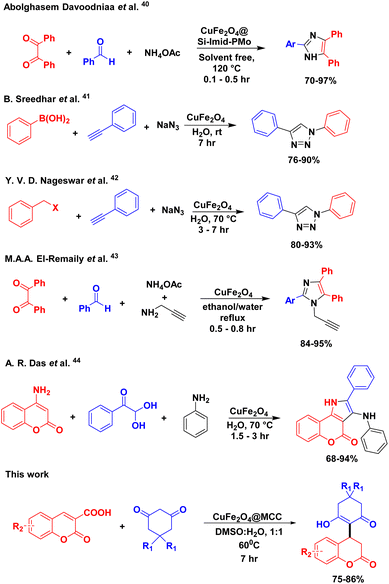
Open Access Article
Open Access ArticleDirect Michael addition/decarboxylation reaction catalyzed by a composite of copper ferrite nanoparticles immobilized on microcrystalline cellulose: an eco-friendly approach for constructing 3,4-dihydrocoumarin frameworks†
Bhupender Kumar a,
Biplob Borah
a,
Biplob Borah a,
J. Nagendra Babu
a,
J. Nagendra Babu b and
L. Raju Chowhan
b and
L. Raju Chowhan *a
*a
aSchool of Applied Material Sciences, Central University of Gujarat, Sector 30, Gandhinagar, Gujarat 382021, India. E-mail: rajuchowhan@gmail.com; rchowhan@cug.ac.in
bDepartment of Chemical Sciences, School for Basic and Applied Sciences, Central University of Punjab, Ghudda, Bathinda, 151401, India
First published on 27th October 2022
Abstract
A composite of copper ferrite oxide nanoparticles immobilized on microcrystalline cellulose (CuFe2O4@MCC) was synthesized. The synthesized composite was characterized by FESEM with EDS-Mapping, TEM, P-XRD, TEM, and BET analysis and investigated for its catalytic activity toward Tandem Michael addition and decarboxylation of coumarin-3-carboxylic acid with cyclic 1,3-diketones to obtain novel 3,4-dihydrocoumarin derivatives. This protocol was established with wide substrate scope and significant yield. The significant characteristics of this methodology are mild reaction conditions, easy setup procedure, non-toxic, and cost-effectiveness. A gram-scale synthesis with low catalyst loading was also demonstrated.
Introduction
In organic synthesis, the construction of active pharmaceutical ingredients containing heteroatoms has been considered a key domain for the development and design of drugs in medicinal and pharmaceutical chemistry, as well as in materials science.1,2 Among diverse oxygen-heterocycles, coumarin (also known as the 2H-chromen-2-one ring) comprises a significant class of structural scaffolds in synthetic organic chemistry with the distinctive benzo-α-pyrone core in its fragment.3 They are frequently found in the fundamental structure of many natural products and synthetic compounds and have been recognized in a large number of pharmaceutically useful components and drug candidates.4 Ningalin B (A), a marine natural product with coumarin at its core, has been known to have immunomodulatory and HIV-1 integrase inhibitory properties.5,6 The natural product (+)-calanolide A (B) isolated from Calophyllum lanigerum was discovered to exhibit anti-HIV activity7–9 whereas (+)-cordatolide A (C) is the key inhibitor of HIV-reverse transcriptase.10 Warfarin (D), another natural product derived from woodruff and lavender with 4-hydroxycoumarin as its key component was used to reduce the blood clotting in the veins, heart, or lungs, (Fig. 1).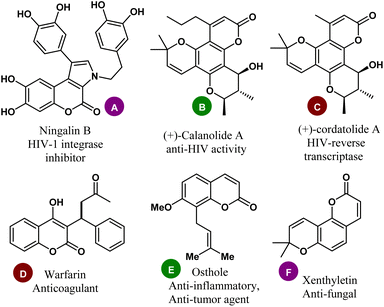 | ||
| Fig. 1 Representative examples of coumarin-based natural products with relevant therapeutic significance. | ||
Aside from natural compounds and their analogs, synthetic coumarin derivatives have become significantly more appealing in recent years for their impressive biological and pharmacological activities, such as antimicrobial,11 antioxidant,12 analgesic,13 anticancer,14–16 and antituberculosis17 properties. The significant potentiality of coumarin and its derivatives in material sciences includes solar energy, laser dyes, optical recording, oxygen sensors, nonlinear optical chromophores, molecular photonic devices, polymers, optical brighteners, and fluorescent probes, chemosensors, whiteners has also been well explored.18–21 Additionally, fluorescent nanoparticles like graphene and carbon quantum dots, which have found extensive use in biological imaging experiments, were synthesized by employing coumarin and its derivatives.22,23 Considering the tremendous therapeutic significance and broad-spectrum chemical landscape of coumarins, the past decades have witnessed immense efforts not just for their synthesis but also Scheme 1 for their utilization as a testing ground in the discovery of new reactions and the synthesis of complex hybrid molecules of medicinal interest.24
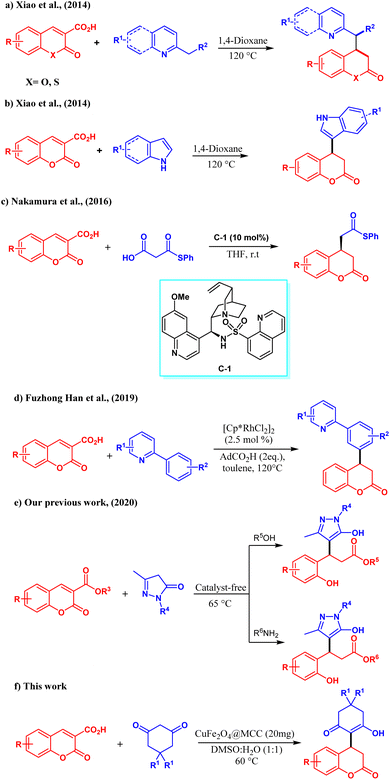
Especially, the exploitation of carboxylic acid/ester functionalized coumarins as Michael acceptors for the assembly of 3,4-dihydrocoumarin has recently gained immense importance. But, due to loosing its aromatic nature, harsh reaction conditions are desired which marks the limitations of the reaction scopes as previously reported works, Michael addition via ring-opening/decarboxylation at high temperature or using organocatalyst.25 In 2014 Xiao et al. reported a lot of work on coumarin, Michael addition followed by decarboxylation of coumarin-3-carboxylic acid with without any catalyst aza arenes for the synthesis of functionalized 3,4-dihydrocoumarin.26 They also disclosed the exploitation of indoles as the nucleophiles for the direct Michael addition with coumarin-3-carboxylic which is followed by decarboxylation to form diverse 3,4-dihydrocoumarin derivatives.27 In 2016 Nakamura et al. described the asymmetric synthesis of functionalized 3,4-dihydrocoumarin using malonic acid half-thioesters and coumarin-3-carboxylic acid in the presence of cinchona derived chiral organocatalyst C-1.25 In 2019 Hu, Han, and co-workers devised Rh(III)-catalyzed a traceless C–H activation strategy for the synthesis of 3,4-hydroxycoumarins by a conjugate addition/decarboxylations of coumarin-3-carboxylic acid and 2-aryl pyridines.28 Encouraged by these promising results, our group also tested the reactivity of carboxylic acid/ester functionalized coumarin towards the reaction with pyrazolones at 60 °C. Changing the solvent system to either water/alcohol or amines in catalyst-free conditions followed tandem conjugate addition/decarboxylation and esterification/amidations to form different pyrazole derivatives.24 Given above most of the previously reported reaction on coumarins leads to ring opening and very few reactions retains in the core structure. On the other hand, in the last decade for the C–C bond formation metal oxide nanoparticles were used in place of the organic base for controlling ring-opening, and side products as well as occurring the addition at normal temperature. Metallic and bimetallic nanoparticles are the most interesting catalysts for C–C bond formation due to having robust qualities especially such as a higher surface area with stability and easy separation.29 These qualities make them appropriate for various applications with a wide range of catalysis.30
However, during the application of metal oxide nanoparticles, some drawbacks are faced like leaching and recovery. In the case of magnetic nanoparticles, agglomeration was observed as a major drawback. To restrain these drawbacks, stabilizers and immobilizers are used to provide a surface.31 Recently, we synthesized Cu2O@MCC and Fe3O4@MCC and studied their catalytic activity towards 1, 3-dipole cycloaddition and Michael addition. The catalytic activity of Cu2O@MCC was studied for the 1,3 dipolar cycloaddition of chalcone/styrylisoxazoles on azomethine ylide of isatin and THIQ.32 Fe3O4@MCC and Fe2O3@MCC were studied for Michael addition of 1,3 diketones on styrylisoxazoles and 1,3-dipole reaction of acetyl oxime/azirine with alkyne respectively.2,33 MCC is used to improve the stability and reactivity of nanoparticles by providing a surface. Due to the distinctive morphology, renewability, lightweight, and nanoscale dimension of MCC, it emerged as an important material for the strengthening of nanocomposite.34–36
In the present work, we synthesized CuFe2O4@MCC and characterized it by FESEM with EDS-Mapping, TEM, P-XRD, TEM, and BET analysis. The synthesized composite has been studied for the reactivity of cyclic 1,3-diketones on coumarin-3-carboxylic acid to obtain novel 3,4-dihydrocoumarins. The obtained products were characterized by IR, NMR, and LCMS. Consequently, for the first time, we report here a direct Michael addition, as well as decarboxylation of coumarin-3-carboxylic acids, and devised the reactivity of cyclic 1,3-diketones.
Results and discussion
Synthesis and characterization of copper ferrite nanoparticles (CuFe2O4) immobilized on microcrystalline cellulose composite
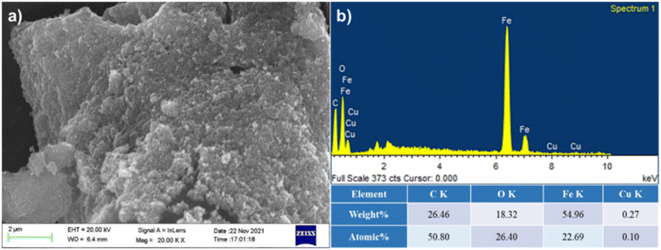
Synthesis of 30% (w/w%) loading of copper ferrite nanoparticles on microcrystalline cellulose nanocomposites was done by using the precipitation technique. In a 500 mL beaker, 1.184 g of copper sulphate pentahydrate and 1.728 g of ferric chloride in 100 mL water were taken and heated at 90 °C with stirring at 600 rpm. After obtaining a homogenous mixture, 2.1 g of microcrystalline cellulose was added to the reaction mixture. For the synthesis of the copper ferrite nanoparticles, an aqueous solution of 2 N NaOH was added at 90 °C. To obtain homogeneity of the composite, addition of aqueous NaOH was done at the rate of 2 mL per minute. The black material was separated and washed with deionized water and methanol. The material was vacuum dried to obtain the famish CuFe2O4@MCC.Field emission scanning electron microscope (FESEM) analysis was done to confirm the binding of CuFe2O4 on MCC. From the FESEM image, we observed heterogenous immobilization of CuFe2O4 on MCC (Fig. 2a). Energy-dispersive X-ray spectroscopy (EDS) spectrum (Fig. 2b) of composite confirms the presence of copper (Cu), carbon(C), iron (Fe), and oxygen (O).
Elemental Mapping of the composite has also sported the presence of carbon and oxygen with both metal iron and copper. As shown in Fig. 3a electron image (3b), mix mapping of composite having all elements of composite, and (3c) and (3d) are for the carbon and oxygen. In Fig. 3(e) and (f) presence of iron and copper was observed.
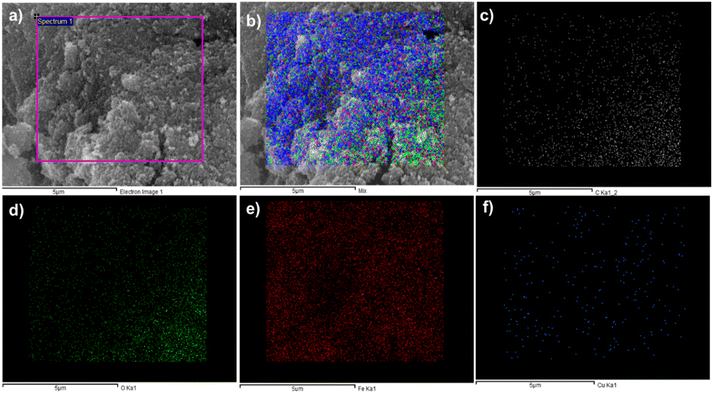 | ||
| Fig. 3 Elemental mapping of CuFe2O4@MCC (a) electron image (b) mix (c) carbon (d) oxygen (e) iron and (f) copper. | ||
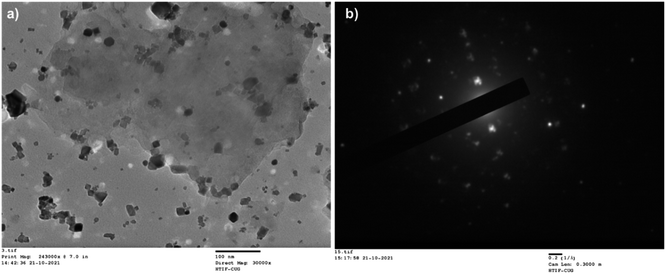
Transmission Electron Microscope (TEM) analysis was done to confirm the immobilization as well as the particle size of CuFe2O4 and the nature of the composite (Fig. 4(a)and (b)).
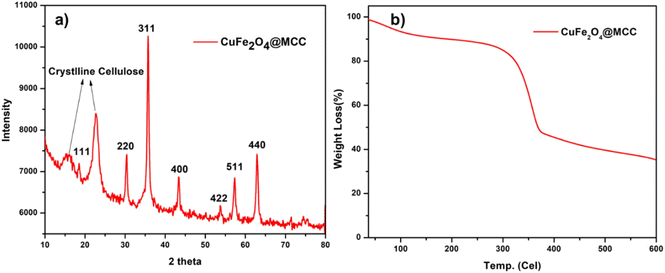
The size of CuFe2O4 nanoparticles was observed less than 50 nm and immobilized on MCC. From SAED pattern confirms the polycrystalline nature of synthesized composite. For the confirmation of CuFe2O4, powder X-ray diffraction was done (Fig. 5a). An X-ray diffraction pattern of CuFe2O4@MCC composite gave sharp reflections at 2θ = 15.82°, and 22.79° for crystalline cellulose with 2θ = 18.51°, 30.64, 35.74°, 43.35°, 53.66, 57.35°, 57.35°, and 62.92 having plane (111), (220), (311), (400), (422), (511), and (440), respectively, which confirms the construction of CuFe2O4 nanoparticles.37 From previous literature, we also confirm that the synthesized composite doesn't contain any iron oxide (Fe3O4, Fe2O3) and copper oxide (Cu2O, CuO) nanoparticles.2,32,33,38,39 Thermogravimetric analysis (TGA) under an inert condition at room temperature to 600 °C with a constant heating rate of 10 °C per minute was done for studying the thermal stability of CuFe2O4@MCC composite (Fig. 5b). 8% degradation was observed from room temperature to 120 °C due to the loss of hydroxy groups and water from cellulose. Another 6% loss of weight was observed up to 277 °C and a sharp degradation of 38% was observed in the temperature range 277 to 375 °C, which carries degradation of microcrystalline cellulose from composite. Therefore, a total of 52 and 65% degradation was observed from 375 to 600 °C respectively. TGA analysis confirms composite is more thermally stable than MCC. Catalysis is a surface phenomenon therefore Brunauer–Emmett–Teller (BET) analysis was done to examine the surface area of the composite CuFe2O4@MCC under inert conditions. The significant results was found with average pore diameter of 28.186 nm and 15.77 m2 g−1 surface area. The surface area of the composite is 12.13 times higher than MCC.35
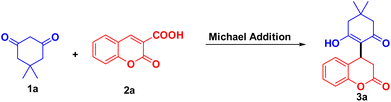
The composite of CuFe2O4 nanoparticles immobilized on MCC was used as a catalyst, and catalytic activity was studied for Michael addition of 5,5-dimethyl-cyclohexane-1,3-dione 1a on coumarin 3-carboxylic 2a. Initially, the reaction was studied without catalyst, with 10 mg and 20 mg of CuFe2O4@MCC in the water at room temperature, for 48 hours and did not observe the change in reactants (Table 1 entries 1–3). The reaction was done in presence of 10 mg of CuFe2O4@MCC and stirred at 50 °C for 12 h to obtain the Michael adduct 3a having 22% yield (Table 1, entry 4). On increase the loading of CuFe2O4@MCC to 15 mg and 20 mg, the significant increase was observed in yield, up to 48 and 56% respectively (Table 1, entries 5 and 6). The reaction temperature increased to 60 °C and a slightly increased in yield was observed (61%). Loading of the composite was increased to 25 mg at a similar reaction condition and didn't get a significant increase in yield (Table 1, entry 8). The optimized reaction condition found that 20 mg of CuFe2O4@MCC composite gives a significant yield at 60 °C for 12 hours in an aqueous medium (Table 1, entry 7). Maintaining the green characteristics of the reaction and reducing the use of organic solvents make easy workup of reaction mixture and separation of the product. As we obtain significant results in our previous reports, the reaction was performed in an aqueous ethanolic solution. In the presence of 20 mg CuFe2O4@MCC composite in EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1
H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) as solvent 65% yield was obtained (Table 1, entry 9), and in the case of EtOH
2) as solvent 65% yield was obtained (Table 1, entry 9), and in the case of EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1
H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) slightly increase in yield was found up to 69% (Table 1, entry 10). Coumarin 3-carboxylic acids are soluble in DMSO, hence, to enhance the reactivity of CuFe2O4@MCC composite and yield of the analytical product we studied that reaction in aqueous dimethyl sulfoxide solution of different concentrations such as 1
1) slightly increase in yield was found up to 69% (Table 1, entry 10). Coumarin 3-carboxylic acids are soluble in DMSO, hence, to enhance the reactivity of CuFe2O4@MCC composite and yield of the analytical product we studied that reaction in aqueous dimethyl sulfoxide solution of different concentrations such as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 1
4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and 1
2, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of DMSO
1 of DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (Table 1, entries 11,12, and 13). We observed an 86% significant yield in 1
H2O (Table 1, entries 11,12, and 13). We observed an 86% significant yield in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of DMSO
1 of DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O at 60 °C for 7 hours in presence of 20 mg of CuFe2O4@MCC composite (Table 1, entry 13). An optimized condition (Table 1, entry 13) was used to delve into substrate scope and found to assist the array of substrates, (Scheme 3, Table 2).
H2O at 60 °C for 7 hours in presence of 20 mg of CuFe2O4@MCC composite (Table 1, entry 13). An optimized condition (Table 1, entry 13) was used to delve into substrate scope and found to assist the array of substrates, (Scheme 3, Table 2).
| Entry | Solvent | Catalyst loading | Temp. (°C) | Time | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction condition: the reaction was conducted using 1 mmol of 5,5-dimethyl-cyclohexane-1,3-dione 1a and 1 mmol of coumarin 3-carboxylic 2a, in 3 mL of solvent with stirring at various temperatures for different times as indicated.b Isolated yield. | |||||
| 1 | H2O | — | RT | 48 h | — |
| 2 | H2O | 10 mg | RT | 48 h | — |
| 3 | H2O | 20 mg | RT | 48 h | — |
| 4 | H2O | 10 mg | 50 | 12 h | 22 |
| 5 | H2O | 15 mg | 50 | 12 h | 48 |
| 6 | H2O | 20 mg | 50 | 12 h | 56 |
| 7 | H2O | 20 mg | 60 | 12 h | 61 |
| 8 | H2O | 25 mg | 60 | 12 h | 62 |
| 9 | EtOH/H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
20 mg | 60 | 7 h | 65 |
| 10 | EtOH/H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
20 mg | 60 | 7 h | 69 |
| 11 | DMSO/H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
20 mg | 60 | 7 h | 68 |
| 12 | DMSO/H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
20 mg | 60 | 7 h | 78 |
| 13 | DMSO/H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
20 mg | 60 | 7 h | 86 |
a Reaction condition: The reaction was conducted with 1.0 mmol of 5,5-dimethyl-cyclohexane-1,3-dione/cyclohexane-1,3-dione (1a–b), 1.0 mmol of coumarin 3-carboxylic acid (2a–h), and CuFe2O4 (20 mg) in aqueous DMSO in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (3 mL). Yields of the isolated product (3a–o). Reaction time: 7 h. 1 (3 mL). Yields of the isolated product (3a–o). Reaction time: 7 h. |
|---|
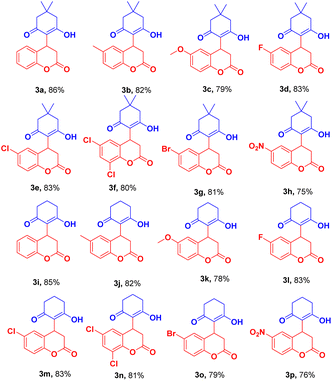 |
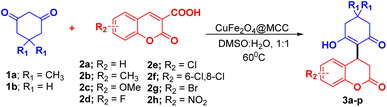
The reaction of dimedone/cyclohexane-1,3-dione (1a/1b) with halo substituted coumarin 3-carboxylic acid such 6-fluoro/chloro/6,8-dichloro/bromo-2-oxo-2H-chromene-3-carboxylic acid afforded the product in excellent yield (Table 2, 3d–g, 3l–o). In the case of nitro substituted coumarin 3-carboxylic acid with 1a/b slightly lower analytical product was observed (Table 2, 3h, 3p).
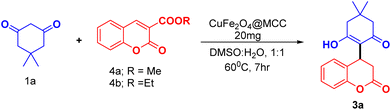
We exploit the reaction of dimedone 1a with coumarin 3-carboxylic ester (4a, 4b) under optimized conditions (Table 1, entry 13). We observed the same product (3a) (Scheme 4) with approximate yield as observed in case of coumarin 3-carboxylic acid. In the case of Scheme 2, the reaction was found to be preceded by decarboxylation whereas coumarin 3-carboxylic 4a/b proceeds by ester hydrolysis followed by decarboxylation.
Scaleup and multicomponent experiment
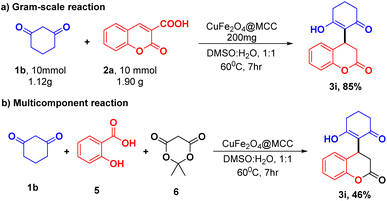
A gram-scale reaction was set up on a 10 mmol scale to validate the applicable protocol. Synthesis of 3a was investigated to find the ability of the established protocol. Appropriately, 10 mmol of 1b and 2a were treated using optimized reaction conditions (Table 1, entry 13) and obtained significant product 3i in 85% yield. In addition to that protocol, the optimized reaction conditions (Table 1, entry 3) were also exploited to perform a multicomponent reaction of 1,3 cyclohexanedione (1b), salicylaldehyde (5), and meldrum acid (6) with CuFe2O4@MCC composite a one-pot approach. Surprisingly, in the optimized condition, we observed the desired product (3i) with a 46% yield (Scheme 5) and on increasing reaction time 12 h, no significant increase in yield was observed.Conclusions
In this report, we study the catalytic activity of CuFe2O4@MCC composite toward Michael Addition of 5,5-dimethyl-cyclohexane-1,3-dione/cyclohexane-1,3-dione and 3-carboxylic coumarins for the synthesis of 3,4-hydroxycoumarins. Synthesis, characterization, and reactivity of composite having two widely available metals with bio-degradable polymer are studied. The catalytic property of CuFe2O4@MCC investigated for nucleophilic addition of 1,3 cyclic diketones on coumarin-3-carboxylic acid in presence of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 solvent systems of H2O
1 solvent systems of H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO. The products were isolated without any purification techniques such as column chromatography with 75–86% yield. Synthesized composite has shown excellent catalytic activity for the construction of 3,4-Dihydrocoumarin frameworks and a comparison study of previously reported methods are shown in Scheme 6.40–44 Due to easy separation of composite as well as significant product, simple and safe process for reaction setup, and moderate reaction time, this protocol also provides the potential as alternative applicability in gram-scale synthesis.
DMSO. The products were isolated without any purification techniques such as column chromatography with 75–86% yield. Synthesized composite has shown excellent catalytic activity for the construction of 3,4-Dihydrocoumarin frameworks and a comparison study of previously reported methods are shown in Scheme 6.40–44 Due to easy separation of composite as well as significant product, simple and safe process for reaction setup, and moderate reaction time, this protocol also provides the potential as alternative applicability in gram-scale synthesis.
Author contributions
Bhupender Kumar; conceptualization-equal, data curation-equal, formal analysis-equal, investigation-equal, writing – original draft-equal Biplob Borah; writing – draft J. Nagendra Babu data curation-equal L. Raju Chowhan; supervision-equal.Conflicts of interest
There are no conflicts to declare.Acknowledgements
BK and BB gratefully acknowledge UGC for a Non-NET fellowship. The authors thank Central Instrumentation Facility of Central University of Gujarat and Central Instrumentation Laboratory, Central University of Punjab for various analyses.Notes and references
- B. Borah, K. D. Dwivedi, B. Kumar and L. R. Chowhan, Arabian J. Chem., 2022, 15, 103654 CrossRef CAS.
- K. D. Dwivedi, B. Kumar, M. S. Reddy, B. Borah, J. Nagendra Babu and L. R. Chowhan, Results Chem., 2021, 3, 100201 CrossRef CAS.
- C. Gleye, G. Lewin, A. Laurens, J.-C. Jullian, P. Loiseau, C. Bories and R. Hocquemiller, J. Nat. Prod., 2003, 66, 690–692 CrossRef CAS PubMed.
- K. P. Barot, S. V Jain, L. Kremer, S. Singh and M. D. Ghate, Med. Chem. Res., 2015, 24, 2771–2798 CrossRef CAS.
- H. Fan, J. Peng, M. T. Hamann and J.-F. Hu, Chem. Rev., 2007, 108, 264–287 CrossRef PubMed.
- C. P. Ridley, M. V. R. Reddy, G. Rocha, F. D. Bushman and D. J. Faulkner, Bioorg. Med. Chem., 2002, 10, 3285–3290 CrossRef CAS.
- T. Ma, Q. Gao, Z. Chen, L. Wang and G. Liu, Bioorg. Med. Chem. Lett., 2008, 18, 1079–1083 CrossRef CAS PubMed.
- T. C. McKee, R. W. Fuller, C. D. Covington, J. H. Cardellina, R. J. Gulakowski, B. L. Krepps, J. B. McMahon and M. R. Boyd, J. Nat. Prod., 1996, 59, 754–758 CrossRef CAS PubMed.
- M. T. Flavin, J. D. Rizzo, A. Khilevich, A. Kucherenko, A. K. Sheinkman, V. Vilaychack, L. Lin, W. Chen, E. M. Greenwood, T. Pengsuparp, J. M. Pezzuto, S. H. Hughes, T. M. Flavin, M. Cibulski, W. A. Boulanger, R. L. Shone and Z.-Q. Xu, J. Med. Chem., 1996, 39, 1303–1313 CrossRef CAS PubMed.
- H. Ranjith, W. Dharmaratne, S. Sotheeswaran, S. Balasubramaniam and E. S. Waight, Phytochem, 1985, 24, 1553–1556 CrossRef.
- A. A. Zaha and A. Hazem, New Microbiol., 2002, 25, 213–222 CAS.
- C. Kontogiorgis and D. Hadjipavlou-Litina, J. Enzyme Inhib. Med. Chem., 2003, 18, 63–69 CrossRef CAS PubMed.
- S. Khode, V. Maddi, P. Aragade, M. Palkar, P. K. Ronad, S. Mamledesai, A. H. M. Thippeswamy and D. Satyanarayana, Eur. J. Med. Chem., 2009, 44, 1682–1688 CrossRef CAS PubMed.
- C.-J. Wang, Y.-J. Hsieh, C.-Y. Chu, Y.-L. Lin and T.-H. Tseng, Cancer Lett., 2002, 183, 163–168 CrossRef CAS.
- M. Kaur, S. Kohli, S. Sandhu, Y. Bansal and G. Bansal, Anticancer Agents Med. Chem., 2015, 15, 1032–1048 CrossRef CAS PubMed.
- A. Thakur, R. Singla and V. Jaitak, Eur. J. Med. Chem., 2015, 101, 476–495 CrossRef CAS PubMed.
- R. S. Keri, S. B. Sasidhar, B. M. Nagaraja and M. A. Santos, Eur. J. Med. Chem., 2015, 100, 257–269 CrossRef CAS PubMed.
- X. Sun, T. Liu, J. Sun and X. Wang, RSC Adv., 2020, 10, 10826–10847 RSC.
- S. R. Trenor, A. R. Shultz, B. J. Love and T. E. Long, Chem. Rev., 2004, 104, 3059–3078 CrossRef CAS PubMed.
- V. F. Traven, A. V Manaev, A. Y. Bochkov, T. A. Chibisova and I. V Ivanov, Russ. Chem. Bull., 2012, 61, 1342–1362 CrossRef CAS.
- D. Cao, Z. Liu, P. Verwilst, S. Koo, P. Jangjili, J. S. Kim and W. Lin, Chem. Rev., 2019, 119, 10403–10519 CrossRef CAS PubMed.
- Ł. Janus, J. Radwan-Pragłowska, M. Piątkowski and D. Bogdał, Int. J. Mol. Sci., 2020, 21(21), 8073 CrossRef.
- Z. Yu, W. Ma, T. Wu, J. Wen, Y. Zhang, L. Wang, Y. He, H. Chu and M. Hu, ACS Omega, 2020, 5, 7369–7378 CrossRef CAS PubMed.
- S. R. Lakshmi, V. Singh and L. R. Chowhan, RSC Adv., 2020, 10, 13866–13871 RSC.
- S. Nakamura, A. Toda, M. Sano, T. Hatanaka and Y. Funahashi, Adv. Synth. Catal., 2016, 358, 1029–1034 CrossRef CAS.
- L. Xu, Z. Shao, L. Wang and J. Xiao, Org. Lett., 2014, 16, 796–799 CrossRef CAS PubMed.
- Z. Shao, L. Xu, L. Wang, H. Wei and J. Xiao, Org. Biomol. Chem., 2014, 12, 2185–2188 RSC.
- F. Han, S. Xun, L. Jia, Y. Zhang, L. Zou and X. Hu, Org. Lett., 2019, 21, 5907–5911 CrossRef CAS PubMed.
- F. Scalambra, P. Lorenzo-Luis, I. de los Rios and A. Romerosa, Coord. Chem. Rev., 2021, 443, 213997 CrossRef CAS.
- D. Pla and M. Gómez, ACS Catal., 2016, 6, 3537–3552 CrossRef CAS.
- (a) S. Sarkar, E. Guibal, F. Quignard and A. K. SenGupta, J. Nanopart. Res., 2012, 14, 715–738 CrossRef; (b) K. D. Dwivedi, S. R. Marri, S. K. Nandigama and R. L. Chowhan, J. Chem. Sci., 2018, 130, 129 CrossRef.
- B. Kumar, J. N. Babu and R. L. Chowhan, Appl. Organomet. Chem., 2022, 1–14 Search PubMed.
- B. Kumar, M. S. Reddy, K. D. Dwivedi, A. Dahiya, J. N. Babu and R. L. Chowhan, Appl. Organomet. Chem., 2021, 1–10 Search PubMed.
- O. A. Battista and P. A. Smith, Ind. Eng. Chem., 2002, 54, 20–29 CrossRef.
- S. Ardizzone, F. S. Dioguardi, T. Mussini, P. R. Mussini, S. Rondinini, B. Vercelli and A. Vertova, Cellulose, 1999, 6, 57–69 CrossRef CAS.
- E. C. Ramires, J. D. Megiatto, A. Dufresne and E. Frollini, Fibers, 2020, 8, 21, DOI:10.3390/fib8040021.
- M. Amir, H. Gungunes, Y. Slimani, N. Tashkandi, H. S. El Sayed, F. Aldakheel, M. Sertkol, H. Sozeri, A. Manikandan, I. Ercan and A. Baykal, J. Supercond. Novel Magn., 2019, 32, 557–564 CrossRef CAS.
- M. Amini, A. Pourvahabi Anbari, S. Ramezani, S. Gautam and K. Hwa Chae, ChemistrySelect, 2016, 1, 4607–4612 CrossRef CAS.
- M. K. Satheeshkumar, E. Ranjith Kumar, C. Srinivas, G. Prasad, S. S. Meena, I. Pradeep, N. Suriyanarayanan and D. L. Sastry, J. Magn. Magn. Mater., 2019, 484, 120–125 CrossRef CAS.
- E. Teymoori, A. Davoodnia, A. Khojastehnezhad and N. Hosseininasab, Q. J. Iran. Chem. Commun., 2019, 7, 174–185 Search PubMed.
- A. S. Kumar, M. A. Reddy, M. Knorn, O. Reiser and B. Sreedhar, Eur. J. Org. Chem., 2013, 4674–4680 CrossRef CAS.
- B. S. P. Anil Kumar, K. Harsha Vardhan Reddy, B. Madhav, K. Ramesh and Y. V. D. Nageswar, Tetrahedron Lett., 2012, 53, 4595–4599 CrossRef CAS.
- M. A. E. A. A. A. El-Remaily and A. M. Abu-Dief, Tetrahedron, 2015, 71, 2579–2584 CrossRef CAS.
- M. Saha, K. Pradhan and A. R. Das, RSC Adv., 2016, 6, 55033–55038 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ra05994k |
| This journal is © The Royal Society of Chemistry 2022 |