Open Access Article
Open Access ArticleStructured copper-hydride nanoclusters provide insight into the surface-vacancy-defect to non-defect structural evolution†
Yizheng
Bao‡
,
Xiaohang
Wu‡
 ,
Bing
Yin‡
,
Xi
Kang
,
Bing
Yin‡
,
Xi
Kang
 ,
Zidong
Lin
,
Huijuan
Deng
,
Haizhu
Yu
,
Zidong
Lin
,
Huijuan
Deng
,
Haizhu
Yu
 ,
Shan
Jin
,
Shan
Jin
 *,
Shuang
Chen
* and
Manzhou
Zhu
*,
Shuang
Chen
* and
Manzhou
Zhu
 *
*
Institutes of Physical Science and Information Technology, Centre for Atomic Engineering of Advanced Materials, Key Laboratory of Structure and Functional Regulation of Hybrid Materials of Ministry of Education, Department of Chemistry, Anhui Province Key Laboratory of Chemistry for Inorganic/Organic Hybrid Functionalized Materials, Anhui University, Hefei, Anhui 230601, China. E-mail: jinshan@ahu.edu.cn; chenshuang@ahu.edu.cn; zmz@ahu.edu.cn
First published on 21st November 2022
Abstract
Exploring the structural evolution of clusters with similar sizes and atom numbers induced by the removal or addition of a few atoms contributes to a deep understanding of structure–property relationships. Herein, three well-characterized copper-hydride nanoclusters that provide insight into the surface-vacancy-defect to non-defect structural evolution were reported. A surface-defective copper hydride nanocluster [Cu28(S-c-C6H11)18(PPh2Py)3H8]2+ (Cu28-PPh2Py for short) with only one C1 symmetry axis was synthesized using a one-pot method under mild conditions, and its structure was determined. Through ligand regulation, a 29th copper atom was inserted into the surface vacancy site to give two non-defective copper hydride nanoclusters, namely [Cu29(SAdm)15Cl3(P(Ph-Cl)3)4H10]+ (Cu29-P(Ph-Cl)3 for short) with one C3 symmetry axis and (Cu29(S-c-C6H11)18(P(Ph-pMe)3)4H10)+ (Cu29-P(Ph-Me)3 for short) with four C3 symmetry axes. The optimized structures show that the 10 hydrides cap four triangular and all six square-planar structures of the cuboctahedral Cu13 core of Cu29-P(Ph-Me)3, while the 10 hydrides cap four triangular and six square-planar structures of the anti-cuboctahedral Cu13 core of Cu29-P(Ph-Cl)3, with the eight hydrides in Cu28-PPh2Py capping four triangular and four square planar-structures of its anti-cuboctahedral Cu13 core. Cluster stability was found to increase sequentially from Cu28-PPh2Py to Cu29-P(Ph-Cl)3 and then to Cu29-P(Ph-Me)3, which indicates that stability is affected by the overall structure of the cluster. Structural adjustments to the metal core, shell, and core–shell bonding model, in moving from Cu28-PPh2Py to Cu29-P(Ph-Cl)3 and then to Cu29-P(Ph-Me)3, enable the structural evolution and mechanism responsible for their physicochemical properties to be understood and provide valuable insight into the structures of surface vacancies in copper nanoclusters and structure–property relationships.
1. Introduction
The nanoclusters continue to receive attention because of their promising catalysis, biosensing, and luminescence applications.1–5 Owing to the quantum size effect and discrete electronic energy levels, the removal or addition of a very small number of atoms from the core or shell structure induces structural defects or structural distortions in the nanocluster that trigger significant changes in performance.6–9 Atom-by-atom regulation can provide insight into the structural-evolution process and the mechanism responsible for how structural change affects performance.10–12 For example, the removal of the center S atom from the Ag14S core in [Ag62S13(SBut)32]4+ (0e) results in the formation of a complete FCC Ag14 unit in [Ag62S12(SBut)32]2+ (4e) (defective structure with a hollow core), in which the presence of free valence electrons leads to photoluminescence quenching.13–15 The traceless removal of two kernel atoms from Au48(m-MBT)26 to give Au46(m-MBT)26 facilitates PL emission (surface defects),16 while a single 28th Ag atom inserted into the interstice (hole vacancy) of the outermost metal layer of the [Ag27(StBu)14(S)2(CF3COO)9(DMAc)4]·DMAc nanocluster gives photoluminescent [Ag28(SAdm)14(S)2(CF3COO)10(H2O)4].17 Consequently, nanoclusters have been used as effective platforms to dissect the structural evolution of a nanomaterial from the perspective of defect chemistry and to understand how this evolution changes its properties, thereby providing rich insight into the relationship between structure and performance.18–23Nowadays, well determined copper-hydride nanoclusters have also been reported, such as the [Cu8H6(μ-dppm)5](PF6)2,24 [Cu9H7(μ-dpmppm)3]X2, [Cu16H14(μ-dpmppm)4]X2,25 [Cu20H11{E2P(OiPr)2}9 (E = S or Se),26,27 [Cu23(PhSe)16(Ph3P)8(H)6]·BF4,28 [Cu25H22(PR3)12]Cl,29,30 [Cu28H15{S2CN(nPr)2}12](PF6),31 [Cu28H20(S2P(OiPr)2)9]−,32 [Cu28H16(dppp)4(RS)4(CF3CO2)8],33 [Cu30H18{E2P(OR)2}12](E = S or Se; R = nPr,iPr or iBu),34 Cu32H20{S2PR2}12],35,36 [Cu53(RCOO)10(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CtBu)20Cl2H18]+,37 [Cu61(StBu)26S6Cl6H14]+ (ref. 38) and [Cu81(PhS)46(tBuNH2)10(H)32]3+.39 And the copper-hydride nanoclusters have drawn increasing research interest because they have aesthetically fascinating molecular structures and are potentially useful in catalysis, hydrogen storage, and photovoltaics applications.8,37,40–43 In contrast to the numerous reports on the structural evolution of gold/silver clusters, examining the structural evolution of copper-hydride clusters remains challenging owing to difficulties associated with synthesizing and crystallizing copper-hydride nanoclusters. A reversible transformation between [Cu7(H){S2CR}6] and [Cu8(H){S2CR}6](PF6), a defect to defect-growth-based copper hydride cluster was reported by the Liu group.44 Metal exchanging Pt4+ ions into [Cu32(PET)24Cl2H8]2− resulted in [Pt2Cu34(PET)22Cl4]2−, an internal-defective nanocluster.45,46 The surface vacancy defective [Cu36H10(PET)24(PPh3)6Cl2] nanoclusters and the hypothetical non-defective half-cubic copper hydride [Cu38H10(SC6H3F2)26(PPh3)8]2+ nanocluster were considered to be other examples of copper-hydride structural evolution that revealed surface metal atom vacancies, ligand defects, and skeletal distortion.47 Although studies on defect clusters have been reported, there are few studies on the effects of surface defects and regrowth on structure and properties because only a few series of copper-hydride clusters are well determined, let alone studies on structural evolution driven in an atom-by-atom manner in a copper-hydride.7,8,44,48,49
CtBu)20Cl2H18]+,37 [Cu61(StBu)26S6Cl6H14]+ (ref. 38) and [Cu81(PhS)46(tBuNH2)10(H)32]3+.39 And the copper-hydride nanoclusters have drawn increasing research interest because they have aesthetically fascinating molecular structures and are potentially useful in catalysis, hydrogen storage, and photovoltaics applications.8,37,40–43 In contrast to the numerous reports on the structural evolution of gold/silver clusters, examining the structural evolution of copper-hydride clusters remains challenging owing to difficulties associated with synthesizing and crystallizing copper-hydride nanoclusters. A reversible transformation between [Cu7(H){S2CR}6] and [Cu8(H){S2CR}6](PF6), a defect to defect-growth-based copper hydride cluster was reported by the Liu group.44 Metal exchanging Pt4+ ions into [Cu32(PET)24Cl2H8]2− resulted in [Pt2Cu34(PET)22Cl4]2−, an internal-defective nanocluster.45,46 The surface vacancy defective [Cu36H10(PET)24(PPh3)6Cl2] nanoclusters and the hypothetical non-defective half-cubic copper hydride [Cu38H10(SC6H3F2)26(PPh3)8]2+ nanocluster were considered to be other examples of copper-hydride structural evolution that revealed surface metal atom vacancies, ligand defects, and skeletal distortion.47 Although studies on defect clusters have been reported, there are few studies on the effects of surface defects and regrowth on structure and properties because only a few series of copper-hydride clusters are well determined, let alone studies on structural evolution driven in an atom-by-atom manner in a copper-hydride.7,8,44,48,49
Herein, we report three novel well-determined Cu-hydride nanoclusters that provide insight into surface-vacancy-defect to non-defect structural evolution: surface-defective [Cu28(S-c-C6H11)18(PPh2Py)3H8]2+ (Cu28-PPh2Py), and non-defective [Cu29(SAdm)15Cl3(P(Ph-Cl)3)4H10](PF6) (Cu29-P(Ph-Cl)3) and Cu29(S-c-C6H11)18(P(Ph-pMe)3)4H10(BF4) (Cu29-P(Ph-Me)3). Structural analyses of the surface vacancy-defective Cu28-PPh2Py and non-defective Cu29-P(Ph-Cl)3/Cu29-P(Ph-Me)3 provide deep insight into how surface vacancy defects structurally evolve into non-defects, including differences in the metal core, number of hydrogen ligands, packing model, metal shell, and core–shell bonding model. Interestingly, Cu29-Ph-pMe has a virtually identical structure to that of the MAg28(SR)18(PR′3)4 silver nanocluster in terms of the number of metal atoms, the thiol and phosphine ligand counts, and the atomic arrangement, despite the presence of hydrogen.50 The structural evolution provides an understanding of how surface vacancy defects structurally evolve and provides valuable insight into the structures of surface vacancies in copper nanoclusters as well as structure–property relationships.
2. Methods
2.1 Chemicals
Tetrakis(acetonitrile)copper(I) tetrafluoroborate ([Cu(CH3CN)4]·BF4, 98%), tetrakis(acetonitrile)copper(I) hexafluorophosphate ([Cu(CH3CN)4]·PF6, 98%), diphenyl-2-pyridylphosphine (PPh2Py, 97%), tri(p-tolyl)phosphine (P(Ph-pMe)3, 98%), tris(4-chlorophenyl)phosphine(P(Ph-pCl)3, 98%), cyclohexyl mercaptan (C6H12S, ≥99%), 1-adamantanethiol (1-AdmSH, 98%), sodium borohydride (NaBH4, 98%), sodium borodeuteride (NaBD4, 98%), acetonitrile (CH3CN), chloroform (CHCl3), chloroform-d (CDCl3), methanol (MeOH), ethanol (EtOH), dichloromethane (CH2Cl2), and n-hexane (Hex) were obtained from commercial sources and used as received.2.2 Synthesis of [Cu28(C6H11S)18(PPh2Py)3(H)8][BF4]2 nanoclusters (Cu28-PPh2Py-H)
[Cu28(C6H11S)18(PPh2Py)3(H)8][BF4]2 was prepared by dissolving [Cu(CH3CN)4]·BF4 (160 mg, 0.51 mmol) in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) CH3CN/CHCl3 (10 mL). After 10 min, PPh2Py (100 mg, 0.40 mmol) was added to the solution, followed by C6H11SH (22 μL, 0.18 mmol), and the mixture was vigorously stirred. After 20 min, a freshly prepared solution of NaBH4 (30 mg, 0.8 mmol, dissolved in 2 mL MeOH) was added dropwise to the aforementioned solution, which immediately turned brown. The mixture was stirred for 5 h at room temperature, at which time a bright orange precipitate of the product had formed at the bottom of the flask. The product was collected and recrystallized from CH2Cl2/Hex over several days at room temperature.
1 (v/v) CH3CN/CHCl3 (10 mL). After 10 min, PPh2Py (100 mg, 0.40 mmol) was added to the solution, followed by C6H11SH (22 μL, 0.18 mmol), and the mixture was vigorously stirred. After 20 min, a freshly prepared solution of NaBH4 (30 mg, 0.8 mmol, dissolved in 2 mL MeOH) was added dropwise to the aforementioned solution, which immediately turned brown. The mixture was stirred for 5 h at room temperature, at which time a bright orange precipitate of the product had formed at the bottom of the flask. The product was collected and recrystallized from CH2Cl2/Hex over several days at room temperature.
2.3 Synthesis of [Cu28(C6H11S)18(PPh2Py)3(D)8][BF4]2 nanoclusters (Cu28-PPh2Py-D)
Cu28-PPh2Py-D was synthesized similarly to Cu28-PPh2Py-H, with the exception that NaBD4 (40 mg, 1 mmol) was used as the reducing agent.2.4 Synthesis of [Cu29(SAdm)15Cl3(P(Ph-Cl)3)4H10](PF6) nanoclusters (Cu29-P(Ph-Cl)3-H)
Cu29-P(Ph-Cl)3-H was prepared by dissolving [Cu(CH3CN)4]·PF6 (100 mg, 0.27 mmol) in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) CH3CN/CHCl3 (10 mL) After 10 min, P(Ph-pCl)3 (100 mg, 0.27 mmol) was added to the solution, followed by 1-AdmSH (50 mg, 0.30 mmol) and the mixture was stirred vigorously. After 20 min, a freshly prepared solution of NaBH4 (60 mg, 1.6 mmol, dissolved in 2 mL MeOH) was added. The mixture was stirred at room temperature for 10 h. The product was collected and recrystallized from CH2Cl2/Hex over several days at room temperature. Although no chloride was used in the synthesis, the capping Cl atoms in Cu29-P(Ph-Cl)3 can be reasonably considered to be derived from the solvent CHCl3, similar to previous reports of some Cl ligand-containing clusters.51,52
1 (v/v) CH3CN/CHCl3 (10 mL) After 10 min, P(Ph-pCl)3 (100 mg, 0.27 mmol) was added to the solution, followed by 1-AdmSH (50 mg, 0.30 mmol) and the mixture was stirred vigorously. After 20 min, a freshly prepared solution of NaBH4 (60 mg, 1.6 mmol, dissolved in 2 mL MeOH) was added. The mixture was stirred at room temperature for 10 h. The product was collected and recrystallized from CH2Cl2/Hex over several days at room temperature. Although no chloride was used in the synthesis, the capping Cl atoms in Cu29-P(Ph-Cl)3 can be reasonably considered to be derived from the solvent CHCl3, similar to previous reports of some Cl ligand-containing clusters.51,52
2.5 Synthesis of [Cu29(SAdm)15Cl3(P(Ph-Cl)3)4D10](PF6) nanoclusters (Cu29-P(Ph-Cl)3-D)
The synthesis of Cu29-P(Ph-Cl)3-D is similar to that of Cu29-P(Ph-Cl)3-H, except that the reducing agent is changed to 64 mg NaBD4 (1.6 mmol).2.6 Synthesis of [Cu29(SC6H11)18((Ph-pMe)3P)4H10][BF4] nanoclusters (Cu29-P(Ph-Me)3-H)
Cu29-P(Ph-Me)3-H was prepared following the procedure used to synthesize Cu28-PPh2Py-H, with the exception that tri(p-tolyl)phosphine ((Ph-pMe)3P) (116 mg, 0.38 mmol) was used instead of PPh2Py (100 mg, 0.40 mmol). Red crystals were obtained by recrystallization from CH2Cl2/Hex over one week.2.7 Synthesis of [Cu29(C6H11S)18((Ph-pMe)3P)4D10][BF4] nanoclusters (Cu29-Ph-pMe-D)
Cu29-P(Ph-pMe)3-D was synthesized similarly to Cu29-P(Ph-Me)3-H, with the exception that NaBD4 (40 mg, 1 mmol) was used instead of NaBH4 (30 mg, 0.8 mmol).2.8 Characterization
All UV-vis spectra of the nanoclusters were recorded on a Metash UV-6000PC UV-vis spectrophotometer, and the samples were dissolved in CH2Cl2 whose background correction was made using a CH2Cl2 blank. X-ray photoelectron spectroscopy (XPS) measurements were performed using a Thermo ESCALAB 250 configured with a monochromated Al Kα (1486.8 eV) 150 W X-ray source, a 0.5 mm circular spot size, a flood gun to counter charging effects, and an analysis chamber base pressure lower than 1 × 10−9 mbar, and the data were collected with an FAT of 20 eV. Electrospray ionization (ESI) mass spectrum was obtained on a Waters XEVO G2-XS QT of mass spectrometer. The samples are dissolved in a mixture solution of CH2Cl2/CH3OH (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), which is directly infused into the chamber at 10 μL min−1 with positive mode. Nuclear magnetic resonance (NMR) analysis was performed on a Bruker Avance spectrometer operating at 400 MHz. CD2Cl2 was used as the solvent to dissolve the crystals of copper-hydride clusters; the residual solvent peak (i.e., 1H at 5.32 ppm) was used as the reference. The clusters were dissolved in CH2Cl2 for the 2H NMR spectra. Data collection for single-crystal X-ray diffraction was carried out using a Stoe Stadivari diffractometer under a liquid nitrogen flow at 150 K, using graphite-monochromatized Cu Kα radiation (λ = 1.54186 Å). Data reductions and absorption corrections were performed using SAINT and SADABS programs.
1), which is directly infused into the chamber at 10 μL min−1 with positive mode. Nuclear magnetic resonance (NMR) analysis was performed on a Bruker Avance spectrometer operating at 400 MHz. CD2Cl2 was used as the solvent to dissolve the crystals of copper-hydride clusters; the residual solvent peak (i.e., 1H at 5.32 ppm) was used as the reference. The clusters were dissolved in CH2Cl2 for the 2H NMR spectra. Data collection for single-crystal X-ray diffraction was carried out using a Stoe Stadivari diffractometer under a liquid nitrogen flow at 150 K, using graphite-monochromatized Cu Kα radiation (λ = 1.54186 Å). Data reductions and absorption corrections were performed using SAINT and SADABS programs.
3. Results and discussion
The Cu28-PPh2Py, Cu29-P(Ph-Cl)3, and Cu29-P(Ph-Me)3 series of copper nanoclusters were directly synthesized using a one-pot method under mild conditions. Briefly, Cu28-PPh2Py was synthesized by dissolving [Cu(CH3CN)4]BF4 in a mixture of acetonitrile (CH3CN) and chloroform (CHCl3) at room temperature, after which C6H11SH and PPh2Py were added, and NaBH4 was used as the reducing agent. The solution turned dark red. The orange crystals were characterized by single-crystal X-ray crystallography (SC-XRC) (Fig. S1†). Cu28-PPh2Py crystallized in the monoclinic P21/a space group (Table S1†). On the other hand, Cu28-PPh3 (Fig. S2†) crystallized in the monoclinic I2/a space group (Table S2†). Cu29-Ph-pMe was obtained when the P(Ph-pMe)3 ligand was used instead of PPh2Py under the same conditions (Fig. S3†). Red Cu29-Ph-pMe crystallized in the trigonal R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) space group (Table S3†).
space group (Table S3†).
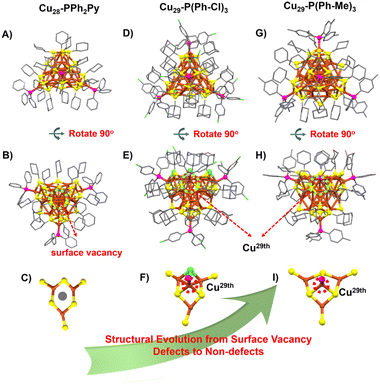
The structures of Cu28-PPh2Py, Cu29-P(Ph-Cl)3, and Cu29-P(Ph-Me)3 are shown in Fig. 1. Cu28-PPh2Py/PPh3 contains 28 Cu atoms, 18 S-c-C6H11 ligands, eight hydrides, and three PPh2Py ligands (Fig. 1A and B). A surface vacancy defect located in the Cu3(SR)6 motif is observed in Cu28-PPh2Py (Fig. 1C). The copper skeletons of Cu29-P(Ph-Cl)3 and Cu29-P(Ph-Me)3 formed (Fig. 1D–I) when the 29th copper atom was inserted into the interstice (surface vacancy site) (Fig. 1F and I). The formed Cu29-P(Ph-Cl)3 possesses 29 Cu atoms, 15-SAdm ligands, 3 Cl atoms, 10 hydrides, and four (P(Ph-Cl)3). Similarly, Cu29-P(Ph-Me)3 possesses 29 Cu atoms, 18 S-c-C6H11 ligands, 10 hydrides, and four P(Ph-pMe)3 ligands, with four P(Ph-pMe)3 units occupying the four vertices of the tetrahedron. As shown in Fig. S4–S6,† the Cu28-PPh2Py framework possesses a C1 symmetry axis, whether observed from the position of the three phosphine ligands or the vacancy (Fig. S4†). On the other hand, the Cu29-P(Ph-Cl)3 framework possesses one C3 symmetry axis, indicative of improved symmetry (Fig. S5†), while the Cu29-P(Ph-Me)3 framework forms a standard tetrahedral configuration with four C3 symmetry axes (Fig. S6†). Once again, the Cu29-Ph-pMe cluster exhibits significantly improved symmetry, affecting the copper-hydride nanocluster's stability. The novel changes observed for the metal core, the number of hydrogen ligands, the packing model, the metal shell, and the core/shell bonding model facilitate a detailed understanding of the surface-vacancy-defect to non-defect structural evolution.
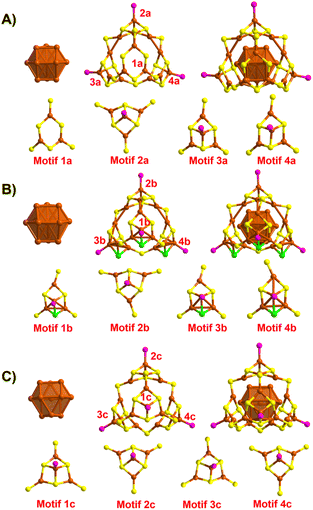
The changes observed during structural evolution can be understood in detail by splitting the structure. The structural anatomy of surface-defective Cu28-PPh2Py is shown in Fig. 2A, which reveals that the Cu13 copper kernel has an anti-cuboctahedral structure. The Cu–Cu distances in the anti-cuboctahedron vary between 2.459 and 2.787 Å, with an average value of 2.605 Å. The anti-cuboctahedron Cu13 is surrounded by a Cu15(SR)18P3 cage shell, which can be viewed as an assembly of a single Cu3(SR6) (Motif 1a) and three Cu4S6P1 motifs (Motifs 2a–4a). While Motifs 2a–4a share the same molecular formula, their geometries differ. In contrast, Motif 1a has surface vacancy defects. Non-defective Cu29-P(Ph-Cl)3 capped by P(Ph-Cl)3, SAdm-, and Cl− and H− ligands was obtained (Fig. 2B) when the 29th copper atom was inserted into the surface vacancy site of Motif 1a. An anti-cuboctahedral Cu13 core, similar to the anti-cuboctahedral Ag13 core in Ag19 and Ag25 (ref. 53) was observed for Cu29-P(Ph-Cl)3 by comparison with the structure of Cu28-PPh2Py. The anti-cuboctahedral Cu13 core is further capped by a Cu16(SR)15P4 shell assembled by using Cu4(SR)6P1 (Motif 2b) and three Cu4(SR)5ClP1 units (Motifs 2a, 2c, and 2d). Because the AdmSH ligand is very sterically hindering, another thiol ligand cannot be accommodated; hence, the small Cl acts as a protective ligand to maintain the stability of the Cu29-P(Ph-Cl)3 structure. Another non-defective Cu29-P(Ph-Me)3 nanocluster capped by P(Ph-Me)3, S-c-C6H11- and H− ligands is shown in Fig. 2C. In contrast to the anti-cuboctahedral Cu13 core of Cu28-PPh2Py and Cu29-P(Ph-Cl)3, the Cu13 core in Cu29-P(Ph-Me)3 is cuboctahedral in structure. The cuboctahedral Cu13 framework was also observed in [Cu13(S2CNnBu2)6(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR)4](PF6).54,55 The Cu–Cu distances in the cuboctahedron vary between 2.457 and 2.878 Å, with an average value of 2.651 Å. The cuboctahedral Cu13 core is surrounded by a Cu16(SR)18P4 shell assembled using four identical Cu4S6P1 motifs (Motifs 1c–4c). The structure of Cu29-P(Ph-Me)3 is consistent with that of a standard tetrahedron, in which the Cu29-P(Ph-Me)3 frame has the same structure as MAg28(SR)18(PR′3)4 (Fig. S7†).
CR)4](PF6).54,55 The Cu–Cu distances in the cuboctahedron vary between 2.457 and 2.878 Å, with an average value of 2.651 Å. The cuboctahedral Cu13 core is surrounded by a Cu16(SR)18P4 shell assembled using four identical Cu4S6P1 motifs (Motifs 1c–4c). The structure of Cu29-P(Ph-Me)3 is consistent with that of a standard tetrahedron, in which the Cu29-P(Ph-Me)3 frame has the same structure as MAg28(SR)18(PR′3)4 (Fig. S7†).
 | ||
| Fig. 2 Structural anatomies of the series of copper nanoclusters. (A) Cu28-PPh2Py, (B) Cu29-P(Ph-Cl)3, and (C) Cu29-P(Ph-Me)3. Color scheme: copper = Cu, yellow = S, purple = P, and green = Cl. | ||
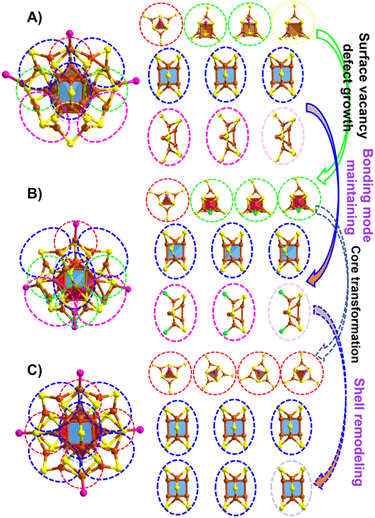
Furthermore, surface-kernel structural transfer provides diverse bonding patterns during structural evolution. First, both the cuboctahedral and anti-cuboctahedral Cu13 cores have eight triangular (Cu3) surfaces and quadrilateral (Cu4) surfaces (Fig. S8†). As shown in Fig. 3A, Cu28-PPh2Py has one Cu4(SR)6P1 motif (marked with a red circle) that covers one triangular Cu3 surface, two Cu4(SR)6P1 motifs (marked with green circles) that cover quadrilateral (Cu4) surfaces, as well as one Cu3(SR)6 motif (marked with a yellow circle) containing the surface vacancy defect that also covers a quadrilateral (Cu4) surface. A vertex Cu–P resulting from the spatial position of the thiol ligand located within the Cu3(SR) ring is the difference between the Cu4(SR)6P1 units (marked with green circles) and the Cu3(SR)6 moiety (marked with a yellow circle). As shown in Fig. S9,† when the carbon tails of the three thiol ligands in the Cu3(SR)6 ring are aligned outwards, the reserved space can support a vertex Cu–P staple insertion to form Cu4(SR)6P1, while there is insufficient reserved space to support a vertex Cu–P staple when one thiol-ligand carbon tail is arranged inward, resulting in surface vacancy defects. This observation reveals that the orientation of the ligand carbon tail affects the formation of surface-vacancy defects. In addition, its bonding mode includes three quadrilateral Cu4 units capped by Cu2(SR)5 shells (marked with blue circles) and three triangular Cu3 units capped by Cu2(SR)5 shells (marked with purple circles). For non-defective Cu29-P(Ph-Cl)3, the Cu4(SR)6P1 motif marked with a red circle also covers a triangular Cu3 surface, and the three Cu4(SR)5ClP1 motifs marked with green circles also cover quadrilateral Cu4 surfaces (Fig. 3B). Three quadrilateral Cu4 moieties capped by Cu2(SR)5 shells (marked with blue circles) and three triangular Cu3 units capped by Cu2(SR)4Cl shells (marked with purple circles) are also present, as observed in the structure of Cu28-PPh2Py. For Cu29-P(Ph-Cl)3, the capping Cl and AdmSH ligands endow enough space to support the insertion of Cu–P to form non-defective clusters (Motif 1a vs. Motif 1b in Fig. 2). Structural evolution from the surface-vacancy-defective Cu28-PPh2Py to non-defective Cu29-P(Ph-Cl)3 involves the growth of defect motifs, with the bonding mode between the core and the motif maintained to a certain degree. The other non-defective Cu29-P(Ph-Me)3 structure contains four Cu4(SR)6P1 motifs that cover four triangular surfaces (Cu3) of cuboctahedral Cu13 (marked with red circles). The spatial arrangement of thiol ligands can better support the insertion of Cu–P, forming four identical Cu4S6P1 motifs. All six Cu2(SR)5 shells cover the quadrilateral Cu4 unit (marked with blue circles) (Fig. 3C).
Structural evolution involves the growth of surface vacancy defects from surface-vacancy-defective Cu28-PPh2Py to non-defective Cu29-P(Ph-Cl)3, accompanied by core and bond pattern maintenance (Fig. 3Avs.Fig. 3B). Structural evolution involves the transformation of the Cu29-P(Ph-Cl)3 core and remodeling of the Cu29-P(Ph-Cl)3 shell, respectively, to form the other non-defective Cu29-P(Ph-Me)3 (Fig. 3Bvs.Fig. 3C). These results provide atomically precise insights into the defect-induced readjustment of the local structure.
Single-crystal X-ray crystallography (SCXRC) revealed both the intramolecular structure and intermolecular packing mode of the metal nanoclusters during structural evolution. Cu28-PPh2Py and Cu29-P(Ph-Cl)3 only exhibit 2H arrangements with “AB” packing sequences (Fig. S10 and S11†), while the crystalline unit cell of Cu29-P(Ph-Me)3 shows a special “ABCDEF” stacking sequence. The Cu29-Ph-pMe nanoclusters in the stacking layer are uniformly arranged and each nanocluster is surrounded by six identical nanoclusters with the same tropism, as shown in Fig. S12.† Such a 6H pattern was previously only observed in Au60 reported by Wu et al.56
The UV-vis spectra of Cu28-PPh2Py, Cu29-P(Ph-Cl)3, and Cu29-P(Ph-Me)3 in CH2Cl2 are shown in Fig. S13.† Several weak absorptions are observed at 256 and 400 nm for Cu28-PPh2Py, at 270, 326, and 415 nm for Cu29-P(Ph-Cl)3, and at 410, 325, and 266 nm for Cu29-P(Ph-Me)3. Because determining the number of hydrogen atoms by SC-XRD is difficult, careful ESI-MS was used to determine the valence and molecular formula of each cluster and the number of hydrides. Positive-mode ESI-MS data for Cu28-PPh2Py, Cu29-P(Ph-Cl)3, and Cu29-P(Ph-Me)3 are shown in Fig. 4. The spectrum of Cu29-Ph-pMe shows a prominent peak corresponding to a +1 charge at m/z = 5144.69 Da, which is attributable to [Cu29(S-c-C6H11)18(P(Ph-pMe)3)4H10]+ (Fig. 4A). The experimentally observed isotope pattern for [Cu29(S-c-C6H11)18(P(Ph-pMe)3)4H10]+ is in good agreement with the calculated pattern (Fig. S14A†). Cu29-P(Ph-Me)3-D was synthesized and subjected to ESI-MS to further confirm the presence and number of hydrides. A prominent peak corresponding to a +1 charge was observed at m/z = 5154.66 Da, which is attributable to [Cu29(S-c-C6H11)18(P(Ph-pMe)3)4D10]+ (Fig. 4B). Accordingly, 10 hydrides are present in the Cu29-P(Ph-Me)3 cluster. Similarly, the ESI-MS spectrum of Cu29-P(Ph-Cl)3 confirmed that it is complex (Fig. 4C and D). First, the prominent peak corresponding to a charge of +1 observed at m/z = 4468.27 Da belongs to [Cu29(SC10H15)15Cl3H10]+ derived from the removal of four P(Ph-Cl)3 ligands from the complete [Cu29(SC10H15)15Cl3(P(Ph-Cl)3)4H10]+, and it is fully consistent with the calculated result (Fig. S14B†). Neighboring peaks at 4433.32 and 4396.33 Da are attributable to [Cu29(SC10H15)15Cl3H10-Cl] and [Cu29(SC10H15)15Cl3H10-2Cl], respectively. The difference of 10 Da between the m/z values of Cu29-P(Ph-Cl)3-H and Cu29-P(Ph-Cl)3-D indicates the presence of 10 hydrogen atoms. By comparison, the ESI-MS spectrum of [Cu28(S-c-C6H11)18(Ph2PyP)3H8]2+ was more complicated, with peaks corresponding to charges of +1, +2, and +3 observed. Specifically, the +1 signal at m/z = 4726.34 Da is attributable to [Cu28(C6H11S)18(C17H14NP)3(H)8 + Cl + CH3CN]+, while that corresponding to a charge of +2 at m/z = 2390.55 Da is attributable to [Cu28(C6H11S)17(C17H14NP)3(H)8 + Cl + (CH3CN)(CH2Cl2)2]2+, consistent with the one thiol moiety in [Cu28(C6H11S)18(C17H14NP)3(H)8]2+ replaced by Cl. The +3 peak at m/z = 1583.35 Da is attributable to [Cu28(S-c-C6H11)18(C17H14NP)3H8 + CuCl]3+ (Fig. 4E–H and isotopic patterns in Fig. S14C–E†). We also synthesized and characterized Cu28-PPh2Py-D. The intervals between the 3+, 2+, and 1+ peaks of Cu28-PPh2Py-H and Cu28-PPh2Py-D were found to be 2.66, 4, and 8, respectively, consistent with the presence of eight hydrogen atoms (i.e., 2.66 × 3/4 × 2/8 × 1). Although no BF4− counterion was found in the unit cell of Cu28-PPh2Py, its presence was confirmed by ESI-MS (Fig. S25†). So, combining these experimental results, none of the three copper hydride clusters have free electrons (28(Cu 4s1) − 18 (SR) − 8 (H-) − 2 (charge) = 0e for Cu28-PPh2Py, 29 (Cu 4s1) − 15 (SR) − 3 (Cl) − 10 (H–) − 1 (charge) = 0e for Cu29-P(Ph-Cl)3 and 29 (Cu 4s1) − 18 (SR) − 10 (H–) − 1 (charge) = 0e for Cu29-P(Ph-Me)3).
 | ||
| Fig. 4 ESI-MS of the series of copper nanoclusters. Positive-mode ESI-MS spectra of (A and B) Cu29-P(Ph-Me)3, (C and D) Cu29-P(Ph-Cl)3, and (E–H) Cu28-PPh2Py. | ||
X-ray photoelectron spectroscopy (XPS) was used to analyze the chemical compositions and copper-charge states in Cu28-PPh2Py, Cu29-P(Ph-Cl)3, and Cu29-P(Ph-Me)3 (Fig. S20–S24†), with the Cu2p, S2p, P2p, F1s, and N1s XPS spectra shown in Fig. S20–S23.† The F1s signals are ascribable to the BF4−/PF6− counter ions. The presence of “BF4−” in the Cu28-PPh2Py nanocluster was also confirmed by ESI-MS (Fig. S25†). Simple XPS cannot accurately determine the specific valence states of copper in copper nanoclusters because the Cu 2p3/2 binding energies of Cu0 and the Cu+ in Cu2S are identical (932.6 eV);57 Therefore, Cu Auger-electron spectroscopy is required to further determine the specific valence states of copper. The Auger spectra of Cu28-PPh2Py, Cu29-P(Ph-Cl)3, and Cu29-P(Ph-Me)3 show only one main Cu LMM peak at 570.69 eV (Fig. S24†), which corresponds to the Cu +1 oxidation state. Moreover, the three clusters exhibited no observable signal near 943 eV, consistent with the absence of Cu(II) in Cu28-PPh2Py, Cu29-P(Ph-Cl)3, and Cu29-P(Ph-Me)3.
The structural evolution from Cu28-PPh2Py to Cu29-P(Ph-Cl)3 and then to Cu29-P(Ph-Me)3 may lead to changes in the positions of the hydrogen atoms. Trying to grow single crystals of suitable quality for neutron diffraction is challenging.26b,27,31,35 So, DFT calculations and HNMR spectra were often used for validating possible plausible locations for hydrogen.26a,38,39,45,47 To determine the hydride locations in Cu28-PPh2Py, Cu29-P(Ph-Cl)3, and Cu29-P(Ph-Me)3, density functional theory (DFT) calculations to optimize their positions based on the X-ray crystallographic structures were performed first.26a,38,39,45,47,58 In previous reports, it was found that for copper clusters, the positions of hydrogens in structures with similar moieties have a certain similarity, for example, the positions of 6 hydrogens in Cu32H20 are found to be similar to their structural positions in Cu20H11.8,26b,35 Inspired by these research reports, and given the number of hydrides and the similarity in Cu@Cu12 kernel structures as well as the motifs of Cu28-PPh2Py, Cu29-P(Ph-Cl)3, Cu29-P(Ph-Me)3 and [Cu25(SPhCl2)18H10]3− (anti-cuboctahedral Cu25), the locations of the hydrides in Cu28-PPh2Py, Cu29-P(Ph-Cl)3, and Cu29-P(Ph-Me)3 were determined referring to that of [Cu25(SPhCl2)18H10]3−, while the positions of hydrogens in Cu25 (Fig. 5A), which served as references, were carefully determined by the Zheng group via HNMR spectroscopy and DFT calculations.59 These optimized structures can remain stable, indicating that the determined hydrogen positions are reasonable. Fig. 5 shows that all hydrides are located around the M13 core. Half of the eight triangular structures and all six square-planar structures of the Cu13 core surface in Cu29-P(Ph-Me)3 are capped with one hydride each (Fig. 5B). Meanwhile, Cu28-PPh2Py shares the same icosahedral structure as Cu25, and its eight hydrides are separated into two groups: four capping the triangular structure and another four capping the square-planar structure of the Cu13 core surface (Fig. 5C). Another Cu29-P(Ph-Cl)3 cluster was obtained, whose Cu@Cu12 core is the same as that of the Cu25 core and Cu28-PPh2Py, which are both cuboctahedral. The ten H atoms of Cu29-P(Ph-Cl)3 cover all square faces and half of the triangular faces (Fig. 5D). The maintained frameworks of the proposed structures of the aforementioned Cu29-P(Ph-Me)3, Cu29-P(Ph-Cl)3, and Cu28-PPh2Py clusters (denoted as Cu29-P(Ph-Me)3T, Cu29-P(Ph-Cl)3T, and Cu28-PPh2PyT) following geometry optimization using the GGA: PBE/DND method and the Dmol3 package60–62 confirmed that these structures are stable (Fig. S19†). The geometric details of the Cu@Cu12 kernels of Cu29-P(Ph-Me)3T, Cu29-P(Ph-Cl)3T, and Cu28-PPh2PyT are listed in Table S4.† The positions of the hydrogens in these three nanoclusters are more intuitively compared in Fig. S15–S18.† All four μ3-H-Cu3 (μ3-H-1/2/3/4-Cu3) units are located in the Cu6(SR)6 ring in Cu25, and three μ4-H-Cu4 (μ3-H-5/6/7-Cu4) moieties are capped by three Cu2(SR)5 motifs, and three μ4-H-Cu4 (μ3-H-8/9/10-Cu4) units are capped by three Cu3(SR)6 units (Fig. S15†). The four μ-H-Cu3 (μ-H-1/2/3/4-Cu3) units in Cu29-P(Ph-Me)3 and Cu28-PPh2Py are also located in their Cu6(SR)6 rings, while three μ-H-Cu3 (μ-H-1/2/3/-Cu3) moieties are located in the Cu6(SR)6 ring of Cu29-P(Ph-Cl)3, and one μ-H-Cu3 moiety is located in the Cu6(SR)3Cl3 ring. Furthermore, six μ-H-Cu4 (μ-H-5/6/7/8/9/10-Cu4) moieties are capped by six Cu2(SR)5 motifs in Cu29-P(Ph-Me)3 (Fig. S16†), three μ-H-Cu4 (μ-H-5/6/7-Cu4) units are capped by three Cu2(SR)5 motifs, and three μ-H-Cu4 (μ-H-8/9/10-Cu4) moieties are capped by three Cu3(SR)5Cl units in the case of Cu29-P(Ph-Cl)3 (Fig. S17†), while three μ-H-Cu4 (μ-H-5/6/7-Cu4) units are capped by three Cu2(SR)5 motifs, and only one μ-H-Cu4 (μ-H-8-Cu4) is capped by Cu3(SR)6 in the case of Cu28-PPh2Py (Fig. S18†). These observations provide a reference for predicting the number and locations of the hydrogen atoms in the nanocluster framework.
Furthermore, the HNMR spectra of these three copper-hydride nanoclusters were carefully obtained via dissolving crystals in the solvent (Fig. S26–S28†). For the 2HNMR of Cu28-PPh2Py-D (Fig. S26B†), four different D atom signal peaks can be observed, with intensity ratios of about 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. Broadened signal peaks can be observed for the D signal in the 2HNMR spectra of Cu29-P(Ph-Me)3-D. And there also were about 4D in the upfield region (low ppm), and about 6D in the low-field region (high ppm) (Fig. S27B†). The deuterium signal ratio at upfield and low-field was 6
3. Broadened signal peaks can be observed for the D signal in the 2HNMR spectra of Cu29-P(Ph-Me)3-D. And there also were about 4D in the upfield region (low ppm), and about 6D in the low-field region (high ppm) (Fig. S27B†). The deuterium signal ratio at upfield and low-field was 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.13 for Cu29-P(Ph-Cl)3-D (Fig. S28B†). Combining the optimized geometry, 2HNMR data, and the peak position of the D in Cu25H10:59 (i) for Cu28-PPh2Py-D, the 4D (3Da + 1Db) in the upfield region (low ppm) can be attributed the H-1, H-2, H-3, and H-4 and the 4D (1Dc + 3Dd) in the low-field region (high ppm) can be attributed to H-5, H-6, H-7, and H-8 (Fig. S17† and the inset of Fig. S26B†); (ii) for Cu29-P(Ph-Me)3-D, the 4D (4Da) in the upfield region (low ppm) can be attributed the H-1, H-2, H-3, and H-4 and the 6D (6Dd) observed in the low-field region (high ppm) can be attributed to H-5, H-6, H-7, H-8, H-9 and H10 (Fig. S16† and the inset of Fig. S27B†). In contrast to four different D atoms (Da, Db, Dc, and Dd) of Cu28-PPh2Py-D, Cu29-P(Ph-Me)3-D has two D atoms, viz., Da and Dd, due to the high symmetry; (iii) for Cu29-P(Ph-Cl)3-D, the 4D (3Da + 1Db) in the upfield region (low ppm) can be attributed the H-1, H-2, H-3, and H-4 and the 6D (3Dc + 3Dd) in the low-field region (high ppm) can be attributed to H-5, H-6, H-7, H-8, H-9 and H-10 (Fig. S18† and the inset of Fig. S28B†). From the information of 2HNMR spectra, the signal peak of hydrogen of can be better attributed. The 46H located between 7 ppm and 9 ppm were assigned to hydrogen from PPh2Py (3 × 14) and 4 Hc/d in the cluster. Due to the influence of N on PPh2Py, part of the hydrogen is significantly shifted, i.e. the hydride resonance (3H) at 8.76 ppm. The signal in the alkane region (206H) was due to the thiol and H-ligands (Cal. 18 × 11 + 4(Ha/Hb) = 204H, and Δ = 2H) (Fig. S26†). In the 1HNMR spectrum of Cu29-P(Ph-Me)3-H, 52H in the aromatic region belonged to the –C6H4R of P(Ph-Me)3 (4 × 4 × 3) and 6 Hd in the cluster, and the 237.63H were attributed to the thiol, H-ligands and –CH3 in P(Ph-Me)3 (Cal. 18 × 11 + 4(Ha) + 4 × 3 × 3 = 238H) (Fig. S27A†). Similarly, 52H in the aromatic region belonged to the –C6H4– of P(Ph-Cl)3 and 6 Hc/d in the cluster, and the 228.12H were attributed to the thiol, H-ligands (Cal. 15 × 15 + 4 (Ha/b) = 229H) (Fig. S28A†).Based on the this information, we can reasonably consider that the hydrogen located in the upfield was the hydrogen (on the cluster surface) covering the Cu3 surface of the Cu13 core, and the hydrogens encapsulated in trigonal prismatic (tp) cages were located in the low-field region, which in turn showed the rationality of predicting the hydrogen positions in the three clusters.
4.13 for Cu29-P(Ph-Cl)3-D (Fig. S28B†). Combining the optimized geometry, 2HNMR data, and the peak position of the D in Cu25H10:59 (i) for Cu28-PPh2Py-D, the 4D (3Da + 1Db) in the upfield region (low ppm) can be attributed the H-1, H-2, H-3, and H-4 and the 4D (1Dc + 3Dd) in the low-field region (high ppm) can be attributed to H-5, H-6, H-7, and H-8 (Fig. S17† and the inset of Fig. S26B†); (ii) for Cu29-P(Ph-Me)3-D, the 4D (4Da) in the upfield region (low ppm) can be attributed the H-1, H-2, H-3, and H-4 and the 6D (6Dd) observed in the low-field region (high ppm) can be attributed to H-5, H-6, H-7, H-8, H-9 and H10 (Fig. S16† and the inset of Fig. S27B†). In contrast to four different D atoms (Da, Db, Dc, and Dd) of Cu28-PPh2Py-D, Cu29-P(Ph-Me)3-D has two D atoms, viz., Da and Dd, due to the high symmetry; (iii) for Cu29-P(Ph-Cl)3-D, the 4D (3Da + 1Db) in the upfield region (low ppm) can be attributed the H-1, H-2, H-3, and H-4 and the 6D (3Dc + 3Dd) in the low-field region (high ppm) can be attributed to H-5, H-6, H-7, H-8, H-9 and H-10 (Fig. S18† and the inset of Fig. S28B†). From the information of 2HNMR spectra, the signal peak of hydrogen of can be better attributed. The 46H located between 7 ppm and 9 ppm were assigned to hydrogen from PPh2Py (3 × 14) and 4 Hc/d in the cluster. Due to the influence of N on PPh2Py, part of the hydrogen is significantly shifted, i.e. the hydride resonance (3H) at 8.76 ppm. The signal in the alkane region (206H) was due to the thiol and H-ligands (Cal. 18 × 11 + 4(Ha/Hb) = 204H, and Δ = 2H) (Fig. S26†). In the 1HNMR spectrum of Cu29-P(Ph-Me)3-H, 52H in the aromatic region belonged to the –C6H4R of P(Ph-Me)3 (4 × 4 × 3) and 6 Hd in the cluster, and the 237.63H were attributed to the thiol, H-ligands and –CH3 in P(Ph-Me)3 (Cal. 18 × 11 + 4(Ha) + 4 × 3 × 3 = 238H) (Fig. S27A†). Similarly, 52H in the aromatic region belonged to the –C6H4– of P(Ph-Cl)3 and 6 Hc/d in the cluster, and the 228.12H were attributed to the thiol, H-ligands (Cal. 15 × 15 + 4 (Ha/b) = 229H) (Fig. S28A†).Based on the this information, we can reasonably consider that the hydrogen located in the upfield was the hydrogen (on the cluster surface) covering the Cu3 surface of the Cu13 core, and the hydrogens encapsulated in trigonal prismatic (tp) cages were located in the low-field region, which in turn showed the rationality of predicting the hydrogen positions in the three clusters.
Surface-defect growth and enhanced overall symmetry affect the stability of copper-hydride nanoclusters. The thermal stabilities of these copper-hydride nanoclusters were explored by storing CH2Cl2 solutions of Cu29-P(Ph-Me)3, Cu29-P(Ph-Cl)3, and Cu28-PPh2Py at room temperature for a week (Fig. S29†). Fig. S29A† reveals that Cu28-PPh2Py starts to decompose after three days, accompanied by precipitation, while some flocs began to form on the fifth day in the Cu29-P(Ph-Cl)3 solution (Fig. S29B†). In contrast, the solution of Cu29-P(Ph-Me)3 remained stable for 5 days (Fig. S29C†). Furthermore, a solution of Cu29-P(Ph-Me)3 in CHCl3 was stable for more than 5 h in an oil bath at 50 °C, while these conditions destroyed both Cu29-P(Ph-Cl)3 and Cu28-PPh2Py (Fig. S30†). In this context, Cu29-P(Ph-Me)3 is the most stable, followed by Cu29-P(Ph-Cl)3 and Cu28-PPh2Py. The metastability exhibited by Cu28-PPh2Py is ascribable to surface vacancy defects that result in incomplete capping of the nanocluster surface. In comparison, Cu29-P(Ph-Me)3, which has a standard tetrahedral structure, is more stable than Cu29-P(Ph-Cl)3.
4. Conclusions
We synthesized and identified three copper hydride nanoclusters through ligand engineering, including [Cu28(S-c-C6H11)18(PPh2Py)3H8](BF4), a surface-vacancy-defective nanocluster, and [Cu29(SAdm)15Cl3(P(Ph-Cl)3)4H10](PF6) and [Cu29(S-c-C6H11)18(P(Ph-pMe)3)4H10](BF4), which are both non-defective nanoclusters. Because there are few reports on the series of the defect- and defect-growth-based copper-hydride clusters, the series of well-characterized copper nanoclusters prepared in this study provides insight into surface-vacancy-defect to non-defect structural evolution. Structural adjustments were observed in the metal core, hydrogen ligand, metal shell, core/shell bonding pattern, and packing mode during the evolution of surface-vacancy-defective Cu28-PPh2Py to non-defective Cu29-P(Ph-Cl)3 and then to the other Cu29-P(Ph-Me)3. Specifically: (i) the anti-cuboctahedral Cu13 core is maintained between Cu28-PPh2Py and Cu29-P(Ph-Cl)3, but changes to a cuboctahedral Cu13 structure in Cu29-P(Ph-Me)3. (ii) The doubly charged Cu28-PPh2Py is embedded with eight hydrides, while singly charged Cu29-P(Ph-Cl)3/Cu29-P(Ph-Me)3 is embedded with 10 hydrides; however, the positions of the hydrogens are very similar in both clusters. (iii) The Cu16(SR)18P4 shell in Cu29-P(Ph-Me)3 contains four identical Cu4S6P1 motifs assembled by sharing six SR ligands, in which four Cu4S6P1 motifs cover the four triangular surfaces (Cu3) of the cuboctahedral Cu13. On the other hand, the Cu16(SR)18P4 shell in Cu29-P(Ph-Cl)3 is an assembly of one Cu4(SR)6P1 motif that covers the triangular surfaces (Cu3) and three Cu4(SR)5ClP1 motifs that cover the quadrilateral surface (Cu4) of the anti-cuboctahedral Cu13. As was observed for Cu29-P(Ph-Cl)3, Cu28-PPh2Py is assembled from one Cu4(SR)6P1 motif that covers the triangular surfaces (Cu3), two Cu4(SR)6P1 motifs that cover the quadrilateral surface (Cu4), and one defective Cu3(SR)6 unit that covers the quadrilateral surface (Cu4), which leads to a complex bonding environment. (iv) Cu29-P(Ph-Me)3 shows a 6H pattern in the crystal lattice, whereas Cu28-PPh2Py and Cu29-P(Ph-Cl)3 only show 2H arrangements. (v) Stability improved in moving from Cu28-PPh2Py to Cu29-P(Ph-Cl)3 and then to Cu29-P(Ph-Me)3, which indicates that surface defects, as well as the symmetry of the overall structure, affect the stability of the copper-hydride cluster. The structural evolution observed in moving from surface-vacancy-defective Cu28-PPh2Py to non-defective Cu29-P(Ph-Cl)3 and then to the other non-defective Cu29-P(Ph-Me)3 enables the structural evolution and the mechanism responsible for the physicochemical properties to be understood and provides valuable insight into the structural surface vacancies in copper nanoclusters and their structure–property relationships.Data availability
All the data supporting this article have been included in the main text and the ESI.†Author contributions
Y. B. and B. Y. carried out the experiments, analyzed the data, and wrote the manuscript. X. K. revised the manuscript. X. W. and H. Y. completed DFT calculations. Z. L. and H. D. assisted in the synthesis of nanoclusters. M. Z., S. C., and S. J. designed the project, analyzed the data, and revised the manuscript.Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
We acknowledge the financial support provided by the National Natural Science Foundation of China (21901001, 21871001, and 21631001), Ministry of Education and Education Department of Anhui Province, andAnhui Provincial Natural Science Foundation (No. 1908085QB54), and acknowledge the technical support of the high-performance computing platform of Anhui University.Notes and references
- R. Jin, C. Zeng, M. Zhou and Y. Chen, Chem. Rev., 2016, 116, 10346–10413 CrossRef CAS PubMed.
- I. Chakraborty and T. Pradeep, Chem. Rev., 2017, 117, 8208–8271 CrossRef CAS PubMed.
- R. Jin, G. Li, S. Sharma, Y. Li and X. Du, Chem. Rev., 2021, 121, 567–648 CrossRef CAS PubMed.
- S. Y. Qian, Z. P. Wang, Z. X. Zuo, X. M. Wang, Q. Wan and X. Yuan, Coord. Chem. Rev., 2022, 451, 214268 CrossRef CAS.
- Y. Xiao, Z. Wu, Q. Yao and J. Xie, Aggregate, 2021, 2, 114–132 CrossRef.
- X. Wei, H. L. Shen, C. Xu, H. Li, S. Jin, X. Kang and M. Z. Zhu, Inorg. Chem., 2021, 60, 5931–5936 CrossRef CAS PubMed.
- M. R. Friedfeld, J. L. Stein, A. Ritchhart and B. M. Cossairt, Acc. Chem. Res., 2018, 51, 2803–2810 CrossRef CAS PubMed.
- R. S. Dhayal, W. E. Van Zyl and C. W. Liu, Acc. Chem. Res., 2016, 49, 86–95 CrossRef CAS PubMed.
- Q. Z. Li, B. Y. Huang, S. Yang, H. Zhang, J. S. Chai, Y. Pei and M. Z. Zhu, J. Am. Chem. Soc., 2021, 143, 15224–15232 CrossRef CAS PubMed.
- Z. Gan, J. Chen, L. Liao, H. Zhang and Z. K. Wu, J. Phys. Chem. Lett., 2018, 9, 204–208 CrossRef CAS PubMed.
- D. Crasto, S. Malola, G. Brosofsky, A. Dass and H. Häkkinen, J. Am. Chem. Soc., 2014, 136, 5000–5005 CrossRef CAS PubMed.
- W. J. Du, S. Deng, S. Chen, S. Jin, Y. Zhen, Y. Pei and M. Z. Zhu, J. Phys. Chem. Lett., 2021, 12, 6654–6660 CrossRef CAS PubMed.
- G. Li, Z. Lei and Q.-M. Wang, J. Am. Chem. Soc., 2010, 132, 17678–17679 CrossRef CAS PubMed.
- S. Jin, S. X. Wang, Y. B. Song, M. Zhou, J. Zhong, J. Zhang, A. D. Xia, Y. Pei, M. Chen, P. Li and M. Z. Zhu, J. Am. Chem. Soc., 2014, 136, 15559–15565 CrossRef CAS PubMed.
- S. Jin, S. X. Wang, L. Xiong, M. Zhou, S. Chen, W. J. Du, A. D Xia, Y. Pei and M. Z. Zhu, Chem. Mater., 2016, 28, 7905–7911 CrossRef CAS.
- Y. Zhou, L. W. Liao, S. L. Zhuang, Y. Zhao, Z. B. Gan, W. M. Gu, J. Li, H. T. Deng, N. Xia and Z. K. Wu, Angew. Chem., Int. Ed., 2021, 60, 8668–8672 CrossRef CAS PubMed.
- J.-S. Yang, Z. Han, X.-Y. Dong, P. Luo, H.-L. Mo and S.-Q. Zang, Angew. Chem., Int. Ed., 2020, 59, 11898–11902 CrossRef CAS PubMed.
- Y. Negishi, T. Nakazak, S. Malol, S. Takano, Y. Niihor, W. Kurashige, S. Yamazoe, T. Tsukuda and H. Häkkine, J. Am. Chem. Soc., 2015, 137, 1206–1212 CrossRef CAS PubMed.
- Y. W. Li and R. Jin, J. Am. Chem. Soc., 2020, 142, 13627–13644 CrossRef CAS PubMed.
- X. Kang, Y. Li, M. Zhu and R. Jin, Chem. Soc. Rev., 2020, 49, 6443–6514 RSC.
- Y. W. Li, M. Zhou, Y. B. Song, T. Higaki, H. Wang and R. C. Jin, Nature, 2021, 594, 380–384 CrossRef CAS PubMed.
- Q. F. Yao, Z. N. Wu, Z. H. Liu, Y. Z. Lin, X. Yuan and J. P. Xie, Chem. Sci., 2021, 12, 99–127 RSC.
- Y.-M. Su, Z. Wang, C.-H. Tung, D. Sun and S. K. Schein, J. Am. Chem. Soc., 2021, 143, 13235–13244 CrossRef CAS PubMed.
- K. Nakamae, M. Tanaka, B. Kure, T. Nakajima, Y. Ura and T. Tanase, Chem.–Eur. J., 2017, 23, 9457–9461 CrossRef CAS PubMed.
- K. Nakamae, T. Nakajima, Y. Ura, Y. Kitagawa and T. Tanase, Angew. Chem., Int. Ed., 2020, 59, 2262–2267 CrossRef CAS PubMed.
- (a) R. S. Dhayal, J.-H. Liao, Y.-R. Lin, P.-K. Liao, S. Kahlal, J.-Y. Saillard and C. W. Liu, J. Am. Chem. Soc., 2013, 135, 4704–4707 CrossRef CAS PubMed; (b) J.-H. Liao, R. S. Dhayal, X. P. Wang, S. Kahlal, J.-Y. Saillard and C. W. Liu, Inorg. Chem., 2014, 53, 11140–11145 CrossRef CAS PubMed.
- R. S. Dhayal, J.-H. Liao, X. Wang, Y.-C. Liu, M.-H. Chiang, S. Kahlal, J.-Y. Saillard and C. W. Liu, Angew. Chem., Int. Ed., 2015, 54, 13604–13608 CrossRef CAS PubMed.
- R.-W. Huang, J. Yin, C. Dong, P. Maity, M. Nejib Hedhili, S. Nematulloev, B. Alamer, A. Ghosh, O. F. Mohammed and O. M. Bakr, ACS Mater. Lett., 2021, 3, 90–99 CrossRef CAS.
- T. A. Nguyen, Z. R. Jones, B. R. Goldsmith, W. R. Buratto, G. Wu, S. L. Scott and T. W. A. Hayton, J. Am. Chem. Soc., 2015, 137, 13319–13324 CrossRef CAS PubMed.
- A. L. Chen, X. Kang, S. Jin, W. J. Du, S. X. Wang and M. Z. Zhu, J. Phys. Chem. Lett., 2019, 10, 6124–6128 CrossRef CAS PubMed.
- A. J. Edwards, R. S. Dhayal, P.-K. Liao, J.-H. Liao, M.-H. Chiang, R. O. Piltz, S. Kahlal, J.-Y. Saillard and C. W. Liu, Angew. Chem., Int. Ed., 2014, 53, 7214–7218 CrossRef CAS PubMed.
- X. H. Liu, H. Shen, Y. Gao, G. C. Deng, H. W. Deng, Y.-Z. Han, B. K. Teo and N. F. Zheng, Chem. Commun., 2022, 58, 7670–7673 RSC.
- Q. L. Guo, B.-L. Han, C.-F. Sun, Z. Wang, Y. W. Tao, J.-Q. Lin, G.-G. Luo, C.-H. Tung and D. Sun, Nanoscale, 2021, 13, 19642–19649 RSC.
- R. Kumar Barik, S.-C. Huo, C.-Y. Wu, T.-H. Chiu, J.-H. Liao, X. P. Wang, S. Kahlal, J.-Y. Saillard and C. W. Liu, Chem.–Eur. J., 2020, 26, 10471–10479 CrossRef PubMed.
- R. S. Dhayal, J.-H. Liao, S. Kahlal, X. Wang, Y.-C. Liu, M.-H. Chiang, W. E. van Zyl, J.-Y. Saillard and C. W. Liu, Chem.–Eur. J., 2015, 21, 8369–8374 CrossRef CAS PubMed.
- R. S. Dhayal, H.-P. Chen, J.-H. Liao, W. E. van Zyl and C. W. Liu, ChemistrySelect, 2018, 3, 3603–3610 CrossRef CAS.
- P. Yuan, R. Chen, X. Zhang, F. Chen, J. Yan, C. Sun, D. Ou, J. Peng, S. Lin, Z. Tang, B. K. Teo, L. S. Zheng and N. F. Zheng, Angew. Chem., Int. Ed., 2019, 58, 835–839 CrossRef CAS PubMed.
- A. Ghosh, R.-W. Huang, B. Alamer, E. Abou-Hamad, M. Nejib Hedhili, O. F. Mohammed and O. M. Bakr, ACS Mater. Lett., 2019, 1, 297–302 CrossRef CAS.
- R. W. Huang, J. Yin, C. Dong, A. Ghosh, M. J. Alhilaly, X. Dong, M. Hedhili, E. Abou-Hamad, B. Alamer, S. Nematulloev, Y. Han, O. Mohammed and O. M. Bakr, J. Am. Chem. Soc., 2020, 142, 8696–8705 CrossRef PubMed.
- A. Baghdasaryana and T. Bürgi, Nanoscale, 2021, 13, 6283–6340 RSC.
- A. Sagadevan, A. Ghosh, P. Maity, O. F. Mohammed, O. M. Bakr and M. Rueping, J. Am. Chem. Soc., 2022, 144, 12052–12061 CrossRef CAS PubMed.
- Q. Tang, Y. J. Lee, D.-Y. Li, W. Choi, C. W. Liu, D. Lee and D.-E. Jiang, J. Am. Chem. Soc., 2017, 139, 9728–9736 CrossRef CAS PubMed.
- C.-Y. Liu, S.-F. Yuan, S. Wang, Z.-J. Guan, D.-E. Jiang and Q.-M. Wang, Nat. Commun., 2022, 13, 2082 CrossRef CAS PubMed.
- P.-K. Liao, C.-S. Fang, A. Edwards, S. Kahlal, J.-Y. Saillard and C. W. Liu, Inorg. Chem., 2012, 51, 6577–6591 CrossRef CAS PubMed.
- S. Lee, M. S. Bootharaju, G. Deng, S. Malola, W. Baek, H. Häkkinen, N. F. Zheng and T. Hyeon, J. Am. Chem. Soc., 2020, 142, 13974–13981 CrossRef CAS PubMed.
- S. Lee, M. S. Bootharaju, G. Deng, S. Malola, H. Häkkine, N. F. Zheng and T. Hyeon, J. Am. Chem. Soc., 2021, 143, 12100–12107 CrossRef CAS PubMed.
- C. W. Dong, R. Wu Huang, C. L. Chen, J. Chen, S. Nematulloev, X. R. Guo, A. Ghosh, B. Alamer, M. N. Hedhili, T. T. Isimjan, Y. Han, O. F. Mohammed and O. M. Bakr, J. Am. Chem. Soc., 2021, 143, 11026–11035 CrossRef CAS PubMed.
- R. P. B. Silalahi, Q. Wang, J.-H. Liao, T.-H. Chiu, Y.-Y. Wu, X. P. Wang, S. Kahlal, J.-Y. Saillard and C. W. Liu, Angew. Chem., Int. Ed., 2022, 61, e202113266 CAS.
- K. K. Chakrahari, J. P. Liao, R. P. Brocha Silalahi, T.-H. Chiu, J.-H. Liao, X. P. Wang, S. Kahlal, J.-Y. Saillard and C. W. Liu, Small, 2021, 17, 2002544 CrossRef CAS PubMed.
- X. Kang, M. Zhou, S. X. Wang, G. D. Sun, M. Z. Zhu and R. C. Jin, Chem. Sci., 2017, 8, 2581–2587 RSC.
- F. Hu, J.-J. Li, Z.-J. Guan, S.-F. Yuan and Q.-M. Wang, Angew. Chem., Int. Ed., 2020, 59, 5312–5315 CrossRef CAS PubMed.
- X. J. Zou, Y. Lv, X. Kang, H. Z. Yu, S. Jin and M. Z. Zhu, Inorg. Chem., 2021, 60, 14803–14809 CrossRef CAS PubMed.
- S. F. Yuan, P. Li, Q. Tang, X.-K. Wan, Z.-A. Nan, D.-E. Jiang and Q.-M. Wang, Nanoscale, 2017, 9, 11405–11409 RSC.
- K. K. Chakrahari, J.-H. Liao, S. Kahlal, Y.-C. Liu, M.-H. Chiang, J.-Y. Saillard and C. W. Liu, Angew. Chem., Int. Ed., 2016, 55, 14704–14708 CrossRef CAS PubMed.
- K. K. Chakrahari, R. P. Brocha Silalahi, J.-H. Liao, S. Kahlal, Y.-C. Liu, J.-F. Lee, M.-H. Chiang, J.-Y. Saillard and C. W. Liu, Chem. Sci., 2018, 9, 6785–6795 RSC.
- Z. B. Gan, J. S. Chen, J. Wang, C. M. Wang, M.-B. Li, C. H. Yao, S. L. Zhuang, A. Xu, L. L. Li and Z. K. Wu, Nat. Commun., 2017, 8, 14739 CrossRef CAS PubMed.
- P. Liu and E. J. Hensen, J. Am. Chem. Soc., 2013, 135, 14032–14035 CrossRef CAS PubMed.
- S. Wang, Z. Wu, S. Dai and D.-E. Jiang, Angew. Chem., Int. Ed., 2021, 60, 12289–12292 CrossRef CAS PubMed.
- C. Sun, N. Mammen, S. Kaappa, P. Yuan, G. Deng, C. Zhao, J. Yan, S. Malola, K. Honkala, H. Häkkinen, B. K. Teo and N. F. Zheng, ACS Nano, 2019, 13, 5975–5986 CrossRef CAS PubMed.
- J. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- B. Delley, J. Chem. Phys., 1990, 92, 508–517 CrossRef CAS.
- B. Delley, J. Chem. Phys., 2000, 113, 7756–7764 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Details of the synthesis process, characterization, and X-ray analysis, and Fig. S1–S30 and Tables S1–S6 offer more details on the nanoclusters. CCDC 2109287, 2109289, and 2166571. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2sc03239b |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2022 |