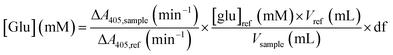Open Access Article
Open Access Article2,5-Furandicarboxaldehyde as a bio-based crosslinking agent replacing glutaraldehyde for covalent enzyme immobilization†
Chiara Danielli ab,
Luuk van Langenb,
Deborah Boesbc,
Fioretta Asaro
ab,
Luuk van Langenb,
Deborah Boesbc,
Fioretta Asaro a,
Serena Anselmid,
Francesca Provenzade,
Monia Renzie and
Lucia Gardossi
a,
Serena Anselmid,
Francesca Provenzade,
Monia Renzie and
Lucia Gardossi *a
*a
aDepartment of Chemical and Pharmaceutical Sciences, University of Trieste, Via L. Giorgieri 1, 34127 Trieste, Italy. E-mail: gardossi@units.it
bViaZym B.V., Molengraaffsingel 10, 2629 JD, Delft, The Netherlands
cDepartment of Biotechnology, Delft University of Technology, Van der Maasweg 9, NL-2629 HZ Delft, The Netherlands
dBioscience Research Center, Via Aurelia Vecchia, 32, 58015 Orbetello, GR, Italy
eDepartment of Life Science (DSV), University of Trieste, Via L. Giorgieri 10, 34127 Trieste, Italy
First published on 14th December 2022
Abstract
In the quest for a bio-based and safer substitute for glutaraldehyde, we have investigated 2,5 diformylfuran (DFF) as bifunctional crosslinking agent for the covalent immobilization of glucoamylase on amino-functionalized methacrylic resins. Immobilization experiments and systematic comparison with glutaraldehyde at four different concentrations for the activation step showed that DFF leads to comparable enzymatic activities at all tested concentrations. Continuous flow experiment confirms a similar long term stability of the immobilized formulations obtained with the two crosslinkers. The NMR study of DFF in aqueous solution evidenced a much simpler behaviour as compared to glutaraldehyde, since no enolic forms can form and only a mono-hydrated form was observed. Unlike in the case of glutaraldehyde, DFF reacts covalently with the primary amino groups via imine bond formation only. Nevertheless, the stability of the covalent immobilization was confirmed also at acidic pH (4.5), most probably because of the higher stability of the imine bonds formed with the aromatic aldehydes. In terms of toxicity DFF has the advantage of being poorly soluble in water and, more importantly, poorly volatile as compared to glutaraldehyde, which displays severe respiratory toxicity. We have performed preliminary ecotoxicity assays using Aliivibrio fischeri, a marine bacterium, evidencing comparable behaviour (below the toxicity threshold) for both dialdehydes at the tested concentrations.
Introduction
The use of enzymes as biocatalysts has several advantages: they have high selectivity, perform reactions in mild conditions and often are able to catalyse transformations not viable through conventional chemistry. Immobilized enzymes, which are insoluble enzyme formulations, present advantages such as applicability in low-water media or continuous flow settings.1–3 For this reason, immobilized enzymes are used in industry in pharmaceutical synthesis, in the food sector, in the cosmetic sector and for the synthesis of fine chemicals.1–4The immobilization of enzymes can be achieved with various techniques, including carrier-free direct cross-linking of protein conglomerates (CLECs, CLEAs), adsorption on solid carriers or covalent binding to solid carriers.5 In this last case, the enzyme is bound to a carrier through residues on the protein surface, usually amine residues.5 Likewise, the carrier needs to have active groups to which the enzyme can bind either directly (such as epoxy groups) or after activation using a bifunctional reagent. Amino-functionalized carriers are examples of this latter case. The most common bifunctional reagent is glutaraldehyde, but several alternatives have been proposed, including carbohydrate-derived dialdehydes.6
Other industrial applications of glutaraldehyde include its use as disinfectant, hardener in X-ray film processing, fixative in tanning, biocide in water treatment, preservative in industrial oils, biocide in sanitary solutions for aircraft and portable toilets, in small quantities as a disinfectant for air ducts, tissue fixative in electron and light microscopy and in histochemistry and biocide in aquaculture. Conversely, the glutaraldehyde market revenue was valued at US$ 450 million in 2021.7
However, the exact behaviour of glutaraldehyde in water, as well as the chemical nature of the bonds it forms with the enzyme and the carrier, is not fully understood.8 In fact, it displays a very complex behaviour in aqueous solution. Thirteen different forms, either hydrated, cyclic, oligomeric or polymeric have been identified, and it is still unclear which of these react with lysine side chains of the protein in enzyme immobilization. In most scientific papers, imine bonds are depicted as responsible for enzyme immobilization with glutaraldehyde, but this could raise questions about the expected reversibility of imine bond at acidic pH. In fact, practice shows that glutaraldehyde yields viable, irreversible, protein cross-linking, applicable in a wide range of conditions. In one study a pyridinium ion form was isolated after reaction of glutaraldehyde with amines, and it was hypothesized that this structure could also be formed during enzyme immobilization.9
In spite of its wide use, the industrial use of glutaraldehyde raises increasing concerns due to its widely documented toxicity.10,11 It has been classified as a candidate substance of very high concern (SVHC) by the European Chemicals Agency,12 and it is regarded fatal if inhaled, toxic if swallowed, and toxic to aquatic life.13 It can also cause severe long-term effects, such as respiratory and skin sensitization. Its highly toxic and environmentally hazardous nature is anticipated to act as a major restraint for the market growth, since the use of this chemical is highly regulated by the government in respective regions, owing to health risks associated with it. Even a 1% solution of glutaraldehyde is poisonous for humans and animals, and products containing more than 0.1% glutaraldehyde solution are labelled as hazardous. Therefore, market players are focusing to lower dependency on glutaraldehyde and to find suitable substitutes, as high level of precaution is needed to reduce occupational and environmental exposure to glutaraldehyde.
In the general quest for finding greener and safer molecules, we have identified 2,5-diformylfuran (DFF) as a bio-based alternative for glutaraldehyde. DFF is a derivative of 5-hydroxymethylfurfural (HMF), which is obtained from the de-hydration of carbohydrates. The oxidation of HMF to DFF can be achieved either by chemical routes14 or by biotransformations, including the use of isolated enzymes or whole cells.15,16
In the present study, we analysed its property as a replacement of glutaraldehyde for enzyme immobilization. We have investigated and directly compared the efficiency of glutaraldehyde and DFF in the covalent immobilization of glucoamylase, an enzyme of large industrial use, on amino-functionalized methacrylic resins. We employed the two di-functional reagents in a wide range of concentrations, up to very low concentrations at which differences in behaviour would be magnified. The resulting immobilized preparations have been compared in a continuous flow experiment, to simulate industrially relevant conditions. The reactivity of DFF towards primary amino groups was investigated by means of NMR spectroscopy, shedding light on the bonds formed in aqueous solution. Moreover, we compared the two crosslinkers in an ecotoxicological study, using marine micro-organisms, since at the present moment only few papers are known dealing with DFF toxicity.17–19 With this work we intend to pave the way for future studies and potential applications of this bio-based difunctional agent.
Experimental
Materials
DFF was chemically synthesized by oxidation of HMF based on a literature procedure.14 The product was obtained with a 99% purity determined by 1H NMR in CDCl3 (see ESI†).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–250
000–250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U g−1 solid) were purchased from Sigma Aldrich.
000 U g−1 solid) were purchased from Sigma Aldrich.Methods
The activated carrier was resuspended in 16 mL of potassium phosphate buffer (25 mM, pH 7, 1.6 mL gcarrier−1). 5 g of Dextrozyme GA (commercial glucoamylase solution; 120 U gcarrier−1 referred to the amount of wet carrier as provided by the manufacturer) were added to the reaction mixture. The mixture was kept shaking at 25 °C for 24 hours. The supernatant was then removed and tested for residual enzyme activity. The immobilized enzyme was rinsed 3 times with 20 mL of demineralized water.
The immobilized enzyme was stored at 4 °C in potassium phosphate buffer (25 mM, pH 7).
For the assay, 100 mg of immobilized glucoamylase were suspended in 10 mL of the maltose solution; the mixture was shaken at room temperature for 1 hour. Every 10 minutes, a sample (50 μL) of the supernatant was taken and diluted 10 times with 0.1 M HCl before glucose analysis.
50 μL of the 10× diluted glucoamylase assay sample were mixed with 2.95 mL of the glucose assay solution in a 4 mL UV-Vis cuvette. The cuvette was mixed by inversion, and the absorbance at 405 nm was monitored for 5 minutes. The variation in absorbance over time was compared to that of a reference solution of 5 mM D-glucose in water. The glucose concentration in each sample was obtained by:
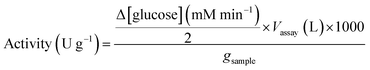
where: Δ[glucose]/2 is the rate of maltose hydrolysis; Vassay is the volume of the assay; 1000 is the conversion factor from mmol to μmol; gsample is the weight of dried immobilized enzyme used for the assay.
After the last activity assay cycle, the supernatant maltose solution was removed by decanting, and the immobilized enzyme sample was rinsed with 3 × 10 mL H2O. The water was then removed, and the enzyme preparation was dried in the vacuum oven (100 °C, 6 h). The weight of the anhydrous immobilized enzyme was used for the activity calculations.
At the end of the experiment, the effluent samples were analysed to determine: (1) glucose concentration (see previous section), (2) enzyme leaching (see following section). After the experiment, the enzymatic preparation was recovered and dried under high vacuum to express all results relating to dry enzyme preparation weight.
For glucose concentration, a negative measure was performed on 50 μL of 10 times diluted maltose assay solution. The glucose concentration in the negative is subtracted from that of all measured samples.
Results and discussion
Behaviour and reactivity of DFF in aqueous solution
In sharp contrast with glutaraldehyde, which has a very complex behaviour in aqueous environment, the behaviour of DFF in water appears to be very simple. DFF has no alpha hydrogen on the aldehyde functions, thus avoiding the formation of enols, whereas GA undergoes aldol-reactions giving rise to aldol-form intermediates leading to different reactivity and contributing to toxic effects.20As evident from the 1H NMR spectrum in D2O (Fig. 1), only two forms are present in aqueous solution. There are two series of signals: two peaks (a and b) correspond to DFF, as expected, while four peaks (1 to 4) belong to its monohydrated diol. Interestingly, only one of the two aldehyde groups is hydrated to diol, as there are no signals from a hypothetic di-hydrated form. The assignations were confirmed by comparison with literature.21
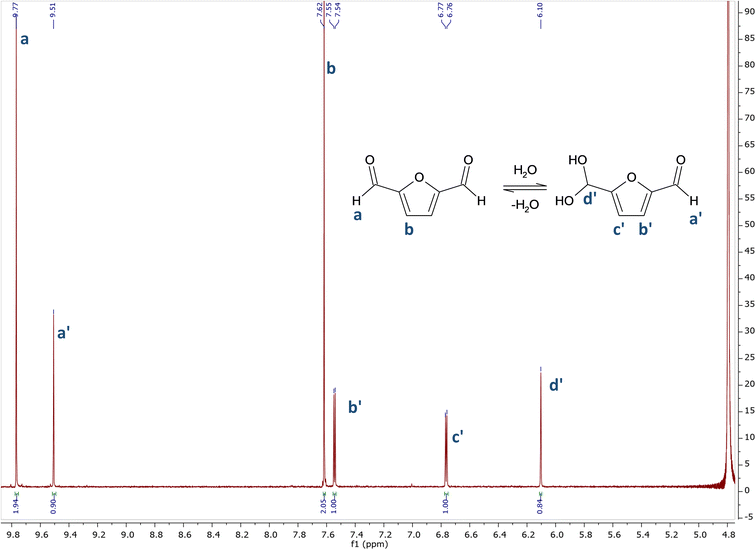 | ||
| Fig. 1 1H NMR spectrum of DFF in D2O. 1H NMR (400 MHz, D2O) δ 9.77 (s), 9.51 (s), 7.62 (s), 7.55 (d, J = 3.6 Hz), 6.77 (d, J = 3.7 Hz), 6.11 (s). | ||
The NMR spectrum in CDCl3 (see ESI†) presents signals only from DFF, confirming that no impurities were present in the starting sample.
On the light of the NMR evidence that the hydration equilibrium is asymmetric and that only one of the two aldehyde groups is hydrated to diol, it was necessary to investigate whether the reaction of DFF with primary amine groups occurs in a symmetrical way. That is the prerequisite for achieving the covalent binding of superficial lysine residues of an enzyme to an amino-functionalized carrier.22
The amine selected for the reaction was n-butylamine (Scheme 1a), which models the Lys side chains of the amino acid residues. The conditions of the model reaction reproduce the protocols typically used for enzyme immobilization: potassium phosphate aqueous buffer, 0.1 M, pH 7; an oily product started forming almost immediately, and the reaction was let to continue for an additional 24 hours. The precipitation of the imine product has similarities with the behaviour of the bond between the enzyme and the solid carrier, which does not participate in the equilibrium of the chemical species in solution. It must be underlined that previous studies found that the thermodynamic equilibrium of chemical reactions can significantly change in favour of the synthetic product when a soluble compound binds to molecules linked to a solid support. That is the case of the formation of amide bonds in solid phase synthesis of peptides catalysed by proteases.23
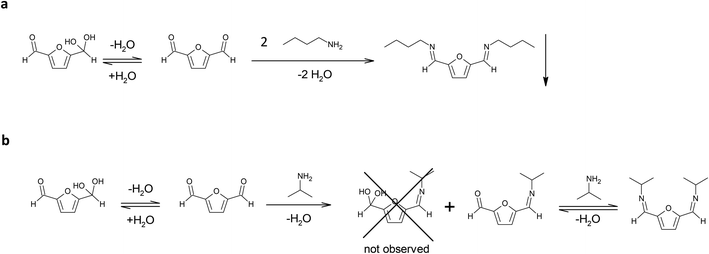 | ||
| Scheme 1 (a) Reaction between DFF and n-butylamine in potassium phosphate buffer, 0.1 M, pH 7. (b) Reaction of DFF with isopropylamine in potassium phosphate buffer, 0.1 M, pH 7. | ||
In these conditions the di-imine derivative (pictured in Scheme 1a), which separated from the solution, was isolated and characterized via 1H NMR (in CDCl3) and 13C NMR (in CDCl3) (see ESI†). The spectra proved that the di-imine structure is symmetrical, further confirming the applicability of DFF as an enzyme crosslinker.
In addition, isopropylamine was chosen as another model amine, because it forms a water-soluble product with DFF. This allows to monitor the reaction in solution for longer times, in contrast to the n-butylamine product that directly separates from the solution. The reaction was conducted in potassium phosphate buffer diluted with deuterated water, in order to follow the reaction via 1H NMR.
After 1 hour, the reaction reached the equilibrium. The 1H NMR spectrum (see ESI†) indicates that the ratio between the mono- and di-imine is 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0. Interestingly, the hypothetical hydrated mono-imine is not present, indicating that the formation of an imine bond shifts the equilibrium of the other aldehyde group towards the non-hydrated form. The spectrum did not change during the following 30 days (see ESI†), reporting the signals from the di-imine derivative and the mono-imine derivative (pictured in Scheme 1b), residual signals of isopropylamine (18% of the initial amount of amine is still free in solution) and no signal of residual DFF in solution. These observations lead to the following conclusions: (i) the formation of the products between isopropylamine, a primary amine, and DFF happens within the first hour of reaction; (ii) after reaching the equilibrium, the only products present in solution at 22 °C are the imine products, even after 1 week; (iii) the di-imine product shows high stability over long storage times, not displaying the formation of side (oxidation) products.
1.0. Interestingly, the hypothetical hydrated mono-imine is not present, indicating that the formation of an imine bond shifts the equilibrium of the other aldehyde group towards the non-hydrated form. The spectrum did not change during the following 30 days (see ESI†), reporting the signals from the di-imine derivative and the mono-imine derivative (pictured in Scheme 1b), residual signals of isopropylamine (18% of the initial amount of amine is still free in solution) and no signal of residual DFF in solution. These observations lead to the following conclusions: (i) the formation of the products between isopropylamine, a primary amine, and DFF happens within the first hour of reaction; (ii) after reaching the equilibrium, the only products present in solution at 22 °C are the imine products, even after 1 week; (iii) the di-imine product shows high stability over long storage times, not displaying the formation of side (oxidation) products.
These findings suggest that the immobilization of enzymes with DFF, that is conducted in similar conditions at room temperature, only involves the formation of Schiff bases between DFF and the protein, and that the resulting imine is stable under immobilization conditions, most probably due to the conjugation of the imine bond with the furan ring and the resonance stabilization. The higher stability of the aromatic imines and the consequent shift of the equilibrium towards the imine products have been already investigated and documented.24 For instance, terephthalaldehyde, has been used as crosslinking agent to form stable gelatine membranes.20
Conveniently, the behaviour of DFF in aqueous environment is more predictable than that of glutaraldehyde, facilitating further studies towards the nature of the stable chemical bond. Moreover, the dosage of this crosslinking agent can be easily determined and any excess prevented because the clearer elucidation of the chemistry of the binding process. Finally, the formation of toxic side products can be excluded due to the absence of enolic equilibria, as in the case of glutaraldehyde.
Glucoamylase from Aspergillus niger
The immobilization study employed glucoamylase from Aspergillus niger as the model enzyme. Glucoamylase belongs to the family of glycoside hydrolases (E.C. 3.2.1.3), which catalyses the cleavage of α-1,4- and β-1,6-glycosidic bonds, starting from the non-reducing end of the polysaccharide chain.25,26 This enzyme was chosen due to its employment on a large scale in industry,27 as well as its easy immobilization on methacrylic carriers.Structurally, glucoamylases are composed by an N-terminal catalytic domain, highly conserved between different organisms, and a smaller, C-terminal starch-binding domain; the two domains are linked by a highly flexible linker region.28
The crystallographic structure of glucoamylase from A. niger was analysed in silico, using existing structures of the catalytic domain (PDB ID: 3EQA) and the starch binding domain (PDB ID: 5GHL), to establish whether covalent binding to the carrier is possible. No structures were found of the whole protein, as the flexible linker portion that connects the two domains breaks during the crystallization process;26 however, for the present study it was deemed sufficient to analyse the isolated domain structures. The analysis concerned the presence of lysine residues on the enzyme surface; both the catalytic (Fig. 2A) and starch binding domain (Fig. 2B) present superficial Lys residues. It is therefore possible, in principle, to covalently immobilize the protein. In conclusion, glucoamylase from Aspergillus niger is suitable for covalent immobilization due to the presence of lysine residues on its surface.
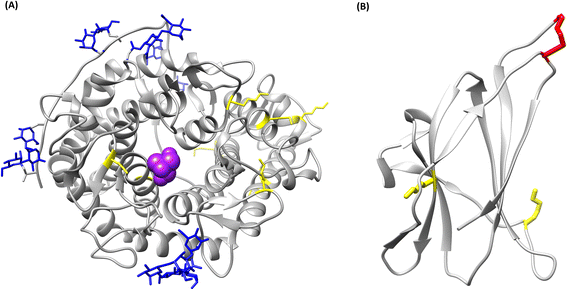 | ||
| Fig. 2 Tridimensional models of the structure of glucoamylase from Aspergillus niger. In yellow, lysine residues; in blue, glycan residues; in purple, TRIS inhibitor in the catalytic pocket; in red, disulfide bonds. (A) Catalytic domain (PDB ID: 3EQA), (B) starch binding domain (PDB ID: 5GHL). | ||
Comparison of DFF and glutaraldehyde: immobilization of glucoamylase on PMMA carrier
Glucoamylase was immobilized on an amino-functionalized poly(methylmethacrylate) carrier, using the following general procedure: (a) activation of the carrier by incubation with the dialdehyde, (b) immobilization by incubation of the activated resin with the enzyme preparation; both steps were carried out in aqueous potassium phosphate buffer (25 mM, pH 7).The activation step was studied using four different amounts of each dialdehyde (200, 20, 2 and 0.2 μmol gcarrier−1), in order to directly compare the efficiency of DFF and glutaraldehyde over a wide range of concentrations. The highest concentration of dialdehyde used for the activation step corresponds to one third of the concentration of amine groups of the methacrylic resin (600 μmol gresin−1), as declared by the producer.
The resulting activated carriers were then incubated with a fixed amount of enzyme (120 U gwet carrier−1). An excess of enzyme was used in the immobilization process, leading to a 20% of residual activity in the supernatant after the procedure (data not shown). Therefore, the observed differences in recovered activity are not ascribable to a shortage of enzyme in the procedure.
The activity of the resulting enzymatic preparations was measured in a standard assay, by recycling the enzyme for four consecutive assays, until a plateau activity value was reached. This plateau value is regarded as the enzymatic activity immobilized on the carrier. The results are presented in Table 1.
| Crosslinker amount (μmol gcarrier−1) | Glutaraldehyde | DFF | ||
|---|---|---|---|---|
| Assay cycle | Activity (U gdry−1) | Assay cycle | Activity (U gdry−1) | |
| a DFF and glutaraldehyde comparison – continuous flow experiment. | ||||
| 200 | 1 | 105 | 1 | 137 |
| 2 | 90 | 2 | 107 | |
| 3 | 86 | 3 | 107 | |
| 4 | 87 | 4 | 107 | |
| 20 | 1 | 214 | 1 | 124 |
| 2 | 121 | 2 | 90 | |
| 3 | 107 | 3 | 83 | |
| 4 | 90 | 4 | 80 | |
| 2 | 1 | 170 | 1 | 118 |
| 2 | 120 | 2 | 96 | |
| 3 | 77 | 3 | 70 | |
| 4 | 76 | 4 | 66 | |
| 0.2 | 1 | 113 | 1 | 119 |
| 2 | 84 | 2 | 95 | |
| 3 | 77 | 3 | 77 | |
| 4 | 75 | 4 | 73 | |
The decrease in activity between the first and the following assay cycles can be explained by the presence of adsorbed, non-covalently bound enzyme on the carrier, and it is comparable between the two crosslinkers.
By comparing the plateau values of the last two cycles, we concluded that the binding efficiency of glutaraldehyde and DFF for enzyme immobilization is comparable at all tested concentrations.
Concerning the different crosslinker concentration, the results (Table 1) show how the immobilized activity remains similar throughout the range of tested concentrations, even with a 1000-fold decrease in the dialdehyde concentration. With such a low concentration of crosslinker, and an excess of enzyme, any difference in crosslinker efficiency would be evident. The activity values are very similar between the two different crosslinking agents, glutaraldehyde and DFF. This observation further supports the conclusion that the binding efficiency of the two crosslinkers is comparable.
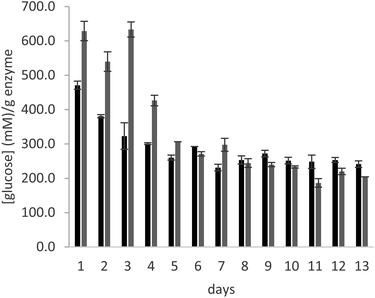
In order to test the behaviour of the immobilized enzyme preparations for longer times, the operational stability of glucoamylase immobilized on a PMMA carrier using either DFF or glutaraldehyde was analysed in a continuous flow experiment, over the course of 13 days. The immobilized enzyme was introduced in a glass column, which was filled with a 25% maltose solution in 10 mM citrate buffer, pH 4.5. A continuous flow of solution was supplied to the column at a rate of 0.15 mL min−1 over the course of the experiment. The enzyme samples used for the experiment are those immobilized with the lowest amount of crosslinker (0.2 μmolaldehyde gwet carrier−1).
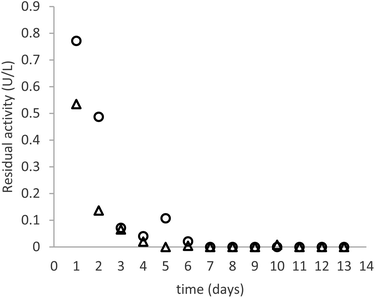
As can be seen in Fig. 3, the productivity of the enzyme decreases significantly in the first three days, stabilizing for later measurements. The trend is comparable in the samples immobilized with both crosslinkers. The cause of this behaviour is elucidated by Fig. 4, which reports the measures of enzymatic activity in the effluent as a consequence of the detachment of some residual non-covalently bound enzyme. Notably, after 2 days of the reaction, no detached enzyme is found in the effluent, and the productivity in glucose remains constant.
In conclusion, the data obtained for long term stability with the continuous flow experiment further demonstrate the efficiency of DFF for the covalent immobilization of glucoamylase on the selected PMMA carrier and confirm its potential use as a more sustainable replacer of glutaraldehyde for this purpose.
Ecotoxicological studies
To the best of our knowledge, the scientific literature reports only three papers dealing with DFF toxicity.17–19 In 2014, Frade et al.17 analysed the toxicity of HMF and twenty among its derivatives, including DFF, on human skin fibroblast cells of line CRL-1502. This is a non-tumour cell line, chosen for its resemblance with human healthy tissues. The cells were incubated with 100–500 μM of the tested compounds, DFF resulted in a cell viability of 32 ± 2% after 72 hours.Another study from the same research group19 examines the toxicity of various platform chemicals, including DFF, in a Microtox assay, following the decrease in luminescence of marine bacteria Aliivibrio fischeri. The assay consisted in exposing A. fischeri to nine different concentrations of the analysed compounds, at a temperature of 15 °C, for 15 and 30 minutes. After the exposure, the luminescence of the sample is analysed, and the EC50 is determined as the concentration corresponding to a 50% decrease in luminescence of the bacteria.
For most of the analysed compounds, the time of exposure does not influence significantly the resulting EC50; an exception is DFF, for which EC50 halves (in other words, toxicity doubles) going from 5 to 30 minutes of exposure. Among the analysed compounds, the authors regard DFF as moderately toxic (EC50 = 100–10 mg L−1).
Lastly, DFF toxicity is briefly mentioned in a work by Martins et al.,18 which examines toxicity of HMF and some of its derivatives towards Aspergillus nidulans. In this paper, IC50 is calculated as the concentration of compound that can inhibit of 50% the growth of A. nidulans, and DFF is briefly mentioned compared to other molecules. Interestingly, in this study it is less toxic (higher IC50) than HMF, unlike what observed in the other papers.
In this paper, we examined the ecotoxicology of DFF, directly comparing it to that of glutaraldehyde. Ecotoxicological tests were performed on Aliivibrio fischeri, in order to compare marine toxicity of DFF as compared to that of glutaraldehyde. Inhibition of natural bioluminescence at the maximum concentration tested was reported in Table 2 as mean value (standard deviation, SD) of experimental replicates. Reported results were corrected according to the DMSO natural toxicity at the concentration used to solubilize tested chemicals (0.5% DMSO in 20 g L−1 NaCl ultrapure water).
| 15 min | 30 min | |||
|---|---|---|---|---|
| Mean (%) | SD (%) | Mean (%) | SD (%) | |
| DFF | 2.31 | 1.13 | 3.76 | 0.32 |
| Glutaraldehyde | 0.41 | 0.35 | 4.37 | 0.33 |
Results highlighted that toxicity of tested chemicals are comparable at the maximum dose tested of 5 mg L−1, closer together and lower of 5% of bioluminescence inhibition after 30 minutes of exposure. Effects lower than 15% are considered not toxic by the Italian law29 and the specific literature.30,31 This is an encouraging result as it demonstrates that DFF can be a valid substitute for traditional aldehyde in terms of eco-compatibility. Although bacteria constitute the first link in the trophic web of aquatic ecosystems, the ecotoxicological assay with A. fischeri is widely used to evaluate the eco-compatibility of chemicals in both freshwater and marine ecosystems as it is highly standardized and repeatable and largely used to evaluate ecotoxicity of chemicals of possible industrial interest.32,33 These results are important to highlight that DFF shows an absence of toxicity under tested conditions for the standardized species used (A. fischeri).
Overall, in terms of toxicity DFF also has the advantage of being poorly soluble in water (about 5 mg mL−1) which decreases its harmful potential in aqueous environment as compared to the fully miscible glutaraldehyde. More importantly, the high boiling point of DFF (276.8 °C at 760 mmHg) makes this crosslinking agent considerably less harmful for human handling as compared to the volatile glutaraldehyde (boiling point 187 °C) that causes severe respiratory toxicity.
Conclusions
DFF was successfully employed as cross-linker for glucoamylase immobilization on amino-functionalized methacrylic carriers. Immobilization experiments and systematic comparison with glutaraldehyde at four different concentrations show that the efficiency of the two crosslinkers is very similar, giving comparable activities at all tested concentrations, even at very low crosslinker concentration for the activation step. Continuous flow experiment confirms that the glucoamylase immobilized with the two crosslinking agents displays comparable activity and long-term stability, with the leaching of residual adsorbed protein during the first three days of the continuous process and then reaching a plateau for the remaining 9 days.NMR studies show that the formation of covalent bonds between DFF and primary amino groups occurs via imine bond formation only, unlike the case of glutaraldehyde where different mechanisms of reaction are possible.8 It is widely known that the formation of an imine – from an amine and an aldehyde – is a reversible reaction which operates under thermodynamic control such that the formation of kinetically competitive intermediates are, in the fullness of time, replaced by the thermodynamically most stable product.34 However, when the glucoamylase was immobilized on DFF activated amino-carriers the stability of the covalent immobilization was confirmed also at acid pH (4.5). The shifting of the equilibrium towards the imine product is probably ascribable to the higher stability of the imine bonds formed with the aromatic aldehydes, as already documented in investigations dealing with terephthalaldehyde.24 The ecotoxicology study of DFF against Aliivibrio fischeri showed a decrease in bioluminescence below the toxicity threshold for both dialdehydes. In terms of toxicity DFF has the advantage of being poorly soluble in water and, more importantly, poorly volatile as compared to glutaraldehyde, which causes severe respiratory toxicity.
The present study paves the way for further investigations aiming at the replacement of glutaraldehyde as crosslinking agent in an array of industrial applications, with the bio-based, less volatile, easy to handle DFF, which has the additional advantage of reacting according to clear and simple reaction mechanisms. The latter feature enables its easier dosage as crosslinking agent while minimizing the chemical routes that might cause toxic effects.
Author contributions
Conceptualization: Chiara Danielli, Luuk van Langen, Lucia Gardossi. Methodology: Chiara Danielli, Luuk van Langen, Deborah Boes, Monia Renzi. Validation: Chiara Danielli, Deborah Boes, Serena Anselmi, Francesca Provenza. Investigation: Chiara Danielli, Deborah Boes, Serena Anselmi, Francesca Provenza, Fioretta Asaro. Writing – original draft preparation: Chiara Danielli, Monia Renzi. Writing – reviewing and editing: Chiara Danielli, Luuk van Langen, Lucia Gardossi, Fioretta Asaro, Monia Renzi. Supervision: Luuk van Langen, Lucia Gardossi. Funding acquisition: Luuk van Langen, Lucia Gardossi.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
We thank prof. Patrizia Nitti for fruitful discussions. We are grateful to Resindion (Binasco, Milano) for providing the methacrylic carrier. This project has received funding from the European Union's Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement no. 860414 (INTERfaces project).Notes and references
- R. Di Cosimo, J. Mc Auliffe, A. J. Poulose and G. Bohlmann, Chem. Soc. Rev., 2013, 42, 6437–6474 RSC.
- M. C. R. Franssen, P. Steunenberg, E. L. Scott, H. Zuilhof and J. P. M. Sanders, Chem. Soc. Rev., 2013, 42, 6491–6533 RSC.
- R. A. Sheldon, Adv. Synth. Catal., 2007, 349, 1289–1307 CrossRef CAS.
- A. Basso and S. Serban, Mol. Catal., 2019, 479, 110607 CrossRef CAS.
- U. Hanefeld, L. Gardossi and E. Magner, Chem. Soc. Rev., 2009, 38, 453–468 RSC.
- R. Schoevaart, A. Siebum, F. Van Rantwijk, R. Sheldon and T. Kieboom, Starch/Staerke, 2005, 57, 161–165 CrossRef CAS.
- Glutaraldehyde Market Overview, https://www.futuremarketinsights.com/reports/glutaraldehyde-market Search PubMed.
- I. Migneault, C. Dartiguenave, M. J. Bertrand and K. C. Waldron, Biotechniques, 2004, 37, 790–802 CrossRef CAS PubMed.
- P. M. Hardy, G. J. Hughes and H. N. Rydon, J. Chem. Soc., Perkin Trans. 1, 1979, 2282–2288 RSC.
- T. Takigawa and Y. Endo, J. Occup. Health, 2006, 48, 75–87 CrossRef CAS PubMed.
- Toxicological Profile, https://www.atsdr.cdc.gov/sites/peer_review/tox_profile_glutaraldehyde.html Search PubMed.
- Glutaraldehyde ECHA Substance Information, https://echa.europa.eu/it/substance-information/-/substanceinfo/100.003.506 Search PubMed.
- Glutaraldehyde ECHA Brief Profile, https://echa.europa.eu/it/brief-profile/-/briefprofile/100.003.506 Search PubMed.
- C. Laugel, B. Estrine, J. Le Bras, N. Hoffmann, S. Marinkovic and J. Muzart, ChemCatChem, 2014, 6, 1195–1198 CAS.
- M. M. Cajnko, U. Novak, M. Grilc and B. Likozar, Biotechnol. Biofuels, 2020, 13, 1–12 CrossRef PubMed.
- A. Millán Acosta, C. Cuesta Turull, D. Cosovanu, M. Núria Sala and R. Canela-Garayoa, ACS Sustain. Chem. Eng., 2021, 9, 14550–14558 CrossRef.
- R. F. M. Frade, J. A. S. Coelho, S. P. Simeonov and C. A. M. Afonso, Toxicol. Res., 2014, 3, 311–314 CrossRef CAS.
- C. Martins, D. O. Hartmann, A. Varela, J. A. S. Coelho, P. Lamosa, C. A. M. Afonso and C. Silva Pereira, Microb. Biotechnol., 2020, 13, 1983–1996 CrossRef CAS PubMed.
- S. P. M. Ventura, P. De Morais, J. A. S. Coelho, T. Sintra, J. A. P. Coutinho and C. A. M. Afonso, Green Chem., 2016, 18, 4733–4742 RSC.
- J. Biscarat, B. Galea, J. Sanchez and C. Pochat-Bohatier, Int. J. Biol. Macromol., 2015, 74, 5–11 CrossRef CAS PubMed.
- J. Carro, P. Ferreira, L. Rodríguez, A. Prieto, A. Serrano, B. Balcells, A. Ardá, J. Jiménez-Barbero, A. Gutiérrez, R. Ullrich, M. Hofrichter and A. T. Martínez, FEBS J., 2015, 282, 3218–3229 CrossRef CAS PubMed.
- S. Cantone, V. Ferrario, L. Corici, C. Ebert, D. Fattor, P. Spizzo and L. Gardossi, Chem. Soc. Rev., 2013, 42, 6262–6276 RSC.
- R. V. Ulijn, N. Bisek, P. J. Halling and S. L. Flitsch, Org. Biomol. Chem., 2003, 1, 1277–1281 RSC.
- E. Kulla and P. Zuman, J. Phys. Chem. A, 2007, 111, 12871–12877 CrossRef CAS PubMed.
- J. Lee and M. Paetzel, Acta Crystallogr., Sect. F: Struct. Biol. Cryst. Commun., 2011, 67, 188–192 CrossRef CAS PubMed.
- C. Roth, O. V. Moroz, A. Ariza, L. K. Skov, K. Ayabe, G. J. Davies and K. S. Wilson, Acta Crystallogr., Sect. D: Struct. Biol., 2018, 74, 463–470 CrossRef CAS PubMed.
- P. W. Tardioli, M. F. Vieira, A. M. S. Vieira, G. M. Zanin, L. Betancor, C. Mateo, G. Fernández-Lorente and J. M. Guisán, Process Biochem., 2011, 46, 409–412 CrossRef CAS.
- J. Sauer, B. W. Sigurskjold, U. Christensen, T. P. Frandsen, E. Mirgorodskaya, M. Harrison, P. Roepstorff and B. Svensson, Biochim. Biophys. Acta, Protein Struct. Mol. Enzymol., 2000, 1543, 275–293 CrossRef CAS PubMed.
- Italian decree by the Ministry for the Environment, D.M 173/2016, https://www.gazzettaufficiale.it/eli/id/2016/09/06/16G00184/sg Search PubMed.
- A. Broccoli, L. Morroni, A. Valentini, V. Vitiello, M. Renzi, C. Nuccio and D. Pellegrini, Aquat. Toxicol., 2021, 237, 105905 CrossRef CAS PubMed.
- M. Piccardo, F. Provenza, S. Anselmi, A. Broccoli, A. Terlizzi and M. Renzi, J. Mar. Sci. Eng., 2021, 9, 1–17 CrossRef.
- S. Bruzzone, C. Chiappe, S. E. Focardi, C. Pretti and M. Renzi, Chem. Eng. J., 2011, 175, 17–23 CrossRef CAS.
- C. Pretti, M. Renzi, S. Ettore Focardi, A. Giovani, G. Monni, B. Melai, S. Rajamani and C. Chiappe, Ecotoxicol. Environ. Saf., 2011, 74, 748–753 CrossRef CAS PubMed.
- M. E. Belowich and J. F. Stoddart, Chem. Soc. Rev., 2012, 41, 2003–2024 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ra07153c |
| This journal is © The Royal Society of Chemistry 2022 |