Open Access Article
Open Access ArticleA green synthesis approach toward large-scale production of benzalacetone via Claisen–Schmidt condensation
Ruolun Sunab,
Chenhui Han*ab and
Jingsan Xu *bc
*bc
aSchool of Chemistry and Chemical Engineering, Inner Mongolia University, Hohhot, Inner Mongolia 010021, P. R. China. E-mail: hanchenhui@imu.edu.cn
bSchool of Chemistry and Physics, Queensland University of Technology, Brisbane, Queensland, Australia. E-mail: jingsan.xu@qut.edu.au
cQUT-MPIKG Joint Laboratory on Nanocatalysis for Sustainable Chemistry, Queensland University of Technology, Australia
First published on 13th October 2022
Abstract
Claisen–Schmidt (CS) condensation between acetone and benzaldehyde with NaOH as the catalyst is a well-recognized pathway for the synthesis of benzalacetone (BA). However, this process is compromised by a side reaction, i.e., a second CS reaction between benzaldehyde and the BA product. In this work, we designed a stirring-induced emulsion synthesis technique for the cyclic and scaling-up production of BA with 99 ± 1% selectivity, without the use of surfactants. In this approach, the water-soluble acetone and NaOH were separated from the oil-soluble benzaldehyde by the organic–aqueous phase interface, such that the CS condensation could only be executed at the liquid interface. The just-formed BA molecules diffuse to the interior of the oil solvent, where any subsequent CS post-reaction is rendered negligible, owing to the absence of NaOH. The oil phase containing the BA molecules can be easily separated from the aqueous solution by stopping stirring and undisturbed standing, allowing for a large-scale production protocol. As a proof of concept, over 1 kg of BA was produced in the laboratory with high yield and purity.
Introduction
The Claisen–Schmidt (CS) condensation reaction occurs between an aldehyde or ketone containing α-hydrogen and an aromatic carbonyl molecule with no α-hydrogen.1,2 Many important chemical compounds are synthesised via the CS reaction in the industries of food, pharmaceuticals, and fine chemicals. One example is the synthesis of benzalacetone (BA) from acetone and benzaldehyde with NaOH as the base catalyst.However, as acetone has two α-Hs which can react with two benzaldehyde molecules, to obtain the mono-condensation product, benzalacetone, is challenging. Dibenzalacetone as a main side-product always generates during the reaction, which lowers the yield of benzalacetone and substantially increases the cost of product isolation. In industry, the most commonly used means for minimizing the generation of dibenzalacetone is increasing the acetone equivalent relative to benzaldehyde, often up to 5 times the dose, leading to a waste of chemical and energy.2 To address this problem, some heterogeneous catalytic systems have been developed, such as using activated carbon, or performing the reaction under supercritical conditions.3,4 Nevertheless, the used acetone was in excess of benzaldehyde in all these cases.
In our previous work, we employed a surfactant (sodium dodecyl sulfate, SDS) stabilized emulsion system to enable the reaction of acetone and benzaldehyde at organic–aqueous phase interfaces to improve the selectivity of benzalacetone.5 In this scenario, the formed benzalacetone molecules diffuse into the oil phase and the post-CS reaction is inhibited owing to the lack of the NaOH catalyst (NaOH dissolved in the water phase). High selectivity and reaction rate are achieved, and in the meantime much lower acetone equivalent is used compared to conventional homogenous reactions.6–9
Nevertheless, the use of SDS surfactant led to difficulties in demulsification and product separation and hence result in a higher cost for the synthesis. Large-scale use of SDS molecules may also cause health and environmental problems.10–12 In this work, we have employed mechanical stirring to generate oil-in-water emulsions such that the CS condensation can be implemented at the liquid interfaces, achieving high selectivity for BA and avoiding the use of surfactant molecules. Since the emulsions are dynamically stabilized, the oil and water phase can separate simply by standing still.
Materials and methods
Materials
Benzaldehyde (99%, Sigma-Aldrich), acetone (99.9%, Sigma-Aldrich), toluene (AR, Fisher Scientific), cyclohexane (99.5%, Sigma-Aldrich), ethanol undenatured (AR, Chem-Supply), benzalacetone (analytical standard, Supelco), trans-dibenzylideneacetone (98%, Sigma-Aldrich), sodium hydroxide (AR, Chem-Supply), calcium chloride (97%, Sigma-Aldrich). All chemicals were used as received without further purification.Methods
(a) Emulsion system: at room temperature, 10 mL (98 mmol) benzaldehyde and 21.8 mL (294 mmol) acetone (taking 3 equiv. as an example) were dissolved in 30 mL cyclohexane and 30 mL ultrapure water, respectively. Then the two solutions were mixed in a 150 mL glass bottle, with an organic–aqueous phase interface formed. After adding 1.5 mL of 4 M NaOH (the NaOH concentration in the water phase was ca. 0.2 M) as catalysts, the mixture was stirred at 1000 rpm to dynamically generate emulsions. After reaction, the resulted oil phase was isolated from the system using a separating funnel and quantitatively analysed by a GC-FID to determine the conversion of benzaldehyde, and the selectivity and yield of benzalacetone using eqn (1)–(3).
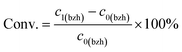 | (1) |
 | (2) |
| Yield = Conv. × Sele. | (3) |
(b) Homogeneous system: similar with the biphasic system, 10 mL (98 mmol) benzaldehyde and 21.8 mL (294 mmol) acetone (taking 3 equiv. as an example) were dissolved in 30 mL cyclohexane and 30 mL ultrapure water, respectively. Then the two solutions were mixed in a 150 mL glass bottle. In this case, there was no organic–aqueous phase interface formed and it was a homogeneous reaction system. All the rest steps were the same as that for the biphasic system.
(c) Scaling up: 0.9 L benzaldehyde and 1.96 L acetone were dissolved in 3.7 L cyclohexane and 2.7 L water, respectively. The two solutions were mixed in a 10 L bottle, with an organic–aqueous phase interface formed. 150 mL of 4 M NaOH was added to the system as the catalyst and then the system was stirred by a mechanical agitator at 2500 rpm to dynamically generate emulsions. The temperature of the reaction system was measured every half hour. After reaction, the oil phase was separated and dried by calcium chloride, resulting a clear oil phase. Then cyclohexane was removed by rotary evaporation to obtain the solid benzalacetone product.
Results and discussion
In a typical emulsion reaction synthesis (entry 1, Table 1), 10 mL of benzaldehyde was dissolved in 30 mL cyclohexane to form the oil phase, and 1 equiv. acetone (with respect to benzaldehyde) and NaOH (0.2 M) were dissolved in the water phase. Then the oil phase was mixed with the water phase by stirring at 1000 rpm to generate emulsions and the CS condensation was executed at the organic–aqueous phase interface. 93 ± 2% benzaldehyde conversion and 99 ± 1% selectivity to BA were reached after 24 h reaction at room temperature. By increasing the acetone equiv. to 3, the reaction can be significant accelerated with the conversion reaching 96 ± 2% after 4 h reaction (selectivity 99 ± 1%, entry 2). It is interesting to note that when the cyclohexane solvent was replaced by toluene, the conversion decreased to 73 ± 2% (entry 3) although the selectivity remained >99%. This should be induced by the offset π–π interactions (quadrupolar interactions) between the benzaldehyde and toluene molecules, resulting in the distribution of benzaldehyde across the inside of toluene droplets and thus much longer diffusion distance is required for benzaldehyde to reach the interface and participate in the reaction.5| Entry | Solvent | Acetone equiv. | T (°C) | t (h) | Conversion (%) | Selectivity (%) |
|---|---|---|---|---|---|---|
| a Benzaldehyde 10 mL, cNaOH = 0.2 M, stirring speed 1000 rpm. The uncertainties shown in the table are estimations according to at least three repetitions of some specific cases. | ||||||
| 1 | 30 mL water + 30 mL cyclohexane | 1 | 25 | 24 | 93 ± 2 | 99 ± 1 |
| 2 | 30 mL water + 30 mL cyclohexane | 3 | 25 | 4 | 96 ± 2 | 99 ± 1 |
| 3 | 30 mL water + 30 mL toluene | 3 | 25 | 4 | 73 ± 2 | 99 ± 1 |
| 4 | 30 mL water + 30 mL ethanol | 1 | 25 | 5 | 97 ± 2 | 80 ± 1 |
| 5 | 30 mL water + 30 mL ethanol | 1 | 45 | 3 | 98 ± 2 | 63 ± 1 |
| 6 | 30 mL water + 30 mL ethanol | 3 | 25 | 1.5 | 98 ± 2 | 92 ± 1 |
For the purpose of comparison, homogenous synthesis in a water/ethanol solvent was also conducted under 1000 rpm stirring. As shown in entries 4–6, high conversion and low selectivity were observed when 1 equiv. acetone was used in the homogenous system. The selectivity can be improved by increasing the acetone equiv. to 3, but still lower than that of the emulsion system. These results clearly demonstrate the superiority of interfacial reaction technique for optimal BA selectivity in CS condensation.
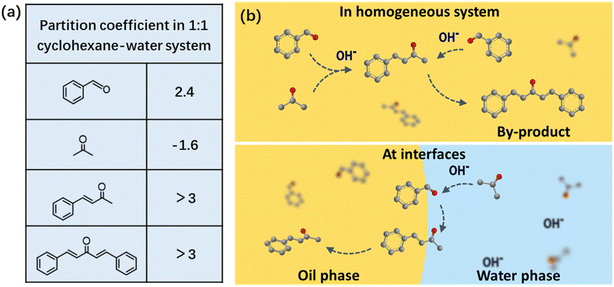
We ascribed the excellent BA selectivity in emulsion system to the steric effect of the organic–aqueous phase interface which blocks the further reacting between acetone's second α-H and benzaldehyde. As shown in Fig. 1, in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 cyclohexane–water system, the partition coefficients of benzaldehyde and acetone were measured to be 2.4 and −1.6, indicating that after phase equilibrium, 99% of benzaldehyde and 97% of acetone exist in the oil and water phases, respectively. Since benzaldehyde molecules contain both hydrophobic groups (benzene rings) and hydrophilic groups (aldehyde groups), they tend to orientate at organic–aqueous phase interfaces with their aldehyde group exposing in the water phase. After reacting with acetone from the water phase at interfaces, the generated benzalacetone diffuses into the bulk oil phase and therefore avoids further side reactions, due to the absence of NaOH in the oil phase.
1 cyclohexane–water system, the partition coefficients of benzaldehyde and acetone were measured to be 2.4 and −1.6, indicating that after phase equilibrium, 99% of benzaldehyde and 97% of acetone exist in the oil and water phases, respectively. Since benzaldehyde molecules contain both hydrophobic groups (benzene rings) and hydrophilic groups (aldehyde groups), they tend to orientate at organic–aqueous phase interfaces with their aldehyde group exposing in the water phase. After reacting with acetone from the water phase at interfaces, the generated benzalacetone diffuses into the bulk oil phase and therefore avoids further side reactions, due to the absence of NaOH in the oil phase.
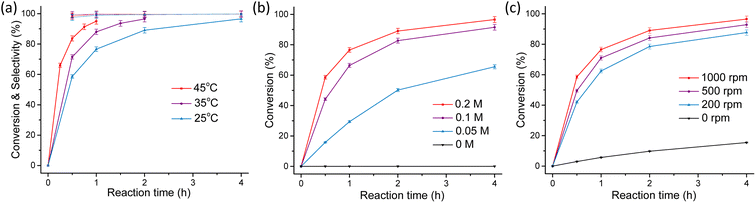
In the next set of experiment, the effect of reaction temperature on the conversion and selectivity was investigated. As shown in Fig. 2a, it is expected that the reaction rate increased with the temperature, with 95 ± 2% conversion reached after 1 h at 45 °C, while it took 2 and 4 h, respectively, for the conversion to reach 95 ± 2% when the reactions were performed at 35 and 25 °C. Nevertheless, it is worth highlighting that the selectivity for BA is all 99 ± 1% for every stage of the reaction (dotted line in Fig. 2a).
The effect of NaOH concentration on the reaction rate was also examined (Fig. 2b). It is found that the CS condensation could not occur without NaOH. As 0.5 M of NaOH was added into the aqueous phase, the reaction was triggered, and a 65 ± 2% conversion was reached after 4 h. The use of 0.1 M of NaOH significantly accelerated the reaction and the conversion reached 91 ± 2% after a 4 h timeframe. Further increase in concentration from 0.1 to 0.2 M had no appreciable effect on the conversion rate after the same time period (96 ± 2% conversion). In this regard, it is important to optimize the concentration of the NaOH catalyst to avoid the waste of chemicals and in the meanwhile to achieve the highest conversion rate.
Since stirring was employed to generate emulsions and thus initiate the CS reaction at the droplet surface, the effect of stirring speed on the reaction rate was studied. As shown in Fig. 2c, when no stirring was applied, the reaction occurred only at the flat organic–aqueous phase interface where the contact area was very limited such that only 15 ± 2% conversion was reached after 4 h reaction. As long as emulsion was formed upon stirring, the reaction demonstrated much higher rate and ca. 90 ± 2% conversion was obtained, because of the dramatically increased organic–aqueous phase interfacial area for the reaction. It is noted that the reaction rate did not improve much upon increased stirring speed (up to 1000 rpm), which should be because the droplets in the emulsion do not get significantly smaller as the speed increased, preventing a significant increase in contact area.
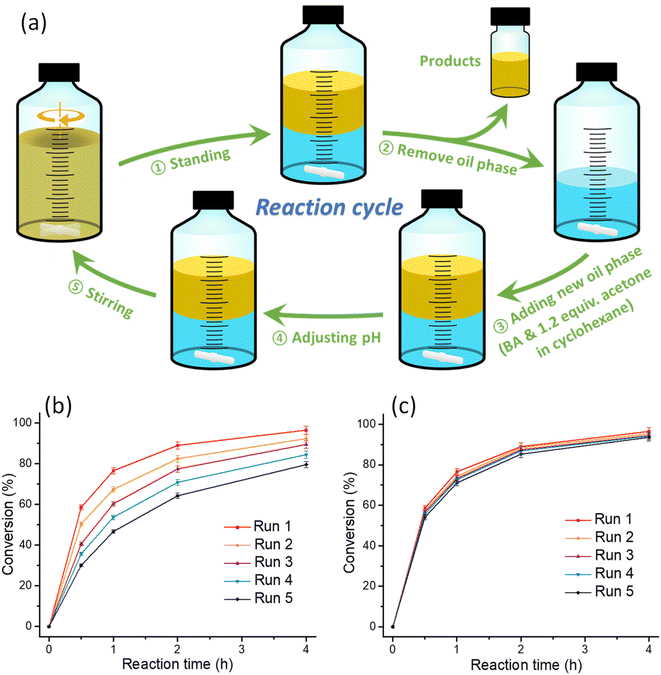
We then designed a protocol to evaluate the recyclability of the emulsion system. As shown in Fig. 3a, after reaction, the vial was left to stand still for 5 min to allow for the separation of the oil and water phase. Then the oil phase containing the BA product was taken out from the vial. In the next reaction cycle, 10 mL benzaldehyde was dissolved in cyclohexane and 1.2 equiv. acetone (0.2 equiv. more acetone was added to compensate the acetone loss in the oil phase) was added to the water phase. Then the reaction was initialized by turning on the stirring. The cycled experiments were carried out for 5 cycles and unfortunately the reaction rate declined with the cycle numbers (Fig. 3b). We figured out that this should be caused by the lowered NaOH concentration as 1 equiv. of water was generated (see the equation in Table 1) in each cycle. To solve this problem, NaOH was added to the water phase to maintain a 0.2 M concentration prior to the execution of the next reaction cycle (step 4). As such, the reaction rate almost remained constant with the cycling (Fig. 3c).
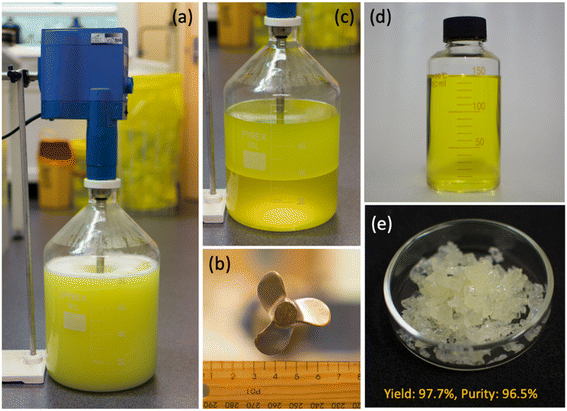
Finally, the emulsion synthesis system shows high feasibility for scale-up production. As shown in Fig. 4a. 10 L liquid system and mechanically stirring was used for the amplified CS synthesis. After 4 h reaction, stirring was stopped to allow for the separation of the water and oil phase (Fig. 4c). Then the yellow-colored oil phase was separated, dried, and rotary evaporated to obtain the BA crystals (Fig. 4d and e), with a yield of 97 ± 2% and a purity of 96%. More detailed monitoring of the synthesis revealed exothermic effect as the reaction proceeded, indicated by the temperature increase from 25 to 35 °C (Fig. 5). On the contrary, no obvious temperature change was observed for the 80 mL system, which should be due to the faster heat dissipation in the small reaction system. We also noted that the reaction rate in the large system was slightly higher than that in the smaller system, probably because of the higher temperature in the former. It is worth mentioning the selectivity remained 99 ± 1% across the whole reaction.
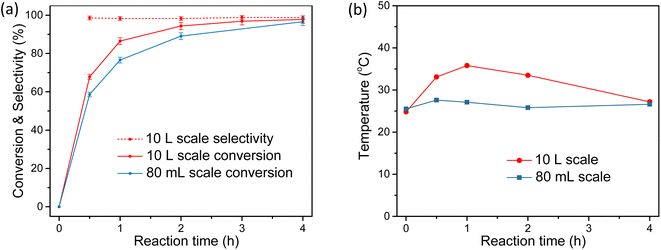 | ||
| Fig. 5 Comparison of the results for different reaction scales. (a) The conversion and selectivity for 10 L and 80 mL reaction scales (the selectivity for 80 mL scale is discussed in detail in Table 1 and Fig. 2a). (b) The temperature variation during the reaction in different scales. Error bars indicate 2% variation of the data, which are estimated according to at least three repetitions of some specific cases. | ||
Conclusion
In summary, we showed that benzalacetone can be synthesized by a green, surfactant-free emulsion technique with high conversion and excellent selectivity. Upon stirring, a dynamically stable emulsion was generated, and the CS condensation reaction was confined at the organic–aqueous phase interface by dissolving acetone and NaOH in water and benzaldehyde in the oil. The just formed BA molecules transported to the interior of the oil droplets and cannot undergo a subsequent CS reaction due to the absence of the NaOH catalyst in the oil phase, resulting in 99 ± 1% selectivity for BA. Furthermore, thanks to the absence of surfactant, the oil phase containing the BA molecule can be facilely separated from the mixture by minutes of standing, enabling cyclic and scaling-up synthesis. In an amplified production, an overall of 1246.9 g of BA was obtained after separation, drying, and distillation, with the yield up to 97 ± 2% and the purity up to 96%. These results indicate the high potential of this method for economical and environmentally friendly production of BA at industrial scale.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the financial support by the Australian Research Council (DP190101607). The faculty of Science and Central Analytical Research Facility (CARF) at QUT are greatly acknowledged for technical assistance.References
- L. Claisen and A. Claparede, Ber. Dtsch. Chem. Ges., 1881, 14, 349–353 CrossRef.
- C. Winter, J. N. Caetano, A. C. Araújo, A. R. Chaves, I. C. Ostroski, B. G. Vaz, C. N. Pérez and C. G. Alonso, Chem. Eng. Sci., 2016, 303, 604–610 CrossRef CAS.
- G. B. Shul'pin, Org. Biomol. Chem., 2010, 8, 4217–4228 RSC.
- S. Mandal, S. Mandal, S. K. Ghosh, A. Ghosh, R. Saha, S. Banerjee and B. Saha, Synth. Commun., 2016, 46, 1327–1342 CrossRef CAS.
- C. Han, H. Xu, E. R. Waclawik, X.-H. Li and J. Xu, Chem. Commun., 2020, 56, 8059–8062 RSC.
- I. T. Baldwin, C. Preston, M. Euler and D. Gorham, J. Chem. Ecol., 1997, 23, 2327–2343 CrossRef CAS.
- D. Kessler and I. T. Baldwin, Plant J., 2007, 49, 840–854 CrossRef CAS PubMed.
- D. Opdyke, Monographs on fragrance raw materials, Food Chem. Toxicol., 1973, 11, 855–876 CrossRef CAS.
- R. Gobato, A. Gobato and D. F. G. Fedrigo, Parana Journal of Science and Education, 2015, 1, 1–10 Search PubMed.
- T. Agner, Acta Derm.-Venereol., 1991, 71, 296–300 CAS.
- A. Nassif, S. C. Chan, F. J. Storrs and J. M. Hanifin, Arch. Dermatol., 1994, 130, 1402–1407 CrossRef CAS.
- H. Löffler and I. Effendy, Contact Dermatitis, 1999, 40, 239–242 CrossRef.
| This journal is © The Royal Society of Chemistry 2022 |