Open Access Article
Open Access ArticlePalladium-catalyzed nucleomethylation of alkynes for synthesis of methylated heteroaromatic compounds†
Xi
Yang
,
Gang
Wang
and
Zhi-Shi
Ye
 *
*
Zhang Dayu School of Chemistry, Dalian University of Technology, Dalian 116024, P. R. China. E-mail: yzhsh1984@dlut.edu.cn
First published on 18th August 2022
Abstract
Herein, we disclosed a novel and efficient palladium-catalyzed nucleomethylation of alkynes for the simultaneous construction of the heteroaromatic ring and methyl group. The 3-methylindoles, 3-methylbenzofurans and 4-methylisoquinolines were obtained in moderate to excellent yields. Notably, this methodology was employed as a key step for synthesis of a pregnane X receptor antagonist, zindoxifene, bazedoxifene and AFN-1252. The kinetic studies revealed that reductive elimination might be the rate-determining step.
Introduction
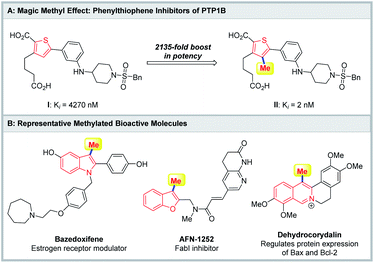
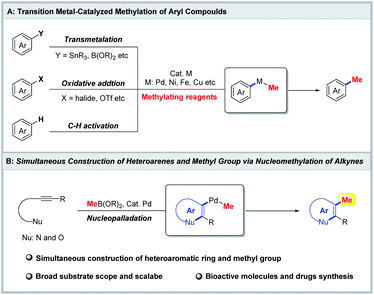
In medicinal chemistry, numerous studies have demonstrated that installation of a methyl group on an aromatic ring can significantly improve the biological activity and pharmacokinetic profile, which is described as the “magic methyl” effect.1 For instance, thiophene PTP1B inhibitor II, in which a methyl group was installed at the 4-position of the thiophene ring, displayed a 2135-fold boost in potency compared with its precursor I (Fig. 1A).2 Additionally, the methylated aromatic ring as a ubiquitous structural unit is widely present in pharmaceuticals, natural products and biological molecules (Fig. 1B).3 Bazedoxifene is a selective estrogen receptor modulator in clinical development for the prevention of postmenopausal osteoporosis.3b AFN-1252 is a potent inhibitor of enoyl-acyl carrier protein reductase (FabI).3f Dehydrocorydalin, which is an alkaloid isolated from traditional Chinese herb Corydalis yanhusuo, regulates protein expression of Bax and Bcl-2.3h As a result, development of an efficient and general strategy for the rapid construction of methylated aromatic compounds would substantially motivate medicinal chemists to explore the “magic methyl” effect in drugs and accelerate the discovery of new drugs.Transition-metal-catalyzed methylation of (hetero)aromatic compounds is undoubtedly a straightforward and powerful strategy for the construction of methylated (hetero)aromatic compounds, and has received considerable attention from the synthetic community in the past few decades.4 Various aromatic compounds assembled with leaving groups, including magnesium,5 zinc,6 tin,7 boron,8 halides,9 and so on,10 have successfully achieved methylation through a transmetalation and oxidative addition process (Scheme 1A). Additionally, in view of the atom-economic and eco-friendly process, transition-metal-catalyzed C–H methylation of (hetero)aromatic compounds has witnessed explosive development (Scheme 1A).4,11 Despite these impressive advances, they have mainly focused on the direct introduction of the methyl group into aromatic rings, while the transformation for simultaneous construction of the (hetero)aromatic ring and methyl group remains unknown.
The transition-metal-catalyzed intramolecular nucleophilic cyclization of alkynes has emerged as an attractive and powerful tool for the construction of heteroaromatic rings including indoles, benzofurans and isoquinolines.12 Consequently, we envisioned that simultaneous construction of the heteroaromatic ring and methyl group could be achieved through nucleometalation/methylation of alkynes (Scheme 1B). To realize this proposal, we had to overcome the following challenges: (1) the transition-metal catalyst promoted the successive nucleophilic cyclization and methylation; (2) competitive protonolysis for the nucleometalation intermediate of alkynes would impede the methylation.13 Herein, we describe a palladium-catalyzed nucleomethylation of alkynes for the simultaneous construction of a heteroaromatic ring and methyl group. The present protocol features mild reaction conditions, broad substrate scope and scalability. Moreover, the method has been used in the synthesis of a pregnane X receptor antagonist, zindoxifene, bazedoxifene and AFN-1252.
Results and discussion
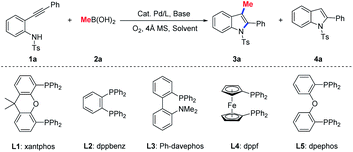
Initially, 2-alkynylanilide 1a and methylboronic acid 2a were chosen as the model substrates to evaluate the feasibility of our hypothesis. Various bases were first examined using Pd(OAc)2 and xantphos (L1) as the catalyst combo (entries 1–4, Table 1). The results showed that the base played a crucial role in the reaction. Using 4-dimethylaminopyridine (DMAP) as base, the competitive protonolysis completely suppressed the methylation (entry 1, Table 1). Delightfully, K2CO3 and K3PO4 successfully promoted the aminopalladation/methylation, affording the desired product 3-methylindole 3a in 81% and 84% yield, respectively (entries 2 and 3, Table 1). Replacement of THF with toluene as solvent resulted in poor reactivity (entry 5, Table 1). The transformation failed when DCM and DMSO were employed as the reaction medium. The further assessment of palladium precursors revealed that Pd(TFA)2 is the best choice and the desired product 3a was isolated in 95% yield (entry 10, Table 1). Additionally, although PdCl2 showed an excellent ratio of 3a/4a, the yield was disappointing (entry 9, Table 1).14 It is worth noting that a significant ligand effect was observed. When Ph-davephos (L3) was employed for this transformation, only 4% yield of the desired product 3a was obtained (entry 12, Table 1). dpephos (L5) also exhibited excellent reactivity with 92% yield (entry 14, Table 1). Moreover, the other methylating reagents, including MeBF3K, trimethylboroxine and MeB(pin), did not show better reactivity than methylboronic acid 2a (entries 15–17, Table 1). Remarkably, the reactivity was maintained very well when the amount of catalyst was reduced to 5 mol% (entry 18, Table 1).| Entry | Pd cat. | L | Solvent | Base | 3a/4a | 3a yield (%) |
|---|---|---|---|---|---|---|
a
1a (0.10 mmol), 2a (0.30 mmol), Pd cat. (10 mol%), L (11 mol%), K3PO4 (1.5 eq.), solvent (2.0 mL), 4 Å MS (100 mg), O2 balloon, 50 °C, 10 h. Yields of 3a and ratios of 3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4a were determined by 1H NMR (with 1,3,5-trimethoxybenzene as internal standard).
b Isolated yield.
c MeB(OH)2 was replaced by MeBF3K.
d MeB(OH)2 was replaced by trimethylboroxine.
e MeB(OH)2 was replaced by MeB(pin).
f Pd(TFA)2 (5 mol%), L1 (5.5 mol%). 4a were determined by 1H NMR (with 1,3,5-trimethoxybenzene as internal standard).
b Isolated yield.
c MeB(OH)2 was replaced by MeBF3K.
d MeB(OH)2 was replaced by trimethylboroxine.
e MeB(OH)2 was replaced by MeB(pin).
f Pd(TFA)2 (5 mol%), L1 (5.5 mol%).
|
||||||
| 1 | Pd(OAc)2 | L1 | THF | DMAP | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0 |
| 2 | Pd(OAc)2 | L1 | THF | K2CO3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05 0.05 |
81 |
| 3 | Pd(OAc)2 | L1 | THF | K3PO4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.12 0.12 |
84 |
| 4 | Pd(OAc)2 | L1 | THF | KOAc | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.66 0.66 |
60 |
| 5 | Pd(OAc)2 | L1 | PhMe | K3PO4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.6 4.6 |
18 |
| 6 | Pd(OAc)2 | L1 | DCM | K3PO4 | N. D. | <5 |
| 7 | Pd(OAc)2 | L1 | DMSO | K3PO4 | N. D. | <5 |
| 8 | Pd(OAc)2 | L1 | 1,4-Dioxane | K3PO4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.27 0.27 |
78 |
| 9 | PdCl2 | L1 | THF | K3PO4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.03 0.03 |
33 |
| 10 | Pd(TFA) 2 | L1 | THF | K 3 PO 4 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 0.03 0.03
|
97/95 |
| 11 | Pd(TFA)2 | L2 | THF | K3PO4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1 |
20 |
| 12 | Pd(TFA)2 | L3 | THF | K3PO4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19.0 19.0 |
4 |
| 13 | Pd(TFA)2 | L4 | THF | K3PO4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.37 0.37 |
46 |
| 14 | Pd(TFA)2 | L5 | THF | K3PO4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.08 0.08 |
92 |
| 15c | Pd(TFA)2 | L1 | THF | K3PO4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.08 0.08 |
56 |
| 16d | Pd(TFA)2 | L1 | THF | K3PO4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.04 0.04 |
60 |
| 17e | Pd(TFA)2 | L1 | THF | K3PO4 | — | — |
| 18 | Pd(TFA) 2 | L1 | THF | K 3 PO 4 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 0.05 0.05
|
95 |
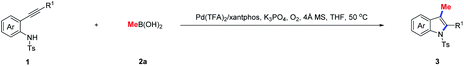
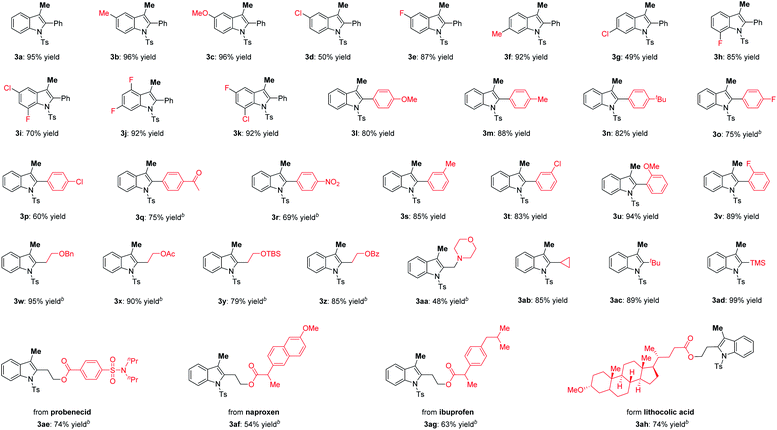
Having identified the optimum conditions, the substrate scope for construction of 3-methylindoles via aminopalladation/methylation of 2-alkynylanilides was evaluated. As illustrated in Table 2, various 2-alkynylanilides 1 reacted smoothly with methylboronic acid 2a to provide the desired 3-methylindoles 3 in moderate to excellent yields. Within the aryl unit of substrate 1, the electronic properties of the substituent at the 4-position had a dramatic effect on the reactivity. For example, the 5-methoxy-3-methylindole 3c was delivered in 96% yield, whereas only 50% yield of 5-chloro-3-methylindole 3d was observed. It is noted that substrate 1 possessing the chloro group at the 6-position of the phenyl ring displayed high reactivity, giving product 3k in 92% yield. When R1 was an aryl group, the 2-alkynylanilides containing methoxy (1l and 1u), methyl (1m and 1s), fluoro (1o and 1v), chloro (1p and 1t), acetyl (1q), and nitro (1r) functional groups were perfectly compatible with the standard conditions, furnishing the desired 3-methylindoles 3l–3v in 60–94% yields. Notably, the effect of steric hindrance had only a marginal influence on the reactivity. For example, the hindered 2-(2-methoxyphenyl)-3-methylindole 3u was afforded in 94% yield. Additionally, when R1 was an alkyl group, the transformation still proceeded smoothly to afford the corresponding products 3w–3ac in satisfactory yield. It is remarkable that the 3-methyl-2-trimethylsilylindole 3ad was achieved in quantitative yield.
Encouraged by the above results, we further applied this aminopalladation/methylation to late-stage modification of medicinal agents. The 2-alkynylanilides obtained from probenecid, naproxen, ibuprofen and lithocholic acid proceeded smoothly to construct the desired products 3ae–3ah in satisfactory yield.15
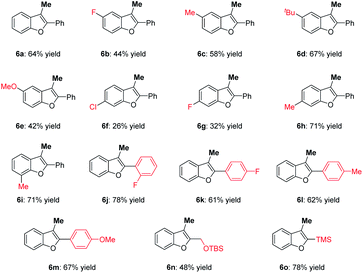
Subsequently, synthesis of the 3-methylbenzofurans through oxypalladation/methylation was explored, and the results are summarized in Table 3. As expected, numerous 2-alkynylphenols 5 successfully underwent oxypalladation/methylation, affording the 3-methylbenzofurans 6 in moderate to good yields. Probably due to the electron effects, introducing the fluoro (6b) and methoxy (6e) groups at the 4-position of the phenyl ring resulted in a diminished yield. Meanwhile, the oxypalladation/methylation of compounds 5h and 5i smoothly occurred to deliver target 3-methylbenzofurans 6h and 6i in 71 and 71% yields, respectively. When R1 was an aryl group, the electron-donating group could improve the reactivity. For instance, 3-methylbenzofuran 6m was furnished in 67% yield. Moreover, substrate 5o bearing the trimethylsilyl group could be successfully converted to the desired product 6o with 78% yield.
| a 5 (0.1 mmol), 2a (0.3 mmol), Pd(TFA)2 (10 mol%), xantphos (11 mol%), K3PO4 (1.5 eq.), 1,4-dioxane (2.0 mL), 4 Å MS (100 mg), O2 balloon, 50 °C, 10 h, isolated yields. |
|---|
|
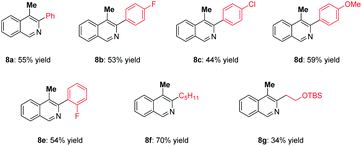
Furthermore, aminopalladation/methylation was conducted to construct 4-methylisoquinolines (Table 4), which are prevalent structural motifs in numerous bioactive molecules and natural products.3g,h,16 Subjecting substrate 7a and methylboronic acid 2a to the standard conditions furnished the desired product 8a in 55% yield. It is noteworthy that halides including F and Cl remained intact under this protocol. Moreover, pentyl-substituted substrate 7f showed good reactivity and 70% yield was achieved. The substrate containing the OTBS group was also a good reaction partner.
| a 7 (0.1 mmol), 2a (0.3 mmol), Pd(TFA)2 (10 mol%), xantphos (11 mol%), K3PO4 (1.5 eq.), 1,4-dioxane (2.0 mL), 4 Å MS (100 mg), O2 balloon, 50 °C, 10 h, isolated yields. |
|---|
|
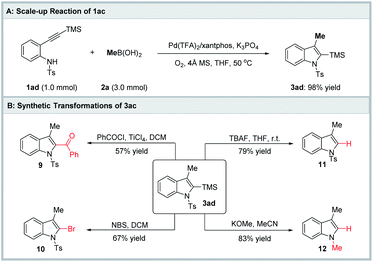
To verify the practical utility of the current protocol, a scale-up experiment and synthetic transformations were carried out (Scheme 2). The aminopalladation/methylation of 2-alkynylanilide 1ad proceeded smoothly to deliver the target 3-methylindole 3ad in 98% yield, demonstrating that the reactivity was perfectly maintained in the scale-up reaction. Besides, titanium promoted the cross-coupling reaction of 3ad with benzoyl chloride affording ketone 9 in 57% yield. Treatment of 3ad with NBS furnished 2-bromoindole 10 in 67% yield. The TMS group of 3ad was removed in the presence of TBAF. Compound 12 was constructed through Ts group deprotection/N-methylation and removal of the TMS group.
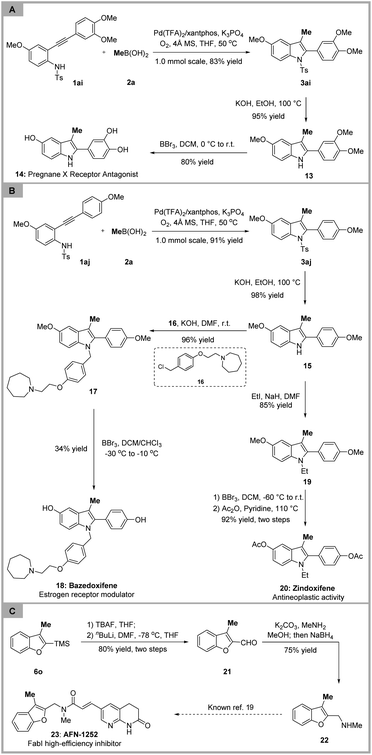
Next, we were keen to perform the synthesis of bioactive molecules and pharmaceutical molecules to further broaden the application of our protocol (Scheme 3). The aminopalladation/methylation of 2-alkynylanilide 1ai and methylboronic acid 2a provided the desired 3-methylindole 3ai in 83% yield, and subsequent deprotection of the Ts group and demethylation of the methoxy group gave compound 14, which is a potent pregnane X receptor antagonist.17 The subjection of 1aj and methylboronic acid to the standard conditions furnished the corresponding product 3aj in 91% yield. The deprotection of the Ts group and N-alkylation with substituted benzyl chloride 16 afforded compound 17. Treatment of compound 17 with BBr3 generated bazedoxifene 18. Additionally, the anti-oestrogen zindoxifene 20, which was identified as a drug for the treatment of hormone-dependent mammary carcinomas,18 could be conveniently constructed from intermediate 15 through N-ethylation, demethylation of the methoxy group and esterification. Besides, the TMS group removal of 6o was followed by formylation and reductive amination to generate compound 22, which was the key intermediate for the construction of AFN-1252 reported in the literature.19
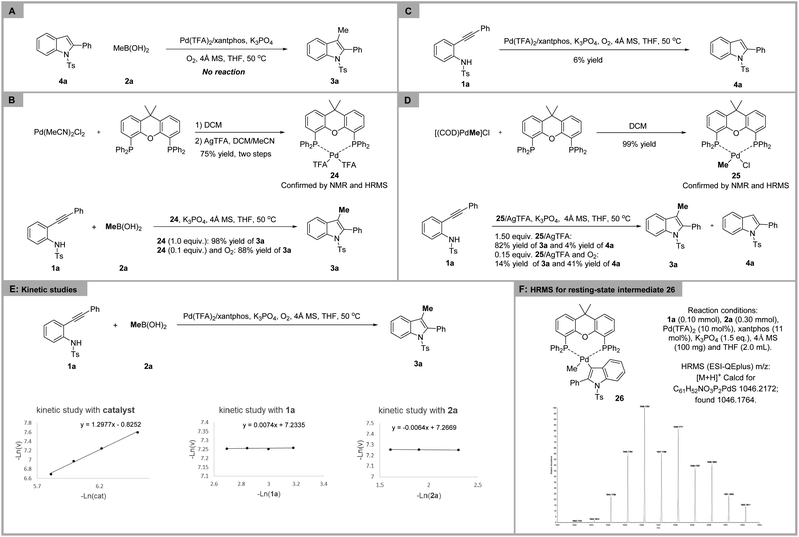
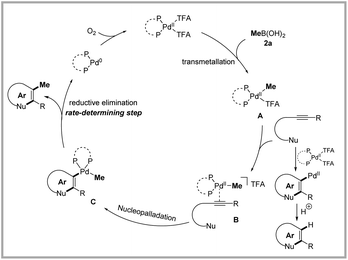
To unveil the mechanistic details of palladium-catalyzed nucleomethylation of alkynes, several control experiments were performed. Treatment of indole 4a with methylboronic acid 2a under the standard conditions failed to achieve 3-methylindole 3a, suggesting that the possibility of palladium-catalyzed C–H methylation of indoles should be ruled out (Scheme 4A). In order to identify the active catalyst, the complex Pd(xantphos)(TFA)2 was synthesized and characterized by NMR and HRMS.20 The palladium complex 24 was found to catalyze the aminopalladation/methylation of 2-alkynylanilide as efficiently as under the standard conditions (Scheme 4B). The subjection of 2-alkynylanilide 1a to the standard conditions without methylboronic acid afforded 4a in only 6% yield, which indicated that the aminopalladation rate of the Pd(TFA)2/xantphos complex might be slow (Scheme 4C). Based on the above results and previous reports,21 we speculated that transmetallation of the Pd(TFA)2/xantphos catalyst and methylboronic acid might take precedence over aminopalladation of 2-alkynylanilide. To further document the above speculation, the Me–Pd complex 25 was synthesized via a method reported in the literature and used for this transformation.22 When the 2-alkynylanilide 1a was treated with 1.50 and 0.15 equivalents of Me–Pd complex 25, 82% and 14% yield of the desired product 3a was obtained, respectively (Scheme 4D).
Kinetic studies were conducted to investigate the rate-determining step (Scheme 4E). The experimental results showed that the reaction order of 2-alkynylanilide 1a and methylboronic acid 2a is zero, indicating that the rate-determining step occurs after the transmetallation of methylboronic acid and aminopalladation of 2-alkynylanilides. According to literature reports, oxidation of Pd(0) to Pd(II) is a kinetically fast process.23 Therefore, the first-order dependence on the catalyst evidenced that reductive elimination might be the rate-determining step. Additionally, the resting-state intermediate 26 was detected by HRMS (Scheme 4F), which further confirmed the above speculation.
Based on these results, the catalytic cycle was proposed in Scheme 5. Transmetallation of the Pd(TFA)2/xantphos catalyst and methylboronic acid gave Me–Pd complex A. Coordination of Me–Pd complex A with the triple bond of the substrate was followed by nucleopalladation to deliver intermediate C, which underwent reductive elimination to deliver the desired product. The palladium(II) catalyst was regenerated by oxidation with O2. Although the transmetallation of the Pd(TFA)2/xantphos catalyst and methylboronic acid as the initial step seems more reasonable, the pathway of nucleopalladation followed by transmetallation should not be entirely dismissed.24
Conclusions
In conclusion, we have successfully developed a palladium-catalyzed nucleomethylation of alkynes, affording a general and facile approach for the construction of 3-methylindoles, 3-methylbenzofurans and 4-methylisoquinolines in moderate to excellent yields. The late-stage modification of bioactive molecules, scaled up reaction and divergent derivatization have been performed to demonstrate the potentially broad applicability of this protocol. It is worth noting that this methodology was employed for synthesis of a pregnane X receptor antagonist, zindoxifene, bazedoxifene and AFN-1252. Preliminary mechanistic studies suggested that reductive elimination might be the rate-determining step. The reaction represents a new strategy for efficient construction of methylated heteroaromatic compounds, which might be potentially useful for organic synthesis and medicinal chemistry.Data availability
All experimental data, and detailed experimental procedures are available in the ESI.†Author contributions
Z.-S. Y. designed the project and directed the study. X. Y. and G. W. carried out the experiments. X. Y., G. W. and Z.-S. Y. analyzed the data and wrote the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the National Natural Science Foundation of China (22071014 and 21801036), Liaoning Revitalization Talents Program (XLYC1907036), and the Fundamental Research Funds for the Central Universities (DUT19TD28) is acknowledged. We thank Bo Wu (DICP) for helpful discussions and manuscript revisions.References
- (a) S. Sun and J. Fu, Bioorg. Med. Chem. Lett., 2018, 28, 3283–3289 CrossRef CAS PubMed; (b) H. Schönherr and T. Cernak, Angew. Chem., Int. Ed., 2013, 52, 12256–12267 CrossRef PubMed; (c) C. S. Leung, S. S. F. Leung, J. Tirado-Rives and W. L. Jorgensen, J. Med. Chem., 2012, 55, 4489–4500 CrossRef CAS PubMed; (d) E. J. Barreiro, A. E. Kümmerle and C. A. M. Fraga, Chem. Rev., 2011, 111, 5215–5246 CrossRef CAS PubMed.
- D. P. Wilson, Z.-K. Wan, W.-X. Xu, S. J. Kinincich, B. C. Follows, D. Joseph-McCarthy, K. Foreman, A. Moretto, J. Wu, M. Zhu, E. Binnun, Y.-L. Zhang, M. Tam, D. V. Erbe, J. Tobin, X. Xu, L. Leung, A. Shilling, S. Y. Tam, T. S. Mansour and J. Lee, J. Med. Chem., 2007, 50, 4681–4698 CrossRef CAS PubMed.
- (a) Y.-J. Wu, in Heterocyclic scaffolds II: Reactions and applications of indoles, ed. G. W. Gribble, Springer-Verlag Berlin Heidelberg, Berlin, 2010, pp. 1–29 Search PubMed; (b) C. P. Miller, M. D. Collini, B. D. Tran, H. A. Harris, Y. P. Kharode, J. T. Marzolf, R. A. Moran, R. A. Henderson, R. H. W. Bender, R. J. Unwalla, L. M. Greenberger, J. P. Yardley, M. A. Abou-Gharbia, C. R. Lyttle and B. S. Komm, J. Med. Chem., 2001, 44, 1654–1657 CrossRef CAS PubMed; (c) E. von Angerer, Cancer Treat. Rev., 1984, 11, 147–153 CrossRef CAS PubMed; (d) H. K. Shamsuzzaman, Eur. J. Med. Chem., 2015, 97, 483–504 CrossRef PubMed; (e) R. J. Nevagi, S. N. Dighe and S. N. Dighe, Eur. J. Med. Chem., 2015, 97, 561–581 CrossRef CAS PubMed; (f) J. A. Karlowsky, N. Kaplan, B. Hafkin, D. J. Hoban and G. G. Zhanel, Antimicrob. Agents Chemother., 2009, 53, 3544–3548 CrossRef CAS PubMed; (g) K. W. Bentley, in The Isoquinoline Alkaloids, Hardwood Academic, Amsterdam, 1998 Search PubMed; (h) J. Lee, E. J. Sohn, S. W. Yoon, C. G. Kim, S. Lee, J. Y. Kim, N. Baek and S. H. Kim, Phytother. Res., 2017, 31, 441–448 CrossRef CAS PubMed; (i) S. Lal and T. J. Snap, Curr. Med. Chem., 2012, 19, 4828–4837 CrossRef CAS PubMed; (j) E. Stempel and T. Gaich, Acc. Chem. Res., 2016, 49, 2390–2402 CrossRef CAS PubMed; (k) J. Mao, Z. Wang, X. Xu, G. Liu, R. Jiang, H. Guan, Z. Zheng and P. J. Walsh, Angew. Chem., Int. Ed., 2019, 58, 11033–11038 CrossRef CAS PubMed; (l) X. Xu, M. Ou, Y.-E. Wang, T. Lin, D. Xiong, F. Xue, P. J. Walsh and J. Mao, Org. Chem. Front., 2022, 9, 2541–2548 RSC.
- (a) D. Aynetdinova, M. C. Callens, H. B. Hicks, C. Y. X. Poh, B. D. A. Shennan, A. M. Boyd, Z. H. Lim, J. A. Leitch and D. J. Dixon, Chem. Soc. Rev., 2021, 50, 5517–5563 RSC; (b) N.-D. Mao, Y. Ye, X.-T. Zhuo, X.-Y. Ye and T. Xie, Tetrahedron, 2021, 96, 132402 CrossRef CAS; (c) J. Huang, Z. Chen and J. Wu, ACS Catal., 2021, 11, 10713–10732 CrossRef CAS; (d) G. Yan, A. J. Borah, L. Wang and M. Yang, Adv. Synth. Catal., 2015, 357, 1333–1350 CrossRef CAS; (e) Y. Chen, Chem. - Eur. J., 2019, 25, 3405–3439 CrossRef CAS PubMed; (f) L. Hu, Y. A. Liu and X. Liao, Synlett, 2018, 29, 375–382 CrossRef CAS.
- (a) T. Hayashi, M. Konishi, Y. Kobori, M. Kumada, T. Higuchi and K. Hirotsu, J. Am. Chem. Soc., 1984, 106, 158–163 CrossRef CAS; (b) P. L. Castle and D. A. Widdowson, Tetrahedron Lett., 1986, 27, 6013–6016 CrossRef CAS.
- K. M. Hossain and K. Takagi, Chem. Lett., 1999, 28, 1241–1242 CrossRef.
- (a) Y. Andersson, A. P. Cheng and B. Långström, Acta Chem. Scand., 1995, 49, 683–688 CrossRef CAS; (b) M. Suzuki, H. Doi, M. Bjorkman, Y. Andersson, B. Långström, Y. Watanabe and R. Noyori, Chem.–Eur. J., 1997, 3, 2039–2042 CrossRef CAS.
- (a) L. J. Goossen, Appl. Organomet. Chem., 2004, 18, 602–604 CrossRef CAS; (b) S. Nakajima, H. Takaya and M. Nakamura, Chem. Lett., 2017, 46, 711–714 CrossRef CAS; (c) Z.-T. He, H. Li, A. M. Haydl, G. T. Whiteker and J. F. Hartwig, J. Am. Chem. Soc., 2018, 140, 17197–17202 CrossRef CAS PubMed; (d) A. M. Haydl and J. F. Hartwig, Org. Lett., 2019, 21, 1337–1341 CrossRef CAS PubMed.
- (a) J. Blum, D. Gelman, W. Baidossi, E. Shakh, A. Rosenfeld, Z. Aizenshtat, B. C. Wassermann, M. Frick, B. Heymer, S. Schutte, S. Wernik and H. Schumann, J. Org. Chem., 1997, 62, 8681–8686 CrossRef CAS; (b) D. Gelman, H. Schumann and J. Blum, Tetrahedron Lett., 2000, 41, 7555–7558 CrossRef CAS; (c) M. Gray, I. P. Andrews, D. F. Hook, J. Kitteringham and M. Voyle, Tetrahedron Lett., 2000, 41, 6237–6240 CrossRef CAS; (d) T. Wang, B. J. Alfonso and J. A. Love, Org. Lett., 2007, 9, 5629–5631 CrossRef CAS PubMed; (e) Y. Nakamura, N. Yoshikai, L. Ilies and E. Nakamura, Org. Lett., 2012, 14, 3316–3319 CrossRef CAS PubMed; (f) L. Hu, X. Liu and X. Liao, Angew. Chem., Int. Ed., 2016, 55, 9743–9747 CrossRef CAS PubMed; (g) Q. Wu, S. Han, X. Ren, H. Lu, J. Li, D. Zou, Y. Wu and Y. Wu, Org. Lett., 2018, 20, 6345–6348 CrossRef PubMed; (h) N. R. Lee, R. T. H. Linstadt, D. J. Gloisten, F. Gallou and B. H. Lipshutz, Org. Lett., 2018, 20, 2902–2905 CrossRef CAS PubMed; (i) S. K. Kariofillis, B. J. Shields, M. A. Tekle-Smith, M. J. Zacuto and A. G. Doyle, J. Am. Chem. Soc., 2020, 142, 7683–7689 CrossRef CAS PubMed; (j) S. K. Kariofillis, S. Jiang, A. M. Żurański, S. S. Gandhi, J. I. M. Alvarado and A. G. Doyle, J. Am. Chem. Soc., 2022, 144, 1045–1055 CrossRef CAS PubMed; (k) Z. Wu, F. Wei, B. Wan and Y. Zhang, J. Am. Chem. Soc., 2021, 143, 4524–4530 CrossRef CAS PubMed; (l) K. M. M. Huihui, J. A. Caputo, Z. Melchor, A. M. Olivares, A. M. Spiewak, K. A. Johnson, T. A. DiBenedetto, S. Kim, L. K. G. Ackerman and D. J. Weix, J. Am. Chem. Soc., 2016, 138, 5016–5019 CrossRef CAS PubMed; (m) P. Zhang, C. Le and D. W. C. MacMillan, J. Am. Chem. Soc., 2016, 138, 8084–8087 CrossRef CAS PubMed.
- (a) B.-T. Guan, S.-K. Xiang, T. Wu, Z.-P. Sun, B.-Q. Wang, K.-Q. Zhao and Z.-J. Shi, Chem. Commun., 2008, 1437–1439 RSC; (b) J. Wang, J. Zhao and H. Gong, Chem. Commun., 2017, 53, 10180–10183 RSC; (c) D. Heijnen, F. Tosi, C. Vila, M. C. A. Stuart, P. H. Elsinga, W. Szymanski and B. L. Feringa, Angew. Chem., Int. Ed., 2017, 56, 3354–3359 CrossRef CAS PubMed; (d) T. Agrawal and S. P. Cook, Org. Lett., 2014, 16, 5080–5083 CrossRef CAS PubMed; (e) M. Tobisu, T. Takahira and N. Chatani, Org. Lett., 2015, 17, 4352–4355 CrossRef CAS PubMed; (f) T. Okita, K. Muto and J. Yamaguchi, Org. Lett., 2018, 20, 3132–3135 CrossRef CAS PubMed; (g) B. Y. Feng, Y. D. Yang and J. S. You, Chem. Sci., 2020, 11, 6031–6035 RSC.
- (a) S. J. Tremont and H. U. Rahman, J. Am. Chem. Soc., 1984, 106, 5759–5760 CrossRef CAS; (b) X. Chen, C. E. Goodhue and J.-Q. Yu, J. Am. Chem. Soc., 2006, 128, 12634–12635 CrossRef CAS PubMed; (c) Y. Zhang, J. Feng and C.-J. Li, J. Am. Chem. Soc., 2008, 130, 2900–2901 CrossRef CAS PubMed; (d) B. Li, Z.-H. Wu, Y.-F. Gu, C.-L. Sun, B.-Q. Wang and Z.-J. Shi, Angew. Chem., Int. Ed., 2011, 50, 1109–1113 CrossRef CAS PubMed; (e) H. Wang, S. Yu, Z. Qi and X. Li, Org. Lett., 2015, 17, 2812–2815 CrossRef CAS PubMed; (f) R. Shang, L. Ilies and E. Nakamura, J. Am. Chem. Soc., 2015, 137, 7660–7663 CrossRef CAS PubMed; (g) S.-J. Chen, G.-P. Lu and C. Cai, RSC Adv., 2015, 5, 70329–70332 RSC; (h) T. Uemura, M. Yamaguchi and N. Chatani, Angew. Chem., Int. Ed., 2016, 55, 3162–3165 CrossRef CAS PubMed; (i) R. Shang, L. Ilies and E. Nakamura, J. Am. Chem. Soc., 2016, 138, 10132–10135 CrossRef CAS PubMed; (j) G. Cera, T. Haven and L. Ackermann, Angew. Chem., Int. Ed., 2016, 55, 1484–1488 CrossRef CAS PubMed; (k) T. Sato, T. Yoshida, H. H. Al Mamari, L. Ilies and E. Nakamura, Org. Lett., 2017, 19, 5458–5461 CrossRef CAS PubMed; (l) K. Polidano, B. D. W. Allen, J. M. J. Williams and L. C. Morrill, ACS Catal., 2018, 8, 6440–6445 CrossRef CAS; (m) Q. Gao, Y. Shang, F. Song, J. Ye, Z.-S. Liu, L. Li, H.-G. Cheng and Q. Zhou, J. Am. Chem. Soc., 2019, 141, 15986–15993 CrossRef CAS PubMed; (n) S. D. Friis, M. J. Johansson and L. Ackermann, Nat. Chem., 2020, 12, 511–519 CrossRef CAS PubMed; (o) B. R. Rosen, L. R. Simke, P. S. Thuy-Boun, D. D. Dixon, J.-Q. Yu and P. S. Baran, Angew. Chem., Int. Ed., 2013, 52, 7317–7320 CrossRef CAS PubMed; (p) W. Liu, X. Yang, Z.-Z. Zhou and C.-J. Li, Chem, 2017, 2, 688–702 CrossRef CAS; (q) J. Jin and D. W. C. MacMillan, Nature, 2015, 525, 87–90 CrossRef CAS PubMed; (r) H. Wang, S. Zhang, Z. Wang, M. He and K. Xu, Org. Lett., 2016, 18, 5628–5631 CrossRef CAS PubMed.
- (a) J. S. S. Neto and G. Zeni, Org. Chem. Front., 2020, 7, 155–210 RSC; (b) J. Li, S. Yang, W. Wu and H. Jiang, Chem.–Asian J., 2019, 14, 4114–4128 CrossRef CAS PubMed; (c) M. Platon, R. Amardeil, L. Djakovitch and J.-C. Hierso, Chem. Soc. Rev., 2012, 41, 3929–3968 RSC; (d) S. Cacchi and G. Fabrizi, Chem. Rev., 2011, 111, PR215–PR283 CrossRef PubMed; (e) K. Krüger, A. Tillack and M. Beller, Adv. Synth. Catal., 2008, 350, 2153–2167 CrossRef; (f) G. R. Humphrey and J. T. Kuethe, Chem. Rev., 2006, 106, 2875–2911 CrossRef CAS PubMed; (g) S. Cacchi and G. Fabrizi, Chem. Rev., 2005, 105, 2873–2920 CrossRef CAS PubMed; (h) Z.-S. Ye, J.-C. Li and G. Wang, Synthesis, 2022, 54, 2133–2147 CrossRef CAS; (i) M. Yamaguchi and K. Manabe, Heterocycles, 2022, 104, 3–26 CrossRef CAS; (j) A. A. Abu-Hashem, H. A. R. Hussein, A. S. Aly and M. A. Gouda, Synth. Commun., 2014, 44, 2285–2312 CrossRef CAS; (k) L. De Luca, G. Nieddu, A. Porcheddu and G. Giacomelli, Curr. Med. Chem., 2009, 16, 1–20 CrossRef CAS; (l) K. C. Majumdar, P. Debnath, N. De and B. Roy, Curr. Org. Chem., 2011, 15, 1760–1801 CrossRef CAS; (m) Y. Yamamoto, I. D. Gridnev, N. T. Patil and T. Jin, Chem. Commun., 2009, 34, 5075–5087 RSC; (n) H. Cedric, D. Etelinda, D. D. Laetitia and B. Tyler, ChemCatChem, 2016, 8, 2912–2915 CrossRef.
- (a) A. Bruneau, K. P. J. Gustafson, N. Yuan, C.-W. Tai, I. Persson, X. Zou and J.-E. Bäckvall, Chem.–Eur. J., 2017, 23, 12886–12891 CrossRef CAS PubMed; (b) M. Yamaguchi and K. Manabe, Org. Lett., 2014, 16, 2386–2389 CrossRef CAS PubMed; (c) B. W. Priv.-Doz, C. Alayrac and L. Tevzadze-Saeftel, Angew. Chem., Int. Ed., 2003, 42, 4257–4260 CrossRef PubMed; (d) K. Dooleweerdt, T. Ruhland and T. Skrydstrup, Org. Lett., 2009, 11, 221–224 CrossRef CAS PubMed; (e) N. Ototake, Y. Morimoto, A. Mokuya, H. Fukaya, Y. Shida and O. Kitagawa, Chem.–Eur. J., 2010, 16, 6752–6755 CrossRef CAS PubMed; (f) E. C. Taylor, A. H. Katz, H. Salgado-Zamora and A. McKillop, Tetrahedron Lett., 1985, 26, 5963–5966 CrossRef CAS; (g) G. Wang, J.-C. Li, Y.-G. Zhou and Z.-S. Ye, Org. Lett., 2021, 23, 802–807 CrossRef CAS PubMed.
- The starting material 2-alkynylanilide 1a was recovered in 37% yield. Additionally, 2,2′-diphenyl-1,1′-ditosyl-1H,1′H-3,3′-biindole was isolated in 6% yield.
- (a) Y. L. Chow, M. Sogame and F. Sato, Sci. Rep., 2016, 6, 38129 CrossRef CAS PubMed; (b) Z.-Z. Ma, W. Xu, N. H. Jensen, B. L. Roth, L.-Y. Liu-Chen and D. Y. W. Lee, Molecules, 2008, 13, 2303–2312 CrossRef CAS; (c) Z.-H. Zhang, Y. Yan, A.-J. Deng, H.-J. Zhang, Z.-H. Li, T.-Y. Yuan, L.-H. Fang, L.-Q. Wu, G.-H. Du and H.-L. Qin, Chin. Chem. Lett., 2018, 29, 131–135 CrossRef CAS.
- When ethylboronic acid was employed for the reaction, trace amounts of the desired product were observed.
- Ž. Hodnik, L. Peterlin Mašič, T. Tomašić, D. Smodiš, C. D'Amore, S. Fiorucci and D. Kikelj, J. Med. Chem., 2014, 57, 4819–4833 CrossRef PubMed.
- R. Stein, M. Dowsett, D. Cunningham, J. Davenport, H. Ford, J.-C. Gazet, E. von. Angerer and R. Coombes, Br. J. Cancer, 1990, 61, 451–453 CrossRef CAS PubMed.
- P. J. Hergenrother, E. J. Geddes, B. S. Drown, S. E. Motika and E. N. Parker, PCT Int. Appl., WO 2019177975 A1, 2019.
- G. Qin, L. Li, J. Li and H. Huang, J. Am. Chem. Soc., 2015, 137, 12490–12493 CrossRef CAS PubMed.
- (a) M. Tian, D. Bai, G. Zheng, J. Chang and X. Li, J. Am. Chem. Soc., 2019, 141, 9527–9532 CrossRef CAS PubMed; (b) Y.-P. He, H. Wu, Q. Wang and J. Zhu, Angew. Chem., Int. Ed., 2020, 59, 2105–2109 CrossRef CAS PubMed.
- The transmetallation experiment of Pd(TFA)2/xantphos and methylboronic acid was conducted, and the Me–Pd(xantphos)–TFA complex was observed by HRMS (see the details in the ESI†), which clarified that transmetallation of complex 24 with methylboronic acid can occur. Additionally, the stable Me–Pd(xantphos)–Cl complex 25 was synthesized via a method reported in the literature: T. L. Andersen, P. Nordeman, H. F. Christoffersen, H. Audrain, G. Antoni and T. Skrydstrup, Angew. Chem., Int. Ed., 2017, 56, 4549–4553 CrossRef CAS PubMed.
- C. Adamo, C. Amatore, I. Ciofini, A. Jutand and H. Lakmini, J. Am. Chem. Soc., 2006, 128, 6829–6836 CrossRef CAS PubMed.
- A. Arcadi, S. Cacchi, G. Fabrizi, A. Goggiamani, A. lazzetti and F. Marinelli, Org. Biomol. Chem., 2013, 11, 545–548 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2sc03294e |
| This journal is © The Royal Society of Chemistry 2022 |