Open Access Article
Open Access ArticleA Ferrier glycosylation/cis-dihydroxylation strategy to synthesize Leishmania spp. lipophosphoglycan-associated βGal(1,4)Man disaccharide†
Dipesh Budhathokia,
Bhavesh Deore a,
M. G. Finnb and
Carlos A. Sanhueza
a,
M. G. Finnb and
Carlos A. Sanhueza *ab
*ab
aDepartment of Pharmaceutical Sciences, St. John's University, 8000 Utopia Parkway, Queens, NY 11439, USA. E-mail: chavezc@stjohns.edu
bSchool of Chemistry and Biochemistry, Georgia Institute of Technology, 901 Atlantic Drive, Atlanta, GA 30306, USA
First published on 4th October 2022
Abstract
The Galβ(1→4)Man disaccharide, found in the cell surface lipophosphoglycan (LPG) of Leishmania species, has been synthesized by a Ferrier glycosylation/cis-dihydroxylation strategy. This stereoselective method proved efficient for synthesizing the target saccharide in good yield. In addition, we prepared two clickable O-glycoside and phospho-glycoside versions of Galβ(1→4)Man to enable conjugation to protein carriers for further immunological and antibody-binding studies.
Introduction
Leishmaniasis is a poverty-associated parasitic disease that imposes a heavy burden on the public health systems of least developed countries, particularly those of South Asia, East Africa, and Latin America. The protozoan Leishmania spp. (Trypanosomatidae), the etiological agent of leishmaniasis, is a vector-borne obligate intracellular parasite transmitted by hematophagous sandflies. More than 20 human-infective Leishmania species have been described to date, together with 70 different types of sandflies (Phlebotomus and Lutzomyia) identified as vectors.1,2 Depending on the infecting Leishmania species, individuals can present one of the three disease phenotypes: self-resolvent cutaneous leishmaniasis (CL), disfiguring mucocutaneous leishmaniasis (MCL), or potentially fatal visceral leishmaniasis (VL).2 Although leishmaniasis is an underreported infection, current estimates for CL range from 700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 1.2 million worldwide cases each year, and yearly VL cases are about 100
000 to 1.2 million worldwide cases each year, and yearly VL cases are about 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000.3 Available anti-leishmaniasis treatments include pentavalent antimonials, liposomal amphotericin B, miltefosine, and paromomycin.4 These drugs are effective in treating leishmaniasis; however, side effects, poor patient compliance, and parasite resistance motivate the search for new and improved drugs. Moreover, the lack of an effective anti-leishmaniasis vaccine to prevent and treat the infection is a critical need.
000.3 Available anti-leishmaniasis treatments include pentavalent antimonials, liposomal amphotericin B, miltefosine, and paromomycin.4 These drugs are effective in treating leishmaniasis; however, side effects, poor patient compliance, and parasite resistance motivate the search for new and improved drugs. Moreover, the lack of an effective anti-leishmaniasis vaccine to prevent and treat the infection is a critical need.
A dense glycocalyx covers the Leishmania spp. cell membrane playing a pivotal role in the parasite's survival and infectivity. The chemical structure of this sugar coat varies according to the parasite species, its life cycle stage, and the host type.5 Fig. 1A shows the structure of Leishmania's lipophosphoglycan (LPG), the predominant membrane-glycan in promastigotes, the human-infective form.6 Its structure comprises a phosphatidylinositol anchor, a conserved oligosaccharide core, a linear phosphoglycan (PG), and a neutral mannose-rich oligosaccharide cap. The chemical structure of LPG varies among Leishmania species. The differences are typically found in the structures of the capping oligosaccharides and the type and sequence of the branching sugars connected to the conserved linear PG, a phospho-polysaccharide comprising the [-6)-β-D-Gal(1→4)α-D-Man-(1→PO3O] repeating unit. For example, in Leishmania donovani, the etiological agent of VL in the Old World, promastigotes PG is unsubstituted and remains linear.7 In contrast, in L. infantum (L. chagasi), the causative agent of New World's VL, PG carries Glcβ(1→3) substitutions.8
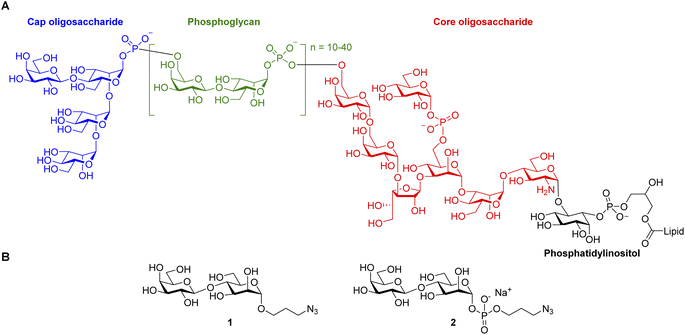 | ||
| Fig. 1 (A) Structure of Leishmania donovani lipophosphoglycan; (B) structures of the clickable Galβ(1→4)Man O- and phospho-glycosides 1 and 2 synthesized in this work. | ||
Given its role as an immune determinant, LPG is an attractive target for developing glycoconjugate vaccine candidates against leishmaniasis.9 The groups of Nikolaev, Vishwakarma, and Seeberger have accomplished the synthesis of different LPG fragments and proved the suitability of protein-conjugated LPG subunits as immunogens.10 However, despite the promising preliminary immunization results,11 an effective anti-leishmaniasis vaccine remains elusive.
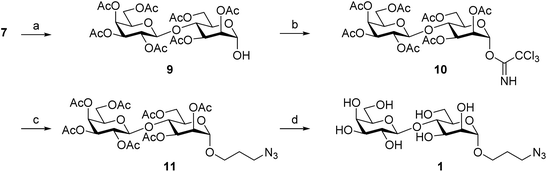
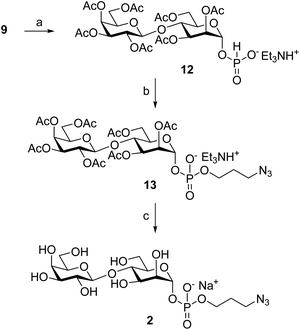
Because of our interest in anti-parasitic immunotherapies12 and the development of virus-like particles (VLP) glycoconjugates to boost anti-carbohydrate immune responses,13 we have centered our attention on LPG to explore VLP-conjugated anti-leishmaniasis vaccine candidates. Here, we report an expeditious synthetic route to prepare Galβ(1→4)Man, a disaccharide exclusively found in the linear phosphoglycan and the capping oligosaccharides of Leishmania's LPG. Two clickable versions of Galβ(1→4)Man (Fig. 1B), namely 3-azidopropyl 4-O-β-D-galactopyranosyl-α-D-mannopyranoside (1) and 3-azidopropyl 4-O-β-D-galactopyranosyl-α-D-mannopyranosyl phosphate (2), were prepared to enable conjugation to the protein carrier and subsequent performance of immunological studies.
Results and discussion
Synthesis of Galβ(1→4)Man disaccharide
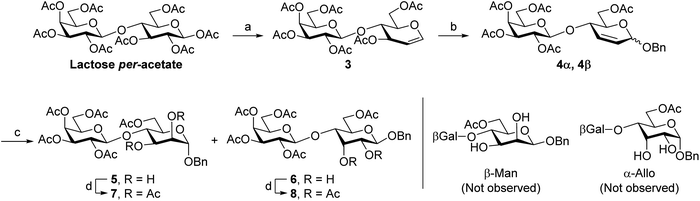
Scheme 1 shows the synthetic strategy to prepare Galβ(1→4)Man. We envisioned the combination of the Ferrier glycosylation14 and osmium-catalyzed cis-dihydroxylation,15 both high-yielding and stereoselective reactions, as a convenient sequence to synthesize the target disaccharide from the inexpensive and readily available starting material D-lactose per-acetate. First. we synthesized the Ferrier glycosyl donor hexa-O-acetyl-D-lactal 3 in 42% yield by adjusting the procedure of Zhao et al.16 which involves forming the lactosyl bromide from lactose per-acetate followed by reduction with Zn dust. The subsequent Ferrier glycosylation14,17 of benzyl alcohol with lactal 3, catalyzed by BF3·Et2O, afforded a mixture of 2,3-unsaturated benzyl glycosides 4α and 4β (α/β = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in 92% yield. The osmium-catalyzed cis-dihydroxylation of the anomeric mixture gave diols 5 (major product) and 6 in excellent yield, which were readily separated by normal-phase flash chromatography. The treatment of diols 5 and 6 with Ac2O in pyridine yielded the respective per-acetates 7 and 8. Given the convenient peak separation in their 1H NMR spectra, we performed the stereochemical configuration analysis of the oxidation adducts on their respective per-acetylated derivatives.
1) in 92% yield. The osmium-catalyzed cis-dihydroxylation of the anomeric mixture gave diols 5 (major product) and 6 in excellent yield, which were readily separated by normal-phase flash chromatography. The treatment of diols 5 and 6 with Ac2O in pyridine yielded the respective per-acetates 7 and 8. Given the convenient peak separation in their 1H NMR spectra, we performed the stereochemical configuration analysis of the oxidation adducts on their respective per-acetylated derivatives.
In the 1H NMR spectrum of acetate 7, derived from the major cis-dihydroxylation product 5, proton H3 (δ 5.32 ppm) appears as a doublet of doublets with coupling constants 3JH3–H4 and 3JH3–H2 of 8.8 and 3.6 Hz respectively.
The large value for the 3JH3–H4 unambiguously identifies a trans-diaxial correlation between H3 and H4. The anomeric proton H1 (δ 4.74 ppm) appears as a doublet with a small 3JH1–H2 (1.8 Hz). These attributes are consistent with an α-mannose configuration.18 In acetate 8, derived from the minor cis-dihydroxylation product 6, H3 (δ 5.76 ppm) appears as a triplet with 3JH3–H4 and 3JH3–H2 values of ∼2.5 Hz, indicating a cis correlation with H4. The proton H1 (δ 4.83 ppm) showed as a doublet with a large 3JH1–H2 value (8.3 Hz), confirming a β-allose configuration for the minor oxidation product.
The results show that the osmium-catalyzed cis-dihydroxylation of disaccharide derivatives 2,3-dideoxy-4-O-β-D-galactopyranosyl-D-erythro-hex-2-enopyranosides exhibit the same stereospecificity reported for the oxidation of the monosaccharide 2,3-dideoxy-D-erythro-hex-2-enopyranoside congeners: oxidation of 2,3-unsaturated α-glycosides leads to the formation of α-mannosides whereas the oxidation of the respective β-anomers yields β-allosides.19
Since the formation of the β-glycoside in the Ferrier glycosylation limits the yield of the target Galβ(1→4)Man disaccharide 5, we attempted to improve the α-stereoselectivity of this reaction by testing different solvents (MeCN, dioxane, THF, and Et2O), common Lewis acid catalysts (FeCl3,20 TMSOTf,21 and I2 (ref. 22)), and Lewis acids reported to afford high α-glycoside yields in Ferrier glycosylations (Y(OTf)3,23 Gd(OTf)3 (ref. 24)). However, in our hands, these attempts did not significantly improve the α-selectivity of this step using D-lactal 3 as a glycosyl donor.
Conclusions
We have synthesized the βGal(1→4)αMan disaccharide, the phospho-polysaccharide repeating unit found in the cell surface lipophosphoglycan of all Leishmania species. The Ferrier glycosylation/cis-dihydroxylation sequence proved efficient in affording the target βGal(1→4)Man core in a stereoselective fashion from readily available lactose per-acetate. Two clickable versions of βGal(1→4)Man, azide-functionalized O-glycoside and phospho-glycoside, were also prepared to enable coupling to protein carriers and immobilization onto surfaces for further immunological and binding studies.Experimental
General information
D-Lactose and all reagents were purchased to the highest purity available and used without previous purification. Yields refer to isolated yields after chromatographic purification and anomeric ratios were determined from the 1H NMR spectra of reaction crudes. Reactions were monitored by thin-layer chromatography (TLC) using SiliCycle SiliaPlate glass-backed TLC 250 mm with F-254 UV indicator. TLC visualization was performed under short wave UV light (254 nm) and charring with 5% H2SO4 in CH3OH. Normal phase flash chromatography was performed using SiliCycle SiliaFlash P60 silica gel (40–63 μm) and mixtures of EtOAc-hexanes and CH2Cl2–MeOH (ACS-grade solvents) as eluents. 1D and 2D NMR spectra were recorded on a Bruker Avance 400 spectrometer at 298 K. 1H NMR chemical shifts are reported in parts per million vs. tetramethylsilane (TMS) internal standard (δH 0.00 ppm). 13C{1H} NMR chemical shifts are reported in parts per million vs. the residual peak of the solvent.28 LCMS data were collected in a Waters e2695 HPLC system coupled to a Waters Acquity QDa single quadrupole mass detector. HRMS data for new compounds were collected in a Waters high-resolution Xevo G2-XS Q-ToF mass spectrometer coupled with an Acquity UPLC H-Class in the positive electrospray ionization mode.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), 1H NMR (500 MHz, CDCl3): 6.35 (dd, J = 1.0 and 6.1 Hz, H-1), 5.34 (t, J = 4.3 Hz, H-3), 5.30 (dd, J = 1.0 and 3.3 Hz, H-4′), 5.13 (dd, J = 7.9 and 10.5 Hz, H-2′), 4.94 (dd, J = 3.4 and 10.5 Hz, H-3′), 4.77 (dd, J = 3.4 and 6.1 Hz, H-2), 4.59 (d, J = 8.0 Hz, H-1′), 4.37 (dd, J = 2.6 and 11.7 Hz, H-6), 4.13 (dd, J = 6.2 and 11.7 Hz, H-6), 4.08 (m, H-6′, H-5), 4.01 (dd, J = 7.3 and 11.2 Hz, H-6′), 3.93 (dd, J = 5.4 and 7.4 Hz, H-4), 3.84 (ddd, J = 1.0, 6.0 and 7.0 Hz, H-5′), 2.09 (s, 3H), 2.05 (s, 3H), 2.02 (s, 3H), 1.99 (s, 3H), 1.98 (s, 3H), 1.91 (s, 3H); APT 13C{1H} NMR (125 MHz, CDCl3): 170.4, 170.4, 170.2, 170.1, 169.9, 169.3, 145.4 (C-1), 101.0 (C-1′), 98.9 (C-2), 74.6 (C-4), 74.1 (C-5), 70.8 (C-3′), 70.7 (C-5′), 68.8 (C-3), 68.8 (C-4), 66.6 (C-4′), 61.8 (C-6), 60.9 (C-6′), 21.1, 20.8, 20.6, 20.6, 20.6, 20.5.
2), 1H NMR (500 MHz, CDCl3): 6.35 (dd, J = 1.0 and 6.1 Hz, H-1), 5.34 (t, J = 4.3 Hz, H-3), 5.30 (dd, J = 1.0 and 3.3 Hz, H-4′), 5.13 (dd, J = 7.9 and 10.5 Hz, H-2′), 4.94 (dd, J = 3.4 and 10.5 Hz, H-3′), 4.77 (dd, J = 3.4 and 6.1 Hz, H-2), 4.59 (d, J = 8.0 Hz, H-1′), 4.37 (dd, J = 2.6 and 11.7 Hz, H-6), 4.13 (dd, J = 6.2 and 11.7 Hz, H-6), 4.08 (m, H-6′, H-5), 4.01 (dd, J = 7.3 and 11.2 Hz, H-6′), 3.93 (dd, J = 5.4 and 7.4 Hz, H-4), 3.84 (ddd, J = 1.0, 6.0 and 7.0 Hz, H-5′), 2.09 (s, 3H), 2.05 (s, 3H), 2.02 (s, 3H), 1.99 (s, 3H), 1.98 (s, 3H), 1.91 (s, 3H); APT 13C{1H} NMR (125 MHz, CDCl3): 170.4, 170.4, 170.2, 170.1, 169.9, 169.3, 145.4 (C-1), 101.0 (C-1′), 98.9 (C-2), 74.6 (C-4), 74.1 (C-5), 70.8 (C-3′), 70.7 (C-5′), 68.8 (C-3), 68.8 (C-4), 66.6 (C-4′), 61.8 (C-6), 60.9 (C-6′), 21.1, 20.8, 20.6, 20.6, 20.6, 20.5.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), 1H NMR (500 MHz, CDCl3): 7.35–7.26 (m, 5H), 6.11 (d, J = 10.2 Hz, H-3), 5.78 (ddd, J = 2.2, 2.2 and 10.2 Hz, H-2), 5.39 (d, J = 2.7 Hz, H-4′), 5.21 (dd, J = 8.0 and 10.4 Hz, H-2′), 5.08 (d, J = 2.2 Hz, H-1), 5.00 (dd, J = 3.4 and 10.4 Hz, H-3′), 4.79 (d, J = 11.8 Hz, OC
3), 1H NMR (500 MHz, CDCl3): 7.35–7.26 (m, 5H), 6.11 (d, J = 10.2 Hz, H-3), 5.78 (ddd, J = 2.2, 2.2 and 10.2 Hz, H-2), 5.39 (d, J = 2.7 Hz, H-4′), 5.21 (dd, J = 8.0 and 10.4 Hz, H-2′), 5.08 (d, J = 2.2 Hz, H-1), 5.00 (dd, J = 3.4 and 10.4 Hz, H-3′), 4.79 (d, J = 11.8 Hz, OC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2Ph), 4.57 (d, J = 8.9 Hz, H-1′), 4.58 (d, J = 11.8 Hz, OC
2Ph), 4.57 (d, J = 8.9 Hz, H-1′), 4.58 (d, J = 11.8 Hz, OC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2Ph), 4.20 (m, H-4, H-6′, H-6 and H-6), 4.11 (m, H-6′ and H-5), 3.92 (dd, J = 6.1 and 6.1 Hz, H-5′), 2.16 (s, 3H), 2.16 (s, 3H), 2.08 (s, 3H), 2.04 (s, 3H), 1.98 (s, 3H); APT 13C{1H} NMR (125 MHz, CDCl3): 170.7, 170.4, 170.3, 170.1, 169.4, 137.7, 131.6 (C-3), 128.4 (2C), 127.9 (2C), 127.8, 127.1 (C-2), 102.2 (d, C-1′), 93.6 (d, C-1), 73.4, 70.9 (C-3′), 70.8 (C-5′), 70.1 (O
2Ph), 4.20 (m, H-4, H-6′, H-6 and H-6), 4.11 (m, H-6′ and H-5), 3.92 (dd, J = 6.1 and 6.1 Hz, H-5′), 2.16 (s, 3H), 2.16 (s, 3H), 2.08 (s, 3H), 2.04 (s, 3H), 1.98 (s, 3H); APT 13C{1H} NMR (125 MHz, CDCl3): 170.7, 170.4, 170.3, 170.1, 169.4, 137.7, 131.6 (C-3), 128.4 (2C), 127.9 (2C), 127.8, 127.1 (C-2), 102.2 (d, C-1′), 93.6 (d, C-1), 73.4, 70.9 (C-3′), 70.8 (C-5′), 70.1 (O![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H2Ph), 68.8 (C-2′), 67.6 (C-5), 66.9 (C-4′), 63.0 (C-6), 61.3 (C-6′), 20.9, 20.7, 20.7, 20.7, 20.6
H2Ph), 68.8 (C-2′), 67.6 (C-5), 66.9 (C-4′), 63.0 (C-6), 61.3 (C-6′), 20.9, 20.7, 20.7, 20.7, 20.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and N-methylmorpholine N-oxide (1.7 g, 12.5 mmol), were dissolved in acetone (40 mL) at room temperature. Then, OsO4 (1.3 ml, 4% sol. in water, 0.20 mmol) was added and the system was maintained for 12 hours at room temperature under vigorous stirring. After that period, TLC showed total consumption of the starting olefin and the predominance of two more polar spots. Then, the volatiles were removed in rotary evaporator and the resulting syrup was purified by normal phase flash chromatography using an isocratic EtOAc/n-Hex (7
1) and N-methylmorpholine N-oxide (1.7 g, 12.5 mmol), were dissolved in acetone (40 mL) at room temperature. Then, OsO4 (1.3 ml, 4% sol. in water, 0.20 mmol) was added and the system was maintained for 12 hours at room temperature under vigorous stirring. After that period, TLC showed total consumption of the starting olefin and the predominance of two more polar spots. Then, the volatiles were removed in rotary evaporator and the resulting syrup was purified by normal phase flash chromatography using an isocratic EtOAc/n-Hex (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) elution system. Chromatographic separation afforded the diol 5 (3.6 g, 5.6 mmol, 53%) as a white foam, the diol 6 (758.3 mg, 1.18 mmol, 11%) as a white foam, and a mixed fraction of 5 and 6 (1.83 g, 5/6 = 12
3) elution system. Chromatographic separation afforded the diol 5 (3.6 g, 5.6 mmol, 53%) as a white foam, the diol 6 (758.3 mg, 1.18 mmol, 11%) as a white foam, and a mixed fraction of 5 and 6 (1.83 g, 5/6 = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The combined isolated yield for this reaction was 92%. Physical data for major derivative 5 (Galβ(1→4)Man): Rf = 0.57 (EtOAc), 1H NMR (500 MHz, CDCl3): 7.30–7.22 (m, 5H), 5.33 (dd, J = 0.7 and 3.4 Hz, H-4′), 5.17 (dd, J = 8.0 and 10.5 Hz, H-2′), 4.93 (dd, J = 3.4 and 10.5, H-3′), 4.88 (d, J = 1.0 Hz, H-1), 4.64 (d, J = 11.8 Hz, 1H), 4.45 (d, J = 8.0 Hz, H-1′), 4.44 (d, J = 11.8 Hz, 1H), 4.22 (dd, J = 2.0 and 11.8 Hz, H-6′), 4.11 (dd, J = 4.8 and 11.6 Hz, H-6), 4.06 (dd, J = 7.6 and 11.3 Hz, H-6), 4.01 (dd, J = 5.5 and 11.7 Hz, H-6′), 4.03 (m, H-2 and H-5′), 3.94 (dd, J = 1.0 and 8.7 Hz, H-3), 3.96 (m, 2H, H-2 and H-5′), 3.88 (dd, J = 3.5 and 8.6 Hz, H-3), 3.82 (ddd, J = 1.6, 5.4, and 9.9 Hz, H-5), 3.67 (dd, J = 8.9 and 9.6 Hz, H-4), 2.10 (s, 3H), 2.06 (s, 3H), 2.03 (s, 3H), 2.02 (s, 3H), 1.91 (s, 3H); APT 13C{1H} NMR (125 MHz, CDCl3): 170.6, 170.2, 169.9, 169.8, 169.4, 136.6, 128.4, 127.9 (x 2Cs), 127.8 (x 2Cs), 101.7 (C-1′), 97.9 (C-1), 80.4, 71.3, 70.7, 70.1, 69.4, 69.2, 68.5, 67.7, 66.7, 62.8, 61.6, 20.8, 20.5, 20.4, 20.4, 20.3; HRMS (ESI/Q-TOF) m/z: [M + Na]+ calcd for C29H38NaO16 665.2058; found 665.2137; physical data for minor derivative 6 (Galβ(1→4)All): Rf = 0.48 (EtOAc/n-Hex, 4
1). The combined isolated yield for this reaction was 92%. Physical data for major derivative 5 (Galβ(1→4)Man): Rf = 0.57 (EtOAc), 1H NMR (500 MHz, CDCl3): 7.30–7.22 (m, 5H), 5.33 (dd, J = 0.7 and 3.4 Hz, H-4′), 5.17 (dd, J = 8.0 and 10.5 Hz, H-2′), 4.93 (dd, J = 3.4 and 10.5, H-3′), 4.88 (d, J = 1.0 Hz, H-1), 4.64 (d, J = 11.8 Hz, 1H), 4.45 (d, J = 8.0 Hz, H-1′), 4.44 (d, J = 11.8 Hz, 1H), 4.22 (dd, J = 2.0 and 11.8 Hz, H-6′), 4.11 (dd, J = 4.8 and 11.6 Hz, H-6), 4.06 (dd, J = 7.6 and 11.3 Hz, H-6), 4.01 (dd, J = 5.5 and 11.7 Hz, H-6′), 4.03 (m, H-2 and H-5′), 3.94 (dd, J = 1.0 and 8.7 Hz, H-3), 3.96 (m, 2H, H-2 and H-5′), 3.88 (dd, J = 3.5 and 8.6 Hz, H-3), 3.82 (ddd, J = 1.6, 5.4, and 9.9 Hz, H-5), 3.67 (dd, J = 8.9 and 9.6 Hz, H-4), 2.10 (s, 3H), 2.06 (s, 3H), 2.03 (s, 3H), 2.02 (s, 3H), 1.91 (s, 3H); APT 13C{1H} NMR (125 MHz, CDCl3): 170.6, 170.2, 169.9, 169.8, 169.4, 136.6, 128.4, 127.9 (x 2Cs), 127.8 (x 2Cs), 101.7 (C-1′), 97.9 (C-1), 80.4, 71.3, 70.7, 70.1, 69.4, 69.2, 68.5, 67.7, 66.7, 62.8, 61.6, 20.8, 20.5, 20.4, 20.4, 20.3; HRMS (ESI/Q-TOF) m/z: [M + Na]+ calcd for C29H38NaO16 665.2058; found 665.2137; physical data for minor derivative 6 (Galβ(1→4)All): Rf = 0.48 (EtOAc/n-Hex, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 1H NMR (400 MHz, CDCl3): 7.35 (m, 5H), 5.39 (d, J = 2.8 Hz, H-4′), 5.24 (dd, J = 8.2 and 10.3 Hz, H-2′), 5.01 (dd, J = 3.3 and 10.5 Hz, H-3′), 4.91 (d, J = 11.8 Hz, 1H, PhCH2O), 4.73 (d, J = 7.8 Hz, H-1), 4.64 (d, J = 11.8 Hz, 1H, PhCH2O), 4.57 (d, J = 8.0 Hz, H-1′), 4.36 (d, J = 2.1 Hz, H-3), 4.32 (dd, J = 1.6 and 12.2 Hz, H-6), 4.21 (dd, J = 7.8 and 11.2 Hz, H-6′), 4.06 (m, 2H, H-6′, H-6), 3.96 (m, 2H, H-5, H-5′), 3.64 (dd, J = 2.3 and 9.5 Hz, H-4), 3.51 (ddd, J = 3.0, 7.6, and 10.0 Hz, H-2), 2.78 (s, 1H, OH), 2.59 (d, J = 7.6 Hz, 1H, OH) 2.17 (s, 3H), 2.11 (s, 3H), 2.08 (s, 3H), 2.07 (s, 3H), 1.99 (s, 3H); APT 13C{1H} NMR (100 MHz, CDCl3): 170.7, 170.5, 170.1, 170.0, 169.5, 137.1, 128.5, 128.1, 127.9, 101.8 (C-1′), 99.5 (C-1), 78.1 (C-4), 71.4 (C-5′), 71.2 (O
1), 1H NMR (400 MHz, CDCl3): 7.35 (m, 5H), 5.39 (d, J = 2.8 Hz, H-4′), 5.24 (dd, J = 8.2 and 10.3 Hz, H-2′), 5.01 (dd, J = 3.3 and 10.5 Hz, H-3′), 4.91 (d, J = 11.8 Hz, 1H, PhCH2O), 4.73 (d, J = 7.8 Hz, H-1), 4.64 (d, J = 11.8 Hz, 1H, PhCH2O), 4.57 (d, J = 8.0 Hz, H-1′), 4.36 (d, J = 2.1 Hz, H-3), 4.32 (dd, J = 1.6 and 12.2 Hz, H-6), 4.21 (dd, J = 7.8 and 11.2 Hz, H-6′), 4.06 (m, 2H, H-6′, H-6), 3.96 (m, 2H, H-5, H-5′), 3.64 (dd, J = 2.3 and 9.5 Hz, H-4), 3.51 (ddd, J = 3.0, 7.6, and 10.0 Hz, H-2), 2.78 (s, 1H, OH), 2.59 (d, J = 7.6 Hz, 1H, OH) 2.17 (s, 3H), 2.11 (s, 3H), 2.08 (s, 3H), 2.07 (s, 3H), 1.99 (s, 3H); APT 13C{1H} NMR (100 MHz, CDCl3): 170.7, 170.5, 170.1, 170.0, 169.5, 137.1, 128.5, 128.1, 127.9, 101.8 (C-1′), 99.5 (C-1), 78.1 (C-4), 71.4 (C-5′), 71.2 (O![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H2Ph), 70.6 (C-2), 70.5 (C-3′), 69.6 (C-5), 69.5 (C-3), 68.4 (C-2′), 66.9 (C-4′), 63.2 (C-6), 61.4 (C-6′), 20.9, 20.6, 20.6, 20.5, 20.5; HRMS (ESI/Q-TOF) m/z: [M + Na]+ calcd for C29H38NaO16 665.2058; found 665.2141.
H2Ph), 70.6 (C-2), 70.5 (C-3′), 69.6 (C-5), 69.5 (C-3), 68.4 (C-2′), 66.9 (C-4′), 63.2 (C-6), 61.4 (C-6′), 20.9, 20.6, 20.6, 20.5, 20.5; HRMS (ESI/Q-TOF) m/z: [M + Na]+ calcd for C29H38NaO16 665.2058; found 665.2141.General procedure for acetylation
Diol, Ac2O (6.0 equiv.), and pyridine (3.0 equiv.) were dissolved in dry CH2Cl2 (5.0 mL mmol−1) at room temperature and stirred until TLC showed total consumption of the starting diol (∼15 min). Then, the mixture was cooled to 0 °C with an ice bath and the excess of Ac2O was quenched by addition of EtOH (30 equiv.). The volatiles were then removed in rotary evaporator and the residue was re-dissolved in EtOAc, transferred to a separation funnel, and washed with H2O followed by 1 M HCl, and NaHCO3. The organic layer was collected, dried over Na2SO4, filtered, and concentrated in rotary evaporator. The crude acetate was purified by normal phase flash chromatography using EtOAc/n-Hex (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 7
1 to 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) gradient as eluent system.
3) gradient as eluent system.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), 1H NMR (400 MHz, CDCl3): 7.31–7.22 (m, 5H), 5.32 (dd, J = 3.6 and 8.8 Hz, H-3), 5.27 (dd, J = 1.0 and 3.4 Hz, H-4′), 5.20 (dd, J = 1.9 and 3.6 Hz, H-2), 5.07 (dd, J = 7.9 and 10.5 Hz, H-2′), 4.89 (dd, J = 3.4 and 10.4 Hz, H-3′), 4.74 (d, J = 1.8 Hz, H-1), 4.62 (d, J = 11.9 Hz, 1H, OC
2), 1H NMR (400 MHz, CDCl3): 7.31–7.22 (m, 5H), 5.32 (dd, J = 3.6 and 8.8 Hz, H-3), 5.27 (dd, J = 1.0 and 3.4 Hz, H-4′), 5.20 (dd, J = 1.9 and 3.6 Hz, H-2), 5.07 (dd, J = 7.9 and 10.5 Hz, H-2′), 4.89 (dd, J = 3.4 and 10.4 Hz, H-3′), 4.74 (d, J = 1.8 Hz, H-1), 4.62 (d, J = 11.9 Hz, 1H, OC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2Ph), 4.48 (d, J = 7.9 Hz, H-1′), 4.46 (d, J = 11.9 Hz, 1H, OC
2Ph), 4.48 (d, J = 7.9 Hz, H-1′), 4.46 (d, J = 11.9 Hz, 1H, OC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2Ph), 4.29 (dd, J = 1.7 and 11.9 Hz, H-6a), 4.10 (m, 2H, H-6b and H-6a′), 3.96 (dd, J = 7.7 and 11.9 Hz, H-6b′), 3.86 (m, 2H, H-5 and H-4), 3.80 (dd, J = 1.0 and 7.7 Hz, H-5′), 2.08 (s, 3H), 2.07 (s, 3H), 2.04 (s, 3H), 1.99 (s, 3H), 1.98 (s, 3H), 1.97 (s, 3H), 1.90 (s, 3H); 13C{1H} NMR (100 MHz, CDCl3): 170.5, 170.4, 170.2, 170.1, 169.8, 169.3, 169.2, 136.2, 128.6 (×2C), 128.2 (×2C), 128.2, 101.1 (C-1), 96.4 (C-1), 74.0, 71.0, 70.4, 69.7, 69.6, 69.6, 69.2, 69.1, 66.6, 62.6, 60.8, 20.9, 20.8, 20.8, 20.6, 20.6, 20.6, 20.5; HRMS (ESI/Q-TOF) m/z: [M + Na]+ calcd for C33H42NaO18 749.2269; found 749.2399.
2Ph), 4.29 (dd, J = 1.7 and 11.9 Hz, H-6a), 4.10 (m, 2H, H-6b and H-6a′), 3.96 (dd, J = 7.7 and 11.9 Hz, H-6b′), 3.86 (m, 2H, H-5 and H-4), 3.80 (dd, J = 1.0 and 7.7 Hz, H-5′), 2.08 (s, 3H), 2.07 (s, 3H), 2.04 (s, 3H), 1.99 (s, 3H), 1.98 (s, 3H), 1.97 (s, 3H), 1.90 (s, 3H); 13C{1H} NMR (100 MHz, CDCl3): 170.5, 170.4, 170.2, 170.1, 169.8, 169.3, 169.2, 136.2, 128.6 (×2C), 128.2 (×2C), 128.2, 101.1 (C-1), 96.4 (C-1), 74.0, 71.0, 70.4, 69.7, 69.6, 69.6, 69.2, 69.1, 66.6, 62.6, 60.8, 20.9, 20.8, 20.8, 20.6, 20.6, 20.6, 20.5; HRMS (ESI/Q-TOF) m/z: [M + Na]+ calcd for C33H42NaO18 749.2269; found 749.2399.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), 1H NMR (400 MHz, CDCl3): 7.33 (m, 5H), 5.76 (t, J = 2.5 Hz, H-3), 5.33 (d, J = 2.6 Hz, H-4′), 5.15 (dd, J = 7.9 and 10.3 Hz, H-2′), 4.98 (dd, J = 3.2 and 10.4 Hz, H-3′), 4.89 (d, J = 12.1 Hz, 1H, OC
2), 1H NMR (400 MHz, CDCl3): 7.33 (m, 5H), 5.76 (t, J = 2.5 Hz, H-3), 5.33 (d, J = 2.6 Hz, H-4′), 5.15 (dd, J = 7.9 and 10.3 Hz, H-2′), 4.98 (dd, J = 3.2 and 10.4 Hz, H-3′), 4.89 (d, J = 12.1 Hz, 1H, OC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2Ph), 4.83 (d, J = 8.3 Hz, H-1), 4.77 (dd, J = 2.5 and 8.2 Hz, H-2), 4.63 (d, J = 12.1 Hz, 1H, OC
2Ph), 4.83 (d, J = 8.3 Hz, H-1), 4.77 (dd, J = 2.5 and 8.2 Hz, H-2), 4.63 (d, J = 12.1 Hz, 1H, OC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2Ph), 4.56 (d, J = 7.8 Hz, H-1′), 4.38 (d, J = 10.1 Hz, H-6), 4.03 (m, 4H, H-5, H-6′, H-6Gal, H-6′Gal), 3.91 (d, J = 6.4 and 6.4 Hz, H-5′), 3.80 (d, J = 2.5 and 9.3 Hz, H-4), 2.12 (s, 3H), 2.11 (s, 3H), 2.08 (s, 3H), 2.07 (s, 3H), 2.06 (s, 3H), 1.98 (s, 3H), 1.97 (s, 3H); 13C{1H} NMR (100 MHz, CDCl3): 170.6, 170.6, 170.1, 169.9, 169.6, 169.6, 169.5, 137.1, 128.3 (×2C), 127.8, 127.6 (×2C), 100.7 (C-1′), 97.4 (C-1), 74.9 (C-4), 70.9 (O
2Ph), 4.56 (d, J = 7.8 Hz, H-1′), 4.38 (d, J = 10.1 Hz, H-6), 4.03 (m, 4H, H-5, H-6′, H-6Gal, H-6′Gal), 3.91 (d, J = 6.4 and 6.4 Hz, H-5′), 3.80 (d, J = 2.5 and 9.3 Hz, H-4), 2.12 (s, 3H), 2.11 (s, 3H), 2.08 (s, 3H), 2.07 (s, 3H), 2.06 (s, 3H), 1.98 (s, 3H), 1.97 (s, 3H); 13C{1H} NMR (100 MHz, CDCl3): 170.6, 170.6, 170.1, 169.9, 169.6, 169.6, 169.5, 137.1, 128.3 (×2C), 127.8, 127.6 (×2C), 100.7 (C-1′), 97.4 (C-1), 74.9 (C-4), 70.9 (O![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H2Ph), 70.8 (C-5′), 70.7 (C-3′), 70.6 (C-5), 70.1 (C-2), 68.9 (C-3), 68.7 (C-2′), 66.8 (C-4′), 62.9 (C-6), 61.3 (C-6′), 20.8, 20.8, 20.6, 20.6, 20.6, 20.5, 20.5; HRMS (ESI/Q-TOF) m/z: [M + Na]+ calcd for C33H42NaO18 749.2269; found 749.2330.
H2Ph), 70.8 (C-5′), 70.7 (C-3′), 70.6 (C-5), 70.1 (C-2), 68.9 (C-3), 68.7 (C-2′), 66.8 (C-4′), 62.9 (C-6), 61.3 (C-6′), 20.8, 20.8, 20.6, 20.6, 20.6, 20.5, 20.5; HRMS (ESI/Q-TOF) m/z: [M + Na]+ calcd for C33H42NaO18 749.2269; found 749.2330.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β = 8
β = 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a colorless syrup. Physical data for major anomer (α): Rf = 0.62 (EtOAc/n-Hex, 19
1) as a colorless syrup. Physical data for major anomer (α): Rf = 0.62 (EtOAc/n-Hex, 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 1H NMR (400 MHz, CDCl3): 5.34 (dd, J = 3.5 and 9.5 Hz, H-3), 5.28 (d, J = 2.5 Hz, H-4′), 5.15 (dd, J = 1.5 and 3.3 Hz, H-2), 5.08 (d, J = 1.5 Hz, H-1), 5.05 (dd, J = 7.8 and 10.4 Hz, H-2′), 4.90 (dd, J = 3.3 and 10.5 Hz, H-3′), 4.49 (d, J = 7.9 Hz, H-1′), 4.38 (dd, J = 3.5 and 13.2 Hz, H-6Man), 4.10 (m, 3H, H-5, H-6Gal, and H-6′Man), 3.96 (dd, J = 7.6 and 11.0 Hz, H-6′Gal), 3.84 (m, 2H, H5′ and H4), 2.09 (s, 3H), 2.07 (s, 3H), 2.06 (s, 3H), 1.99 (s, 3H), 1.99 (s, 3H), 1.97 (s, 3H), 1.90 (s, 3H); 13C{1H} NMR (100 MHz, CDCl3): 170.8, 170.5, 170.3, 170.2, 170.1, 169.6, 169.3, 100.9 (C-1′), 91.8 (C-1), 74.2 (C-4), 70.9 (C-3′), 70.4 (C-5′), 70.3 (C-2), 69.2 (C-2′), 69.1 (C-3), 68.9 (C-5), 66.7 (C-4′), 62.6 (C-6), 60.9 (C-6′), 20.9, 20.9, 20.8, 20.6, 20.6, 20.6, 20.5; HRMS (ESI/Q-TOF) m/z: [M + Na]+ calcd for C26H36NaO18 659.1799; found 659.1939.
1), 1H NMR (400 MHz, CDCl3): 5.34 (dd, J = 3.5 and 9.5 Hz, H-3), 5.28 (d, J = 2.5 Hz, H-4′), 5.15 (dd, J = 1.5 and 3.3 Hz, H-2), 5.08 (d, J = 1.5 Hz, H-1), 5.05 (dd, J = 7.8 and 10.4 Hz, H-2′), 4.90 (dd, J = 3.3 and 10.5 Hz, H-3′), 4.49 (d, J = 7.9 Hz, H-1′), 4.38 (dd, J = 3.5 and 13.2 Hz, H-6Man), 4.10 (m, 3H, H-5, H-6Gal, and H-6′Man), 3.96 (dd, J = 7.6 and 11.0 Hz, H-6′Gal), 3.84 (m, 2H, H5′ and H4), 2.09 (s, 3H), 2.07 (s, 3H), 2.06 (s, 3H), 1.99 (s, 3H), 1.99 (s, 3H), 1.97 (s, 3H), 1.90 (s, 3H); 13C{1H} NMR (100 MHz, CDCl3): 170.8, 170.5, 170.3, 170.2, 170.1, 169.6, 169.3, 100.9 (C-1′), 91.8 (C-1), 74.2 (C-4), 70.9 (C-3′), 70.4 (C-5′), 70.3 (C-2), 69.2 (C-2′), 69.1 (C-3), 68.9 (C-5), 66.7 (C-4′), 62.6 (C-6), 60.9 (C-6′), 20.9, 20.9, 20.8, 20.6, 20.6, 20.6, 20.5; HRMS (ESI/Q-TOF) m/z: [M + Na]+ calcd for C26H36NaO18 659.1799; found 659.1939.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 1H NMR (400 MHz, CDCl3): 8.68 (s, 1H), 6.15 (d, J = 2.0 Hz, H-1), 5.37 (m, 2H, H-2, H-3), 5.29 (dd, J = 0.8 and 3.3 Hz, H-4′), 5.09 (dd, J = 8.0 and 10.5 Hz, H-2′), 4.91 (dd, J = 3.4 and 10.5 Hz, H-3′), 4.52 (d, J = 7.8 Hz, H-1′), 4.37 (dd, J = 1.9 and 11.9 Hz, H-6), 4.12 (dd, J = 6.1 and 11.1 Hz, H-6′), 4.10 (dd, J = 5.2 and 11.1 Hz, H-6), 4.05 (m, H-5), 3.97 (dd, J = 7.7 and 11.2 Hz, H-6′), 3.93 (dd, J = 9.6 and 9.6 Hz, H-4), 3.83 (dd, J = 7.4 and 7.4 Hz, H-5′), 2.11 (s, 3H), 2.09 (s, 3H), 2.04 (s, 3H), 1.99 (s, 3H), 1.98 (s, 3H), 1.98 (s, 3H), 1.91 (s, 3H). APT 13C{1H} NMR (100 MHz, CDCl3): 170.4, 170.4, 170.2, 170.1, 169.5, 169.3, 169.2, 160.0 (
1), 1H NMR (400 MHz, CDCl3): 8.68 (s, 1H), 6.15 (d, J = 2.0 Hz, H-1), 5.37 (m, 2H, H-2, H-3), 5.29 (dd, J = 0.8 and 3.3 Hz, H-4′), 5.09 (dd, J = 8.0 and 10.5 Hz, H-2′), 4.91 (dd, J = 3.4 and 10.5 Hz, H-3′), 4.52 (d, J = 7.8 Hz, H-1′), 4.37 (dd, J = 1.9 and 11.9 Hz, H-6), 4.12 (dd, J = 6.1 and 11.1 Hz, H-6′), 4.10 (dd, J = 5.2 and 11.1 Hz, H-6), 4.05 (m, H-5), 3.97 (dd, J = 7.7 and 11.2 Hz, H-6′), 3.93 (dd, J = 9.6 and 9.6 Hz, H-4), 3.83 (dd, J = 7.4 and 7.4 Hz, H-5′), 2.11 (s, 3H), 2.09 (s, 3H), 2.04 (s, 3H), 1.99 (s, 3H), 1.98 (s, 3H), 1.98 (s, 3H), 1.91 (s, 3H). APT 13C{1H} NMR (100 MHz, CDCl3): 170.4, 170.4, 170.2, 170.1, 169.5, 169.3, 169.2, 160.0 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif)
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH), 101.4 (C-1′), 94.5 (C-1), 90.5 (
NH), 101.4 (C-1′), 94.5 (C-1), 90.5 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) Cl3), 74.2 (C-4), 71.5 (C-5), 71.0 (C-3′), 70.5 (C-5′), 69.2 (C-3), 69.2 (C-2′), 67.9 (C-2), 66.5 (C-4′), 62.2 (C-6), 60.8 (C-6′), 20.8, 20.8, 20.8, 20.7, 20.7, 20.6, 20.6; HRMS (ESI/Q-TOF) m/z: [M + Na]+ calcd for C28H36Cl3NNaO18 802.0896; found 802.0851.
Cl3), 74.2 (C-4), 71.5 (C-5), 71.0 (C-3′), 70.5 (C-5′), 69.2 (C-3), 69.2 (C-2′), 67.9 (C-2), 66.5 (C-4′), 62.2 (C-6), 60.8 (C-6′), 20.8, 20.8, 20.8, 20.7, 20.7, 20.6, 20.6; HRMS (ESI/Q-TOF) m/z: [M + Na]+ calcd for C28H36Cl3NNaO18 802.0896; found 802.0851.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 to 3
7 to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) gradient as eluent system. Glycoside 11 (240.0 mg, 0.33 mmol, 70%) was obtained as a colorless oil. Rf = 0.43 (EtOAc/n-Hex, 7
2) gradient as eluent system. Glycoside 11 (240.0 mg, 0.33 mmol, 70%) was obtained as a colorless oil. Rf = 0.43 (EtOAc/n-Hex, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), 1H NMR (500 MHz, CDCl3): 5.28 (dd, J = 1.0 and 3.4 Hz, H-4′), 5.25 (dd, J = 3.6 and 9.1 Hz, H-3), 5.15 (dd, J = 1.9 and 3.6 Hz, H-2), 5.07 (dd, J = 7.9 and 10.5 Hz, H-2′), 4.90 (dd, J = 3.4 and 10.4 Hz, H-3′), 4.68 (d, J = 1.9 Hz, H-1), 4.48 (dd, J = 8.0 Hz, H-1′), 4.35 (dd, J = 1.2 and 11.9 Hz, H-6Man), 4.12 (dd, J = 6.0 and 11.1 Hz, H-6′Man), 4.11 (dd, J = 1.3 and 11.6 Hz, H-6Gal), 3.96 (dd, J = 7.8 and 11.1 Hz, H-6′Gal), 3.82 (m, 3H, H-4, H-5, and H-5′), 3.71 (m, 1H), 3.43 (m, 1H), 3.35 (t, J = 6.5 Hz, 2H), 2.09 (s, 3H), 2.07 (s, 3H), 2.06 (s, 3H), 2.00 (s, 3H), 1.99 (s, 3H), 1.97 (s, 3H), 1.91 (s, 3H), 1.79 (m, 2H); APT 13C{1H} NMR (125 MHz, CDCl3): 170.5, 170.4, 170.2, 170.1, 169.8, 169.3, 169.2, 101.2 (C-1′), 97.4 (C-1), 74.4, 70.9 (C-3′), 70.4, 69.6 (C-2), 69.4 (C-3), 69.2 (C-2′), 69.1, 66.6 (C-4′), 64.8 (O
3), 1H NMR (500 MHz, CDCl3): 5.28 (dd, J = 1.0 and 3.4 Hz, H-4′), 5.25 (dd, J = 3.6 and 9.1 Hz, H-3), 5.15 (dd, J = 1.9 and 3.6 Hz, H-2), 5.07 (dd, J = 7.9 and 10.5 Hz, H-2′), 4.90 (dd, J = 3.4 and 10.4 Hz, H-3′), 4.68 (d, J = 1.9 Hz, H-1), 4.48 (dd, J = 8.0 Hz, H-1′), 4.35 (dd, J = 1.2 and 11.9 Hz, H-6Man), 4.12 (dd, J = 6.0 and 11.1 Hz, H-6′Man), 4.11 (dd, J = 1.3 and 11.6 Hz, H-6Gal), 3.96 (dd, J = 7.8 and 11.1 Hz, H-6′Gal), 3.82 (m, 3H, H-4, H-5, and H-5′), 3.71 (m, 1H), 3.43 (m, 1H), 3.35 (t, J = 6.5 Hz, 2H), 2.09 (s, 3H), 2.07 (s, 3H), 2.06 (s, 3H), 2.00 (s, 3H), 1.99 (s, 3H), 1.97 (s, 3H), 1.91 (s, 3H), 1.79 (m, 2H); APT 13C{1H} NMR (125 MHz, CDCl3): 170.5, 170.4, 170.2, 170.1, 169.8, 169.3, 169.2, 101.2 (C-1′), 97.4 (C-1), 74.4, 70.9 (C-3′), 70.4, 69.6 (C-2), 69.4 (C-3), 69.2 (C-2′), 69.1, 66.6 (C-4′), 64.8 (O![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H2CH2CH2N3), 62.5 (C-6), 60.8 (C-6′), 48.1 (OCH2CH2
H2CH2CH2N3), 62.5 (C-6), 60.8 (C-6′), 48.1 (OCH2CH2![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H2N3), 28.6 (OCH2
H2N3), 28.6 (OCH2![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H2CH2N3), 20.9, 20.8, 20.8, 20.7, 20.6, 20.6, 20.5; HRMS (ESI/Q-TOF) m/z: [M + Na]+ calcd for C29H41N3NaO18 742.2283; found 742.2336.
H2CH2N3), 20.9, 20.8, 20.8, 20.7, 20.6, 20.6, 20.5; HRMS (ESI/Q-TOF) m/z: [M + Na]+ calcd for C29H41N3NaO18 742.2283; found 742.2336.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), 1H NMR (400 MHz, CD3OD): 4.78 (s, H-1), 4.36 (d, J = 7.4 Hz, H-1′), 3.90 (m, 4H), 3.81 (m, 4H), 3.73 (dd, J = 4.3 and 11.5 Hz, H-6), 3.63 (m, 2H), 3.53 (m, 3H), 3.41 (m, 2H), 1.88 (m, 2H); 13C{1H} NMR (100 MHz, CD3OD): 103.8 (C-1′), 100.0 (C-1), 76.9, 75.8, 73.4, 71.7, 71.2, 70.1, 69.8, 69.0, 64.1, 61.2, 60.7, 48.1, 28.5; HRMS (ESI/Q-TOF) m/z: [M + Na]+ calcd for C15H27N3NaO11 448.1543; found 448.1654.
4), 1H NMR (400 MHz, CD3OD): 4.78 (s, H-1), 4.36 (d, J = 7.4 Hz, H-1′), 3.90 (m, 4H), 3.81 (m, 4H), 3.73 (dd, J = 4.3 and 11.5 Hz, H-6), 3.63 (m, 2H), 3.53 (m, 3H), 3.41 (m, 2H), 1.88 (m, 2H); 13C{1H} NMR (100 MHz, CD3OD): 103.8 (C-1′), 100.0 (C-1), 76.9, 75.8, 73.4, 71.7, 71.2, 70.1, 69.8, 69.0, 64.1, 61.2, 60.7, 48.1, 28.5; HRMS (ESI/Q-TOF) m/z: [M + Na]+ calcd for C15H27N3NaO11 448.1543; found 448.1654.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 to 1
24 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) containing 1% Et3N as mobile phase. Purification afforded H-phosphonate 12 (409.1 mg, 0.50 mmol, 42%) as a white solid. Rf = 0.50 (MeOH/CH2Cl2, 1
9) containing 1% Et3N as mobile phase. Purification afforded H-phosphonate 12 (409.1 mg, 0.50 mmol, 42%) as a white solid. Rf = 0.50 (MeOH/CH2Cl2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), 1H NMR (400 MHz, CDCl3): 6.92 (d, J = 638 Hz, P–
4), 1H NMR (400 MHz, CDCl3): 6.92 (d, J = 638 Hz, P–![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) ), 5.47 (dd, 3JH1–H2 = 2.3 and 3JH–P = 10.5 Hz, H-1), 5.32 (dd, J = 3.4 and 9.5 Hz, H-3), 5.29 (d, J = 3.0 Hz, H-4′), 5.21 (m, H-2), 5.07 (dd, J = 7.4 and 10.0 Hz, H-2′), 4.89 (dd, J = 3.5 and 10.0 Hz, H-3′), 4.47 (d, J = 10.5 Hz, H-1′), 4.35 (dd, J = 3.2 and 12.0 Hz, H-6Man), 4.12 (m, 3H, H-5, H-6Gal, and H-6′Man), 3.96 (dd, J = 7.7 and 11.0 Hz, H-6′Gal), 3.84 (t, J = 9.3 Hz, H-5′), 3.82 (dd, J = 7.2 and 7.2 Hz, H-4), 3.02 (q, J = 7.3 Hz, 6H, N(C
), 5.47 (dd, 3JH1–H2 = 2.3 and 3JH–P = 10.5 Hz, H-1), 5.32 (dd, J = 3.4 and 9.5 Hz, H-3), 5.29 (d, J = 3.0 Hz, H-4′), 5.21 (m, H-2), 5.07 (dd, J = 7.4 and 10.0 Hz, H-2′), 4.89 (dd, J = 3.5 and 10.0 Hz, H-3′), 4.47 (d, J = 10.5 Hz, H-1′), 4.35 (dd, J = 3.2 and 12.0 Hz, H-6Man), 4.12 (m, 3H, H-5, H-6Gal, and H-6′Man), 3.96 (dd, J = 7.7 and 11.0 Hz, H-6′Gal), 3.84 (t, J = 9.3 Hz, H-5′), 3.82 (dd, J = 7.2 and 7.2 Hz, H-4), 3.02 (q, J = 7.3 Hz, 6H, N(C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2CH3)3), 2.09 (s, 3H), 2.06 (s, 3H), 2.05 (s, 3H), 1.99 (s, 3H), 1.98 (s, 3H), 1.94 (s, 3H), 1.91 (s, 3H), 1.30 (t, J = 7.3 Hz, 9H, N(CH2C
2CH3)3), 2.09 (s, 3H), 2.06 (s, 3H), 2.05 (s, 3H), 1.99 (s, 3H), 1.98 (s, 3H), 1.94 (s, 3H), 1.91 (s, 3H), 1.30 (t, J = 7.3 Hz, 9H, N(CH2C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3)3); 13C{1H} NMR (100 MHz, CDCl3): 170.6, 170.4, 170.2, 170.1, 169.8, 169.6, 169.1, 101.1 (C-1′), 92.6 (d, 2JC–P = 3.7 Hz, C-1), 74.0 (C-5′), 70.9 (C-3′), 70.4 (C-4), 70.1 (d, 3JC–P = 7.2 Hz, C-2), 69.9 (C-5), 69.4 (C-3), 69.1 (C-2′), 66.7 (C-4′), 62.4 (C-6), 60.8 (C-6′), 45.7 (N(
3)3); 13C{1H} NMR (100 MHz, CDCl3): 170.6, 170.4, 170.2, 170.1, 169.8, 169.6, 169.1, 101.1 (C-1′), 92.6 (d, 2JC–P = 3.7 Hz, C-1), 74.0 (C-5′), 70.9 (C-3′), 70.4 (C-4), 70.1 (d, 3JC–P = 7.2 Hz, C-2), 69.9 (C-5), 69.4 (C-3), 69.1 (C-2′), 66.7 (C-4′), 62.4 (C-6), 60.8 (C-6′), 45.7 (N(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H2CH3)3), 20.8, 20.8, 20.8, 20.6, 20.6, 20.6, 20.5, 8.6 (N(CH2
H2CH3)3), 20.8, 20.8, 20.8, 20.6, 20.6, 20.6, 20.5, 8.6 (N(CH2![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)3); 31P NMR (162 MHz, CDCl3): −0.10 (dd, 1JP–H = 638 Hz, 3JP–H1 = 9.8 Hz); HRMS (ESI/Q-TOF) m/z: [M − H]− calcd for C26H36O20P 699.1543; found 699.1545.
H3)3); 31P NMR (162 MHz, CDCl3): −0.10 (dd, 1JP–H = 638 Hz, 3JP–H1 = 9.8 Hz); HRMS (ESI/Q-TOF) m/z: [M − H]− calcd for C26H36O20P 699.1543; found 699.1545.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1.0 mL) was added. TLC showed the oxidation of H-phosphonate to phosphate completes in less than 30 minutes. The reaction was then quenched by addition of 10% Na2S2O3 (6.0 mL), transferred to a separation funnel, and extracted with CHCl3. The organic layer was then successively washed with brine, and 1 M TEAB buffer. The collected organic fraction was then dried over Na2SO4, filtered, and concentrated in rotary evaporator. The resulting crude was purified by normal phase flash chromatography using a MeOH/CH2Cl2 gradient (1
1, 1.0 mL) was added. TLC showed the oxidation of H-phosphonate to phosphate completes in less than 30 minutes. The reaction was then quenched by addition of 10% Na2S2O3 (6.0 mL), transferred to a separation funnel, and extracted with CHCl3. The organic layer was then successively washed with brine, and 1 M TEAB buffer. The collected organic fraction was then dried over Na2SO4, filtered, and concentrated in rotary evaporator. The resulting crude was purified by normal phase flash chromatography using a MeOH/CH2Cl2 gradient (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 to 1
99 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) containing 1% of Et3N. This procedure afforded the phosphodiester 13 (30.2 mg, 0.03 mmol, 30%) as a white solid. Rf = 0.5 (MeOH/CH2Cl2, 15
9) containing 1% of Et3N. This procedure afforded the phosphodiester 13 (30.2 mg, 0.03 mmol, 30%) as a white solid. Rf = 0.5 (MeOH/CH2Cl2, 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85), 1H NMR (400 MHz, CDCl3): 11.9 (br s, 1H, HN+(CH2CH3)3), 5.41 (dd, 3JH1–H2 = <1.0 Hz and 3JH1–P = 7.9 Hz, H-1), 5.31 (dd, J = 3.5 and 9.4 Hz, H-3), 5.28 (d, J = 3.3 Hz, H-4′), 5.24 (s, H-2), 5.07 (dd, J = 8.2 and 10.4 Hz, H-2′), 4.89 (dd, J = 3.2 and 10.4 Hz, H-3′), 4.47 (d, J = 8.0 Hz, H-1′), 4.36 (d, J = 10.9 Hz, H-6Man), 4.12 (m, 3H, H-5, H-6′Man, H-6Gal), 3.95 (dd, J = 7.9 and 11.0 Hz, H-6′Gal), 3.92 (m, 2H, OC
85), 1H NMR (400 MHz, CDCl3): 11.9 (br s, 1H, HN+(CH2CH3)3), 5.41 (dd, 3JH1–H2 = <1.0 Hz and 3JH1–P = 7.9 Hz, H-1), 5.31 (dd, J = 3.5 and 9.4 Hz, H-3), 5.28 (d, J = 3.3 Hz, H-4′), 5.24 (s, H-2), 5.07 (dd, J = 8.2 and 10.4 Hz, H-2′), 4.89 (dd, J = 3.2 and 10.4 Hz, H-3′), 4.47 (d, J = 8.0 Hz, H-1′), 4.36 (d, J = 10.9 Hz, H-6Man), 4.12 (m, 3H, H-5, H-6′Man, H-6Gal), 3.95 (dd, J = 7.9 and 11.0 Hz, H-6′Gal), 3.92 (m, 2H, OC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2CH2CH2N3), 3.87 (t, J = 9.7 Hz, H-4), 3.81 (dd, J = 6.8 and 8.0 Hz, H-5′), 3.37 (t, J = 6.9 Hz, OCH2CH2C
2CH2CH2N3), 3.87 (t, J = 9.7 Hz, H-4), 3.81 (dd, J = 6.8 and 8.0 Hz, H-5′), 3.37 (t, J = 6.9 Hz, OCH2CH2C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2N3), 3.04 (m, 6H, HN+(CH2CH3)3), 2.09 (s, 3H), 2.06 (s, 3H), 2.06 (s, 3H), 1.99 (s, 3H), 1.98 (s, 3H), 1.94 (s, 3H), 1.91 (s, 3H), 1.83 (m, 2H, OCH2C
2N3), 3.04 (m, 6H, HN+(CH2CH3)3), 2.09 (s, 3H), 2.06 (s, 3H), 2.06 (s, 3H), 1.99 (s, 3H), 1.98 (s, 3H), 1.94 (s, 3H), 1.91 (s, 3H), 1.83 (m, 2H, OCH2C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2CH2N3), 1.33 (t, J = 7.3 Hz, 9H, HN+(CH2CH3)3). APT 13C{1H} (100 MHz, CDCl3): 170.5, 170.4, 170.2, 170.1, 169.8, 169.6, 169.1, 101.1 (C-1′), 93.4 (d, 2JC1–P = 6.0 Hz, C-1), 73.8 (C-5′), 70.9 (C-3′), 70.4 (C-4), 69.8 (d, 2JC2–P = 6.8 Hz, C-2), 69.8 (C-5), 69.5 (C-3), 69.1 (C-2), 66.6 (C-4′), 62.7 (d, 2JC1–P = 5.3 Hz, OCH2CH2CH2N3), 62.5 (C-6), 60.8 (C-6′), 48.2 (OCH2CH2CH2N3), 45.8 (HN+(CH2CH3)3), 30.1 (d, 2JC–P = 7.4 Hz, OCH2CH2CH2N3), 20.9, 20.9, 20.8, 20.6, 20.6, 20.6, 20.5, 8.6 (HN+(CH2CH3)3); 31P NMR (162 MHz, CDCl3): −3.09 (d, 3JP–H1 = 6.9 Hz); HRMS (ESI/Q-TOF) m/z: [M − H]− calcd for C29H41N3O21P 798.1976, found 798.1972.
2CH2N3), 1.33 (t, J = 7.3 Hz, 9H, HN+(CH2CH3)3). APT 13C{1H} (100 MHz, CDCl3): 170.5, 170.4, 170.2, 170.1, 169.8, 169.6, 169.1, 101.1 (C-1′), 93.4 (d, 2JC1–P = 6.0 Hz, C-1), 73.8 (C-5′), 70.9 (C-3′), 70.4 (C-4), 69.8 (d, 2JC2–P = 6.8 Hz, C-2), 69.8 (C-5), 69.5 (C-3), 69.1 (C-2), 66.6 (C-4′), 62.7 (d, 2JC1–P = 5.3 Hz, OCH2CH2CH2N3), 62.5 (C-6), 60.8 (C-6′), 48.2 (OCH2CH2CH2N3), 45.8 (HN+(CH2CH3)3), 30.1 (d, 2JC–P = 7.4 Hz, OCH2CH2CH2N3), 20.9, 20.9, 20.8, 20.6, 20.6, 20.6, 20.5, 8.6 (HN+(CH2CH3)3); 31P NMR (162 MHz, CDCl3): −3.09 (d, 3JP–H1 = 6.9 Hz); HRMS (ESI/Q-TOF) m/z: [M − H]− calcd for C29H41N3O21P 798.1976, found 798.1972.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 to 1
99 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluent system. This procedure afforded the deprotected azido-derived Galβ(1→4)αMan phospho-glycoside 2 (33.7 mg, 0.06 mmol, 67%) as a white solid. 1H NMR (400 MHz, CD3OD): 5.34 (dd, 3JH1–H2 = 1.5 Hz, 3JH1–P = 7.9 Hz, H-1), 4.25 (d, J = 7.5 Hz, H-1′), 3.85 (m, 2H), 3.83 (m, 2H), 3.79 (m, 5H), 3.70 (m, 3H), 3.61 (dd, J = 4.1 and 11.5 Hz, H-6), 3.50 (dd, J = 4.1 and 7.3 Hz, 1H), 3.45 (dd, J = 7.6 and 9.7 Hz, 1H), 3.40 (d, J = 2.6 Hz, H-4′), 3.36 (m, 4H), 1.79 (m, 2H), 1.26 (t, J = 7.2 Hz, 3H); APT 13C{1H} (100 MHz, CD3OD): 103.9 (C-1′), 96.1 (d, 2JC1–P = 5.5 Hz, C-1), 76.6, 75.7, 73.3, 72.6, 71.1, 70.5 (d, JC–P = 8.6 Hz), 69.1, 69.0, 62.3 (d, JC–P = 5.8 Hz, OCH2CH2CH2N3), 61.3, 60.4, 52.2, 29.7 (d, JC–P = 7.6 Hz, OCH2CH2CH2N3); 31P NMR (162 MHz, CD3OD): −1.82 (q, J = 7.1 Hz); HRMS (ESI/Q-TOF) m/z: [M − H]− calcd for C15H27N3O14P 504.1236; found 504.1239.
1) as eluent system. This procedure afforded the deprotected azido-derived Galβ(1→4)αMan phospho-glycoside 2 (33.7 mg, 0.06 mmol, 67%) as a white solid. 1H NMR (400 MHz, CD3OD): 5.34 (dd, 3JH1–H2 = 1.5 Hz, 3JH1–P = 7.9 Hz, H-1), 4.25 (d, J = 7.5 Hz, H-1′), 3.85 (m, 2H), 3.83 (m, 2H), 3.79 (m, 5H), 3.70 (m, 3H), 3.61 (dd, J = 4.1 and 11.5 Hz, H-6), 3.50 (dd, J = 4.1 and 7.3 Hz, 1H), 3.45 (dd, J = 7.6 and 9.7 Hz, 1H), 3.40 (d, J = 2.6 Hz, H-4′), 3.36 (m, 4H), 1.79 (m, 2H), 1.26 (t, J = 7.2 Hz, 3H); APT 13C{1H} (100 MHz, CD3OD): 103.9 (C-1′), 96.1 (d, 2JC1–P = 5.5 Hz, C-1), 76.6, 75.7, 73.3, 72.6, 71.1, 70.5 (d, JC–P = 8.6 Hz), 69.1, 69.0, 62.3 (d, JC–P = 5.8 Hz, OCH2CH2CH2N3), 61.3, 60.4, 52.2, 29.7 (d, JC–P = 7.6 Hz, OCH2CH2CH2N3); 31P NMR (162 MHz, CD3OD): −1.82 (q, J = 7.1 Hz); HRMS (ESI/Q-TOF) m/z: [M − H]− calcd for C15H27N3O14P 504.1236; found 504.1239.Conflicts of interest
The authors declare no competing financial interests or personal relationships that could have influenced the performance of this work.Acknowledgements
C. A. S. thanks the SJU's Office of Grants and Sponsored Research for Seed Grant FY23 and the Department of Pharmaceutical Sciences for generous starting funds. D. B. and B. D. thanks the Department of Pharmaceutical Sciences for graduate assistantship. We thank Dr Fereshteh Zandkarimi, director of the Mass Spectrometry Core Facilities at Columbia University, for facilitating access to HRMS.References
- CDC, Epidemiology and Risk factors, https://www.cdc.gov/parasites/leishmaniasis/epi.html, accessed on May 8th, 2022 Search PubMed.
- M. Boelaert and S. Sundar, 47 – Leishmaniasis, in Manson's Tropical Infectious Diseases (Twenty-third Edition), ed. J. Farrar, P. J. Hotez, T. Junghanss, G. Kang, D. Lalloo and N. J. White, W.B. Saunders, London, 2014, pp 631–651 Search PubMed
.
- S. Mann, K. Frasca, S. Scherrer, A. F. Henao-Martínez, S. Newman, P. Ramanan and J. A. Suarez, A Review of Leishmaniasis: Current Knowledge and Future Directions, Current Tropical Medicine Reports, 2021, 8(2), 121–132 CrossRef
.
-
(a) J. B., B. M. and K. Chanda, An Overview on the Therapeutics of Neglected Infectious Diseases—Leishmaniasis and Chagas Diseases, Front. Chem., 2021, 9(37), 622286 Search PubMed
; (b) N. K. Copeland and N. E. Aronson, Leishmaniasis: treatment updates and clinical practice guidelines review, Curr. Opin. Infect. Dis., 2015, 28(5), 426–437 CrossRef CAS
; (c) B. Monge-Maillo and R. López-Vélez, Therapeutic Options for Visceral Leishmaniasis, Drugs, 2013, 73(17), 1863–1888 CrossRef CAS PubMed
; (d) N. Singh, M. Kumar and R. K. Singh, Leishmaniasis: current status of available drugs and new potential drug targets, Asian Pac. J. Trop. Med., 2012, 5(6), 485–497 CrossRef CAS
.
-
(a) M. J. McConville and M. A. J. Ferguson, The structure, biosynthesis and function of glycosylated phosphatidylinositols in the parasitic protozoa and higher eukaryotes, Biochem. J., 1993, 294(2), 305–324 CrossRef CAS PubMed
; (b) R. R. de Assis, I. C. Ibraim, P. M. Nogueira, R. P. Soares and S. J. Turco, Glycoconjugates in New World species of Leishmania: polymorphisms in lipophosphoglycan and glycoinositolphospholipids and interaction with hosts, Biochim. Biophys. Acta Gen. Subj., 2012, 1820(9), 1354–1365 CrossRef PubMed
; (c) J. M. Coelho-Finamore, V. C. Freitas, R. R. Assis, M. N. Melo, N. Novozhilova, N. F. Secundino, P. F. Pimenta, S. J. Turco and R. P. Soares, Leishmania infantum: Lipophosphoglycan intraspecific variation and interaction with vertebrate and invertebrate hosts, Int. J. Parasitol., 2011, 41(3), 333–342 CrossRef CAS PubMed
; (d) M. J. McConville, L. F. Schnur, C. Jaffe and P. Schneider, Structure of Leishmania lipophosphoglycan: inter- and intra-specific polymorphism in Old World species, Biochem. J., 1995, 310(3), 807–818 CrossRef CAS PubMed
.
-
(a) M. J. McConville, S. J. Turco, M. A. Ferguson and D. L. Sacks, Developmental modification of lipophosphoglycan during the differentiation of Leishmania major promastigotes to an infectious stage, EMBO J., 1992, 11(10), 3593–3600 CrossRef CAS PubMed
; (b) S. J. Turco and A. Descoteaux, The Lipophosphoglycan of Leishmania Parasites, Annu. Rev. Microbiol., 1992, 46(1), 65–92 CrossRef CAS PubMed
.
- D. L. Sacks, P. F. Pimenta, M. J. McConville, P. Schneider and S. J. Turco, Stage-specific binding of Leishmania donovani to the sand fly vector midgut is regulated by conformational changes in the abundant surface lipophosphoglycan, J. Exp. Med., 1995, 181(2), 685–697 CrossRef CAS
.
- R. P. P. Soares, M. E. Macedo, C. Ropert, N. F. Gontijo, I. C. Almeida, R. T. Gazzinelli, P. F. P. Pimenta and S. J. Turco, Leishmania chagasi: lipophosphoglycan characterization and binding to the midgut of the sand fly vector Lutzomyia longipalpis, Mol. Biochem. Parasitol., 2002, 121(2), 213–224 CrossRef CAS
.
-
(a) G. F. Späth, L. Epstein, B. Leader, S. M. Singer, H. A. Avila, S. J. Turco and S. M. Beverley, Lipophosphoglycan is a virulence factor distinct from related glycoconjugates in the protozoan parasite Leishmania major, Proc. Natl. Acad. Sci. U.S.A., 2000, 97(16), 9258–9263 CrossRef PubMed
; (b) F. H. Routier, A. V. Nikolaev and M. A. J. Ferguson, The preparation of neoglycoconjugates containing inter-saccharide phosphodiester linkages as potential anti-Leishmania vaccines, Glycoconjugate J., 1999, 16(12), 773–780 CrossRef CAS PubMed
; (c) H. Moll, G. F. Mitchell, M. J. McConville and E. Handman, Evidence of T-cell recognition in mice of a purified lipophosphoglycan from Leishmania major, Infect. Immun., 1989, 57(11), 3349–3356 CrossRef CAS
; (d) D. G. Russell and J. Alexander, Effective immunization against cutaneous leishmaniasis with defined membrane antigens reconstituted into liposomes, J. Immunol., 1988, 140(4), 1274 CAS
; (e) M. J. McConville, A. Bacic, G. F. Mitchell and E. Handman, Lipophosphoglycan of Leishmania major that vaccinates against cutaneous leishmaniasis contains an alkylglycerophosphoinositol lipid anchor, Proc. Natl. Acad. Sci. U.S.A., 1987, 84(24), 8941–8945 CrossRef CAS PubMed
.
-
(a) G. Sureshkumar and S. Hotha, AuBr3 mediated glycosidations: synthesis of tetrasaccharide motif of the Leishmania donovani lipophosphoglycan, Glycoconjugate J., 2012, 29(4), 221–230 CrossRef CAS PubMed
; (b) A. J. Ross, O. V. Sizova and A. V. Nikolaev, Parasite glycoconjugates. Part 16: synthesis of a disaccharide and phosphorylated di- and tri-saccharides from Leishmania lipophosphoglycan, Carbohydr. Res., 2006, 341(11), 1954–1964 CrossRef CAS PubMed
; (c) D. Ruhela and R. A. Vishwakarma, A facile and novel route to the antigenic branched phosphoglycan of the protozoan Leishmania major parasite, Tetrahedron Lett., 2004, 45(12), 2589–2592 CrossRef CAS
; (d) D. Ruhela and R. A. Vishwakarma, Efficient synthesis of the antigenic phosphoglycans of the parasite, Chem. Commun., 2001,(19), 2024–2025 RSC
; (e) M. C. Hewitt and P. H. Seeberger, Solution and Solid-Support Synthesis of a Potential Leishmaniasis Carbohydrate Vaccine, J. Org. Chem., 2001, 66(12), 4233–4243 CrossRef CAS
; (f) M. Upreti and R. A. Vishwakarma, Synthesis of the phosphodisaccharide repeat of antigenic lipophosphoglycan of Leishmania donovani parasite, Tetrahedron Lett., 1999, 40(13), 2619–2622 CrossRef CAS
; (g) A. P. Higson, Y. E. Tsvetkov, M. A. J. Ferguson and A. V. Nikolaev, The synthesis of Leishmania major phosphoglycan fragments, Tetrahedron Lett., 1999, 40(52), 9281–9284 CrossRef CAS
; (h) P. Higson, A. E. Tsvetkov, Y. A. J. Ferguson and M. V. Nikolaev, Parasite glycoconjugates. Part 8.1 chemical synthesis of a heptaglycosyl triphosphate fragment of Leishmania mexicana lipo- and proteo-phosphoglycan and of a phosphorylated trisaccharide fragment of Leishmania donovani surface lipophosphoglycan, J. Chem. Soc., Perkin Trans. 1, 1998, 16, 2587–2596 RSC
; (i) V. Nikolaev, A. M. Watt, G. A. J. Ferguson and M. S. Brimacombe, Parasite glycoconjugates. Part 6.1 chemical synthesis of phosphorylated penta- and hepta-saccharide fragments of Leishmania major antigenic lipophosphoglycan, J. Chem. Soc., Perkin Trans. 1, 1997,(6), 969–980 RSC
; (j) A. V. Nikolaev, T. J. Rutherford, M. A. J. Ferguson and J. S. Brimacombe, Parasite glycoconjugates. Part 4. Chemical synthesis of disaccharide and phosphorylated oligosaccharide fragments of Leishmania donovani antigenic lipophosphoglycan, J. Chem. Soc., Perkin Trans. 1, 1995, 16, 1977–1987 RSC
.
-
(a) C. Anish, C. E. Martin, A. Wahlbrink, C. Bogdan, P. Ntais, M. Antoniou and P. H. Seeberger, Immunogenicity and Diagnostic Potential of Synthetic Antigenic Cell Surface Glycans of Leishmania, ACS Chem. Biol., 2013, 8(11), 2412–2422 CrossRef CAS PubMed
; (b) M. E. Rogers, O. V. Sizova, M. A. J. Ferguson, A. V. Nikolaev and P. A. Bates, Synthetic Glycovaccine Protects against the Bite of Leishmania-Infected Sand Flies, J. Infect. Dis., 2006, 194(4), 512–518 CrossRef
; (c) X. Liu, S. Siegrist, M. Amacker, R. Zurbriggen, G. Pluschke and P. H. Seeberger, Enhancement of the Immunogenicity of Synthetic Carbohydrates by Conjugation to Virosomes: A Leishmaniasis Vaccine Candidate, ACS Chem. Biol., 2006, 1(3), 161–164 CrossRef CAS
.
-
(a) G. M. Rodrigues da Cunha, M. A. Azevedo, D. S. Nogueira, M. d. C. Clímaco, E. Valencia Ayala, J. A. Jimenez Chunga, R. J. Y. La Valle, L. M. da Cunha Galvão, E. Chiari, C. R. N. Brito, R. P. Soares, P. M. Nogueira, R. T. Fujiwara, R. Gazzinelli, R. Hincapie, C.-S. Chaves, F. M. S. Oliveira, M. G. Finn and A. F. Marques, α-Gal immunization positively impacts Trypanosoma cruzi colonization of heart tissue in a mouse model, PLoS Neglected Trop. Dis., 2021, 15(7), e0009613 CrossRef CAS PubMed
; (b) A. P. V. Moura, L. C. B. Santos, C. R. N. Brito, E. Valencia, C. Junqueira, A. A. P. Filho, M. R. V. Sant'Anna, N. F. Gontijo, D. C. Bartholomeu, R. T. Fujiwara, R. T. Gazzinelli, C. S. McKay, C. A. Sanhueza, M. G. Finn and A. F. Marques, Virus-like Particle Display of the α-Gal Carbohydrate for Vaccination against Leishmania Infection, ACS Cent. Sci., 2017, 3(9), 1026–1031 CrossRef CAS
.
-
(a) M. M. Alam, C. M. Jarvis, R. Hincapie, C. S. McKay, J. Schimer, C. A. Sanhueza, K. Xu, R. C. Diehl, M. G. Finn and L. L. Kiessling, Glycan-Modified Virus-like Particles Evoke T Helper Type 1-like Immune Responses, ACS Nano, 2021, 15(1), 309–321 CrossRef CAS PubMed
; (b) Z. Yin, M. Comellas-Aragones, S. Chowdhury, P. Bentley, K. Kaczanowska, L. BenMohamed, J. C. Gildersleeve, M. G. Finn and X. Huang, Boosting Immunity to Small Tumor-Associated Carbohydrates with Bacteriophage Qβ Capsids, ACS Chem. Biol., 2013, 8(6), 1253–1262 CrossRef CAS PubMed
.
- R. J. Ferrier, W. G. Overend and A. E. Ryan, The reaction between 3,4,6-tri-O-acetyl-D-glucal and p-nitrophenol, J. Chem. Soc., 1962, 3667–3670 RSC
.
-
(a) H. C. Kolb, M. S. VanNieuwenhze and K. B. Sharpless, Catalytic Asymmetric Dihydroxylation, Chem. Rev., 1994, 94(8), 2483–2547 CrossRef CAS
; (b) K. B. Sharpless, W. Amberg, Y. L. Bennani, G. A. Crispino, J. Hartung, K. S. Jeong, H. L. Kwong, K. Morikawa and Z. M. Wang, The osmium-catalyzed asymmetric dihydroxylation: a new ligand class and a process improvement, J. Org. Chem., 1992, 57(10), 2768–2771 CrossRef CAS
.
- J. Zhao, S. Wei, X. Ma and H. Shao, A simple and convenient method for the synthesis of pyranoid glycals, Carbohydr. Res., 2010, 345(1), 168–171 CrossRef CAS
.
-
(a) R. J. Ferrier and N. Prasad, Unsaturated carbohydrates. Part IX. Synthesis of 2,3-dideoxy-α-D-erythro-hex-2-enopyranosides from tri-O-acetyl-D-glucal, J. Chem. Soc. C, 1969, 4, 570–575 RSC
; (b) A. M. Gómez, F. Lobo, C. Uriel and J. C. López, Recent Developments in the Ferrier Rearrangement, Eur. J. Org. Chem., 2013, 32, 7221–7262 CrossRef
; (c) G. Descotes and J.-C. Martin, Sur l'isomérisation du 1,5-anhydro-3,4,6-tri-O-benzyl-1,2-didésoxy-d-arabino-hex-1-énitol on présence d'acides de Lewis, Carbohydr. Res., 1977, 56(1), 168–172 CrossRef CAS
.
- J. Ø. Duus, C. H. Gotfredsen and K. Bock, Carbohydrate Structural Determination by NMR Spectroscopy: Modern Methods and Limitations, Chem. Rev., 2000, 100(12), 4589–4614 CrossRef CAS
.
-
(a) I. Fukuhara, R. Matsubara and M. Hayashi, Selective Synthesis of Some Aminosugars via Catalytic Aminohydroxylation of Protected 2,3-Unsaturated d-Gluco- and d-Galacto-2-hexenopyranosides, J. Org. Chem., 2020, 85(14), 9179–9189 CrossRef CAS
; (b) P. V. Murphy, J. L. O'Brien and A. B. Smith, Stereospecific synthesis of β-d-allopyranosides by dihydroxylation of β-d-erythro-2,3-dideoxyhex-2-enopyranosides, Carbohydr. Res., 2001, 334(4), 327–335 CrossRef CAS PubMed
; (c) Z. J. Liu, M. Zhou, J. M. Min and L. H. Zhang, Syntheses of 5′-O-glycosylnucleosides, Tetrahedron: Asymmetry, 1999, 10(11), 2119–2127 CrossRef CAS
; (d) T. M. B. de Brito, L. P. da Silva, V. L. Siqueira and R. M. Srivastava, Synthesis of 2,3-unsaturated 4-Amino Sugars and Cyclohexyl 2,3-Di-O-Acetyl-4,6-Di-O-Methyl-α-D-Manno-Pyranoside from Cyclohexyl 4,6-Di-O-Acetyl-2,3-Dideoxy-α-D-erythro-Hex-2-Enopyranoside, J. Carbohydr. Chem., 1999, 18(6), 609–616 CrossRef CAS
; (e) R. W. Friesen and S. J. Danishefsky, On the use of the haloetherification method to synthesize fully functionalized disaccharides, Tetrahedron, 1990, 46(1), 103–112 CrossRef CAS
; (f) H. H. Baer and L. Siemsen, Synthesis of 2-amino-2-deoxy- and 3-amino-3-deoxy-α-d-mannopyranosyl α-d-mannopyranoside by sequential osmylations of a dienic disaccharide, Carbohydr. Res., 1986, 146(1), 63–72 CrossRef CAS
; (g) A. M. Gomez, F. Lobo, S. Miranda and J. C. Lopez, A Survey of Recent Synthetic Applications of 2,3-Dideoxy-Hex-2-enopyranosides, Molecules, 2015, 20(5), 8357–8394 CrossRef CAS
; (h) M. Fukudome, T. Shiratani, Y. Nogami, D.-Q. Yuan and K. Fujita, Shortcut Synthesis of β-Cyclomannin from β-Cyclodextrin, Org. Lett., 2006, 8(25), 5733–5736 CrossRef CAS
.
- C. Masson, J. Soto and M. Bessodes, Ferric Chloride: a New and Very Efficient Catalyst for the Ferrier Glycosylation Reaction, Synlett, 2000, 9, 1281–1282 Search PubMed
.
- R. D. Dawe and B. Fraser-Reid, α-C-Glycopyranosides from Lewis acid catalysed condensations of acetylated glycals and enol silanes, J. Chem. Soc., Chem. Commun., 1981, 22, 1180–1181 RSC
.
- M. Koreeda, T. A. Houston, B. K. Shull, E. Klemke and R. J. Tuinman, Iodine-catalyzed Ferrier Reaction 1. A Mild and Highly Versatile Glycosylation of Hydroxyl and Phenolic Groups1, Synlett, 1995, 1, 90–92 CrossRef
.
- P. Chen and S. Li, Y(OTf)3 as a highly efficient catalyst in Ferrier Rearrangement for the synthesis of O- and S-2,3-unsaturated glycopyranosides, Tetrahedron Lett., 2014, 55(42), 5813–5816 CrossRef CAS
.
- P. Chen and J. Su, Gd(OTf)3 catalyzed preparation of 2,3-unsaturated O-, S-, N-, and C-pyranosides from glycals by Ferrier Rearrangement, Tetrahedron, 2016, 72(1), 84–94 CrossRef CAS
.
-
(a) Q. Wang, T. R. Chan, R. Hilgraf, V. V. Fokin, K. B. Sharpless and M. G. Finn, Bioconjugation by Copper(I)-Catalyzed Azide-Alkyne [3 + 2] Cycloaddition, J. Am. Chem. Soc., 2003, 125(11), 3192–3193 CrossRef CAS
; (b) V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes, Angew. Chem., Int. Ed., 2002, 41(14), 2596–2599 CrossRef CAS
; (c) H. C. Kolb, M. G. Finn and K. B. Sharpless, Click Chemistry: Diverse Chemical Function from a Few Good Reactions, Angew. Chem., Int. Ed., 2001, 40(11), 2004–2021 CrossRef CAS
; (d) R. Huisgen, 1,3-Dipolar Cycloadditions. Past and Future, Angew. Chem., Int. Ed., 1963, 2(10), 565–598 CrossRef
.
- G. Zemplén and E. Pacsu, Über die Verseifung acetylierter Zucker und verwandter Substanzen, Ber. Dtsch. Chem. Ges., 1929, 62(6), 1613–1614 CrossRef
.
- D. V. Yashunsky and A. V. Nikolaev, Hydrogenphosphonate synthesis of sugar phosphomonoesters, J. Chem. Soc., Perkin Trans. 1, 2000,(8), 1195–1198 RSC
.
- H. E. Gottlieb, V. Kotlyar and A. Nudelman, NMR Chemical Shifts of Common Laboratory Solvents as Trace Impurities, J. Org. Chem., 1997, 62(21), 7512–7515 CrossRef CAS PubMed
.
- S. Kopitzki, K. J. Jensen and J. Thiem, Synthesis of Benzaldehyde-Functionalized Glycans: A Novel Approach Towards Glyco-SAMs as a Tool for Surface Plasmon Resonance Studies, Chem.–Eur. J., 2010, 16(23), 7017–7029 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: 1H and 13C{1H} NMR spectra for synthesized compounds. See https://doi.org/10.1039/d2ra05158c |
| This journal is © The Royal Society of Chemistry 2022 |