Open Access Article
Open Access ArticleColorimetric and fluorescent detection of synthetic cathinones in oral fluid with meso-aryl BODIPYs and Cu(II)†
Jordi Hernández-Contrerasa,
Margarita Parra abc,
Salvador Gil
abc,
Salvador Gil abc,
Ana M. Costero
abc,
Ana M. Costero abc,
Pau Arroyoab,
Félix Sancenónacd,
Ramón Martínez-Máñez
abc,
Pau Arroyoab,
Félix Sancenónacd,
Ramón Martínez-Máñez acd,
José A. Sáez
acd,
José A. Sáez *ab and
Pablo Gaviña
*ab and
Pablo Gaviña *abc
*abc
aInstituto Interuniversitario de Investigación de Reconocimiento Molecular y Desarrollo Tecnológico (IDM), Universitat Politècnica de València, Universitat de València, Doctor Moliner 50, Burjassot, Valencia, 46100, Spain. E-mail: pablo.gavina@uv.es; Jose.A.Saez@uv.es
bDepartamento de Química Orgánica, Universitat de València, Doctor Moliner 50, Burjassot, Valencia, 46100, Spain
cCIBER de Bioingeniería, Biometariales y Nanomedicina (CIBER-BBN), Spain
dDepartamento de Química, Universitat Politècnica de València, Camino de Vera s/n, Valencia 46022, Spain
First published on 20th October 2022
Abstract
Synthetic cathinones are a class of new psychoactive substances whose consumption has increased a lot and is widespread throughout the world. Thus, there is currently a need for rapid and simple detection of these drugs. In particular, detection of synthetic cathinones in oral fluid in drivers can be of great importance in preventing traffic accidents. Herein, we report two probes, based on BODIPY derivatives combined with Cu(II), which are able to detect these drugs both in water and in oral fluid, by changes in color and fluorescence. The determined limits of detection for ephedrone (as a model drug) are lower than the usual concentrations in saliva after intake of this type of drug. The sensing mechanism seems to be related to the cathinone induced reduction of Cu(II) to Cu(I) with concomitants changes in the BODIPY structure.
Introduction
Cathinone is a psychoactive compound obtained from Catha edulis (khat) leaves. Synthetic compounds based on the cathinone structure are a group of drugs, also known as “bath salts” whose consumption has increased a lot and is widespread throughout the world. These compounds belong to the group of New Psychoactive Substances (NPS). The most commonly consumed are: 3,4-methylenedioxypyrovalerone (MDPV), 4-methylmethcathinone (mephedrone), 4-methcathinone (ephedrone), methylone (beta-keto-MDMA), ethylone (beta-keto-3,4-methylenedioxy-N-ethylamphetamine, MDEA), 4-methoxymethcathinone, 4-fluoromethcathinone and butylone (Chart 1).1–3The most important effects of these compounds are alertness, talkativeness, euphoria or sexual arousal among others. However, in case of overdose, dehydration, breakdown of skeletal muscle tissue into the bloodstream, and kidney failure can be produced and can even lead to death. These effects are observed between 30–45 min after the intake and generally last for 1 to 3 h.4
Driving after drug consumption is highly dangerous because driving skills are affected which very often leads to vehicle crashes. Therefore, preventive detection of drugs in drivers is of crucial interest not only for police forces but also for drivers themselves. In this sense, the use of cheap, reliable and easy-to-use chromogenic sensors to detect synthetic cathinones (SCs) in oral fluid is of great interest.5
Although some sensors and probes for cathinones have been reported, this is still an emerging field requiring continuous improvement. Some of these probes exhibit rapid detection but require a complicated sample pretreatment. For example, Shen and col. have synthesized a molecular imprinted polymer for the detection of cathinones in urine based on aggregation induced emission (AIE), with a limit of detection (LoD) of 0.34 μM, but the urine sample requires pretreatment.6 Bovine serum albumin-stabilized gold nanoclusters have also been developed and carbon quantum dots (C-dot) functionalized chromatography papers as photoluminescent systems with potential for screening of these drugs in crime sites have also been reported. However, they require concentrations in the mM range.7,8 Finally, several biosensors have been prepared but they are not selective to cathinones.9
It has been described in the literature that cathinone derivatives are capable of reducing Cu(II) to Cu(I) giving rise to colour changes in Cu(II)-neocuproine complexes.10,11 On the other hand, it is widely recognized that BODIPY derivatives possess valuable optical characteristics, such as intense absorption and fluorescence transitions in the visible spectral region, high molar absorption coefficients and fluorescence quantum yields, good stability and independence from pH.12,13 Considering these facts we decided to synthesize two probes, based on BODIPY derivatives combined with Cu(II) (Chart 1), to detect cathinones both in water and in oral fluid. The sensing mechanism would be based on the ability of cathinones to reduce Cu(II) to Cu(I) and on the different behaviour of the BODIPY core in presence of Cu(I) or Cu(II). The different substitution on the phenyl group at the meso position of the BODIPY core should induce different chemical properties interesting for the sensing process.
Experimental
Materials
The reagents employed in the synthesis were acquired from Sigma Aldrich and used without further purification. 1H NMR and 13C NMR spectra were registered with a Bruker Avance 300 MHz spectrometer, all of them referenced to solvent peak, CDCl3. All photophysical analysis was carried out in air-equilibrated THF at 298 K, unless otherwise specified. UV-vis absorption spectra were recorded with a PerkinElmer λ40 spectrophotometer or Shimadzu UV-2600 using quartz cells with path length of 1.0 cm. The estimated experimental error was 2 nm on the band maximum. Mass spectrometry spectra were carried out with a TripleTOFTM 5600 LC/MS/MS System, with 2 gas sources (both to 35 psi), 450 °C and ion gas voltage of 5500 V. Plot2 was the program used to plot titrations.Synthesis of compound 2
2 mL (19.4 mmol) of 2,4-dimethyl-1H-pyrrole were dissolved in 150 mL of DCM dried under Ar atmosphere. Then, 1.2 mL (11 mmol) of benzoyl chloride were added and the mixture was stirred overnight at room temperature. Then, 10 mL (70.5 mmol) of NEt3 were added. After stirring for 30 minutes, 10 mL (81.4 mmol) of BF3OEt2 were added dropwise in an ice-water bath and the stirring was kept at room temperature overnight. Then, the solvent was removed and the crude was purified by column chromatography using hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DCM 8
DCM 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 as eluent. Compound 2 was obtained as a green solid (600 mg, 16% yield). 1H NMR (300 MHz, CDCl3) δ 1.35 (s, 6H), 2.53 (s, 6H), 5.96 (s, 2H), 7.25 (m, 2H), 7.34 (m, 3H).
2 as eluent. Compound 2 was obtained as a green solid (600 mg, 16% yield). 1H NMR (300 MHz, CDCl3) δ 1.35 (s, 6H), 2.53 (s, 6H), 5.96 (s, 2H), 7.25 (m, 2H), 7.34 (m, 3H).
Sensing experiments
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) solution of both 1 and Cu(II). Then, 300 μL of the reaction mixture were added. The reaction mixture was prepared with increasing quantities of ephedrone 5 mM solution in water until the saturation point was reached. In each case, an incubation period of 2 min at reflux temperature was established.
5) solution of both 1 and Cu(II). Then, 300 μL of the reaction mixture were added. The reaction mixture was prepared with increasing quantities of ephedrone 5 mM solution in water until the saturation point was reached. In each case, an incubation period of 2 min at reflux temperature was established.Results and discussion
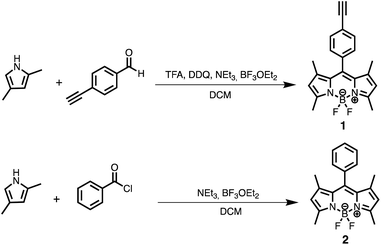
The synthesis of BODIPYs 1 and 2 is shown in Scheme 1. Compound 1 was prepared following the procedure described in the literature14 whereas compound 2 was prepared through a new optimized procedure from benzoyl chloride that avoids the usual treatment with DDQ and TFA (Scheme 1).Solutions of these dyes in THF showed the characteristic optical properties of BODIPY compounds: an intense absorption band at 501 nm and 503 nm with a shoulder at 480 nm and 470 nm for compounds 1 and 2, respectively. In addition, both of them show a strong fluorescence emission at 511 nm. To study the behavior of 1 and 2 in the presence of Cu(II) and Cu(I), the corresponding UV-vis and fluorescence emission spectra in THF/H2O (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
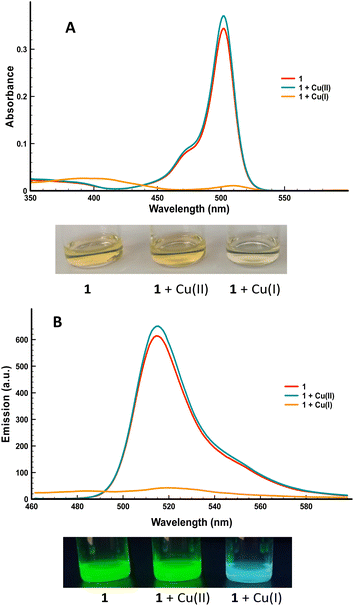
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) were registered. The UV-vis spectra of both probes did not show any appreciable changes in the presence of 1 equiv. of Cu(AcO)2 neither at room temperature nor under reflux. However, different results were observed when CuBr (1 equiv.) was added to the solution of 1 or 2. When the solutions were left at room temperature, no changes were observed in the corresponding spectra. However, when the solutions of the dyes and Cu(I) were heated to reflux for 2–3 minutes, strong changes were observed in both, the absorption and the emission spectra of the solutions (see Fig. 1 for compound 1).
5) were registered. The UV-vis spectra of both probes did not show any appreciable changes in the presence of 1 equiv. of Cu(AcO)2 neither at room temperature nor under reflux. However, different results were observed when CuBr (1 equiv.) was added to the solution of 1 or 2. When the solutions were left at room temperature, no changes were observed in the corresponding spectra. However, when the solutions of the dyes and Cu(I) were heated to reflux for 2–3 minutes, strong changes were observed in both, the absorption and the emission spectra of the solutions (see Fig. 1 for compound 1).
Fig. 1 shows the changes observed in the UV-vis and emission spectra of compound 1, after refluxing the solution for 2 min in the presence of Cu(I) or Cu(II) (for compound 2 see ESI†). As can be seen in Fig. 1A, no changes in the UV-vis absorption spectrum of 1 were observed in the presence of Cu(II), whereas the characteristic absorption band of the BODIPY core at ca. 500 nm disappeared when the solution was heated for 2 min in presence of Cu(I). This change could be observed naked-eye as a loss of color of the solution. Additionally, both cations induced different behavior in the corresponding fluorescence spectra (Fig. 1B): again Cu(II) did not induce any change, whereas Cu(I) gave rise to a total quenching of the fluorescence emission of the dye.
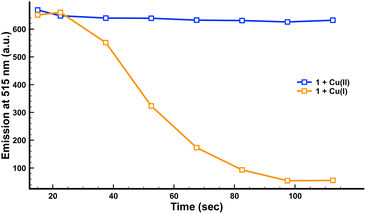
The evolution with time of the absorption and emission spectra of the dyes, with both copper cations, was studied to establish the optimal experimental conditions (see Fig. 2 for fluorescence studies with compound 1; for UV data of compound 1 and UV and fluorescence data of compound 2, see ESI†).
As can be seen in Fig. 2, 1 did not show any significant changes in its fluorescence intensity in the presence of Cu(II) over this two-minute period. By contrast, in the presence of Cu(I) a clear quenching of the fluorescence was observed after less than two minutes. The same behaviour was observed for compound 2 in the presence of Cu(I).
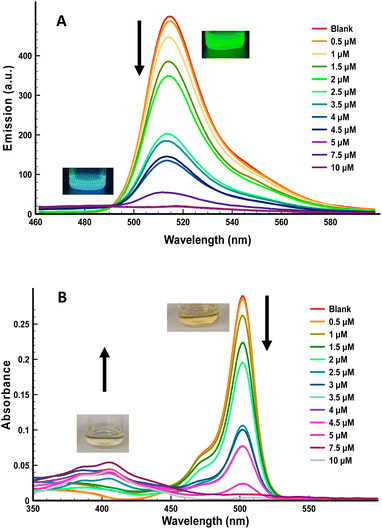
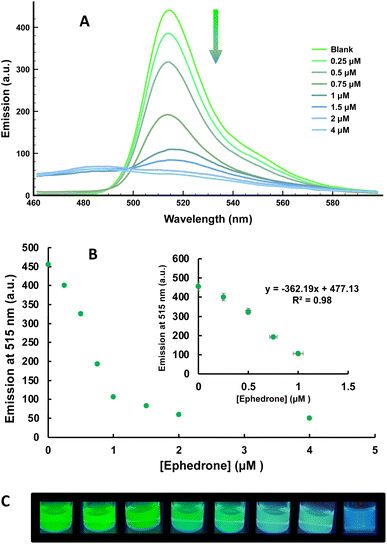
Based on this behavior and on the ability of cathinones to reduce Cu(II) to Cu(I) we decided to explore the chromogenic and fluorescent response of THF solutions of 1 and 2 containing 1 equiv. of Cu(II), in the presence of increasing amounts of ephedrone, as a model of a SC. As can be observed in Fig. 3 for compound 1 (for compound 2, see ESI†), important changes in both, the UV-vis and the fluorescence spectra of the dye were observed in the presence of ephedrone, which can be ascribed to the reduction of Cu(II) to Cu(I) by the SC. These changes in the absorption and emission spectra of the dye + Cu(II) showed a linear dependence with the amount of added ephedrone, which allows the system to be used as a sensor for this drug. In addition, these changes can be observed naked eye, either under natural light or under a common UV lamp (Fig. 3). Control experiments heating a solution of compound 1 with this cathinone, in absence of Cu(II), did not show changes neither in its UV-vis nor in its fluorescence spectra (see ESI†).
From the titration experiments carried out, the LoD could be determined using the expression: LoD=(3·Sb)/m, where Sb is the blank standard deviation and m the slope. The value determined from fluorescence measurements was 0.05 μM of ephedrone for compound 1 (Fig. S8†). This value is considerably lower than the amount necessary to induce severe effects in drinks.15
At this point we considered interesting to get some insight into the mechanism of the sensing process. Regarding the spectroscopic behavior, we observed the complete disappearance of the characteristic BODIPY core UV-vis absorption band at ca. 501 when solutions of compounds 1 or 2 (50 μM in THF: water 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
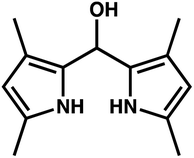
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 solution) where refluxed for 2 min, either in the presence of Cu(I) or in the presence of a mixture of Cu(AcO)2 and SC. Concomitantly, a new, much less intense band at 405 nm appear. This fact suggests that a new compound with a lower conjugation is generated, which also explains the observed quenching of the fluorescence emission of the dye. To gain information about this compound, these solutions were studied by HRMS. The major peak in the mass spectra of solutions of either 1 or 2 and Cu(II) in the presence of ephedrone, was observed at m/z 235.169 ([M + NH3]+) and was attributed to compound 3 (Fig. 4). This dipyrromethane derivative 3 would explain both the hypsochromic shift in the absorption spectra and the quenching of the fluorescence.
5 solution) where refluxed for 2 min, either in the presence of Cu(I) or in the presence of a mixture of Cu(AcO)2 and SC. Concomitantly, a new, much less intense band at 405 nm appear. This fact suggests that a new compound with a lower conjugation is generated, which also explains the observed quenching of the fluorescence emission of the dye. To gain information about this compound, these solutions were studied by HRMS. The major peak in the mass spectra of solutions of either 1 or 2 and Cu(II) in the presence of ephedrone, was observed at m/z 235.169 ([M + NH3]+) and was attributed to compound 3 (Fig. 4). This dipyrromethane derivative 3 would explain both the hypsochromic shift in the absorption spectra and the quenching of the fluorescence.
The generation of compound 3 would involve different transformations on the BODIPY dye: elimination of the aryl substituent at the meso position, incorporation of a water molecule in this position, and elimination of the BF2 bridge. A tentative mechanism for the formation of this compound would start with an electron transfer from Cu(I) to the BODIPY core to generate a radical-anion, followed by homolytic cleavage at the meso position leading to a BODIPY anion and an aryl radical. In this sense, Chattopadhyay reported the photochemical removal of the phenyl group in 8-phenyl BODIPY through a homolytic mechanism.16 The involvement of free radicals in the sensing process was demonstrated by carrying out the reaction in presence of hydroquinone. Under these conditions the reaction did not take place. With respect to the BF2 removal, Ravikanth et al. reported the Lewis acid assisted decomplexation of F-BODIPYs to dipyrrins.17 Thompson et al. also reported the obtention of free-base dipyrrins from F-BODIPYs using KOH in a t-BuOH/H2O mixture. In this case, the potassium cation assisted the substitution of the fluoride by the alkoxide generated in situ.18,19 We can speculate that, in our case the copper cation would assist the substitution of both fluorides by water molecules or solvent molecules, in a similar way as the potassium cation or the Lewis acid, leading to the corresponding free-base dipyrrin. Finally, a conjugated addition of water at the meso position, probably also catalysed by the copper cation, would lead to compound 3. Further theoretical studies to support this mechanism will be carried out.
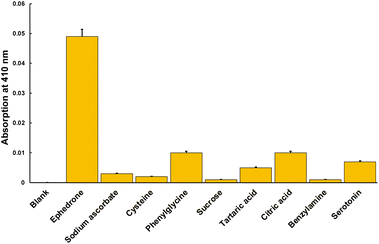
To explore the possibility of applying this method in the near future, to the detection of cathinones in different matrices, such as beverages or oral fluid, several possible interferents that could be found in these matrices where evaluated. We included acids (tartaric and citric acids), thiols (cysteine), reducing species (sodium ascorbate) and sugars (sucrose). Amines (phenylglycine, benzylamine, serotonin) were also studied due to the similarity of their structure to cathinones. As can be seen in the Fig. 4, the response towards ephedrone is around 5 times higher than that of the interferents (Fig. 5).
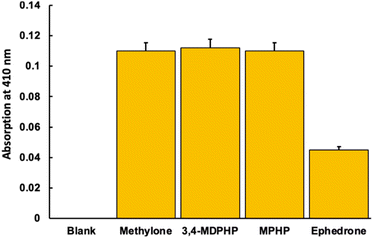
Importantly, other common SCs were also studied in order to confirm the versatility of the method. Thus, measurements with methylone, 3,4-MDPHP and MPHP were carried out. The obtained results showed that the probe was able to detect the presence of these SCs, frequently used as abuse drugs, with even better sensibility than for ephedrone (Fig. 6).
As a proof of the applicability of the method, we decided to explore the utility of the sensor to detect cathinones in oral fluid. The possibility of detecting these drugs in oral fluid in a rapid and easy way, would be of great importance for preventing traffic accidents. For this purpose, saliva samples were spiked with ephedrone (5 mM). Pure saliva was also used for comparison (Blank). Fluorescence titration experiments with increasing amounts of ephedrone (Fig. 7) were carried out and a linear response in the 0–1 μM concentration range was observed, allowing to appreciate the presence of SC by naked eye at concentrations above 1 μM (Fig. 7C). A limit of detection of 0.16 μM (32 ng mL−1) for ephedrone was obtained for probe 1 + Cu(II), which is lower than the expected concentration in oral fluid after their consumption (ca. 150–300 ng L−1).20 We noticed that the experiments in saliva samples had even better response than in water samples. Recovery experiments carried out with cathinone in saliva are summarized in Table S1† resulting in around 90% recovery (see ESI†).
Conclusions
Two new probes based on a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of a meso-aryl BODIPY dye and Cu(II) have been prepared and their ability to detect the presence of SCs in water and in oral fluid has been studied. Upon heating for 2 min the probe in the presence of cathinones, strong changes in the UV-vis and the emission spectra of the probes are observed. This effect seems to be related to the ability of cathinones to reduce Cu(II) to Cu(I) and to the structural changes induced by Cu(I) on the BODIPY core under the experimental conditions. In fact, the same changes in the UV-vis and fluorescence spectra of the BODIPYs were observed either in the presence of Cu(I) or in the presence of cathinone and Cu(II). A LoD for ephedrone of 0.05 μM in water and 0.16 μM (32 ng mL−1) in oral fluid was determined for probe 1 + Cu(II). Also a visual LOD of ca. 1 μM ephedrone in oral fluid could be observed. The applicability of the probe for the detection of other related synthetic cathinones such as methylone, 3,4-MDPHP and MPHP has been demonstrated, along with a low interference from compounds that may be present in different matrices. All this paves the way for the use of this methodology in the development of rapid tests to detect the presence of cathinones in complex matrices such as oral fluid or even beverages.
1 mixture of a meso-aryl BODIPY dye and Cu(II) have been prepared and their ability to detect the presence of SCs in water and in oral fluid has been studied. Upon heating for 2 min the probe in the presence of cathinones, strong changes in the UV-vis and the emission spectra of the probes are observed. This effect seems to be related to the ability of cathinones to reduce Cu(II) to Cu(I) and to the structural changes induced by Cu(I) on the BODIPY core under the experimental conditions. In fact, the same changes in the UV-vis and fluorescence spectra of the BODIPYs were observed either in the presence of Cu(I) or in the presence of cathinone and Cu(II). A LoD for ephedrone of 0.05 μM in water and 0.16 μM (32 ng mL−1) in oral fluid was determined for probe 1 + Cu(II). Also a visual LOD of ca. 1 μM ephedrone in oral fluid could be observed. The applicability of the probe for the detection of other related synthetic cathinones such as methylone, 3,4-MDPHP and MPHP has been demonstrated, along with a low interference from compounds that may be present in different matrices. All this paves the way for the use of this methodology in the development of rapid tests to detect the presence of cathinones in complex matrices such as oral fluid or even beverages.
Author contributions
Conceptualization: A. M. C., P. G., J. A. S.; methodology: J. H. C., M. P., S. G., P. A., F. S.; investigation: J. H. C., P. A.; funding acquisition: P. G., A. M. C., R. M. M.; supervision: P. G., A. M. C., J. A. S; writing original draft: J. H. C., A. M. C., M. P., S. G.; writing-review & editing: P. G., J. A. S., R. M. M., F. S.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the Spanish Government, MCIN/AEI/10.13039/501100011033 and “ERDF A way of making Europe” (fund RTL2018-100910-B-C42), the Spanish Ministry of Health, Consumer Affairs and Social Welfare (Project 2020I040 PNSD 2020) and Generalitat Valenciana (PROMETEO 2018/024) for financial support. SCSIE (Universitat de València) is gratefully acknowledged for all the equipment employed. NMR was registered at the U26 facility of ICTS “NAMBIOSIS” at the Universitat of València.Notes and references
- J. P. Smith, O. B. Sutcliffe and C. E. Banks, Analyst, 2015, 140, 4932, 10.1039/c5an00797f.
- J. Soares, V. M. Costa, M. de L. Bastos, F. Carvalho and J. P. Capela, Archives of Toxicology, 2021, 95, 2895, DOI:10.1007/s00204-021-03083-3Z.
- K. Kesha, C. L. Boggs, M. G. Ripple, C. H. Allan, B. Levine, R. Jufer-Phipps, S. Doyon, P. L. Chi and D. R. Fowler, J. Forensic Sci., 2013, 58, 1654, DOI:10.1111/1556-4029.
- B. Correia, J. Fernandes, M. J. Botica, C. Ferreira and A. Quintas, Medicines, 2022, 9, 19, DOI:10.3390/medicines9030019.
- N. M. Morato, V. Pirro, P. W. Fedick and R. G. Cooks, Anal. Chem., 2019, 91, 7450, DOI:10.1021/acs.analchem.9b01637.
- Y. Yan, L. Jiang, S. Zhang, X. Shen and C. Huang, Biosens. Bioelectron., 2022, 205, 114113, DOI:10.1016/j.bios.2022.114113.
- Y.-T. Yen, T.-Y. Chen, C.-Y. Chen, C.-L. Chang, S.-C. Chyueh and H.-T. Chang, Sensors, 2019, 19, 3554, DOI:10.3390/s19163554.
- Y.-T. Yen, Y.-S. Lin, T.-Y. Chen, S.-C. Chyueh and H.-T. Chang, R. Soc. Open Sci., 2019, 6, 191017, DOI:10.1098/rsos.191017.
- Y. Luo, H. Yu, O. Alkhamis, Y. Liu, X. Lou, B. Yu and Y. Xiao, Anal. Chem., 2019, 91, 7199, DOI:10.1021/acs.analchem.9b00507.
- A. M. Al-Obaid, S. A. Al-Tamrah, F. A. Aly and A. A. Alwarthan, J. Pharm. Biomed. Anal., 1998, 17, 321, DOI:10.1016/S0731-7085(97)00203-3.
- M. Philp, R. Shimmon, M. Tahtouh and S. Fu, Forensic Chem., 2016, 1, 39, DOI:10.1016/j.forc.2016.06.001.
- A. M. Costero, M. Parra, S. Gil and P. Gaviña, BODIPY Core as Signaling Unit in Chemosensor Design, Chapter 2 in “BODIPY Dyes-A Privilege Molecular Scaffold with Tunable Properties”, IntechOpen, ISBN 978-1-78985-082-6, 2019 Search PubMed.
- G. Ulrich, R. Ziessel and A. Harriman, Angew. Chem., Int. Ed., 2008, 47, 1184–1201, DOI:10.1002/anie.200702070.
- Y. Chen, J. Zhao, H. Guo and L. Xie, J. Org. Chem., 2012, 77, 2192, DOI:10.1021/jo202215x.
- A. Grela, L. Gautama and M. D. Coleb, Forensic Sci. Int., 2018, 292, 50, DOI:10.1016/j.forsciint.2018.08.034.
- S. Mula, A. K. Ray, M. Banerjee, T. Chaudhuri, K. Dasgupta and S. Chattopadhyay, J. Org. Chem., 2008, 73, 2146–2154, DOI:10.1021/jo702346s.
- V. Lakshmi, T. Chatterjee and M. Ravikanth, Eur. J. Org. Chem., 2014, 2105, DOI:10.1002/ejoc.201301662.
- D. A. Smithen, A. E. G. Baker, M. Offman, S. M. Crawford, T. S. Cameron and A. Thompson, J. Org. Chem., 2012, 77, 3439, DOI:10.1021/jo3002003.
- R. L. Gapare and A. Thompson, Chem. Commun., 2022, 58, 7351, 10.1039/d2cc02362h.
- E. Papaseit, E. Olesti, C. Pérez-Mañá, M. Torrens, F. Fonseca, M. Grifell, M. Ventura, R. de la Torre and M. Farré, Pharmaceuticals, 2021, 14, 100, DOI:10.3390/ph14020100.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ra05188e |
| This journal is © The Royal Society of Chemistry 2022 |