Open Access Article
Open Access ArticleCopper-promoted direct sulfenylation of C1–H bonds in 4-aryl pyrrolo[1,2-a]quinoxalines†
Thuy T. Caabc,
Khanh T. M. Le ab,
Son N. T. Phan
ab,
Son N. T. Phan ab,
Huy H. Nguyen
ab,
Huy H. Nguyen ab,
Huy X. Le
ab,
Huy X. Le ab,
Nam T. S. Phan
ab,
Nam T. S. Phan ab and
Tung T. Nguyen
ab and
Tung T. Nguyen *ab
*ab
aFaculty of Chemical Engineering, Ho Chi Minh City University of Technology (HCMUT), 268 Ly Thuong Kiet, District 10, Ho Chi Minh City, Vietnam. E-mail: tungtn@hcmut.edu.vn
bVietnam National University Ho Chi Minh City, Linh Trung Ward, Thu Duc District, Ho Chi Minh City, Vietnam
cFaculty of Basic Sciences, University of Medicine and Pharmacy at Ho Chi Minh City, Ho Chi Minh City, Vietnam
First published on 7th December 2022
Abstract
Methods for direct functionalization of C(sp2)–H bonds in pyrrolo[1,2-a]quinoxalines have witnessed emerging development over the last decade. Herein we report a new tactic to afford a selective sulfenylation of 4-aryl pyrrolo[1,2-a]quinoxalines with diaryl disulfides. The reactions proceeded in the presence of a copper catalyst and potassium iodide promoter. Functionalities including nitro, ester, amide, methylthio, and halogen groups were all tolerated. Our method offers a convenient route to obtain highly substituted pyrrolo[1,2-a]quinoxalines-based thioethers in moderate to good yields.
Pyrrolo[1,2-a]quinoxalines are important motifs ubiquitously found in medicinally relevant molecules or functional materials.1 Recent studies feature new methods to develop the library of substituted pyrrolo[1,2-a]quinoxalines, which were traditionally limited to Pictet–Spengler-type annulation products.2 Perhaps intangible benefits such as convenient diversification and short synthesis would be considered should direct functionalization of C–H bonds in pyrrolo[1,2-a]quinoxalines be successful. Thus far, methods to forge new carbon–carbon and carbon-heteroatom bonds in pyrrolo[1,2-a]quinoxalines have been reported.3 One of the earliest examples presented a C1–thiocyanation and selenocyanation of pyrrolo[1,2-a]quinoxaline C–H bonds.3a Until now, the functionalization of C1–H bonds has been extensively studied, except one example for C3–H iodination.3c Mechanistically, high selectivity toward C1–H bonds was obtained following the electrophilic substitution. Given that certain successes are achieved, more examples are still expected with respect to better practicality and more general scope of substrates.
Carbon–sulfur bonds are often found in synthetically and practically useful molecules.4 While the cross coupling of (pseudo)halides and thiols to afford C–S bonds has been precedented, it suffers from inevitable pre-functionalization. Thus, sulfenylation of C–H bonds would be beneficial from the atom- and step-economy standpoints.5 Following our continuing interest,3d herein we develop a method to afford thioethers via the direct sulfenylation of C1–H bonds in pyrrolo[1,2-a]quinoxalines with diaryl disulfides. In comparison to thiophenols, diaryl disulfides would offer advantages including odorless and easily handling solids. Activation of S–S bonds to afford radical or electrophilic sulfur-containing adducts could be feasibly obtained in the presence of cheap and commercial iodide sources.6 As such, we hypothesized that electrophilic sulfenylation of C1–H bonds in pyrrolo[1,2-a]quinoxalines is possible should disulfides be combined with suitable iodide and oxidant. It should be noted that the thioethers reported in our study are possibly obtained using a previously developed method,3a albeit in a lengthy sequence of hydrolysis and cross coupling.
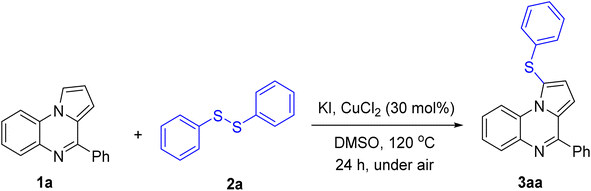
We started our investigation by studying the reaction of 4-phenyl pyrrolo[1,2-a]quinoxaline 1a and diphenyl disulfide 2a (Table 1). The product 3aa was obtained in 73% isolated yield using CuCl2 catalyst, KI promoter, DMSO solvent, under air, at 120 °C (entry 1).7 The regioselectivity was confirmed by comparing the coupling constant of protons on C2 and C3 with those previously reported regarding the C1–H functionalization.3a,h The reaction was easy to scale up, as 70% yield of 3aa was isolated in a 5 mmol scale run. Omitting CuCl2 gave an extremely low yield of 3aa (entry 2), confirming the crucial role of the copper salt. Attempts to increase the yield of 3aa by using other copper salts were unsuccessful (entries 3–5). Iron(III) chloride was inferior to CuCl2 (entry 6). Some alternative iodides could be used, albeit affording 3aa in relatively lower yields (entries 7 and 8). The reaction in the presence of I2 gave 3aa in 45% yield (entry 9). Without KI, only a small amount of 3aa was obtained after column chromatography (entry 10). Sulfenylation of 1a with 2a under argon gave 60% yield of 3aa (entry 11), implying the role of air as the co-oxidant. Lastly, replacing DMSO by other solvents gave low to moderate yields of 3aa (entries 12–14).
| Entry | Variation from standard conditions | Yield of 3aa (%) |
|---|---|---|
| a Reaction conditions: 1a (0.1 mmol), 2a (0.05 mmol), CuCl2 (0.03 mmol), KI (0.05 mmol), DMSO (1 mL), 120 °C, 24 h, under air. Isolated yields.b 5 mmol scale. | ||
| 1 | None | 73, 70b |
| 2 | Without CuCl2 | <10 |
| 3 | CuI instead of CuCl2 | 38 |
| 4 | Cu(OAc)2 instead of CuCl2 | 27 |
| 5 | CuBr2 instead of CuCl2 | 53 |
| 6 | FeCl3 instead of CuCl2 | 55 |
| 7 | nBu4NI instead of KI | 72 |
| 8 | NaI instead of KI | 64 |
| 9 | I2 instead of KI | 45 |
| 10 | Without KI | <10 |
| 11 | Under argon | 60 |
| 12 | DMF instead of DMSO | 25 |
| 13 | 1,4-Dioxane instead of DMSO | 30 |
| 14 | Toluene instead of DMSO | 31 |
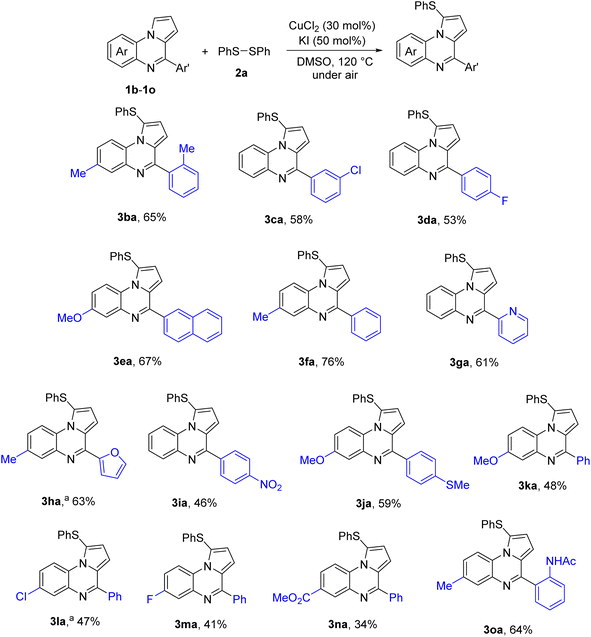
Next scope of pyrrolo[1,2-a]quinoxalines was explored. The result is shown in Scheme 1. Overall, moderate to good yields of diaryl thioethers were obtained regardless of electronic properties of pyrrolo[1,2-a]quinoxalines. Functionalities such as fluoro (3da, 3ma), chloro (3ca, 3la), nitro (3ia), methylthio (3ja), ester (3na), and amide (3oa) groups were all compatible with reaction conditions. Heterocyclic substrates such as those containing pyridine (3ga) and furan (3ha) were also competent substrates.
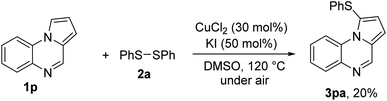
We questioned whether functionalization at C4 was crucial for successful sulfenylation. Thus, coupling of pyrrolo[1,2-a]quinoxaline 1p with diphenyl disulfide 2a was attempted (Scheme 2). As only 20% yield of the product 3pa was obtained, substitution at C4–H bond with aromatics was important. At this stage, using C4-alkyl substituted pyrrolo[1,2-a]quinoxalines have not been examined.
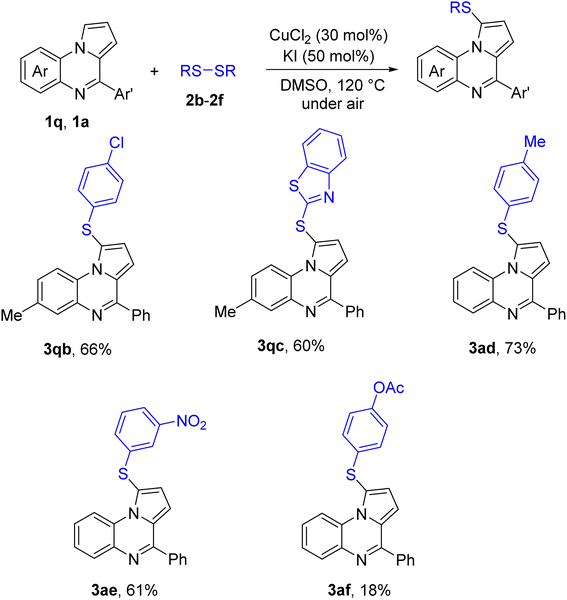
Other diaryl disulfides rather than 2a were also attempted. The result is shown in Scheme 3. Notably, use of di-heteroaryl disulfides such as 2c did not affect the sulfenylation. A low yield of the sulfenylation product was obtained with respect to the acetoxylated disulfide (3af). Dialkyl disulfides were inactive toward the reaction conditions.
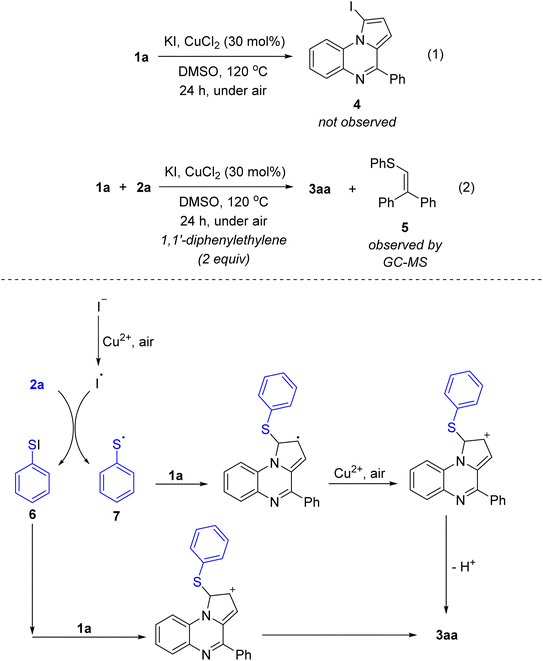
We next run some control experiments to have a better understanding of reaction mechanism (Scheme 4). In the absence of disulfide 2a, no iodination of C1–H bond in 1a was observed (eqn (1)). This result implied that the sequence of electrophilic iodination followed by copper catalyzed activation of carbon–iodine bond was unlikely involved the reaction mechanism. Next, the addition of the radical quencher 1,1′-diphenylethylene (2 equivalents) did not completely suppress the sulfenylation (eqn (2)). Yet the small amount of vinyl sulfide 5 could be detected by GC-MS. Replacing diphenyl disulfide 2a with thiophenol to couple with 1a did not give the product 3aa, revealing that thiophenol should not be the key sulfide source. Based on these results, a possible mechanism was proposed as that shown in Scheme 4. The iodide salt was oxidized in the presence of CuCl2 and air to afford iodide radical, followed by the reaction with diphenyl disulfide 2a to yield the adduct 6 and phenylthio radical 7. Electrophilic substitution of 1a with 6 followed by re-aromatization would afford the sulfenylation product 3aa. Alternatively, radical addition of 7 to 1a followed by oxidation and re-aromatization would also yield 3aa. We envisaged that in the absence of air, DMSO could play a significant role in the oxidation steps.
Conclusions
In conclusion, we have developed a method for direct sulfenylation of C1–H bonds in pyrrolo[1,2-a]quinoxalines with diaryl disulfides. The reactions featured mild conditions and excellent compatibility with a wide range of functionalities. Early thoughts on mechanism revealed a direct electrophilic sulfenylation which may include the formation of phenylthio radical intermediates.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge Ho Chi Minh City University of Technology (HCMUT) for supporting this study.Notes and references
- Selected examples: (a) J. Guillon, I. Forfar, M. Mamani-Matsuda, V. Desplat, M. Saliège, D. Thiolat, S. Massip, A. Tabourier, J.-M. Léger, B. Dufaure, G. Haumont, C. Jarry and D. Mossalayi, Bio. Med. Chem., 2007, 15, 194 CrossRef CAS PubMed; (b) H. de Lucio, J. García-Marín, P. Sánchez-Alonso, J. C. García-Soriano, M. Á. Toro, J. J. Vaquero, F. Gago, R. Alajarín and A. Jiménez-Ruiz, Eur. J. Med. Chem., 2022, 227, 113915 CrossRef CAS PubMed; (c) C. Biswas, K. N. Krishnakanth, J. J. Lade, A. C. Chaskar, A. Tripathi, P. Chetti, V. R. Soma and S. S. K. Raavi, Chem. Phys. Lett., 2019, 730, 638 CrossRef CAS.
- Selected reviews: (a) A. A. Kalinin, L. N. Islamova and G. M. Fazleeva, Chem. Heterocycl. Compd., 2019, 55, 584 CrossRef CAS; (b) H. X. Le and T. T. Nguyen, ChemistrySelect, 2022, 7, e202200166 CAS.
- (a) Z. Yang, J. He, Y. Wei, W. Li, P. Liu, J. Zhao and Y. Wei, Org. Biomol. Chem., 2020, 18, 9088 RSC; (b) Y. Li, Z. Yang, Y. Liu, Y. Liu, Y. Gu and P. Liu, Mol. Catal., 2021, 511, 111747 CrossRef CAS; (c) Y. Liu, Y. Wei, Z. Yang, Y. Li, Y. Liu and P. Liu, Org. Biomol. Chem., 2021, 19, 5191 RSC; (d) H. X. Le, T. N. B. Hoang, T. H. Tran, C. T. D. Nguyen, L. N. T. Chiem, N. T. S. Phan and T. T. Nguyen, Tetrahedron Lett., 2021, 67, 152879 CrossRef CAS; (e) Z. Yang, J. He, Y. Wei, W. Li and P. Liu, Org. Biomol. Chem., 2020, 18, 3360 RSC; (f) Y. Li, Y. Liu, D. Hao, C. Li, Y. Liu, Y. Gu, L. Vaccaro and P. Liu, Tetrahedron, 2022, 105, 132610 CrossRef CAS; (g) D. Hao, Z. Yang, Y. Liu, Y. Li, Y. Liu and P. Liu, J. Mol. Struct., 2022, 1267, 133636 CrossRef CAS; (h) D. Hao, Z. Yang, Y. Liu, Y. Li, C. Li, Y. Gu, L. Vaccaro, J. Liu and P. Liu, Org. Biomol. Chem., 2022, 20, 847 RSC.
- Selected recent reviews: (a) D. Kaiser, I. Klose, R. Oost, J. Neuhaus and N. Maulide, Chem. Rev., 2020, 119, 8701 CrossRef PubMed; (b) P. Annamalai, K.-C. Liu, S. S. Badsara and C.-F. Lee, Chem. Rec., 2021, 21, 3674 CrossRef CAS PubMed; (c) E. Azzi, A. Lanfranco, R. Moro, A. Deagostino and P. Renzi, Synthesis, 2021, 53, 3440 CrossRef CAS.
- Selected recent examples: (a) P. Gandeepan, J. Koeller and L. Ackermann, ACS Catal., 2017, 7, 1030 CrossRef CAS; (b) R. Kajiwara, K. Takamatsu, K. Hirano and M. Miura, Org. Lett., 2020, 22, 5915 CrossRef CAS PubMed; (c) S. Kathiravan, P. Anaspure, T. Zhang and I. A. Nicholls, Org. Lett., 2021, 23, 3331 CrossRef CAS PubMed; (d) S. Vásquez-Céspedes, A. Ferry, L. Candish and F. Glorius, Angew. Chem., Int. Ed., 2015, 54, 5772 CrossRef PubMed; (e) Z. Song, C. Ding, S. Wang, Q. Dai, Y. Sheng, Z. Zheng and G. Liang, Chem. Commun., 2020, 56, 1847 RSC.
- Selected examples for activation of ArXXAr (X = S or Se) by iodide: (a) A. Mathuri, M. Pramanik, A. Parida and P. Mal, Org. Biomol. Chem., 2021, 19, 8539 RSC; (b) Y. Ren, B. Xu, Z. Zhong, C. U. Pittman, Jr. and A. Zhou, Org. Chem. Front., 2019, 6, 2023 RSC.
- Please see the ESI for more details.†.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ra05078a |
| This journal is © The Royal Society of Chemistry 2022 |