Open Access Article
Open Access ArticleLithium sensors based on photophysical changes of 1-aza-12-crown-4 naphthalene derivatives synthesized via Buchwald–Hartwig amination†
Haneul Kim and
Byungjin Koo *
*
Department of Polymer Science and Engineering, Dankook University, Yongin, Gyeonggi 16890, Republic of Korea. E-mail: bkoo@dankook.ac.kr
First published on 8th November 2022
Abstract
Lithium detection is of great significance in many applications. Lithium-sensing compounds with high selectivity are scarce and, if any, complicated to synthesize. We herein report a novel yet simple compound that can detect lithium ions in an organic solvent through changes in absorbance and fluorescence. Naphthalene functionalized with 1-aza-12-crown-4 (1) was synthesized via one step from commercially available 1-bromonaphthalene through Buchwald–Hartwig amination. In order to obtain a structure–property relationship, we also synthesized two other compounds that are structurally similar to 1, wherein the compounds 2 and 3 include an imide moiety (an electron acceptor) and do not include a 1-aza-12-crown-4 unit, respectively. Upon the addition of lithium ions, compound 1 displayed a clear isosbestic point in the absorption spectra and a new peak in the fluorescence spectra, whereas the compounds 2 and 3 indicated miniscule and no spectroscopic changes, respectively. 1H NMR titration studies and the calculated optimized geometry from density functional theory (DFT) indicated the lithium binding on the aza-crown. The calculated limit of detection (LOD) was 21 μM. The lithium detection with 1 is selective among other alkali metals (Na+, K+, and Cs+). DFT calculation indicated that the lone pair electrons in the nitrogen atom of 1 is delocalized yet available to bind lithium, whereas the nitrogen lone pair electrons of 2 showed significant intramolecular charge transfer to the imide acceptor, resulting in a high dipole moment, and thus were unavailable to bind lithium. This work elucidates the key design parameters for future lithium sensors.
1. Introduction
For advanced electronic technology,1,2 lithium has served as one of the most important elements in the past few decades. Due to the surging demand fpr lithium-ion batteries used in personal electronics and electric vehicles, lithium extraction technology is garnering tremendous attention for sustainable production of lithium. Lithium is extracted from primary sources (e.g., lithium-rich brine) as well as from secondary sources (e.g., spent lithium-ion batteries), wherein organic solvents such as PC-88A serve as extractants that acquire lithium from aqueous solution.3–5 To determine the lithium concentration in the extracted organic phase, generally ranging from a few to hundreds of millimolar concentrations, instruments such as ICP-OES (inductively coupled plasma-optical emission spectroscopy) are currently employed.6–8 However, these instruments require prolonged sample preparation steps and measurement time. Therefore, methods that enable more efficient and prompt determination of lithium concentrations need to be developed.Various lithium sensing strategies, such as electrochemical detection,9–12 fiber-based sensors,13 a flow-through optode,14 and photophysical (absorbance and fluorescence) transduction,15–19 have been developed. Among them is the photophysical method that exhibits superior utility owing to the apparent optical changes without interpretation of complex data, fast measurement time, and facile sample preparation procedures. The working principles of these spectroscopic lithium sensors are grounded on chelation-induced photophysical changes,20,21 including intramolecular charge transfer (ICT),22 photoinduced electron transfer (PET),23 Forster resonance energy transfer (FRET),24 and excited-state intramolecular proton-transfer (ESIPT).25 To build the optical sensors, chemists design a sensor that consists of two parts: an ionophore for selective lithium binding and a chromophore/fluorophore for optical changes.21,26 While various lithium-chelating ligands have been developed including porphyrins,27,28 phenanthrolines,29 and calixarenes,30,31 crown ethers and their derivatives32–35 are known to show strong affinity to lithium over sodium and potassium, particularly in the case of 12-crown-4 and 14-crown-4 given the cavity size. Chromo- and fluorophores are small molecular dyes, such as acenes,36 squaraine dyes,37 BODIPY,38,39 coumarins,16,40 quinoxaline,41 and naphthalimides.17 However, these probes functionalized with crown ethers often possess complex molecular structures that require multiple synthetic steps to prepare.
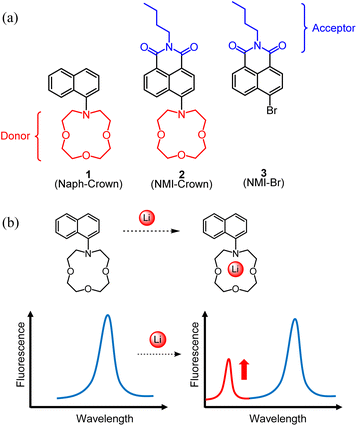
Herein, we report a structurally simple lithium sensor (1, referred to as Naph-Crown) containing naphthalene with 1-aza-12-crown-4 (Fig. 1a). In spite of the simplicity, this molecule has never been reported. To understand the structure–property relationship, we also designed and synthesized the compounds 2 (NMI-Crown, wherein NMI stands for naphthalene monoimide) and 3 (NMI-Br) (Fig. 1a). Our initial hypothesis was that the compound 2 was believed to undergo ICT upon photo-excitation with the appearance of a longer absorption band (ICT band), which would disappear if lithium were bound to the crown ether. However, we observed minute absorbance and fluorescence changes. Unexpectedly, the compound 1 exhibited unequivocal ratiometric absorbance changes and the generation of a new fluorescence peak (Fig. 1b). The sensor was not responsive to Na+, K+, and Cs+ in both absorbance and fluorescence, presenting selectivity among alkali metals. An additional advantage of the sensor 1 is that it can be readily synthesized only one step from a commercially available starting material through Buchwald–Hartwig amination, suggesting the great possibility of practical applications and commercialization.
2. Materials and methods
2.1 General
Chemicals were purchased from Sigma-Aldrich, TCI, Daejung Chemicals, Samchun Chemicals, and Duksan Chemicals without further purification unless noted otherwise. All reactions were carried out under argon with standard Schlenk techniques. Dry solvents were prepared with activated 4 Å molecular sieves. All 1H NMR and 13C NMR are reported in ppm on a JEOL ECS 400 MHz NMR spectrometer. 1H NMR is referenced to a chloroform peak (δ = 7.26 ppm) or an acetonitrile peak (δ = 1.94 ppm) for NMR titration studies with lithium. The multiplicity is reported as follows: s = singlet, d = doublet, dd = doublet of doublet, t = triplet, m = multiplet or unresolved, br = broad. Coupling constants J are reported in Hz. 13C NMR is referenced to chloroform (δ = 77.16 ppm). High-resolution mass spectrometry (HRMS) using electrospray ionization (ESI) in a positive mode was performed with AB SCIEX Q-TOF 5600. UV-vis spectra were recorded on a Scinco Mega U600 UV-vis spectrophotometer at room temperature. Fluorescence measurements were performed at room temperature with a Hitachi F-7000 (150 W xenon lamp).2.2 Synthesis of 1 (Naph-Crown)
To the flame-dried Schlenk flask, 1-bromonaphthalene (0.97 mmol, 1.00 equiv., 200 mg), 1-aza-12-crown-4 (1.11 mmol, 1.14 equiv., 193 mg), Pd2(dba)3 (0.0097 mmol, 0.01 equiv., 9 mg), DavePhos (0.058 mmol, 0.06 equiv., 23 mg), and NaOtBu (1.35 mmol, 1.4 equiv., 130 mg) were dissolved in 1.7 mL of dry toluene, and this mixture was degassed with argon. The reaction mixture was heated and stirred at 90 °C for 25 h. After cooling down to room temperature, the solvent was evaporated in vacuo. The mixture was purified with column chromatography (hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 1
ethyl acetate = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), producing a dark yellow oil (185 mg, 63% yield). 1H NMR (400 MHz, CDCl3) δ 8.66 (d, J = 8.2 Hz, 1H), 7.82 (dd, J = 7.4, 1.8 Hz, 1H), 7.56 (d, J = 8.1 Hz, 1H), 7.54–7.43 (m, 2H), 7.40 (t, J = 7.8 Hz, 1H), 7.23 (d, J = 7.4 Hz, 1H), 3.83–3.73 (m, 12H), 3.46 (t, J = 4.8 Hz, 4H). 13C NMR (101 MHz, CDCl3) δ 149.21, 135.11, 130.79, 128.11, 125.89, 125.63, 125.40, 124.77, 123.71, 117.89, 71.55, 71.05, 70.18, 54.66. HRMS (ESI) calculated for C18H24NO3 ([M + H]+) 302.1751; found, 302.1747.
1), producing a dark yellow oil (185 mg, 63% yield). 1H NMR (400 MHz, CDCl3) δ 8.66 (d, J = 8.2 Hz, 1H), 7.82 (dd, J = 7.4, 1.8 Hz, 1H), 7.56 (d, J = 8.1 Hz, 1H), 7.54–7.43 (m, 2H), 7.40 (t, J = 7.8 Hz, 1H), 7.23 (d, J = 7.4 Hz, 1H), 3.83–3.73 (m, 12H), 3.46 (t, J = 4.8 Hz, 4H). 13C NMR (101 MHz, CDCl3) δ 149.21, 135.11, 130.79, 128.11, 125.89, 125.63, 125.40, 124.77, 123.71, 117.89, 71.55, 71.05, 70.18, 54.66. HRMS (ESI) calculated for C18H24NO3 ([M + H]+) 302.1751; found, 302.1747.
2.3 Synthesis of 2 (NMI-Crown)
To the flame-dried Schlenk flask, N-butyl-4-bromo-1,8-naphthalimide (0.60 mmol, 1.00 equiv., 200 mg), 1-aza-12-crown-4 (0.68 mmol, 1.14 equiv., 120 mg), Pd2(dba)3 (0.006 mmol, 0.01 equiv., 5.5 mg), DavePhos (0.036 mmol, 0.06 equiv., 14 mg), and NaOtBu (0.840 mmol, 1.4 equiv., 81 mg) were dissolved in 1.5 mL of dry toluene, and this mixture was degassed with argon. The reaction mixture was heated and stirred at 100 °C for 20 h. After cooling down to room temperature, the solvent was evaporated in vacuo. The mixture was purified with column chromatography (dichloromethane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 1
ethyl acetate = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), producing a dark yellow solid (75 mg, 29% yield). Melting point 60–61 °C. 1H NMR (400 MHz, CDCl3) δ 9.09 (d, J = 8.5 Hz, 1H), 8.58 (d, J = 7.2 Hz, 1H), 8.49 (d, J = 8.2 Hz, 1H), 7.68 (t, J = 8.1 Hz, 1H), 7.31 (br, 1H), 4.17 (t, J = 7.8 Hz, 2H). 3.90–3.55 (m, 16H), 1.76–1.62 (m, 2H), 1.50–1.37 (m, 2H), 0.96 (t, J = 7.3 Hz). 13C NMR (101 MHz, CDCl3) δ 164.68, 164.10, 132.07, 131.99, 131.25, 130.48, 127.10, 125.41, 122.93, 115.61, 71.31, 71.26, 69.12, 54.35, 40.08, 30.32, 20.47, 13.95. HRMS (ESI) calculated for C24H31N2O5 ([M + H]+) 427.2227; found, 427.2226.
1), producing a dark yellow solid (75 mg, 29% yield). Melting point 60–61 °C. 1H NMR (400 MHz, CDCl3) δ 9.09 (d, J = 8.5 Hz, 1H), 8.58 (d, J = 7.2 Hz, 1H), 8.49 (d, J = 8.2 Hz, 1H), 7.68 (t, J = 8.1 Hz, 1H), 7.31 (br, 1H), 4.17 (t, J = 7.8 Hz, 2H). 3.90–3.55 (m, 16H), 1.76–1.62 (m, 2H), 1.50–1.37 (m, 2H), 0.96 (t, J = 7.3 Hz). 13C NMR (101 MHz, CDCl3) δ 164.68, 164.10, 132.07, 131.99, 131.25, 130.48, 127.10, 125.41, 122.93, 115.61, 71.31, 71.26, 69.12, 54.35, 40.08, 30.32, 20.47, 13.95. HRMS (ESI) calculated for C24H31N2O5 ([M + H]+) 427.2227; found, 427.2226.
2.4 Synthesis of 3 (NMI-Br)
To the flame-dried Schlenk flask, 4-bromo-1,8-naphthalic anhydride (3.6 mmol, 1.00 equiv., 1 g) was added and dissolved in 8.3 mL of ethylene glycol. n-Butylamine (5.4 mmol, 1.5 equiv., 0.53 mL) was added, and the mixture was stirred at 160 °C for 12 h. After cooling down to room temperature, 2 M HCl (aq.) was added, and the mixture was extracted three times with dichloromethane. The combined organic phase was dried over MgSO4, filtered, and concentrated in vacuo. The crude mixture was purified with column chromatography (hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 4
ethyl acetate = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), producing an off-yellow solid (1.07 g, 89% yield). Melting point 99 °C. 1H NMR (400 MHz, CDCl3) δ 8.67 (dd, J = 7.3, 1.2 Hz, 1H), 8.58 (dd, J = 8.7, 1.3 Hz, 1H), 8.42 (d, J = 7.8 Hz, 1H), 8.05 (d, J = 7.8 Hz, 1H), 7.86 (dd, J = 8.6, 7.4 Hz, 1H), 4.18 (t, J = 7.8 Hz, 2H), 1.76–1.66 (m, 2H), 1.51–1.39 (m, 2H), 0.98 (t, J = 7.3 Hz, 3H).
1), producing an off-yellow solid (1.07 g, 89% yield). Melting point 99 °C. 1H NMR (400 MHz, CDCl3) δ 8.67 (dd, J = 7.3, 1.2 Hz, 1H), 8.58 (dd, J = 8.7, 1.3 Hz, 1H), 8.42 (d, J = 7.8 Hz, 1H), 8.05 (d, J = 7.8 Hz, 1H), 7.86 (dd, J = 8.6, 7.4 Hz, 1H), 4.18 (t, J = 7.8 Hz, 2H), 1.76–1.66 (m, 2H), 1.51–1.39 (m, 2H), 0.98 (t, J = 7.3 Hz, 3H).
2.5 DFT calculation
The ORCA 5.0 software program42 was used to calculate frontier molecular orbitals. The gas-phase ground state molecular geometry optimizations were carried out in B3LYP hybrid exchange–correlation functional43 with the 6-31G* basis set.44 Cartesian coordinates of optimized structures of the compounds 1, 2, and 1 + LiCl were included in ESI.† Avogadro software program was used to display HOMO and LUMO.2.6 Photophysical analysis
Naph-Crown, NMI-Crown, and NMI-Br were dissolved in acetonitrile (ACN) at a concentration of 0.01 or 0.025 mM (for absorption) and 0.1 mM (for fluorescence). Lithium perchlorate with a concentration of 100 mM was prepared in ACN. For the UV-vis and fluorescence experiments, 2 mL of the solution was aliquoted into a quartz cuvette, and the lithium solution was subsequently added. The experiments with other alkali metals were performed in an identical way with perchlorate salts. For potassium and cesium, the saturated solutions were used.3. Results and discussion
We employed naphthalene as a chromo/fluorophore and 1-aza-12-crown-4 as a selective ligand for lithium. Although various naphthalene-based dyes have been developed,45 Naph-Crown (1) in this work has never been reported. This molecule possesses a nitrogen atom that is moderately conjugated to the naphthalene ring (Fig. 1a). To understand the effect of lithium chelation and ratiometric sensor responses, we additionally prepared an ICT-type molecule, NMI-Crown (2). Generally, ICT-type sensors are known to exhibit clear ratiomentric sensor responses,46–49 although our result indicated disparate behaviors (vide infra). The NMI-Br (3) lacking the crown ether was selected as a control molecular sensor.The syntheses of the compounds 1, 2, and 3 were shown in Scheme 1. For the synthesis of 1, Buchwald–Hartwig cross-coupling reactions were considered,50,51 and previously, N-aryl-aza-crown ethers were prepared using a DavePhos ligand with high yields.52 Hence, we used a similar Buchwald–Hartwig condition. The reaction was begun with a commercially available 1-bromonaphthalene that reacted with 1-aza-12-crown-4 in the presence of Pd2(dba)3 (1 mol%), DavePhos (6 mol%), and NaOtBu (1.4 equiv.) in toluene. The isolated yield was 63%. For the synthesis of 2, 4-bromo-1,8-naphthalic anhydride was treated with n-butylamine to form 3 containing a naphthalene monoimide (NMI) skeleton, followed by the Buchwald–Hartwig amination with the same condition as in 1, resulting in the production of 2 with the isolated yield of 29%. The compounds 1 and 2 were characterized by 1H NMR, 13C NMR, and HRMS.
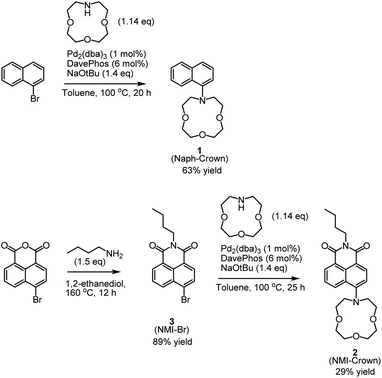 | ||
| Scheme 1 Synthetic procedures to prepare the compounds 1, 2, and 3 through Buchwald–Hartwig amination. | ||
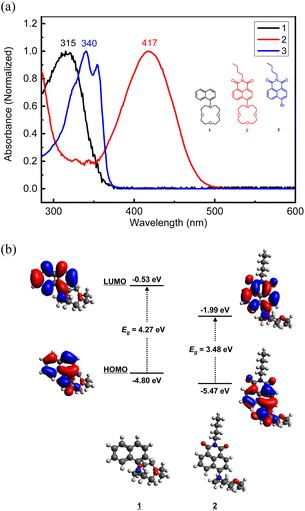
The UV-vis spectra of each compound were measured in acetonitrile (Fig. 2a). Naph-Crown (1) showed the absorption maximum at 315 nm, which was red-shifted about 40 nm from naphthalene.53 This red-shift may be attributed to the extended conjugation from the nitrogen atom in the naphthalene ring. NMI-Crown (2), having an acceptor imide moiety, exhibited the absorption maximum at 417 nm, which was far red-shifted from 1 by more than 100 nm. This large red-shift is related to a strong ICT characteristic of the donor–acceptor type molecule 2. Density functional theory (DFT) calculation in B3LYP functional with 6-31G* basis set indicated the lowered HOMO (from −4.80 to −5.47 eV) and LUMO (from −0.53 eV to −1.99 eV) due to the electron-withdrawing group (Fig. 2b). In addition, the shape of LUMO of 2 was shifted to the imide moiety and the dipole moments of 1 and 2 were calculated to be 2.78 D and 7.18 D, respectively, suggesting the formation of strong ICT upon photoexcitation. NMI-Br (3), wherein the crown ether was replaced with a bromine atom, exhibited the absorption maximum at 340 nm, which was far blue-shifted from 2 owing to the diminished ICT character.
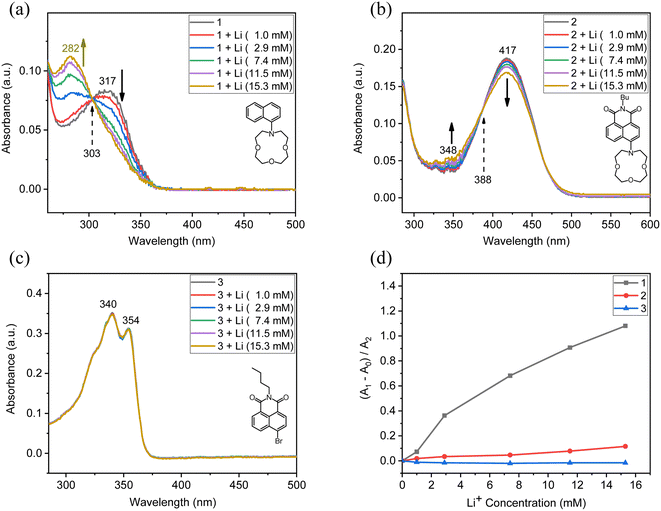
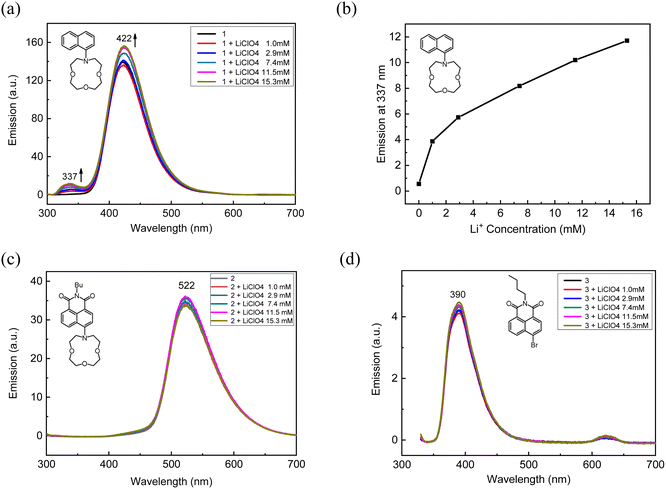
To investigate the lithium binding effect, we measured UV-vis spectra upon the addition of lithium salt solutions (lithium perchlorate) in each compound (Fig. 3). We used acetonitrile as a model organic solvent. The concentration of the compounds was kept at 0.01 mM, and the lithium salt concentration of 100 mM in acetonitrile was prepared. Upon the addition of the lithium solution to Naph-Crown (1), we observed the decrease of the peak at 317 nm, while a new peak at 282 nm was generated (Fig. 3a). A clear isosbestic point was observed at 303 nm, indicating that two species are responsible for this change. The blue-shifted new peak at 282 nm could be attributed to the naphthalene absorption without the conjugation of the lone pair electrons on the nitrogen atom owing to the chelation to lithium. The binding constant (K) of the compound 1 with lithium was calculated based on the following equation, ΔA = [D]Δε[M]/((1/K) + [M]),54 wherein ΔA, [D], [M], Δε, and K represent the absorption difference at a particular wavelength upon the addition of metal ions, the dye concentration (herein Naph-Crown), the metal ion concentration, the change in extinction coefficient upon the addition of metal ions, and the binding constant, respectively. The resulting plot produced the binding constant of 127 M−1 for the association between the compound 1 and lithium (Fig. S1†). In the case of NMI-Crown (2), there was a miniscule ratiometric change in which the absorption at 417 nm was slightly decreased with the increased signal at 348 nm (Fig. 3b). An isosbestic point at 388 nm was observed. On the contrary to the previous reports on ICT-type sensors with excellent properties,46–49 we found that in our case the strong ICT feature leading to reduced basicity of nitrogen may be detrimental to sensing properties. For NMI-Br (3) without the crown ether, the absorption spectra presented no changes upon the lithium addition, demonstrating that the crown ether is necessary for sensor responses (Fig. 3c). We calculated the relative absorbance change rate, (A1 − A0)/A2, and plotted this rate (y-axis) over the lithium concentration (x-axis), wherein A0, A1, and A2 represent the decreasing absorption (at 317, 417, and 354 nm in each of 1, 2, and 3), the increasing absorption (at 282, 348, and 340 nm, ditto), and the initial absorption without lithium (at 317, 417, and 354 nm, ditto), respectively (Fig. 3d). The graph explicitly represents the high sensitivity of 1 (black curve) in comparison to 2 (red) and 3 (blue).
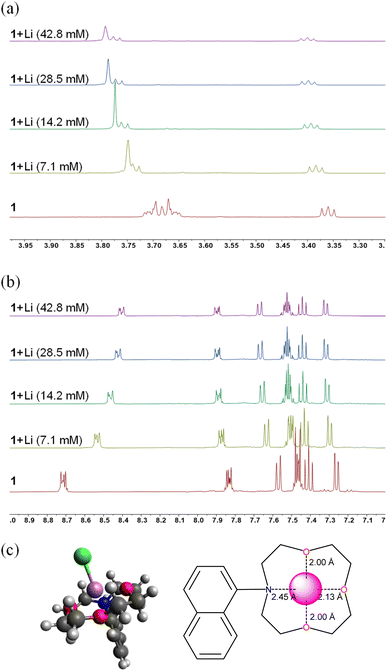
To confirm the binding of lithium to a crown-ether moiety on 1, we performed the 1H NMR titration studies (referenced to CD3CN at 1.94 ppm) and DFT calculation (Fig. 4). The 1H chemical shifts of the crown-ether moiety near 3.3–3.8 ppm exhibited significant peak broadening with the downward chemical shift (Fig. 4a), implying that there may be fast associative/dissociative equilibrium between the lithium and the crown-ether, which could not be differentiated in the NMR time scale. The peak broadening was relatively insignificant in the naphthyl unit (Fig. 4b). The DFT calculation of the compound 1 and lithium (chloride as anion for charge neutrality) in B3LYP/6-31G* produced the optimized geometry (Fig. 4c), wherein the lithium (purple) is coordinated to four heteroatoms of the crown-ether with the distances of 2.00–2.45 Å. In addition, in order to examine the effect of cyclic etherial ligands, we synthesized the n-butylamine-substituted NMI (NMI–NH–Bu). This compound was not responsive to lithium ions (Fig. S2a†), further demonstrating that aza-crown-ether is necessary for lithium chelation.
A theoretical limit of detection (LOD) was estimated using the following equation: LOD = 3(RMSnoise/slope),55 wherein RMSnoise represents a root-mean-square noise that was calculated by taking ten blank measurements of the compound 1 (detailed procedure was described in ESI†). The slope was obtained in the concentration–signal plot (black curve in Fig. 3d). The calculated LOD was 21 μM. Although this LOD is higher than the previously reported LOD values (e.g., LOD of 5 μM from a naphthalene diimide derivative with two crown ethers),17 our sensor can cover a wide range of applications that require lithium detection, along with the structural simplicity.
Next, fluorescence measurements were executed with lithium ions (Fig. 5). Naph-Crown (1) with lithium indicated a new peak at 337 nm (Fig. 5a), which was increasing with higher lithium concentrations (Fig. 5b). We suppose that this emission could be generated from a locally excited (LE) state that is present when the lone pair electrons of the nitrogen atom in 1 is bound to lithium. Although it appeared that lithium was bound to 1 significantly given the UV-vis spectra, we still observed the large emission of 1 at 422 nm. It is worth mentioning that no self-quenching occurred at this concentration of 0.1 mM (Fig. S3†). The compound 2 has a longer-wavelength emission at 521 nm owing to the strong CT state. In contrast to 1, no new peak was observed with the lithium addition (Fig. 5c). In the compound 3, no significant emission changes were observed, repeatedly demonstrating that crown ether is necessary for lithium binding (Fig. 5d).
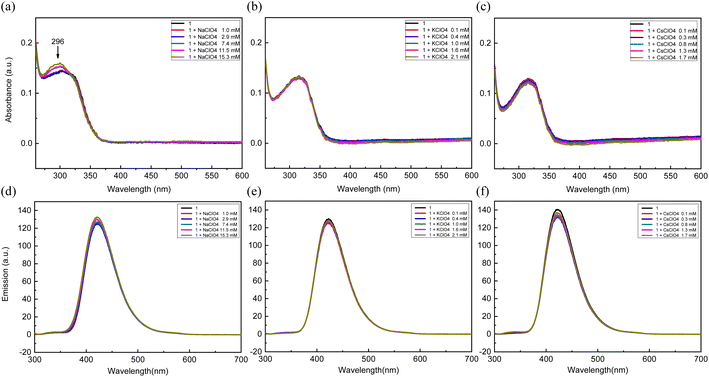
Having confirmed the absorption and emission changes with lithium in 1, we performed selectivity tests with Na+, K+, and Cs+ having the identical perchlorate counterions. In most cases, we found that the compound 1 was not responsive to the three alkali metal ions based on both absorption and emission spectra (Fig. 6), suggesting the excellent selectivity of the sensor 1 among alkali metals. It is worth noting that the sodium cation generated a weak new absorption signal at 296 nm (Fig. 6a). This is perhaps due to the weak interaction between the aza-crown-ether and sodium cation, as reported previously.56,57 To test the binding of lithium in the presence of other alkali metals, lithium and sodium with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio were mixed and added to the receptor 1. A clear isosbestic point at 303 nm was still observed (Fig. S4†), presenting that the binding of lithium was effective in the presence of non-interacting alkali metals. A similar behavior was observed with potassium ions as well (Fig. S4†).
1 molar ratio were mixed and added to the receptor 1. A clear isosbestic point at 303 nm was still observed (Fig. S4†), presenting that the binding of lithium was effective in the presence of non-interacting alkali metals. A similar behavior was observed with potassium ions as well (Fig. S4†).
4. Conclusions
In this study, we report the synthesis of the molecular sensor 1 that can selectively detect lithium ions through photophysical changes. The compound 1, possessing a naphthalene skeleton with 1-aza-12-crown-4, was synthesized through the Buchwald–Hartwig amination, requiring only one synthetic step from a commercially available starting material. Upon the addition of lithium, the sensor 1 indicated a ratiometric absorption change as well as generation of a new emission peak owing to the lithium binding. LOD was estimated to be 21 μM. Other alkali metals, including Na+, K+, and Cs+, were rarely responsive. NMR studies and DFT calculations revealed the binding of lithium to the aza-crown ether on 1. The compound 2, having an imide acceptor and giving rise to the ICT, showed unnoticeable photophysical changes, suggesting that the strong ICT may not be beneficial to the fabrication of lithium sensor, in contrast to the conventional knowledge. Based on this structure–property relationship, we are currently synthesizing other lithium-sensing chromophores working in visible light which can be recognized by naked eyes for practical applications.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (2020R1G1A1102161). The Department of Polymer Science and Engineering was supported through the Research-Focused Department Promotion & Interdisciplinary Convergence Research Project as a part of the University Innovation Support Program for Dankook University in 2022. This work was also supported by Korea Institute for Advancement of Technology (KIAT) grant funded by the Korea Government (MOTIE) (P0002007, HRD Program for Industrial Innovation).References
- J. B. Goodenough and K.-S. Park, The Li-Ion Rechargeable Battery: A Perspective, J. Am. Chem. Soc., 2013, 135(4), 1167–1176 CrossRef CAS PubMed.
- J. R. Geddes, S. Burgess, K. Hawton, K. Jamison and G. M. Goodwin, Long-Term Lithium Therapy for Bipolar Disorder: Systematic Review and Meta-Analysis of Randomized Controlled Trials, Am. J. Psychiatry, 2004, 161(2), 217–222 CrossRef PubMed.
- P. Meshram, B. D. Pandey and T. R. Mankhand, Extraction of lithium from primary and secondary sources by pre-treatment, leaching and separation: a comprehensive review, Hydrometallurgy, 2014, 150, 192–208 CrossRef CAS.
- B. Swain, Recovery and recycling of lithium: a review, Sep. Purif. Technol., 2017, 172, 388–403 CrossRef CAS.
- G. Liu, Z. Zhao and A. Ghahreman, Novel approaches for lithium extraction from salt-lake brines: a review, Hydrometallurgy, 2019, 187, 81–100 CrossRef CAS.
- L. Zhang, L. Li, D. Shi, X. Peng, F. Song, F. Nie and W. Han, Recovery of lithium from alkaline brine by solvent extraction with β-diketone, Hydrometallurgy, 2018, 175, 35–42 CrossRef CAS.
- A. Pálsdóttir, C. A. Alabi and J. W. Tester, Characterization of 14-Crown-4 Ethers for the Extraction of Lithium from Natural Brines: Synthesis, Solubility Measurements in Supercritical Carbon Dioxide, and Thermodynamic Modeling, Ind. Eng. Chem. Res., 2021, 60(21), 7926–7934 CrossRef.
- B. Koo, L. E. Sofen, D. J. Gisch, B. Kern, M. A. Rickard and M. B. Francis, Lithium-Chelating Resins Functionalized with Oligoethylene Glycols toward Lithium-Ion Battery Recycling, Adv. Sustainable Syst., 2021, 5(2), 2000230 CrossRef CAS.
- A. L. Suherman, B. Rasche, B. Godlewska, P. Nicholas, S. Herlihy, N. Caiger, P. J. Cowen and R. G. Compton, Electrochemical Detection and Quantification of Lithium Ions in Authentic Human Saliva Using LiMn2O4-Modified Electrodes, ACS Sens., 2019, 4(9), 2497–2506 CrossRef CAS PubMed.
- A. Bakhtiarvand Bakhtiari, B. Mohammadi, P. Moazzam, D. Didehroshan and A. Razmjou, An Evolving Insight into the Progress of Material Design for Membrane-Based Electrochemical Lithium Ion Detection, Adv. Mater. Interfaces, 2021, 8(19), 2100571 CrossRef CAS.
- M. Nel-lo, Ò. Ferrer, S. Colominas and J. Abellà, Lithium sensors based on Li6La3Ta1.5Y0.5O12 and Li6BaLa2Ta2O12 garnet electrolytes for molten lead alloys, Sens. Actuators, B, 2021, 339, 129831 CrossRef CAS.
- E. Zdrachek and E. Bakker, Potentiometric Sensing, Anal. Chem., 2021, 93(1), 72–102 CrossRef CAS PubMed.
- M. N. Sweilam, J. R. Varcoe and C. Crean, Fabrication and Optimization of Fiber-Based Lithium Sensor: A Step toward Wearable Sensors for Lithium Drug Monitoring in Interstitial Fluid, ACS Sens., 2018, 3(9), 1802–1810 CrossRef CAS PubMed.
- M. I. Albero, J. A. Ortuño, M. S. García, M. Cuartero and M. C. Alcaraz, Novel flow-through bulk optode for spectrophotometric determination of lithium in pharmaceuticals and saliva, Sens. Actuators, B, 2010, 145(1), 133–138 CrossRef CAS.
- K. Hiratani, M. Kaneyama, Y. Nagawa, E. Koyama and M. Kanesato, Synthesis of [1]Rotaxane via Covalent Bond Formation and Its Unique Fluorescent Response by Energy Transfer in the Presence of Lithium Ion, J. Am. Chem. Soc., 2004, 126(42), 13568–13569 CrossRef CAS PubMed.
- D. Citterio, J. Takeda, M. Kosugi, H. Hisamoto, S.-i. Sasaki, H. Komatsu and K. Suzuki, pH-Independent Fluorescent Chemosensor for Highly Selective Lithium Ion Sensing, Anal. Chem., 2007, 79(3), 1237–1242 CrossRef CAS PubMed.
- R. V. Hangarge, D. D. La, M. Boguslavsky, L. A. Jones, Y. S. Kim and S. V. Bhosale, An Aza-12-crown-4 Ether-Substituted Naphthalene Diimide Chemosensor for the Detection of Lithium Ion, ChemistrySelect, 2017, 2(35), 11487–11491 CrossRef CAS.
- T. Gunnlaugsson, B. Bichell and C. Nolan, Fluorescent PET chemosensors for lithium, Tetrahedron, 2004, 60(27), 5799–5806 CrossRef CAS.
- J.-H. Dong, C. Yang, H.-Q. Ding, P.-J. Xing, F.-Y. Zhou, H. Tian, X. Liu, H.-T. Zheng, S.-H. Hu and Z.-L. Zhu, Development of a Portable Method for Serum Lithium Measurement Based on Low-Cost Miniaturized Ultrasonic Nebulization Coupled with Atmospheric-Pressure Air-Sustained Discharge, Anal. Chem., 2021, 93(39), 13351–13359 CrossRef CAS PubMed.
- B. Valeur and I. Leray, Design principles of fluorescent molecular sensors for cation recognition, Coord. Chem. Rev., 2000, 205(1), 3–40 CrossRef CAS.
- E. Villemin and O. Raccurt, Optical lithium sensors, Coord. Chem. Rev., 2021, 435, 213801 CrossRef CAS.
- Z. R. Grabowski, K. Rotkiewicz and W. Rettig, Structural Changes Accompanying Intramolecular Electron Transfer: Focus on Twisted Intramolecular Charge-Transfer States and Structures, Chem. Rev., 2003, 103(10), 3899–4032 CrossRef PubMed.
- A. P. De Silva, T. S. Moody and G. D. Wright, Fluorescent PET (Photoinduced Electron Transfer) sensors as potent analytical tools, Analyst, 2009, 134(12), 2385–2393 RSC.
- J. Fan, M. Hu, P. Zhan and X. Peng, Energy transfer cassettes based on organic fluorophores: construction and applications in ratiometric sensing, Chem. Soc. Rev., 2013, 42(1), 29–43 RSC.
- A. C. Sedgwick, L. Wu, H.-H. Han, S. D. Bull, X.-P. He, T. D. James, J. L. Sessler, B. Z. Tang, H. Tian and J. Yoon, Excited-state intramolecular proton-transfer (ESIPT) based fluorescence sensors and imaging agents, Chem. Soc. Rev., 2018, 47(23), 8842–8880 RSC.
- M. Kamenica, R. R. Kothur, A. Willows, B. A. Patel and P. J. Cragg, Lithium Ion Sensors, Sensors, 2017, 17, 10 CrossRef PubMed.
- R. A. Richards, K. Hammons, M. Joe and G. M. Miskelly, Observation of a Stable Water-Soluble Lithium Porphyrin, Inorg. Chem., 1996, 35(7), 1940–1944 CrossRef CAS.
- M. Tabata, J. Nishimoto and T. Kusano, Spectrophotometric determination of lithium ion using a water-soluble octabromoporphyrin in aqueous solution, Talanta, 1998, 46(4), 703–709 CrossRef CAS PubMed.
- S. O. Obare and C. J. Murphy, A Two-Color Fluorescent Lithium Ion Sensor, Inorg. Chem., 2001, 40(23), 6080–6082 CrossRef CAS PubMed.
- M. McCarrick, S. J. Harris and D. Diamond, Assessment of three azophenol calix[4]arenes as chromogenic ligands for optical detection of alkali metal ions, Analyst, 1993, 118(9), 1127–1130 RSC.
- O. Santoro, M. R. J. Elsegood, S. J. Teat, T. Yamato and C. Redshaw, Lithium calix[4]arenes: structural studies and use in the ring opening polymerization of cyclic esters, RSC Adv., 2021, 11(19), 11304–11317 RSC.
- C. J. Pedersen, Cyclic polyethers and their complexes with metal salts, J. Am. Chem. Soc., 1967, 89(10), 2495–2496 CrossRef CAS.
- B. P. Czech, D. A. Babb, B. Son and R. A. Bartsch, Functionalized 13-crown-4, 14-crown-4, 15-crown-4, and 16-crown-4 compounds: synthesis and lithium ion complexation, J. Org. Chem., 1984, 49(25), 4805–4810 CrossRef CAS.
- A. F. Danil de Namor, J. C. Y. Ng, M. A. Llosa Tanco and M. Salomon, Thermodynamics of Lithium–Crown Ether (12-crown-4 and 1-Benzyl-1-aza-12-crown-4) Interactions in Acetonitrile and Propylene Carbonate. The Anion Effect on the Coordination Process, J. Phys. Chem., 1996, 100(34), 14485–14491 CrossRef.
- R. E. C. Torrejos, G. M. Nisola, H. S. Song, L. A. Limjuco, C. P. Lawagon, K. J. Parohinog, S. Koo, J. W. Han and W.-J. Chung, Design of lithium selective crown ethers: synthesis, extraction and theoretical binding studies, Chem. Eng. J., 2017, 326, 921–933 CrossRef CAS.
- A. Caballero, R. Tormos, A. Espinosa, M. D. Velasco, A. Tárraga, M. A. Miranda and P. Molina, Selective Fluorescence Sensing of Li+ in an Aqueous Environment by a Ferrocene–Anthracene-Linked Dyad, Org. Lett., 2004, 6(24), 4599–4602 CrossRef CAS PubMed.
- S.-H. Kim, S.-K. Han, S.-H. Park, C.-M. Yoon and S.-R. Keum, Novel fluorescent chemosensor for Li+ based on a squarylium dye carrying a monoazacrown moiety, Dyes Pigm., 1999, 43(1), 21–25 CrossRef CAS.
- M. Kollmannsberger, K. Rurack, U. Resch-Genger and J. Daub, Ultrafast Charge Transfer in Amino-Substituted Boron Dipyrromethene Dyes and Its Inhibition by Cation Complexation: A New Design Concept for Highly Sensitive Fluorescent Probes, J. Phys. Chem. A, 1998, 102(50), 10211–10220 CrossRef CAS.
- Y. Ando, Y. Hiruta, D. Citterio and K. Suzuki, A highly Li+-selective glass optode based on fluorescence ratiometry, Analyst, 2009, 134(11), 2314–2319 RSC.
- M.-S. Peng and J. Cai, Synthesis and fluorescence of crown ethers containing coumarin, Dyes Pigm., 2008, 79(3), 270–272 CrossRef CAS.
- M. Homocianu, A. M. Ipate, D. Homocianu, A. Airinei and C. Hamciuc, Metal ions sensing properties of some phenylquinoxaline derivatives, Spectrochim. Acta, Part A, 2019, 215, 371–380 CrossRef CAS PubMed.
- F. Neese, Software update: The ORCA program system—Version 5.0, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2022, e1606 Search PubMed.
- A. D. Becke, Density-functional thermochemistry. III. The role of exact exchange, J. Chem. Phys., 1993, 98(7), 5648–5652 CrossRef CAS.
- W. J. Hehre, R. Ditchfield and J. A. Pople, Self—Consistent Molecular Orbital Methods. XII. Further Extensions of Gaussian—Type Basis Sets for Use in Molecular Orbital Studies of Organic Molecules, J. Chem. Phys., 1972, 56(5), 2257–2261 CrossRef CAS.
- M. Al Kobaisi, S. V. Bhosale, K. Latham, A. M. Raynor and S. V. Bhosale, Functional Naphthalene Diimides: Synthesis, Properties, and Applications, Chem. Rev., 2016, 116(19), 11685–11796 CrossRef PubMed.
- L. Ding, Z. Zhang, X. Li and J. Su, Highly sensitive determination of low-level water content in organic solvents using novel solvatochromic dyes based on thioxanthone, Chem. Commun., 2013, 49(66), 7319–7321 RSC.
- P. Kumar, R. Kaushik, A. Ghosh and D. A. Jose, Detection of Moisture by Fluorescent Off-On Sensor in Organic Solvents and Raw Food Products, Anal. Chem., 2016, 88(23), 11314–11318 CrossRef CAS PubMed.
- H.-L. Qian, C. Dai, C.-X. Yang and X.-P. Yan, High-Crystallinity Covalent Organic Framework with Dual Fluorescence Emissions and Its Ratiometric Sensing Application, ACS Appl. Mater. Interfaces, 2017, 9(29), 24999–25005 CrossRef CAS PubMed.
- K. Imato, T. Enoki and Y. Ooyama, Development of an intramolecular charge transfer-type colorimetric and fluorescence sensor for water by fusion with a juloidine structure and complexation with boron trifluoride, RSC Adv., 2019, 9(54), 31466–31473 RSC.
- J. F. Hartwig, Evolution of a Fourth Generation Catalyst for the Amination and Thioetherification of Aryl Halides, Acc. Chem. Res., 2008, 41(11), 1534–1544 CrossRef CAS PubMed.
- P. Ruiz-Castillo and S. L. Buchwald, Applications of Palladium-Catalyzed C–N Cross-Coupling Reactions, Chem. Rev., 2016, 116(19), 12564–12649 CrossRef CAS PubMed.
- X.-X. Zhang and S. L. Buchwald, Efficient Synthesis of N-Aryl-Aza-Crown Ethers via Palladium-Catalyzed Amination, J. Org. Chem., 2000, 65(23), 8027–8031 CrossRef CAS PubMed.
- I. B. Berlman, 6—Graphs, in Handbook of Fluorescence Spectra of Aromatic Molecules, ed. I. B. Berlman, Academic Press, 2nd edn, 1971, pp. 107–415 Search PubMed.
- S. J. K. Pond, O. Tsutsumi, M. Rumi, O. Kwon, E. Zojer, J.-L. Brédas, S. R. Marder and J. W. Perry, Metal-Ion Sensing Fluorophores with Large Two-Photon Absorption Cross Sections: Aza-Crown Ether Substituted Donor−Acceptor−Donor Distyrylbenzenes, J. Am. Chem. Soc., 2004, 126(30), 9291–9306 CrossRef CAS PubMed.
- J. Li, Y. Lu, Q. Ye, M. Cinke, J. Han and M. Meyyappan, Carbon Nanotube Sensors for Gas and Organic Vapor Detection, Nano Lett., 2003, 3(7), 929–933 CrossRef CAS.
- I.-H. Chu, H. Zhang and D. V. Dearden, Macrocyclic chemistry in the gas phase: intrinsic cation affinities and complexation rates for alkali metal cation complexes of crown ethers and glymes, J. Am. Chem. Soc., 1993, 115(13), 5736–5744 CrossRef CAS.
- J. Warnock Samuel, R. Sujanani, S. Zofchak Everett, S. Zhao, J. Dilenschneider Theodore, G. Hanson Kalin, S. Mukherjee, V. Ganesan, D. Freeman Benny, M. Abu-Omar Mahdi and M. Bates Christopher, Engineering Li/Na selectivity in 12-Crown-4–functionalized polymer membranes, Proc. Natl. Acad. Sci. U. S. A., 2021, 118(37), e2022197118 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ra05746h |
| This journal is © The Royal Society of Chemistry 2022 |