T. Junkers, Polym. Chem., 2014, 5, 3053–3060 RSC.
- T. Zhang, T. Chen, I. Amin and R. Jordan, Polym. Chem., 2014, 5, 4790–4796 RSC.
- G. M. Miyake and J. C. Theriot, Macromolecules, 2014, 47, 8255–8261 CrossRef CAS.
- A. Ohtsuki, L. Lei, M. Tanishima, A. Goto and H. Kaji, J. Am. Chem. Soc., 2015, 137, 5610–5617 CrossRef CAS.
- X. Pan, M. Lamson, J. Yan and K. Matyjaszewski, ACS Macro Lett., 2015, 4, 192–196 CrossRef CAS PubMed.
- X. Qiao, Q. Hao, M. Chen, G. Shi, Y. He and X. Pang, ACS Appl. Mater. Interfaces, 2022, 14, 21555–21563 CrossRef CAS PubMed.
- C. Kütahya, Y. Zhai, S. Li, S. Liu, J. Li, V. Strehmel, Z. Chen and B. Strehmel, Angew. Chem., Int. Ed., 2021, 60, 10983–10991 CrossRef PubMed.
- S. Dadashi-Silab, K. Kim, F. Lorandi, G. Szczepaniak, S. Kramer, L. Peteanu and K. Matyjaszewski, ACS Macro Lett., 2022, 11, 376–381 CrossRef CAS PubMed.
- W. Zhang, J. He, C. Lv, Q. Wang, X. Pang, K. Matyjaszewski and X. Pan, Macromolecules, 2020, 53, 4678–4684 CrossRef CAS.
- C. Tian, P. Wang, Y. Ni, L. Zhang, Z. Cheng and X. Zhu, Angew. Chem., Int. Ed., 2020, 59, 3910–3916 CrossRef CAS PubMed.
- J. Li, M. Chen, X. Lin, Q. Li, W. Zhang, G. Jin, X. Pan, J. Zhu and X. Zhu, ACS Macro Lett., 2020, 9, 1799–1805 CrossRef CAS PubMed.
- H. Zhao, H. Li, C. Tian, L. Zhang and Z. Cheng, Macromol. Rapid Commun., 2021, 42, 2100211 CrossRef CAS PubMed.
- C. Kütahya, C. Schmitz, V. Strehmel, Y. Yagci and B. Strehmel, Angew. Chem., Int. Ed., 2018, 57, 7898–7902 CrossRef PubMed.
- C. Kütahya, N. Meckbach, V. Strehmel, J. S. Gutmann and B. Strehmel, Chem.–Eur. J., 2020, 26, 10444–10451 CrossRef.
- Q. Ma, W. Wang, L. Zhang and H. Cao, Macromol. Rapid Commun., 2022, 43, 2200122 CrossRef CAS.
- M. L. Allegrezza and D. Konkolewicz, ACS Macro Lett., 2021, 10, 433–446 CrossRef CAS PubMed.
- Q. Fu, T. G. McKenzie, S. Tan, E. Nam and G. G. Qiao, Polym. Chem., 2015, 6, 5362–5368 RSC.
- J. Li, C. Ding, Z. Zhang, J. Zhu and X. Zhu, React. Funct. Polym., 2017, 113, 1–5 CrossRef CAS.
- N. Li, D. Ding, X. Pan, Z. Zhang, J. Zhu, C. Boyer and X. Zhu, Polym. Chem., 2017, 8, 6024–6027 RSC.
- K. Parkatzidis, N. P. Truong, M. N. Antonopoulou, R. Whitfield, D. Konkolewicz and A. Anastasaki, Polym. Chem., 2020, 11, 4968–4972 RSC.
- K. Parkatzidis, H. S. Wang, N. P. Truong and A. Anastasaki, Chem, 2020, 6, 1575–1588 CAS.
- S. Shanmugam, J. Xu and C. Boyer, Chem. Sci., 2015, 6, 1341–1349 RSC.
- L. Shen, Q. Lu, A. Zhu, X. Lv and Z. An, ACS Macro Lett., 2017, 6, 625–631 CrossRef CAS PubMed.
- C. Wu, H. Chen, N. Corrigan, K. Jung, X. Kan, Z. Li, W. Liu, J. Xu and C. Boyer, J. Am. Chem. Soc., 2019, 141, 8207–8220 CrossRef CAS PubMed.
- C. Wu, S. Shanmugam, J. Xu, J. Zhu and C. Boyer, Chem. Commun., 2017, 53, 12560–12563 RSC.
- J. Xu, C. Fu, S. Shanmugam, C. J. Hawker, G. Moad and C. Boyer, Angew. Chem., Int. Ed., 2017, 56, 8376–8383 CrossRef CAS PubMed.
- J. Xu, S. Shanmugam, C. Fu, K.-F. Aguey-Zinsou and C. Boyer, J. Am. Chem. Soc., 2016, 138, 3094–3106 CrossRef CAS.
- J. Xu, S. Shanmugam, H. T. Duong and C. Boyer, Polym. Chem., 2015, 6, 5615–5624 RSC.
- Y. Yang and Z. An, Polym. Chem., 2019, 10, 2801–2811 RSC.
- M. Chen, M. J. MacLeod and J. A. Johnson, ACS Macro Lett., 2015, 4, 566–569 CrossRef CAS.
- F. Zhou, R. Li, X. Wang, S. Du and Z. An, Angew. Chem., Int. Ed., 2019, 58, 9479–9484 CrossRef CAS PubMed.
- Z. Wu, K. Jung, C. Wu, G. Ng, L. Wang, J. Liu and C. Boyer, J. Am. Chem. Soc., 2022, 144, 995–1005 CrossRef CAS PubMed.
- Z. Wu, K. Jung and C. Boyer, Angew. Chem., Int. Ed., 2020, 59, 2013–2017 CrossRef CAS PubMed.
- H. Cao, G. Wang, Y. Xue, G. Yang, J. Tian, F. Liu and W. Zhang, ACS Macro Lett., 2019, 8, 616–622 CrossRef CAS PubMed.
- S. Shanmugam, J. Xu and C. Boyer, Angew. Chem., Int. Ed., 2016, 128, 1048–1052 CrossRef.
- C. Ding, J. Wang, W. Zhang, X. Pan, Z. Zhang, W. Zhang, J. Zhu and X. Zhu, Polym. Chem., 2016, 7, 7370–7374 RSC.
- S. Shanmugam, J. Xu and C. Boyer, J. Am. Chem. Soc., 2015, 137, 9174–9185 CrossRef CAS PubMed.
- S. Xu, G. Ng, J. Xu, R. P. Kuchel, J. Yeow and C. Boyer, ACS Macro Lett., 2017, 6, 1237–1244 CrossRef CAS PubMed.
- S. Xu, J. Yeow and C. Boyer, ACS Macro Lett., 2018, 7, 1376–1382 CrossRef CAS.
- S. Allison-Logan, Q. Fu, Y. Sun, M. Liu, J. Xie, J. Tang and G. G. Qiao, Angew. Chem., Int. Ed., 2020, 59, 21392–21396 CrossRef CAS PubMed.
- J. Xu, K. Jung and C. Boyer, Macromolecules, 2014, 47, 4217–4229 CrossRef CAS.
- M. Chen, M. Zhong and J. A. Johnson, Chem. Rev., 2016, 116, 10167–10211 CrossRef CAS PubMed.
- Y. Gu, P. Lin, C. Zhou and M. Chen, Sci. China: Chem., 2021, 64, 1039–1046 CrossRef CAS.
- G. Ng, P. Judzewitsch, M. Li, C. W. Pester, K. Jung and C. Boyer, Macromol. Rapid Commun., 2021, 42, 2100106 CrossRef CAS PubMed.
- G. Ng, K. Jung, J. Li, C. Wu, L. Zhang and C. Boyer, Polym. Chem., 2021, 12, 6548–6560 RSC.
- Q. Quan, H. Wen, S. Han, Z. Wang, Z. Shao and M. Chen, ACS Appl. Mater. Interfaces, 2020, 12, 24319–24327 CrossRef CAS PubMed.
- K. Jiang, S. Han, M. Ma, L. Zhang, Y. Zhao and M. Chen, J. Am. Chem. Soc., 2020, 142, 7108–7115 CrossRef CAS PubMed.
- N. Corrigan, J. Yeow, P. Judzewitsch, J. Xu and C. Boyer, Angew. Chem., Int. Ed., 2019, 58, 5170–5189 CrossRef CAS PubMed.
- T.-M. Liu, J. Conde, T. Lipiński, A. Bednarkiewicz and C.-C. Huang, NPG Asia Mater., 2016, 8, e295 CrossRef CAS.
- J. Elisseeff, K. Anseth, D. Sims, W. McIntosh, M. Randolph, M. Yaremchuk and R. Langer, Plast. Reconstr. Surg., 1999, 104, 1014–1022 CrossRef CAS.
- H. Lee, S. Chung, M.-G. Kim, L. P. Lee and J. Y. Lee, Adv. Healthcare Mater., 2016, 5, 1638–1645 CrossRef CAS.
- J. Elisseeff, K. Anseth, D. Sims, W. McIntosh, M. Randolph and R. Langer, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 3104–3107 CrossRef CAS.
- J. Yeow, S. Shanmugam, N. Corrigan, R. P. Kuchel, J. Xu and C. Boyer, Macromolecules, 2016, 49, 7277–7285 CrossRef CAS.
- M. C. M. C. de Toledo, A. d. S. Abreu, J. A. Carvalho, J. A. R. Ambrósio, D. d. S. Godoy, B. C. dos Santos Pinto, M. Beltrame Junior and A. R. Simioni, Photodiagn. Photodyn. Ther., 2020, 32, 101966 CrossRef CAS PubMed.
- A. E. Chekwube, B. George and H. Abrahamse, J. Photochem. Photobiol., A, 2020, 204, 111811 CrossRef CAS PubMed.
- S. Alpugan, D. Topkaya, D. Atilla, V. Ahsen, J. H. Niazi and F. Dumoulin, J. Porphyrins Phthalocyanines, 2017, 21, 887–892 CrossRef CAS.
- W. S. G. Medina, N. A. G. d. Santos, C. Curti, A. C. Tedesco and A. C. d. Santos, Chem.-Biol. Interact., 2009, 179, 402–406 CrossRef CAS PubMed.
- J. D. Spikes, J. E. van Lier and J. C. Bommer, J. Photochem. Photobiol., A, 1995, 91, 193–198 CrossRef CAS.
- J. W. Owens and M. Robins, J. Porphyrins Phthalocyanines, 2001, 5, 460–464 CrossRef CAS.
- K. Zhang, M. Xiao, L. Zhang, Y. Chen and J. Tan, ACS Macro Lett., 2022, 11, 716–722 CrossRef CAS PubMed.
- J. Tan, H. Sun, M. Yu, B. S. Sumerlin and L. Zhang, ACS Macro Lett., 2015, 4, 1249–1253 CrossRef CAS PubMed.
- D. Liu, W. Cai, L. Zhang, C. Boyer and J. Tan, Macromolecules, 2020, 53, 1212–1223 CrossRef CAS.
- J. He, Y. Chen, L. Zhang and J. Tan, Chin. Chem. Lett., 2022 DOI:10.1016/j.cclet.2022.03.067.
- N. J. W. Penfold, J. Yeow, C. Boyer and S. P. Armes, ACS Macro Lett., 2019, 8, 1029–1054 CrossRef CAS PubMed.
- S. L. Canning, G. N. Smith and S. P. Armes, Macromolecules, 2016, 49, 1985–2001 CrossRef CAS PubMed.
- N. J. Warren and S. P. Armes, J. Am. Chem. Soc., 2014, 136, 10174–10185 CrossRef CAS.
- Y. Ding, Q. Zhao, L. Wang, L. Huang, Q. Liu, X. Lu and Y. Cai, ACS Macro Lett., 2019, 8, 943–946 CrossRef CAS PubMed.
- Y. Jiang, N. Xu, J. Han, Q. Yu, L. Guo, P. Gao, X. Lu and Y. Cai, Polym. Chem., 2015, 6, 4955–4965 RSC.
- L. Cao, Q. Zhao, Q. Liu, L. Ma, C. Li, X. Wang and Y. Cai, Macromolecules, 2020, 53, 2220–2227 CrossRef CAS.
- Y. Ma, P. Gao, Y. Ding, L. Huang, L. Wang, X. Lu and Y. Cai, Macromolecules, 2019, 52, 1033–1041 CrossRef.
- Q. Yu, Y. Ding, H. Cao, X. Lu and Y. Cai, ACS Macro Lett., 2015, 4, 1293–1296 CrossRef CAS PubMed.
- J. Yeow and C. Boyer, Adv. Sci., 2017, 4, 1700137 CrossRef.
- A. Chemtob, A. Rannée, L. Chalan, D. Fischer and S. Bistac, Eur. Polym. J., 2016, 80, 247–255 CrossRef CAS.
- E. Lobry, F. Jasinski, M. Penconi, A. Chemtob, C. Ley, C. Croutxé-Barghorn, E. Oliveros, A. M. Braun and A. Criqui, Macromol. Chem. Phys., 2014, 215, 1201–1211 CrossRef CAS.
- Y. Rahmawan, L. Xu and S. Yang, J. Mater. Chem. A, 2013, 1, 2955–2969 RSC.
- F. Jasinski, E. Lobry, A. Chemtob, C. Croutxé-Barghorn and A. Criqui, Macromol. Chem. Phys., 2013, 214, 1669–1676 CrossRef CAS.
- T. G. Gantchev, J. E. van Lier and D. J. Hunting, Radiat. Phys. Chem., 2005, 72, 367–379 CrossRef CAS.
- T. G. Gantchev and J. E. van Lier, Chem. Phys. Lett., 2003, 369, 627–634 CrossRef CAS.
- A. D. De Grey, DNA Cell Biol., 2002, 21, 251–257 CrossRef CAS.
- B. H. Bielski, Photochem. Photobiol., 1978, 28, 645–649 CrossRef CAS.
- M. E. Alberto, B. C. De Simone, G. Mazzone, E. Sicilia and N. Russo, Phys. Chem. Chem. Phys., 2015, 17, 23595–23601 RSC.
- L. B. Josefsen and R. W. Boyle, Met.-Based Drugs, 2008, 2008, 276109 Search PubMed.
- E. Corey and W. C. Taylor, J. Am. Chem. Soc., 1964, 86, 3881–3882 CrossRef CAS.
- W. F. Smith, J. Am. Chem. Soc., 1972, 94, 186–190 CrossRef CAS.
- G. Cocquet, P. Rool and C. Ferroud, J. Chem. Soc., Perkin Trans. 1, 2000, 2277–2281, 10.1039/B001114M.
- R. Bernstein and C. S. Foote, J. Phys. Chem. A, 1999, 103, 7244–7247 CrossRef CAS.
- R.-h. Liu, S.-y. Fu, H.-y. Zhan and L. A. Lucia, Ind. Eng. Chem. Res., 2009, 48, 9331–9334 CrossRef CAS.
- D. B. Kell, Biochim. Biophys. Acta, Rev. Bioenerg., 1979, 549, 55–99 CrossRef CAS.
- A. D. N. J. de Grey, Bioelectrochem. Bioenerg., 1999, 49, 43–50 CrossRef CAS PubMed.
- K. Matyjaszewski and R. Poli, Macromolecules, 2005, 38, 8093–8100 CrossRef CAS.
- E. H. Krenske, E. I. Izgorodina and M. L. Coote, in Controlled/Living Radical Polymerization, American Chemical Society, 2006, ch. 28, vol. 944, pp. 406–420 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: UV-vis spectrum of zinc tetrasulfonated phthalocyanine (ZnPcS4−), kinetics of photopolymerization, 1H NMR spectra of polymers, molecular weight distributions determined by GPC, DLS derived intensity-based size distributions of polymeric nanoparticles prepared by PISA (Fig. S1–S29, Tables S1–S13), experiments of trapping ROS (Scheme S1), and DFT calculations. See https://doi.org/10.1039/d2sc03952d |
| This journal is © The Royal Society of Chemistry 2022 |