Open Access Article
Open Access ArticlePillar[n]arene–calix[m]arene hybrid macrocyclic structures
Zhaona Liu†
a,
Bing Li†
b,
Leqian Song†
b and
Huacheng Zhang
 *b
*b
aMedical School, Xi'an Peihua University, Xi'an 710125, Shaanxi, China. E-mail: zhaonaliu@peihua.edu.cn
bSchool of Chemical Engineering and Technology, Xi'an Jiaotong University, Xi'an, Shaanxi 710049, China. E-mail: zhanghuacheng@xjtu.edu.cn
First published on 3rd October 2022
Abstract
To reserve planar chirality, enhance molecular recognition, and build advanced self-assemblies, hybrid macrocyclic hosts containing rigid pillar[n]arene and flexible calix[m]arene were designed, prepared and investigated for interesting applications. This review summarizes and discusses different synthetic strategies for constructing hybrid macrocyclic structures. Pillar[n]arene dimer with rigid aromatic double bridges provided the possibility of introducing calix[m]arene cavities, where the planar chirality was reserved in the structure of pillararene. The capacity for molecular recognition was enhanced by hybrid macrocyclic cavities. Interestingly, the obtained pillar[n]arene–calix[m]arene could self-assemble into “channels” and “honeycomb” in both the solid state and solution phase as well as donate the molecular architecture as the wheel for the formation of mechanically interlocked molecules, such as rotaxane. In addition, the pillar[n]arene and calix[m]arene could also be coupled together to produce pillar[n]arene embeded 1,3-alternate and cone conformational calix[m]arene derivatives, which could catalyze the oxidative polymerization of aniline in aqueous solutions. Except for building hybrid cyclophanes by covalent bonds, weak supramolecular interactions were used to prepare pillar[n]arene–calix[m]arene analogous composites with other pillar-like pillar[n]pyridiniums and calix-like calix[m]pyrroles, exhibiting reasonable performances in enhancing molecular recognition and trapping solvent molecules.
1 Introduction
To mimic natural and biological processes,1–3 such as protein–glucose interactions,4–8 macrocycles9–11 were designed and prepared as specialized host molecules12,13 for molecular recognition14 by weak supramolecular interactions.15 To date, various macrocycles16–18 with various physiochemical characteristics have been reported. Among them, cyclophanes19–21 with repeated aromatic moieties, such as pillar[n]arene22–28 and calix[m]arene29–34 (Scheme 1), have attracted considerable academic and practical attention.35 For example, owing to the presence of multivalent modification sites,36 the structural skeletons of both pillar[n]arene and calix[m]arene could be directly used as the backbone for embedding valuable functional groups for various applications to water-soluble37 self-assembled amphiphiles,38–41 advanced hierarchical architectures,42,43 such as metal organic frameworks,44–48 as well as energy storage and batteries.49,50Actually, owing to the presence of similar aromatic oligomeric structures, the development of novel structures and applications of pillar[n]arene51 has always resembled those of calix[m]arene.52 However, because of different substitution sites on aromatic rings, pillar[n]arene and calix[m]arene53 always exhibit different topologies and morphologies in molecular conformation and configuration,54 as well as diverse behaviors in particular physiochemical characteristics.55 For example, from the molecular geometry and shape perspective, the flexible structures of calix[m]arene could result in an adaptable cavity for diverse guests,56 while the rigid conformational structures of pillar[n]arene can produce unique planar chirality.57
It is important to compare these two attractive analogues of aromatic macrocycles in terms of their structural and physiochemical behaviors.58 Several investigation methods have been developed for this purpose; for example, computational studies59 such as density functional theory (DFT)60 have been employed in investigating the diverse physiochemical properties of pillar[n]arene and calix[m]arene for different performances and applications, such as host–guest complexation.60 Furthermore, a direct comparison between these two cyclophanes in practical experiments was also carried out.61,62 Pillar[n]arene and calix[m]arene demonstrated their advantages. Thus, it aroused another academic interest in combining the molecular structure and characteristics of pillar[n]arene and calix[m]arene with the art of organic synthesis.63 The integration of pillar[n]arene and calix[m]arene into hybrid host molecules not only enriches the family of hybrid macrocycles but also combines their features, such as flexible architectures and planar chirality.64–67
In this review, the preparation and application of pillar[n]arene–calix[m]arene hybrid macrocyclic compounds are summarized and discussed. Different classic organic synthesis methods68 were employed during the preparation, producing pillar[n]arene dimer with aromatic double bridges, coupled with pillar[n]arene–calix[m]arene hybrid hosts, and noncovalent interaction-based pillar[n]arene–calix[m]arene resembling systems. Pillar[n]arene dimer with rigid aromatic double bridges possessing resembled calix[m]arene cavities could reserve the planar chirality and include dumbbell guests69,70 for fabricating mechanically interlocked molecules.71,72 In addition, the pillar[n]arene and calix[m]arene could also be covalently coupled to produce pillar[n]arene-containing 1,3-alternate and cone conformational calix[m]arene derivatives for catalyzing the oxidative polymerization of aniline.73 Furthermore, weak supramolecular interactions have been used to prepare pillar[n]arene–calix[m]arene analogous composites using pillar[n]pyridiniums74/calixarene and calix[m]pyrrole75,76/pillararene to enhance molecular recognition and trapping solvent molecules.77 Finally, we consider possible scientific and technical issues and new challenges, as well as provide brief and primary suggestions for future studies to be conducted on pillar[n]arene–calix[m]arene hybrid macrocycles.
2 Preparation of pillar[n]arene–calix[m]arene hybrid macrocyclic structures
2.1 Merging structures of pillar[n]arene and calix[m]arene
Based on the art of organic synthesis,78 both the direct modification of pillar[n]arene with calix[m]arene and the functionalization of calix[m]arene with pillar[n]arene were challenging because of several organic experimental issues, such as the diverse solubilities of the two cyclophanes in reaction solutions,79 as well as the multivalent reactive sites80 embedded in their structures. Thus, the current rigid skeleton of pillar[n]arene was initially considered the structural basis, and heteroatoms, such as oxygen-atom on the hydroquinone moieties of pillar[n]arene, were further used as a bridge to incorporate extra aromatic subunits via meta positions, producing an additional calix-like cyclophane cavity. Such hybrid macrocycles have particular structural characteristics, e.g., “fused” hybrid macrocyclic cavities “double-bridged”81 by aromatic rigid linkers with unique planar chirality.82 Furthermore, it provides the opportunity to raise another supramolecular issue, i.e., the fabrication of mechanically interlocked molecules.82For example,83 1,4-dimethoxypillar[4]arene[1]hydroquinone P1 (Scheme 2) was used as the previous piece to react with the “bridging group”—1,5-difluoro-2,4-dinitrobenzene F1 (Scheme 2) in the presence of triethylamine as the catalyst in THF solutions under N2 atmosphere to produce 2,4-dinitro-5-fluoro-phenyl difunctionalized pillar[5]arene P2 (Scheme 2) using the classic nucleophilic aromatic substitution (SNAr) yielding 90%. Then, P2 was dissolved in DMF to promote its solubility under K2CO3 condition and prepare another nitrophenyl difunctionalized pillar[5]arene P3 (Scheme 2), yielding 99%. Interestingly, the vapor diffusion method was applied to synthesize the single crystal structures of P2 and P3 using hexane, heptane and dichloromethane, revealing that tubular and honeycombed self-assemblies84 were formed in the solid state with solvent molecules encapsulated inside these structures (Fig. 1).83
 | ||
| Scheme 2 The synthetic strategy that produces a significant previous piece of P2/P5 and P3 in high yields using the nucleophilic substitution of P1/P4 with F1.83 | ||
 | ||
| Fig. 1 Single crystal structures of P2 in stick representation indicate (a) a column-like tubular supramolecular self-assembly with (b) noncovalent interactions, as highlighted in green, as well as (c) honeycombed self-assemblies owing to the possession of π–π stacking interactions and C–H⋯π hydrogen bonds, where hydrogen atoms were omitted for clarity.83 | ||
Considering the above organic experiment of preparing significant previous pieces of conformationally locked A1/A2 difunctionalized pillar[5]arene P2 and P3 (Scheme 2), the pillar[5]arene/calix[4]arene-containing fused hybrid macrocyclic compound—oxacalix[4]arene-bridged pillar[5]arene dimers PC1–PC3 with diverse planar chirality (Scheme 3) were further obtained using two different synthesis strategies, such as the one-pot reaction between pillar[4]arene[1]hydroquinone P4 and F1 (Scheme 3) in DMSO in the presence of K2CO3 at room temperature and two-step approach with P5 (Scheme 3) as the intermediate compound in relatively improved yields.85 Interestingly, PC1 and PC2 were a pair of enantiomers, as confirmed by chiral high performance liquid chromatography (HPLC), and PC3 (Fig. 2) was the achiral meso compound of PC1/PC2 as revealed by 1H NMR and electrospray ionization high resolution mass spectrometry (ESI-HRMS).
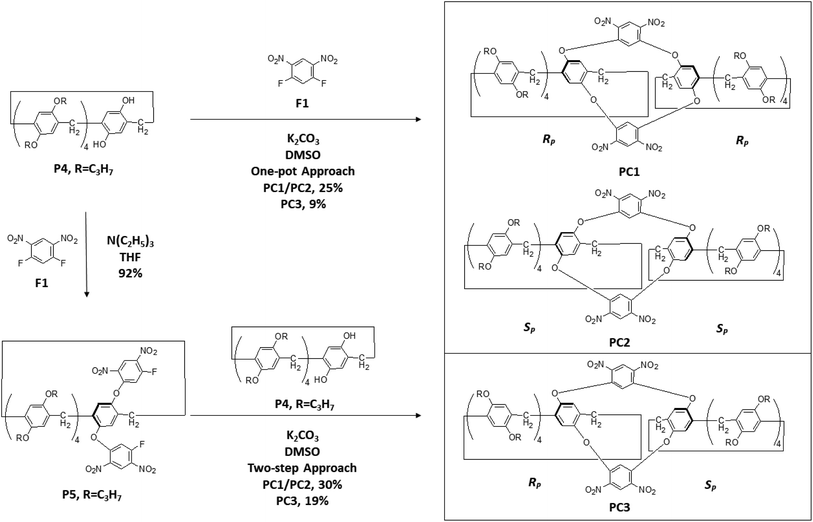 | ||
| Scheme 3 Two synthesis routes from the starting molecule P4 with the assistance of F1 to prepare diverse oxacalix[4]arene-bridged pillar[5]arene dimers, such as PC1, PC2 and PC3, with different planar chirality. P5 is an important intermediate compound in one synthesis strategy.85 | ||
 | ||
| Fig. 2 Single crystal structures of PC3 in the stick representation of (a) top view, (b) side view and (c) packing model showing a self-assembled channel-like architecture. The configurations of the two pillar[5]arenes possessed Rp and Sp rings. Solvent molecules and hydrogen atoms were omitted for clarity.85 | ||
Fused hybrid macrocyclic hosts are good candidates for preparing advanced molecular architectures, such as mechanically interlocked molecules. Conformationally fixed double-bridged pillar[5]arene–calix[4]arene hybrid structures were further used as the “wheel” subunit for constructing planar chirality-containing [2]rotaxanes,86,87 such as PC4, PC5, PC8 and PC9 (Scheme 4), as well as [3]rotaxanes, such as PC6, PC7 and PC10 (Scheme 4), using the “stopper” moieties-possessing functional molecules F2 and F3 (Scheme 4) to form the “axle” subunit via the threading/stoppering method (Fig. 3).85
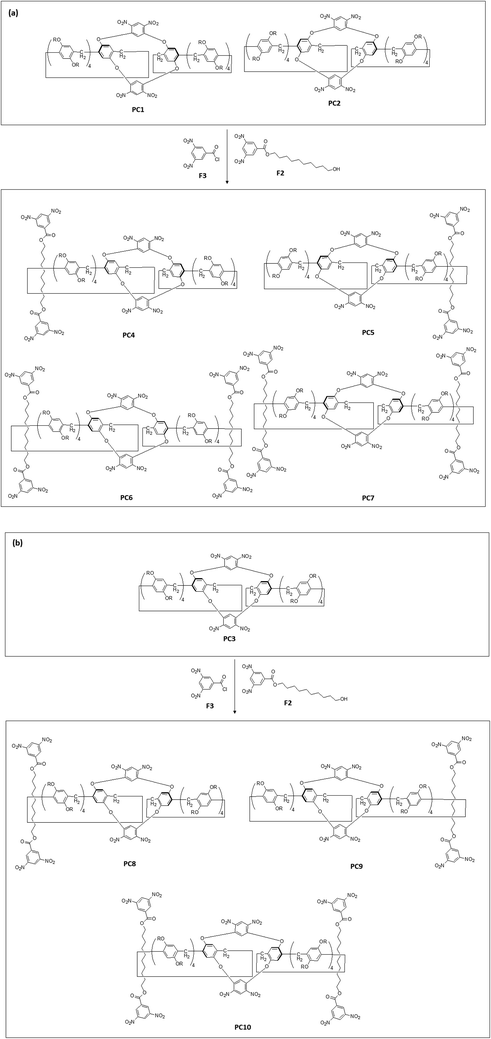 | ||
| Scheme 4 Schematic illustration of the synthesis of diverse planar chirality-containing pillar[5]arene-based [2]rotaxanes, such as PC4, PC5, PC8 and PC9, as well as [3]rotaxanes, such as PC6, PC7 and PC10, by utilizing previous pieces, such as double-bridged pillar[5]arene derivatives, including PC1, PC2 and PC3, as well as functional reagents, including stopper-containing F2 and F3 for the formation of axle subunits.85 | ||
 | ||
| Fig. 3 Single crystal structures of a pair of planar chiral enantiomers, PC6 and PC7. Hydrogen atoms were omitted for clarity.85 | ||
2.2 Coupling independent structures of pillar[n]arene and calix[m]arene
Except for expanding the current structural skeleton of pillar[n]arene to build extra calix-like cavities, two independent structures of pillar[n]arene and calix[m]arene can be coupled via classic organic reactions. For example, tetraacyl chlorides-containing p-tert-butylthiacalix[4]arene C1 (Scheme 5) could be integrated with amino monofunctionalized pillar[5]arene derivative P6 (Scheme 5) in dichloromethane in the presence of Hünge's base under the argon atmosphere through acylation reaction, preparing pillar[5]arene–calix[4]arene hybrid macrocycles, such as 1,3-alternate conformational calixarene-containing PC11 (Scheme 5) and cone conformational calixarene-containing PC12 (Scheme 5), yielding 58% and 65%, respectively.88 Interestingly, the combination of pillar[5]arene and thiacalix[4]arene into a hybrid macrocyclic system not only allowed the integration of the diverse capacities of molecular recognition towards valuable guest molecules, such as aniline and aniline/p-toluenesulfonic acid, but also promoted the oxidative polymerization of aniline in aqueous p-toluenesulfonic acid solutions by increasing the yield and molecular weight of emeraldine. | ||
| Scheme 5 Synthesis route for producing PC11 and PC12 by coupling thiacalix[4]arene C1 and pillar[5]arene P6.88 | ||
2.3 Integrating pillar-like and calix-like aromatic cyclic oligomers
In addition to the direct coupling of pillar[n]arene and calix[m]arene into hybrid macrocyclic structures, other pillar-like and calix-like macrocycles, such as heteroatom-containing ones, were employed to resemble fused cyclophanes. Owing to the introduction of heteroatoms, the capacity for molecular recognition generated from fused macrocycles was enhanced.For example, carboxylate89,90 difunctionalized pillar[5]arene P7 (Scheme 6) was reacted with the calix-like nitrogen-rich macrocycle—calix[4]pyrrole C2 (Scheme 6) via an esterification reaction in the presence of catalytic amounts of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI) and 4-dimethylaminopyridine (DMAP), leading to the formation of pillar[5]arene–calix[4]pyrrole PC13 (Scheme 6) with two calix[4]pyrrole subunits on both ends yielding 50%.91 Owing to the possession of nitrogen-rich calix[4]pyrrole structures and conformational fixed pillar[5]arene moieties, PC13 exhibited enhanced inclusion ability for chiral anion salts, such as chiral (R/S)-mandelate anionic guests as tetramethylammonium salts, by a series of weak supramolecular interactions, including hydrogen bonding, cation–π, π–π stacking and C–H⋯π interactions. The association constants (Ka) were determined in the range of 5.15 × 104 to 2.25 × 105 M−1.
 | ||
| Scheme 6 Synthesis of PC13 using P7 and C2.91 | ||
Particularly, the introduction of heteroatoms embedded in the macrocyclic structures provided the possibility of combining pillar-like and calix-like cyclic aromatic oligomers via noncovalent interactions, i.e., the heteroatom-containing macrocycles acting as the guest molecule to be included by the other cavity via supramolecular interactions, such as electrostatic forces. For example,92 cationic pillar[n]pyridiniums, such as the rigid square-shaped tetramer P8 and the flexible roughly hexagonal hexamer P9 (Scheme 7), were encapsulated by anionic p-sulfonatocalix[4]arene C3 (Scheme 7) in the solid state and aqueous solution phase by electrostatic attractions, and anion⋯π+, anion⋯π+⋯anion and π⋯π+ interactions, leading to the co-assemblies of oppositely charged hybrid macrocycles (Fig. 4 and 5).
 | ||
| Scheme 7 Chemical structures of P8, P9 and C3.92 | ||
 | ||
| Fig. 4 Expanded asymmetric unit of 1/2 P8·C3 (A), where H2O was trapped inside the center of P8 (B) by a series of weak supramolecular interactions (C–E).92 Copyright© 2022 by The Royal Society of Chemistry. | ||
 | ||
| Fig. 5 Asymmetric unit of 1/2 P9·C3 (A), where H2O was trapped inside P9 (B) by a series of weak supramolecular interactions (C–E).92 Copyright© 2022 by The Royal Society of Chemistry. | ||
3 Overview and outlook
In conclusion, the design, preparation and application of pillar[n]arene–calix[m]arene hybrid macrocyclic structures were discussed and summarized in this review. To overcome the challenges of the art of organic synthesis, various strategies were employed to fabricate hybrid cyclophanes. First, the structural skeletons of pillar[n]arene and calix[m]arene were fused via the formation of a pillararene dimer93 with double aromatic bridges by incorporating the architectures of calixarene under the SNAr reaction conditions, leading to the self-assembly of hybrid macrocycles in the solid and solution phases, the reservation of planar chirality of pillararene, and the enhancement of molecular recognition by hybrid cavities. Interestingly, double-bridged pillar[n]arene dimers further provided the pillar[n]arene cavity as the wheel to integrate with “dumb-bell”-like linear molecules, affording mechanically interlocked molecules, such as [2]/[3]rotaxanes, with planar chirality. Additionally, classic organic synthetic methods, such as acylation reaction, were further carried out to directly combine independent units of pillar[n]arene and calix[m]arene, producing two types of pillar[5]arene–calix[4]arene hybrid macrocyles with 1,3-alternate and cone conformations, which were observed to have the capacity to catalyze the oxidative polymerization of aniline in aqueous solutions. Furthermore, pillar-like cyclophanes, such as pillar[n]pyridiniums, and calix-like cyclophanes, such as calix[m]pyrrole, were also used for the fabrication of hybrid macrocyclic systems with calixarene and pillararene, respectively, to resemble pillar[n]arene–calix[m]arene composites, where both covalent bonds and noncovalent interactions acted as the driving forces. Particularly, these analogous systems exhibited reasonable performances in enhancing molecular recognition and trapping solvent molecules in the solution and solid phases.Numerous studies on the fabrication and application of pillararene–calixarene hybrid macrocycles are still attractive for future researchers, needing attention from related scientists in synthesis chemistry, physical chemistry and material science.
First, the efficient integration of pillar[n]arene and calix[m]arene together as well as the effective separation of enantiopure hybrid macrocycles94 are still challenges in organic synthesis chemistry. In particular, owing to the possession of pillararene and the introduction of larger functional groups, the planar chirality of pillararene granted future applications for hybrid macrocycles but made the separation and purification of such compounds more difficult.95
Second, other sized pillar-like and calix-like aromatic macrocycles should be employed in the fabrication of such hybrid cyclophanes to enrich the family of pillar[n]arene/calix[m]arene-based fused cyclophanes.96 Larger cavities of fused hybrid macrocycles not only enlarge the possibilities of complexing diverse functional guest molecules,97 but also provide significant backbones and building blocks for constructing advanced functional structures, such as self-assemblies and mechanically interlocked molecules.98,99 Future applications should be further explored based on functional architectures with covalent bonds, mechanical forces and supramolecular interactions.100
Third, a computational study is highly recommended for proposing and investigating pillararene–calixarene hybrid macrocycle-based supramolecular materials,101,102 such as the study of different behaviors of host–guest inclusions, as well as the competition and cooperation between the two hybrid macrocycles.103
Abbreviations
| EDCI | 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride |
| ESI-HRMS | Electrospray ionization high resolution mass spectrometry |
| DMAP | 4-Dimethylaminopyridine |
| HPLC | High performance liquid chromatography |
| Ka | Association constants |
| SNAr | Nucleophilic aromatic substitution |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
H. Z. and Z. L. would like to thank Dr Nathan L. Strutt for his kind help and patient guide to pillararene chemistry during their academic visit to Northwestern University. H. Z. and Z. L. acknowledge the financial support from the “Young Talent Support Plan” (No. 050700-71240000000046) of Xi'an Jiaotong University, Xi'an Peihua University, Natural Science Foundation of Shaanxi Province (No. 2021JM-006 and 2021JQ-863).References
- G. Yang, L. Wu, G. Chen and M. Jiang, Chem. Commun., 2016, 52, 10595–10605 RSC.
- X. Sun, W. Zhai, J. S. Fossey and T. D. James, Chem. Commun., 2016, 52, 3456–3469 RSC.
- W. Li, A. T. Bockus, B. Vinciguerra, L. Isaacs and A. R. Urbach, Chem. Commun., 2016, 52, 8537–8540 RSC.
- R. C. Bunn, M. A. Jensen and B. C. Reed, Mol. Biol. Cell, 1999, 10, 819–832 CrossRef CAS PubMed.
- N. C. Raiha, O. Somersalo, C. G. Nordstrom and C. E. Raiha, Acta Paediatr., 1962, 135, 171–177 CrossRef CAS PubMed.
- Y. You, F. Liu, K. J. Du, G. B. Wen and Y. W. Lin, J. Mol. Model., 2014, 20, 2358 CrossRef PubMed.
- K. Ludin, R. Jiang and M. Carlson, Proc. Natl. Acad. Sci., 1998, 95, 6245–6250 CrossRef CAS PubMed.
- K. Amaike, T. Tamura and I. Hamachi, Chem. Commun., 2017, 53, 11972–11983 RSC.
- E. M. Driggers, S. P. Hale, J. Lee and N. K. Terrett, Nat. Rev. Drug Discovery, 2008, 7, 608–624 CrossRef CAS PubMed.
- A. K. Yudin, Chem. Sci., 2015, 6, 30–49 RSC.
- M. J. Strauss, A. M. Evans, E. K. Roesner, R. J. Monsky, M. I. Bardot and W. R. Dichtel, Acc. Mater. Res., 2022, 3(9), 935–947 CrossRef CAS.
- Q. Mou, Y. Ma, X. Jin, D. Yan and X. Zhu, Chem. Commun., 2016, 52, 11728–11743 RSC.
- J. Teyssandier, S. D. Feyter and K. S. Mali, Chem. Commun., 2016, 52, 11465–11487 RSC.
- K. Kellett, J. H. Broome, M. Zloh, S. B. Kirton, S. Fergus, U. Gerhard, J. L. Stair and K. J. Wallace, Chem. Commun., 2016, 52, 7474–7477 RSC.
- A. Brown and P. D. Beer, Chem. Commun., 2016, 52, 8645–8658 RSC.
- M. Mahjoubin-Tehran, P. T. Kovanen, S. Xu, T. Jamialahmadi and A. Sahebkar, Pharmacol. Ther., 2020, 214, 107620 CrossRef CAS.
- H. Bai, J. Wang, C. U. Phan, Q. Chen, X. Hu, G. Shao, J. Zhou, L. Lai and G. Tang, Nat. Commun., 2021, 12, 759 CrossRef CAS PubMed.
- S. Mehta, V. Bongcaron, T. K. Nguyen, Y. Jirwanka, A. Maluenda, A. P. G. Walsh, J. Palasubramaniam, M. D. Hulett, R. Srivastava, A. Bobik, X. Wang and K. Peter, Small, 2022, 18, 2270169 CrossRef.
- B. Gao, L. L. Tan, N. Song, K. Li and Y. W. Yang, Chem. Commun., 2016, 52, 5804–5807 RSC.
- F. Jia, H. Y. Wang, D. H. Li, L. P. Yang and W. Jiang, Chem. Commun., 2016, 52, 5666–5669 RSC.
- S. T. Schneebeli, N. L. Strutt, C. Cheng and J. F. Stoddart, in Pillararenes, The Royal Society of Chemistry, 2016, pp. 278–307, 10.1039/9781782622321-00278.
- L. Chen, Y. Cai, W. Feng and L. Yuan, Chem. Commun., 2019, 55, 7883–7898 RSC.
- Y. Y. Chen, X. M. Jiang, G. F. Gong, H. Yao, Y. M. Zhang, T. B. Wei and Q. Lin, Chem. Commun., 2021, 57, 284–301 RSC.
- Q. Li, H. Zhu and F. Huang, Trends Chem., 2020, 2, 850–864 CrossRef CAS.
- M. Xue, Y. Yang, X. Chi, Z. Zhang and F. Huang, Acc. Chem. Res., 2012, 45, 1294–1308 CrossRef CAS PubMed.
- T. Ogoshi, T. a. Yamagishi and Y. Nakamoto, Chem. Rev., 2016, 116, 7937–8002 CrossRef CAS PubMed.
- T. Ogoshi, T. Kakuta and T. a. Yamagishi, Angew. Chem., Int. Ed., 2019, 58, 2197–2206 CrossRef CAS PubMed.
- P. J. Cragg and K. Sharma, Chem. Soc. Rev., 2012, 41, 597–607 RSC.
- V. Guérineau, M. Rollet, S. Viel, B. Lepoittevin, L. Costa, P. Saint-Aguet, R. Laurent, P. Roger, D. Gigmes, C. Martini and V. Huc, Nat. Commun., 2019, 10, 113 CrossRef.
- A. Yousaf, S. A. Hamid, N. M. Bunnori and A. A. Ishola, Drug Des., Dev. Ther., 2015, 9, 2831–2838 CAS.
- A. M. Hussain, U. M. Ashraf, G. Muhammad, N. M. Tahir and N. A. S. Bukhari, Curr. Pharm. Des., 2017, 23, 2377–2388 CrossRef.
- C. Chen, X. Ni, H. W. Tian, Q. Liu, D. S. Guo and D. Ding, Angew. Chem., Int. Ed., 2020, 59, 10008–10012 CrossRef CAS PubMed.
- Y. Zhou, H. Li and Y. W. Yang, Chin. Chem. Lett., 2015, 26, 825–828 CrossRef CAS.
- Y. Y. Wang, Y. Kong, Z. Zheng, W. C. Geng, Z. Y. Zhao, H. Sun and D. S. Guo, Beilstein J. Org. Chem., 2019, 15, 1394–1406 CrossRef CAS PubMed.
- D. Xia, P. Wei, B. Shi and F. Huang, Chem. Commun., 2016, 52, 513–516 RSC.
- A. Tikad, H. Fu, C. M. Sevrain, S. Laurent, J. F. Nierengarten and S. P. Vincent, Chem.–Eur. J., 2016, 22, 13147–13155 CrossRef CAS PubMed.
- W. Cheng, H. Tang, R. Wang, L. Wang, H. Meier and D. Cao, Chem. Commun., 2016, 52, 8075–8078 RSC.
- K. Yang, Y. Pei, J. Wen and Z. Pei, Chem. Commun., 2016, 52, 9316–9326 RSC.
- Y. Chang, C. Hou, J. Ren, X. Xin, Y. Pei, Y. Lu, S. Cao and Z. Pei, Chem. Commun., 2016, 52, 9578–9581 RSC.
- W. Jiang, Y. Liu, C. Yu, S. Li, Y. Li and Y. Zhou, Chem. Commun., 2016, 52, 8223–8226 RSC.
- H. Zhang, Z. Liu and Y. Zhao, Chem. Soc. Rev., 2018, 47, 5491–5528 RSC.
- S. Fang, M. Wang, Y. Wu, Q. H. Guo, E. Li, H. Li and F. Huang, Chem. Sci., 2022, 13, 6254–6261 RSC.
- J. Cao, Y. Wu, Q. Li, W. Zhu, Z. Wang, Y. Liu, K. Jie, H. Zhu and F. Huang, Chem. Sci., 2022, 13, 7536–7540 RSC.
- J. Tian, L. Chen, D. W. Zhang, Y. Liu and Z. T. Li, Chem. Commun., 2016, 52, 6351–6362 RSC.
- X. Zhang, Z. Zhang, J. Boissonnault and S. M. Cohen, Chem. Commun., 2016, 52, 8585–8588 RSC.
- Y. Zhang, T. G. Zhan, T. Y. Zhou, Q. Y. Qi, X. N. Xu and X. Zhao, Chem. Commun., 2016, 52, 7588–7591 RSC.
- L. Bai, B. Tu, Y. Qi, Q. Gao, D. Liu, Z. Liu, L. Zhao, Q. Li and Y. Zhao, Chem. Commun., 2016, 52, 3003–3006 RSC.
- H. Zhang, Z. Liu, F. Xin and Y. Zhao, Coord. Chem. Rev., 2020, 420, 213425 CrossRef CAS.
- M. H. Li, X. Y. Lou and Y. W. Yang, Chem. Commun., 2021, 57, 13429–13447 RSC.
- S. Cao, L. Zhou, C. Liu, H. Zhang, Y. Zhao and Y. Zhao, Biosens. Bioelectron., 2021, 181, 113164 CrossRef CAS PubMed.
- Y. Wang, G. Ping and C. Li, Chem. Commun., 2016, 52, 9858–9872 RSC.
- Y. Jia, Y. Fang, Y. Li, L. He, W. Fan, W. Feng, Y. Yang, J. Liao, N. Liu and L. Yuan, Talanta, 2014, 125, 322–328 CrossRef CAS PubMed.
- F. Zhang, Y. Sun, D. Tian, W. S. Shin, J. S. Kim and H. Li, Chem. Commun., 2016, 52, 12685–12693 RSC.
- N. Hontama, Y. Inokuchi, T. Ebata, C. Dedonder-Lardeux, C. Jouvet and S. S. Xantheas, J. Phys. Chem. A, 2010, 114, 2967–2972 CrossRef CAS PubMed.
- J. H. Lee, J. Park, J. W. Park, H. J. Ahn, J. Jaworski and J. H. Jung, Nat. Commun., 2015, 6, 6650 CrossRef CAS.
- C. X. Yu, F. L. Hu, J. G. Song, J. L. Zhang, S. S. Liu, B. X. Wang, H. Meng, L.-L. Liu and L.-F. Ma, Sens. Actuators, B, 2020, 310, 127819 CrossRef CAS.
- J. F. Chen, J. D. Ding and T. B. Wei, Chem. Commun., 2021, 57, 9029–9039 RSC.
- S. Cao, H. Zhang, Y. Zhao and Y. Zhao, eScience, 2021, 1, 28–43 CrossRef.
- S. Wang, Y. Yin, J. Gao, X. Lianga and H. Shi, J. Braz. Chem. Soc., 2021, 32, 1963–1971 CAS.
- S. R. Peerannawar and S. P. Gejji, Comput. Theor. Chem., 2013, 1015, 44–51 CrossRef CAS.
- D. D. Zheng, D. Y. Fu, Y. Wu, Y. L. Sun, L. L. Tan, T. Zhou, S. Q. Ma, X. Zha and Y. W. Yang, Chem. Commun., 2014, 50, 3201–3203 RSC.
- J. Fernández-Rosas, B. Gómez-González, M. Pessêgo, P. Rodríguez-Dafonte, M. Parajó and L. Garcia-Rio, Supramol. Chem., 2016, 28, 464–474 CrossRef.
- W. B. Hu, W. J. Hu, Y. A. Liu, J. S. Li, B. Jiang and K. Wen, Chem. Commun., 2016, 52, 12130–12142 RSC.
- H. Zhang and J. Han, Org. Biomol. Chem., 2020, 18, 4894–4905 RSC.
- Z. Liu, H. Zhang and J. Han, Org. Biomol. Chem., 2021, 19, 3287–3302 RSC.
- Z. Liu, L. Zhou, H. Zhang and J. Han, Org. Biomol. Chem., 2022, 20, 4278–4288 RSC.
- Z. Liu, Z. Li, B. Li, L. Zhou, H. Zhang and J. Han, Polymers, 2022, 14, 1777 CrossRef CAS PubMed.
- A. Milo, Isr. J. Chem., 2018, 58, 131–135 CrossRef CAS.
- S. Yamazoe, H. Shimogawa, S. Sato, J. D. Esko and M. Uesugi, Chem. Biol., 2009, 16, 773–782 CrossRef CAS PubMed.
- H. Zhang, J. Shen, Z. Liu, Y. Bai, W. An and A. Hao, Carbohydr. Res., 2009, 344, 2028–2035 CrossRef CAS PubMed.
- J. E. M. Lewis, M. Galli and S. M. Goldup, Chem. Commun., 2017, 53, 298–312 RSC.
- L. Chen, X. Sheng, G. Li and F. Huang, Chem. Soc. Rev., 2022, 51, 7046–7065 RSC.
- M. Cheng, C. Yao, Y. Cao, Q. Wang, Y. Pan, J. Jiang and L. Wang, Chem. Commun., 2016, 52, 8715–8718 RSC.
- S. Kosiorek, B. Rosa, T. Boinski, H. Butkiewicz, M. P. Szymański, O. Danylyuk, A. Szumna and V. Sashuk, Chem. Commun., 2017, 53, 13320–13323 RSC.
- A. Kim, R. Ali, S. H. Park, Y. H. Kim and J. S. Park, Chem. Commun., 2016, 52, 11139–11142 RSC.
- J. Lee, N. W. Waggoner, L. Polanco, G. R. You, V. M. Lynch, S. K. Kim, S. M. Humphrey and J. L. Sessler, Chem. Commun., 2016, 52, 8514–8517 RSC.
- Y. Yan, J. Huang and B. Z. Tang, Chem. Commun., 2016, 52, 11870–11884 RSC.
- K. C. Nicolaou, Proc. R. Soc. A, 2014, 470, 20130690 CrossRef CAS PubMed.
- Y. Sun, J. Wang and Y. Yao, Chem. Commun., 2017, 53, 165–167 RSC.
- N. L. Löw, E. V. Dzyuba, B. Brusilowskij, L. Kaufmann, E. Franzmann, W. Maison, E. Brandt, D. Aicher, A. Wiehe and C. A. Schalley, Beilstein J. Org. Chem., 2012, 8, 234–245 CrossRef PubMed.
- S. Chao, Z. Shen, Y. Pei and Z. Pei, Chem. Commun., 2021, 57, 10983–10997 RSC.
- K. Kato, S. Fa, S. Ohtani, T. H. Shi, A. M. Brouwer and T. Ogoshi, Chem. Soc. Rev., 2022, 51, 3648–3687 RSC.
- M. Xue, X. Xu, J. An, J. Wang, Y. Yang and Y. Liu, RSC Adv., 2016, 6, 11488–11491 RSC.
- Q. Wang, Z. Li, D. D. Tao, Q. Zhang, P. Zhang, D. P. Guo and Y. B. Jiang, Chem. Commun., 2016, 52, 12929–12939 RSC.
- K. Wan, S. C. Gao, X. Fang, M. Y. Xu, Y. Yang and M. Xue, Chem. Commun., 2020, 56, 10155–10158 RSC.
- T. Ogoshi, T. Akutsu, Y. Shimada and T. a. Yamagishi, Chem. Commun., 2016, 52, 6479–6481 RSC.
- X. B. Hu, L. Chen, W. Si, Y. Yu and J. L. Hou, Chem. Commun., 2011, 47, 4694–4696 RSC.
- D. N. Shurpik, L. S. Yakimova, V. V. Gorbachuk, D. A. Sevastyanov, P. L. Padnya, O. B. Bazanova, I. d. K. Rizvanov and I. I. Stoikov, Org. Chem. Front., 2018, 5, 2780–2786 RSC.
- Y. Liu, F. Zhou, F. Yang and D. Ma, Org. Biomol. Chem., 2019, 17, 5106–5111 RSC.
- S. Kosiorek, N. Rad and V. Sashuk, ChemCatChem, 2020, 12, 2776–2782 CrossRef CAS.
- Y. Lv, C. Xiao and C. Yang, New J. Chem., 2018, 42, 19357–19359 RSC.
- K. Kravets, M. Kravets, H. Butkiewicz, S. Kosiorek, V. Sashuk and O. Danylyuk, CrystEngComm, 2022, 24, 2213–2216 RSC.
- R. Puttreddy, N. K. Beyeh, E. Kalenius, R. H. A. Ras and K. Rissanen, Chem. Commun., 2016, 52, 8115–8118 RSC.
- X. Yang, S. Y. Wong, D. K. Bwambok, M. B. J. Atkinson, X. Zhang, G. M. Whitesides and A. S. Myerson, Chem. Commun., 2014, 50, 7548–7551 RSC.
- N. L. Strutt, H. Zhang and J. F. Stoddart, Chem. Commun., 2014, 50, 7455–7458 RSC.
- N. Li, J. J. Liu, J. W. Sun, B. X. Dong, L. Z. Dong, S. J. Yao, Z. Xin, S. L. Li and Y. Q. Lan, Green Chem., 2020, 22, 5325–5332 RSC.
- B. Mohan and H. K. Sharma, Inorg. Chim. Acta, 2019, 486, 63–68 CrossRef CAS.
- Y. Chen, H. Q. Tao, Y. H. Kou, H. Meier, J. L. Fu and D. R. Cao, Chin. Chem. Lett., 2012, 23, 509–511 CrossRef CAS.
- S. H. Li, H. Y. Zhang, X. Xu and Y. Liu, Nat. Commun., 2015, 6, 7590 CrossRef PubMed.
- X. B. Hu, Z. Chen, L. Chen, L. Zhang, J. L. Hou and Z. T. Li, Chem. Commun., 2012, 48, 10999–11001 RSC.
- D. Brynn Hibbert and P. Thordarson, Chem. Commun., 2016, 52, 12792–12805 RSC.
- I. K. Petrushenko, N. I. Tikhonov and K. B. Petrushenko, Phys. E, 2021, 130, 114719 CrossRef CAS.
- H. Zuilhof, A. C. H. Sue and J. Escorihuela, J. Org. Chem., 2021, 86, 14956–14963 CrossRef PubMed.
Footnote |
| † Three authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |