Open Access Article
Open Access ArticleRecovery and utilization of crude glycerol, a biodiesel byproduct
Yujia Liua,
Biqi Zhong a and
Adeniyi Lawal*b
a and
Adeniyi Lawal*b
aSchool of Chemical Engineering and Light Industry, Guangdong University of Technology, Guangzhou, 510006, China
bNew Jersey Center for MicroChemical Systems, Department of Chemical Engineering and Materials Science, Stevens Institute of Technology, Castle Point on Hudson, Hoboken, NJ 07030, USA. E-mail: adeniyi.lawal@stevens.edu
First published on 30th September 2022
Abstract
Biodiesel production has increased significantly in the past decade because it has been demonstrated to be a viable alternative and renewable fuel. Consequently, the production of crude glycerol, the main byproduct of the transesterification of lipids to biodiesel, has risen as well. Therefore, the effective recovery and utilization of crude glycerol can provide biodiesel with additional value. In this review, we first summarized the state-of-the-art progress on crude glycerol recovery and purification. Subsequently, numerous approaches have been reviewed for the utilization of crude glycerol, including use as animal feeds, for combustion, anaerobic fermentation, and chemical conversion. Finally, an extensive discussion and outlook is presented in relation to the techniques and processes in the chemical conversion of crude glycerol.
1. Introduction
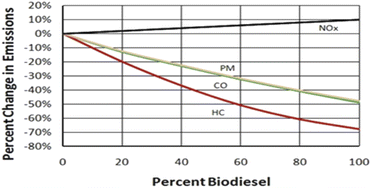
Biodiesel is a renewable fuel produced domestically from animal fats, vegetable oils, or recycled restaurant waste cooking oil and grease. It is nontoxic and biodegradable and is a clean burning alternative to petroleum diesel. Biodiesel is a liquid fuel, commonly referred to as B100. Similar to petroleum diesel, biodiesel is used in fuel compression-ignition engines.1The use of biodiesel as a vehicle fuel enhances energy safety, improves public health and the environment, and provides safety benefits.1,2 Biodiesel can directly replace or expand the supply of conventional petroleum diesel for use in conventional diesel engines. In comparison to petroleum diesel, biodiesel used in conventional diesel engines reduces significant exhaust emissions of carbon monoxide (CO), sulfates, unburned hydrocarbons (HC), polycyclic aromatic hydrocarbons (PAHs), nitrated PAHs, and particulate matter (PM). B100 has optimal emission reductions but lower levels of blends also have advantages. B20 (20 wt% biodiesel and 80 wt% petroleum diesel) has been proven to reduce PM emissions by 10%, CO by 11%, and unburned HC by 21% (Fig. 1) in older engines. Biodiesel increases the cetane number of the fuel while improving the lubricity of the fuel. Biodiesel is non-toxic and non-flammable; thus, it is safer than petroleum diesel and causes less damage to the environment if spilled or released into the environment. Biodiesel has a flash point above 130 °C compared to petroleum diesel's flash point of approximately 52 °C. Biodiesel can be safely handled, stored, and transported.3
The raw materials for biodiesel production are used cooking oil, vegetable oil, yellow grease, and tallow. The production process undergoes a transesterification process that converts oils and fats into chemicals called long-chain mono alkyl esters or biodiesel. In simple terms, 100 pounds of fat or oil are reacted with 10 pounds of a short-chain alcohol (usually methanol) to form 100 pounds of biodiesel and 10 pounds of glycerol using a catalyst (usually sodium hydroxide [NaOH] or rarely, potassium hydroxide [KOH]) to speed up the reaction process. Current research is focused on developing microalga as a potential biodiesel feedstock since it has less competition with oil crops for land availability.4
The global biodiesel industry has seen a steady growth trend over the past decade, with profitable production facilities located in advanced economies everywhere from coast to coast. The industry reached a significant milestone in 2009 when its production exceeded the 15 billion liter mark for the first time. A current market study from Trusted Business Insights in 2021 (ref. 5) shows that the global glycerol market size was $1.5 billion in 2020 and is expected to grow at a compound annual growth rate (CAGR) of 3.9% from 2021 to 2027. The biodiesel source segment accounted for the largest revenue share in 2019 at over 59.1%.
Fig. 2 shows the projected biodiesel and crude glycerol productions from 2003 to 2020 (ref. 6). The total industrial production substantially exceeded the 2013 biodiesel requirement under the Federal Renewable Fuel Standard and was sufficient to meet the requirements of most advanced biofuels.5
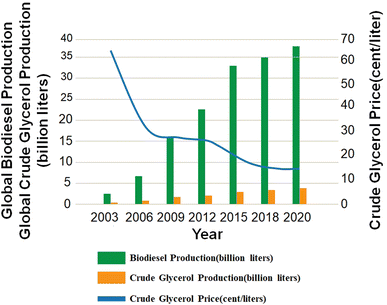 | ||
| Fig. 2 Global biodiesel and crude glycerol production from 2003 to 2020 (ref. 6). | ||
Crude glycerol has a profound impact on the future development of the biodiesel industry since biodiesel production will generate about 10 wt% of the product as the byproduct crude glycerol. According to the latest report of the Organization for Economic Co-operation and Development (OECD), the world biodiesel production was about 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 799 million liters in 2020 and the world biodiesel market is expected to reach the 49
799 million liters in 2020 and the world biodiesel market is expected to reach the 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 882 million liters in 2030,7 which infers that about 4 billion gallons of crude glycerol will be produced. An excess of crude glycerol as a byproduct in biodiesel production will affect the refined glycerol market. Hence, there is a need to develop an economically attractive and sustainable process that utilizes this crude glycerol.
882 million liters in 2030,7 which infers that about 4 billion gallons of crude glycerol will be produced. An excess of crude glycerol as a byproduct in biodiesel production will affect the refined glycerol market. Hence, there is a need to develop an economically attractive and sustainable process that utilizes this crude glycerol.
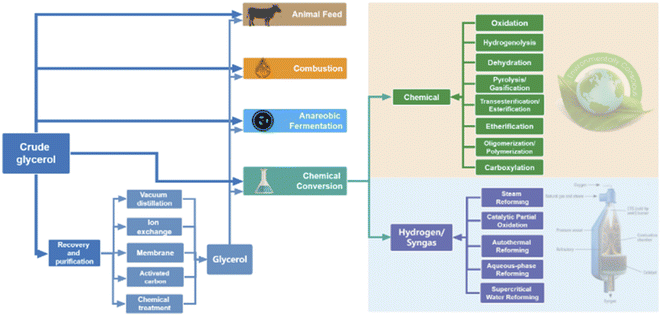
In this review, we first summarize the state-of-the-art technologies and processes on crude glycerol recovery and purification. Subsequently, numerous approaches are discussed for the utilization of crude glycerol, including use as animal feeds, for combustion, anaerobic fermentation, and chemical conversion (shown in Scheme 1).
2. Characteristics of crude glycerol
Table 1 shows that glycerol can be divided into three main categories, namely, crude glycerol, purified glycerol, and commercially synthesized glycerol. The properties of crude glycerol and purified glycerol differ greatly from each other, but the differences between purified and synthesized glycerol are minimal. Actually, purified glycerol is usually prepared of a quality close to that of commercial synthetic glycerol.| Parameter | Crude glycerol | Purified glycerol | Commercially synthesized glycerol |
|---|---|---|---|
| Glycerol content (%) | 60–80 | 99.1–99.8 | 99.2–99.98 |
| Moisture content (%) | 1.5–6.5 | 0.11–0.8 | 0.14–0.29 |
| Ash content (%) | 1.5–2.5 | 0.054 | <0.002 |
| Soap content (%) | 3.0–5.0 | 0.56 | N/A |
| Acidity (pH) | 0.7–1.3 | 0.10–0.16 | 0.04–0.07 |
| FAME | Residue | Residue | Residue |
| Color (APHA) | Dark | 34–45 | 1.8–10.3 |
| Chloride (ppm) | ND | 1.0 | 0.6–9.5 |
Depending on the potential end use and purity, purified glycerol can be classified into three grades (Table 2). Purified glycerol produced from biodiesel byproducts is typically traded in the market at 99.5–99.7% purity. As expected, however, the purity of crude glycerol from biodiesel production is far below that of purified glycerol.
| Grade | Type of glycerol | Preparation and application |
|---|---|---|
| Grade-I | Technical grade ∼99.5% | Prepared by the synthetic process and used as a base material for various chemicals but not for food or pharmaceutical formulations |
| Grade-II | USP grade 96–99.5% | Prepared from vegetable oil sources or animal fat, applicable to food, pharmaceuticals, and cosmetics |
| Grade-III | Kosher or USP/FCC grade 99.5–99.7% | Prepared from vegetable oil sources, suitable for use in kosher foods and drinks |
The results of elemental analysis of crude glycerol obtained from biodiesel production are summarized in Table 3,10 showing that carbon, balanced oxygen, and hydrogen are the main elemental components. With high value of carbon in crude glycerol allows it to be a renewable energy source that can be used for different applications.
| Elements | wt% |
|---|---|
| Carbon (C) | 52.77 |
| Balance oxygen (O) | 36.15 |
| Hydrogen (H) | 11.08 |
| Nitrogen (N) | <0.0001 |
| Sulfur (S) | — |
3. Crude glycerol recovery and purification
A variety of practicable techniques and methods are available for recovering crude glycerol from transesterification reaction, such as centrifugation, bleaching, and chemical treatment. In chemical treatment, the neutralization reaction using a strong acid to remove the catalyst and soaps is the most common method used in the pretreatment process of crude glycerol.11 The salts produced from neutralization can be removed by decantation and filtration. The bleaching procedure effectively reduces the large amount of free fatty acids (FFA), odor, and pigmented compounds (e.g., carotenoids and chlorophyll) contained in crude glycerol.13 Maximum yields can be achieved using bleaching recovery techniques for glycerol14 since bleaching not only recovers the glycerol but also saponifies the free triglycerides.15In the third recovery process, crude glycerol is first recovered by centrifugation, and then any contaminated soap is converted to acid or salt by treatment with hydrochloric acid.16,17 Water and methanol in the glycerol phase can be separated by distillation through a simple distillation process18 and the glycerol layer can be neutralized with caustic soda. Following product recovery, the process of glycerol purification is implemented. Although a variety of purification techniques are available, as shown in Fig. 3, purifying crude glycerol to the level of purity required for food or pharmaceutical grade is expensive.19
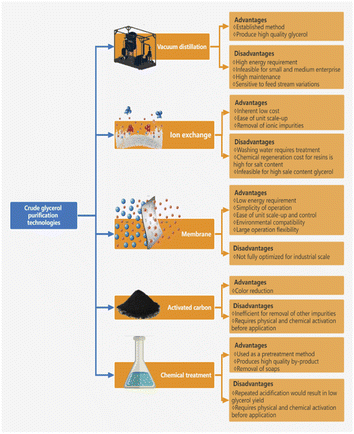 | ||
| Fig. 3 Summary of glycerol purification technologies.12 | ||
4. Utilization of glycerol
The main utilization of glycerol in 2020 is shown in Fig. 4. The top category is the application in pharmaceutical industries, personal oral care products, and cosmetics since glycerol is an ideal ingredient in preventing moisture loss. Owing to the large amount of excess glycerol produced, in addition to its traditional uses in the pharmaceutical and personal care sectors, new opportunities have emerged in recent years to convert glycerol into value-added chemicals. Numerous approaches for utilizing crude glycerol have been investigated, including use as animal feeds, for combustion, anaerobic fermentation, and chemical conversion.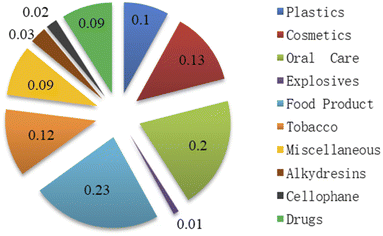 | ||
| Fig. 4 End use of glycerol in 2020 (ref. 5). | ||
4.1. Animal feeds
Glycerol has been used to feed animals since the 1970s.20 However, the supply of glycerol restricted its use as animal feed.21 The growth of corn prices and excess of crude glycerol led to a renewed enthusiasm to study the use of crude glycerol in animal feeds. Nevertheless, crude glycerol needs to be effectively purified before it can be used in animal feed.El-Hawarry et al.22 utilized glycerol, molasses, and starch as carbon sources for rearing Nile tilapia to form different bioflocs. Under low stocking density, the whole-body protein and lipid content in bioflocs formed with glycerol shows the highest value.
Louvado et al.23 conducted a comparative trial for feeding Dicentrachus labrax with or without the addition of refined glycerol as a supplement. Their results showed that the addition of refined glycerol as a supplement during feeding did not affect the composition of the fish's intestinal bacterial colonies but reduced the amino acid catabolism.
To decrease enteric methane emissions of cattle, Karlsson et al.24 evaluated the impact of replacing wheat starch (200 g kg−1 of Dry Matter) with refined glycerol in a grass silage and barley-based Total Mixed Rations (TMR) on feed intake, milk production, and methane emissions since glycerol can provide the energy required for milk production without increasing intestinal methane production. Their results indicated that replacing wheat starch (200 g kg−1 of DM) with refined glycerol in a grass silage and barley-based TMR increased CH4 emissions and Dry Matter Intake (DMI) with no effect on CH4/DMI or ECM yield.
4.2. Combustion
The simplest method of utilizing crude glycerol is its combustion as a fuel because this utilization requires no purification whatsoever. Compared to crude oil (∼42.3 MJ kg−1),25 natural gas (∼52.2 MJ kg−1), and bituminous coal (∼30.2 MJ kg−1),26 glycerol has an intermediate calorific level (∼16 MJ kg−1), but it has not yet been utilized as a fuel.27,28 The challenges of combusting crude glycerol are: (1) the intermediate calorific value prevents traditional burners from maintaining a stable flame;29 (2) it features viscosity at room temperature30 and is difficult to atomize with traditional nebulizers; (3) it is a flame retardant, so it is difficult to be burned;31 (4) the salt content can cause corrosion of burner nozzles and combustion systems;9 (5) acrolein can be obtained by the combustion of glycerol32 at low temperature (between 280 and 300 °C).There is also water present in crude glycerol, which causes difficulties in combustion. Moreover, the auto-ignition temperature of crude glycerol is 370 °C, which is quite high compared to gasoline (280 °C) and kerosene (210 °C).33 Standard combustion produces a self-sustaining flame with a single spark; however, glycerol will not spontaneously combust under these conditions. The droplets of glycerol passing through an open flame will combust, but the energy released is not sufficient to maintain a sustained combustion reaction. Co-burning with other more easily ignited fuels will aid the ignition process and maintain the flame. Therefore, special burners have been designed for the co-combustion of crude glycerol with other fuels.34,35
Metzger29 employed a modified burner system to combust methylated glycerol, demethylated glycerol, and laboratory grade glycerol. The study used a 7 kW swirl burner and an adiabatic combustion chamber to improve combustion and flame stability.
Setyawan et al.36 compared crude glycerol with biodiesel, pure glycerol, petroleum diesel, and ethanol in order to investigate the ignition and burning characteristics of a single drop of crude glycerol. At the same temperature, the total combustion time and ignition delay time of crude glycerol ranked second to pure glycerol, while the combustion rate was the largest. The results show that impurities, mainly water and methanol, have a profound effect on the combustion performance of crude glycerol.
The co-combustion of crude glycerol with other renewable liquids is also a viable option since it does not contribute to CO2 emissions or increase the concentration of harmful products (SO2, NOx, and CO) of combustion.37,38 Szwaja et al.37 burned glycerol mixed with ethanol in a spark ignition reciprocating engine to analyze the toxic content, combustion thermodynamics, and engine performance in the exhaust gases emitted by combustion.
Combustion with other fuels is the simplest way to utilize crude glycerol and it does not rely on any purification. Nevertheless, it has its own technological limitations, including problems of high auto-ignition temperatures and corrosion caused by the presence of salts.
4.3. Anaerobic fermentation
A few technologies39,40 are being sought on the basis of biological and chemical conversions to add value to crude glycerol. A number of microorganisms can be used naturally to produce methane through anaerobic digestion using purified glycerol as their sole source of carbon and energy.41 Unlike chemical conversion, biotransformation can transform glycerol into a bulk product devoid of high pressure and/or temperature. Compared to aerobic fermentation, anaerobic fermentation is also advantageous in terms of lower operating costs and capital. Rhamnolipids are the most extensively studied biosurfactants nowadays.42 Zhao et al.43 used different substrates for the anaerobic growth of P. aeruginosa strains to synthesize rhamnolipids. They found that glycerol as a substrate was the only way to synthesize rhamnolipids during the anaerobic growth of P. aeruginosa strains, and the air–water surface tension reduced from 72.6 mN m−1 to less than 29 mN m−1. Li et al.44 isolated a biohydrogen-producing strain named Enterobacter aerogenes EB-06, and the yield coefficient reached 1.07 mmol H2 per mol glycerol under the optimal conditions.At current crude glycerol price of45 $1.07 per gal (10 cents per lb), glycerol is used as a substitute for sugar in the production of fuels and chemicals through microbial fermentation. Compared to sugar, the use of glycerol fermentation for fuel production and chemical reduction has many advantages.46 One advantage is that the high reduction of carbon atoms in glycerol produces higher fuel yields and reduces the chemicals in glycerol. The conversion of glycerol to the pyruvate or glycolytic intermediates phosphoenolpyruvate (PEP) produces twice the amount of reducing equivalents from glucose or xylose metabolism. Thus, fermentative metabolism will be able to obtain higher fuel yields and fewer chemicals from glycerol than those obtained from common sugars, such as xylose or glucose.36
4.4. Chemical conversion
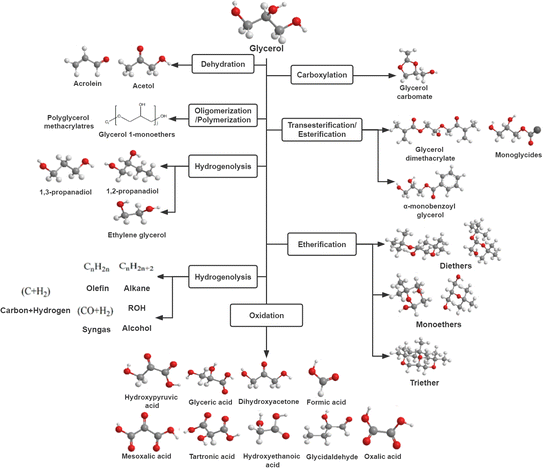 | ||
| Fig. 5 Probable catalytic pathways for the conversion of glycerol into useful chemicals.31 | ||
Glycerol catalytic oxidation, which uses oxidizing agents including air, produces larger quantities of products such as glyceric acid, dihydroxyacetone, and formic acid. The reaction pathway for the selective oxidation of glycerol is complex, generating different C3 products (dihydroxyacetone (DHA), glyceric acid (GLYA), and tartronic acid (TA)), C2 products (oxalic acid (OXA) and glycolic acid (GLYCA)), and even C1 products (formic acid (FA)).48 As can be seen, propane based on glycerol is superior to the conventional propane production method in the production of acrylic acid. In the case of the former, the acid-catalyzed elimination of water is the first process to obtain acrolein and subsequent oxidation to acrylic acid, whereas two oxidation steps with different catalysts are required for the latter.49 The catalytic oxidation of glycerol is mainly performed using noble metals such as Pt, Pd, Au, and bimetallic Pt-, Pd-, or Au-based catalysts. Along with the improvement of product selectivity and catalytic activity, several studies have focused on improving the selectivity of the desired products and catalytic activity under base-free conditions.50 Ayman El Roz et al.51 performed experiments on the oxidation of glycerol to form glyceraldehyde under alkali-free conditions using catalysts loaded with Pt on different supports in a batch reactor to investigate the impact on the reaction of different supports. The experimental results showed that Pt loading on γ-Al2O3 had the highest catalyst activity but the selectivity of this catalyst for glyceraldehyde decreased significantly with increasing reaction time. In contrast, Pt/SiO2 had the highest selectivity for glyceraldehyde.
As shown in Fig. 5, glycerol can be used to yield ethylene glycol, 1,2-propanediol, and 1,3-propanediol via hydrogenolysis. In general, the hydrogenolysis of glycerol utilizes a homogeneous base (i.e., Ca(OH)2, NaOH, and Ba(OH)2) and a supported transition metal catalyst (i.e., Pd, Pt, Ru, Ni, or Cu) to catalyze this process, which can selectively break the C–C and/or C–O bonds.52–54 Gong et al.55 employed hydrotalcite-derived catalysts to selectively hydrolyze glycerol to 1,2-propanediol in aqueous phase and under alkali-free conditions. Their results showed that the Co2–Ca4–Al3 catalyst reached 100% optimal glycerol conversion and 90.5% 1,2-propanediol selectivity in glycerol hydrogenolysis. An extensive study has shown that bifunctional catalysts with Brønsted acid sites and metal could increase selective glycerol hydrolysis to 1,3-propanediol, where H2 was dissociated at the metal sites and glycerol was activated at the acid sites.56 Currently, tungsten-containing bifunctional catalysts have shown superior performance in the selective hydrogenolysis of glycerol to 1,3-propanediol, and Wu et al.57 summarized in detail the role of various tungsten-containing bifunctional catalysts, including tungsten species and metal active sites for the selective production of 1,3-propanediol from glycerol for its hydrolysis.
The catalytic dehydration of propanetriol resulted in the formation of acetonol and acrolein, as demonstrated in Fig. 5. Acetol is formed by removing a water molecule and then undergoing double bond rearrangement. The removal of another water molecule leads to acrolein formation. Dehydration needs to performed at a temperature of 280 to 350 °C.58 Acetol can be produced from glycerol with both heterogeneous and homogeneous catalysts.59 Basu and Sen.60 summarized the recent progress on common and typical catalysts used in the catalytic synthesis of acetone alcohols from glycerol, including noble metals and transition-based metals. On the other hand, the gas-phase dehydration of glycerol to acrolein is also a hot research topic in recent years. Abdullah A.61 summarized the recent advances in glycerol-catalyzed dehydration to acrolein, including the improved performance of various catalysts and prospects for commercialization and scale-up of green acrolein.
The catalytic pyrolysis of glycerol can form syngas via a pathway for producing many reaction intermediates. Shahirah et al.62 synthesized 3%La–20%Ni/77%α-Al2O3 catalyst for the pyrolysis of glycerol to form syngas, and characterized its physiochemical properties. In their results, the highest glycerol conversion reached 36.96% at 1073 K and the H2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO ratios of syngas were constantly lower than 2.0. Batista et al.63 applied sodium and activated vermiculites as catalysts for glycerol pyrolysis. Their results showed that the use of catalysts increased the conversion of the glycerol pyrolysis process. The gasification of glycerol also can form syngas, alkane, and olefin with gasification agents such as O2, steam, and CO2.64 Almeida et al.65 employed alumina particles as bed material in a down-flow fixed-bed reactor to gasify glycerol with steam as the oxidizing agent.
CO ratios of syngas were constantly lower than 2.0. Batista et al.63 applied sodium and activated vermiculites as catalysts for glycerol pyrolysis. Their results showed that the use of catalysts increased the conversion of the glycerol pyrolysis process. The gasification of glycerol also can form syngas, alkane, and olefin with gasification agents such as O2, steam, and CO2.64 Almeida et al.65 employed alumina particles as bed material in a down-flow fixed-bed reactor to gasify glycerol with steam as the oxidizing agent.
The transesterification/esterification of glycerol can produce monoglycerides, α-monobenzoyl glycerol, and glycerol dimethacrylate. Glycerol in transesterification reactions is performed with alkaline catalysts with fatty methyl esters, whereas the esterification of glycerol is performed with fatty acids.66 Mou et al.67 studied the esterification of glycerol with acetic acid on hydrophobic polymer-based solid acid to produce glycerol diacetate (DAG) and glycerol triacetate (TAG) as petrol fuel additives.
The catalytic etherification of glycerol can produce several useful fuel additives, for example, mono-, di-, and tri-ethers, which are transformed by the reactive etherification of glycerol with alcohols or alkenes. Chiosso et al.68 studied the catalytic performance of carbonaceous system (Ccs) functionalized with –SO3H groups in the etherification of refined (Gly) and crude glycerol (GlyC) with benzyl alcohol (BA).
As shown in Fig. 5, the oligomerization reactions of glycerol can be performed to yield polyglycerol and polyglycerol esters. A number of applications of low molecular-weight oligomers in polymer production, food industry, and cosmetics have stimulated researchers' interest in the study of glycerol-catalyzed oligomerization. Barros et al.69 used low-cost dolomite catalyst in glycerol oligomerization to produce diglycerol and triglycerol, and evaluated the catalytic performance with different reaction parameters.
The carboxylation of glycerol can generate glycerol carbonate. Glycerol carbonate is extensively applied in the cosmetic and pharmaceutical industries, as well as a source of electrolytes for lithium-ion batteries and as an intermediate in polymer synthesis.70 Hu et al.71 studied the catalytic performance of cobalt-based zeolitic imidazolate framework-67 (ZIF-67) under different reaction parameters for the process of glycerol carboxylation.
Glycerol can also be oxidized by biocatalysts such as microorganisms and enzymes. Dihydroxyacetone (DHA) is a chemical product that is widely used in the cosmetic industry for the manufacture of artificial sunscreens.72 Table 4 lists a variety of studies on the production of DHA from glycerol.
| Author | Remarks |
|---|---|
| de la Morena et al.73 | They utilized Gluconobacter oxydans ATCC 621 as the biocatalyst to convert glycerol into dihydroxyacetone |
| Ripoll et al.74 | Agar-immobilized Gluconobacter oxydans NBRC 14819 (Gox) was the best heterogeneous biocatalyst, reaching a quantitative production of 50 g L−1 of DHA from glycerol solely in the presence of water |
| Jain et al.75 | Using genetic engineering techniques to modify genes in Escherichia coli (E. coli) aimed at increasing DHA production, achieving a maximum theoretical yield of 6.60 g L−1 DHA |
The electrocatalytic process does not need traditional chemical oxidants (Table 5). Biotransformation and catalysis are the two major approaches to convert crude glycerol into various chemicals. There are prospects for producing lipids, citric acid, butanol, and monoglycerides from crude glycerol. However, there is still room for the further development of many of these technologies before they can be incorporated into biorefineries and be economically efficient and operationally feasible.
| Author | Remarks |
|---|---|
| Zhou and Shen76 | Oxidation of glycerol to DHA catalyzed by the PtAuPdAg catalyst in alkaline solution; the HPLC results show that the DHA selectivity was 79.6% |
| Huang et al.77 | Cobalt borate was used as a catalyst to increase the yield of glycerol oxidation to C3 chemicals, resulting in 67% DHA in the liquid product and an average yield of 90 mmol m−2 h−1 |
| Tran et al.78 | Manganese oxide (MnO2) was utilized as a catalyst for the electrocatalytic glycerol oxidation, which reached the selectivity of 46% for DHA |
| Liu et al.79 | They developed a photoelectrochemical system based on nanoporous BiVO4, producing 56 mmol gcatalyst per h of DHA at a potential of 1.2 V vs. RHE under AM 1.5 illumination (100 mW cm−2) |
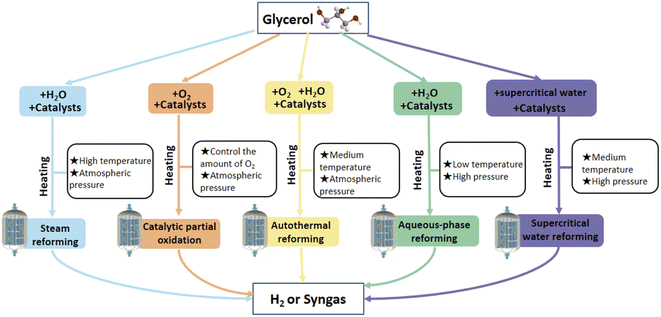
4.4.2.1. Steam reforming. Steam reforming (SR) is a proven technique that can be carried out at atmospheric pressure. Czermik et al.80 first investigated SR of a mixture of fatty acid methyl esters and crude glycerol in a fluidized bed reactor using a commercial nickel catalyst. The yield of H2 was as high as 76% at a reaction temperature of 1123 K and a steam-to-carbon (S/C) ratio of 2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 3
1 to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. From then on, there has been a growing number of research articles and recent studies on SR of glycerol that have generated a strong interest in the SR of glycerol, as shown in Table 6.
1. From then on, there has been a growing number of research articles and recent studies on SR of glycerol that have generated a strong interest in the SR of glycerol, as shown in Table 6.
| Author | Metal catalysts | Support | Remarks |
|---|---|---|---|
| Wang et al.81 | At 450 °C and ambient pressure | ||
| Ni–N | CeO2–C | Conversion = 82.3%, H2 selectivity = 14% | |
| Ni–N | CeO2–P | Conversion = 100%, H2 selectivity = 44.7% | |
| Ni–Cl | CeO2–C | Conversion = 96.9%, H2 selectivity = 34.2% | |
| Ni–Cl | CeO2–P | Conversion = 100%, H2 selectivity = 38.7% | |
| Ni–N: nickel nitrate hexahydrate as nickel sources | |||
| Ni–Cl: Nickel chloride hexahydrate as nickel sources | |||
| C: Calcined in a muffle furnace at 550 °C for 4 h | |||
| P: Glow discharge plasma for 2 h | |||
| Zhou et al.82 | At temperature of 400 °C | ||
| Ni–Co | CNT | Conversion of glycerol was 95.7% with Ni(I)Co(I)/CNTs >92.2%, Ni(0)Co(0)/CNTs >85.7%, Ni(I)Co(0)/CNTs >78.3% with Ni(0)Co(0)/CNTs. i: in the cave; o: on the external surface | |
| Jing et al.83 | At temperature of 450 °C | ||
| Ni | Ce0.1–Al | Conversion = 86.7%, H2 selectivity = 71.3% | |
| Ce0.3–Al | Conversion = 92.1%, H2 selectivity = 78.2% | ||
| Ce0.5–Al | Conversion = 98.5%, H2 selectivity = 81.8% | ||
| Ce0.7–Al | Conversion = 97.4%, H2 selectivity = 82.9% | ||
| Ce0.9–Al | Conversion = 96.8%, H2 selectivity = 70.1% | ||
| Wang et al.84 | At temperature of 700 °C | ||
| Ni | Attapulgite | Conversion = 90.2%, H2 selectivity = 61.8% | |
| Ni–Co | Conversion = 94.5%, H2 selectivity = 65.5% | ||
| Ni–Cu | Conversion = 98.1%, H2 selectivity = 64.0% | ||
| Ni–Zn | Conversion = 93.4%, H2 selectivity = 63.0% | ||
| Zhang et al.85 | At temperature of 450 °C | ||
| Ni | Zr–Al-c | Conversion = 96.1%, H2 selectivity = 90.9% | |
| Zr–Al-ch | Conversion = 95.9%, H2 selectivity = 88.4% | ||
| Zr–Al-u | Conversion = 99.2%, H2 selectivity = 97.7% | ||
| Zr–Al-uh | Conversion = 99.3%, H2 selectivity = 87.9% u: urea homogeneous precipitation | ||
| uh: Combining the homogeneous precipitation and hydrothermal treatment | |||
| c: Co-precipitation method | |||
| ch: Hydrothermal treatment | |||
| Shejale and Yadav86 | At temperature of 773 K | ||
| Ni–Cu | La2O3–MgO | Conversion = 84.5% | |
| Ni–Co | Conversion = 70.1% | ||
| Gao et al.87 | At temperature of 630 °C | ||
| Ni | Coal fly ash | Conversion = 90%, H2 selectivity = 42% | |
| FA1 | Conversion = 95%, H2 selectivity = 34% | ||
| FA2 | Conversion = 99%, H2 selectivity = 83% | ||
| FA3 | Conversion = 100%, H2 selectivity = 83% | ||
| FA4 | FA1 and FA2 collected from Canada, FA3 collected from India, FA4 collected from China | ||
| Veiga et al.88 | At temperature of 630 °C | ||
| Ni | La2O3–CeO2 | Conversion = 99.7% | |
| La2O3–ZrO2 | Conversion = 99.7% | ||
| La2(Ce0.5Zr0.5)O7 | Conversion = 99.9% |
4.4.2.2. Catalytic partial oxidation. Catalytic partial oxidation (CPO) was firstly reported by Ashcraft89,90 and Schmidt91,92 groups in the 1990s, having fast start-up and response times and, due to its exothermic properties, can be designed to be sustainable without an external heat source through proper insulation.93 Glycerol can be oxidized utilizing a noble metal catalyst, such as Pt, Pd, Rh, and Au. This process generates H2, CO, CO2, H2O, and larger hydrocarbons, olefins such as ethylene.94 Wang95 investigated the thermodynamics of hydrogen production by the partial oxidation of glycerol using the Gibbs free energy minimization method and concluded that at the optimal reaction conditions of 1000–1100 K, O2/C3H8O3 molar ratios of 0.4–0.6, 1 atm, the H2 yield reached 78.93–87.31% with almost complete glycerol conversion. The partial oxidation of glycerol alone has rarely been studied few because of steam, which is generated in combustion reactions. This can also be explained by the fact that much less H2 is produced during the CPO of glycerol only. After the addition of steam, the autothermal reforming reaction of CPO becomes possible, as described in the following section.
4.4.2.3. Autothermal reforming. To improve H2 production, co-feeding glycerol with steam under CPO conditions is called autothermal reforming (ATR).
ATR can be performed with a variety of feed combinations for effective thermal management. In order to maintain a self-sustaining operation, the heat of process would be less than zero.
Liu et al.96 showed the sustainable ATR of glycerol with a dual layer monolith catalyst and performed thermodynamic analysis by Aspen Plus (shown in Fig. 6). Their conclusion was that almost 100% conversion of glycerol to H2, CO2, CO, and CH4, essentially equilibrated at a temperature of 650 °C. In addition, Liu and Lawal96 replaced the model compounds with actual crude glycerol for ATR and calculated the results in comparison with those calculated using Aspen Plus simulations. Their non-chemometric simulation method does not require the selection of a probable set of reactions and produces results that fit the experimental data very well. The overall gaseous carbon yield was up to 98% at S/C ratio of 1, O2/C ratio of 0.7, and 750 °C.
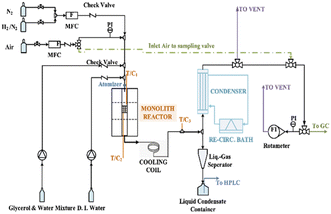 | ||
| Fig. 6 Schematic of experimental set-up.96 | ||
 | ||
| Fig. 7 Schematic of the experiment for enhanced H2 production through the aqueous-phase reforming of glycerol.110 | ||
Experimental results show that at low temperatures and O2/C molar ratios, a more efficient catalyst for water gas shift reaction is needed to obtain the gas equilibrium molar composition.
Schmidt et al.97,98 performed the ATR of glycerol/water mixtures and glycerol on H2 and syngas using catalysts of Pt and Rh coated on monoliths to study the effect on the production of H2 and syngas within millisecond contact times. They developed a process that did not require a mixer preheater or any upstream to remove the huge cost of thermal input associated with glycerol evaporation and glycerol homogeneous decomposition. Glycerol was injected into the reaction system by means of a nebulizer, where 10–100 μm droplets of glycerol were sprayed directly onto the surface of the ignited catalyst.
At high temperature, noble metal catalysts commonly experience catalyst sintering. Rennard et al.96 conducted catalyst stability tests, and no coke fouling was observed for glycerol ATR running over 400 hours on Rh–Ce/CeO2/Al2O3 catalysts. Nevertheless, Liu and Lin99 studied the ATR of glycerol using Pt/LaMnO3 and Pt catalysts in a 28 hour test and the average particle size of Pt particles was found to increase by one order of magnitude after the reaction. Moreira R. et al.100 compared ATR technology with other reforming technologies, outlining aspects such as catalysts and kinetics, suggesting how ATR technology can be improved in the future.
4.4.2.4. Aqueous-phase reforming. The reaction of the reactants with water to produce hydrogen can also take place in the aqueous phase, where aqueous phase reforming occurs. The aqueous phase reforming (APR) of sugar substrates was first performed by Dumesic et al.101 using platinum-based catalysts at a pressure of 20–25 bar and temperature of 200–250 °C. They investigated the APR of glycerol, ethylene glycol, sorbitol, and glucose as feedstocks. Glycerol belongs to the same carbohydrate group such as sugar; therefore, it is vital to learn about the basic aspects of the study. The reaction products in this study were H2, CO2, CH4, and higher chain alkane, and they used swing absorption technology to extract and purify H2 from the product stream.102 The use of noble metal catalysts for APR has been studied further, as shown in Table 7.
| Author | Metal catalysts | Support | Remarks |
|---|---|---|---|
| Larimi and Khorasheh103 | Pt–Rh | Al2O3 | The crude glycerol conversion reached 43.1%, while the pure glycerol conversion was 93.5% |
| Wu et al.104 | Ni–Cu | CeO2 | The addition of Cu promoted the water–gas shift (WGS) reaction and inhibited the production of methane, which increased the H2 production rate from 125.08 to 195.57 μmol min−1 g cat−1 |
| Guo et al.105 | Pt | γ-Al2O3 | Pt/Al2O3: H2 yield of 18.3%, Pt1Ni1/Al2O3: H2 yield of 17.0% |
| Pt–Ni | Pt1Co1/Al2O3: H2 yield of 20.7%, Pt1Cu1/Al2O3: H2 yield of 13.1% | ||
| Pt–Co | Pt1Fe0.5/Al2O3: H2 yield of 18.8%, Pt1Fe0.75/Al2O3: H2 yield of 22.2% | ||
| Pt–Cu | Pt1Fe1/Al2O3: H2 yield of 30.1%, Pt1Fe2/Al2O3: H2 yield of 21.9% | ||
| Pt–Fe | Pt1Fe3/Al2O3: H2 yield of 19.6% | ||
| Entezary and Kazemeini106 | Pt | CeO2–Al2O3 | In a structured catalyst microreactor, the conversion of glycerol reached 75.3% and selectivity toward hydrogen production reached 92.4% |
| Bastan and Kazemeini107 | Ni | Al2O3–MgO | At 2% Ni loading, the catalyst showed the highest activity with 92% total conversion and 76% hydrogen selectivity |
| Alvear et al.108 | Pt–Pd | Mesoporous carbon | At temperature of 225 °C |
| Conversion = 70%, H2 selectivity ≈90%, CO2 selectivity ≈90%, alkane selectivity ≈10% | |||
| Fasolini et al.109 | Pt | TiO2 | Glycerol conversion reached 66% with 27% H2, 17% CO2, and 33% 1,2-propanediol selectivity at 225 °C and 3 h |
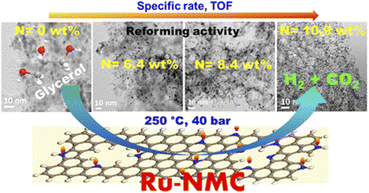
| Gogoi et al.110 | Ru | Nitrogen-doped mesoporous carbons (NMCs) | The 5% Ru-NMC-3 catalyst (Fig. 7) with 10.9% N content performed best with 92% glycerol conversion and 88.5% H2 selectivity |
| Pt–Ru |
4.4.2.5. Supercritical water reforming. Among the different energy-added pathways for excess glycerol in biodiesel production, supercritical water reforming (SCWR) is a developmental technique of great interest.111,112 Ortiz et al.113 investigated the SCWR of glycerol in a tubular fixed-bed reactor using a Ru/Al2O3 catalyst. The experimental results showed that when using Ru/Al2O3 catalysts, glycerol conversion was very high (>99%) at 600 °C and above but low (<50%) at 500 and 550 °C. Bogdan et al.114 investigated the transformation of ethanol, glycerol, glucose, and sorbitol with supercritical water. The conversion of glycerol increased with increased operating temperature from 500 °C to 700 °C and reached 100% at 700 °C.
5. Conclusion
Crude glycerol has been well accepted as an attractive sustainable resource. New studies are desired to add value to this byproduct to further develop biodiesel production. The simplest method of utilizing crude glycerol is its combustion as a fuel since this utilization does not require any purification. However, it has its own technical challenges since glycerol has a high auto-ignition temperature and the corrosion problems caused by the presence of salts. Biotransformation and catalysis are the two major pathways for the conversion of crude glycerol into different chemicals. There are prospects for the production of lipids, citric acid, butanol, and monoglycerides from crude glycerol. Nevertheless, there is still room for the further development of many of these technologies before they can be incorporated into biorefineries and be economically efficient and operationally feasible.The thermoconversion of crude glycerol to H2 or syngas offers the opportunity to reduce our reliance on fossil fuels. Heterogeneous catalysis is essential for the conversion of crude glycerol to H2 or syngas. However, there is still much potential for improvement in existing methods. For instance, steam reforming needs designing catalysts to operate at low temperatures. Catalytic partial oxidation and autothermal reforming need to be designed with temperature-durable catalysts.
In the field of animal feed and anaerobic fermentation, crude glycerol needs to be effectively purified before it can be used. In fact, impurities in crude glycerol can also strongly influence the conversion of glycerol into other products. In some biotransformation processes, contaminants can inhibit the growth of cell and fungus, resulting in lower product yields and rates. Moreover, impurities can poison the catalyst, increase the coke yield, and affect the product yield of catalytic conversion. Therefore, the purification technology of crude glycerol is of great significance for the in-depth application of crude glycerol in various fields.
Although purified glycerol is more widely used in applications than crude glycerol, purified glycerol incurs added costs. It is noteworthy that most studies have focused on pure glycerol instead of actual biodiesel byproduct. From this perspective, there is still room to improve the suitability of crude glycerol for further applications. Various model compounds of crude glycerol should be studied as raw materials, thus reducing the cost of purification and expanding the application of crude glycerol. For example, in the field of anaerobic fermentation, research could be conducted to find microorganisms that can tolerate impurities in crude glycerol with good fermentation results. Because of the complex composition of crude glycerol, the thermal conversion of crude glycerol to H2 or syngas requires the development of a suitable reactor with an active and tolerant catalyst for the reaction mechanism.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 22008036).References
- http://www.afdc.energy.gov/fuels/biodiesel_basics.html.
- M. A. Ahmad, A. Letchumanan, S. Samsuri, W. N. A. Mazli and J. M. Saad, Bioresour. Bioprocess., 2021, 8, 54 CrossRef.
- http://www.afdc.energy.gov/fuels/biodiesel_benefits.html.
- F. Saladini, N. Patrizi, F. M. Pulselli, N. Marchettini and S. Bastianoni, Renewable Sustainable Energy Rev., 2016, 66, 221–227 CrossRef.
- https://www.trustedbusinessinsights.com/details/glycerol-market-2020-and-forecast-2021-2027.
- N. M. Saifuddin, R. Hussein and M. Ong, Green Chem. Lett. Rev., 2018, 11, 135–157 CrossRef.
- OECD, and Food and A. O. o. t. U. Nations, Biodiesel projections: Production and use, 2021 Search PubMed.
- H. Hassan, T. L. Ooi and A. Salmiah, J. Oil Palm Res., 2003, 15, 1–5 Search PubMed.
- P. L. Kenkel and R. B. Holcomb, Feasibility of on-farm or small scale oilseed processing and biodiesel production, 2008 Search PubMed.
- Y. D. You, J. L. Shie, C. Y. Chang, S. H. Huang, C. Y. Pai, Y. H. Yu and C. F. H. Chang, Energy Fuels, 2008, 22, 182–189 CrossRef CAS.
- M. S. Ardi, M. K. Aroua and N. A. Hashim, Renewable Sustainable Energy Rev., 2015, 42, 1164–1173 CrossRef CAS.
- L. R. Kumar, S. K. Yellapu, R. D. Tyagi and X. Zhang, Bioresour. Technol., 2019, 293, 122155 CrossRef CAS PubMed.
- M. Yuliana, L. Trisna, F. Sari and V. B. Lunardi, J. Environ. Chem. Eng., 2021, 9, 105239 CrossRef CAS.
- A. U. Israel, I. B. Obot and J. E. Asuquo, J. Chem., 2008, 5, 940–945 CAS.
- M. Grant and R. Hawkins, The concise lexicon of environmental terms: with a list of scientific abbreviations and glossary of acronyms, Wiley, Chichester, 1995 Search PubMed.
- K. C. Yong, T. L. Ooi, K. Dzulkefly, W. M. Z. W. Yunus and A. H. Hazimah, J. Oil Palm Res., 2001, 13, 1–6 CAS.
- M. J. Haas, A. J. McAloon, W. C. Yee and T. A. Foglia, Bioresour. Technol., 2006, 97, 671–678 CrossRef CAS PubMed.
- S. Chozhavendhan, R. Praveen Kumar, S. Elavazhagan, B. Barathiraja, M. Jayakumar and S. J. Varjani, in Waste to Wealth, ed. R. R. Singhania, R. A. Agarwal, R. P. Kumar and R. K. Sukumaran, Springer Singapore, Singapore, 2018, pp. 65–82, DOI:10.1007/978-981-10-7431-8_4.
- M. Hasheminejad, M. Tabatabaei, Y. Mansourpanah, M. K. far and A. Javani, Bioresour. Technol., 2011, 102, 461–468 CrossRef CAS PubMed.
- L. J. Fisher, J. D. Erfle, G. A. Lodge and F. D. Sauer, Canadian Veterinary Journal La Revue Veterinaire Canadienne, 1973, 53, 289–296 CAS.
- B. Kerr, W. Dozier and K. Bregendahl, Proceedings of the 23rd Annual Carolina Swine Nutrition Conference, 2007 Search PubMed.
- W. N. El-Hawarry, R. M. Shourbela, Y. G. Haraz, S. A. Khatab and M. A. O. Dawood, Aquaculture, 2021, 542, 736919 CrossRef CAS.
- A. Louvado, F. J. R. C. Coelho, M. Palma, L. C. Tavares, R. O. A. Ozorio, L. Magnoni, I. Viegas and N. C. M. Gomes, Appl. Microbiol. Biotechnol., 2020, 104, 8439–8453 CrossRef CAS PubMed.
- J. Karlsson, M. Ramin, M. Kass, M. Lindberg and K. Holtenius, J. Dairy Sci., 2019, 102, 7927–7935 CrossRef CAS PubMed.
- E. Alakangas, in Fuel Flexible Energy Generation, ed. J. Oakey, Woodhead Publishing, Boston, 2016, pp. 59–96 Search PubMed.
- https://www.engineeringtoolbox.com/fuels-higher-calorific-values-d_169.html.
- N. Rahmat, A. Z. Abdullah and A. R. Mohamed, Renewable Sustainable Energy Rev., 2010, 14, 987–1000 CrossRef CAS.
- M. D. Bohon, B. A. Metzger, W. P. Linak, C. J. King and W. L. Roberts, Proc. Combust. Inst., 2011, 33, 2717–2724 CrossRef CAS.
- M. Hájek and F. Skopal, Bioresour. Technol., 2010, 101, 3242–3245 CrossRef PubMed.
- A. Muelas, P. Remacha, A. Pina, J. Barroso, A. Sobrino, D. Aranda, N. Bayarri, C. Estevez and J. Ballester, Exp. Therm. Fluid Sci., 2020, 114 CrossRef CAS.
- T. Valliyappan, N. N. Bakhshi and A. K. Dalai, Bioresour. Technol., 2008, 99, 4476–4483 CrossRef CAS.
- N. Striugas, Laboratory of Combustion Processes, Kaunas, Lithuania, 2010 Search PubMed.
- C. Mota, B. P. Pinto and A. D. Lima, Glycerol: a versatile renewable feedstock for the chemical industry, 2017 Search PubMed.
- M. Stelmachowski, Ecol. Chem. Eng. S, 2011, 18, 9–30 CAS.
- D. T. Johnson and K. A. Taconi, Environ. Prog., 2007, 26, 338–348 CrossRef CAS.
- H. Y. Setyawan, M. M. Zhu, Z. Z. Zhang and D. K. Zhang, Energy, 2016, 113, 153–159 CrossRef CAS.
- S. Szwaja, M. Gruca and M. Pyrc, Ecol. Chem. Eng. S, 2022, 226, 107085 CAS.
- A. Bala-Litwiniak and H. Radomiak, Energy Sources, Part A, 2016, 38, 2510–2516 CrossRef CAS.
- B. Liu, K. Christiansen, R. Parnas, Z. Xu and B. Li, Int. J. Hydrogen Energy, 2013, 38, 3196–3205 CrossRef CAS.
- R. Gallardo, M. Alves and L. R. Rodrigues, Biomass Bioenergy, 2014, 71, 134–143 CrossRef CAS.
- Z. Wang, J. Zhuge, H. Fang and B. A. Prior, Biotechnol. Adv., 2001, 19, 201–223 CrossRef CAS.
- M. M. Muller, J. H. Kugler, M. Henkel, M. Gerlitzki, B. Hormann, M. Pohnlein, C. Syldatk and R. Hausmann, J. Biotechnol., 2012, 162, 366–380 CrossRef PubMed.
- F. Zhao, Y. T. Wu, Q. Z. Wang, M. Y. Zheng and Q. F. Cui, Microb. Cell Fact., 2021, 20, 185 CrossRef CAS PubMed.
- Y. F. Li, Y. Q. Qiu, X. Zhang, M. L. Zhu and W. S. Tan, Bioresour. Bioprocess., 2019, 6, 15 CrossRef CAS.
- S. Ahmed, D. Papadias, II.A.3 Hydrogen from Glycerol: A Feasibility Study, 2010 Search PubMed.
- S. S. Yazdani and R. Gonzalez, Curr. Opin. Biotechnol., 2007, 18, 213–219 CrossRef CAS PubMed.
- Z. Chun-Hui, N. B. Jorge, F. Yong-Xian and G. Q. Lu, Chem. Soc. Rev., 2008, 37, 527–549 RSC.
- A. Villa, N. Dimitratos, C. E. Chan-Thaw, C. Hammond, L. Prati and G. J. Hutchings, Acc. Chem. Res., 2015, 48, 1403–1412 CrossRef CAS PubMed.
- T. Ohara, T. Sato, N. Shimizu, G. Prescher and H. Greim, Acrylic Acid and Derivatives, Ullmann's Encyclopedia of Industrial Chemistry, 2003 Search PubMed.
- Z. He, X. Ning, G. Yang, H. Wang, Y. Cao, F. Peng and H. Yu, Catal. Today, 2021, 365, 162–171 CrossRef CAS.
- A. El Roz, P. Fongarland, F. Dumeignil and M. Capron, Front. Chem., 2019, 7, 156 CrossRef CAS PubMed.
- H. Liu, Z. Huang, H. Kang, X. Li, C. Xia, J. Chen and H. Liu, Appl. Catal., B, 2018, 220, 251–263 CrossRef CAS.
- X. Wang, A. K. Beine, P. J. C. Hausoul and R. Palkovits, ChemCatChem, 2019, 11, 4123–4129 CrossRef CAS.
- T. S. de Andrade, M. M. V. M. Souza and R. L. Manfro, Renewable Energy, 2020, 160, 919–930 CrossRef CAS.
- H. H. Gong, X. G. Zhao, X. Y. Li, M. Y. Chen, Y. Ma, J. Fang, X. J. Wei, Q. P. Peng and Z. S. Hou, ACS Sustainable Chem. Eng., 2021, 9, 2246–2259 CrossRef CAS.
- L. Gong, Y. Lu, Y. Ding, R. Lin, J. Li, W. Dong, T. Wang and W. Chen, Appl. Catal., A, 2010, 390, 119–126 CrossRef CAS.
- F. L. Wu, H. F. Jiang, X. H. Zhu, R. Lu, L. Shi and F. Lu, ChemSusChem, 2021, 14, 569–581 CrossRef CAS PubMed.
- M. J. Antal, W. S. L. Mok and G. N. Richards, Carbohydr. Res., 1990, 199, 91–109 CrossRef CAS.
- B. C. M. Morales and B. A. O. Quesada, Catal. Today, 2021, 372, 115–125 CrossRef CAS.
- S. Basu and A. K. Sen, ChemBioEng Rev., 2021, 8, 633–653 CrossRef CAS.
- A. Abdullah, A. Zuhairi Abdullah, M. Ahmed, J. Khan, M. Shahadat, K. Umar and M. A. Alim, J. Cleaner Prod., 2022, 341, 130876 CrossRef CAS.
- M. N. N. Shahirah, J. Gimbun, S. S. Lam, Y. H. Ng and C. K. Cheng, Renewable Energy, 2019, 132, 1389–1401 CrossRef CAS.
- L. M. B. Batista, F. A. Bezerra, J. L. F. Oliveira, A. M. D. Araujo, V. J. Fernandes, A. S. Araujo, A. D. Gondim and A. P. M. Alves, J. Therm. Anal. Calorim., 2019, 137, 1929–1938 CrossRef CAS.
- P. Basu, Biomass gasification pyrolysis & torrefaction, 2013 Search PubMed.
- A. Almeida, R. Pilao, A. Ribeiro, E. Ramalho and C. Pinho, Energy Fuels, 2019, 33, 9942–9948 CrossRef CAS.
- F. Jérôme, Y. Pouilloux and J. Barrault, ChemSusChem, 2008, 1, 586–613 CrossRef.
- R. L. Mou, X. J. Wang, Z. Q. Wang, D. Y. Zhang, Z. L. Yin, Y. Lv and Z. Wei, Fuel, 2021, 302, 121175 CrossRef CAS.
- M. E. Chiosso, M. L. Casella and A. B. Merlo, Catal. Today, 2021, 372, 107–114 CrossRef CAS.
- F. J. S. Barros, J. A. Cecilia, R. Moreno-Tost, M. F. de Oliveira, E. Rodriguez-Castellon, F. M. T. Luna and R. S. Vieira, Waste Biomass Valorization, 2020, 11, 1499–1512 CrossRef CAS.
- N. A. Razali, M. Conte and J. McGregor, Catal. Lett., 2019, 149, 1403–1414 CrossRef CAS.
- C. C. Hu, M. Yoshida, H. C. Chen, S. Tsunekawa, Y. F. Lin and J. H. Huang, Chem. Eng. Sci., 2021, 235, 116451 CrossRef CAS.
- Y. Zheng, X. Chen and Y. Shen, Chem. Rev., 2008, 108, 5253–5277 CrossRef CAS PubMed.
- S. de la Morena, M. Wojtusik, V. E. Santos and F. Garcia-Ochoa, Catalysts, 2020, 10, 101 CrossRef CAS.
- M. Ripoll, E. Jackson, J. A. Trelles and L. Betancor, J. Biotechnol., 2021, 340, 102–109 CrossRef CAS PubMed.
- V. K. Jain, C. J. Y. Tear and C. Y. Lim, Enzyme Microb. Technol., 2016, 86, 39–44 CrossRef CAS PubMed.
- Y. F. Zhou and Y. Shen, J. Appl. Electrochem., 2021, 51, 79–86 CrossRef CAS.
- X. Huang, Y. Zou and J. Jiang, ACS Sustainable Chem. Eng., 2021, 9, 14470–14479 CrossRef.
- G. S. Tran, T. G. Vo and C. Y. Chiang, J. Catal., 2021, 404, 139–148 CrossRef CAS.
- D. Liu, J. C. Liu, W. Z. Cai, J. Ma, H. B. Yang, H. Xiao, J. Li, Y. J. Xiong, Y. Q. Huang and B. Liu, Nat. Commun., 2019, 10, 1779 CrossRef PubMed.
- S. Czernik, R. French, C. Feik and E. Chornet, Ind. Eng. Chem. Res., 2002, 41, 4209–4215 CrossRef CAS.
- B. Wang, Y. Xiong, Y. Han, J. Hong, Y. Zhang, J. Li, F. Jing and W. Chu, Appl. Catal., B, 2019, 249, 257–265 CrossRef CAS.
- H. Zhou, S. Liu, F. Jing, S.-Z. Luo, J. Shen, Y. Pang and W. Chu, Ind. Eng. Chem. Res., 2020, 59, 17259–17268 CrossRef CAS.
- F. Jing, S. Liu, R. Wang, X. Li, Z. Yan, S. Luo and W. Chu, Renewable Energy, 2020, 158, 192–201 CrossRef CAS.
- Y. Wang, M. Chen, Z. Yang, T. Liang, S. Liu, Z. Zhou and X. Li, Appl. Catal., A, 2018, 550, 214–227 CrossRef CAS.
- Y. Zhang, C. Yong, Z. Yan, S. Luo, F. Jing and W. Chu, Int. J. Hydrogen Energy, 2020, 45, 22448–22458 CrossRef CAS.
- A. D. Shejale and G. D. Yadav, Ind. Eng. Chem. Res., 2018, 57, 4785–4797 CrossRef CAS.
- K. Gao, O. A. Sahraei and M. C. Iliuta, Appl. Catal., B, 2021, 291, 119958 CrossRef CAS.
- S. Veiga, M. Romero, R. Faccio, D. Segobia, H. Duarte, C. Apesteguia and J. Bussi, Catal. Today, 2020, 344, 190–198 CrossRef CAS.
- P. D. F. Vernon, M. L. H. Green, A. K. Cheetham and A. T. Ashcroft, Catal. Today, 1992, 13, 417–426 CrossRef CAS.
- A. T. Ashcroft, A. K. Cheetham, J. S. Foord, M. L. H. Green, C. P. Grey, A. J. Murrell and P. D. F. Vernon, Nature, 1990, 344, 319–321 CrossRef CAS.
- D. A. Hickman and L. D. Schmidt, Science, 1993, 259, 343–346 CrossRef CAS PubMed.
- D. A. Hickman and L. D. Schmidt, J. Catal., 1992, 138, 267–282 CrossRef CAS.
- K. L. Hohn and Y.-C. Lin, ChemSusChem, 2009, 2, 927–940 CrossRef CAS PubMed.
- M. Huff and L. D. Schmidt, J. Phys. Chem., 1993, 97, 11815–11822 CrossRef CAS.
- W. J. Wang, Fuel Process. Technol., 2010, 91, 1401–1408 CrossRef CAS.
- Y. Liu, R. Farrauto and A. Lawal, Chem. Eng. Sci., 2013, 89, 31–39 CrossRef CAS.
- D. C. Rennard, J. S. Kruger, B. C. Michael and L. D. Schmidt, Ind. Eng. Chem. Res., 2010, 49, 8424–8432 CrossRef CAS.
- P. J. Dauenhauer, J. R. Salge and L. D. Schmidt, J. Catal., 2006, 244, 238–247 CrossRef CAS.
- S.-K. Liu and Y.-C. Lin, Ind. Eng. Chem. Res., 2012, 51, 16278–16287 CrossRef CAS.
- R. Moreira, F. Bimbela, L. M. Gandia, A. Ferreira, J. L. Sanchez and A. Portugal, Renewable Sustainable Energy Rev., 2021, 148, 111299 CrossRef CAS.
- R. D. Cortright, R. R. Davda and J. A. Dumesic, Nature, 2002, 418, 964–967 CrossRef CAS PubMed.
- P. R. d. l. Piscina and N. Homs, Chem. Soc. Rev., 2008, 37, 2459–2467 RSC.
- A. Larimi and F. Khorasheh, Int. J. Hydrogen Energy, 2019, 44, 8243–8251 CrossRef CAS.
- K. Wu, B. L. Dou, H. Zhang, D. S. Liu, H. S. Chen and Y. J. Xu, Fuel, 2022, 308, 122014 CrossRef CAS.
- Y. Guo, X. H. Liu and Y. Q. Wang, Ind. Eng. Chem. Res., 2019, 58, 2749–2758 CrossRef CAS.
- B. Entezary and M. Kazemeini, Int. J. Hydrogen Energy, 2018, 43, 21777–21790 CrossRef CAS.
- F. Bastan and M. Kazemeini, Biomass Convers. Biorefin., 2020, 1–10 Search PubMed.
- M. Alvear, A. Aho, I. L. Simakova, H. Grenman, T. Salmi and D. Y. Murzin, Chem. Eng. J., 2020, 398, 125541 CrossRef CAS.
- A. Fasolini, E. Lombardi, T. Tabanelli and F. Basile, Nanomaterials, 2021, 11, 1175 CrossRef CAS PubMed.
- P. Gogoi, N. Kanna, P. Begum, R. C. Deka, C. V. V. Satyanarayana and T. Raja, ACS Catal., 2020, 10, 2489–2507 CrossRef CAS.
- S. Guo, L. Guo, J. Yin and H. Jin, J. Supercrit. Fluids, 2013, 78, 95–102 CrossRef CAS.
- E. Markočič, B. Kramberger, J. G. van Bennekom, H. Jan Heeres, J. Vos and Ž. Knez, Renewable Sustainable Energy Rev., 2013, 23, 40–48 CrossRef.
- F. J. G. Ortiz, F. J. Campanario, P. G. Aguilera and P. Ollero, Energy, 2016, 96, 561–568 CrossRef.
- V. I. Bogdan, A. E. Koklin, A. N. Kalenchuk, N. V. Maschenko, T. V. Bogdan and L. M. Kustov, Biomass Bioenergy, 2020, 143, 105849 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |