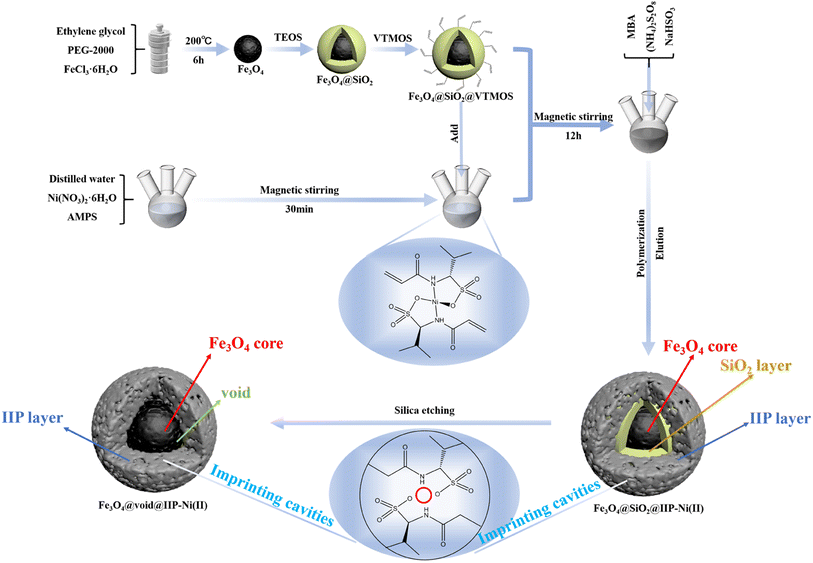
Open Access Article
Open Access ArticleSelective removal and recovery of Ni(II) using a sulfonic acid-based magnetic rattle-type ion-imprinted polymer: adsorption performance and mechanisms†
Weiye Zhang a,
Xiujun Dengb,
Siqing Yea,
Yan Xiaa,
Lingling Lia,
Weili Lia and
Hongxing He
a,
Xiujun Dengb,
Siqing Yea,
Yan Xiaa,
Lingling Lia,
Weili Lia and
Hongxing He *a
*a
aYunnan Key Laboratory of Food Safety Testing Technology, Kunming University, Kunming 650214, China. E-mail: hxhe0212@kmu.edu.cn
bSchool of Chemistry and Chemical Engineering, Yunnan Key Laboratory of Metal-Organic Molecular Materials and Device, Kunming University, Kunming 650214, China
First published on 1st December 2022
Abstract
It is significant to selectively remove Ni(II) ions from wastewater. A novel sulfonic acid-based magnetic rattle-type ion-imprinted polymer (Fe3O4@void@IIP-Ni(II)) was designed by taking advantage of the strong interaction between Ni(II) and sulfonic acid groups. Green polymerization was used to synthesize Fe3O4@void@IIP-Ni(II), which was then investigated using SEM, TEM, FT-IR, VSM, TGA, EDS, and XPS. The adsorption results indicated that the prepared imprinted material had a short adsorption equilibrium time (10 min), good magnetic responsiveness (about 5 seconds) and high adsorption capacity (44.64 mg g−1) for Ni(II) at the optimal pH of 6.0. The removal rate of Ni(II) was up to 99.97%, and the adsorption process was spontaneous and endothermic, following the pseudo-secondary kinetic model and Langmuir model. The selectivity coefficients of the imprinted material were 4.67, 4.62, 8.94 and 9.69 for Ni(II)/Co(II), Ni(II)/Cu(II), Ni(II)/Pb(II) and Ni(II)/Zn(II), respectively. The regeneration and application of the imprinted material in actual water samples have been verified. Moreover, the mechanism of selective adsorption for Ni(II) was investigated by FTIR, XPS and density functional theory (DFT) calculation. The results showed that the imprinted sorbent has a strong binding ability with Ni(II), and the adsorption of Ni(II) on Fe3O4@void@IIP-Ni(II) was the result of the co-coordination of O atoms of the sulfonic acid groups and N atoms of –N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups in AMPS with Ni(II).
O groups in AMPS with Ni(II).
1. Introduction
Heavy metals are important pollutants causing potential threats. The threat of heavy metal pollution is that they cannot be decomposed by microorganisms and tend to accumulate in living organisms.1,2 Nickel is a typical heavy metal that mainly exists in the form of Ni(II) in electroplating, mining, battery, and other industrial wastewater. A small amount of nickel is beneficial to the human body, but high levels of Ni(II) can damage the central nervous system and lead to a variety of skin diseases and even cancers.3,4 Different countries and organizations have strict regulations on the discharge limits of nickel-containing wastewater and the content of nickel in drinking water. For example, the maximum allowable discharge concentration of nickel-containing wastewater in China is 1.0 mg L−1, and the permissible limit of nickel ions in drinking water recommended by the World Health Organization is 20 μg L−1.5,6 On the other hand, as a more expensive metal, the recovery of nickel is also very valuable, because it not only saves operating costs, but also reduces the total nickel usage, thus improving environmental safety. As a result, it is essential to develop a method that can efficiently and selectively separate and recover Ni(II) from wastewater.So far, several methods used to remove Ni(II) include adsorption, chemical precipitation, membrane separation and ion exchange.7–10 Among these methods, adsorption has been widely used because of its merits of facile operation, cost-effectiveness and high efficiency.11 However, traditional adsorbents lack the selectivity separation for specific metal ions, which limits their application in specific metal recovery.
In recent years, ion imprinting polymers (IIPs) have attracted extensive attention due to their selective recognition of template ions. Compared to traditional imprinting techniques, surface imprinting has the advantages of fast adsorption kinetics, good selectivity and high adsorption capacity.12,13 This technology requires various matrix materials to play a supporting role in the imprinting process. Currently, commonly used matrix materials include silica particles, graphene oxide (GO), chitosan (CTS), carbon nanotubes (CNTs) and Fe3O4 microspheres.14–18 Due to the magnetic properties of Fe3O4 microspheres, its magnetic separation technology can well solve the problem of solid–liquid separation of imprinting materials in waste liquid, which has a great development prospect.19 Recently, a rattle-type magnetic IIPs was constructed. Rattle-type structure is a core@void@shell structure. It can be described as a movable core encapsulated in a shell.20 As an important extension of core–shell polymers, rattle-type polymers are mostly synthesized by a templating strategy in which the middle layer and the outer shell are prepared on a prefabricated core and the middle layer is subsequently removed. The large cavity volume between the inner core and the outer shell of the rattle-type polymers provides sufficient space for the loading of target ions.21–23 The rattle-type magnetic imprinted polymer has not only magnetic sensitivity, high specific surface area, low density and higher adsorption capacity, but also has good selectivity for template ions.24 Up to now, theoretical calculations based on density functional theory (DFT) have been used to design IIPs and reveal the adsorption mechanism.25–27 According to previous work, sulfonic acid-based imprinted polymers have good adsorption selectivity for Ni(II).28 Unfortunately, there are few reports on the affinity mechanism between Ni(II) and sulfonic acid groups by DFT calculations.
Herein, a novel sulfonic acid-based magnetic rattle-type Ni(II) ion-imprinted polymer was synthesized by green polymerization using water as porogen and AMPS as water-soluble functional monomer to selectively remove and recover Ni(II) from aqueous solution. The selective adsorption properties for Ni(II) were studied by batch adsorption experiments. It was characterized by FT-IR, SEM, TEM, EDS, VSM and TG, and the adsorption mechanism for Ni(II) was discussed by FT-IR, XPS and DFT calculations.
2. Experimental
2.1 Reagents
HCl and NaOH were purchased from Kolon Chemical Co. Ltd (Chengdu, China). Ni(NO3)2·6H2O, Co(NO3)2·7H2O, Cu(NO3)2·2H2O, Zn(NO3)2·7H2O and Pb(NO3)2 were purchased from Beijing Chemical Plant (Beijing, China). 2-Acryloyl-2-methylpropionic acid (AMPS) was purchased from Aladdin Biochemical Technology Co. (Shanghai, China). Ammonium persulfate and sodium bisulfate were purchased from Windship Chemical Reagent Technology Co. (Tianjin, China). N,N′-Methylenebisacrylamide (MBA) was purchased from Maclean Biochemical Technology Co. (Shanghai, China). All aqueous solutions were prepared by deionized water.2.2 Synthesis of magnetic Ni(II) ion-imprinted polymer (Fe3O4@SiO2@IIP-Ni(II) and Fe3O4@void@IIP-Ni(II))
The synthesis of Fe3O4@SiO2@NIP-Ni(II) adsorbent was roughly the same as the above steps, except that Ni(NO3)26H2O was not introduced and HCl was not used for washing.
Fe3O4@void@NIP-Ni(II) spheres were prepared using the same procedure.
2.3 Batch adsorption experiments
Batch adsorption experiments were performed by adding a certain amount of sorbent to a certain volume of metal ion solution, and the mixture was shaken for a certain time at 25 °C. The concentrations of all metal ions were determined by ICP-OES. Each adsorption experiment was conducted in triplicate. The adsorption capacity was calculated according to the following eqn (1).29
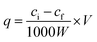 | (1) |
The effect of pH on Ni(II) adsorption was examined by adding 20 mg sorbent into 20 mL 100 mg L−1 Ni(II) ion solution with varying pH values at 25 °C for 2 h. The pH of the solution was adjusted by HCl and NaOH solution. The effect of contact time on Ni(II) adsorption was determined by adding 50 mg sorbent into 50 mL 100 mg L−1 Ni(II) ion solution at different contact times at 25 °C and pH 6.0 for 60 min. Samples were taken out from the solution at various time intervals until saturated adsorption was reached. To evaluate the effect of initial concentrations on Ni(II) adsorption, 20 mg sorbent was added to 20 mL Ni(II) ion solution with varying concentrations (25–200 mg L−1) at 25 °C and pH 6.0 for 2 h.
In the selective adsorption experiment, 20 mg adsorbent was added to 20 mL mixed solution containing 5 mg L−1 Ni2+, Co2+, Cu2+, Zn2+and Pb2+, and vibrated at 25 °C for 2 h. Finally, the ion concentration in the filtrate was detected by ICP-OES. The distribution ratio (D), the selectivity coefficient (k) and the relative selectivity coefficient (k′) were calculated according to the following eqn (2)–(4).15
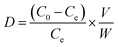 | (2) |
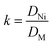 | (3) |
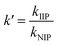 | (4) |
2.4 Theoretical calculation
Density functional theory calculations were performed utilizing the Gaussian 16 W, the geometry optimization of the functional group and metal ion complexes were performed at PBE1PBE (solvent = water, D3 BJ)/def2svp level, and def2tzvp basis set was used to calculate the adsorption energy (Ead).27 The adsorption energy (Ead) between AMPS and the different metal ions was calculated by eqn (5).| Ead = E(total) − E(AMPS) − E(adsorbates) | (5) |
3. Results and discussion
3.1. Characterization of absorbents
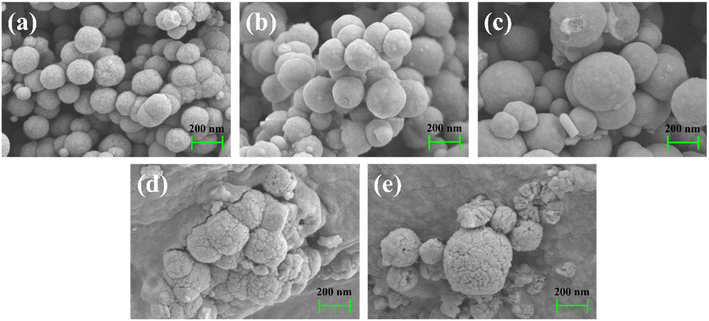 | ||
| Fig. 2 SEM images of (a) Fe3O4 (b) Fe3O4@SiO2 (c) Fe3O4@SiO2@VTMOS (d) Fe3O4@SiO2@IIP-Ni(II) and (e) Fe3O4@void@IIP-Ni(II). | ||
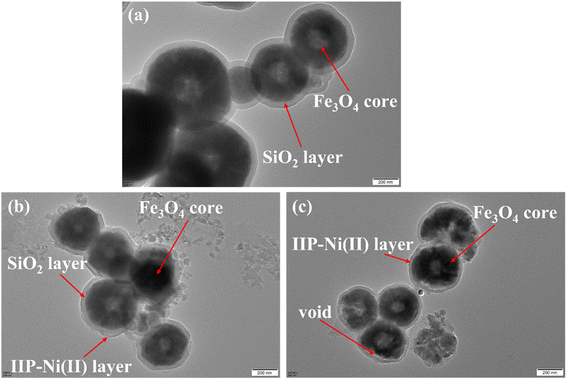
Fig. 3 shows the TEM images of Fe3O4@SiO2, Fe3O4@SiO2@IIP-Ni(II) and Fe3O4@void@IIP-Ni(II). The dark Fe3O4 core can be clearly seen in each image. As shown in Fig. 3(a), the light-colored thin layer outside the Fe3O4 core is coated with silica gel. In Fig. 3(b), a thin layer appears outside the silica gel layer, which is the imprinted thin layer grafted onto the surface of Fe3O4@SiO2. Fig. 3(c) shows the Fe3O4@SiO2@IIP-Ni(II) treated with HF solution. Compared with Fig. 3(b), it can be seen that there is only one continuous thin layer outside the Fe3O4 core, and a hollow structure is formed between the thin layer and the core. It is the result of HF acid etching of the silica layer while retaining only the imprinted layer.
In addition, EDS spectra of Fe3O4@void@IIP-Ni(II) before and after Ni(II) ion adsorption are shown in Fig. S1.† Before and after Ni(II) adsorption, the content of S was relatively high, 6.78% (wt%) and 6.09% (wt%), respectively, while the content of Si is relatively low, 0.06% (wt%) and 0.26% (wt%), respectively. The high content of the former is mainly due to the introduction of S in the sulfonic acid group on the functional monomer AMPS. It can also proved that AMPS was successfully introduced into the adsorbent. The low content of the latter is mainly due to the etching of SiO2 by HF solution, which further confirms the generation of hollow structures. The obvious signal of Ni changed considerably before and after adsorption, with the Ni content changing from 0 (wt%) to 4.47 (wt%). The former indicated that Ni(II) elution was complete after the imprinting process, while the latter confirmed the existence of the adsorption properties of Fe3O4@void@IIP-Ni(II).
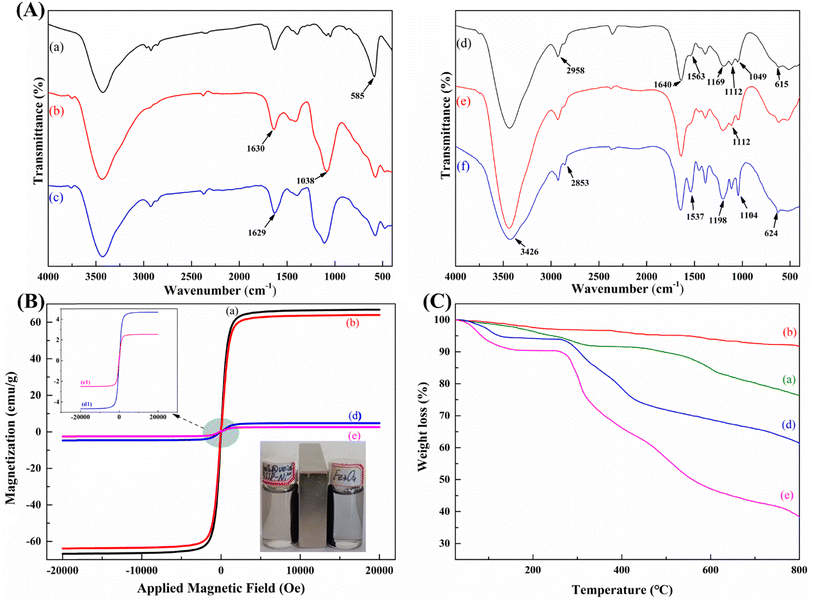
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration. This proved that the double bond was successfully grafted onto the surface of Fe3O4@SiO2 (Fig. 4A(c)).31 As shown in Fig. 4A(d), 2958 cm−1 was the stretching vibration of C–H, 1640 cm−1 was the stretching vibration of C
C stretching vibration. This proved that the double bond was successfully grafted onto the surface of Fe3O4@SiO2 (Fig. 4A(c)).31 As shown in Fig. 4A(d), 2958 cm−1 was the stretching vibration of C–H, 1640 cm−1 was the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, 1169 cm−1, 1049 cm−1 and 615 cm−1 were the stretching vibration of S
O, 1169 cm−1, 1049 cm−1 and 615 cm−1 were the stretching vibration of S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and the asymmetric stretching vibration of S–O, respectively,32 1563 cm−1 was the characteristic peak of N–H.33 The appearance of these peaks indicated that the polymer AMPS-co-MBA was successfully grafted onto the Fe3O4@SiO2@VTMOS surface. In the IR spectrum of Fe3O4@void@IIP-Ni(II) shown in Fig. 4A(e), compared with Fig. 4A(d), the intensity of the Si–O peak at 1112 cm−1 was weakened, which proved the shedding of the silicon shell and the formation of the rattle-type structure.
O and the asymmetric stretching vibration of S–O, respectively,32 1563 cm−1 was the characteristic peak of N–H.33 The appearance of these peaks indicated that the polymer AMPS-co-MBA was successfully grafted onto the Fe3O4@SiO2@VTMOS surface. In the IR spectrum of Fe3O4@void@IIP-Ni(II) shown in Fig. 4A(e), compared with Fig. 4A(d), the intensity of the Si–O peak at 1112 cm−1 was weakened, which proved the shedding of the silicon shell and the formation of the rattle-type structure.
3.2 Adsorption performance
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, the amount of template ion and functional monomer was less, and the number of imprinting sites was less, resulting in smaller adsorption capacity.35 The adsorption capacity increased at the dosage ratio of 2
4, the amount of template ion and functional monomer was less, and the number of imprinting sites was less, resulting in smaller adsorption capacity.35 The adsorption capacity increased at the dosage ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, and reached a maximum at the dosage ratio is 2
4, and reached a maximum at the dosage ratio is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6. Whereas, the adsorption capacity gradually decreased as the dosage of cross-linking agent increased from 6 mmol to 10 mmol. This is because the increase of crosslinkers makes the surface of the imprinted polymer dense, resulting in the encapsulation of the imprinted sites and the difficulty of elution of template ions.36 Overall, optimal amounts of template (Ni(II)), monomer (AMPS) and crosslinker (MBA) were 2 mmol, 4 mmol and 6 mmol, respectively.
6. Whereas, the adsorption capacity gradually decreased as the dosage of cross-linking agent increased from 6 mmol to 10 mmol. This is because the increase of crosslinkers makes the surface of the imprinted polymer dense, resulting in the encapsulation of the imprinted sites and the difficulty of elution of template ions.36 Overall, optimal amounts of template (Ni(II)), monomer (AMPS) and crosslinker (MBA) were 2 mmol, 4 mmol and 6 mmol, respectively.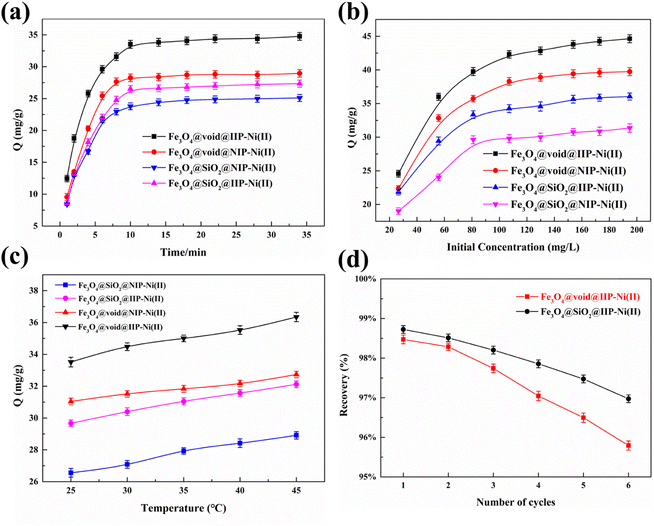 | ||
| Fig. 5 Effect of time (a), Ni(II) initial concentration (b), temperature and (c) desorption and reusability (d) on the adsorption. | ||
To further investigate the adsorption kinetic mechanism of imprinted materials, pseudo-first-order kinetics and pseudo-second-order kinetics models were used for the linear fitting of data. The linear form of the pseudo-first-order kinetics and the pseudo-second-order kinetics models are expressed as follows eqn (6) and (7).12
ln(qe − qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe − k1t qe − k1t
| (6) |
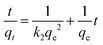 | (7) |
Fig. S4† present the plots of pseudo-first-order and pseudo-second-order kinetic models of the four adsorbents. The kinetic parameters of the two models are shown in Table 1. The results showed that the correlation coefficients fitted by the pseudo-second-order kinetic model (R22) were better than those of the pseudo-first-order kinetic model (R12), and they are 0.9977, 0.9983, 0.9989 and 0.9982, respectively. Besides, the qe value fitted by pseudo-second-order kinetic was closer to the experimental value. It can be concluded that the adsorption of Ni(II) ion onto Fe3O4@void@IIP-Ni(II), Fe3O4@void@NIP-Ni(II), Fe3O4@SiO2@IIP-Ni(II) and Fe3O4@SiO2@NIP-Ni(II) was a chemical adsorption process.
| Adsorption kinetic model | qe,exp | Pseudo-first-order kinetic models | Pseudo-second-order kinetic models | ||||
|---|---|---|---|---|---|---|---|
| k1 | qe,cal | R12 | k2 | qe,cal | R22 | ||
| Fe3O4@void@IIP-Ni(II) | 27.35 | 0.1428 | 11.81 | 0.8905 | 0.01741 | 29.33 | 0.9977 |
| Fe3O4@void@NIP-Ni(II) | 25.10 | 0.1323 | 9.884 | 0.8850 | 0.02152 | 26.71 | 0.9983 |
| Fe3O4@SiO2@IIP-Ni(II) | 34.77 | 0.1275 | 12.55 | 0.8714 | 0.01821 | 36.58 | 0.9989 |
| Fe3O4@SiO2@NIP-Ni(II) | 28.96 | 0.1679 | 10.64 | 0.8640 | 0.01991 | 30.77 | 0.9982 |
To better study the adsorption mechanism of adsorbent, the data were fitted by Langmuir and Freundlich isotherm models. The linear expressions of the two models were as follows eqn (8) and (9).12
Langmuir isotherm:
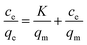 | (8) |
Freundlich isotherm:
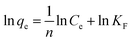 | (9) |
The plots of adsorption isotherm models are presented in Fig. S5,† and the parameters of two models are given in Table 2. It can be seen that Langmuir model can better describe the adsorption mechanism of Ni(II) on imprinted materials. The correlation coefficients of Fe3O4@void@IIP-Ni(II), Fe3O4@void@NIP-Ni(II), Fe3O4@SiO2@IIP-Ni(II) and Fe3O4@SiO2@NIP-Ni(II) were 0.9982, 0.9973, 0.9988 and 0.9965, respectively. In addition, the maximum adsorption capacity fitted by Langmuir model was closer to the experimental value. As a result, Langmuir model may be utilized to describe the adsorption process, indicating that Ni(II) ion adsorption on the adsorbents was a monolayer adsorption on the adsorbent surface.
| Adsorption isothermal model | qe,exp | Langmuir adsorption isotherm | Freundlich adsorption isotherm | ||||
|---|---|---|---|---|---|---|---|
| KL | qm | RL2 | KF | n | RF2 | ||
| Fe3O4@void@IIP-Ni(II) | 44.64 | 23.97 | 50.85 | 0.9982 | 10.61 | 3.513 | 0.8830 |
| Fe3O4@void@NIP-Ni(II) | 39.75 | 22.01 | 44.90 | 0.9973 | 9.977 | 3.627 | 0.8657 |
| Fe3O4@SiO2@IIP-Ni(II) | 36.05 | 19.42 | 39.95 | 0.9988 | 10.64 | 4.137 | 0.8892 |
| Fe3O4@SiO2@NIP-Ni(II) | 31.37 | 20.58 | 34.84 | 0.9965 | 8.893 | 4.020 | 0.8837 |
The relevant thermodynamic parameters were calculated to clarify the thermodynamic mechanism of the adsorption process. The Gibbs free energy (ΔG), enthalpy (ΔH) and entropy (ΔS) were evaluated as follows eqn (10) and (11).4
ΔG = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(1000Kc) ln(1000Kc)
| (10) |
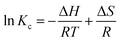 | (11) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kc versus 1/T were utilized to obtain the values of ΔH and ΔS as shown in Fig. S6,† the calculated values of thermodynamic parameters are reported in Table S2.†
Kc versus 1/T were utilized to obtain the values of ΔH and ΔS as shown in Fig. S6,† the calculated values of thermodynamic parameters are reported in Table S2.†
The negative values of ΔG suggested the feasibility and spontaneity for Ni(II) adsorption, the positive values of ΔH revealed that the adsorption process was endothermic and the higher temperature could accelerate reaction kinetics to promote adsorption. Notably, the obtained ΔH value was between 28 and 46, which can be inferred that the adsorption of Ni(II) on adsorbents was mainly driven by coordination interaction, supplemented by electrostatic interaction.38 As for ΔS, the positive value indicated an increase in the randomness of adsorption process, which may be due to the release of water molecules on the adsorbent during Ni(II) adsorption.
3.3 Selectivity and reusability
To investigate the adsorption selectivity of four adsorbents for trace Ni(II), the mixed solutions of Ni(II), Co(II), Cu(II), Zn(II) and Pb(II) with initial concentration of 5 mg L−1 were selected. The results showed that Fe3O4@void@IIP-Ni(II) exhibited an excellent adsorption performance for Ni(II) than Co(II), Cu(II), Zn(II) and Pb(II) ions, and the removal rate of for Ni(II) was as high as 99.97%, close to 100% (shown in Fig. 6). Meanwhile, Fe3O4@void@IIP-Ni(II) showed higher removal efficiency for all metal ions than that of Fe3O4@SiO2@IIP-Ni(II), which was mainly due to the formation of the rattle-type structure by silicon corrosion, thus greatly enhancing the adsorption capacity. Remarkably, whether the silicon layer was etched or not, both IIPs and NIPs showed the highest removal efficiency for Ni(II), while the removal efficiency for Zn(II) was not high. To explore those experimental results, the binding energies of different metal ions to AMPS were calculated using the DFT method, and the calculated results are shown in Fig. 7. It can be seen that the calculated results are in high agreement with the experimental results, and the binding energies of AMPS with these metal ions are Ni(II), Co(II), Cu(II), Pb(II) and Zn(II) from high to low. This means that AMPS has good affinity with Ni(II) in aqueous solution, and the selected monomer AMPS is suitable for the adsorption of Ni(II).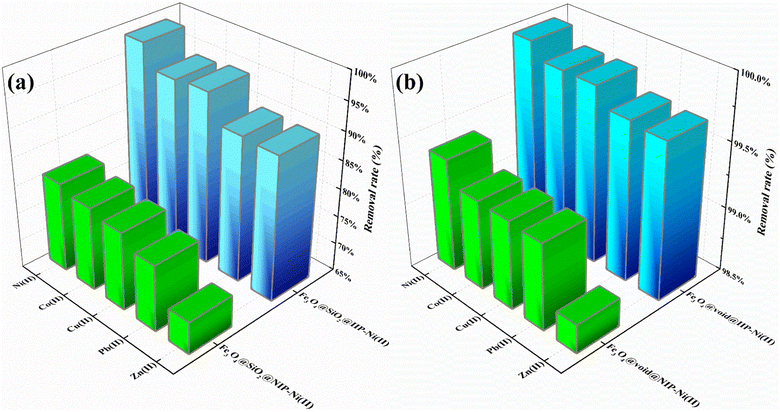 | ||
| Fig. 6 Removal rate of ions in mixed solution by (a) Fe3O4@SiO2@IIP-Ni(II) and Fe3O4@SiO2@NIP-Ni(II); (b) Fe3O4@void@IIP-Ni(II) and Fe3O4@void@NIP-Ni(II). | ||
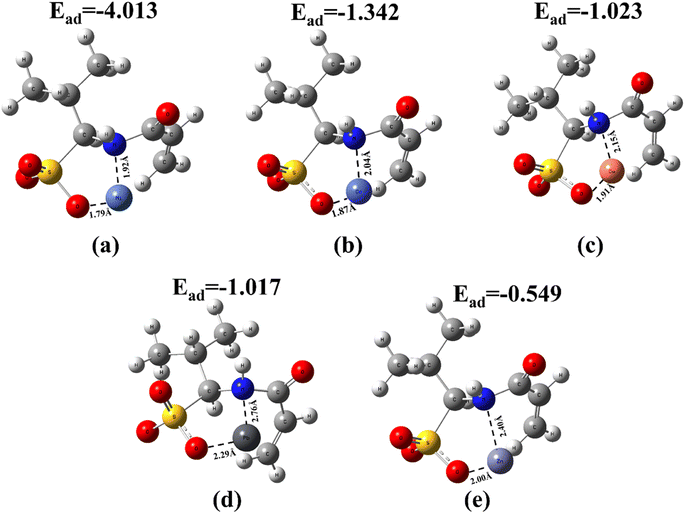 | ||
| Fig. 7 Binding energies of different ions to AMPS (a) AMPS-Ni(II), (b) AMPS-Co(II), (c) AMPS-Cu(II), (d) AMPS-Pb(II), (e) AMPS-Zn(II). | ||
From the perspective of k values as listed in Table S3,† the values of NIPs with or without silicon etching were consistent with the calculation results by DFT method; however, the k values of IIPs were partially inconsistent with those of DFT, for example, kNi(II)/Co(II) was slightly larger than kNi(II)/Cu(II) for Fe3O4@void@IIP-Ni(II), which was mainly due to the imprinting effect. Notably, the k values of Ni(II)/Co(II), Ni(II)/Cu(II), Ni(II)/Pb(II), and Ni(II)/Zn(II) for Fe3O4@void@IIP-Ni(II) were 4.67, 4.62, 8.94, and 9.69, respectively, which were all greater than 1, indicating that the imprinted adsorbent had a high selectivity for Ni(II).
The reusability of the sorbent is one of the most critical parameters for its practical application. The reuse properties of two imprinted adsorbents was explored by six adsorption–desorption experiments using 2 mol L−1 HCl solution as the eluent. The results are shown in Fig. 5d. It can be seen that after six cycles, the recovery of Ni by both imprinted adsorbents was still above 95%. The results showed that two magnetic Ni(II) imprinted adsorbents had good reusability.
3.4 Adsorption mechanism
To further elaborate the adsorption mechanism, the chemical states of functional groups before and after Ni adsorption by Fe3O4@void@IIP-Ni(II) were investigated by FT-IR, XPS and and DFT calculation. The FT-IR results of the adsorbents before and after adsorption are shown in Fig. 4A, where Fig. 4A(f) shows Fe3O4@void@IIP-Ni(II) without Ni(II) adsorption, and Fig. 4A(e) shows Fe3O4@void@IIP-Ni(II) after Ni(II) adsorption. Compared with Fig. 4A(e), the absorbance of N-H at 3426 cm−1 was significantly enhanced, and the absorbance of N–H at 1537 cm−1 decreased and shifted. In addition, similar changes were observed for S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 1198 cm−1, 1044 cm−1 and S–O bond at 624 cm−1. It can be inferred that the adsorption of Ni(II) is mainly due to the coordination between –NH and –SO3H of AMPS and Ni(II) in the imprinted adsorbent.
O at 1198 cm−1, 1044 cm−1 and S–O bond at 624 cm−1. It can be inferred that the adsorption of Ni(II) is mainly due to the coordination between –NH and –SO3H of AMPS and Ni(II) in the imprinted adsorbent.
The XPS results further clarified the changes of functional groups of Fe3O4@void@IIP-Ni(II) before and after adsorption of Ni(II) (Fig. 8). Fig. 8a shows the XPS survey spectrum before and after Ni(II) adsorption. The appearance of the Ni 2p peak after adsorption indicated that Ni(II) has been successfully absorbed. The high-resolution XPS spectrum of O 1s (Fig. 8b) has two peaks at 531.66 eV and 530.21 eV, which were –SO3H and –N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in AMPS, respectively.39 After the adsorption of Ni(II), the binding energy of these groups changed and shifted to 532.43 eV and 531.40 eV, respectively, which means that these groups participated in the adsorption of Ni(II). As for the N1s (Fig. 8c), a significant difference was observed before and after the adsorption of Ni(II). Before adsorption, N 1s had two peaks at 400.24 eV and 398.52 eV, which were ascribed to –NH2+R and –NHR groups respectively. After adsorption, a new peak appeared at 399.94 eV, indicating the existence of chelation between Ni(II) and the nitrogen-containing groups, where the lone pair electrons of N formed coordination bonds with Ni(II).40 The XPS spectrum of S 2p is shown in Fig. 8d. Two apparent peaks at 168.78 eV and 167.48 eV corresponded to –SO3H and –SO3− groups of the sulfonic acid group in AMPS, respectively.41 After adsorption, the binding energies of the two groups were increased to 169.49 eV and 168.34 eV, respectively, which indicated that Ni(II) coordinated with sulfonic acid group. As a result, it can be speculated that the adsorption of Ni(II) on Fe3O4@void@IIP-Ni(II) was the result of the co-coordination of O atoms of the sulfonic acid groups and N atoms of –N–C
O in AMPS, respectively.39 After the adsorption of Ni(II), the binding energy of these groups changed and shifted to 532.43 eV and 531.40 eV, respectively, which means that these groups participated in the adsorption of Ni(II). As for the N1s (Fig. 8c), a significant difference was observed before and after the adsorption of Ni(II). Before adsorption, N 1s had two peaks at 400.24 eV and 398.52 eV, which were ascribed to –NH2+R and –NHR groups respectively. After adsorption, a new peak appeared at 399.94 eV, indicating the existence of chelation between Ni(II) and the nitrogen-containing groups, where the lone pair electrons of N formed coordination bonds with Ni(II).40 The XPS spectrum of S 2p is shown in Fig. 8d. Two apparent peaks at 168.78 eV and 167.48 eV corresponded to –SO3H and –SO3− groups of the sulfonic acid group in AMPS, respectively.41 After adsorption, the binding energies of the two groups were increased to 169.49 eV and 168.34 eV, respectively, which indicated that Ni(II) coordinated with sulfonic acid group. As a result, it can be speculated that the adsorption of Ni(II) on Fe3O4@void@IIP-Ni(II) was the result of the co-coordination of O atoms of the sulfonic acid groups and N atoms of –N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups in AMPS with Ni(II).
O groups in AMPS with Ni(II).
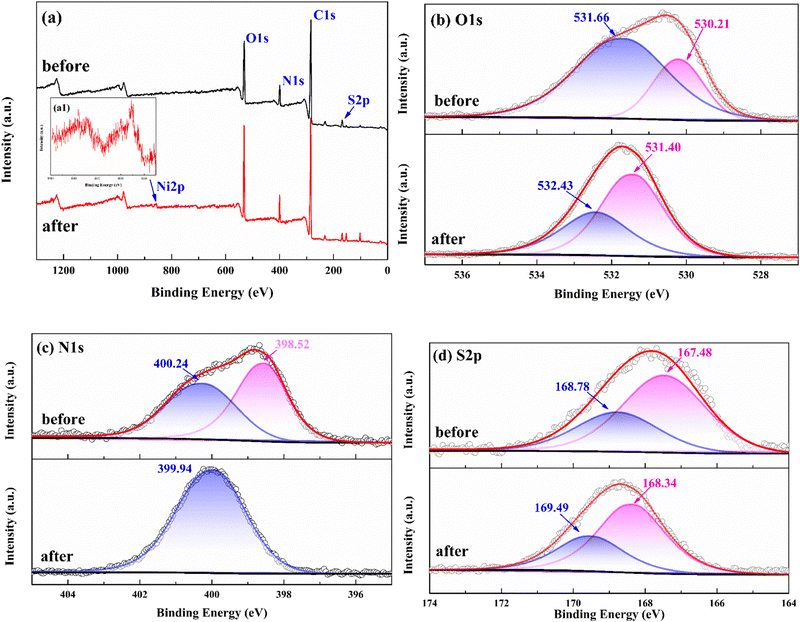 | ||
| Fig. 8 XPS survey spectrum of IIP before and after Ni(II) adsorption (a), and high-resolution XPS of O 1s (b), N 1s (c), and S 2p (d) of IIP before and after adsorption of Ni(II). | ||
The adsorption mechanism was further studied by DFT calculation. The optimized structures of the complexes formed by the functional groups with Ni(II) are shown in Fig. S6.† It can be seen from the binding energy that when Ni(II) coordinates with the N atom on the AMPS and the O atom on the sulfonic acid group (−4.013 eV), the complex structure is more stable than that with two O atoms on the sulfonic acid group (−3.863 eV). To further verify the chelating sites of the AMPS− molecule, the Fukui functions (f−) of the coordination atoms was also calculated (Fig. 9a and Table S4†). The results showed that the N atom of –N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group and the O atom of S–O group on AMPS molecule had higher f− values (0.2744 and 0.1135, respectively), indicating that the N atom and the O atom of AMPS molecule are the preferred chelating sites for Ni(II), and they are more likely to lose electrons and be attacked by Ni(II). This calculation result is in well agreement with the XPS result.
O group and the O atom of S–O group on AMPS molecule had higher f− values (0.2744 and 0.1135, respectively), indicating that the N atom and the O atom of AMPS molecule are the preferred chelating sites for Ni(II), and they are more likely to lose electrons and be attacked by Ni(II). This calculation result is in well agreement with the XPS result.
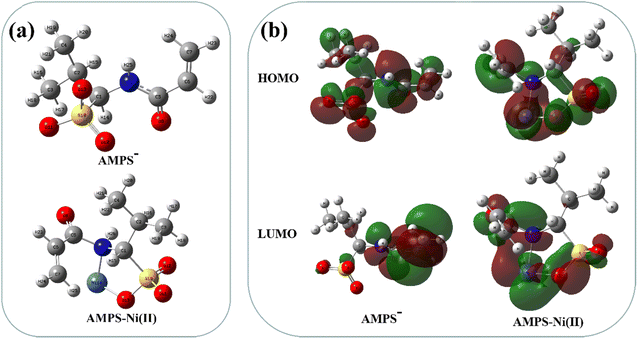 | ||
| Fig. 9 Structural optimization of AMPS− and AMPS-Ni(II) (a), and contour plots of HOMO and LUMO of AMPS− and AMPS-Ni(II) (b). | ||
The contour plots of highest occupied molecular orbital (HOMO) and lowest unoccupied orbital (LUMO) of AMPS− and AMPS-Ni(II) are shown in Fig. 9b. The HOMO of AMPS− before adsorption was distributed on each atom, while after adsorption of Ni(II), the HOMO was concentrated near Ni(II), N and sulfonic acid groups, and their adjacent atoms. The LOMO of AMPS− was distributed in the positions of double bonds and N atoms of AMPS, after the adsorption of Ni(II), it was concentrated in the attachment of Ni atoms, double bonds and sulfonic acid groups. The changes of the HOMO and LUMO before and after adsorption confirms the charge transfer in the adsorption process.42 The adsorption mechanism can also be reflected by the change of bond length and Wiberg bond order before and after adsorption, and the results are listed in Table S5.† It can be found that after the adsorption of Ni(II), the bond lengths of S–O bonds on the sulfonic acid groups and C–N bonds near the N atoms lengthened, and their Wiberg bond order weakened. This is due to the fact that the transfer of electrons from O and N atoms to the vacant orbitals of Ni(II) after the adsorption of Ni(II).43
3.5 Application in real water sample
The prepared magnetic rattle-type materials were applied to collect Ni(II) in real lake water sample from Tingxin lake of Kunmming University (Kunming, China). The nickel content in the lake water samples founded 0.47 μg L−1. When 100 mg Fe3O4@void@IIP-Ni(II) or Fe3O4@void@NIP-Ni(II) was added to 10 mg L−1 50 mL of water sample and shaken at 25 °C for 1 h, the initial concentration of Ni(II) detected by ICP-OES was 10.58 mg L−1. After sorbents treatment, the amount of residual Ni(II) was almost zero, and the removal efficiency of Ni(II) in the actual water sample was close to 100%. The results validated the suitability of the magnetic rattle-type materials to remove Ni(II) from actual water.4. Conclusion
In the present study, a novel magnetic rattle-type ion-imprinted polymer (Fe3O4@void@IIP-Ni(II)) was prepared for selective adsorption of Ni(II). The maximum adsorption capacity is 44.64 mg g−1 at the optimal pH of 6.0, and the adsorption reaches equilibrium in about 10 min. The removal rate of Ni(II) in a trace amount of mixed solution is as high as 99.97%, and Fe3O4@void@IIP loaded with Ni(II) can be easily separated from water solution in the presence of an applied magnetic field. The selectivity coefficients (k) for Ni(II)/Zn(II) is as high as 9.69, which is ascribed to the imprinting effect. Fe3O4@void@IIP-Ni(II) could be reused to adsorb Ni(II) from aqueous solutions, and the recovery of Ni(II) ions on Fe3O4@void@IIP-Ni(II) was maintained at about 95% after 6 adsorption–desorption cycles. The adsorption process of Ni(II) on Fe3O4@void@IIP-Ni(II) can be well described by Langmuir and Pseudo-secondary kinetic models, it was an endothermic, entropy-increasing and spontaneous process. The results of FT-IR, XPS and DFT calculations showed that O atoms of the sulfonic acid groups and N atoms of –N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups in AMPS played a dominant role in the adsorption of Ni(II), and the functional monomer AMPS has a high coordination ability for Ni(II). Furthermore, the imprinted polymer can also be used to adsorb trace Ni(II) in real water samples. To sum up, Fe3O4@void@IIP-Ni(II) proves to be a suitable adsorbent for the selective removal of trace Ni(II) from mixed solutions due to its magnetic properties and easy separation and recovery from the solution, which is benefit to energy saving, environmental protection and reuse. This work proposes a new method for guiding the adsorption mechanism and determining the adsorption configuration of imprinted adsorbents using DFT calculations.
O groups in AMPS played a dominant role in the adsorption of Ni(II), and the functional monomer AMPS has a high coordination ability for Ni(II). Furthermore, the imprinted polymer can also be used to adsorb trace Ni(II) in real water samples. To sum up, Fe3O4@void@IIP-Ni(II) proves to be a suitable adsorbent for the selective removal of trace Ni(II) from mixed solutions due to its magnetic properties and easy separation and recovery from the solution, which is benefit to energy saving, environmental protection and reuse. This work proposes a new method for guiding the adsorption mechanism and determining the adsorption configuration of imprinted adsorbents using DFT calculations.
Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was financially supported by the Applied Basic Research Project of Yunnan Province (No. 202001BA070001-192, 202001BA070001-120, ZX20220099), the Scientific Research Project of Kunming University (No. YJL20025), and the Scientific Research Fund of Education Department of Yunnan Province (No. 2019J0566, 2022Y747).Notes and references
- P. Shao, D. Liang, L. Yang, H. Shi, Z. Xiong, L. Ding, X. Yin, K. Zhang and X. Luo, J. Hazard. Mater., 2020, 387, 121676 CrossRef CAS PubMed
.
- B. Gupta, A. Mishra, R. Singh and I. S. Thakur, Environ. Technol. Innovation, 2021, 21, 101278 CrossRef CAS
.
- W. Liu, M. Zhang, X. Liu, H. Zhang, J. Jiao, H. Zhu, Z. Zhou and Z. Ren, Ind. Eng. Chem. Res., 2020, 59, 6033–6042 CrossRef CAS
.
- H. X. He, Q. Gan and C. G. Feng, Chin. J. Polym. Sci., 2018, 36, 462–471 CrossRef CAS
.
- X. Shen and X. Chen, Sep. Purif. Technol., 2019, 223, 88–95 CrossRef CAS
.
- M. A. Islam, M. R. Awual and M. J. Angove, J. Environ. Chem. Eng., 2019, 7, 103305 CrossRef CAS
.
- Z. Yao, P. Shao, D. Fang, J. Shao, D. Li, L. Liu, Y. Huang, Z. Yu, L. Yang, K. Yu and X. Luo, Chem. Eng. J., 2022, 427, 131470 CrossRef CAS
.
- T. Kim, T. K. Kim and K. D. Zoh, J. Water Process Eng., 2020, 33, 101109 CrossRef
.
- J. Lu, Y. Qin, Y. Wu, M. Meng, Y. Yan and C. Li, Environ. Sci.: Water Res. Technol., 2019, 5, 1626–1653 RSC
.
- A. Ma, A. Abushaikha, S. J. Allen and G. Mckay, Chem. Eng. J., 2019, 358, 1–10 CrossRef CAS
.
- W. Liu, Z. An, L. Qin, M. Wang, X. Liu and Y. Yang, Chem. Eng. J., 2021, 411, 128477 CrossRef CAS
.
- M. Li, C. Feng, M. Li, Q. Zeng and Q. Gan, Hydrometallurgy, 2015, 154, 63–71 CrossRef CAS
.
- P. He, H. Zhu, Y. Ma, N. Li, X. Niu, M. Wei and J. Pan, Chem. Eng. J., 2019, 367, 55–63 CrossRef CAS
.
- P. Yang, H. Cao, D. Mai, T. Ye, X. Wu, M. Yuan, J. Yu and F. Xu, React. Funct. Polym., 2020, 151, 104569 CrossRef CAS
.
- L. Hou, C. Yang, X. Rao, L. Hu and X. Zhu, Colloids Surf., A, 2021, 625, 126949 CrossRef CAS
.
- E. Kazemi, S. Dadfarnia, A. Shabani and M. Ranjbar, Food Chem., 2017, 237, 921–928 CrossRef CAS PubMed
.
- Z. Cheng, H. Lyu, B. Shen, J. Tian, Y. Sun and C. Wu, Chemosphere, 2022, 288, 132581 CrossRef CAS PubMed
.
- X. Li, X. Ma, R. Huang, X. Xie, L. Guo and M. Zhang, J. Sep. Sci., 2018, 41, 2837–2845 CrossRef CAS PubMed
.
- S. Buyuktiryaki, R. Kecili and C. Hussain, TrAC, Trends Anal. Chem., 2020, 127, 115893 CrossRef CAS
.
- X. Wang, J. Feng, Y. Bai, Q. Zhang and Y. Yin, Chem. Rev., 2016, 116, 10983–11060 CrossRef CAS PubMed
.
- W. Zhao, H. Chen, Y. Li, L. Li, M. Lang and J. Shi, Adv. Funct. Mater., 2008, 18, 2780–2788 CrossRef CAS
.
- R. Yang, Y. Liu, X. Yan, S. Liu and H. Zheng, J. Mater. Chem. A, 2016, 4, 9807–9815 RSC
.
- R. Yang, Y. Liu, X. Yan and S. Liu, Talanta, 2016, 161, 114–121 CrossRef CAS PubMed
.
- J. P. Fan, J. X. Yu, X. M. Yang, X. H. Zhang, T. T. Yuan and H. L. Peng, Chem. Eng. J., 2018, 337, 722–732 CrossRef CAS
.
- J. Lu, Y. Y. Qin, Y. L. Wu, M. N. Chen and Y. Yan, Chem. Eng. J., 2021, 417, 128085 CrossRef CAS
.
- W. Lai, K. Zhang, P. Shao, L. Yang, L. Ding, S. G. Pavlostathis, H. Shi, L. Zou, D. Liang and X. Luo, J. Hazard. Mater., 2019, 379, 120791 CrossRef CAS PubMed
.
- C. Ni, Q. Liu, Z. Ren, H. Hu, B. Sun, C. Liu, P. Shao, L. Yang, S. G. Pavlostathis and X. Luo, J. Environ. Chem. Eng., 2021, 9, 106701 CrossRef CAS
.
- H. He, Q. Gan and C. Feng, RSC Adv., 2017, 7, 15102–15111 RSC
.
- Z. Chang, L. Yang, K. Zhang, W. Hu, C. Ni, P. Shao, H. Shi, K. Yu and X. Luo, Chem. Eng. J., 2021, 416, 129144 CrossRef CAS
.
- Y. Cheng, J. Nie, J. Li, H. Liu, Z. Yan and L. Kuang, Food Chem., 2019, 287, 100–106 CrossRef CAS PubMed
.
- M. Hussain, T. Rehan, K. W. Goh, S. I. Shah, A. Khan, L. C. Ming and N. Shah, Polymers, 2022, 14, 2681 CrossRef CAS PubMed
.
- Y. H. Gad, Radiat. Phys. Chem., 2008, 77, 1101–1107 CrossRef CAS
.
- N. Sun, R. Lei, J. Xu, S. C. Kundu, Y. Cai, J. Yao and Q. Ni, J. Mater. Sci., 2019, 54, 3319–3330 CrossRef CAS
.
- F. Goudarzi and P. Hejazi, React. Funct. Polym., 2019, 143, 104322 CrossRef CAS
.
- Y. Cui, W. Kang, L. Qin, J. Ma, X. Liu and Y. Yang, Chem. Eng. J., 2020, 397, 125480 CrossRef CAS
.
- H. Hu, Z. Ren, Y. Xi, L. Fang, D. Fang, L. Fang, P. Shao, H. Shi, K. Yu and X. Luo, Chem. Eng. J., 2021, 420, 129611 CrossRef CAS
.
- R. D. Harter, Soil Sci. Soc. Am. J., 1983, 47, 47–51 CrossRef CAS
.
- Y. Chen, X. Ma and J. Peng, Carbohydr. Polym., 2021, 271, 118435 CrossRef CAS PubMed
.
- T. Lu, Y. Zhu, Y. Qi, W. Wang and A. Wang, Int. J. Biol. Macromol., 2018, 106, 870–877 CrossRef CAS PubMed
.
- J. Wang and F. Liu, Chem. Eng. J., 2014, 242, 117–126 CrossRef CAS
.
- J. Ma, Y. Zhang, Y. Tang, Y. Wei, Y. Liu and C. Liu, Water Sci. Technol., 2018, 78, 982–990 CrossRef CAS PubMed
.
- B. Zhang, Y. Niu, L. Li, W. Xu, H. Chen, B. Yuan and H. Yang, Microchem. J., 2019, 151, 104220 CrossRef CAS
.
- M. Li, C. Zhao, Q. Feng, J. Feng and X. Meng, Chem. J. Chin. Univ., 2021, 42, 3680–3691 Search PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ra06918k |
| This journal is © The Royal Society of Chemistry 2022 |