Open Access Article
Open Access ArticleDesigning red-fluorescent superparamagnetic nanoparticles by conjugation with gold clusters
Agne Mikalauskaitea,
Marijus Pleckaitis b,
Giedre Grincienea,
Vitalijus Karabanovasb and
Arunas Jagminas
b,
Giedre Grincienea,
Vitalijus Karabanovasb and
Arunas Jagminas *a
*a
aState Research Institute Center for Physical Sciences and Technology, Sauletekio Ave. 3, LT-10254, Vilnius, Lithuania. E-mail: arunas.jagminas@ftmc.lt
bBiomedical Physics Laboratory, National Cancer Institute, LT-08406, Vilnius, Lithuania
First published on 9th December 2022
Abstract
Photoluminescent (PL) metal and metal oxide nanoclusters (NCs), with a size of just several nanometers, are a separate class of nanomaterials abundant with new attractive optical, physical, and chemical properties and biocompatibility. However, the synthesis of PL magnetic NCs via attachment of PL NCs to iron oxide-based nanoparticles (NPs) is still problematic. Motivated by this, herein, we report the development of a microwave-driven conjugation approach of red-fluorescent gold nanoclusters (BSA@AuNCs) to superparamagnetic NPs. Synthesized CoFe2O4@AuNCs possess strong photoluminescence in water and ethanol media as well as good colloidal and optical stability, and magnetization response. High-resolution transmission electron microscopy (HRTEM), steady-state and time-resolved photoluminescence spectroscopy, X-ray powder diffraction (XRD), and magnetic measurements from ambient to cryogenic temperatures were applied for structural characterization and evaluation of optical and magnetic properties of the synthesized species.
Introduction
Photoluminescent metal nanoclusters consisting of several to tens of atoms, are ultra-small NPs, with size comparable with the Fermi wavelength of an electron, ca. ≤2 nm, that demonstrate intriguing physical and chemical properties.1–5 For chemical synthesis of NCs, various ligands from phosphines6 and thiols7 to proteins8–10 have been proposed. Protein-functionalized NCs of noble metals most often exhibit a strong photoluminescence signal in the visible spectral region, have high quantum yield,11 and excellent photostability for more than one month.12 However, upon attaching them to the surface of a magnetic NP, the red PL of AuNCs usually is completely quenched.Superparamagnetic iron oxide-based NPs are commonly used magnetic nanocarriers for various biomedical applications, such as hyperthermia and MRI contrast agents, biosensors, biolabels, drug transporters, etc.13 To prevent aggregation, they are covered with biocompatible shell of dextran, chitosan, poly(vinyl) alcohol, polyethylene oxides, proteins,14–17 whereas to endue them with a novel surface chemistry, the subsequent attachment of desired molecules are required.18,19 The attachment ways of Au0 NPs to magnetic iron oxide NPs producing hybrid plasmodia nanocomposites20–22 have been reported in several papers. Several efforts have been also made during the past decade seeking to fabricate fluorescent magnetic nanocrystals and NPs. The proposed synthesis protocols, however, required multiple steps by synchronized procedures. For example, Desmettre et al.23 reported a way for fabrication of fluorescent magnetite NPs in a NIR region by covering them with copolymer shell stabilized with the azICG (azide-terminated indocyanine green) dye. However, low fluorescence quantum yield24 and binding to proteins25 reduced the applicability of azICG dye functionalized magnetite NPs in diagnostic technologies. Recently, dye-free luminescent magnetic NPs have been fabricated by coating with blue luminescent polymer, ca. branched polyethyleneimine fluorophores.26,27 However, the fluorescence of dye molecules and quantum dots (QDs) in the visible wavelengths range usually is significantly reduced when attached to the iron oxide surface due to a strong absorption of UV and visible light. In 2018, Sony et al. synthesized superparamagnetic and fluorescent NPs composed of magnetite (Fe3O4) core and gold cluster shell by conjugation with positively charged erlotinib drug molecules. For this, arginine amino acid was attached to the surface of magnetite core via prolonged sonication. Red-fluorescent Au@BSA NCs, synthesized separately, were linked to the magnetite core and stabilized with erlotinib by the next processing steps.28 However, in this case as in others, the saturation magnetization value of superparamagnetic NPs reduces after covering with fluorescent shell.
In this study, we present a new route for engineering of red-photoluminescent magnetic SPION-type NPs by conjugation with the BSA-based AuNCs. In contrast to previous reports, the synthesized magnetic NPs after conjugation with gold nanoclusters possess a higher saturation magnetization value when compared with individual ferrite NPs, and show excellent PL stability for weeks. We envisage that these NPs have prospective applications in the dual-mode diagnosis, and therapy.
Experimental
Materials
HAuCl4·4H2O, CoCl2, FeCl3, PBS buffer, BSA (96%) and amino acids: D,L-cysteine (Cys, C3H7NO2S, 96%), L-arginine (Arg, C6H14N4O2), L-lysine (Lys, C6H14N2O2), D,L-methionine (Met), and L-glutamine (Glu, C5H10N2O3, 99.5%) were purchased from Sigma-Aldrich and used without any further purification. BSA powder, was obtained from Sigma-Aldrich. Chemically pure NaOH was purchased from Posh SA, Poland and purified by over saturation of aqueous solution. Distilled water was used throughout all tests. Dialysis tubes SnakeSkin™ were purchased from Thermo Sci. (MWCO, 10 kDa, Rockford, IL, USA).Syntheses of ferrite and magnetite NPs
Cobalt ferrite (CoFe2O4) NPs were synthesized from an alkaline aqueous solution containing 0.0275 mol L−1 CoCl2 and 0.05 mol L−1 FeCl3, and 0.05–0.2 mol L−1 of amino acid as chelating agent. NaOH aqueous solution was used to adjust the pH of solution to 12.2. The syntheses were conducted in the Teflon line autoclave at 130 °C for up to 10 h. The products were collected by centrifugation and magnetic collection, then rinsed and dried at 60 °C overnight.Synthesis of luminescent Co ferrite NPs
The following procedure has been designed for formation of red-photoluminescent magnetic nanoparticles. Briefly, 100 μg of CoFe2O4 NPs were added to 5 mL 2-(N-morpholino)ethanesulfonic acid buffer (MES) at a pH 6.3 and sonicated for 30 min. In other glass, 0.25 g of bovine serum albumin (BSA) were dissolved in aqueous 5 mL of HAuCl4 (10 mmol L−1) solution. Subsequently, both solutions were mixed, and pH adjusted to 12.4 by dropwise addition of 1 mol L−1 NaOH solution. The obtained solution was transferred to a 35 mL microwave vial and quickly heated by microwave radiation (160 W) at 60 °C for 1 h. After reaction, the solution was cooled down naturally to room temperature. Obtained products were collected by centrifugation and washed with deionized water. Ultrasmall NPs were collected using a permanent magnet. Up to five experiments were carried out to account for data discrepancy. The purification of NP solutions was performed by dialysis against distilled water for 12 h using a dialysis membrane SnakeSkin™.Characterization
The morphology of as-synthesized NPs was studied by TEM FEI Tecnai F20X-TWIN, operated at an accelerating voltage of 200 kV. The NPs subjected to observations were dispersed in EtOH on a carbon-coated Ni grid. The size distribution histograms of NPs were estimated using the ImageJ software. Phase analysis of ferrite NPs was analyzed using X-ray powder D8 diffractometer (Bruker AXC, Germany) equipped with a Göbel mirror with rotating Cu anode as a primary beam monochromator for CuKα radiation in the range from 10° to 75° with a step size 0.02° and a counting time 8 s per step. Phase identifications were performed using database PDF4+ and the size of crystallites was estimated by the Halder–Wagner (H–W) approximation. UV-vis absorption spectra were recorded using a Shimadzu 2450 spectrometer and the photoluminescence spectra were recorded on an Edinburgh F900 fluorescence spectrometer (Edinburgh instruments Ltd, UK) equipped with a picosecond pulsed diode laser EPL-470 employed for excitation of samples at λex = 470 nm and λex = 375 nm.Fluorescence decay measurements were acquired by a time-correlated single-photon counting technique using FLS 920 spectrometer (Edinburgh instruments Ltd) equipped with a single photon photomultiplier detector (S900-R) (Hamamatsu, Japan) and diode laser (λ = 405 nm, pulse length – <200 ps, pulse repetition rate – 200 kHz) for excitation of CoFe2O4@AuNC and Fe3O4@AuNC solutions in PBS. Photoluminescence decay was measured by collecting 1000 counts at the peak emission wavelength (∼700 nm) of the CoFe2O4@AuNCs PL band. Average fluorescence lifetime was calculated using eqn (1):
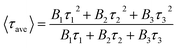 | (1) |
The quantum yield (QY) of photoluminescent NPs formation inside microwave reactor was calculated by comparing the PL intensities of the sample to that of a standard fluorescent dye, PD17, in ethanol with QY 97–99%. Magnetization measurements were accomplished versus applied magnetic field at 4 K to 300 K temperature using a vibrating sample magnetometer calibrated by a Ni sample.
Results and discussion
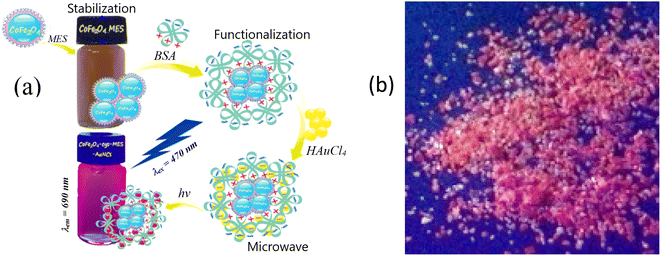
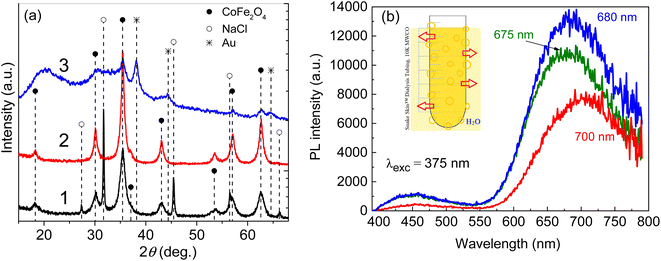
Fig. 1 depicts the synthesis scheme of magnetic-fluorescent nanoparticles designed in this study. As reported in the Experimental section, two-step synthesis was used for fabrication of ultrasmall cobalt ferrite NPs stabilized with amino acid and the subsequent their conjugation with BSA driven red-luminescent gold nanoclusters. The XRD pattern of hydrothermally synthesized Co ferrite NPs from an alkaline Co(II) and Fe(III) chloride solutions with cysteine amino acid, implied formation of CoFe2O4 containing crystalline NaCl as side phase (Fig. 2a).Closer inspection of this XRD spectrum within 2Θ range from 10° to 75° reveals the presence of all main diffraction peaks at 30.08°, 35.44°, 43.06°, and 62.58°, characteristic to CoFe2O4 planes (220), (311), (400), and (440), respectively, listed in the DB card no. 01-083-4766. The inclusion of crystalline NaCl species in the as-grown ferrite species is clearly seen from the diffraction peaks at 2Θ angles 31.70°, 45.45°, and 56.47° positions (Fig. 2a, plot 1), corresponding to the planes of NaCl (200), (220), and (222), respectively (DB card no. 01-085-8594). Note that upon rinsing in water pure CoFe2O4 product was obtained (Fig. 2a, pattern 2).
The attaching of gold to CoFe2O4 species upon the subsequent processing in the microwave reactor was also verified by XRD pattern depicted in Fig. 2a (plot 3) since the typical diffraction peaks from the main gold planes (111), (200), and (220) at 2Θ angles 38.18°, 44.39°, and 64.58°, respectively, are viewed (DB card no. 00-004-0784).
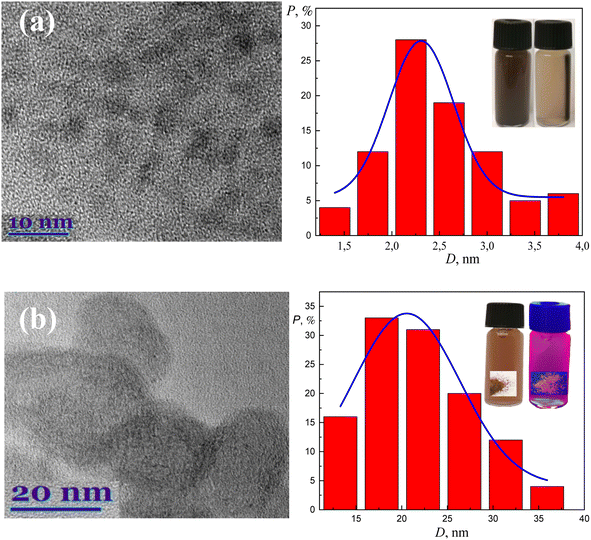
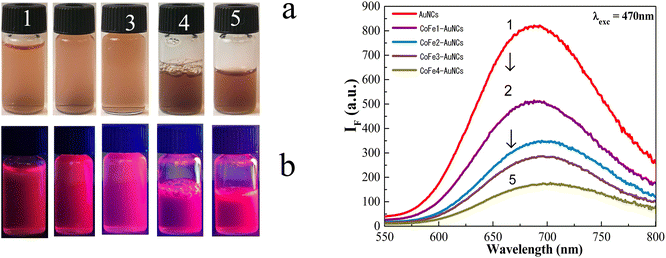
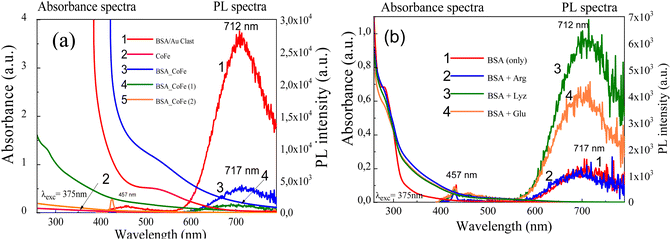
The formation and attachment of gold nanoclusters to CoFe2O4 NPs was verified further by photoluminescence measurements of as-synthesized and dialyzed CoFe2O4@AuNCs NPs (Fig. 2b). From the HRTEM observations (Fig. 3a), the average size of as-formed CoFe2O4 NPs stabilized with D,L-cysteine under the adapted herein hydrothermal synthesis conditions is about 2.4 nm (Fig. 3a). The dialysis of photoluminescent CoFe2O4@AuNCs species resulted in the enhancement of PL and blue shift of PL peak from 700 nm to 680 nm (Fig. 2b) most likely due to the decrease in size, which is typical for gold nanoclusters. It was, however, determined that using subsequent microwave-assisted treatment to conjugate ferrite NPs with Au@BSA nanoclusters, resulted in tenfold increase in the nanoprobes size (Fig. 3b). It was established that fluorescence intensity and stability of NPs depended on the concentration of CoFe2O4–Cys NPs inserted in the microwave synthesis reactor (Fig. 4). Under blue light excitation, the synthesized hybrid-type NPs emitted at around 700 ± 10 nm, which is quite similar to the PL of pure Au@BSA nanoclusters (Fig. 5a).8 Based on the spherical Jellium model,29 we can suspect the presence of Au27–Au29 clusters entrapped inside the hybrid particle. The PL spectra presented in Fig. 5, show that PL of the BSA gold nanocluster solutions synthesized together with Co ferrite NPs is almost 6 times weaker than Au@BSA nanoclusters alone (Fig. 5a, spectra 1 and 3). We determined here that the addition of amino acids to the synthesis solution of hybrid species can enhance (lysine, glutamine) or not influence (arginine) PL intensity (Fig. 5b). The reasons for such influence are not clear and will be studied in a separate study.
Noteworthy that after leaving solutions for three weeks in water or PBS buffer at 4 °C, hybrid CoFe2O4 NPs preserved fluorescent properties. Moreover, the conjugated Au@BSA clusters to Co ferrite NPs remained fluorescent after two–three weeks, even in 5–6% intense compared to as-formed ones.
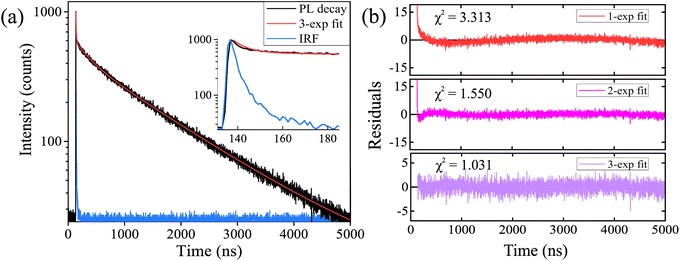
Time-resolved fluorescence spectroscopy measurements were made using 405 nm pulsed diode laser and are presented in Fig. 6a. Decay of photoluminescence at ∼700 nm was fitted with the tri-exponential tail-fitting model.30 A fast exponential component (τ1) was in the range of ∼5.8 ns and could be attributed to the instrument response function. An intermediate component (τ2) had a lifetime of ∼268 ns and a long-lifetime component (τ3) was ∼1495 ns.
They resulted in an average PL lifetime of ∼1423 ns. Mono- and bi-exponential tail-fitting models were inaccurate, and the best fit for CoFe2O4@AuNCs in PBS was achieved with the tri-exponential model. Quality of the fits is represented by residuals and χ2, which were 3.313, 1.550, and 1.031 for mono-, bi-, and tri-exponential fits, respectively (Fig. 6b). In our previous studies, we demonstrated that BSA31 or human blood plasma protein-stabilized32 gold nanoclusters alone had similar optical properties as do herein reported CoFe2O4@AuNCs. Yang et al. showed that noble metal nanoclusters exhibit metal-centered and ligand-centered emissions, which could be attributed to the shorter PL lifetimes in the range of nanoseconds and longer lifetimes in the range of microseconds, respectively.33 This could explain the bi-exponential nature of CoFe2O4@AuNCs photoluminescence. Long-lived excited state of Au NCs photoluminescence creates highly favorable conditions for the generation of various reactive oxygen species, making it possible to use gold nanoclusters as photosensitizers for photodynamic therapy.31,33
The zeta-potential (ξ) of hydrothermally synthesized Au@BSA nanoclusters (≅−16.6 eV), as well as CoFe2O4–Cys NPs, hybridized with these NCs was found to be negative. The magnitude of ξ-potential measured for hybrid NPs is larger than that of entrapped Au@BSA nanoclusters. From the experimental results (Table 1), the ξ-potential value of hybrid NPs depends on the amount of ferrite NPs added to the microwave reactor to synthesize fluorescent NPs. Consequently, the ξ-potential of hybridized NPs designed from 2.4 nm-sized CoFe2O4–Cys NPs and BSA-driven gold nanoclusters vary with the entrapped amount of CoFe2O4–Cys NPs increasing to ≅−26 eV for probes containing 10–15 mg mL−1 of ferrite (Table 1). Further increase in the concentration of ferrite NPs just slightly influenced their ξ-potential after hybridization. However, the aqueous solutions containing more than 20 mg mL−1 of NPs tend to agglomerate (see samples no 4 and no 5 in Fig. 4). Noticing, the highest ξ-potential values should be ascribed to the most stable NPs.
| No. | NP | Conc. (mg mL−1) | ζ (eV) |
|---|---|---|---|
| 0 | AuNC sol. | 0 | −16.6 |
| 1 | CoFe2O4@AuNC | 2.43 | −18.6 |
| 2 | CoFe2O4@AuNC | 7.28 | −25.7 |
| 3 | CoFe2O4@AuNC | 10.52 | −26.3 |
| 3 | CoFe2O4@AuNC | 14.56 | −25.5 |
| 4 | CoFe2O4@AuNC | 21.83 | −23.5 |
| 5 | CoFe2O4@AuNC | 29.13 | −22.0 |
| 6 | CoFe2O4@AuNC | 38.82 | −28.1 |
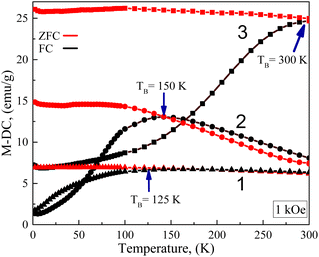
Magnetic properties of as-synthesized ultrasmall (remained in the synthesis solution after centrifugation) and 2.4 nm-sized CoFe2O4–Cys NPs before and after hybridization with gold NCs were studied in the temperature range from 300 K to 4 K under zero-field-cooling (ZFC) and field cooling (FC) conditions at an applied field of 1 kOe. Fig. 7 shows the ZFC/FC curves and superparamagnetic blocking temperature (TB) increase from 125 K to 300 K, clearly identifying by the peak positions at the ZFC branch for ultrasmall (125 K) and 2.4 nm-sized (150 K), and hybrid (fluorescent) (300 K) NPs, respectively. TB increase we ascribed to the size increase of analyzed NPs as in ref. 30.
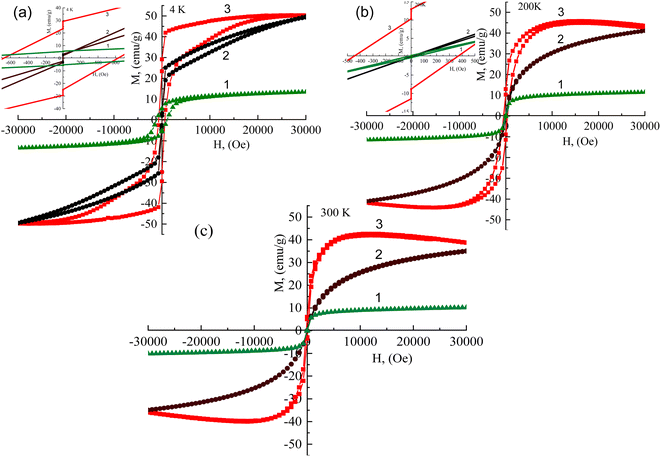
The magnetic hysteresis curves obtained for 2.4 nm-sized cobalt ferrite NPs synthesized in this study with cysteine before and after hybridization with Au@BSA nanoclusters at various temperatures are depicted in Fig. 8. From these, all samples have open hysteresis at T = 4 K, while at T = 200 K only ensembled NPs preserve their hysteretic behavior. At ambient temperature (T = 300 K), however, all NPs become superparamagnetic in accordance with the ZFC/FC curves in Fig. 7. Moreover, the MS value of ferrite NPs increased after conjugation with Au@BSA nanoclusters to 42.58 emu g−1 at T = 300 K. Therefore, it can be concluded that the best superparamagnetic behavior and stability possessed Au@BSA entrapped 2.4 nm-sized CoFe2O4–Cys NPs, which saturation magnetization value approximated to 34.9 emu g−1. Note that a slow MS increase to saturation value characteristic for ferrite NPs before hybridization at T = 300 K (Fig. 8c) compared to MS(H) plots run at other temperatures also implied the good magnetic properties of this sample. The presence of a loop for hybrid NPs at 4 K, its decrease at 200 K, and its disappearance at ambient temperatures we linked to the core spins coupled with the frozen disordered surface spins.34 Despite the spherical structure of gold clusters entrapped with 2.4 nm-sized CoFe2O4–Cys NPs in tenfold larger ensembles, their reduced remanent MR/MS values at 4 K and 200 K equaled to 0.59 and 0.22, respectively, which at 4 K is close to the theoretical value of 0.5 expected for no interacting uniaxial single-domain particles with the easy axis randomly oriented.35 The saturated magnetization, MS, blocking temperature, TB, coercive field, HC, and remanent magnetization, MR, values for investigated herein magnetic Co ferrite NPs are presented in Table 2.
| Samples | MS (emu g−1) | TB (K) | HC (Oe) | MR (emu g−1) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 4 K | 200 K | 300 K | 200 K | 4 K | 4 K | 200 K | 300 K | ||
| Ultrasmall CoFe2O4 NPs | 13.44 | 11.48 | 10.17 | 125 | — | — | — | — | — |
| 2.4 nm-sized CoFe2O4 NPs | 49.6 | 41.1 | 34.9 | 150 | — | — | — | — | — |
| Au@BSA CLs-coated NPs | 50.32 | 45.82 | 42.58 | 300 | 800 | 1200 | 30 | 10 | — |
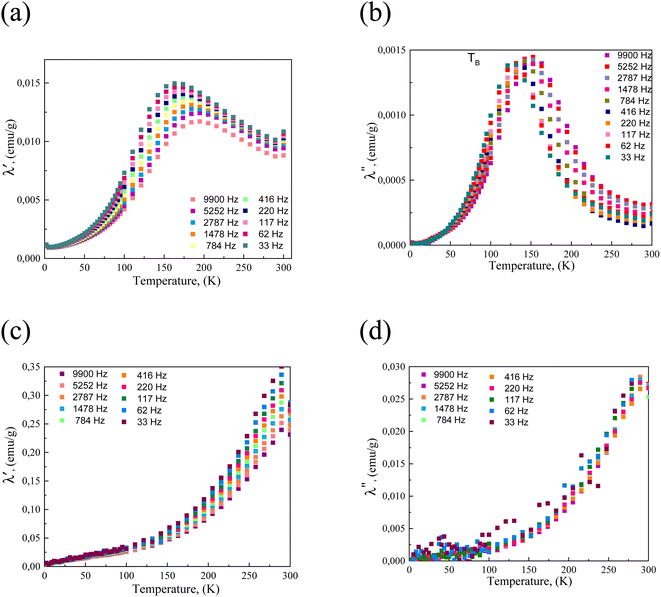
The temperature-dependent magnetic susceptibility plots for ultrasmall, 2.4 nm-sized CoFe2O4–Cys and hybrid-type CoFe2O4–Au@BSANCs NPs at various external alternating current (ac) magnetic field frequencies within 33 Hz to 9.9 kHz range are shown in Fig. 9. As seen, the magnetic susceptibility of the 2.4 nm-sized CoFe2O4–Cys NPs initially increases and reaches a maximum value in a vicinity of the blocking temperature at about 150–175 K. For these NPs above the TB, the susceptibility (χ) follows the modified Curie-law: χ = χ0 + C/T. The shapes of temperature-dependent susceptibility plots for the same Co ferrite NPs after conjugation with gold NCs change significantly (Fig. 9c and d), indicating TB increase to 300 K and higher most likely due to the hybrid NPs size increase compatible with TEM observations (Fig. 3b).
Conclusions
We developed the conjugation way of superparamagnetic cobalt ferrite NPs with red-photoluminescent gold nanoclusters by microwave-assisted treatment. From the TEM observations, zeta-potentials, and magnetic measurements of the synthesized hybrid-type NPs, we concluded that they are composed of several 2.4 nm-sized CoFe2O4 NPs and gold nanoclusters forming tenfold larger assembles with high stability as well as enhanced magnetic and fluorescent properties. The novel red-photoluminescent superparamagnetic NPs with an average lifetime of about 1.4 μs could play a pivotal role in early disease diagnostics and molecular bioimaging.Author contributions
A. J. conceived this study and wrote the manuscript. A. M., M. P., and G. G. performed the experiments, V. K. critically reviewed and edited the manuscript. All authors have read and approved the final manuscript.Conflicts of interest
The authors indicate there are no conflicts to declare.Acknowledgements
Dr Agne Mikalauskaite acknowledges a fellowship from the Research Council of Lithuania and the Ministry of Foreign Affairs and International Cooperation.References
- H. T. Sun and Y. Sakka, Luminescent metal nanoclusters: controlled synthesis and functional applications, Sci. Technol. Adv. Mater., 2014, 15, 014205 CrossRef CAS PubMed.
- P. L. Xavier, K. Chaudhari, P. K. Verma, S. K. Pal and T. Pradeep, Luminescent quantum clusters of gold in transferrin family protein, lactoferrin exhibiting FRET, Nanoscale, 2010, 2, 2769–2776 Search PubMed.
- R. C. Jin, Quantum sized, thiolate-protected gold nanoclusters, Nanoscale, 2010, 2, 343–362 RSC.
- L. Shang, S. J. Dong and G. U. Nienhaus, Ultra-small fluorescent metal nanoclusters: synthesis and biological applications, Nano Today, 2011, 6, 401–418 Search PubMed.
- S. Choi, R. M. Dickson and J. H. Yu, Developing luminescent silver nanodots for biological applications, Chem. Soc. Rev., 2012, 41, 1867–1891 RSC.
- M. Osawa, M. Hoshino, M. Akita and T. Wada, Synthesis and characterization of phenanthryl-phosphine gold complex: observation of room temperature, Inorg. Chem., 2005, 44, 1157–1159 CrossRef CAS PubMed.
- A. C. Templeton, W. P. Wuelfing and R. W. Murray, Monolayer protected cluster molecules, Acc. Chem. Res., 2000, 33, 27–36 CrossRef CAS PubMed.
- J. Xie, Y. Zheng and J. Y. Ying, Protein-directed synthesis of highly fluorescent gold nanoclusters, J. Am. Chem. Soc., 2009, 131, 888–889 CrossRef CAS PubMed.
- M. B. Dickerson, K. H. Sandhage and R. R. Naik, Protein- and peptide-directed syntheses of inorganic materials, Chem. Rev., 2008, 108, 4935–4978 CrossRef CAS PubMed.
- X. L. Guevel, N. Daum and N. M. Schneider, Synthesis and characterization of human transferrin stabilized gold nanoclusters, Nanotechnology, 2011, 22, 275103 CrossRef PubMed.
- I. Nandi, S. Chall, S. Chowdhury, T. Mitra, S. S. Roy and K. Chattopadhyay, Protein fibril-templated biomimetic synthesis of highly fluorescent gold nanoclusters and their applications in cysteine sensing, ACS Omega, 2018, 3, 7703–7714 CrossRef CAS PubMed.
- A. K. Gupta and M. Gupta, Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications, Biomaterials, 2005, 26, 3995–4021 CrossRef CAS PubMed.
- W. H. Suh, K. S. Suslick, G. D. Stucky and Y. H. Suh, Nanotechnology, nanotoxicology, and neuroscience, Prog. Neurobiol., 2009, 87, 133–170 CrossRef CAS PubMed.
- X. Zhao and J. M. Harris, J. Pharm. Sci., 1998, 87, 1450–1458 CrossRef CAS PubMed.
- R. Hao, R. J. Xing, Z. C. Xu, Y. L. Hou, S. Gao and S. H. Sun, Synthesis, functionalization, and biomedical applications of multifunctional magnetic nanoparticles, Adv. Mater., 2010, 22, 2729–2742 CrossRef CAS PubMed.
- M. Anbarasu, M. Anandan, E. Chinnasamy, V. Gopinath and K. Balamurugan, Synthesis and characterization of polyethylene glycol (PEG) coated Fe3O4 nanoparticles by chemical co-precipitation method for biomedical applications, Spectrochim. Acta, Part A, 2015, 135, 536–539 CrossRef CAS PubMed.
- S. A. Corr, Y. P. Rakovich and Y. K. Guriko, Multifunctional Magnetic Fluorescent Nanocomposites for Biological Applications, Nanoscale Res. Lett., 2008, 3, 87–104 CrossRef CAS.
- R. D. Rutledge, C. L. Warner, J. W. Pittman, R. S. Addleman, M. Engelhard, W. Chouyyok and M. G. Warner, Thiol-Ene Induced Diphosphonic Acid Functionalization of Superparamagnetic Iron Oxide Nanoparticles, Langmuir, 2010, 26, 12285–12292 CrossRef CAS PubMed.
- J. L. Lyon, D. A. Fleming, M. B. Stone, P. Schiffer and M. E. Williams, Synthesis of Fe oxide core/Au shell nanoparticles by iterative hydroxylamine seeding, Nano Lett., 2004, 4, 719–723 CrossRef CAS.
- T. A. Larson, J. Bankson, J. Aaron and K. Sokolov, Hybrid plasmonic magnetic nanoparticles as molecular specific agents for MRI/optical imaging and photothermal therapy of cancer cells, Nanotechnology, 2007, 18, 325101 CrossRef.
- A. Mikalauskaitė, R. Kondrotas, G. Niaura and A. Jagminas, Gold-Coated Cobalt Ferrite Nanoparticles via Methionine Induced Reduction, J. Phys. Chem. C, 2015, 119(30), 17398–17407 CrossRef.
- M. D. Daniele, L. M. Shoughnessy, R. Roeder, A. Childress, Y. P. Bandera and S. Foulger, Magnetic Nanoclusters Exhibiting Protein-Activated Near Infrared Fluorescence, ACS Nano, 2013, 7(1), 203–213 CrossRef CAS PubMed.
- T. Desmettre, J. M. Devoisselle and S. Mordon, Fluorescence properties and metabolic features of indocyanine green (ICG) as related to angiography, Surv. Ophthalmol., 2000, 45, 15–27 CrossRef CAS PubMed.
- R. Simmons and R. J. Shephard, Does indocyanine green obey Beer's law?, J. Appl. Physiol., 1971, 30, 502–507 CrossRef CAS PubMed.
- T. J. Muckle, Plasma proteins binding of indocyanine green, Biochem. Med., 1976, 15, 17–21 CrossRef CAS PubMed.
- R. Khodadust, O. Unal and H. Y. Acar, Theranostic potential of self-luminescent branched polyethyleneimine-coated superparamagnetic iron oxide nanoparticles, Beilstein J. Nanotechnol., 2022, 13, 82–95 CrossRef CAS PubMed.
- R. Kas, E. Sevinc, U. Topan and H. Y. Acar, A universal method for the preparation of magnetic and luminescent hybrid nanoparticles, J. Phys. Chem. C, 2010, 114, 7758–7766 CrossRef CAS.
- J. Nebu, J. S. Anjali Devi, R. S. Aparna, K. Abha and G. Sony, Erlotinib conjugated gold nanoclusters enveloped magnetic iron oxide nanoparticles – a targeted probe for imaging pancreatic cancer cells, Sens. Actuators, 2018, 257, 1035–1043 CrossRef CAS.
- J. Zheng, C. W. Zhang and R. M. Dickson, Highly fluorescent, water soluble, size-tunable gold quantum dots, Phys. Rev. Lett., 2004, 93, 077402 CrossRef PubMed.
- C. Liu, A. J. Rondinone and Z. J. Zhang, Synthesis of magnetic spinel ferrite CoFe2O4 nanoparticles from ferrite salt and characterization of the size-dependent superparamagnetic properties, Pure Appl. Chem., 2000, 72, 37–45 CrossRef CAS.
- V. Poderys, G. Jarockyte, S. Bagdonas, V. Karabanovas and R. Rotomskis, Protein-stabilized gold nanoclusters for PDT: ROS and singlet oxygen generation, J. Photochem. Photobiol., B, 2020, 204, 111802 CrossRef CAS PubMed.
- G. Jarockyte, V. Poderys, V. Barzda, V. Karabanovas and R. Rotomskis, Blood plasma stabilized gold nanoclusters for personalized tumor theranostics, Cancers, 2022, 14(8), 1887 CrossRef CAS PubMed.
- T. Yang, S. Dai, H. Tan, Y. Zong, Y. Liu, K. Zhang, P. Wu, S. Zhang, J. Xu and Y. Tian, Mechanism of Photoluminescence in Ag Nanoclusters: Metal-Centered Emission versus Synergistic Effect in Ligand-Centered Emission, J. Phys. Chem. C, 2019, 123(30), 18638–18645 CrossRef CAS.
- M. Muroi, R. Street, P. G. McCormick and J. Amighian, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 63, 184414 CrossRef.
- E. C. Stoner and E. P. Wohlfart, Philos. Trans. R. Soc. London, Ser. A, 1948, 240, 599–607 CrossRef.
| This journal is © The Royal Society of Chemistry 2022 |