Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
KMnCuTe2: a layered antiferromagnetic semiconductor with long metal–metal distance†
Fan Suna,
Zhao Liua,
Jiawei Lina,
Jun Dengb,
Zhongnan Guo *a and
Wenxia Yuan
*a and
Wenxia Yuan *a
*a
aDepartment of Chemistry, School of Chemistry and Biological Engineering, University of Science and Technology Beijing, Beijing, 100083, China. E-mail: guozhongn@ustb.edu.cn; wxyuanwz@163.com
bResearch & Development Centre for Functional Crystals, Beijing National Laboratory for Condensed Matter Physics, Institute of Physics, Chinese Academy of Sciences, Beijing 100190, China
First published on 11th October 2022
Abstract
The magnetic semiconductor in a two-dimensional system is a major subject for both theoretical and experimental investigations. Here we report the synthesis of a new quaternary manganese chalcogenide KMnCuTe2, which shows layered structure and antiferromagnetic (AFM) semiconducting features. Single crystals of KMnCuTe2 were obtained using a self-flux method and based on single-crystal X-ray diffraction, KMnCuTe2 adopts the ThCr2Si2-type structure composed of edge-sharing tetrahedral layers separated by K+ cations. The Mn and Cu atoms randomly distribute in the centre of tetrahedral units. Attributed to the large radius of Te, KMnCuTe2 has large lattice parameters (a = 4.3115(3) Å and c = 14.9360(20) Å), leading to a long metal–metal distance (3.049 Å) in the tetrahedral layers. Based on the experiments and theoretical calculations, KMnCuTe2 exhibits a G-type AFM interaction with the transition temperature at around 225 K and an indirect semiconducting nature with the band gap of 0.95 eV. The magnetic semiconducting property of KMnCuTe2 is unique in AMnMCh2 systems (A = Li, Na, K, M = Cu, Ag and Ch = S, Se, Te), which could be associated with the large metal–metal distance. Our work not only highlights the role of metal–metal interactions on regulating the properties of ThCr2Si2-type compounds, but also provides a feasible strategy to obtain the layered magnetic semiconductor.
Introduction
The ThCr2Si2-type structure is a very common one for ternary inorganic compounds. In the last decade, the transition metal chalcogenides with ThCr2Si2-type structure have attracted intense attention attributed to the discovery of Fe-based high-temperature superconductors.1–3 From the crystal structure point of view, the ThCr2Si2-type iron chalcogenides are composed of edge-sharing FeCh4 tetrahedral layers (Ch = S, Se, Te) with the alkali metal intercalated in between the layers.4 Stimulated by the fascinating Fe-based ThCr2Si2-type compounds, the Ni- and Co-based analogues have also been fully investigated, and exhibit heavy-fermion behavior and long-range magnetic order, respectively.5–9 Interestingly, the ThCr2Si2-type structure can also be formed in transition metal chalcogenides by mixing the metal site in tetrahedral layers, largely enriching the compositional diversity of this material family. In these “metal-mixed” phases, the transition metal ions are partially substituted by monovalent metal ions M+, forming a series of quaternary metal chalcogenides including AFeMCh2, ACoMCh2, AMnMCh2, ACrMCh2 and AZnMCh2 (A = K, Rb, Cs, M = Cu, Ag, Li and Ch = S, Se, Te).10–24 Recently, the alkaline-earth metal chalcogenides KMgCuSe2 and KMgCuTe2 with ThCr2Si2-type structure were also synthesized by our group.25 Given the similar chemical composition with the Fe-based high-temperature superconductors, the KFeAgTe2 was described in more details focusing on the interactions between its crystal structure, magnetic order and electronic structure.12,13,26 Very recently, the intertwined magnetic order and nematic orders, which were considered as the feature of Fe-based high-temperature superconductors, were observed in KFeAgTe2.27 A exotic spin-nematic state induced by a small strain was also observed in this layered iron telluride.28The layered manganese chalcogenide AMnMCh2 (A = K, Rb, Cs, M = Cu, Ag and Ch = S, Se, Te) are also intriguing materials, not only as a similar specimen to Fe-based superconductors in electronic structure but also as a potential two-dimensional (2D) magnetic system. It was reported that some members in AMnMCh2 family show the spin-glass state while the others are paramagnets.16–18 However, the long-range magnetic order such as antiferromagnetic (AFM) interaction has not been reported yet in this Mn-based quaternary family. It has been revealed that the lattice parameters, especially the metal–metal distances in ab plane, play a key role on determining the magnetism and transport properties for ThCr2Si2-type materials.18,22 Synthesizing the new member in AMnMCh2 family with large metal–metal distance could be expected to realize the unique physical property.
In this work, we report the single-crystal growth of a new member in AMnMCh2 family, KMnCuTe2. It is indicated that the title compound adopts the ThCr2Si2-type structure that is comprised of edge-sharing [Mn(Cu)Te4] tetrahedral layers with intercalated K+ cations. The Mn and Cu atoms randomly distribute in the centre of tetrahedron with the ratio of Mn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu close to 1
Cu close to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 due to the similar radii of Mn and Cu. Due to the large radius of Te, KMnCuTe2 has much larger lattice parameters (a = 4.3115(3) Å and c = 14.9360(20) Å) compared to the sulfides and selenides in AMnMCh2, leading to a long metal–metal distance in tetrahedral layers (3.049 Å). Based on the experiments and theoretical calculations, we demonstrate that KMnCuTe2 exhibits unique magnetic semiconducting behavior with an indirect band gap (0.95 eV) and G-type AFM interaction.
1 due to the similar radii of Mn and Cu. Due to the large radius of Te, KMnCuTe2 has much larger lattice parameters (a = 4.3115(3) Å and c = 14.9360(20) Å) compared to the sulfides and selenides in AMnMCh2, leading to a long metal–metal distance in tetrahedral layers (3.049 Å). Based on the experiments and theoretical calculations, we demonstrate that KMnCuTe2 exhibits unique magnetic semiconducting behavior with an indirect band gap (0.95 eV) and G-type AFM interaction.
Experimental section
Synthesis
Single-crystals of KMnCuTe2 were grown by self-flux method. Powders of Mn (99.99%, Aladdin), Cu (99.9%, Aladdin) and Te (99.9%, Aladdin) were mixed with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio and ground in an agate mortar. The raw materials were cold-pressed into disks with 10 mm diameter under 200 kg cm−2 uniaxial stress and put into the alumina crucibles together with K ingot (97%, SINOPHARM) as predetermined compositions. The total mass of the samples was around 2 g. The alumina crucibles were sealed into the quartz tubes under vacuum and then firstly heated to 473 K and held for 12 hours for the pre-reaction of potassium and Te. After pre-reaction, the samples were re-ground and cold-pressed again. All the manipulations were carried out inside an argon-filled glove box (O2 < 1 ppm) in order to prevent the sample oxidation. The disks were heated to 1273 K in 12 hours, held for 48 hours, and then cooled down to 874 K with the cooling rate of 4 K per hour before cooling to room temperature. It should be mentioned that the crucibles was shook for several times during the heating process of synthesis to obtain the homogeneous phase.
2 molar ratio and ground in an agate mortar. The raw materials were cold-pressed into disks with 10 mm diameter under 200 kg cm−2 uniaxial stress and put into the alumina crucibles together with K ingot (97%, SINOPHARM) as predetermined compositions. The total mass of the samples was around 2 g. The alumina crucibles were sealed into the quartz tubes under vacuum and then firstly heated to 473 K and held for 12 hours for the pre-reaction of potassium and Te. After pre-reaction, the samples were re-ground and cold-pressed again. All the manipulations were carried out inside an argon-filled glove box (O2 < 1 ppm) in order to prevent the sample oxidation. The disks were heated to 1273 K in 12 hours, held for 48 hours, and then cooled down to 874 K with the cooling rate of 4 K per hour before cooling to room temperature. It should be mentioned that the crucibles was shook for several times during the heating process of synthesis to obtain the homogeneous phase.
Characterization
Powder X-ray diffraction (PXRD) were collected on a PANalytical diffractometer (X'Pert PRO MRD) equipped with Cu Kα radiation (λ = 1.5406 Å) operation at 40 kV and 40 mA. Single crystal X-ray diffraction (SCXRD) was performed on a Bruker D8 Venture diffractometer at 50 kV and 1.4 mA with Mo Kα radiation (λ = 0.71073 Å). All data were collected at room temperature under nitrogen flow. The crystal structure of KMnCuTe2 was solved (using direct method) and refined using Jana 2006 package.29 Morphology of the samples was investigated via scanning electron microscopy (SEM, Hitachi S-4800), and the component analysis was made by energy dispersive X-ray spectroscopy (EDS). The result for each sample was obtained based on the average of 10 sets of data. The magnetization susceptibility was measured using a vibrating sample magnetometer (VSM, Quantum Design) from 300 to 10 K. The electrical resistivity of single crystal samples was measured via four-probe method using a physical property measurement system (PPMS, Quantum Design). The specific heat capacity was also measured using PPMS. The optical diffuse reflectance measurements were performed at room temperature using a Shimadzu UV-3600 UV-vis-NIR spectrometer and BaSO4 was used as the reference of 100% reflectance. The absorption data were calculated based on the obtained reflectance spectrum according to the Kubelka–Munk equation, α/S = (1 − R)2/(2R), where R is the reflectance and α and S are the absorption and scattering coefficients, respectively.30Calculation
All calculations were performed in the density functional theory (DFT) using Vienna ab into simulation package (VASP), and the projector-augmented wave (PAW) scheme and Perdew–Burke–Ernzerhof generalized gradient approximation (PBE-GGA) were used for the exchange correlation functional.31–33 The wave functions were expanded into plane-wave up to a cutoff energy of 450 eV.34 We built a 2 × 2 × 1 supercell to simulate the different magnetic structures, and a Monkhorst–Pack k-mesh of 4× 4 × 2 was used for sampling the first Brillouin zone in the self-consistent calculation.35 The structures were relaxed with an energy convergence criterion of 10−6 eV, ensuring that the maximum force on an atom was <0.03 eV Å−1. Considering the strong correlations of 3d electrons of Cu and Mn, on-site Hubbard U for 3d orbitals with U = 5 eV was adopted.36Results and discussion
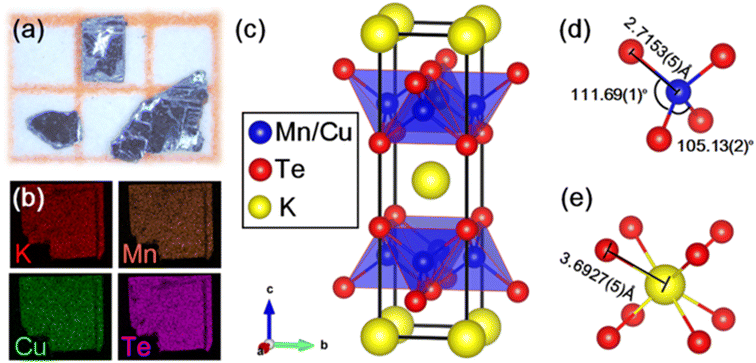
We successfully synthesized a new quaternary manganese chalcogenide KMnCuTe2, which is a new member in AMnMCh2 (A = K, Rb, Cs, M = Cu, Ag and Ch = S, Se, Te) family.16–18 Using self-flux method, single crystal sheets were obtained by cleaving the crystal grains as shown in Fig. 1a, and the products show black color and are about 1 mm in size. Elemental mapping in Fig. 1b confirms the homogeneous distribution of K, Mn, Cu and Te in sample. Chemical analysis by EDS shows that the atomic ratio of K![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn
Mn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu
Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Te is 0.97
Te is 0.97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.96
0.96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.98
0.98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, which is close to the stoichiometry.
2, which is close to the stoichiometry.
The single-crystal of KMnCuTe2 was picked directly from the product for SCXRD measurement. The obtained crystallographic information and structural parameters are listed in Tables S1–S3.† It can be seen that the final refinement gives very low weight agreement factors Rint, R and Rw, suggesting that our structure solution is satisfactory. KMnCuTe2 crystallizes in the ThCr2Si2-type structure, which adopts a body-centered tetragonal lattice with the space group of I4/mmm (No. 139). The lattice parameters are refined to be a = 4.3115(3) Å and c = 14.9360(20) Å, comparable to the analogous tellurides including KFeCuTe2, KFe0.85Ag1.15Te2, CsMn1.18Ag0.64Te2 and CsFe0.72Ag1.28Te2.12,20,23,24 As shown in Fig. 1c, the structure shows very typical layered nature, which is composed of the edge-sharing [Mn(Cu)Te4] tetrahedral layer extending in the ab plane and the potassium ions are located in the [KTe8] cubic sites between the layers. The Mn and Cu atoms are randomly distributed in the centre of tetrahedral units (shown in Fig. 1c) and this mixed occupation is mainly due to the similar radii of Mn and Cu. The [Mn(Cu)Te4] tetrahedron is compressed along the ab plane with the four Te–Mn(Cu)–Te angles across the ab plane being 111.69(1)° and the other two Te–Mn(Cu)–Te angles on the same side being 105.13(2)°, as shown in Fig. 1d. The Mn(Cu)–Te bond length is refined as 2.7153(5) Å, which is larger than Fe(Cu)–Te bond length (2.6344(8) Å) in the analogue, KFeCuTe2.20 The distance between K+ and the neighboring Te2− is 3.6927(5) Å (Fig. 1e), implying the negligible interaction between the alkali metals and the transition telluride layers. The refined composition from SCXRD is KMn0.96Cu1.04Te2, which is in good agreement with the EDS result. The PXRD pattern of the powder sample from grinding the crystals matches well with the simulated one from SCXRD, as shown in Fig. S1.† We found that KMnCuTe2 is chemically stable in ambient condition, with the PXRD pattern basically unchanged after exposure in air for 7 days (Fig. S1†).
The crystal structure of KMnCuTe2 is also related to that of the BaMxTe2 system (M = Cu and Ag) reported by Jana et al.37 Both KMnCuTe2 and BaMxTe2 are composed of the edge-sharing tetrahedral layers, in which the Mn(Cu)–Te bond length is slightly smaller than the Cu–Te bonds in BaCu0.43Te2 (2.72–2.74 Å).37 Different from the alternating stacking of K+ and [Mn(Cu)Te4] layers in KMnCuTe2, the [CuxTe4] tetrahedral layers are separated by both Ba2+ cations and Te square planes in BaCuxTe2. A modulated structure was also observed in BaMxTe2 due to the distorted Te square nets, but no modulated structure has been found in AMnMCh2 system yet.
As we mentioned, KMnCuTe2 is a new member in AMnMCh2 family, and ascribing to the large radius of Te, both a and c lattice constants of KMnCuTe2 are much larger than those of the sulfides and selenides in AMnMCh2, as shown in Table 1. Only the reported CsMn1.18Ag0.64Te2 shows larger lattice parameters than KMnCuTe2, but the related physical property was not described.23 The metal–metal distance in ab plane, which equals to 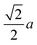 , is also listed in Table 1. The Mn(Cu)–Mn(Cu) distance is 3.049 Å in KMnCuTe2 (Fig. 2), which is much longer that those in KMnCuSe2 (2.893 Å) and KMnCuS2 (2.800 Å),16,17 and is also longer than the Mn(Ag)–Mn(Ag) bond in KMnAgSe2 (3.004 Å).18 This demonstrates a very weak interaction between the metal atoms in tetrahedral layers in KMnCuTe2. It has been widely believed that the structure parameter, especially the metal–metal distance, plays an important role on the magnetic and transport properties of AMnMCh2 compounds. With the relatively large metallic distance in layer, KMnCuTe2 provides an important platform to understand the structure–property relationship of AMnMCh2 materials, which will be discussed in details below.
, is also listed in Table 1. The Mn(Cu)–Mn(Cu) distance is 3.049 Å in KMnCuTe2 (Fig. 2), which is much longer that those in KMnCuSe2 (2.893 Å) and KMnCuS2 (2.800 Å),16,17 and is also longer than the Mn(Ag)–Mn(Ag) bond in KMnAgSe2 (3.004 Å).18 This demonstrates a very weak interaction between the metal atoms in tetrahedral layers in KMnCuTe2. It has been widely believed that the structure parameter, especially the metal–metal distance, plays an important role on the magnetic and transport properties of AMnMCh2 compounds. With the relatively large metallic distance in layer, KMnCuTe2 provides an important platform to understand the structure–property relationship of AMnMCh2 materials, which will be discussed in details below.
| Composition | a (Å) | c (Å) | Metal–metal distances (Å) | MT | Ref. |
|---|---|---|---|---|---|
| KMnCuS2 | 3.9596(6) | 13.297(3) | 2.800 | SG | 16 |
| RbMnCuS2 | 4.0001(3) | 13.624(2) | 2.828 | SG | 16 |
| CsMnCuS2 | 4.0514(3) | 14.147(2) | 2.865 | SG | 16 |
| KMnCuSe2 | 4.0912(8) | 13.893(4) | 2.893 | SG | 17 |
| RbMnCuSe2 | 4.1283(6) | 14.169(3) | 2.919 | PM | 17 |
| CsMnCuSe2 | 4.1902(5) | 14.686(3) | 2.963 | PM | 17 |
| KMnAgSe2 | 4.2486(3) | 13.943(2) | 3.004 | SG | 18 |
| KMnCuTe2 | 4.3115(3) | 14.9360(20) | 3.049 | AFM | This work |
| CsMn1.18Ag0.64Te2 | 4.520(2) | 15.484(8) | 3.196 | Not mentioned | 23 |
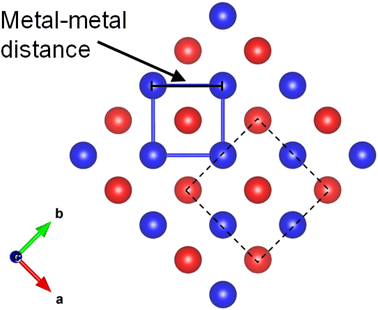 | ||
| Fig. 2 The metal–metal distance along the layer direction in ThCr2Si2-type compounds. The dash square is the unit cell of the structure. | ||
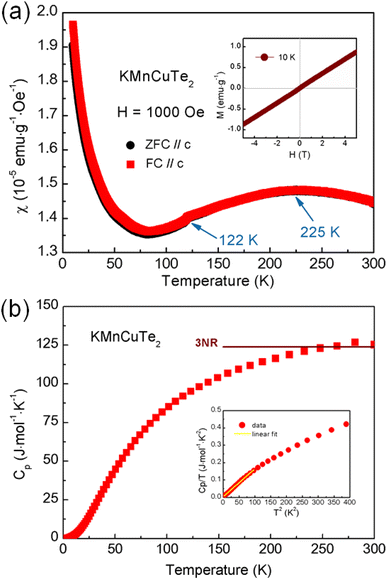
To investigate the influence of the large lattice parameters on magnetic properties, we performed the measurements on temperature dependent magnetic susceptibility (M–T curve) of KMnCuTe2. As shown in Fig. 3, the magnetic susceptibility shows a broad transition at around 225 K, suggesting a low-dimensional AFM interaction. Meanwhile, a kink was also observed at about 122 K in the M–T curve. In high temperature range, the magnetic susceptibility deviates from the Curie–Weiss law, implying the complex magnetic interactions between Mn2+ ions at high temperatures. The absence of the bifurcate between the zero-field cooling (ZFC) and field cooling (FC) curves in low temperature range rules out the spin-glass behavior, different from magnetic states of KMnCuSe2 and KMnCuS2. It is worth noting that although this low-dimensional AFM ordering is unique in AMnMCh2 family, the similar magnetic behavior was observed in some layered transition metal oxychalcogenides such as Ba2CoO2Ag2Se2.38 The Curie–Weiss-like upturn in low temperature range is presumably due to the paramagnetic impurities on the surface of KMnCuTe2 crystal. The magnetization loop at 10 K is also shown in inset of Fig. 3a, which exhibit linear dependence on the field (H) with basically no hysteresis, further confirming the AFM ordering in this compound.
In our previous work, it was observed that the magnetism in Fe-based AFeMCh2 family is mainly determined by the a lattice constant, where the long-range AFM order can only be maintained with the a axis larger than 4.19 Å while the spin-glass behavior turns to appear with the a axis smaller than this critical value.22 Interestingly, the AFM interaction observed in KMnCuTe2 demonstrates the similar regulation rule of the a lattice parameter over the magnetic behavior of AMnMCh2 compounds with the critical value as ∼4.3 Å (Table 1), implying the uniform mechanism of the structural modulation on magnetic coupling in ThCr2Si2-type material family with different transition metals.
The heat capacity versus T (Cp–T curve) was plotted in Fig. 3b. However, there is no clear anomaly observed in Cp–T curve at the AFM transition temperature (225 K). This phenomenon could be corresponding to the broad transition in M–T curve and suggests that the magnetic entropies above transition temperature decreases gradually during the cooling process. As shown in the inset of Fig. 3b, the Cp(T)/T versus T2 shows a non-linear characteristic at low temperature (below 20 K). By fitting the data below 10 K with the expression: Cp/T = γ + βT2, where γ is the Sommerfeld coefficient, the fitted γ and β were obtained as 3.35 mJ mol−1 K−2 and 1.60 mJ mol−1 K−4, respectively. According to the formula θD = (12π4NR/β)1/3, the Debye temperature θD is estimated to be about 182 K.
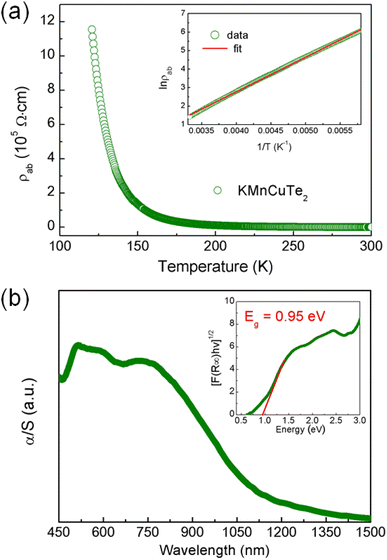
The variation of in-plane resistivity with temperature for KMnCuTe2 crystal is shown in Fig. 4a. Clearly it exhibits a semiconducting behavior from 120 K to 300 K. The resistivity data obey the thermally activated behavior ρ = ρ0 exp(Ea/kBT) as shown in inset, and the activation energy Ea is calculated as 0.16 eV. The electrical resistivity at room temperature is around 408 Ω cm, which is much larger than those of KMnCuSe2 and KMnCuS2. It is generally believed that the telluride shows smaller resistivity than its analogous selenide and sulfide due to the smaller electronegativity of Te. We indicate that the abnormal rules in KMnCuTe2 compared to KMnCuSe2 and KMnCuS2 could be resulted from the long metal–metal distance, which weakens the metallic orbital overlap between Mn(Cu) atoms and significantly increases the in-plane resistivity. It is noticed that in-plane resistivity of KMnCuTe2 is also larger than those of KFeCuTe2 and KFeAgTe2, suggesting the significant effect of transition metal species on electrical properties of ThCr2Si2-type compounds.12,13,20
The UV-vis diffuse reflectance spectrum was collected to show the optical property of KMnCuTe2 crystals. To extract the band gap, the reflectance data were transformed to pseudo-absorption data using Kubelka–Munk equation as described in the experimental section (Fig. 4b). The optical band gap was extracted using the indirect model as suggested by the band structure calculation described below, and the obtained band gap (Eg) is 0.95 eV, which is consistent with the black color of the crystal. Hence it can be concluded that KMnCuTe2 is an antiferromagnetic semiconductor with the narrow band gap.
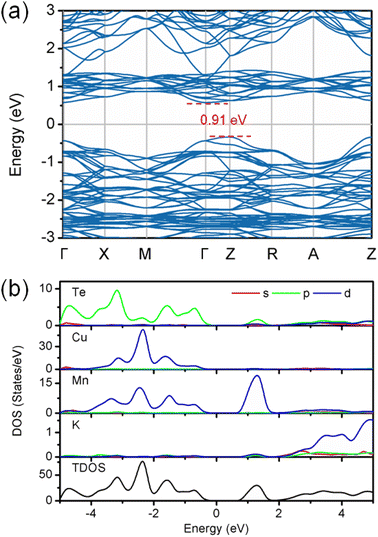
The density functional theory calculations were also carried out to better understand the nature of magnetism and electronic structure of KMnCuTe2. Three AFM structures, including A-type, C-type and G-type were built in a 2 × 2 × 1 supercell as shown in Fig. 5, and the calculation suggested the G-type AFM as the energy favorable ground state of KMnCuTe2. It should be mentioned that the energy difference to the A-type AFM state is only 0.024 meV per f.u., suggesting that the A-type AFM can also be the possible ground state of KMnCuTe2. With the G-type AFM structure, the electronic band structure and density of states of KMnCuTe2 were calculated. As shown in Fig. 6a, the valence band maximum (VBM) is located at Z point while the conduction band minimum (CBM) is located at Γ, indicating that KMnCuTe2 is an indirect semiconductor. The calculated band gap is 0.91 eV, in consistent with the experimental result (0.95 eV). Based on the partial electronic density of states (DOS) shown in Fig. 6b, the valence band of KMnCuTe2 near the Fermi level (EF) is contributed by the orbitals of Mn, Cu and Te, while the conduction band near the band edge is mainly composed of the 3d orbitals of Mn.
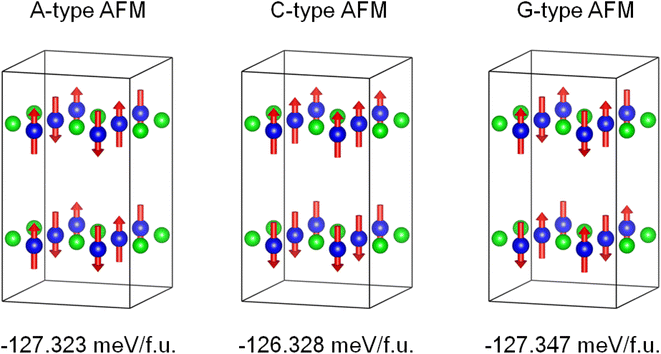 | ||
| Fig. 5 The calculated energies of different AFM structures of KMnCuTe2 in a 2 × 2 × 1 supercell, showing that the G-type AFM is the energy favorable ground state of KMnCuTe2. | ||
Conclusion
Here we report a new quaternary chalcogenide KMnCuTe2 which exhibits layered structure and antiferromagnetic semiconducting behavior. As a new member in AMnMCh2 (A = K, Rb, Cs, M = Cu, Ag and Ch = S, Se, Te) material family, KMnCuTe2 adopts the ThCr2Si2-type structure with mixed Mn and Cu occupation. The refined lattice parameters (a = 4.3115(3) Å and c = 14.9360(20) Å) and resulted metal–metal distance (3.049 Å) are larger than the sulfides and selenides in AMnMCh2. KMnCuTe2 shows a long-range G-type antiferromagnetic interaction with the transition temperature at around 225 K, very unique in the AMnMCh2 system. The resistivity and diffuse reflectance spectra indicate that KMnCuTe2 is an indirect semiconductor with the optical band gap as 0.95 eV. These results shed light on the relationship between crystal structure and physical properties of ThCr2Si2-type materials.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
The financial supports by the National Natural Science Foundation of China (No. 51872028 and 51832010) are gratefully acknowledged.References
- J. G. Guo, S. F. Jin, G. Wang, S. C. Wang, K. X. Zhu, T. T. Zhou, M. He and X. L. Chen, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 82, 180520 CrossRef.
- T. P. Ying, X. L. Chen, G. Wang, S. F. Jin, T. T. Zhou, X. F. Lai, H. Zhang and W. Y. Wang, Sci. Rep., 2012, 2, 1–7 Search PubMed.
- T. P. Ying, X. L. Chen, G. Wang, S. F. Jin, X. F. Lai, T. T. Zhou, H. Zhang, S. J. Shen and W. Y. Wang, J. Am. Chem. Soc., 2013, 135, 2951–2954 CrossRef CAS PubMed.
- Z. N. Guo, F. Sun and W. X. Yuan, Cryst. Growth Des., 2017, 17, 2238–2253 CrossRef CAS.
- J. R. Neilson, A. Llobet, A. V. Stier, L. Wu, J. J. Wen, J. Tao, Y. M. Zhu, Z. B. Tesanovic, N. P. Armitage and T. M. McQueen, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 86, 054512 CrossRef.
- J. R. Neilson and T. M. McQueen, J. Am. Chem. Soc., 2012, 134, 7750–7757 CrossRef CAS PubMed.
- G. Huan, M. Greenblatt and M. Croft, Eur. J. Inorg. Chem., 1989, 26, 193–220 CAS.
- J. H. Yang, B. Chen, H. D. Wang, Q. H. Mao, M. Imai, K. Yoshimura and M. H. Fang, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 88, 064406 CrossRef.
- Z. N. Guo, H. H. Zhang, D. Wang, B. L. Han, S. F. Jin and W. X. Yuan, Dalton Trans., 2016, 45, 8248–8252 RSC.
- C. Mujica, J. Páez and J. Llanos, Mater. Res. Bull., 1994, 29, 263–268 CrossRef CAS.
- M. Oledzka, K. V. Ramanujachary and M. Greenblatt, Mater. Res. Bull., 1996, 31, 1491–1499 CrossRef CAS.
- H. C. Lei, E. S. Bozin, K. Wang and C. Petrovic, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 84, 060506 CrossRef.
- H. Ryu, H. C. Lei, B. Klobes, J. B. Warren, R. P. Hermann and C. Petrovic, Phys. Rev. B: Condens. Matter Mater. Phys., 2015, 91, 174517 CrossRef.
- D. D. Yuan, N. Liu, K. K. Li, S. F. Jin, J. G. Guo and X. L. Chen, Inorg. Chem., 2017, 56, 13187–13193 CrossRef CAS PubMed.
- M. Oledzka, J. G. Lee, K. V. Ramanujachary and M. Greenblatt, J. Solid State Chem., 1996, 127, 151–160 CrossRef CAS.
- M. Oledzka, J. G. Lee, K. V. Ramanujachary and M. Greenblatt, Mater. Res. Bull., 1997, 32, 889–898 CrossRef CAS.
- M. Oledzka, K. V. Ramanujachary and M. Greenblatt, Mater. Res. Bull., 1998, 33, 855–866 CrossRef CAS.
- X. F. Lai, S. F. Jin, X. Chen, T. T. Zhou, T. P. Ying, H. Zhang and S. J. Shen, Mater. Express, 2014, 4, 343–348 CrossRef CAS.
- H. R. Heulings IV, J. Li and D. M. Proserpio, Main Group Met. Chem., 1998, 21, 225–230 Search PubMed.
- F. Sun, Z. N. Guo, N. Liu, K. Lin, D. Wang and W. X. Yuan, Dalton Trans., 2017, 46, 3649–3654 RSC.
- X. N. Sun, Y. P. Song, J. Deng, S. F. Jin and X. L. Chen, Dalton Trans., 2019, 48, 17572–17578 RSC.
- B. B. Wang, Z. N. Guo, F. Sun, J. Deng, J. W. Lin, D. Wu and W. X. Yuan, J. Solid State Chem., 2019, 272, 126–130 CrossRef CAS.
- E. J. Wu, M. A. Pell and J. A. Ibers, Acta Crystallogr., 1996, C52, 2384–2385 CAS.
- J. Li, H. Y. Guo and R. A. Yglesias, Chem. Mater., 1995, 7, 599–601 CrossRef CAS.
- Z. N. Guo, Z. Liu, J. Deng, J. W. Lin, Z. J. Zhang, X. Han, F. Sun and W. X. Yuan, J. Alloys Compd., 2021, 883, 160820 CrossRef CAS.
- R. Ang, K. Nakayama, W. G. Yin, T. Sato, H. C. Lei, C. Petrovic and T. Takahashi, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 88, 155102 CrossRef.
- Y. Song, H. B. Cao, B. C. Chakoumakos, Y. Zhao, A. F. Wang, H. C. Lei, C. Petrovic and R. J. Birgeneau, Phys. Rev. Lett., 2019, 122, 087201 CrossRef CAS PubMed.
- Y. Song, D. S. Yuan, X. Y. Lu, Z. J. Xu, E. Bourret-Courchesne and R. J. Birgeneau, Phys. Rev. Lett., 2019, 123, 247205 CrossRef CAS PubMed.
- V. Petříček, M. Dušek and L. Palatinus, Z. Kristallogr. – Cryst. Mater., 2014, 229, 345–352 CrossRef.
- L. F. Gate, Appl. Opt., 1974, 13, 236–238 CrossRef CAS PubMed.
- G. Kresse and J. Furthmüller, Comput. Mater. Sci., 1996, 6, 15–50 CrossRef CAS.
- G. Kresse and J. Furthmüller, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169 CrossRef CAS PubMed.
- J. P. Predew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef PubMed.
- G. Kresse and D. Joubert, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 1758–1775 CrossRef CAS.
- H. J. Monkhorst and J. D. Pack, Phys. Rev. B: Condens. Matter Mater. Phys., 1975, 13, 5188–5192 CrossRef.
- A. Jain, G. Hautier, S. P. Ong, C. J. Moore, C. C. Fischer, K. A. Persson and G. Ceder, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 84, 045115 CrossRef.
- S. Jana, M. Ishtiyak, G. Panigrahi, J. Prahash, A. Mesbah, S. Gueddida, S. Lebegue, C. D. Malliakas and J. A. Ibers, Inorg. Chem., 2020, 59, 12276–12285 CrossRef CAS PubMed.
- T. T. Zhou, Y. M. Wang, S. F. Jin, D. D. Li, X. F. Lai, T. P. Ying, H. Zhang, S. J. Shen, W. J. Wang and X. L. Chen, Inorg. Chem., 2014, 53, 4154–4160 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Crystal data, atomic coordinates, displacement parameters and anisotropic displacement parameters of KMnCuTe2 (Tables S1–S3) and PXRD patterns in Fig. S1. CCDC 2193743. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2ra04789f |
| This journal is © The Royal Society of Chemistry 2022 |