Let's frame it differently – analysis of instructors’ mechanistic explanations
Julia
Eckhard
 a,
Marc
Rodemer
a,
Marc
Rodemer
 b,
Axel
Langner
b,
Axel
Langner
 a,
Sascha
Bernholt
a,
Sascha
Bernholt
 *b and
Nicole
Graulich
*b and
Nicole
Graulich
 *a
*a
aJustus-Liebig-University Giessen, Institute of Chemistry Education, Heinrich-Buff-Ring 17, D-35392 Giessen, Germany. E-mail: nicole.graulich@didaktik.chemie.uni-giessen.de
bIPN – Leibniz Institute for Science and Mathematics Education, Olshausenstraße 62, D-24118 Kiel, Germany. E-mail: bernholt@ipn.uni-kiel.de
First published on 17th September 2021
Abstract
Research in Organic Chemistry education has revealed students’ challenges in mechanistic reasoning. When solving mechanistic tasks, students tend to focus on explicit surface features, apply fragmented conceptual knowledge, rely on rote-memorization and, hence, often struggle to build well-grounded causal explanations. When taking a resource perspective as a lens, students’ difficulties may arise from either an unproductive or a missing activation of cognitive resources. Instructors’ explanations and their guidance in teaching situations could serve as a lynchpin to activate these resources. Compared to students’ challenges in building mechanistic explanations in Organic Chemistry, little is known about instructors’ explanations when solving mechanistic tasks and how they shape their targeted explanations for students in terms of the construction and embedding of cause–effect rationales. This qualitative study aims to contribute to the growing research on mechanistic reasoning by exploring instructors’ explanatory approaches. Therefore, we made use of the framing construct, intended to trigger certain frames with explicit instruction. Ten Organic Chemistry instructors (university professors and lecturers) were asked to solve case comparison tasks while being prompted in two scenarios: an expert frame and a teaching frame. Our analysis shows that there is a shift from instructors’ mechanistic explanations in the expert frame towards more elaborated explanations in the teaching frame. In the teaching frame, contrary to what might be expected, complete cause–effect relationships were not always established and instructors differed in how they shaped their explanations. Additional explanatory elements were identified in both frames and their shift in use is discussed. Comparing approaches between frames sheds light on how instructors communicate mechanistic explanations and allows us to derive implications for teaching Organic Chemistry.
Introduction
In a time of global challenges, conspiracy theories, and fake news, the ability to construct well-grounded explanations can be regarded as a necessary skill. The importance of constructing explanations is not only evident in the growing body of literature in which the nature and types of explanations (Hempel, 1965; Achinstein, 1983; Salmon, 1984) and the structure of explanations and arguments (Toulmin, 2003) are discussed, but also in recent science curricula. The construction of scientific explanations promotes the understanding of ideas and claims made in science (e.g., Driver et al., 2000; Duschl and Osborne, 2002).When considering scientific explanations, mechanistic explanations are of interest as they entail the prediction and explanation of phenomena (Machamer et al., 2000; Craver and Darden, 2013). Building mechanistic explanations requires accounting for how and why a phenomenon comes about, and consideration of the interaction of factors leading to a specific phenomenon (Machamer et al., 2000). The entities of a mechanism with their properties, activities, and specific organization (temporal and spatial) need to be regarded (Machamer et al., 2000; Craver and Darden, 2013), and integration and shifting between levels is often required, i.e., different granularities in a description – macroscopic, submicroscopic or symbolic – must be considered (Gilbert and Treagust, 2009; Krist et al., 2019).
Focusing on students’ mechanistic explanations in the context of Organic Chemistry reveals that students often rely on explicit instead of implicit features (Bhattacharyya and Bodner, 2005; Strickland et al., 2010; Anzovino and Bretz, 2016; Graulich and Bhattacharyya, 2017). They are known to use simplified pattern recognition, rote-memorization, and heuristics (Maeyer and Talanquer, 2013; Graulich and Bhattacharyya, 2017; Galloway et al., 2019). These strategies often result in unsuccessful solutions and hinder meaningful learning. Hence, difficulties in mechanistic reasoning can be attributed to challenges in appropriately inferring implicit information and building causal relationships.
Recent studies of students constructing mechanistic explanations further underline the fact that they often struggle to make claims acknowledging causal relationships. Moreira et al. (2019a), for instance, revealed that fewer than half of the students in their study generated causal explanations; they typically relied on re-describing entities and properties without attempts to build causal links between them. Similar observations were made by Weinrich and Talanquer (2016), who found that students often relied on descriptive and relational accounts by considering properties or activities without associating them in a causal manner and without describing phenomena “[…] as sequential chains of events with causes and effects” (Weinrich and Talanquer, 2016, p. 400), which would correspond to a linear causal account.
As findings of prior research underline, the ability to build causal relationships can be regarded as crucial to explain, predict, and understand mechanistic processes. Accordingly, there is a need to promote the understanding of causality in teaching mechanisms. As instructors influence how students approach problems, and reason while constructing scientific explanations (e.g., Russ, 2018; Moreira et al., 2019b), the role of instructors as lynchpins has to be acknowledged. In this regard, little is known about instructors’ approaches when solving and explaining mechanistic problems and how they shape their explanations when targeting explanations for students. On the one hand, it is assumed that instructors, as experts in their domain, have great and varied content knowledge, which is organized in high-order knowledge structures. This enables them to activate resources in a way that reflects the task demands (diSessa and Sherin, 1998; Talanquer, 2018a). On the other hand, instructors, as teachers, are expected to possess pedagogical content knowledge (Shulman, 1986). They are required to appropriately transform knowledge of a domain for the teaching context.
Instructors as a lynchpin for constructing mechanistic explanations
The influence that instructors and teachers have on student learning and achievement is widely recognized (Hattie, 2008). Instructors’ impact on the development of students’ reasoning skills has been increasingly appreciated over recent years, e.g., with regard to teachers’ instructional strategies such as making the rationale of scientific explanations explicit or defining scientific explanation (e.g., Osborne et al., 2004; McNeill and Krajcik, 2008).The influence instructors have on students’ ability to construct and apply mechanistic explanations is highlighted in chemistry education as well. Talanquer (2018b), for example, stated “Chemistry instructors at all educational levels should foster students’ ability to spontaneously and productively apply chemical mechanisms in their sense-making and meaning-making activities regardless of the context” (Talanquer, 2018b, p. 1906).
Instructors do not only play a crucial role on what and how is being learned but how to assess learners’ abilities. As Stowe and Cooper (2019) state “[…] assessments should not simply ask for recall of facts but rather for use or knowledge to predict, explain, and/or model phenomena.” (Stowe and Cooper, 2019, p. 601).
An instructor's teaching of mechanisms and the influence on students’ reasoning was recently analyzed by Moreira and colleagues (2019b). In their study, teacher–student-interactions as well as modes of reasoning (Sevian and Talanquer, 2014) used by the students and the instructor were analyzed. In the observed lesson, the instructor emphasized simple causal explanations. After the lesson, a trend towards students constructing simple causal explanations from both directions (i.e., from both more simplistic modes of reasoning and more sophisticated modes of reasoning towards simple causal explanations) was observed. Hence, the instructor's modes of reasoning, i.e., her explanations, influenced students’ expressed reasoning (Moreira et al., 2019b). Therefore, the instructor can be regarded as a lynchpin for the construction of students’ mechanistic explanations.
As instructors are one of the key factors when learning Organic Chemistry at the university level, it is important to investigate how they verbalize and transform their content knowledge of mechanisms into mechanistic explanations in a teaching context, compared to their more typical professional context as domain experts. We use the framing construct, which acknowledges that a certain frame (i.e., interpretation of a situation) triggers particular behavior (Goffman, 1974; Tannen, 1993; MacLachlan and Reid, 1994), to explore how instructors make use of causes and effects when explaining mechanistic tasks in different frames, and how they combine their cause–effect rationales with further explanatory elements. By elucidating instructors’ mechanistic explanations, we aim to infer implications for teaching to promote causal accounts in mechanistic reasoning.
Theoretical framework
Causality as a core element of mechanistic reasoning
To characterize mechanistic reasoning in chemistry contexts, several authors suggested frameworks to analyze students’ explanations (e.g., Sevian and Talanquer, 2014; Becker et al., 2016; Cooper et al., 2016; Caspari et al., 2018). These frameworks offer valuable approaches to conceptualize mechanistic reasoning through consideration of mechanistic aspects such as explicit and implicit properties, statics and dynamics in a mechanism, consideration of cause–effect relations, and weighing of multiple variables. Commonalities are evident, such as that arguments or mechanistic explanations categorized at the most elaborated level go beyond a pure description of a mechanism, capture the idea of process-orientation, and include aspects of causality. The latter can be considered one of the core elements of mechanistic reasoning. Early approaches that characterize causality in mechanistic reasoning describe a causal mechanism as an explanation of “the process by which a cause brings about an effect” (Koslowski, 1996, p. 13). This is in line with the assumption of Russ et al. (2009) who claim that reasoning about causality itself through identifying causal factors is not sufficient to generate mechanistic explanations. The “how ‘X’ brings about ‘Y’” (Russ et al., 2009, p. 881) needs to be considered together with the process regarding the interacting causal factors, which requires the consideration of causes and effects.Although frameworks analyzing mechanistic reasoning in chemistry contexts include causality as a major focus, they have different approaches to complexity or elaborateness in students’ responses. Sevian and Talanquer (2014) developed a framework that considers four modes of reasoning, in which causal aspects are described in the higher reasoning modes, e.g., in the linear causal mode by inferring explicit and implicit properties, identifying organization, connections and interactions between entities, and building linear cause–effect relations (Sevian and Talanquer, 2014). Cooper and colleagues differentiate between the causal and mechanistic part of a causal mechanistic explanation (Becker et al., 2016; Cooper et al., 2016; Crandell et al., 2018). Their causal-mechanistic-reasoning framework allows to analyze whether students take into account what is happening, how it is happening, and why it is happening, by categorizing students’ utterances into descriptive (what), mechanistic (what and how), causal (what and why), and causal mechanistic answers (what, how, and why) (Cooper et al., 2016; Crandell et al., 2018). Becker et al. (2016) further analyzed student explanations in various dimensions: non-canonical or canonical, and non-electrostatic or electrostatic. They consider if an explanation includes references to the underlying mechanism (Becker et al., 2016). The framework developed by Caspari and colleagues (2018) characterized the structure of comparative mechanistic reasoning with different levels of complexity of cause–effect relations (Caspari et al., 2018).
Besides different approaches to complexity or elaborateness in students’ responses, the frameworks also differ in the graduations of causality in their coding schemes: they differ in how specific the description of causes and effects in student responses should be, and which grain size the explanation requires to be causal. In comparative mechanistic reasoning (Caspari et al., 2018), for an explanation to be considered causal a cause and an effect must always be verbalized on an electronic level. The causal-mechanistic-reasoning framework (Cooper et al., 2016) requires reasoning to be explained one scalar level below the phenomenon, whereas in the modes of reasoning the granularity of a causal answer is not defined. Hence, a causal answer could entail a cause–effect relation at varying levels of granularity, e.g., including reasoning about explicit structural features or implicit concepts (Sevian and Talanquer, 2014; Bodé et al., 2019).
Although the frameworks that characterize mechanistic reasoning in chemistry contexts seem to differ, they all consider causality of explanations as some answer to “the why” question, entailing a description of causal factors and their effects. Hence, based on the notion of causality, causes and effects can be regarded as a core element of mechanistic explanation.
Framing as a lens to elicit variations in resource activation
The resource-based framework (Hammer et al., 2005) defines knowledge and the ability to reason about something “as comprised of many fine-grained resources that may be activated or not in any particular context” (Hammer et al., 2005, p. 92). This context-dependency of resource activation can be attributed to individuals having different expectations of how to (inter)act (Tannen, 1993). To ascertain one's own expectations, one answers the question “What is it that's going on here?” (Goffman, 1974, p. 8) to frame a situation (Goffman, 1974; Tannen, 1993; MacLachlan and Reid, 1994). Hutchison and Hammer (2010) stated that “[…] framing shapes how people experience the situation, form expectations, and make choices” (Hutchison and Hammer, 2010, p. 509). Accordingly, an individual's framing of a context determines their resource activation and can thereby influence the engagement in situations differently. Hammer et al. (2005) exemplified this by noting that “[a] student may frame a physics problem as an opportunity for sense making, or an occasion for rote use of formulas” (Hammer et al., 2005, p. 98). Thus, the engagement in constructing explanations can be attributed to different framings of the instruction. In chemistry contexts, framing has been used to investigate students’ argumentative discourse in a laboratory activity in terms of triggering a certain frame with explicit instruction, e.g., by explicit deployment of task operators (predict-verify frame vs. observe-infer frame) (Petritis et al., 2020). Results showed that framing affects the engagement in argumentation as well as the sophistication and completeness of arguments, e.g., in terms of which chemical concepts were used and if connections were made between evidence and claims (Petritis et al., 2020). The induction of different frames due to different contextual features in prompts was further realized by Slominski et al. (2020) to analyze researchers’ or instructors’ (i.e., experts from biology, physics, and engineering departments) reasoning approaches (Slominski et al., 2020).In summary, one can state that individuals’ approaches or activation of resources in a situation depend on individuals’ framing of a situation, which is influenced by experience but also by explicit instruction (i.e., prompting). Comparing explanations of two differently framed prompts allows the effect of each prompt to be elicited and changes made when shifting from one frame to the other. Therefore, in this study we made use of framing to elicit instructors’ variations of explanatory approaches when solving mechanistic tasks in two differently prompted settings. By asking the instructors to explain for themselves/think-aloud we aimed at triggering an “expert frame” to elicit explanations, which they would give spontaneously to explain the mechanistic tasks for themselves without a teaching context. By changing the explicit instruction to a request to explain to students, we intended to trigger a “teaching frame” in which explanations are shaped to intentionally target fictitious students.
Research goals and questions
As the frameworks that characterize mechanistic reasoning were mainly used to analyze students’ reasoning, little is known about instructors’ approaches when explaining mechanistically. Instructors can be regarded as lynchpins to promote the building of scientific explanations in students, and thus, we wanted to investigate how they shape their mechanistic explanations in order to generate implications for teaching Organic Chemistry. We wanted further insight into instructors’ construction of cause–effect rationales. Specifically, we were interested in whether causes and effects are verbalized in relation to each other and whether different grain sizes are used in the rationales, i.e., whether implicit information is used in addition to explicit features. Not much is known about how instructors verbalize logical sequences to make a claim when explaining mechanistic tasks and how they make use of the given representations in different frames. Further, we were interested in how instructors combine their cause–effect rationale with additional explanatory elements in an explanation, especially when targeting their explanations at students. This focus is intended to broaden the analysis of how cause–effect rationales are embedded by the instructors. Hence, we set out to analyze how different frames – an expert and a teaching frame – influenced instructors’ explanatory approaches. The following research questions guided our investigation:RQ 1: How do instructors make use of causes and effects when explaining case comparisons in an expert frame and in a teaching frame?
RQ 2: How does the use of causes and effects in instructors’ mechanistic explanations change from an expert frame to a teaching frame?
RQ 3: How is the rationale combined with additional explanatory elements in the different frames?
Methods
Context and participants
The research was conducted at three German universities during the summer term of 2018. Ten instructors volunteered to participate in the study – nine male and one female (no other gender was mentioned). Seven of the ten instructors were leaders of Organic Chemistry research groups; the remaining three were affiliated to such research groups. All of the instructors were professors or lecturers teaching Organic Chemistry (beginner) courses following a traditional curriculum on a regular basis. Their teaching experience ranged from 11 to 30 years, with an average of 21 years. To protect the instructors’ identity, they are given pseudonyms in this publication, randomly chosen from a list of the most common English surnames.The research design followed ethical standards and all the participants were provided with information about their rights and the handling of the data (IRB approval is not a requirement at German universities). Participants were assured that they could opt out at any time during data collection. Furthermore, informed written consent was obtained prior to the data collection permitting the transcription of the interviews by the research team and/or a professional transcription service, data analysis conducted by the research team, and the use of the data for conferences and publications. The interviews were conducted in German and quotes were translated for this publication.
Instrument
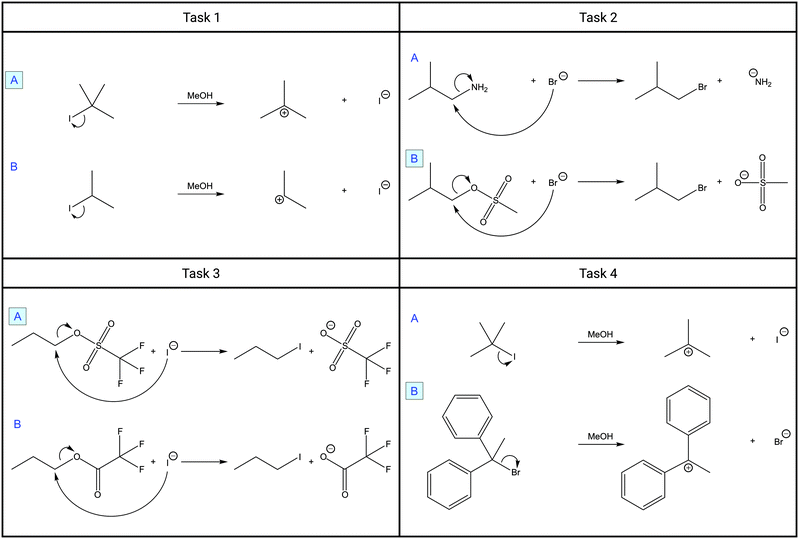
To elicit instructors’ reasoning, purposefully designed case comparisons were used as instruments (Graulich and Schween, 2018) (Fig. 1). These case comparison tasks have been shown to be a useful tool to elicit deeper reasoning and to weigh variables through comparison (Alfieri et al., 2013). Such tasks have been used in the context of Organic Chemistry. For example, Watts et al. (2021) analyzed resources activated by students when reasoning mechanistically. Bodé et al. (2019) investigated students’ ability to construct scientific arguments, and Caspari et al. (2018) analyzed the levels of complexity of relations that students constructed. Graulich and Bhattacharyya (2017) examined the categorization behavior of undergraduate students. Although these studies focused on students’ mechanistic reasoning, we used case comparisons to elicit instructors’ mechanistic explanations. The case comparisons used in this study address nucleophilic substitution reactions (Fig. 1), which are covered in beginner lectures of Organic Chemistry. The two cases, A and B, were purposefully contrasted in each of the tasks. They differed in one or two explicit differences, such as different leaving groups or backbones of substrates, which influenced the reaction rate. The instructors were asked to decide which reaction (of the two reactions shown) runs faster and to explain the reasons for their decision in two different frames, i.e., to explain spontaneously for themselves and to explain for a fictitious student. This type of question, i.e., to determine the faster reaction, is a typical mechanistic question proposed by Goodwin (2003) for Organic Chemistry. To draw a conclusion about which reaction is faster, one needs to consider which of the two reactions has a lower activation energy, which in turn is dependent on the potential energy of the transition states. Such conclusions about the transition states can be derived by inferring information from the structural formulas (Goodwin, 2003). Thereby one “[…] needs to form a bridge between structure and energy” (Goodwin, 2003, p. 144), i.e., structural and energetic accounts must be connected (Goodwin, 2003; Goodwin, 2008; Caspari et al., 2018).For example, in task 1 the explicit difference of the structural formulas lies in the number of alkyl residues, i.e., tertiary versus secondary alkyl substrate. When answering which reaction is faster, one would be asked to build a cause–effect rationale: when the leaving group departs, the higher number of alkyl substituents leads to more donation of electron density of σC–H-orbitals into the adjacent forming pz-orbital and thus a better weakening of the partially formed positive charge in the transition state of A compared to B. Based on these structural considerations about the cause (hyperconjugation of alkyl residues) and effect (charge weakening) in the transition state, one can infer a claim about the activation energy or which reaction proceeds faster, i.e., reaction A is being faster due to a lower potential energy of the transition state compared to B. Thus, an explanation to a case comparison can be expected to entail several causal relations, i.e., structural causes and resulting effects on structural changes during the reaction within the structural account. Furthermore, connecting links between the structural and energetic account can be expected to be established (Goodwin, 2003).
Tasks 1 and 4 both show the mechanistic step of a leaving group departure. In each case, a leaving group leaves an alkyl substrate in methanol under the formation of a carbocation and a negatively charged leaving group. Tasks 1 and 4 differ in the nature of the substrate, i.e., tertiary (2-iodo-2-methylpropane) vs. secondary alkyl substrate (2-iodopropane) and tertiary alkyl substrate (2-iodo-2-methylpropane) vs. tertiary phenyl substrate (1-bromo-1,1-diphenylethane). Additionally, in task 4, the cases differ in the nature of the leaving group (iodide vs. bromide); therefore, this task has two explicit differences, i.e., substrate backbones and leaving groups differ. Tasks 2 and 3 display a bimolecular nucleophilic substitution reaction (SN2), in which a halide attacks the substrate and simultaneously the leaving group departs. In both tasks, the cases differ in the nature of the leaving group, i.e., amide (azanide) vs. mesylate (methanesulfonate) and triflate (trifluoromethanesulfonate) vs. trifluoroacetate.
As linking structural causes and their effects in the structural account has been found to be challenging for students (Caspari et al., 2018), in this study, we focus on instructors’ structural account. Thereby we especially focus on how they make use of information inferred from the structural formulas and how they shape their explanations to make claims about energy.
Data collection
To collect the instructors’ explanation within two frames, we conducted semi-structured interviews within a larger project on solving case comparisons in Organic Chemistry (parts published in Rodemer et al., 2020). The instructors were interviewed individually and asked to solve case comparisons in two differently prompted scenarios. The case comparisons were displayed on a screen in the same order for each instructor. The instructors could decide for themselves when to move on to the next task. The interviews were recorded with an audio recording device.In the first scenario, the instructors were asked to first mentally solve the task, by deciding which reaction occurs faster, and to explain their choice afterwards. We call this scenario the expert frame. Under this frame, the given prompt was: “In the following you will see the same reactions as before (while mentally solving the task). Describe the reactions and the differences between A and B. Let us know which reaction runs faster and explain the reasons for your decision.” With this prompt, we wanted to trigger a spontaneous solving behavior to elicit how the instructors would solve a task for themselves. This prompt was specified during the interview by asking the instructors to explain for themselves or a colleague, i.e., to give an explanation for a knowledge level on an equal footing.
In the second scenario, the instructors were asked to explain the case comparison to a (fictitious) typical student who (hypothetically speaking) would participate in an Organic Chemistry I lecture of the instructor. We call this second scenario the teaching frame, in which we used the following prompt: “In the following you will see the same reactions as before (during spontaneously solving the task). Please explain which of the reactions runs faster to a student with low prior knowledge who takes part in your Organic Chemistry I course.” The instructors went through all of the case comparisons at first in the expert frame, and were then given the second prompt of the teaching frame. Only instructors’ direct responses to the tasks were included in the data analysis to capture their initial approach to explain the cases.
Data analysis
The interviews were transcribed verbatim and implemented into the coding software MAXQDA for qualitative content analysis (Saldaña, 2016). To further analyze the data, we followed four distinctive steps (Fig. 2).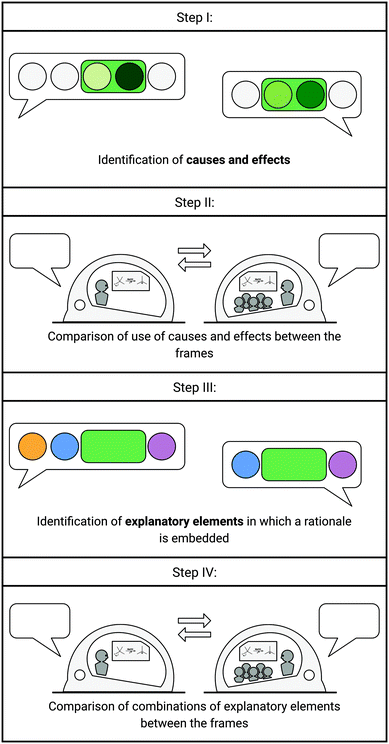 | ||
| Fig. 2 Steps of the analysis. Green circles represent causes and effects. The green rectangle represents the rationale. Colored circles represent explanatory elements. | ||
Step I: identification of causes and effects
In the first step of analysis, the cause–effect rationales entailed in the structural account were analyzed. When explaining mechanistic problems, the rationale involves the part of the explanation in which reasons for the why are expressed. In each rationale, we were looking for verbalized structural causes and their effects within instructors’ structural account. As each reaction step has an inherent cause and a resulting effect, one would expect that a rationale includes a cause, such as a property of an entity that leads to an effect in the process. A rationale, for example, could entail reasoning about the electron donating effect of the alkyl groups (cause) that weakens the positive charge of a resulting carbocation (effect). To identify which structural causes and resulting effects are used by instructors, we adapted aspects of the coding approach used for comparative mechanistic reasoning proposed by Caspari et al. (2018), as parts of their coding entailed the characterization of causes and effects. Caspari et al. (2018) differentiated between the use of explicit structural differences or implicit† structural properties used to verbalize a cause, and if either no effect, a non-electronic, or an electronic effect on a change in the process was described in a relation. Given our research focus on two differently framed situations, we used an adapted version of cause and effect categories to characterize instructors’ use of causes and effects as the core of a structural account without coding complexity of relations.Besides identifying causes and effects, we also identified if causes and effects are combined (i.e., complete cause–effect rationales) or if either causes or effects are mentioned separately (i.e., incomplete cause–effect rationales). The counting of the rationales was done according to their content. For example, if an explanation for task 2 entailed reasoning about mesomeric effects and, in addition, about the acid strength to support a claim, two rationales were counted.
We used three coding categories to characterize the grain size of causes: explicit, implicit, and implicit-electronic. The assignment of these coding categories was determined by the use or non-use of implicit properties (Table 1). An explicit cause was given when an explicit structural difference, such as explicit surface features of the structural formulas, was used as a cause. The code implicit cause was given when an implicit structural property or concept (i.e., which denominates a property) was used as a cause. The code implicit-electronic cause was applied when an implicit structural property was named and verbalized by referring to the electronic structure.
| Code | Code description | Example (referring to task 1) | |
|---|---|---|---|
| Cause | Explicit | Participant refers to what is explicitly visible/to parts of the representation without inferring an implicit structural property | “…because we have a tertiary carbon backbone as a substrate, instead of a secondary.” |
| Implicit | Participant uses an implicit structural property/names a concept | “…because of hyperconjugation.” | |
| Implicit-electronic | Participant refers to the implicit-electronic structure | “…because with the tertiary carbocation ion there is more hyperconjugation due to more electron-pushing groups” | |
| Effect | Non-electronic | Participant verbalizes an effect of a structural cause without elaborating on the electronic level | “…the product in A is more stable.” |
| Electronic | Participant verbalizes an effect of a structural cause with elaborating on the electronic level/charge distribution | “…which leads to the positive charge being weakened.” | |
The analysis further focused on the structural effects on the course of the reaction. We used two coding categories to capture the differences in how a resulting effect was described: non-electronic or electronic. The assignment of the codes was determined by its reference to the electronic level, i.e., when the effect of the implicit structural property was further explained using a reference to electron movement, overlap of orbitals, or charge distribution (Table 1). Instructors verbally expressed effects often as “being faster”. However, these statements were not considered as effects in our coding, as they cannot be considered as structural effects directly related to a structural cause. We considered these statements as a part of a claim about energy that the task required. To us it was of interest how the structural account, i.e., structural causes and their effects, was verbalized in an explanation to make a claim about energy.
Step II: comparison of use of causes and effects between frames
To compare the use of causes and effects between the frames, an exact test of symmetry (multinomial exact test) was performed. This test was chosen to determine the change or relatedness of frequency distributions of codes found in both frames, e.g., to determine whether implicit causes in the expert frame were verbalized in the same way in the teaching frame or to determine whether there is a shift towards the other cause code categories. Therefore, the data were grouped by participant, task, and content of the rationale (concept) to identify if rationales (i.e., causes and effects) appeared similarly, i.e., referring to the same content in both frames. If a code was found within this grouping in one frame and no matching code was found in the other frame, it was coded with “none” in the other frame. Moreover, pairwise post hoc comparisons were performed to further draw conclusions about significance.Step III: identification of explanatory elements in which a rationale is embedded
To characterize additional explanatory elements used by the instructors to accompany their rationales in mechanistic explanations, we derived three inductive categories. The additional explanatory elements included the description of the process, the verbalization of general statements and rules, and an outline of their problem-solving approach (Table 2). The code description of the process was chosen when the displayed reaction process was referred to, e.g., if an instructor verbalized the formation of a charge or the bond breaking and formation.| Code | Code description | Example (referring to task 1) | |
|---|---|---|---|
| Explanatory elements | Description of the process | Participant refers to the displayed reaction process | “We see two reactions here as competitive reactions in terms of speed. The reactions concern the leaving of identical iodide leaving groups from an alkyl; one is secondary and the other one tertiary…” |
| General statement | Participant verbalizes generalizations and rules | “The reactivity for nucleophilic substitution reactions increases in general, in the case of SN1, from primary, to secondary, to tertiary…” | |
| Approach | Participant verbalizes an approach to solve the task | “Now we have to look at the carbocations and weigh the stabilization of them against each other.” |
The code general statement included the general description of properties (e.g., of functional groups in general) and statements that are verbalized in a rule-based manner (e.g., talking about the reactivity of entities in general). As instructors often explicitly stated how they approached a task, the code approach was derived. This code includes verbalization of strategies or problem-solving steps, such as hints to solve the task, i.e., what to look for, what (and how) to compare, consider, weigh, or to distinguish something.
Step IV: comparison of combinations of explanatory elements between the frames
In a fourth step, we analyzed how the explanatory elements were used in mechanistic explanations by the ten instructors while solving the four tasks in the expert and teaching frame. As we were interested in how instructors embed their rationales, we took a closer look at the sequential use of explanatory elements in their explanations, i.e., how and if rationale, description of the process, general statement, and approach were used and combined in their explanations. Accordingly, we identified transitions made between the elements while explaining. Furthermore, we determined how often an explanatory element was used at the beginning of an explanation.With the coding of causes and effects and the coding of additional explanatory elements, a complete categorization of the transcripts was achieved. During the qualitative analysis, the coding schemes (i.e., causes and effects, explanatory elements) were constantly discussed between the authors to ensure that coding decisions faithfully represented the data. For inter-rater reliability, the first and the third author coded a random sample of 20% of the data independently. For the causes and effects coding rubric (Table 1), a Cohen's kappa coefficient of 0.88 was obtained and for the explanatory elements coding rubric (Table 2) a Cohen's kappa coefficient of 0.93 was calculated, indicating high agreement and reliability for both coding rubrics (Rädiker and Kuckartz, 2019).
Results and discussion
Here the mechanistic explanations of the instructors in two frames are compared and structured according to our three research questions. We focus on the comparison between an expert frame and a teaching frame to shed light on the potential flexibility of instructors when explaining mechanistic tasks. The use of framing illustrates that depending on the prompt, different expectations of an explanation can be triggered. In the following, we first focus on the characterization of structural causes and their effects. The second part reports how the use of causes and effects changed from one frame to the other. In the third part, explanatory elements used by the instructors are illustrated and it is shown how they were combined with the cause–effect rationales in both frames.RQ 1: How do instructors make use of causes and effects when explaining case comparisons in an expert frame and in a teaching frame?
Causes and effects used in both frames
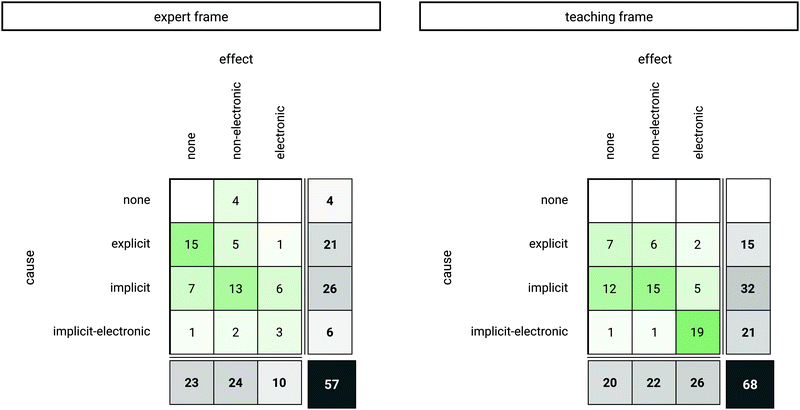
The distribution of causes and effects in instructors’ structural account reveals differences between the frames; hence, differently shaped rationales were stated in both frames (Fig. 3). When prompted to solve the four case comparisons in the expert frame (Fig. 3, left), the ten instructors verbalized a total of 57 rationales. The causes used in these rationales differed with regard to the extent the instructors used implicit information, such as implicit properties. In the expert frame, we identified 21 explicit causes, 26 implicit causes, and 6 implicit-electronic causes. Furthermore, 24 rationales entailed non-electronic effects and 10 rationales included electronic effects. As one would expect causes and effects to be verbalized relating to each other, we further analyzed if complete cause–effect rationales were formed. In 4 rationales, causes were missing as only an effect of a property was mentioned without referring to a cause of this effect, whereas in 23 rationales, an effect of a mentioned cause was not stated. Hence, nearly half of the rationales formed by the instructors in the expert frame were incomplete cause–effect rationales, stating either causes or effects and not both in relation, which corresponds to the prompt of the frame, which was to explain to themselves or a colleague. Beside instructors’ use of structural causes and effects in the expert frame, we wanted to elicit how the instructors’ cause–effect rationales are shaped when asked to target their explanation for a learner; if then, for instance, causes and effects are used more often in a fine-grained manner (e.g., considering the electronic level) and if the construction of complete cause–effect rationales increases. When solving case comparisons in the teaching frame, the instructors used a total of 68 rationales to explain their reasoning (Fig. 3, right). The identified causes of the rationales were 15 explicit causes, 32 implicit causes, and 21 implicit-electronic causes. The identified effects of the rationales in the teaching frame were 22 non-electronic effects and 26 electronic effects. As in the expert frame, causes and effects were not always mentioned together in a rationale. In contrast to the expert frame, each rationale entailed a cause. However, in the teaching frame, nearly a third of the instructors’ cause–effect rationales can be considered incomplete, as in 20 out of 68 rationales the effects of mentioned causes were missing.It is noticeable that 57 rationales were mentioned in the expert frame while 68 rationales were mentioned in the teaching frame (Fig. 3). Prompting the instructors to explain to a fictitious student increased the verbalization of cause–effect rationales, as compared to the expert frame showing the possibility of adaption of the instructors to different addressees.
Cause and effect combinations in the expert frame
The most common combinations of causes and effects in rationales found in the expert frame (Fig. 3, left) show that instructors often relied on structural features, i.e., explicit causes to back up their claim without mentioning an effect of a structural cause.Instructor Franklin's rationale of task 4 illustrates this finding. In his explanation, he described the type of reaction and the backbones of the substrates in a bullet point manner without further elaborating on the effect of a structural cause, e.g., a weakening of charge.
Instructor Franklin: SN1 substitution. Tertiary substrate versus twice benzylic tertiary substrate. B is faster by orders of magnitude. (Task 4, expert frame)
Instructor Franklin compared the representational features (tertiary and secondary substrate), without referring to implicit properties, which corresponds to an explicit cause. Instructor Franklin verbalized “being faster” as a claim about energy which he linked to an explicit structural difference. He did not state further effects of structural causes, in terms of how the explicit differences of the backbones influence the reaction process. Thus, no effect of a structural cause was verbalized and coded within his explanation.
Verbalizing causes without effects, as found in instructor Franklin's statement, was identified in 40% of the rationales in the expert frame. This finding does not seem surprising if one considers the framing. In the expert frame, the explanations of the instructors appear to be short or abbreviated, which can be interpreted as instructors taking into account the knowledge of their addressees, i.e., themselves or colleagues and thereby reflecting an expert's existing knowledge in Organic Chemistry mechanisms. Accordingly, instructors assume that addressees have the skills to infer implicit concepts and effects on the process from the comparison of structural features (e.g., tertiary vs. secondary backbone). As literature suggest, experts are able to recognize important features, being able to make use of their structured cognitive resources, i.e., their links to concepts (e.g., Bedard and Chi, 1992). The instructors, thus, stated explicit structural differences as the linkage to certain concepts occurred naturally to them, respectively their addresses.
Other rationales (56%) went beyond the consideration of structural differences and included implicit information, such as naming of implicit structural properties or naming of concepts. These implicit causes were used most frequently in combination with non-electronic effects (Fig. 3 left), as illustrated by the explanation of instructor Evans for task 1.
Instructor Evans: Yes, it's about splitting off from a leaving group and then a secondary and tertiary substrate form. In addition, the tertiary will almost certainly go better because it is better stabilized through inductive effects. (Task 1, expert frame)
In his statement, instructor Evans acknowledged the explicit difference of the backbones (tertiary and secondary substrate) and further mentioned the inductive effects of the tertiary alkane as an implicit property (implicit cause). He also mentioned the resulting increase in stabilization, which was coded as a non-electronic effect. Instructor Evans’s explanation could not be categorized as the most elaborated category of a cause, as a reference to the level of electrons was missing, e.g., the positive inductive effects resulting from electron donation of the methyl groups, which would be categorized as an implicit-electronic cause. Implicit-electronic causes were used the least by the instructors in the expert frame.
Looking at use of effects in the expert frame, it was found that nearly half (24 out of 57) of the rationales entailed non-electronic effects, e.g., see instructor Evans’s rationale for task 1. In such rationales, effects were verbalized without a reference to the level of electrons, i.e., often stating a stabilization without explaining how an implicit property electronically influences the course of a reaction. However, almost a fifth of the rationales entailed effects that were electronic in nature, e.g., when instructors mentioned a weakening or distribution of a positive charge.
In the expert frame in general, the instructors’ explanations were characterized by stating comparisons based on explicit structural features and naming of implicit structural properties to back up their claim without referring to the electronic level or inferring effects of structural causes. This shortened use of causes and effects in the expert frame resulted in nearly half of the rationales being incomplete, i.e., in 23 out of the 57 rationales an effect of a cause was missing and in 4 out of the 57 rationales a cause of an effect was not mentioned. One could easily interpret these findings as a lack of sophistication, as a focus on explicit features mirrors the findings from studies based on student interviews. While students often focus on the surface of the representation and overlook implicit structural features (Weinrich and Talanquer, 2016; Graulich et al., 2019; Moreira et al., 2019a), the instructors’ behavior of verbalizing explicit structural features and implicit information (while neglecting the electronic level) to back up a claim could rather be linked to their expert-like behavior. Literature suggests that Organic Chemistry experts, like the interviewed instructors, are able to engage in multivariate thinking due to internalized concepts and to conceptualize processes at a different representational levels (Bhattacharyya, 2008). Thus, the fact that the instructors often verbalized explicit features in the expert frame does not imply that they did not use their internalized concepts in their reasoning and do not have access to these resources. The verbalization of their reasoning was influenced by the prompt of the expert frame; accordingly, the framing may have led them to make use of their routinized expert response behavior that they use within their professional context and anticipate an expert's existing knowledge in Organic Chemistry. This behavior could be interpreted as not fully elaborating the “highest resolution” of reasoning, e.g., by not mentioning cause–effect relations with a depth in grain size, or solely naming causes without effects. Talanquer (2013) commented on chemists’ ability to make effective decisions, noting that they “rely on the thoughtful application of a variety of empirical generalizations used as heuristics or rules-of-thumb to make quick decisions” (Talanquer, 2013, p. 836). With this expert-like manner, they might have been able to go efficiently to the next task by quickly generating a claim.
The fact that the instructors did not verbalize their mechanistic consideration in detail in the expert frame, i.e., on the grain size of electrons, could result from the instructors not considering this level of resolution to be appropriate or necessary in this context. Similarly, Weinrich and Talanquer (2016) concluded from their work on mechanistic reasoning: “that more advanced knowledge may lead individuals to build less sophisticated but more targeted and productive explanations” (Weinrich and Talanquer, 2016, p. 403). Since the framing of the prompt led the instructors to solve the task spontaneously, without further requirements for how elaborated the explanation should be, the instructors may have considered their explanations to be productive for this given context of explaining for themselves. Thus, verbalizing less detailed knowledge and using more short-cuts seemed reasonable to the instructors in this frame.
Cause and effect combinations in the teaching frame
As shown in Fig. 3 (right) for the teaching frame, the most frequent cause–effect rationale was the combination of implicit-electronic causes in combination with electronic effects. This finding can be illustrated by the excerpt of instructor Davis explaining task 4 in the teaching frame. In his rationale, instructor Davis recognized the explicit difference in the different backbones of the products (tert-butyl carbocation vs. diphenylethyl carbocation). He inferred implicit information and thereby considered electronic properties. He described how the p-orbitals can overlap and donate electron density to the empty p-orbital of the cation (implicit-electronic cause). He used this implicit-electronic property as a cause for an electronic effect by verbalizing how this leads to a delocalization of the positive charge and sharing of electron deficiency.Instructor Davis: […] The second reaction is faster. This is due to the stability of the generated cation. The biphenyl substituted cation is more stable than the tertiary butyl cation, which we see in reaction A. And this is due to the two phenyl substituents. They contribute to the stability of this cation by donating electron density of the p-orbitals. The empty p-orbital of this cation [in B] can overlap with the p-orbitals of both phenyl rings and thus… positive charge is delocalized over 13 C-centers [carbon atoms]. Then each of them [the carbon atoms] has to carry only a 13th of the electron deficit load. Yes, this is what makes this cation so stable. […] (Task 4, teaching frame, excerpt)
In his rationale, instructor Davis not only named concepts and implicit properties, but described what this property means on a submicroscopic level, e.g., the ability of phenyl substituents to donate electron density of the p-orbitals. Furthermore, concerning his verbalization of the effect of such a cause, he referred to delocalization and weakening of charge (electronic effect), which accounts for the stability of the diphenylethyl cation. As he elaborated on how the stability comes about by referring to the submicroscopic level, instructor Davis gave the term “stable” a greater depth of meaning. As his statement exemplifies, in rationales entailing the combination of implicit-electronic causes and electronic effects, concept designations and implicit properties were not only used as buzzwords, but were actually explained in an electronic and process-oriented way.
The second most frequent combination of causes and effects in instructors’ rationales in the teaching frame was the use of implicit causes in combination with non-electronic effects. The excerpt of instructor Miller, as he explained task 1, exemplifies how implicit causes were used, often in terms of mentioning concepts, and in combination with non-electronic effects. As instructor Miller mentioned the inductive effect of the methyl groups, he referred to the implicit properties of an entity and thus the cause in his rationale was categorized as implicit. Furthermore, he mentioned how this structural cause leads to an increase in stability of the carbocation (non-electronic effect).
Instructor Miller: […] in one case it's a tertiary carbocation that forms, in the upper example, in the lower example it's a secondary carbocation. So, that's the difference. So the question of which of the two carbocations forms faster is related to stability. The more stable the carbocation, the faster it will form. And on the grounds of the +I effect of the methyl groups, which is stronger in the upper case than in the lower case, because we have three methyl groups at the top, which have a +I effect, and only two methyl groups with a +I effect at the bottom, therefore the upper carbocation is more stable and will also form faster. (Task 1, teaching frame)
Instructor Miller named the positive inductive effects in his rationale but did not further elaborate this concept at an electronic level, i.e., describing the electron donation of the methyl groups. He then related this cause to an increased stability without mentioning what this means on the electronic level. This excerpt exemplifies a major finding, as nearly half of the rationales in the teaching frame entailed implicit causes, without referring to electronic properties and interactions on the electronic level.
The combination of implicit causes without an effect were found as often as the latter combination. This can be showcased by the quote of instructor Evans, while solving task 1 in the teaching frame. In his statement, he recognized the explicit difference of the different backbones of the substrates (secondary vs. tertiary), and referred to the inductive effect of the methyl groups (implicit cause), without further mentioning how these inductive effects influence the course of the reaction.
Instructor Evans: […] Due to the inductive effects by the three methyl groups, which have a stronger impact than in the lower case with the two methyl groups, we have a faster reaction progression in the case of a tertiary system. […] (Task 1, teaching frame)
Instructor Evans relied on naming inductive effects to back up his claim, without further explicating the effect, thus building an incomplete cause–effect rationale. Instructor Evans, among others, verbalized a qualitative statement of the inductive effects by stating that they have “a stronger impact”. He did not further elaborate on this statement or provided an electronic effect to his structural cause, thus, missing to form a complete cause–effect relation.
Summarizing the instructors’ explanations in the teaching frame, it is apparent that they often interpreted the prompt to explain to a student in a way that meant they elaborated more of what they might have not explicitly verbalized in the expert frame, which tributes to an increased number of rationales that could be identified in the teaching frame. Further, the instructors most frequently established causal relationships by verbalizing implicit-electronic causes with electronic effects. This increased depth in grain size in instructors’ cause–effect rationales could be interpreted as an intention of instructors to provide a more detailed explanation and to support students to understand the mechanistic processes. As Ramsey (2008) states: “We have to be told where and why the electrons are moving in order for the mechanism to be explanatory.” (Ramsey, 2008, p. 974). However, not all rationales stated in the teaching frame entailed an electronically justified cause–effect rationale, which would correspond to a high complexity of relation (Caspari et al., 2018).
The findings show how instructors made use of causes and effect in the different frames. Further, the question arises where a change in elaboration of causes and effects has taken place.
RQ 2: How does the use of causes and effects in instructors’ mechanistic explanations change from an expert frame to a teaching frame?
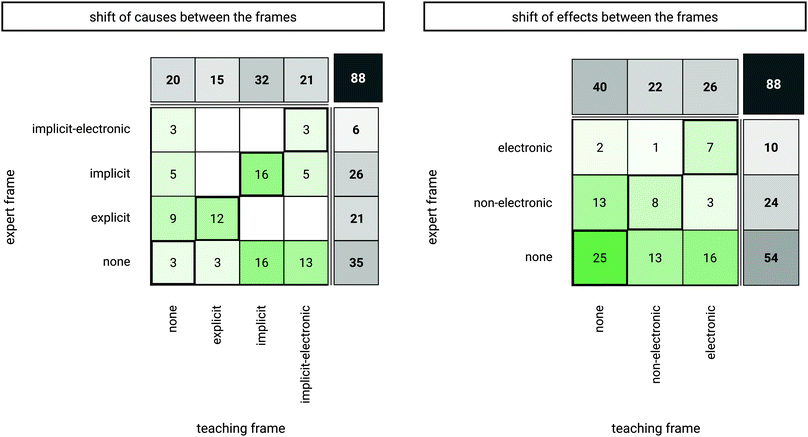
We further analyzed how the frequency distributions of the cause and effect codes from both frames relate, in terms of which codes relating to the same concept, and respectively content, can be identified in both frames and which codes differ. With regard to the 57 rationales verbalized in the expert frame and the 68 rationales verbalized in the teaching frame, only 37 rationales that were related to a particular concept appeared in both frames. Consequently, 51 rationales referring to particular concepts appeared in only one of the two frames (20 rationales in the expert frame and 31 rationales in the teaching frame). Rationales (i.e., causes and effects) that were mentioned in only one frame were coded with “none” in the other frame, resulting in a total of 88 cause and effect codes in both frames. Based on these codes, we analyzed how each coded category appeared in both frames, e.g., if all implicit codes of the expert frame occurred the same way (also as implicit codes relating to the same concept in the teaching frame) or if they were not mentioned, or shifted in their elaborateness in the teaching frame (Fig. 4).
Overall, the findings show a tendency towards an increase in grain size in both cause and effect codes, an increase in the use of causes and effects, and multiple rationales being verbalized in the teaching frame with different conceptual approaches.
In more detail, based on an exact test of symmetry (multinomial exact test), the cross table of cause codes (Fig. 4, left) can be considered non-symmetric (p < 0.001), indicating a significant change in the distribution of codes from the expert to the teaching frame with a large effect (Cohen's g = 0.29, (Cohen, 1988)). With regard to the absolute values, more implicit as well as implicit-electronic causes were mentioned in the teaching frame compared to the expert frame (Fig. 4, left). More precisely, it was found that 34 cause codes occurred equally in both frames (Fig. 4, left, sum of counters in diagonal tiles). In the teaching frame, 32 mentions of causes were found that were not mentioned in the expert frame (Fig. 4, left, bottom row; 3 counters at none/explicit, 16 counters at none/implicit, 13 counters at none/implicit-electronic), which refers to an increase in the use of causes in the teaching frame. Furthermore, as the cross table shows, 5 of the implicit causes used in the expert frame were verbalized as implicit-electronic causes in the teaching frame, hence, their elaborateness increased.
The cross table of effect codes (Fig. 4, right) is also non-symmetric (p < 0.001), again indicating a significant change in the distribution of codes from the expert to the teaching frame with a medium effect (Cohen's g = 0.17). Pairwise post hoc comparisons indicate that this change can be attributed predominantly to the increased use of electronic effects in the teaching frame (p < 0.01, Cohen's g = 0.39). In detail, 40 effect codes were verbalized likewise in both frames (Fig. 4, right, sum of counters in diagonal tiles). In the teaching frame, 29 mentions of effects were found in the teaching frame that were not mentioned in the expert frame (Fig. 4, right, bottom row, 13 counters at none/non-electronic; 16 counters at none/electronic), which accounts for an increase in the use of effects in the teaching frame. Furthermore, while 1 electronic effect description in the expert frame occurred as a non-electronic effect in the teaching frame, 3 non-electronic effects occurred in the category of electronic effects in the teaching frame, hence, elaborateness increased.
The exact test of symmetry underlines the fact that there is a shift towards higher elaborated causes in the teaching frame (Fig. 4, left). Whereas, for the use of effects (Fig. 4, right), the number 25 in the lower left corner at none/none indicates that many rationales of the instructors entailed structural causes without a reference to effects of the reaction in the expert frame as well as in the teaching frame. Thus, the results for the effect codes indicate, however, a shift towards naming more effects in the teaching frame, especially electronic effects.
Robinson's explanation when solving task 2 in the expert and teaching frames clearly exemplifies a frame shift. Comparing his explanation in both frames shows differences in the usage and grain size of causes and effects. When solving task 2 in the expert frame, instructor Robinson states the explicit difference between the reactions, i.e., the differing leaving groups: amide and mesylate. To back up his claim for reaction B being faster, instructor Robinson used a non-electronic effect, talking about the stabilization of the leaving group, without mentioning an implicit property as a cause for this stabilization or describing how the stabilization takes place.
Instructor Robinson: So the lower one is faster because here we have two different leaving groups. One is an amine and then we have the amide that leaves. In the other one, it is the mesylate that leaves and in the mesylate, of course… well the leaving group is much better stabilized and the amide is just not [stabilized]. (Task 2, expert frame)
While solving task 2 in the teaching frame, instructor Robinson backed up his claim differently compared to the expert frame. In the first part of his explanation, he argued about what makes mesylate the more stabilized and therefore more favorable leaving group. He mentioned the mesomeric effect of mesylate, having electron pairs being able to “flip over” (implicit-electronic cause), and described how this results in the charge being distributed over the oxygen atoms (electronic effect).
Instructor Robinson: Right. If we compare the reactions here, the difference is the leaving group. We simply have an amine at the top [reaction A] and mesylate at the bottom [reaction B]. This means that even if both reactions run by a SN2 mechanism, we compare the different quality of the leaving group and thus we see how stabilized the leaving group is when it is split off. This means that we have the amide at the top [reaction A], accordingly, NH2minus, while we have the mesylate at the bottom [reaction B]. In the lower case, the negative charge can be distributed over all three oxygen atoms by simply flipping over the electron pairs. That means there is a strong mesomeric effect present and as you know, charged species are… the more you can distribute their charge… or the more atoms you can distribute it to, the more favorable. […] (Task 2, teaching frame, part 1 of his explanation)
In contrast to the expert frame, instructor Robinson verbalized implicit-electronic properties for mesylate being the more favorable leaving group and described an effect of that structural cause including interactions on the electronic level. The frame shift, thus, resulted in a more elaborated cause–effect rationale for task 2. If we now look at the rest of his explanation when solving task 2 in the teaching frame, instructor Robinson added a conceptually different rationale to his first rationale by mentioning the acid/base properties of the leaving groups (implicit cause).
Instructor Robinson: […] This can also be directly correlated with the pKavalue. That means, the stronger the corresponding acid to the anion, the better the anion can be considered as leaving group. And if you now look at the pKavalue of the amine and compare it with the corresponding sulfonic acid… then you would immediately see that the amine is much less acidic, because amines react as a base after all and from this you can deduce that mesylate is the better leaving group and should be faster in B. (Task 2, teaching frame, part 2 of his explanation)
In his further elaboration, instructor Robinson related the aforementioned cause–effect rationale and leaving group ability to the acid/base-strength. In this part of his explanation, he used implicit properties (pKa values as a measure of acid strength, implicit cause). The differences in the completeness of the cause–effect rationales – as well as differences in the grain size of causes and effects – become apparent when comparing the frame shift. Frequent naming of implicit-electronic causes with electronic effects and implicit causes without resulting effects in the teaching frame were contrasted with the expert frame where explicit causes were often mentioned without an effect or with non-electronic effects.
Furthermore, instructor Robinson chose two different conceptual approaches in the teaching frame to underpin his claim. This increase in use of causes and effects in the teaching frame in contrast to the expert frame clearly shows that some instructors build multiple cause–effect rationales (cf.Table 3 in the Appendix) with additional conceptual approaches due to the frame shift.
In tasks in which more than one explicit structural feature differed (task 4) and the leaving group ability was discussed (tasks 2, 3, 4) instructors tended to use multiple rationales, i.e., multiple conceptual approaches to underpin their claim. For example, like instructor Robinson's statement shows, the claim about energy was often asserted by two conceptual approaches: (1) a leaving group's capacity to distribute charge via resonance and (2) a statement about acidity. Noticeable is how the instructors were engaged in connecting their rationales. As instructors Robinson's explanation shows, he connects his two rationales by stating the mesomeric effect (cause) and the charge distribution in one anion (effect) “can also be directly correlated with the pKa value” and relates to the corresponding acidity to the anion. By combining his rationales, he provided reasoning how acid/base-strength is related to mesomeric effects by referring to the capacity of charge distribution. He did not only provide two conceptual approaches but also showed how these are connected via the electronic structure, e.g., capacity to accommodate charge, which is often found when instructors used multiple rationales.
Another example of an instructor's frame shift, which is comparable to instructor Robinson's explanation, can be characterized by looking at instructor Carter's explanations of task 3. In his explanation in the expert frame, he builds a cause–effect rationale stating that the difference in acidity (implicit cause) leads to a better stabilization of the trifluoromethanesulfonate ion (non-electronic effect).
Instructor Carter: In both cases, the reactions are SN2 reactions, without any doubts. The good nucleophile substitutes two leaving groups. However, the lower leaving group trifluoroacetate is still a bit worse than the upper leaving group, the trifluorosulfonate [trifluoromethanesulfonate]. This is because the trifluorosulfonate ion [trifluoromethanesulfonate] is even better stabilized than the trifluoroacetate ion and is therefore the weaker base. Conversely, trifluoroacetic acid is a stronger acid than trifluoromethanesulfonic acid, so the corresponding acid of the upper leaving group, is the stronger acid than trifluoroacetic acid. (Task 3, expert frame)
In his explanation in the expert frame, he uses one conceptual approach (acid/base properties) (cf.Table 3). In the teaching frame, he adds another rationale by referring to resonance and the formation of contributing structures leading to a stabilization of the negatively charged leaving group. In this way, he provides more meaning to the used notion of “stability” relating it to the possibility of charge distribution.
Instructor Carter: The upper reaction is faster because the forming negatively charged leaving group is better stabilized via resonance than in the lower case. In the upper case, you can formulate contributing structures [resonance structures]. In the lower case, there would only be two. And the possibility to formulate contributing structures which actually goes hand in hand, generally speaking, with stability of entities. One can check this by looking up the acidity of the corresponding acids. The corresponding acid in the upper case would be trifluoromethanesulfonic acid. And this is a stronger acid than trifluoroacetic acid. So, because of the fundamentally lower basicity and higher stability of the upper leaving group, the upper reaction is faster. (Task 3, teaching frame)
By referring to resonance and acid properties, he, hence, uses two conceptual approaches in the teaching frame (cf.Table 3).
As a counterexample, we can consider instructor Davis, who only states one rationale in the teaching frame and names acid/base properties of the entities, which was coded as implicit cause.
Instructor Davis: […] The ability to be a good leaving group correlates with acid strength. Ammonia is a base, so we know, a very bad acid. Methanesulfonic acid, as the name implies, is a real acid, which means the stronger acid is methanesulfonic acid, so that is the better leaving group. Which meands reaction 2, B, is got to go much faster. (Task 2, teaching frame)
In his statement instructor Davis relates the leaving group ability to the acid/base strength (implicit cause). Compared to instructor Robinson, he did not relate the leaving group ability to charge distribution, thus, he did not state an effect within his rationale and further only chose one conceptual approach, neglecting, e.g., the mesomeric effects occurring in mesylate.
On the one hand, thus, one finds more elaborated cause–effect rationales in the teaching frame compared to the expert frame. This result shows instructors’ adaptability and underlines the fact that they are able to adjust their explanation depending on the framing. Considering causes and effects on a deeper grain size further highlights the instructors’ tendency to step away from descriptive explanations, as implicit properties, the electronic structure of entities and its change in the course of the process are often included in their rationales in the teaching frame.
On the other hand, one finds an increase in use of causes and effects, which accounts for instructors often building multiple cause–effect rationales in the teaching frame. The latter appears to be a promising observation when thinking about providing students different conceptual approaches to a problem (cf.Table 3 in the Appendix). Results of a study looking at students’ reasoning in mechanistic tasks by Bodé and colleagues (2019), show that incorrect claims were often stated when few connections among concepts were established (Bodé et al., 2019). This suggests that strengthening links among concepts might be supported by instructors verbalizing multiple conceptual approaches.
However, in the teaching frame, even though instructors targeted their explanations for learners, in one third of their rationales causal relationships in terms of verbalizing structural causes in combination with their effects were neglected. Mentioning such causal relationships can be considered crucial for students in teaching contexts of mechanistic reasoning (Machamer et al., 2000; Cooper, 2015; Crandell et al., 2018). At the same time, the instructors may have implicitly considered more than they explicitly expressed when building their explanation and verbalizing the grain size of causes and effects. Bodé and colleagues (2019) stated in this regard that “an expert may have the ability to continue expanding their explanation or argument to ever decreasing levels of granularity, the actual granularity of their scientific explanation or argument, even a multicomponent, causal one, will depend on their context and purpose” (Bodé et al., 2019, p. 1072). This may also account for the findings in our study.
This aspect of flexibly adjusting an explanation was as well brought up by instructor Smith in a side comment, after the expert frame:
Instructor Smith: If you ask about the speed of the reaction, then you would have to work with the activation energies and I cannot just say ‘the more stable cation is formed, therefore it reacts faster’. It depends on which level of explanation I adapt. So, in school, that is certainly how you would explain it. Um. In an introductory organic chemistry course, I would work with energy diagrams to correlate the transition state with the product, in this case [referring to task 1] the cation, according to the Hammond postulate for this exothermic step.
He expressed his awareness that his brief statement given in the expert frame needs to be adapted to the respective level of knowledge of the learner by stating more implicit information, e.g., on the reaction process. He then discussed the energetic process of the reaction in relation to structural properties, when providing his explanations in the teaching frame. Beside the trend of making more implicit information explicit due to the frame shift in our study, there is still great variety in instructors’ use of cause–effect rationales in these two frames over all tasks. The latter points to the necessity of supporting instructors to become aware of how they are forming explanations and when to purposefully change the explanation in different contexts. Whereas some instructors decided to use more implicit information with regard to the frame shift, others did not change the way they verbalized their cause–effect rationales between the frames. Instructor Jones, for example, does not elaborate on the cause he uses in the expert frame explaining task 3 in the teaching frame. In both frames, he refers to the quality of the leaving groups to make a claim, which was coded as an explicit cause.
Instructor Jones: Here the leaving group differs. Once a trifluoroacetate in case B. And in case A a trifluoromethanesulfonate. Both are SN2 reactions. The trifluoromethanesulfonate is the better leaving group than the trifluoroacetate. And therefore, A will proceed faster than B. (Task 3, expert frame)
In the teaching frame, he uses the same cause as in the expert frame.
Instructor Jones: Here we have a case of a primary substrate with two different leaving groups, the nucleophile is the same, the leaving group is once a sulfonate and once a carboxylate, the residues with the CF3-group are also still the same, which means we have to distinguish between sulfonate and carboxylate. Sulfonate is the clearly more stable anion and the better leaving group and that means that case A is faster than case B. (Task 3, teaching frame)
Compared to the expert frame, he added a non-electronic effect as he claims the trifluoromethanesulfonate is being more stable. Even though he does not use more implicit properties as a cause in the teaching frame compared to the expert frame, he forms a complete cause–effect rationale, describes how one should approach the task (“we have to distinguish”) and adapts his language to the frame. In comparison to the expert frame, he no longer uses a sophisticated nomenclature of the leaving groups, but the name of the functional groups, thus, building a bridge towards generalization or possibly simplification for a fictitious student. As the example of instructor Jones illustrates, it is not only worth looking at causes and effects, but also further aspects of the given explanation that might change through a frame shift, which are possibly influencing learning.
RQ 3: How is the rationale combined with additional explanatory elements in the different frames?
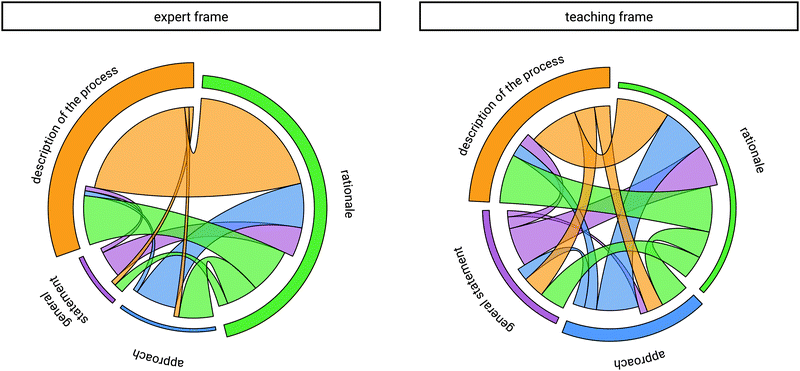
Explanatory elements identified in both frames
After discussing instructors’ use of causes and effects, we analyzed how cause–effect rationales are embedded in the explanatory context when the framing changes. The inductive content analysis of instructors’ explanations revealed three additional categories of explanatory elements, besides the rationale, i.e., description of the process, general statement, and approach (Fig. 5).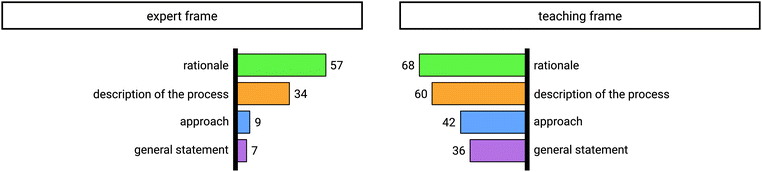 | ||
| Fig. 5 Identified explanatory elements in instructors’ explanations for 40 tasks in the expert frame (left) and for 40 tasks in the teaching frame (right). | ||
In the expert frame 57 rationales were verbalized by the instructors and in the teaching frame 68 rationales were mentioned. The identified explanatory element of description of the process was categorized 34 times in the expert frame and 60 times in the teaching frame. Furthermore, the category of approach was found 9 times in the expert frame and 42 times in the teaching frame. General statements were categorized 7 times in the expert frame, whereas they were mentioned 36 times in the teaching frame.
Comparing the use of explanatory elements, an increase from the expert frame to the teaching frame is apparent. This does not seem surprising if one assumes that the instructors adapted their initial explanation due to shifting towards a teaching context. When explaining in the teaching frame they tended to include additional elements in their explanation, influenced by what they thought might be beneficial in a learning context.
Embedding of rationales with explanatory elements in both frames
To further analyze how instructors embed their rationales, we took a closer look at the combination with the explanatory elements and analyzed their sequential use (Fig. 6).In the expert frame, the rationale was often accompanied by a description of the process (Fig. 6, left, width of yellow and green chords transitioning between description of the process and rationale). Thus, the beginning of an explanation often started with the description of the process (Fig. 6, left, height of the yellow node) followed by the verbalization of the rationale. This common occurrence is illustrated with instructor Lewis's explanation, as he explained task 1 in the expert frame. He started his explanation with a short description of the mechanistic step of leaving group departure, i.e., which was categorized as description of the process.
Instructor Lewis: We have two reactions, in which an iodide leaves an alkane, forming a positively charged carbon atom. […] (Task 1, expert frame, description of the process)
After the description of the leaving group departure, he verbalized his rationale, in which he stated an explicit cause (i.e., tertiary vs. secondary carbocation) and a non-electronic effect (i.e., better stability).
Instructor Lewis: […] The difference is that it is a secondary and a tertiary [carbocation]. In the upper case, the positive charge is significantly better stabilized than in the lower one, the rest is the same. The upper one should be formed faster because of the better stability. (Task 1, expert frame, rationale)
Compared to the frequent combination of description of the process together with the rationales in the expert frame, the categories approach and general statement were combined less frequently with the rationales (Fig. 6, left, widths of the blue and purple chords between the respective explanatory elements and the rationale). As the expert frame was meant to explain to colleagues or themselves, it is not surprising that the instructors tend to connect their rationale only with few explanatory elements. These findings again indicate a pragmatic approach in the expert frame, as the instructors focused on the description of what was going on and stating their reason, when spontaneously solving a case comparison, rather than mentioning their problem-solving approach or general rules and statements.
Similar to the expert frame, the rationale in the teaching frame was often accompanied by a description of the process (Fig. 6, right, width of the yellow and green chords between the description of the process and rationale). Further, the description of the process was also often the beginning of an explanation (Fig. 6, right, height of the yellow node). When explaining to a fictitious student, rationales were typically accompanied by general statements and problem-solving approaches (Fig. 6, right, blue and purple chords between the respective explanatory elements).
Instructor Lewis's explanation of task 1 in the teaching frame illustrates this usage and frequent sequence of explanatory elements in explanations. In his explanation, he started with a description of the process as he referred to the departure of the leaving group and the formation of a carbocation.
Instructor Lewis: It is about the separation of the identical iodide leaving group from an alkyl, one is secondary, one is tertiary. An energetically much higher carbocation is formed. This means that the carbocation is then an intermediate stage, an intermediate product. […] (Task 1, teaching frame, description of the process)
After describing the leaving group departure, he stated an approach, by describing what one needs to consider first.
Instructor Lewis: […] And now let us first consider the energy of these two intermediates: […] (Task 1, teaching frame, approach)
After he expressed where the focus must be placed first to solve this task, he went on to add a general statement about how stabilization of compounds is achieved in general via electron donation.
Instructor Lewis: […] It is an electron-deficient compound. In general, stabilization is achieved by residues that can supply electrons in some way; be it through mesomeric effects or inductive effects. […] (Task 1, teaching frame, general statement)
He stated his rationale by considering inductive effects of the methyl groups (implicit cause) being able to stabilize the positive charge (non-electronic effect).
Instructor Lewis: […] We do not have mesomeric effects, we only have inductive effects through the methyl groups. Above we have three methyl groups, which could stabilize this electron deficiency compound, this positive charge, below we have only two. […] (Task 1, teaching frame, rationale)
Instructor Lewis's explanation exemplifies a common sequence of explanatory elements in the teaching frame, in which first the reaction process was introduced, then an approach to solve the task was stated and a general statement was inferred before giving the rationale. As chords (and their widths) between the explanatory elements as well as the height of the outermost ring (node) in Fig. 6 (right) indicate, additional sequences and combinations of explanatory elements could be identified and, thus, the rationales were embedded differently.
Beside the description of the process, the problem-solving approach was frequently used at the beginning of an explanation (Fig. 6, right, height of the blue node). Instructor Miller, for instance, used an approach at the beginning of his explanation for task 2 in the teaching frame.
Instructor Miller: Well, to determine which reaction is faster, we have to rely on the quality of the leaving group. […] (Task 2, teaching frame, approach)
In his statement, instructor Miller started with an approach of how one can tackle the question to answer which of the two reactions proceeds faster. Then, he made a general statement by correlating the stability of a leaving group with the stabilization of the negative charge.
Instructor Miller: […] And it is like this: the more stable a leaving group, the better the negative charge can be stabilized. […] (Task 2, teaching frame, general statement)
He then went on by stating an approach of how one can find out which of the leaving group is the better stabilized one, i.e., looking at the structure.
Instructor Miller: […] And you can tell this either by looking at the structure and recognizing that […] (Task 2, teaching frame, approach)
Afterwards, he stated his rationale by acknowledging the (de)localization of charge. Compared to instructor Lewis in the teaching frame, instructor Miller did not start his statement with a description of the process but with an approach to the problem. He guided a fictitious student through the task by explicitly explaining a strategy to solve the tasks, using forms of direct address several times (“we”/”you”).
As shown by the explanations of instructors Lewis and Miller in the teaching frame, instructors used general statements and explained their approach frequently. Through general statements and rules, instructors might have intended to structure their explanation by first verbalizing something general and afterwards giving a specific example of how the general statement referred to the given task, e.g., “The reactivity for SN1 reactions increases from primary to secondary to tertiary alkyl groups. In this case we have a tertiary butyl cation versus…”. However, it is known that students tend to overgeneralize general statements and rules, e.g., overgeneralizing definitions of reactions when classifying reactions (Stains and Talanquer, 2008) or when applying the octet rule (e.g., Taber, 1998; Luxford and Bretz, 2014). The overemphasis of general statements in teaching situations may promote the reliance of rule-based reasoning, focusing on a single variable, in cases in which multiple variable thinking would be necessary (Kraft et al., 2010; Talanquer, 2014). By stating approaches, the instructors might have intended to guide learners or to highlight their approach by explicitly verbalizing ways to come to a problem solution. However, it should be noted that the identified approaches of the instructors differ. While some of the verbalized approaches might be potentially helpful for learners by stating the concepts or train of thoughts necessary to make a claim, others may not, as they trigger a pattern recognition based on representational features (“looking at the structure and recognizing that…”) and might therefore promote heuristic reasoning. Both frames also revealed the frequent use of the description of the process. In teaching contexts, this could be beneficial for learning, as it goes beyond a static description of entities (e.g., a product-oriented approach) and could support reasoning about the dynamic processes of mechanisms.
The characterization of explanatory elements provided further information on how the instructors constructed their explanation in the expert and teaching frames, and how they embedded the cause–effect rationale. While the rationale in the expert frame was frequently accompanied by a description of the process, in the teaching frame general statements and approaches accompanied the rationale more often and more transitions were made between the explanatory elements. The expert frame was found to trigger short-cut response behavior, while the teaching frame led to stating additional explanatory elements that instructors might believe to be necessary in a teaching situation. When explaining to fictitious students, the instructors integrated more approaches and general statements in their explanations. Although more explanatory elements were found in the teaching frame compared to the expert frame, this does not mean that the verbalization of an explanatory element would necessarily result in a benefit for learning. While some generalizations made through general statements could lead to a better structuring of an explanation, they could also promote an overgeneralization. Hence, general statements that regard implicit causes and effects (e.g., relating stability to charge distribution like instructor Lewis did solving task 1 in the teaching frame) might be more beneficial in a teaching context than general statements regarding the explicit level (e.g., designating the reactivity order based on the backbones of the substrates).
It remains to be seen whether certain explanatory elements, combinations, or transitions between them support students’ learning in a more meaningful way than others do. At this point, the mere and multiple naming of explanatory elements should not be regarded as helpful per se, as qualitative differences within the codes could be identified (e.g., in how approaches are verbalized).
The differences found in the use of the explanatory elements in both frames, thus, may not seem surprising, as framing is considered to be an individual process and each instructor gave individual explanations of what they felt was appropriate in the given fictitious context (Berland and Hammer, 2012). To be more certain how to meet the prompt, the instructors often asked about the requirements of the situation of the respective frames. For example, instructor Miller asked for a feedback on his brief explanation after stating his answer to task 1 in the expert frame:
Instructor Miller: That was a very brief explanation. Is that enough for you? So, I mean, that would now be my explanation for a colleague, so to speak. I mean to a student I would explain: You see the same leaving group. It is a SN1 reaction… [goes on more in depth with his explanation]. So that would now be the explanation for a student, so to speak, in an organic chemistry beginner course.
Interviewer: Exactly, that [explaining to a fictitious student] will take place in the next scenario.
By explicitly ascertain the requirements, the instructors were able to frame the situation respectively, which allowed us to draw conclusions about their shift in the explanations.
In addition, the framing was specifically ascertained by the interviewers, e.g., by asking whether this explanation was shaped for themselves or a colleague, respectively a student with low prior knowledge.
At the beginning of the teaching frame, instructors further mentioned to aim at giving an explanation in a didactic way. After giving the prompt of the teaching frame, instructor Lewis, for example, ensured himself if there is time for doing so.
Interviewer: Now we go on with your explanation [in the teaching frame].
Instructor Lewis: Okay. But I still have time to prepare for it, to explain in a didactically meaningful way?
Interviewer: Of course. But the task format is the same like before [in the expert frame].
Instructor Lewis: Okay, sure. Still, I think there [in the expert frame] I was thinking more for myself, so to speak, and not thinking about how to get there in a meaningful way.
His statement further indicates that his framing has shifted from “thinking for himself” to aiming at thinking and explaining in a structured, meaningful way in the teaching frame, which was intended by the prompts. As illustrated before, instructor Lewis intentionally, thus, shaped his explanation to show how to come about to make a claim – “how to get there”, e.g., by considering the process and verbalizing approaches.
Limitations
A limiting factor of this study is that task effects were not considered in the analysis, i.e., if small changes in the task design, such as differing substrates or differing leaving groups, change the shape of the explanation. This analysis would have been based on a very small sample size and thus we decided to consider the four tasks together. Another limitation is the number of instructors interviewed. This was limited due to the rank sought for the study, i.e., Organic Chemistry research group leader and/or lecturer for Organic Chemistry beginner courses. Another limiting factor may result from the fictitious situation (e.g., explain to a fictitious student) in which we placed the instructors. Direct addresses to students in the teaching frame (see instructor Miller, task 2, teaching frame) show that the instructors often adapted their explanation specifically to students, i.e., they intentionally shaped their explanation in this way. Our data, however, were not collected in an authentic situation. The study therefore does not cover the social aspects or the spontaneity of a teaching situation. Investigations of how instructors react in authentic teaching settings would be useful to gain further insights into their construction of explanations. Furthermore, our prompt was to direct instructors’ explanation to a fictitious student with low prior knowledge. We did not collect any data about students’ prior knowledge in the classes of the instructors. Not regarding specific students’ prior knowledge in our prompt was meant to create a consistent set of conditions for all of the instructors. Our focus of analysis therefore drew attention to each instructors’ interpretation to the same prompt. Analyzing instructors’ explanations targeted for different prior knowledge of students remains part of further investigations. In this regard, specific emphasis could be placed on how students respond to the use of multiple conceptual approaches and whether these prove to be beneficial to learning. Furthermore, to analyze an individual instructor's framing in detail one could investigate how an instructor would embed an explanation in the teaching context, e.g., what would have been conceptually covered prior to instruction by the instructor.The instructors were prompted in a way to frame a situation – first to explain spontaneously and then to explain to a fictitious student with low prior knowledge. These prompts were used for all four tasks. This may have influenced the generation of explanations going from one task to the other, as the instructors first had to complete the tasks in the expert frame and then again in the teaching frame. Although we have not observed a pattern that indicates such a sequence effect, it may have influenced instructors’ decisions. Further, the task format of comparing two reactions in case comparisons, compared to common predict-the-product task, might have influenced instructors’ approaches. However, the instructors self-reported at the end of the interview to use case comparisons in their classes, when teaching reactivity orders of single entities and did not report to have had struggled with the task format.
Additionally, to answer our research questions, we focused on the most common trends of instructors’ mechanistic explanations triggered by a frame shift. Therefore, how individual instructors performed and whether individual explanations remained stable across frames were not described. However, this layer might provide further insights on instructors’ approaches.
It can further be assumed that most instructors were unfamiliar with the demands and structure of mechanistic reasoning described in chemistry education literature. The categories of causes and effects defined in our study were not directly communicated to the instructors. Accordingly, they verbalized their explanations in a way which they thought might be adequate – which was what we intended by promoting a certain frame. For example, the pure verbalization of a stability difference was consistently categorized as a non-electronic effect. The instructors, of course, could have implied either reasoning about an electronic stabilization (structural account) or stability in terms of a thermodynamic attribution (energetic account). Further prompting could have revealed the core of the possibly implied variety of meanings of some terms, but would have additionally biased the intended framing and might have influenced the subsequent tasks.
Conclusions and implications
In this study, instructors’ explanations of case comparison tasks in two differently prompted scenarios were the focus. We designed our instrument to tap into instructors’ abilities to adapt their explanation to a specific context or frame (Tannen, 1993; Hammer et al., 2005) – an expert frame and a teaching frame.Our findings revealed that the expert frame led the instructors to favor a short-cut explanatory approach, which was shown, e.g., in verbalizing causes without an effect. Further, they often did not involve the electronic structure and electronic interactions in their cause–effect rationales. These findings correspond to instructors taking the knowledge of their addressees into account, i.e., anticipating an expert's existing knowledge in Organic Chemistry mechanisms and abilities to infer implicit information. In the teaching frame, the rationales of the instructors were frequently more elaborated compared to the expert frame, as more implicit information was verbalized and often multiple and complete cause–effect rationales were established, which is a promising finding and showcases instructors’ adaptivity. Not all of the explanations given in the teaching frame, however, entailed an electronically justified cause–effect rationale, which would correspond to a high complexity of a relation. As Caspari and colleagues (2018) stated concerning the complexity of relations, “A more complex relation [with an electronically justified cause and effect] is not necessarily any better (or worse) than a less complex one. The advantage of a less complex relation is that it can be more easily communicated than a complex relation and enables faster decision making” (Caspari et al., 2018, p. 1130). Thus, by stating less complex cause–effect rationales, the instructors could have attempted to facilitate understanding by communicating their reasoning in a less complex way. If one takes another look at the verbalized causes in the expert frame as well as when the instructors specifically targeted the explanation for a fictitious student, it was found that causes were often verbalized as a pure naming of implicit properties, i.e., rather as verbalisms and buzzwords (e.g., “is a weak base, therefore […]”). In teaching contexts, students might adapt these vague verbalizations, which might hinder their meaningful learning and should be treated with caution. It has been shown that students tend to rely on verbalisms and therefore tend to engage in rote learning without having a deep understanding of the verbalism (Bhattacharyya, 2008). Furthermore, breaking down explanations to, e.g., “good or bad” leaving groups or “strong or weak” acids in a teaching situation may cause learners to remain in dualistic thinking rather than considering multiple variables and reaction conditions (Popova and Bretz, 2018). As Popova and Bretz (2018) showed in their study of Organic Chemistry students’ perceptions of leaving groups, less than half of the students were able to refer to the electronic and structural features that characterize a leaving group as “good”. In their explanation, students found it difficult to name the implicit properties and to use them in a meaningful way to draw a conclusion. They typically relied on reciting examples of good and bad leaving groups that were presented during their lecture (Popova and Bretz, 2018). This suggests that the mere naming of implicit properties or concepts as buzzwords in a teaching context may encourage the use of heuristics in students as well, in terms of verbalisms that cover up the lack of deep understanding of chemical concepts.
We found that in the teaching frame instructors often verbalized multiple rationales with different conceptual approaches. In a teaching context, it would be valuable to explain how different concepts can be related. What was found in our data was promising as instructors often connected different conceptual approaches of rationales, e.g., resonance and acidity/basicity, in drawing back references to the (change of the) electronic structure, e.g., the capacity to accommodate charge.
Further, our results showed that in both frames the instructors often verbalized their final answer to the case comparisons as a pure qualitative statement, i.e., “has a stronger impact”, “is better”, “is weaker”. In doing so, instructors did not address the effect of a structural cause of the reaction on a structural level and remain vague about the source of this qualitative difference. In teaching context, a pure statement of qualitative differences could result in students memorizing these differences without deeper understanding. Purposefully verbalizing the effect on the structural change allows to fill this missing link between the structural cause and the claim about energy or qualitative differences. Hence, if instructors attempt to explain a change in energy in a mechanistic task, the structural cause with its effect on the structural change needs to be considered in order to make a causally founded claim (Goodwin, 2003; Caspari et al., 2018). To explain a case comparison that asks for a comparison in reaction rates, structural account and energetic account have to be connected by referring to both, cause and effect, in the structural account. While we have shown that instructors are able to shift between levels of details and grain size in an explanation, in the case of students, it may not be the same question of deciding which grain size of an explanation to use or being aware of it. Rather, it may reflect their actual understanding. Building well-grounded cause–effect relations needs support, for instance through explicit prompting or scaffolding. Students can be engaged in considering the electronic structure and building more elaborate relations, even in situations in which transfer of knowledge is needed (Caspari and Graulich, 2019; Crandell et al., 2020). This emphasizes the importance of instructors’ roles in purposefully supporting students to consider causes and effects with a reasonable grain size when teaching and verbalizing mechanistic reasoning.
The further analysis of instructors’ mechanistic explanations revealed the use of explanatory elements, i.e., description of the process, general statements, and approaches. These elements were used differently to embed the rationales in both frames. For example, while some of the verbalized approaches state the concepts or train of thoughts beneficial for problem-solving, others could trigger pattern recognition when only superficial features of a reaction are noted to be taken into consideration. A supportive approach could therefore rather be verbalized by stating how and where to derive implicit properties from the explicit features of a mechanism. Thereby, the formation of cause–effect rationales could be supported in terms of explicitly verbalizing the necessity to use implicit properties as structural cause for an effect to be able to make claims about energy. Hence, it remains to be investigated whether a certain embedding of rationales and use of explanatory elements (of a certain quality) is beneficial to promote students’ mechanistic reasoning. Further research could be conducted taking the learning gains of students into account when they are “confronted” with a certain explanation.
Based on the analysis of instructors’ mechanistic explanations, it was possible to pose implications, which consider students’ challenges in building well-grounded explanations reported in the literature, e.g., students’ challenges in building cause–effect relations (Weinrich and Talanquer, 2016; Caspari et al., 2018; Moreira et al., 2019a) or in inferring implicit information (Bhattacharyya and Bodner, 2005; Strickland et al., 2010; Anzovino and Bretz, 2016). Overall, we are aware that instructors’ explanations are not solely responsible for learning and explanations constructed by students. An instructor's elaborate, causal explanation presented to students does not mean that this explanation is transferred to students’ minds and that in turn the students are able to construct causal explanations themselves. Nevertheless, as students’ challenges when reasoning mechanistically are well researched and the impact of an instructor's expressed mechanistic reasoning on students’ mechanistic reasoning has already been shown (Moreira et al., 2019b), instructors should be aware of their important role in the learning process. Based on our findings, the following aspects should be considered when instructors engage in mechanistic reasoning in teaching contexts: (1) formation of complete rationales in which structural causes and associated effects are verbalized; (2) when using multiple rationales, i.e., multiple conceptual approaches, interrelating links should be made in terms of considering (the change of) the electronic structure; (3) when stating general statements focusing less on generalizations based on explicit differences, rather generalizable implicit-electronic relations should be stated; (4) making problem-solving approaches explicit. These aspects have the potential to be supportive when teaching Organic Chemistry, yet they must always be adapted to the specific student cohort's needs and prior knowledge. Hence, instructors should be aware of the complexity of the cause–effect relation, i.e., the grain size of causes and effects, they verbalize and of what the untargeted verbalization of additional explanatory elements might trigger in learners (e.g., structuring vs. overgeneralization; teaching problem-solving strategies vs. promoting pattern recognition). Our study revealed that instructors are engaged in customizing their explanation according to the frame, which illustrates their flexibility in adjusting or elaborating their mechanistic explanation. Whereas in the past, students’ challenges were often the focus in studies, our study showed it is worth considering instructors’ explanatory approaches.
Conflicts of interest
There are no conflicts to declare.Appendix
Acknowledgements
The authors would like to thank the German Research Foundation DFG (Deutsche Forschungsgemeinschaft) for funding this research (project number: 329801962). We also thank the instructors who voluntarily participated in the study, as well as the members of the Graulich research group and Parchmann research group for fruitful discussions and support in data collection, especially Gyde Asmussen who supported us throughout the whole project. This publication is part of the first author's doctoral (Dr rer. nat.) thesis in the Faculty of Biology and Chemistry at the Justus-Liebig-University Giessen, Germany.References
- Achinstein P., (1983), The nature of explanation, Oxford University Press on Demand.
- Alfieri L., Nokes-Malach T. J. and Schunn C. D., (2013), Learning through case comparisons: A meta-analytic review, Educ. Psychol., 48, 87–113.
- Anzovino M. E. and Bretz S. L., (2016), Organic chemistry students' fragmented ideas about the structure and function of nucleophiles and electrophiles: A concept map analysis, Chem. Educ. Res. Pract., 17, 1019–1029.
- Becker N., Noyes K. and Cooper M., (2016), Characterizing students’ mechanistic reasoning about London dispersion forces, J. Chem. Educ., 93, 1713–1724.
- Bedard J. and Chi M. T., (1992), Expertise, Curr. Dir. Psychol. Sci., 1, 135–139.
- Berland L. K. and Hammer D., (2012), Framing for scientific argumentation, J. Res. Sci. Teach., 49, 68–94.
- Bhattacharyya G., (2008), Who am I? What am I doing here? Professional identity and the epistemic development of organic chemists, Chem. Educ. Res. Pract., 9, 84–92.
- Bhattacharyya G. and Bodner G. M., (2005), “It gets me to the product”: How students propose organic mechanisms, J. Chem. Educ., 82, 1402–1407.
- Bodé N. E., Deng J. M. and Flynn A. B., (2019), Getting past the rules and to the WHY: Causal mechanistic arguments when judging the plausibility of organic reaction mechanisms, J. Chem. Educ., 96, 1068–1082.
- Caspari I. and Graulich N., (2019), Scaffolding the structure of organic chemistry students’ multivariate comparative mechanistic reasoning, Int. J. Phys. Chem. Educ., 11, 31–43.
- Caspari I., Kranz D. and Graulich N., (2018), Resolving the complexity of organic chemistry students' reasoning through the lens of a mechanistic framework, Chem. Educ. Res. Pract., 19, 1117–1141.
- Cohen J., (1988), Statistical power analysis for the behavioural sciences, 2nd edn, Hillsdale, NJ: Lawrence Erlbaum Associates.
- Cooper M. M., (2015), Why ask why? J. Chem. Educ., 92, 1273–1279.
- Cooper M. M., Kouyoumdjian H. and Underwood S. M., (2016), Investigating students’ reasoning about acid–base reactions, J. Chem. Educ., 93, 1703–1712.
- Crandell O. M., Kouyoumdjian H., Underwood S. M. and Cooper M. M., (2018), Reasoning about reactions in organic chemistry: Starting it in general chemistry, J. Chem. Educ., 96, 213–226.
- Crandell O. M., Lockhart M. A. and Cooper M. M., (2020), Arrows on the page are not a good gauge: Evidence for the importance of causal mechanistic explanations about nucleophilic substitution in organic chemistry, J. Chem. Educ., 97, 313–327.
- Craver C. F. and Darden L., (2013), In search of mechanisms: Discoveries across the life sciences, University of Chicago Press.
- diSessa A. A. and Sherin B. L., (1998), What changes in conceptual change? Int. J. Sci. Educ., 20, 1155–1191.
- Driver R., Newton P. and Osborne J., (2000), Establishing the norms of scientific argumentation in classrooms, Sci. Educ., 84, 287–312.
- Duschl R. A. and Osborne J., (2002), Supporting and promoting argumentation discourse in science education, Stud. Sci. Educ., 38, 39–72.
- Galloway K. R., Leung M. W. and Flynn A. B., (2019), Patterns of reactions: A card sort task to investigate students’ organization of organic chemistry reactions, Chem. Educ. Res. Pract., 20, 30–52.
- Gilbert J. K. and Treagust D. F., (2009), Multiple representations in chemical education, Dodrecht: Springer, pp. 333–350.
- Goffman E., (1974), Frame analysis: An essay on the organization of experience, Harvard University Press.
- Goodwin W. M., (2003), Explanation in organic chemistry, Ann. N.Y. Acad. Sci., 988, 141–153.
- Goodwin W. M., (2008), Structural formulas and explanation in organic chemistry, Found. Chem., 10, 117–127.
- Graulich N. and Bhattacharyya G., (2017), Investigating students' similarity judgments in organic chemistry, Chem. Educ. Res. Pract., 18, 774–784.
- Graulich N. and Schween M., (2018), Concept-oriented task design: Making purposeful case comparisons in organic chemistry, J. Chem. Educ., 95, 376–383.
- Graulich N., Hedtrich S. and Harzenetter R., (2019), Explicit versus implicit similarity – exploring relational conceptual understanding in organic chemistry, Chem. Educ. Res. Pract., 20, 924–936.
- Hammer D., Elby A., Scherr R. E. and Redish E. F., (2005), in Mestre J. P. (ed.), Transfer of learning from a modern multidisciplinary perspective, Grennwich, CT: Information Age Publishing, ch. 3, vol. 89, pp. 89–119.
- Hattie J., (2008), Visible learning: A synthesis of over 800 meta-analyses relating to achievement, Routledge.
- Hempel C. G., (1965), Aspects of scientific explanation and other essays in the philosophy of science, New York: Free Press.
- Hutchison P. and Hammer D., (2010), Attending to student epistemological framing in a science classroom, Sci. Educ., 94, 506–524.
- Koslowski B., (1996), Theory and evidence: The development of scientific reasoning, MIT Press.
- Kraft A., Strickland A. M. and Bhattacharyya G., (2010), Reasonable reasoning: Multi-variate problem-solving in organic chemistry, Chem. Educ. Res. Pract., 11, 281–292.
- Krist C., Schwarz C. V. and Reiser B. J., (2019), Identifying essential epistemic heuristics for guiding mechanistic reasoning in science learning, J. Learn. Sci., 28, 160–205.
- Luxford C. J. and Bretz S. L., (2014), Development of the bonding representations inventory to identify student misconceptions about covalent and ionic bonding representations, J. Chem. Educ., 91, 312–320.
- Machamer P., Darden L. and Craver C. F., (2000), Thinking about mechanisms, Philos. Sci., 67, 1–25.
- MacLachlan G. L. and Reid I., (1994), Framing and interpretation, Carlton, Vic.: Melbourne University Press.
- Maeyer J. and Talanquer V., (2013), Making predictions about chemical reactivity: Assumptions and heuristics, J. Res. Sci. Teach., 50, 748–767.
- McNeill K. L. and Krajcik J., (2008), Scientific explanations: Characterizing and evaluating the effects of teachers' instructional practices on student learning, J. Res. Sci. Teach., 45, 53–78.
- Moreira P., Marzabal A. and Talanquer V., (2019a), Using a mechanistic framework to characterise chemistry students' reasoning in written explanations, Chem. Educ. Res. Pract., 20, 120–131.
- Moreira P., Marzabal A. and Talanquer V., (2019b), Investigating the effect of teacher mediation on student expressed reasoning, Chem. Educ. Res. Pract., 20, 606–617.
- Osborne J., Erduran S. and Simon S., (2004), Enhancing the quality of argumentation in school science, J. Res. Sci. Teach., 41, 994–1020.
- Petritis S. J., Kelley C. and Talanquer V., (2020), Exploring the impact of the framing of a laboratory experiment on the nature of student argumentation, Chem. Educ. Res. Pract., 105–121.
- Popova M. and Bretz S. L., (2018), Organic chemistry students’ understandings of what makes a good leaving group, J. Chem. Educ., 95, 1094–1101.
- Rädiker S. and Kuckartz U., (2019), Analyzing qualitative data with MAXQDA, Switzerland: Springer International Publishing.
- Ramsey J. L., (2008), Mechanisms and their explanatory challenges in organic chemistry, Philos. Sci., 75, 970–982.
- Rodemer M., Eckhard J., Graulich N. and Bernholt S., (2020), Decoding case comparisons in organic chemistry: Eye-tracking students’ visual behavior, J. Chem. Educ., 3530–3539.
- Russ R. S., (2018), Characterizing teacher attention to student thinking: A role for epistemological messages, J. Res. Sci. Teach., 55, 94–120.
- Russ R. S., Coffey J. E., Hammer D. and Hutchison P., (2009), Making classroom assessment more accountable to scientific reasoning: A case for attending to mechanistic thinking, Sci. Educ., 93, 875–891.
- Saldaña J., (2016), The coding manual for qualitative researchers, Los Angeles: Sage Publications Limited.
- Salmon W. C., (1984), Scientific explanation and the causal structure of the world, Princeton, NJ: Princeton University Press.
- Sevian H. and Talanquer V., (2014), Rethinking chemistry: A learning progression on chemical thinking, Chem. Educ. Res. Pract., 15, 10–23.
- Shulman L. S., (1986), Those who understand: Knowledge growth in teaching, Educ. Res., 15, 4–14.
- Slominski T., Fugleberg A., Christensen W. M., Buncher J. B. and Momsen J. L., (2020), Using framing as a lens to understand context effects on expert reasoning, CBE—Life Sci. Educ., 19, ar48.
- Stains M. and Talanquer V., (2008), Classification of chemical reactions: Stages of expertise, J. Res. Sci. Teach., 45, 771–793.
- Stowe R. L. and Cooper M. M., (2019), Assessment in chemistry education, Isr. J. Chem., 59, 598–607.
- Strickland A. M., Kraft A. and Bhattacharyya G., (2010), What happens when representations fail to represent? Graduate students’ mental models of organic chemistry diagrams, Chem. Educ. Res. Pract., 11, 293–301.
- Taber K. S., (1998), An alternative conceptual framework from chemistry education, Int. J. Sci. Educ., 20, 597–608.
- Talanquer V., (2013), Chemistry education: Ten facets to shape us, J. Chem. Educ., 90, 832–838.
- Talanquer V., (2014), Chemistry education: Ten heuristics to tame, J. Chem. Educ., 91, 1091–1097.
- Talanquer V., (2018a), in Science education research and practice in Asia-Pacific and beyond, Singapore: Springer, pp. 39–52.
- Talanquer V., (2018b), Importance of understanding fundamental chemical mechanisms, J. Chem. Educ., 95, 1905–1911.
- Tannen D., (1993), Framing in discourse, Oxford University Press on Demand.
- Toulmin S. E., (2003), The uses of argument (updated edition), Cambridge: Cambridge university press.
- Watts F. M., Zaimi I., Kranz D., Graulich N. and Shultz G. V., (2021), Investigating students’ reasoning over time for case comparisons of acyl transfer reaction mechanisms, Chem. Educ. Res. Pract.
- Weinrich M. L. and Talanquer V., (2016), Mapping students' modes of reasoning when thinking about chemical reactions used to make a desired product, Chem. Educ. Res. Pract., 17, 394–406.
Footnote |
| † The term “implicit” refers to the inferences made from the representational level, i.e., inferring meaning/conceptual knowledge |
| This journal is © The Royal Society of Chemistry 2022 |