 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Calcium hydroxide recycled from waste eggshell resources for the effective recovery of fluoride from wastewater†
Wenjing Chen *,
Yuanyue Wu,
Zhiyin Xie,
Yiyuan Li,
Weitai Tang and
Jinbei Yu
*,
Yuanyue Wu,
Zhiyin Xie,
Yiyuan Li,
Weitai Tang and
Jinbei Yu
College of Resources and Environment, Chengdu University of Information Technology, No.24 Xuefu Road, Shuangliu District, Chengdu, 610225, China. E-mail: wenjingchen88@126.com; Fax: +86 28 85966913; Tel: +86 28 85966913
First published on 4th October 2022
Abstract
In the hunt of waste recovery pathways, eggshells emerged as a potential adsorbent for fluoride because they contain plenty of calcium. However, as the main component, calcite has weak interaction with fluoride. In this study, calcium hydroxide was derived from waste eggshells successfully by an aging treatment with moisture for fluoride recovery from water. The X-ray diffraction (XRD) and infrared spectroscopy (FT-IR) analyses indicate that CaO in calcined egg shells (AEG900) is completely converted to calcium hydroxide. The adsorption experiments showed that the adsorption capacity of AEG900 for fluoride was improved by nearly 29.21% compared with the calcined eggshells without the aging treatment. In the batch experiment, the temperature effect is the most significant for the adsorption process, and nearly a half increment of removal rate is achieved by increasing the temperature by 30 °C. Further research revealed that the adsorption process fitted well with the pseudo-second order model and the Langmuir–Freundlich isotherm model, with a maximum adsorption capacity of 370.15 mg g−1. Moreover, precipitation was regarded as the main step for fluoride removal mechanism based on the calculated results of the surface complexation model. X-ray photoelectron spectroscopy (XPS) results showed that the stable fluorite formed in situ of AEG900 avoids calcium loss in water. Finally, AEG900 was applied in fluoride removal with real-life groundwater and industrial wastewater, and the results showed that the final fluoride concentration could meet the WHO requirement and industrial wastewater discharge standard.
1. Introduction
Fluoride generated from natural, anthropogenic, and industrial activities is widespread in different water sources. Especially, flouride will lead to different health effects for humans depending on the concentration. As an essential trace element for bone mineralization and enamel formation, fluoride is beneficial for strengthening the bone and teeth of young children at the level of 0.5 to 1.0 mg L−1 in drinking water.1 However, the excessive ingestion of fluoride will cause dental fluorosis, skeletal fluorosis, and other diseases; hence, the World Health Organization (WHO) suggests fluoride concentration in drinking water below 1.5 mg L−1.2,3 Recently, most community water systems in the US have made more serious requirements for fluoride concentration, which has been changed from 0.7 ∼ 1.2 mg L−1 to 0.7 mg L−1. However, with increasing industrial activities, particularly in the semiconductor manufacturing and smelting industry, thousands of mg L−1 of fluoride is discharged into natural water.4,5 More than 30 mg L−1 in groundwater is detected, which is hazardous for water environment safety. Moreover, according to the statistics from Jadhav et al., China and India are most threatened by fluoride, which nearly occupy 48.30% and 36.62% population of the selected countries.6After realizing the reason for fluorosis, tremendous research studies have been carried out to keep fluoride in water at an acceptable level.7,8 The common treatment methods for fluoride removal include chemical precipitation/coagulation, membrane separation, ion exchange, and adsorption. Precipitation/coagulation can be traced back to around 90 years ago. The famous Nalgonda process was developed to remove fluoride in drinking water, which included two steps, the formation of insoluble fluoride with lime and the coagulation treatment with alum. This process is of low cost and has high removal effectiveness, in which the initial concentration of fluoride with 109 mg L−1 in groundwater can be reduced to 8 mg L−1 after 35 min.9 However, in over 10 years of practical applications, some serious drawbacks were found in the Nalgonda process, especially the negative effect of alum and excessive sludge.10 Membrane technology is considered a reliable method for fluoride removal in recent years, of which reverse osmosis has become popular to supply safe drinking water and over 95% of fluoride can be removed by membrane separation, and the effluent concentration can be under 0.3 mg L−1.11 As a relatively new technique, membrane separation involves high costs for skillful operators, film materials, and maintenance, which presents challenges for small-scale water treatment systems. Based on the US Environmental Protection Agency (EPA) report, the removal rate of fluoride can be up to 100% by the adsorption process, which is recognized as one of the best available technologies for fluoride control.12
As the EPA recommended adsorbent, activated alumina is widely used to remove fluoride and has been used in large-scale systems.13,14 Although this process owns high selectivity and low cost, to achieve a high adsorption performance, a narrow pH range of 5.0–6.0 is required in this process because activated alumina will dissolve at pH < 5, and excessive aluminum will remain in the effluent causing secondary pollution.15 At pH > 7, hydroxide and other anions will be more easily adsorbed than fluoride.16 On the other hand, while many synthesized adsorbents (mesoporous or modified alumina) display a high removal rate of fluoride, their preparation methods are relatively complex and expensive.17 Moreover, few commercial alumina samples can reduce fluoride concentration under the safety limit for drinking water. For example, as Dayananda reported, the commercial alumina only could remove 39% fluoride in water with the initial fluoride concentration of 5 mg L−1 after 10 h.18 Hence, numerous adsorbents have been developed in recent years to achieve a high-efficient, low-cost, and stable adsorption process for fluoride removal, in which bio-adsorbent is a potential candidate.
Bio-adsorbents are derived from industrial and agricultural wastes, which is a mode of resource utilization for wastes. Eggshells are abundant food waste from daily life, the food industry, and poultry farm. Apart from their use as fertilizers and feed additives, there are still a large number of eggshells without appropriate disposal.19 Currently, EPA has ranked eggshells at 15th as one of the environmental problem makers, and more than 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 dollars has been paid for the landfill disposal of eggshells in the USA every year.20 Due to being rich in calcium carbonate and a natural porous structure, eggshells have a potential application to adsorb heavy metals,21 organic dyes,22 and anions.23 As a calcium-based material, eggshells own high fluoride affinity and biocompatibility. In 2012, Bhaumik et al. firstly tried to use eggshells to adsorb fluoride with 5 to 20 mg L−1 in water, and the maximum fluoride adsorption capacity was 1.09 mg g−1.24 They found that the chemical adsorption acted as the main step, and a lower pH would release more Ca2+ from calcite on the eggshell surface to benefit fluoride adsorption. While eggshells display a significant advantage for fluoride removal over other bio-adsorbents, there are fewer studies on this system. In 2021, Lee et al. restarted this topic, and they found that the CaCO3 in natural eggshells changed to CaO by thermal treatment, 70% fluoride could be removed within 15 min, and the maximum adsorption capacity of thermally treated eggshells could reach 258.28 mg g−1.25 After reviewing the relevant studies about the waste eggshell recycling as bio-adsorbent, most studies were focused on discussing CaO as the main active component to capture anions in water.26 In fact, CaO as a solid component in the calcined eggshells is unstable and hygroscopic, leading to an exothermic reaction with water and causing calcium ion loss in water.
000 dollars has been paid for the landfill disposal of eggshells in the USA every year.20 Due to being rich in calcium carbonate and a natural porous structure, eggshells have a potential application to adsorb heavy metals,21 organic dyes,22 and anions.23 As a calcium-based material, eggshells own high fluoride affinity and biocompatibility. In 2012, Bhaumik et al. firstly tried to use eggshells to adsorb fluoride with 5 to 20 mg L−1 in water, and the maximum fluoride adsorption capacity was 1.09 mg g−1.24 They found that the chemical adsorption acted as the main step, and a lower pH would release more Ca2+ from calcite on the eggshell surface to benefit fluoride adsorption. While eggshells display a significant advantage for fluoride removal over other bio-adsorbents, there are fewer studies on this system. In 2021, Lee et al. restarted this topic, and they found that the CaCO3 in natural eggshells changed to CaO by thermal treatment, 70% fluoride could be removed within 15 min, and the maximum adsorption capacity of thermally treated eggshells could reach 258.28 mg g−1.25 After reviewing the relevant studies about the waste eggshell recycling as bio-adsorbent, most studies were focused on discussing CaO as the main active component to capture anions in water.26 In fact, CaO as a solid component in the calcined eggshells is unstable and hygroscopic, leading to an exothermic reaction with water and causing calcium ion loss in water.
Herein, we attempted to recycle Ca(OH)2 from calcined eggshells and explore their application as a bio-adsorbent for fluoride removal in water. To our best knowledge, this is the first study reporting Ca(OH)2 as the main active component, which is derived from waste eggshells and their application in fluoride removal. The prepared eggshells with different treatment temperatures and aging treatment with moisture were characterized by different physicochemical methods to reveal the effect of composition and structural changes. Then, the batch experiments were conducted to discuss the factors influencing the fluoride removal by eggshells with Ca(OH)2 as the main component and found that temperature significantly affected the removal rate rather than pH, as reported in the literature. Moreover, using the equilibrium model calculation and XPS analysis, the fluoride removal mechanism and the contribution of adsorption and precipitation by Ca(OH)2 based eggshells were proposed. Finally, the adsorption tests were conducted with real-life industrial wastewater and groundwater to prove their potential for different application backgrounds for fluoride removal.
2. Experimental section
2.1 Materials
Details of the information for materials are described in ESI.†2.2 Preparation of the adsorbent materials
The collected eggshells were washed to remove grease and other impurities, and the eggshell membrane was peeled and dried at 100 °C for 24 h. Then, eggshells were ground into small pieces and sieved by a 200-mesh screen to obtain an eggshell powder with a particle size of around 70 μm, as shown in Fig. 1(a). The sieved eggshells were used in the adsorption experiment as the contrast sample named EG. Then, the eggshell powder was heated to 500, 600, 700, 800, and 900 °C in the muffle furnace under air atmosphere for 4 h. As shown in Fig. 1(b)–(f), the obtained samples were denoted as ES500, ES600, ES700, ES800, and ES900, respectively. All calcined samples were stored in a dryer before use in the adsorption experiments, and ES800 and ES900 were selected for further treatment. The aging treatment was conducted in moisture, in which ES800 and ES900 were stored in a box with a relative humidity of 70% for one week. It is worth noting that before the sample characterization and adsorption experiments, the aged samples were dried at 80 °C for 24 h to remove free water. | ||
| Fig. 1 The digital photo of raw eggshells (a), eggshells after heating at 500 (b), 600 (c), 700 (d), 800 (e) and 900 °C (g), and heated eggshells after aging (f) and (h). | ||
2.3 Adsorption performance
 | (1) |
 | (2) |
Langmuir model:
 | (3) |
Freundlich model:
| qe = KFCe1/n | (4) |
Langmuir–Freundlich model:
 | (5) |
Pseudo-first-order model:
| qt = qe[1 − exp(k1t)] | (6) |
Pseudo-second-order model:
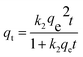 | (7) |
2.4 Characterization method
Scanning electron microscopy (SEM, Phenom™ ProX Desktop SEM) was used to observe the surface morphology of eggshells after thermal treatment. The specific surface area and pore size of eggshells were measured by the Accelerated Surface Area and Porosimetry System (SSA-4200, Beijing Builder, China) and calculated based on the Brunauer–Emmett–Teller (BET) and Barrett–Joyner–Halenda (BJH) methods. The Fourier transform infrared spectroscopy (FT-IR, Nicolet is 50, USA) was employed to measure the functional groups on eggshells, and the tablet method with KBr was adopted with the measuring wavelength from 400 to 4000 cm−1. The X-ray diffraction (XRD) analysis was conducted to determine the crystal structure and components of the sample by a multi-function diffractometer (DX-2700BH, Haoyuan, China), equipped with a copper (Cu Kα) radiation (λ = 1.541 Å) scanning from 10° to 80°. The synchronous thermal analysis (TGA/DSC1, Mettler Toledo, Switzerland) was used to show the mass change of eggshells after thermal treatment at different temperatures. The measurement temperature was from 30 to 1000 °C with an increased rate of 20 °C min−1 under air atmosphere. Moreover, X-ray photoelectron spectroscopy (XPS, Thermo Scientific K-Alpha, UK) of eggshells before and after fluoride adsorption was measured.3. Results and discussions
3.1 Screening of adsorbent
The effects of calcination temperature and humidity on the preparation of adsorbents are discussed in this section. As shown in Fig. 1, the adsorbents were different visually with different treatment temperatures. With the increase in calcination temperature, the color of eggshells becomes darker and darker until it is black below 700 °C. However, below 800 °C, the color of the powder will turn white, and all of them become white below 900 °C. Aging treatment also can affect the color of the powder, and the white color disappears in AES800. Those color changes reflect the change of components in eggshells after the treatment. Fig. 2(a) shows the TGA curve of eggshells, and a slight mass variation happens before 600 °C with the weight loss of 3.88%, which indicates organic matter in eggshells has become charred, and some of them escaped as CO2. Then, a big drop of mass loss is found at about 42.55% until 800 °C, which means the thermal decomposition of CaCO3 occurs according to eqn (8). After 800 °C, the eggshell mass remains stable, meaning the decomposition of CaCO3 has been completed. Moreover, a distinct inverted peak occurs between 700 to 850 °C in the DSC curve (Left axis in Fig. 2(a)), which is ascribed to the endothermic decomposition of CaCO3. It is worth pointing out that the total weight loss of eggshells was 46.43% in this study, which is higher than in a reported work with 40%.23 Different calcination environments could cause this difference. In this study, air was chosen because of easier operation, and organic matter would become CO2 instead of char.| CaCO3(s) + heat → CaO(s) + CO2(g) | (8) |
Fig. 2(b) shows the fluoride adsorption on eggshells with different treatment processes. The equilibrium concentration of fluoride decreases from 590.11 to 317.33 mg L−1 by increasing the calcination temperature to 900 °C. Obviously, natural eggshells and calcined eggshells obtained below 600 °C are not feasible for fluoride removal, and only 3.68% fluoride is removed for ES600. However, when the calcination temperature increases from 700 to 900 °C, the adsorption capacity of eggshells escalates from 89.07 mg g−1 (ES700) to 282.67 mg g−1 (ES900), which implies that the component change of eggshells may play the main role for the improvement of fluoride adsorption. A similar tendency is also found in Lee's work.25 In their study, the adsorption capacity of ES800 (18.54 mg g−1) was similar to ES900 (18.56 mg g−1); however, in our study, the adsorption capacity of ES900 is obviously larger than that of ES800 (230.42 mg g−1). Apart from the effect of different calcination conditions, BET results could explain this discrepancy to a certain extent. Indeed, the specific surface area (SSA) of ES900 (2.785 m2 g−1) was more than twice that of ES800 (1.08 m2 g−1), which could provide more active sites for fluoride. Moreover, aged eggshells display higher adsorption capacity for fluoride, which increases to 333.70 mg g−1 for AES800 and 365.24 mg g−1 for AES900, respectively. This enhancement is considerable, in which the equilibrium concentration drops to 234.76 mg L−1 for AES900, meaning 61% fluoride could be removed even with a high initial concentration. Eqn (9) is the main reaction that happens in the aging stage in eggshells, where CaO adsorbing moisture from air would convert to hydrated lime, which is more favorable for fluoride removal.
| CaO(s) + H2O(g) → Ca(OH)2(s) | (9) |
In order to identify the components formed in the eggshells at different calcination temperatures, XRD patterns of each adsorbent were obtained, as shown in Fig. 3(a). The results confirm that when the calcination temperature is below 700 °C, the major component in the samples is calcite (JCPDS No. 05-0586). When eggshells are heated under 800 °C, lime is formed in ES800 (JCPDS No. 37-1497). By comparing the XRD patterns of ES800 and ES900, it can be deduced that almost all CaCO3 converts to CaO at 900 °C because only the pattern of lime can be found in the ES900 XRD pattern, and the same result was reported in Risso's study, who attempted to obtain CaO catalysts from eggshells at 900 °C.28 Unlike that described in Lee's study, Ca(OH)2 is not present in fresh ES800 and ES900 due to strict dry storage conditions.25 Conversely, Ca(OH)2 (JCPDS No. 04-0733) is formed after storing in humid air due to eqn (9), and CaO almost disappears in aged samples, as shown in Fig. 3(b). Moreover, the proportion of Ca(OH)2 in AES900 is obviously higher than that in AES800, which could be the reason for a higher adsorption capacity for fluoride for AES900, as mentioned in Fig. 2(b). Panagiotou et al. suggested that Ca(OH)2 was more soluble than CaO or CaCO3, so soluble Ca in water would be more reactive with the target ions, and the reason for the removal of ions was precipitation rather than adsorption.29 To prove that the adsorption happened on eggshells, the XRD result of AES900 after fluoride adsorption to equilibrium is shown in Fig. 3(b), and CaF2 (JCPDS No. 35-0816) can be found from the main diffraction patterns, which indicates that the ion exchange happens and generates fluorite on the surface of AES900 based on eqn (10).30 Moreover, the peak of Ca(OH)2 almost disappears, but the peak of CaCO3 can still be found in the XRD spectrum of AES900 after adsorption. Hence, Ca(OH)2 plays the main role in fluoride removal, and aging treatment with humid air is very important to improve the adsorption capacity of calcined eggshells.
| Ca(OH)2(s) + 2F− → CaF2(s) + 2OH− | (10) |
 | ||
| Fig. 3 XRD patterns of heated eggshells with different temperatures (a) and aged eggshells before and after adsorption of fluoride (b). | ||
To obtain more information about the structure of adsorbents, the FT-IR spectra of adsorbents thermally treated above 800 °C were measured, as shown in Fig. 4(a). Before adsorption, the exhibited adsorption peaks include 3633, 3440, 1411, 1050, and 871 cm−1 for all samples. Among them, the sharp peak at 3633 cm−1 and the broad peak at 3440 cm−1 are attributed to the bending vibrations of –OH from Ca(OH)2 and crystalline hydrate, respectively.31,32 A higher relative intensity peak at 3633 cm−1 can be found in the aged samples but not in calcined samples at 800 or 900 °C, which is in agreement with the XRD result that more Ca(OH)2 is generated by aging treatment. The peaks at 1411 and 1050 cm−1 and the peaks at 709 and 2513 cm−1 in ES800 and AES800 belong to the vibrations of C–O bonds.33 Moreover, the peaks at 2976, 2859, and 1791 cm−1 are attributed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds from CO32−, and the above-mentioned bands indicate the presence of CaCO3 in ES800 and AES800.31 However, the peaks belonging to CaCO3 are not found in the sample calcined 900 °C, which could be explained by a lower relative peak intensity. Moreover, the sharp bands at 871 cm−1 correspond to the Ca–O bond, and the broad peaks at 498 cm−1 are from the Ca
O bonds from CO32−, and the above-mentioned bands indicate the presence of CaCO3 in ES800 and AES800.31 However, the peaks belonging to CaCO3 are not found in the sample calcined 900 °C, which could be explained by a lower relative peak intensity. Moreover, the sharp bands at 871 cm−1 correspond to the Ca–O bond, and the broad peaks at 498 cm−1 are from the Ca![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds.34 After adsorption, the peak of –OH bond at 3633 cm−1 disappears in AES900, which indicates that the active site of Ca–OH on the surface reacted with F−. The peak of Ca–O bond at 871 cm−1 becomes weak and the peak of Ca
O bonds.34 After adsorption, the peak of –OH bond at 3633 cm−1 disappears in AES900, which indicates that the active site of Ca–OH on the surface reacted with F−. The peak of Ca–O bond at 871 cm−1 becomes weak and the peak of Ca![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds disappears and these indicate the reaction happened between Ca and F. The change of AES900 before and after adsorption confirms the key role of Ca(OH)2 for fluoride adsorption again.35 Furthermore, the SEM image of AES900 was taken, as shown in Fig. 4(b). After calcination, the pore size of the eggshell powder became smaller (<5 μm), which is also reflected in the BET data (SSA increased from 0.515 to 2.785 m2 g−1). Moreover, the common pores from natural eggshells could not be found in the SEM image because the average pore size was 12.8 nm for AES900.36
O bonds disappears and these indicate the reaction happened between Ca and F. The change of AES900 before and after adsorption confirms the key role of Ca(OH)2 for fluoride adsorption again.35 Furthermore, the SEM image of AES900 was taken, as shown in Fig. 4(b). After calcination, the pore size of the eggshell powder became smaller (<5 μm), which is also reflected in the BET data (SSA increased from 0.515 to 2.785 m2 g−1). Moreover, the common pores from natural eggshells could not be found in the SEM image because the average pore size was 12.8 nm for AES900.36
 | ||
| Fig. 4 FT-IR spectra for different adsorbents and AEG900 after adsorbed fluoride (a) and SEM morphological images for AEG900 (b). | ||
Moreover, the final pH and stability of adsorbents also were discussed, and the results are listed in Table S1.† During the adsorption experiment, the initial pH was around 6.66, and the final pH was around 10.51 for natural eggshells and eggshells with calcination temperature below 700 °C. There is no obvious difference in the final pH for eggshells with or without aging treatment, which are all around 12.01. The final pH increase is a common phenomenon in Ca-based materials for fluoride removal and also happens in the case of sepiolite.37 The stability of adsorbents was evaluated by measuring the weight loss of adsorbents during the adsorption experiment and calculated as TDS (%) shown in Table S1.† TDS displayed the proportion of weight loss of adsorbents to raw adsorbents after adsorption of fluoride. From the data in Table S1,† it is clear that the stability of natural eggshells and calcined eggshells obtained below 700 °C are very weak, and more than 50% weight loss happened in the fluoride adsorption process. Combined with pH change, it could be deduced that CaCO3 as the main component in these materials would dissolve in water with a weak acidic condition (pH = 6.66) causing mass loss, and the increase in the final pH reflects the consumption of H+ during the dissolution of calcite.38,39 Moreover, eggshells obtained by thermal treatment above 800 °C show higher stability. The values of TDS for EG800 and EG900 are 14.33% and 10%, respectively. As the main reason, CaO in those materials reacts with water to form Ca(OH)2, and meanwhile, Ca dissolves in water, causing mass loss, and Santos et al. gave a similar explanation for the weight loss of EG800.23 In contrast, the stability of aged eggshells is the best, and the values of TDS are 4.67% and 3.00% for AEG800 and AEG900, respectively. In this system, the reaction between fluoride and Ca(OH)2 might have a major role, and forming CaF2 covered on the surface of eggshells would prevent Ca loss.
Hence, above all, AES900 displays an excellent adsorption capacity and better stability for the removal of high concentrations of fluoride in water, and the adsorption performance of AEG900 with different effects will be discussed in the next section comprehensively.
3.2 Batch adsorption experiments
Fig. 5(b) shows the effect of temperature on fluoride adsorption. With increasing temperature from 10 to 40 °C, the removal rate of fluoride increases from 35.0 to 87.44%. This increment is significant (nearly 52.44%) within a 30 °C increase compared with other studies.43,44 This is probably because the increasing temperature could increase the diffusion of fluoride to the adsorbent and enhance the reaction between Ca(OH)2 and F−.
Fig. 5(c) shows the effect of initial pH for fluoride adsorption and final pH in solution. With an increase in pH, the adsorption capacity increases from 348.89 mg g−1 at pH 3, peaking at 364.77 mg g−1 at pH 5 and then decreases to 333.86 mg g−1 at pH 11. This tendency is similar to Saini's work and their plots of the speciation curve of fluoride in solution at different pH could be helpful in understanding this phenomenon.43 Firstly, at pH below 3, more than half of fluoride will present as HF, which will further form a stable association to reduce the concentration of free fluoride ions.45 When pH increases to 5, most of the fluoride will present as free fluoride ions, which is favorable for reaction with the adsorbent. On the other hand, in weak acidic conditions, more active sites will explore and carry a positive charge, which will capture fluoride ions easily by electrostatic attraction.39 At a basic pH, while all the fluorides are present as free ions, the adsorption capacity still decreases, which is mainly due to the physical changes on the surface of AES900. In alkaline conditions, the surface of the adsorbent will carry a negative charge, which will repel the fluoride ions.39 Moreover, OH− can act as a competitor for fluoride adsorption, and more OH− will occupy the active sites, leading to a decrease in adsorption capacity for fluoride.46
Apart from fluoride ions, other ions also exist in the natural water and also can adsorb on eggshells acting as a competitor in the fluoride adsorption process. Herein, chloride, bicarbonate, sulfate, and hydrogen phosphate as typical competitive ions were discussed. Fig. 5(d) shows the effects of other ions on the adsorption of fluoride. It can be found that co-existing ions have negative effects on fluoride adsorption and the adsorption capacity of fluoride decreases in the order of HPO42− > HCO3− > SO42− > Cl−, which is similar to other studies.25,47 Among them, the negative effects of chloride and sulfate are not significant, and the adsorption capacities of fluoride only decrease by 2.42% and 5.77%, respectively. Bicarbonate and hydrogen phosphate have remarkable inhibitive effects on fluoride adsorption, and the adsorption capacities of fluoride decrease by about 36.32% and 89.06%, respectively. The main reason for the competitive adsorption of bicarbonate is the increase in pH, which is adjustable before the adsorption process. However, the negative effect of hydrogen phosphate is inescapable, and to ensure the feasibility of fluoride removal, dephosphorization treatment for the inflow is necessary.
 | ||
| Fig. 6 Kinetic model fitting results for fluoride adsorption by AES900. ([F−]0 = 600 mg L−1, [adsorbent] = 1 g L−1, temperature = 20 °C and pH at neutral). | ||
| Model | Pseudo-first order model | Pseudo-second order model | ||||
|---|---|---|---|---|---|---|
| k1 (min−1) | qe (mg g−1) | R2 | k2 (g mg−1 min−1) | qe (mg g−1) | R2 | |
| Parameters | 0.0767 | 337.834 | 0.923 | 0.000378 | 353.171 | 0.989 |
Moreover, the experimental data is fitted in isotherm models, including Langmuir, Freundlich, and Langmuir–Freundlich models, as shown in Fig. 7(b), and the obtained parameters are listed in Table 2. Clearly, the Freundlich model is not suitable for describing the fluoride adsorption onto AES900 with R2 > 0.87. While the R2 of fitting the Langmuir curve is higher than 0.94, the simulated qm is 380.93 mg g−1 with a 4.11% relative error for the experimental value. The fitting result by the Langmuir–Freundlich model has the highest correlation (R2 > 0.96), and the calculated qm,LF only has a 1.37% relative error for experimental value. Langmuir–Freundlich model is favorable for heterogeneous systems with the variable density function, which is determined by nLF.48 If nLF equals 1, the adsorbent material is homogeneous, and the Langmuir–Freundlich model is reduced to the Langmuir model. In this study, nLF is 0.34, indicating AEG900 is a heterogeneous material to adsorb fluoride.
| Model | Langmuir | Freundlich | Langmuir–Freundlich | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| qm (mg g−1) | KL (L mg−1) | R2 | KF (mg g−1) | n (g L−1) | R2 | qm,LF (mg g−1) | KLF (L mg−1) | nLF | R2 | |
| Parameters | 380.93 | 0.04 | 0.94 | 105.46 | 4.85 | 0.87 | 370.15 | 0.04 | 0.34 | 0.96 |
3.3 Removal mechanism analysis
 | ||
| Fig. 8 Equilibrium mass distribution of fluoride between the dissolved and precipitated phase predicted by Visual MINTEQ 3.1. | ||
| Initial fluoride concentration (mg L−1) | Experimental result | Visual MINTEQ prediction | Precipitated fluoridea (mg L−1) | Adsorbed fluorideb (mg L−1) | |
|---|---|---|---|---|---|
| Final fluoride concentration (mg L−1) | Total dissolved fluoride (mg L−1) | Total precipitated fluoride (mg L−1) | |||
| a Calculated from initial fluoride concentration minus final fluoride concentration and the calculated adsorbed fluoride concentration.b Calculated from predicted total dissolved fluoride minus final fluoride concentration in the experiment. If the result is negative, the value of adsorbed fluoride is regarded as 0. | |||||
| 5 | 2.12 | 2.01 | 2.99 | 2.88 | 0.00 |
| 10 | 4.77 | 2.02 | 7.98 | 5.23 | 0.00 |
| 50 | 7.53 | 2.16 | 47.85 | 42.47 | 0.00 |
| 100 | 12.42 | 2.40 | 97.61 | 87.58 | 0.00 |
| 200 | 23.50 | 3.66 | 196.35 | 176.50 | 0.00 |
| 300 | 35.15 | 48.33 | 251.69 | 251.67 | 13.18 |
| 400 | 84.03 | 148.07 | 251.96 | 251.93 | 64.04 |
| 500 | 150.43 | 248.06 | 251.98 | 251.94 | 97.63 |
| 600 | 250.75 | 348.06 | 252.00 | 251.94 | 97.31 |
| 700 | 349.13 | 448.06 | 252.00 | 251.94 | 98.93 |
| 800 | 451.47 | 548.06 | 252.00 | 251.95 | 96.58 |
| 900 | 551.62 | 648.07 | 252.00 | 251.93 | 96.45 |
Moreover, as shown in Fig. 9(b), the change of C 1s spectra for AEG900 before and after adsorption are slight, and the two peaks belong to CaCO3 and C–C at 289.26 and 284.98 eV, respectively. For the O 1s spectra in Fig. 9(d), the peaks of metal carbonate and metal hydroxide can be decomposed with the binding energies at 531.34 and 532.88 eV, respectively, for raw AEG900. However, after the removal of fluoride, the metal hydroxide peak disappeared in the O 1s spectra, implying the dissociation of hydroxide accompanied by calcium dissolution from AEG900 in water. This conclusion is consistent with the FT-IR result of AEG900, as shown in Fig. 3, in which a sharp peak of –OH groups at 3633 cm−1 disappeared after the removal of fluoride. In conclusion, XPS analysis indicates that a strong interaction occurs between calcium and fluoride to form a stable component on the adsorbent, in which Ca(OH)2 is consumed preferentially compared with CaCO3.
3.4 Evaluation of fluoride removal in real-life water
To show the feasibility of AEG900 in different application environments, the fluoride removal experiments were conducted with different concentration levels of fluoride in deionized water, real groundwater, and industrial wastewater, and the results are shown in Fig. 10. According to the experimental results, we can find that when the dosage of AEG900 is 3 g L−1, the final concentration of fluoride can reach 0.83 mg L−1, meeting the guidelines of WHO (1.5 mg L−1). To investigate its feasibility for low fluoride concentration removal, the same dosage of AEG900 was used to treat fluoride with an initial concentration of 10 mg L−1, and the final fluoride is 1.43 mg L−1. A satisfying result can be obtained, while the low initial concentration will limit the driving force for the interaction between fluoride ions and adsorbents.Additionally, AEG900 was applied to remove fluoride in different real water samples. The groundwater was collected in Chengdu city, China, and the industrial water was collected from the photovoltaic industry, and the detailed characterizations are listed in Table S4.† Based on the results of pre-experiments, the optimum conditions were chosen for different water samples, and the removal rates of fluoride were 99.77% for industrial wastewater and 75.54% for groundwater. It is clear that AEG900 is efficient in treating fluoride in groundwater to meet the WHO standards. For photovoltaic wastewater, lime precipitation is the most mature method for fluoride removal, but the final fluoride concentration is commonly in the range of 20 to 100 mg L−1, which is higher than the emission standard (15 mg L−1).51 However, in this study, by in situ precipitation on AEG900, the lower final fluoride concentration can be achieved without a turbidity increase in wastewater.
To sum up, AEG900 is feasible for fluoride removal within a wide range of fluoride concentrations and can reduce fluoride to the permissible concentration suggested by WHO. It also can be used in different application scenarios, and by adjusting the adsorption condition, it is feasible for industrial wastewater or groundwater treatment.
3.5 Comparison of other adsorbents reported in the literature
To confirm the superiority of AEG900, the fluoride adsorption experiment was conducted under the same condition as Lee's study.25 The adsorption capacity is 56.84 mg g−1 for AEG900 (F−]0 = 200 mg L−1 and [adsorbent] = 3.33 g L−1), which is nearly 3 times that of eggshells treated under 800 °C for 4 h as Lee et al. reported (18.54 mg g−1). Moreover, the equilibrium adsorption capacities of fluoride by different adsorbents in the literature are plotted in Fig. 11. To make the comparison meaningful, the adsorption capacities were compared with different dosages of adsorbents and the initial concentration of fluoride higher than 100 mg L−1 was discussed in these studies. Obviously, AEG900 owns outstanding adsorption capacity for fluoride removal, especially compared with other absorbents recovered from waste. For example, under similar conditions, the adsorption capacity of AEG900 (370.15 mg g−1) was nearly three times that of nepheline prepared from kaolinite (125.0 mg g−1).44 Moreover, compared with other calcium-based materials, AEG900 also shows higher adsorption performance. For example, the adsorption capacity of CaSO4·2H2O was 128.08 mg g−1 with 1.5 g L−1 dosage, which is even less than the adsorption capacity of AEG900 for fluoride with 3 g L−1 dosage.32 Besides, some synthetic adsorbents displayed higher adsorption performance than AEG900, such as calcium hydroxide nanorods (450 mg g−1). Compared with AEG900 recovered from eggshells, nano-size calcium hydroxide with higher SSA and the content of Ca(OH)2 is favorable for the adsorption and precipitation of fluoride in water, but meanwhile, the higher preparation cost and the risk of nanoparticle leaching remains.46 Hence, AEG900, with its low-cost, easy preparation method, and high fluoride removal efficiency, displays application potential for fluoride removal.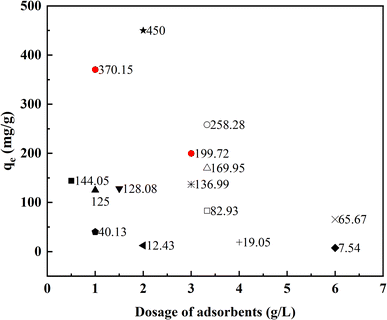 | ||
| Fig. 11 Comparation of the adsorption capacity of fluoride removal by different adsorbents with different dosages. (■: 3D porous metal oxide;52 □: Mytilus coruscus shells;53 ▲: nepheline;44 ▼: CaSO4·2H2O;32 ◆: Zr(IV)-loaded grape pomace;54 ○: calcined eggshells;25 ×: porous calcium silicate hydrates;35 ★: calcium hydroxide nanorods;46 +: charcoals;55 △: Sepiolite;37 ◀: tea powder-Zr;56 ◇: CeCO3OH nanospheres; ☆: CaO20@Al2O3;42●: in this study. | ||
Conclusion
In this study, a modified preparation method was proposed to improve fluoride removal in an aqueous solution by Ca(OH)2 derived from calcined eggshells. By an aging treatment in a humid environment, CaO as the main active component in calcined eggshells could convert to Ca(OH)2 to realize a great degree of improvement for fluoride removal, wherein the fluoride removal rate of EG900 increased nearly by 29.21%. The single-factor experiments demonstrate that AEG900 owns the characteristic of calcium-based adsorbents for fluoride removal, and the adsorption equilibrium process was well fitted with the Langmuir–Freundlich isotherm models with the maximum adsorption capacity of 370.15 mg g−1. Based on the adsorbent material characterization, surface complexation model analysis, and adsorption performance results, the adsorption and precipitation both contribute to the fluoride removal mechanism, in which the contribution of precipitation is bigger. Fluorite as a product is formed in situ, which avoids increased turbidity in the lime precipitation method. Moreover, satisfactory results of fluoride removal by AEG900 in different initial fluoride concentrations, real-life groundwater, and photovoltaic industrial wastewater were achieved, and the final fluoride could reach the requirement of WHO and the discharge standard of industrial wastewater. Overall, by aging treatment, calcined eggshells were endowed with outstanding defluorination capacity, which could be applied as a potential adsorbent for fluoride removal.Author contributions
Wenjing Chen: conceptualization, methodology, writing-original draft preparation, supervision. Yuanyue Wu: conceptualization, writing-review & editing. Zhiyin Xie: methodology, data curation. Yiyuan Li: writing-review & editing. Weitai Tang: formal analysis. Jinbei Yu: project administration.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to acknowledge the financial support of Natural Science Foundation of Sichuan Province (2022NSFSC1238), Research Foundation of Chengdu University of Information Technology (KYTZ202117), and Innovation and Entrepreneurship program for University Student in Sichuan Province (202110621041).References
- B.-W. Gu, C.-G. Lee and S.-J. Park, Application of response surface methodology and semi-mechanistic model to optimize fluoride removal using crushed concrete in a fixed-bed column, Environ. Technol., 2018, 39, 616–627 CrossRef CAS.
- O. World Health, Guidelines for drinking-water quality, World Health Organization, Geneva, 2011 Search PubMed.
- M. Vithanage and P. Bhattacharya, Fluoride in the environment: sources, distribution and defluoridation, Environ. Chem. Lett., 2015, 13, 131–147 CrossRef CAS.
- EPA, National Primary Drinking Water Regulations; Announcement of the Results of EPA's Review of Existing Drinking Water Standards and Request for Public Comment and/or Information on Related Issues, 2017 Search PubMed.
- K. Cherukumilli, T. Maurer, J. N. Hohman, Y. Mehta and A. J. Gadgil, Effective Remediation of Groundwater Fluoride with Inexpensively Processed Indian Bauxite, Environ. Sci. Technol., 2018, 52, 4711–4718 CrossRef CAS PubMed.
- S. V. Jadhav, E. Bringas, G. D. Yadav, V. K. Rathod, I. Ortiz and K. V. Marathe, Arsenic and fluoride contaminated groundwaters: A review of current technologies for contaminants removal, J. Environ. Manage., 2015, 162, 306–325 CrossRef CAS PubMed.
- L. Huang, Z. Luo, X. Huang, Y. Wang, J. Yan, W. Liu, Y. Guo, S. R. Babu Arulmani, M. Shao and H. Zhang, Applications of biomass-based materials to remove fluoride from wastewater: A review, Chemosphere, 2022, 301, 134679 CrossRef CAS PubMed.
- K. Wan, L. Huang, J. Yan, B. Ma, X. Huang, Z. Luo, H. Zhang and T. Xiao, Removal of fluoride from industrial wastewater by using different adsorbents: A review, Sci. Total Environ., 2021, 773, 145535 CrossRef CAS PubMed.
- E. J. Reardon and Y. Wang, A Limestone Reactor for Fluoride Removal from Wastewaters, Environ. Sci. Technol., 2000, 34, 3247–3253 CrossRef CAS.
- Y. Z. Zhu, D. W. Liu, Z. Y. Liu and Y. F. Li, Impact of aluminum exposure on the immune system: A mini review, Environ. Toxicol. Pharmacol., 2013, 35, 82–87 CrossRef CAS.
- D. Dolar, K. Košutić and B. Vučić, RO/NF treatment of wastewater from fertilizer factory — removal of fluoride and phosphate, Desalination, 2011, 265, 237–241 CrossRef CAS.
- EPA, The Drinking Water Treatability Database, 2010 Search PubMed.
- G. J. Millar, S. J. Couperthwaite, L. A. Dawes, S. Thompson and J. Spencer, Activated alumina for the removal of fluoride ions from high alkalinity groundwater: New insights from equilibrium and column studies with multicomponent solutions, Sep. Purif. Technol., 2017, 187, 14–24 CrossRef CAS.
- S. I. Alhassan, L. Huang, Y. He, L. Yan, B. Wu and H. Wang, Fluoride removal from water using alumina and aluminum-based composites: A comprehensive review of progress, Crit. Rev. Environ. Sci. Technol., 2021, 51, 2051–2085 CrossRef CAS.
- S. George, P. Pandit and A. B. Gupta, Residual aluminium in water defluoridated using activated alumina adsorption – Modeling and simulation studies, Water Res., 2010, 44, 3055–3064 CrossRef CAS PubMed.
- R. C. M. Meenakshi, Fluoride in drinking water and its removal, J. Hazard. Mater., 2006, 137, 456–463 CrossRef CAS PubMed.
- W. Yang, C. Li, S. Tian, L. Liu and Q. Liao, Influence of synthesis variables of a sol-gel process on the properties of mesoporous alumina and their fluoride adsorption, Mater. Chem. Phys., 2020, 242, 122499 CrossRef CAS.
- D. Dayananda, V. R. Sarva, S. V. Prasad, J. Arunachalam and N. N. Ghosh, A Simple Aqueous Solution Based Chemical Methodology for Preparation of Mesoporous Alumina: Efficient Adsorbent for Defluoridation of Water, Part. Sci. Technol., 2015, 33, 8–16 CrossRef CAS.
- G. De Angelis, L. Medeghini, A. M. Conte and S. Mignardi, Recycling of eggshell waste into low-cost adsorbent for Ni removal from wastewater, J. Cleaner Prod., 2017, 164, 1497–1506 CrossRef CAS.
- S. Owuamanam and D. Cree, Progress of Bio-Calcium Carbonate Waste Eggshell and Seashell Fillers in Polymer Composites: A Review, J. Compos. Sci., 2020, 4, 70 CrossRef CAS.
- D. Shi, H. Tong, M. Lv, D. Luo, P. Wang, X. Xu and Z. Han, Optimization of hydrothermal synthesis of hydroxyapatite from chicken eggshell waste for effective adsorption of aqueous Pb(II), Environ. Sci. Pollut. Res., 2021, 28, 58189–58205 CrossRef CAS PubMed.
- M. A. Abdel-Khalek, M. K. Abdel Rahman and A. A. Francis, Exploring the adsorption behavior of cationic and anionic dyes on industrial waste shells of egg, J. Environ. Chem. Eng., 2017, 5, 319–327 CrossRef CAS.
- A. F. Santos, A. L. Arim, D. V. Lopes, L. M. Gando-Ferreira and M. J. Quina, Recovery of phosphate from aqueous solutions using calcined eggshell as an eco-friendly adsorbent, J. Environ. Manage., 2019, 238, 451–459 CrossRef CAS PubMed.
- R. Bhaumik, N. K. Mondal, B. Das, P. Roy, K. C. Pal, C. Das, A. Baneerjee and J. k. Datta, Eggshell Powder as an Adsorbent for Removal of Fluoride from Aqueous Solution: Equilibrium, Kinetic and Thermodynamic Studies, E-J. Chem., 2012, 9, 790401 Search PubMed.
- J.-I. Lee, S.-H. Hong, C.-G. Lee and S.-J. Park, Fluoride removal by thermally treated egg shells with high adsorption capacity, low cost, and easy acquisition, Environ. Sci. Pollut. Res., 2021, 28, 35887–35901 CrossRef CAS PubMed.
- P. Photiou and I. Vyrides, Calcined eggshells in anaerobic digestion: Buffering acidification in AD and evaluating end products from phosphate adsorption as soil conditioners, J. Environ. Chem. Eng., 2022, 10, 107957 CrossRef CAS.
- G. P. Jeppu and T. P. Clement, A modified Langmuir–Freundlich isotherm model for simulating pH-dependent adsorption effects, J. Contam. Hydrol., 2012, 129–130, 46–53 CrossRef CAS PubMed.
- R. Risso, P. Ferraz, S. Meireles, I. Fonseca and J. Vital, Highly active Cao catalysts from waste shells of egg, oyster and clam for biodiesel production, Appl. Catal., A, 2018, 567, 56–64 CrossRef CAS.
- E. Panagiotou, N. Kafa, L. Koutsokeras, P. Kouis, P. Nikolaou, G. Constantinides and I. Vyrides, Turning calcined waste egg shells and wastewater to Brushite: Phosphorus adsorption from aqua media and anaerobic sludge leach water, J. Cleaner Prod., 2018, 178, 419–428 CrossRef CAS.
- S. Budyanto, Y.-L. Kuo and J. C. Liu, Adsorption and precipitation of fluoride on calcite nanoparticles: A spectroscopic study, Sep. Purif. Technol., 2015, 150, 325–331 CrossRef CAS.
- S. Ullah, A. G. Al-Sehemi, M. Mubashir, A. Mukhtar, S. Saqib, M. A. Bustam, C. K. Cheng, M. Ibrahim and P. L. Show, Adsorption behavior of mercury over hydrated lime: Experimental investigation and adsorption process characteristic study, Chemosphere, 2021, 271, 129504 CrossRef CAS PubMed.
- S. Shao, B. Ma, Y. Chen, W. Zhang and C. Wang, Behavior and mechanism of fluoride removal from aqueous solutions by using synthesized CaSO4·2H2O nanorods, Chem. Eng. J., 2021, 426, 131364 CrossRef CAS.
- Y. Wei, H. Xu, S. Xu, H. Su, R. Sun, D. Huang, L. Zhao, Y. Hu, K. Wang and X. Lian, Synthesis and characterization of calcium carbonate on three kinds of microbial cells templates, J. Cryst. Growth, 2020, 547, 125755 CrossRef CAS.
- M. Galván-Ruiz, J. Hernández, L. Baños, J. Noriega-Montes and M. E. Rodríguez-García, Characterization of Calcium Carbonate, Calcium Oxide, and Calcium Hydroxide as Starting Point to the Improvement of Lime for Their Use in Construction, J. Mater. Civ. Eng., 2009, 21, 694–698 CrossRef.
- W. Guan and X. Zhao, Fluoride recovery using porous calcium silicate hydrates via spontaneous Ca2+ and OH− release, Sep. Purif. Technol., 2016, 165, 71–77 CrossRef CAS.
- O. Eskikaya, M. Gun, R. Bouchareb, Z. Bilici, N. Dizge, R. Ramaraj and D. Balakrishnan, Photocatalytic activity of calcined chicken eggshells for Basic Red 2 and Reactive Red 180 decolorization, Chemosphere, 2022, 135210 CrossRef CAS PubMed.
- J.-I. Lee, S.-H. Hong, C.-G. Lee and S.-J. Park, Experimental and model study for fluoride removal by thermally activated sepiolite, Chemosphere, 2020, 241, 125094 CrossRef CAS PubMed.
- E. L. Sjöberg and D. T. Rickard, Calcite dissolution kinetics: Surface speciation and the origin of the variable pH dependence, Chem. Geol., 1984, 42, 119–136 CrossRef.
- I. V. Dolgaleva, I. G. Gorichev, A. D. Izotov and V. M. Stepanov, Modeling of the Effect of pH on the Calcite Dissolution Kinetics, Theor. Found. Chem. Eng., 2005, 39, 614–621 CrossRef CAS.
- S. Moriyama, K. Sasaki and T. Hirajima, Effect of calcination temperature on Mg–Al bimetallic oxides as sorbents for the removal of F− in aqueous solutions, Chemosphere, 2014, 95, 597–603 CrossRef CAS PubMed.
- Q. Zhou, X. Lin, B. Li and X. Luo, Fluoride adsorption from aqueous solution by aluminum alginate particles prepared via electrostatic spinning device, Chem. Eng. J., 2014, 256, 306–315 CrossRef CAS.
- D. Dayananda, V. R. Sarva, S. V. Prasad, J. Arunachalam and N. N. Ghosh, Preparation of CaO loaded mesoporous Al2O3: Efficient adsorbent for fluoride removal from water, Chem. Eng. J., 2014, 248, 430–439 CrossRef CAS.
- A. Saini, P. H. Maheshwari, S. S. Tripathy, S. Waseem and S. R. Dhakate, Processing of rice straw to derive carbon with efficient de-fluoridation properties for drinking water treatment, Journal of Water Process Engineering, 2020, 34, 101136 CrossRef.
- H. Wang, Q. Feng, K. Liu, Z. Li, X. Tang and G. Li, Highly efficient fluoride adsorption from aqueous solution by nepheline prepared from kaolinite through alkali-hydrothermal process, J. Environ. Manage., 2017, 196, 72–79 CrossRef CAS PubMed.
- B. Nayak, A. Samant, R. Patel and P. K. Misra, Comprehensive Understanding of the Kinetics and Mechanism of Fluoride Removal over a Potent Nanocrystalline Hydroxyapatite Surface, ACS Omega, 2017, 2, 8118–8128 CrossRef CAS PubMed.
- M. Chaudhary and A. Maiti, Defluoridation by highly efficient calcium hydroxide nanorods from synthetic and industrial wastewater, Colloids Surf., A, 2019, 561, 79–88 CrossRef CAS.
- A. Saini, P. H. Maheshwari, S. S. Tripathy, S. Waseem, A. Gupta and S. R. Dhakate, A novel alum impregnated CaO/carbon composite for de-fluoridation of water, Groundwater for Sustainable Development, 2021, 14, 100622 CrossRef.
- E. Turiel, C. Perez-Conde and A. Martin-Esteban, Assessment of the cross-reactivity and binding sites characterisation of a propazine-imprinted polymer using the Langmuir–Freundlich isotherm, Analyst, 2003, 128, 137–141 RSC.
- G. El Diwani, S. K. Amin, N. K. Attia and S. I. Hawash, Fluoride pollutants removal from industrial wastewater, Bull. Natl. Res. Cent., 2022, 46, 143 CrossRef.
- L. Wang, C. Di, T. Li, Y. Chun and Q. Xu, Preparation and catalytic behavior of biomorphic calcium oxide/carbon solid base materials, Catal. Sci. Technol., 2015, 5, 5185–5195 RSC.
- B. Palahouane, N. Drouiche, S. Aoudj and K. Bensadok, Cost-effective electrocoagulation process for the remediation of fluoride from pretreated photovoltaic wastewater, J. Ind. Eng. Chem., 2015, 22, 127–131 CrossRef CAS.
- X. Wang, H. Pfeiffer, J. Wei, J. Dan, J. Wang and J. Zhang, 3D porous Ca-modified Mg-Zr mixed metal oxide for fluoride adsorption, Chem. Eng. J., 2022, 428, 131371 CrossRef CAS.
- J.-I. Lee, J.-K. Kang, S.-H. Hong, C.-G. Lee, S. Jeong and S.-J. Park, Thermally treated Mytilus coruscus shells for fluoride removal and their adsorption mechanism, Chemosphere, 2021, 263, 128328 CrossRef CAS.
- Y. Zhang and K. Huang, Grape pomace as a biosorbent for fluoride removal from groundwater, RSC Adv., 2019, 9, 7767–7776 RSC.
- E. Tchomgui-Kamga, E. Ngameni and A. Darchen, Evaluation of removal efficiency of fluoride from aqueous solution using new charcoals that contain calcium compounds, J. Colloid Interface Sci., 2010, 346, 494–499 CrossRef CAS PubMed.
- H. Cai, L. Xu, G. Chen, C. Peng, F. Ke, Z. Liu, D. Li, Z. Zhang and X. Wan, Removal of fluoride from drinking water using modified ultrafine tea powder processed using a ball-mill, Appl. Surf. Sci., 2016, 375, 74–84 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: The ESI† is available online. See https://doi.org/10.1039/d2ra05209a |
| This journal is © The Royal Society of Chemistry 2022 |






