Open Access Article
Open Access ArticleSelf-fused concatenation of interferon with enhanced bioactivity, pharmacokinetics and antitumor efficacy†
Jin Hu‡
 *a,
Jianquan Shi‡b,
Yeshuang Yuan‡c,
Shengjie Lia,
Bo Zhanga,
Haitao Dongd,
Qing Zhonga,
Qiu Xiea,
Xiaoyin Baie and
Yingxing Lia
*a,
Jianquan Shi‡b,
Yeshuang Yuan‡c,
Shengjie Lia,
Bo Zhanga,
Haitao Dongd,
Qing Zhonga,
Qiu Xiea,
Xiaoyin Baie and
Yingxing Lia
aDepartment of Medical Research Center, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing 100730, China. E-mail: ncuskhujin@163.com
bDepartment of Intensive Care Unit, Beijing Chest Hospital, Capital Medical University, Beijing Tuberculosis and Thoracic Tumor Research Institute, Beijing 101149, China
cDepartment of Rheumatology, Beijing Hospital, National Center of Gerontology, Institute of Geriatric Medicine, Clinical Immunology Center, Graduate School of Peking Union Medical College, Chinese Academy of Medical Sciences, Peking Union Medical College, Beijing 100730, China
dDepartment of Stomatology, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing 100730, China
eDepartment of Gastroenterology, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing 100730, China
First published on 4th October 2022
Abstract
We report an easy but universal protein modification approach, self-fused concatenation (SEC), to biosynthesize a set of interferon (IFN) concatemers with improved in vitro bioactivity, in vivo pharmacokinetics and therapeutic efficacy over the monomeric IFN, and the results can be positively enhanced by the concatenated number of self-fused proteins.
Protein therapeutics have clinically been proven to be effective in treating many diseases due to their high specificity and biological activity.1–4 However, they have conspicuous limitations such as quick renal clearance, poor stability and strong immunogenicity during their use, thus requiring frequent administrations to achieve therapeutic effects, which in turn increases the chance of dramatically oscillating concentrations in blood and adverse effects as well as poor patient compliance.1,5,6 Many strategies, such as dissolving in appropriate buffers,7 loading in nanocarriers,8–10 site-specific mutation,11,12 immobilization in functional materials,13 modification with polymers,14,15 etc., can effectively improve the stability and drug delivery. Covalent conjugation of poly(ethylene glycol) (PEG) on the surface of protein therapeutics, named PEGylation, is a type of important strategy to extend the half-life of biomolecules.16 Various proteins, such as interferon α-2a (IFNα-2a), L-asparaginase, adenosine deaminase and tumor necrosis factor alpha have successfully been conjugated with PEG to enhance their therapeutic properties.17–19 Genetically fusing proteins with long-acting human serum albumin (HSA) is another conventional strategy, and various HSA-fused proteins such as IFNα, antihemophilic factor, recombinant factor IX and anti-vascular endothelial growth factor have been approved by the Food and Drug Administration (FDA) for clinical use.20–23 Nevertheless, both approaches require the introduction of exogenous molecules, resulting in inevitable immunogenicity, reduced biological activity and potential toxicity induced by the extra moiety.24–27
In order to avoid the unpredictable influences caused by the introduction of macromolecules, we developed an easy but universal protein modification approach, self-fused concatenation (SEC), to modify model protein, green fluorescence protein (GFP). We successfully synthesized a set of GFP concatemers using this protein fusion technique.28 The concatemers displayed larger hydrodynamic radius and remarkable improvement in in vivo tumor retention over the monomeric GFP. However, this technique has never been applied in therapeutic proteins to display its potential as an alternative of protein modification in the field of drug delivery, since the biosynthesis and modification conditions of therapeutics are much stricter than model proteins.
IFNs α are a group of cytokines that possess various biological effects, including antiviral, antitumor and immunomodulatory activities in patients with defined types of viral and cancer diseases.29,30 It has already been approved by FDA (Food and Drug Administration) for clinical application since 1986. However, IFN α has a low molecular weight (∼20 kDa) and short in vivo half-life (4–8 h), which requires frequent administrations to achieve sustained high concentrations, thus resulting in severe side effects and poor compliance.31–33
Herein, as the first case, we successfully biosynthesized IFN concatemers via SEC in prokaryotic expression system and systematically studied the effects of modification on the pharmaceutical profiles of IFN. The circulating half-lives of trimer (3 × IFN) and dimer (2 × IFN) of IFN were 10.6- and 4.80-fold longer than that of monomer (1 × IFN), respectively. 3 × IFN and 2 × IFN displayed better inhibition ability on tumor growth in ovarian cancer inoculated mice, with 2.11- and 1.44-fold longer of median survival time of mice than that of 1 × IFN. Moreover, the concatenated number could positively enhance the in vitro bioactivity, in vivo pharmacokinetics and therapeutic efficacy of IFN. These findings demonstrated that SEC would be a promising alternative to optimize the pharmaceutic profiles of protein/peptide therapeutics for clinical application.
To obtain concatenated IFNs, we designed and constructed three recombinant plasmids containing the sequence of monomer (1 × IFN), dimer (2 × IFN) and trimer (3 × IFN) of IFN. A 6 × His tag was fused at the C-terminus for nickel-nitrilotriacetic acid immobilized metal affinity chromatography (Ni-NTA IMAC) purification (Fig. 1a). The natural IFN, 1 × IFN, and the self-fused proteins, 2 × IFN and 3 × IFN, were successfully overexpressed in Escherichia coli (E. coli) after induced with isopropyl-β-D-1-thiogalactopyranoside (IPTG) and purified via Ni-NTA IMAC in high yield (∼80 mg per liter of bacteria culture medium) (Fig. S1–S3†). Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) showed only a single band around the molecular weight (MW) of approximately 20, 40 and 60 kDa that corresponded to 1 × IFN, 2 × IFN and 3 × IFN, respectively (Fig. 1b). The accurate MW was further detected by liquid chromatography-electrospray ionization mass spectrometry (LC-ESI-MS). The MWs of 1 × IFN, 2 × IFN and 3 × IFN were 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 092.0, 40
092.0, 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 930.0 and 60
930.0 and 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 470.0 kDa, which were in accordance with the theoretical value of 20
470.0 kDa, which were in accordance with the theoretical value of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 092.0, 40
092.0, 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 930.6 and 60
930.6 and 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 470.0 kDa, respectively (Fig. 1c). Dynamic light scattering (DLS) showed that 3 × IFN and 2 × IFN possessed hydrodynamic radii (Rh) of 6.857 and 4.612 nm, much larger than that of 1 × IFN (2.759 nm) (Fig. 1d). The secondary structural conformations of IFN concatemers detected by circular dichroism (CD) spectroscopy exhibited the identical signature of α-helix with a typical doublet at 208/222 nm, and the far-UV scans were in consistent with each other (Fig. 1e). These results indicated that IFN concatemers were successfully synthesized via SEC and the physicochemical properties were well retained after modification.
470.0 kDa, respectively (Fig. 1c). Dynamic light scattering (DLS) showed that 3 × IFN and 2 × IFN possessed hydrodynamic radii (Rh) of 6.857 and 4.612 nm, much larger than that of 1 × IFN (2.759 nm) (Fig. 1d). The secondary structural conformations of IFN concatemers detected by circular dichroism (CD) spectroscopy exhibited the identical signature of α-helix with a typical doublet at 208/222 nm, and the far-UV scans were in consistent with each other (Fig. 1e). These results indicated that IFN concatemers were successfully synthesized via SEC and the physicochemical properties were well retained after modification.
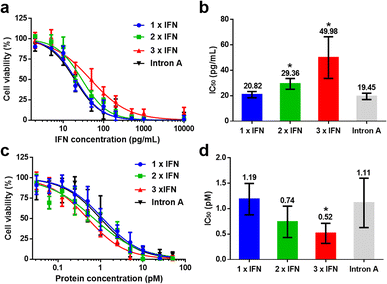
To evaluate the biological activity of IFN concatemers, we quantified the cytotoxicity of proteins against human Burkitt's B lymphoma line under the same mass or molar concentrations. As expected, the monomeric IFN exhibited the best bioactivity per unit of IFN (Fig. 2a), with the half maximal inhibitory concentration of 20.82 pg mL−1, whereas 2 × IFN and 3 × IFN possessed the retention activity of 70.91% and 41.66%, with the IC50 value of 29.36 and 49.98 mg mL−1, respectively (Fig. 2b and Table S1†). However, the bioactivity per protein was significantly enhanced with the increased fusing number of IFN concatemers (Fig. 2c). Particularly, the IC50 values of 2 × IFN and 3 × IFN were 0.74 and 0.52 pM, which were 1.6 × and 2.3 × fold higher than that of 1× IFN (1.19 pM) per protein after calculation (Fig. 2d and Table S2†). We also assessed the cytotoxicity of Intron A, a commercial recombinant interferon that has been applied in clinical, it exhibited similar anti-tumor activity comparing with 1 × IFN (Fig. 2). The results revealed that the increased bioactivity induced by the number of IFN outperformed the reduced activity caused by the steric hindrance of fused macromolecule. As the commercialized techniques, PEGylation and HSA fusion, require the introduction of inactive macromolecules that reduce the biological activity. For example, the bioactivity retentions of PEGylated and HSA-fused IFN were only 7% (ref. 34) and 1% (ref. 35) in previously reported literature. The data suggested that SEC could well improve the bioactivity of IFN via self-fusion of the active protein, which was dramatically superior than the existing methods.
Since natural IFN is limited by the rapid rate of blood clearance, we then quantified the pharmacokinetic behaviour of IFN concatemers in the mice model. Upon intravenous injection, plasma samples were collected at selected times and the levels of IFN were detected. The data were fitted with a two-compartment model and the pharmacokinetic parameters were summarized in Table S3.† Obviously, 3 × IFN showed a much slower elimination rate than 2 × IFN, and 2 × IFN exhibited a much slower rate than 1 × IFN (Fig. 3). The distribution (t1/2α) and terminal (t1/2β) half-life of 3 × IFN was 0.854 h and 13.3 h, which was 2.08-fold and 10.6-fold longer than that of 1 × IFN (t1/2α = 0.411 h, t1/2β = 1.26 h); the distribution and terminal half-life of 2 × IFN was 0.618 h and 6.05 h, which was1.50-fold and 4.80-fold longer than that of 1 × IFN. Moreover, the area under the curves (AUCs) of 3 × IFN and 2 × IFN were 1.36 and 0.474 μg L−1 h−1, which were 6.38 and 2.23-fold than that of 1 × IFN (0.213 μg L−1 h−1). The data indicated that the pharmacokinetic of IFN concatemers is positively correlated with the concatenated number of proteins.
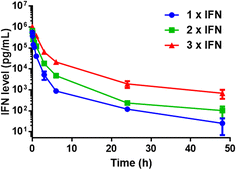 | ||
| Fig. 3 In vivo pharmacokinetics of IFN concatemers. Data are shown as the mean ± standard deviation (n = 3, *P < 0.05 for 3 × IFN vs. 1 × IFN). | ||
We further investigated the antitumor efficacy of IFN concatemers in human OVCAR-3 ovarian cancer xenograft in nude mice. All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Peking Union Medical College (PUMC) Hospital and approved by the Ethics Review Board of PUMC Hospital. Before carrying out the animal study, we tested the in vitro bioactivity towards OVCAR-3 cells. The IC50s of 1 × IFN, 2 × IFN and 3 × IFN of IFN-equivalent were 230.3, 389.8 and 599.7 ng mL−1, respectively (Fig. S4†), indicating IFN concatemers can kill OVCAR-3 cells. Next, mice with a mean tumor volume of 20 mm3 were injected with IFN concatemers at an IFN-equivalent dose of 1 mg kg−1 every three days. 1 × IFN slightly inhibited tumor growth comparing with saline, whereas 2 × IFN exhibited relatively better antitumor ability than 1 × IFN. In contrast, 3 × IFN completely inhibited the tumor growth during treatment (Fig. 4a and S5†). The median survival times for the mice treated with 3 × IFN and 2 × IFN were 85.5 and 58.5 days, which were 2.11- and 1.44-fold longer than that of the mice treated with 1 × IFN (40.5 days), respectively (Fig. 4b). Besides, hematoxylin and eosin (H&E) staining showed that the tumors of the mice treated with saline and 1 × IFN were composed of tightly packed cells, and the tumors of the mice treated with 2 × IFN and 3 × IFN existed extensive vacuoles, indicating serious damage to tumor cells (Fig. 4c). Collectively, these results suggested that increasing the MW of IFN concatemers would remarkably enhance the antitumor efficacy, which could be attributed to the improved pharmacokinetics and bioactivity.
We finally assessed the biological safety of IFN concatemers. The body weight of mice did not decrease during treatment (Fig. S6†). H&E staining of hearts, livers and kidneys after treatment demonstrated that there was no noticeable damage in histological level (Fig. S7†). In addition, the clinical hematological markers such as white blood cells (WBC), red blood cells (RBC), platelets (PLT) and hemoglobin (HGB) of mice treated with IFN concatemers were comparable to those of mice treated with saline (Fig. S8†), as well as the biochemical parameters of lactate dehydrogenase (LDH) and creatine kinase isoenzymes (CK-MB) for heart function, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) for liver function, creatinine (CRE) and blood urea nitrogen (BUN) for kidney function (Fig. S9†). Taken together, the data indicated that SEC modification for protein did not induce the systemic toxicity of IFN.
In summary, we synthesized IFN concatemers using SEC technique to enhance the in vitro biological activity and in vivo pharmaceutical properties of IFN without the introduction of exogenous macromolecules. We have genetically engineered a series of IFN concatemers in prokaryotic expression system and studied the tandem number effect on the properties of IFN. Such a kind of study leads to several findings that are vital for the development of protein therapeutics: (i) concatenated IFNs exhibited increased biological activity relative to the unmodified IFN; (ii) IFN concatemers possessed excellent circulating half-life, with 10.6- and 4.80-fold longer for 3 × IFN and 2 × IFN as compared with 1 × IFN; (iii) IFN concatemers displayed improved in vivo anti-tumor activity, resulting in 2.11- and 1.44-fold longer of median survival time of mice treated with 3 × IFN and 2 × IFN than that of 1 × IFN. These data also demonstrated that the concatenated number of self-fused protein would increase the in vitro bioactivity, in vivo pharmacokinetics and therapeutic efficacy of IFN.
The limitation of this study is the difference between animal study and clinical treatment. The determination of pharmacokinetics and in vivo anti-tumor efficiency in mice were not completely accurate. This is because the experimental mice were immune-deficient that lacked antibody responses and partial immune activations, which could have some impacts on the in vivo behaviours of IFN concatemers (including immunogenicity and immune response), especially for long-term treatment. However, the drawback partially influenced the results but could not change the conclusion, since the experimental results of IFN concatemers were greatly enhanced comparing with unmodified IFN. Taken together, this study indicated that SEC might be a promising modification alternative to optimize the pharmaceutic profiles of protein/peptide agents without the introduction of exogenous molecules.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Dr Li Jin and the MS Facility Center of Biomedical Analysis, Tsinghua University for performing the LC-ESI-MS measurements. This work was financially supported by grants from the National Natural Science Foundation of China (Grant No. 21805311 to J. Hu and 82271831 to B. Zhang), National High Level Hospital Clinical Research Funding (Grant No. 2022-PUMCH-A-141 to H. Dong, 2022-PUMCH-A-206 to Q. Xie, 2022-PUMCH-A-074 to X. Bai) and Youth Program of Peking Union Medical College Hospital Foundation (No. pumch201910847 to H. Dong).Notes and references
- A. M. Wagner, M. P. Gran and N. A. Peppas, Acta Pharm. Sin. B, 2018, 8, 147–164 CrossRef PubMed.
- L. A. Sharpe, A. M. Daily, S. D. Horava and N. A. Peppas, Expet Opin. Drug Deliv., 2014, 11, 901–915 CrossRef CAS.
- M. Y. Yu, J. Wu, J. J. Shi and O. C. Farokhzad, J. Control Release, 2016, 240, 24–37 CrossRef CAS PubMed.
- D. Shao, M. Li, Z. Wang, X. Zheng, Y. H. Lao, Z. Chang, F. Zhang, M. Lu, J. Yue, H. Hu, H. Yan, L. Chen, W. F. Dong and K. W. Leong, Adv. Mater., 2018, e1801198 CrossRef PubMed.
- J. H. Ko and H. D. Maynard, Chem. Soc. Rev., 2018, 47, 8998–9014 RSC.
- Y. Rui, D. R. Wilson, J. Choi, M. Varanasi, K. Sanders, J. Karlsson, M. Lim and J. J. Green, Sci. Adv., 2019, 5, eaay3255 CrossRef CAS PubMed.
- M. C. Manning, D. K. Chou, B. M. Murphy, R. W. Payne and D. S. Katayama, Pharm. Res., 2010, 27, 544–575 CrossRef PubMed.
- M. M. Joseph, S. R. Aravind, S. K. George, R. K. Pillai, S. Mini and T. T. Sreelekha, Eur. J. Pharm. Biopharm., 2015, 93, 183–195 CrossRef CAS PubMed.
- P. T. Sujai, M. M. Joseph, G. Saranya, J. B. Nair, V. P. Murali and K. K. Maiti, Nanoscale, 2020, 12, 6971–6975 RSC.
- M. Y. Yu, J. Wu, J. J. Shi and O. C. Farokhzad, J. Control Release, 2016, 240, 24–37 CrossRef CAS PubMed.
- Y. Ravikumar, S. P. Nadarajan, T. H. Yoo, C. Lee and H. Yun, Trends Biotechnol., 2015, 33, 462–470 CrossRef CAS PubMed.
- X. Guo, T. Yan, J. Rao, X. Yue, X. Pei, J. Deng, W. Sun, W. Yang, B. Zhang and J. Xie, Biomolecules, 2021, 11, 761 CrossRef CAS PubMed.
- A. A. Caparco, D. R. Dautel and J. A. Champion, Small, 2022, 18, e2106425 CrossRef PubMed.
- C. A. Stevens, K. Kaur and H. A. Klok, Adv. Drug Deliv. Rev., 2021, 174, 447–460 CrossRef CAS PubMed.
- B. Mukherjee, S. D. Karmakar, C. M. Hossain and S. Bhattacharya, Protein Pept. Lett., 2014, 21, 1121–1128 CrossRef CAS PubMed.
- F. M. Veronese and A. Mero, BioDrugs, 2008, 22, 315–329 CrossRef CAS.
- A. Grigoletto, A. Mero, H. Yoshioka, O. Schiavon and G. Pasut, J. Drug Target., 2017, 25, 856–864 CrossRef CAS PubMed.
- A. Grigoletto, A. Mero, K. Maso and G. Pasut, Methods Enzymol., 2017, 590, 317–346 CAS.
- G. Pasut, M. Sergi and F. M. Veronese, Adv. Drug Deliv. Rev., 2008, 60, 69–78 CrossRef CAS.
- R. J. Melder, B. L. Osborn, T. Riccobene, P. Kanakaraj, P. Wei, G. X. Chen, D. Stolow, W. G. Halpern, T. S. Migone, Q. Wang, K. J. Grzegorzewski and G. Gallant, Cancer Immunol. Immunother., 2005, 54, 535–547 CrossRef CAS PubMed.
- H. S. Chung, J. Y. Oh, S. B. Yoo, S. M. Lee and H. S. Cho, Regul. Pept., 2011, 170, 1–3 CrossRef CAS.
- K. Maso, I. M. Montagner, A. Grigoletto, O. Schiavon, A. Rosato and G. Pasut, Eur. J. Pharm. Biopharm., 2019, 142, 49–60 CrossRef CAS PubMed.
- G. M. Subramanian, M. Fiscella, A. Lamouse-Smith, S. Zeuzem and J. G. McHutchison, Nat. Biotechnol., 2007, 25, 1411–1419 CrossRef CAS PubMed.
- K. Knop, R. Hoogenboom, D. Fischer and U. S. Schubert, Angew. Chem., Int., 2010, 49, 6288–6308 CrossRef CAS PubMed.
- K. Sroda, J. Rydlewski, M. Langner, A. Kozubek, M. Grzybek and A. F. Sikorski, Cell. Mol. Biol. Lett., 2005, 10, 37–47 CAS.
- D. Levin, H. A. Lagasse, E. Burch, S. Strome, S. Tan, H. Jiang, Z. E. Sauna and B. Golding, J. Thromb. Haemostasis, 2017, 15, 721–734 CrossRef CAS PubMed.
- Y. Hashimoto, T. Shimizu, Y. Mima, A. S. Abu Lila, T. Ishida and H. Kiwada, Toxicol. Appl. Pharmacol., 2014, 277, 30–38 CrossRef CAS PubMed.
- J. Hu, J. Shi, B. Zhang, S. Li and H. Dong, Biochem. Biophys. Rep., 2021, 28, 101112 CAS.
- L. Bracci, E. Proietti and F. Belardelli, Ann. N. Y. Acad. Sci., 2007, 1112, 256–268 CrossRef CAS PubMed.
- J. Sprooten, P. Agostinis and A. D. Garg, Int. Rev. Cell Mol. Biol., 2019, 348, 217–262 CAS.
- R. J. Wills, Clin. Pharmacokinet., 1990, 19, 390–399 CrossRef CAS PubMed.
- J. Hu, G. Wang, X. Liu and W. Gao, Adv. Mater., 2015, 27, 7320–7324 CrossRef CAS.
- S. J. Bell, C. M. Fam, E. A. Chlipala, S. J. Carlson, J. Lee, M. S. Rosendahl, D. H. Doherty and G. N. Cox, Bioconjug. Chem., 2008, 19, 299–305 CrossRef CAS PubMed.
- Y. S. Wang, S. Younger, J. Bausch, R. Zhang, C. McNemar and D. F. Wyss, Biochemistry, 2000, 28, 10634–10640 CrossRef PubMed.
- Y. S. Huang, Z. Chen, Z. Y. Yang, T. Y. Wang, L. Zhou, J. B. Wu and L. F. Zhou, Eur. J. Pharm. Biopharm., 2007, 67, 301–308 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra04978c |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |