Open Access Article
Open Access ArticleTemplate and interfacial reaction engaged synthesis of CeMnOx hollow nanospheres and their performance for toluene oxidation†
Yuhua Zhenga,
Jing Zhouc,
Xi Zengb,
Dandan Hua,
Fang Wangb and
Yanbin Cui *a
*a
aState Key Laboratory of Multiphase Complex Systems, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China. E-mail: ybcui@ipe.ac.cn
bKey Laboratory of Cleaner Production and Integrated Resource Utilization of China National Light Industry, Beijing Technology and Business University, Beijing 100048, China
cSchool of Chemical Engineering, Shenyang University of Chemical Technology, Shenyang 110142, Liaoning, China
First published on 13th September 2022
Abstract
A series of well-dispersed CeMnOx hollow nanospheres with uniform diameter and thickness were synthesized by a novel approach combining the template method and interfacial reaction. A SiO2 template was used as a hard template for preparation of SiO2@CeO2 nanospheres by solvothermal reaction. SiO2@CeMnOx could be formed after KMnO4 was reacted with SiO2@CeO2 by interfacial reaction between MnO4− and Ce3+. Among all the prepared catalysts, CeMnOx-3 with a moderate content of Mn (15 wt%) exhibited the lowest temperature for complete combustion of toluene (280 °C). Moreover, it showed high stability for 36 h with toluene conversion above 97.7% and good water tolerance with 5 vol% H2O. With characterization, we found that the reaction between Ce and Mn in the Ce–Mn binary oxides gave rise to increased Ce3+ and oxygen vacancies, which led to the formation of enhanced reducibility and more surface-absorbed oxygens (O22−, O2− and O−), and improved the catalytic performance further.
1 Introduction
As one of the primary sources of air pollutants, volatile organic compounds (VOCs) are not only significant contributors to smog formation, but also can cause environmental pollution and bring great negative effects to human health.1,2 Some VOCs have been classified as carcinogens, such as benzene, toluene, dichloromethane, and dichloroethane.3 Therefore, the effective removal of VOCs has been an important research topic in environmental governance.Numerous methods have been used to eliminate VOCs, including incineration, absorption, adsorption, condensation, and catalytic oxidation.4–6 Among them, catalytic oxidation has been recognized as one of the most effective and economical ways for abating VOCs, as it has the advantages of low degradation temperature, high removal efficiency, and no secondary pollution.4,7 To realize the industrial application of catalytic oxidation, the catalysts need to be developed extensively. So far, two types of traditional catalysts were researched deeply, including supported noble metals (such as Au, Pd, and Pt)8,9 and transition metal oxides.10,11 Although the former exhibits high activity and COx selectivity at low temperatures, the high cost and high sensitivity to water vapor, sulfur and chlorine compounds limit their applications. Transition metal oxides, such as Co3O4,11,12 NiO,13 MnOx,14 CuO,15 and their composite oxides,16–18 possess the advantages of low cost and high toxicity resistance. However, the reaction temperature for these catalysts is relatively high, which will affect their stability and increase energy costs.
In addition to these two types of catalysts, CeO2 is also reported as an effective catalyst for the catalytic oxidation of VOCs as it has high oxygen storage capacity caused by its excellent redox properties. In recent years, CeO2 with different morphologies and structures was researched for VOCs oxidation.19,20 The catalytic activity of CeO2 in the VOCs oxidation process can be improved by adding other elements to form composites, such as Mn, Cu, Ni and Co. The increment in catalytic performance may originate from the synergistic effect between CeO2 and other metal oxides. The oxygen vacancy due to different electric states between Ce4+ and Mx+ is another essential factor for the enhanced activity.16,21 Thereinto, Ce–Mn composite oxides with tunable structure and size have been often used for VOCs oxidation because of the low costs, improved catalytic performances, and high thermal stability. For example, Wang et al. synthesized CeO2–MnOx and proved that the synergistic effect between Mn and Ce is the decisive factor on its excellent activity for benzene oxidation.22 Zhao et al. prepared a series of CeaMnOx microspheres with arbutus-like hierarchical structure by coprecipitation. The Ce0.03MnOx showed best catalytic activity for toluene oxidation with high stability and water resistance, due to the Mn4+ species and abundant surface oxygen.23
As 3D nano/micro-structured materials (including hollow nanospheres) possess high surface area and low density, they exhibit some advantages superior to corresponding bulk materials, which are beneficial for their application in catalysis, drug delivery, supercapacitors and so on.23–27 Although little progress has been made in terms of synthesis and catalytic performance of CeO2–MnOx hollow nanospheres, the as-obtained materials could not possess homogeneous structure, including uniform morphology, diameter, and interior architecture.28,29 In this paper, we prepared well-defined CeMnOx hollow nanospheres with different amounts of MnOx by the combination of template method and interfacial reaction. Toluene was chosen as the target contaminant for catalytic oxidation. By tuning the content of MnOx in the catalysts, the effect of Ce/Mn ratio on catalytic activity was evaluated. Our results demonstrated that CeMnOx-3 with moderate content of Mn (15 wt%) showed low temperature for complete combustion of toluene (280 °C) and high stability for 36 h with toluene conversion above 97.7%. It also exhibited good water tolerance and recyclability under the condition of 5 vol% H2O. Based on the study of catalysts, including structure, morphology, element states, and catalytic performance, the synergistic effect between Ce and Mn was proposed to play a critical role in the improvement of catalytic performance.
2 Experimental
The detailed Experimental section is described in the ESI.†3 Results and discussion
3.1 Composition and structure
XRD was conducted to characterize the crystalline structures of as-prepared catalysts. As displayed in Fig. 1, the peaks for all the samples are similar and could be indexed to fluorite CeO2 (JCPDS no. 34-0394) and no diffraction peak of MnOx species is observed. Meanwhile, compared with the prominent reflection peaks (111 and 220) of CeO2, a slight shift to higher Bragg angles is observed for the CeMnOx binary oxides. For the radius of Mn3+ (0.064 nm) and Mn4+ (0.060 nm) are smaller than those of Ce3+ (0.134 nm) and Ce4+ (0.114 nm), the spacing between crystal planes (d) of CeO2 decreased when the manganese ions substituted cerium ions. Therefore, the value of θ for CeMnOx is calculated to be higher than that of CeO2 from Bragg's law: 2d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = kλ.30 This shift also illustrates that Mn has successfully migrated into the CeO2 lattice through interfacial reaction due to the close contact between Ceδ+ and Mnδ+ during the calcination process. The introduction of Mn species could modify the crystallization of CeO2, and form Mn–Ce solid solution. The difference in ionic radius means that the defects in the CeO2 lattice are easily formed, including oxygen vacancies that can provide active centers for the creation of reactive oxygen species.31
θ = kλ.30 This shift also illustrates that Mn has successfully migrated into the CeO2 lattice through interfacial reaction due to the close contact between Ceδ+ and Mnδ+ during the calcination process. The introduction of Mn species could modify the crystallization of CeO2, and form Mn–Ce solid solution. The difference in ionic radius means that the defects in the CeO2 lattice are easily formed, including oxygen vacancies that can provide active centers for the creation of reactive oxygen species.31
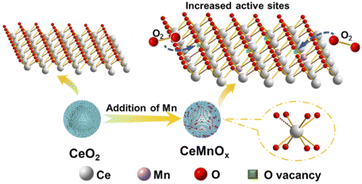
The synthesis process of CeMnOx composite included three main steps and was illustrated in Scheme 1. Firstly, homogeneous SiO2@CeO2 was synthesized by the solvothermal method.32–34 In this step, uniform SiO2 spheres with a diameter of about 250 nm (Fig. S1 in the ESI†) were used as templates, and CeO2 nanoparticles were covered on the surface of SiO2 spheres by the self-assembly method. From XRD in Fig. S2,† we can see that the nanoparticles on SiO2 were CeO2 even though the broad diffraction peaks illustrate its low crystallinity, indicating CeO2 could be obtained just after the solvothermal treatment of Ce(NO3)3 directly. In the second step, SiO2@CeO2 was dispersed in KMnO4 solution to coat the MnOx layer. Although most of Ce in SiO2@CeO2 existed in the form of Ce4+ with high valence, there was still a small amount of Ce3+ coming from the transformation of Ce4+ due to the excellent redox behavior of CeO2. KMnO4 with strong oxidation ability would oxidize this tiny amount of Ce3+ to CeO2 and be reduced to MnOx itself. This reaction can also be indirectly observed by the color change of the reaction system from purple to dark brown, corresponding to the color of MnO4− and MnOx, respectively. In addition, the rough surface formed by the accumulation of CeO2 nanoparticles in the first step could increase the contact between KMnO4 and Ce(III) and accelerate the surface interaction. At last, the SiO2 core was etched with NaOH solution and CeMnOx hollow spheres were obtained.
ICP-AES was used to ascertain the amount of Mn in the CeMnOx catalysts. The actual contents of Mn in CeMnOx catalysts increase with the concentration of KMnO4 solution. It should be noticed that the preparation method involves the oxidation of Ce3+ on the surface of CeO2 with KMnO4 to obtain MnOx. As the amount of Ce3+ in CeO2 is fixed, the content of MnOx reaches saturation for CeMnOx-4 (19.0%), which is close to sample 5, 18.90%.
Raman spectra of catalysts CeO2 and the CeMnOx are shown in Fig. 2. The band at 460 cm−1 is assigned to the triply degenerated F2g active mode of CeO2, which reflects the symmetric breathing pattern of oxygen atoms around cerium ions in the fluorite CeO2.35 With the increase of Mn contents, the F2g band shifts to lower wavenumbers (red shift) gradually, suggesting the variations of Ce–O bonding symmetry. The red shifts may attribute to the lattice contraction36,37 and consequent oxygen vacancies,38 which both originated from the partial incorporation of Mn ions with smaller radius into fluorite CeO2. No Raman peaks for MnOx species (e.g. Mn3O4) could be observed in the CeMnOx catalysts, which reveals that the MnOx in the binary oxides is highly dispersed, in accordance with XRD results.39–41
N2 adsorption–desorption isotherms of CeO2 and CeMnOx are displayed in Fig. 3. All the catalysts present type II isotherms with H3-type hysteresis loops. According to IUPAC classification, these isotherms suggest the presence of slit-shaped micropores in the samples. The Brunauer–Emmett–Teller (BET) surface areas of the catalysts in Table 1 show an obvious downtrend (from 107.2 to 23.8 m2 g−1) opposite to that of Mn content, indicating the addition of Mn leads to a more compact structure, which could be confirmed by SEM images in the following section.
As CeMnOx-3 exhibited the best catalytic activity among all the CeMnOx catalysts, it was chosen as an example to study the morphologies by SEM and the images of other samples were given in Fig. S3.† The SiO2@CeMnOx-3 before NaOH treatment are composed of large-scale uniform and well-dispersed microspheres with 250–400 nm in diameter (Fig. 4a). The surfaces of the microspheres are rough and consists of small particles, indicating that CeO2 nanoparticles has deposited on the SiO2 templates successfully. After removing of SiO2 templates with NaOH treatment, CeMnOx-3 hollow spheres can be obtained (Fig. 4b), which inherit the morphologies of their precursors well. A few cracked spheres in Fig. 4b and c reveal their hollow features clearly. A high magnified SEM image of a ruptured CeMnOx-3 microsphere in Fig. 4d reveals that the thickness of the hollow sphere is about 50 nm and the sphere is composed of abundant nanoparticles, contributing to a high specific surface area. For SiO2@CeO2 before NaOH treatment, the surfaces were composed of nanoparticles with 20 nm in diameter (Fig. S3a†). With careful observation, we can see that the SiO2@CeO2 nanospheres are loosely structured, with some areas showing a tendency to delaminate, indicating the weak interaction between the nanoparticles. For this reason, the shells of CeO2 hollow sphere are fragile and easily to be broken after NaOH treatment, which is indicated by many broken spheres in Fig. S3b.† With the increase of Mn content, the ratio of broken spheres decreases and the surface of the spheres gets smoother and more compact, indicating the interaction between nanoparticles is enhanced, which is also the reason for the downward trend in the specific surface area (Fig. S3c–f†).
TEM images in Fig. 5a, e and i confirm the hollow character of the CeMnOx catalysts with uniform diameter and thickness, in consistent with the SEM images. From the TEM images of CeMnOx-1 in Fig. 5b and c, we can see some large nanocrystals with high crystallinity on the surface. For CeMnOx-3, large nanoparticles on the surface disappear and the structure of the sphere gets puffy (Fig. 5f and g). The thickness of the hollow sphere increases obviously from CeMnOx-3 to CeMnOx-5, and the structure becomes more compact (Fig. 5j). For the HRTEM images of the three catalysts, there is apparent lattice fringe with a spacing of about 0.31 nm, corresponding to the (111) plane of face-centered cubic CeO2. No lattice fringe indexed to MnOx species can be observed, but there are some areas with amorphous structure (marked with red circles in Fig. 5c, g and k), which attributes to part of Mn species that have not entered the CeO2 lattice. We can see that there are more amorphous MnOx particles that well dispersed on the surface of CeMnOx-3 than those in CeMnOx-1. But with a further increase of Mn content, CeMnOx-5 is nearly covered by a layer of amorphous MnOx particles, illustrating the high amount of Mn (Fig. 5k). The diffraction points and rings in SAED in Fig. 5d prove the coexistence of pure CeO2 and few CeMnOx with low crystallinity. For CeMnOx-3, the diffraction points that indexed to CeO2 was hard to be observed, further confirming the formation of a large amount of amorphous MnOx on the surface of CeMnOx solid solution (Fig. 5h). The diffraction points disappeared totally for CeMnOx-5, caused by the coverage of the MnOx layer on CeO2. The structural differences would affect the catalytic activity (Fig. 5l).
3.2 Chemical states of the catalysts
The chemical compositions of CeO2 and CeMnOx were investigated by XPS (Fig. 6). Ce 3d spectra (Fig. 6a) are fitted to eight peaks by deconvolution and labelled as U and V, contributing to 3d5/2 and 3d3/2 spin–orbit components of Ce, respectively.42,43 These peaks marked with V, V′′, V′′′, U, U′′ and U′′′ are attributed to the Ce4+ species while V′ and U′ are assigned to the Ce3+ species. The relative content of Ce3+ can be calculated by Ce3+/(Ce3+ + Ce4+) based on the peak area and the results are shown in Table 2. The relative surface Ce3+ content increases slightly, reaches a maximum for CeMnOx-3. According to literatures, toluene catalytic oxidation on CeO2 and MnOx usually conforms to Mars–van-Krevelen (MvK) mechanism, in which oxygen atoms on the surface of the catalysts are key factors as they participate in the reaction.38 So, the concentrations of oxygen vacancies and surface adsorbed oxygen species on the catalysts should be investigated deeply. Generally, nonstoichiometric Ce3+ species in CeO2 leads to the generation of crystal defects and oxygen vacancies to maintain electrostatic balance following the reaction: 4Ce4+ + O2− → 4Ce4+ + 0.5O2 + 2e−/□ → 2Ce4+ + 2Ce3+ + 0.5O2 + □ (□ represents oxygen vacancy).38,44–47 Therefore, higher Ce3+ content indicates more oxygen vacancies, contributing to superior redox properties and excellent catalytic activity. The corresponding XPS spectra of Mn 2p for CeMnOx are deconvoluted into three main peaks with binding energies at 640.5, 641.7 and 643.0 eV (Fig. 6b), which are assigned to the spin–orbital of Mn2+, Mn3+ and Mn4+ species, respectively. The results in Table 2 show that CeMnOx-3 has the largest concentration of Mn with a lower valence state (52.5%). The existence of more Mn2+ and Mn3+ also imply the formation of more oxygen vacancies, similar to the effect of Ce3+. | ||
| Fig. 6 XPS spectra of Ce 3d (a), Mn 2p (b) and O 1s (c) for CeO2 and CeMnOx-1, CeMnOx-3 and CeMnOx-5. | ||
All the O 1s spectra in Fig. 6c can be deconvoluted into three peaks located at about 529.6, 530.5, and 533.1 eV, attributing to the lattice oxygen (OI), adsorbed oxygen with low coordination (such as O22−, O2− and O−, denoted as OII), and carbonates (OIII), respectively.40,48 The percentages of adsorbed oxygen for all the catalysts exhibit the same trend with Ce3+ and Mnδ+ with lower valence states, consistent with the conclusion that Ce and Mn species with lower valence states are benefit for increasing the amount of surface adsorbed oxygen.49 Strong interaction between Ce and Mn weakens the Ce–O bonds located at the interface of MnOx and CeO2, and increases the mobility of lattice oxygen. As a result, oxygen vacancies can be easily formed and surface adsorbed oxygen species are enhanced consequently, which are benefit for toluene oxidation according to the MvK mechanism.
3.3 Reducibility of the catalysts
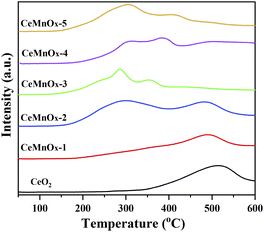
Redox properties of the catalysts were investigated by H2-TPR and the curves were exhibited in Fig. 7. CeO2 exhibits a reduction peak at about 520 °C, which can be ascribed to the consumption of H2 by CeO2.50 After the introduction of Mn, the reduction peaks of CeMnOx-1 and CeMnOx-2 shift to the lower temperature continually, implying that the introduction of Mn ions can improve the reduction reducibility of CeO2 significantly. It should be noted that a broad band of H2 consumption for CeMnOx-2 began to occur at about 300 °C, which can be assigned to the reduction of MnOx species.36 With the increase of Mn content, CeMnOx-3 exhibit three peaks at about 230, 285 and 354 °C, corresponding to the reduction of Mn4+ to Mn3+, Mn3+ to Mn2+ and partial reduction of surface ceria, respectively.50–52 For CeMnOx-4 and CeMnOx-5, even the peaks shift to high temperature again, they are still much lower than that of pure CeO2. It is reported that –Mn–O–Ce– bond of CeMnOx hollow spheres can reduce the oxygen vacancy formation energy remarkably, and then strengthen the mobility of oxygen species from the bulk to the surface when compared with pure ceria.53 These results indicate that the addition of an appropriate amount of Mn into the CeO2 lattice can lead to high mobility and XPS results. Combined with all these results above, it is reasonable to speculate that the CeMnOx can exhibit better promote the release of the lattice oxygen, inconsistent with the catalytic activity than pure CeO2, and CeMnOx-3 should be the best one.3.4 Catalytic activity
The catalytic oxidation of toluene over the hollow structured CeMnOx catalyst is present in Fig. 8a. In general, the activities of all catalysts reveal a similar positive correlation with the reaction temperature. CeO2 shows the lowest catalytic activity, whose T50 and T90 are as high as 310 and 369 °C, respectively. After the introduction of Mn, all the CeMnOx exhibit better performance than CeO2, with the following order: CeMnOx-3 > CeMnOx-4 ≈ CeMnOx-5 > CeMnOx-2 > CeMnOx-1 (deducing by T50 and T90, marked with red lines in Fig. 8a). This result demonstrates that the introduction of Mn can improve the catalytic activities due to the synergistic effect between Ce and Mn. Especially for CeMnOx-3, the complete conversion temperature of toluene is only 280 °C, while the conversion is only 8.9% for CeO2 at the same temperature.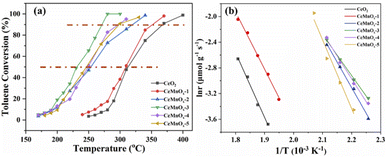 | ||
| Fig. 8 (a) Toluene conversions as a function of reaction temperature and (b) Arrhenius linear fit plots for toluene oxidation over the catalysts. | ||
It is reported that with excess oxygen, the catalytic oxidation of toluene follows first-order kinetics with the toluene concentration.34,54 When the toluene conversion is less than 20%, the ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) r dependence of 1/T can be used to calculate apparent activation energies (Fig. 8b). For clarification, the reaction rates and activation energies calculated from the slope of the linear fit of the Arrhenius scatter plots were listed in Table 3. The reaction rate over CeMnOx-3 is 0.141 μmol g−1 s−1, the highest among these catalysts. Ea for CeMnOx-3 is the lowest and increased with the order: CeMnOx-3 < CeMnOx-4 < CeMnOx-2 < CeMnOx-5 < CeMnOx-1 < CeO2. The highest reaction rate and lowest Ea value for CeMnOx-3 both confirm its excellent performance on toluene oxidation.
r dependence of 1/T can be used to calculate apparent activation energies (Fig. 8b). For clarification, the reaction rates and activation energies calculated from the slope of the linear fit of the Arrhenius scatter plots were listed in Table 3. The reaction rate over CeMnOx-3 is 0.141 μmol g−1 s−1, the highest among these catalysts. Ea for CeMnOx-3 is the lowest and increased with the order: CeMnOx-3 < CeMnOx-4 < CeMnOx-2 < CeMnOx-5 < CeMnOx-1 < CeO2. The highest reaction rate and lowest Ea value for CeMnOx-3 both confirm its excellent performance on toluene oxidation.
3.5 Structure–activity relationships
The activation ability of oxygen molecules for catalysts is an essential factor in evaluating their catalytic activity. It has been proved that the surface oxygen vacancies are favourable for the adsorption and activation of O2.37 Therefore, the catalysts with high oxygen vacancies may exhibit good catalytic performance. XPS spectra (Fig. 6) confirm that after the addition of Mn, the content of Ce3+ species increase and give rise to more oxygen vacancies, favourable for the activation of O2. Accordingly, O 1s spectra of CeMnOx confirm the existence of more surface-absorbed oxygens compared with CeO2, which would increase the redox property of CeMnOx catalysts significantly. According to the MvK mechanism, Olatt (O2−) on the surface of the catalyst are the active species. After being consumed of O2− in the oxidation, they can be regenerated by migration of electrophilic surface-absorbed oxygens (O22−, O2− and O−) into oxygen vacancies with the dissociation of O2 in the feed continually (Scheme 2). Therefore, CeMnOx-3 with the most Ce3+ species and surface-absorbed oxygens showed the best catalytic activity. However, when Mn continues to increase, the surface MnOx species would increase while the Ce3+ content decreases gradually (Table 2). From Fig. 5k, there was even a layer of amorphous MnOx that covered the solid solution CeMnOx, inhibiting the contact between –Mn–O–Ce– and O2. Therefore, the surface-absorbed oxygens and catalytic activity decreased correspondingly.3.6 The stability test and water resistance
The stability of the catalysts is also a critical factor for practical application. Fresh CeMnOx-3 was tested for the toluene conversion (inlet concentration 1000 ppm) as a function of time on stream at 280 °C. As shown in Fig. 9a, the catalyst was relatively stable for 10 h, with 100% toluene conversion. In the next 10 h, the conversion only exhibits a slight decrease, and is stable at 99%. After reacting for 20 h, the reaction system was cooled to room temperature and reheated to 200 °C for reusability test. It can be seen that in the second cycle, there is no apparent change in toluene conversion. Even after the third test, the toluene conversion can maintain 97.7%. Obviously, the catalyst has excellent catalytic stability and reusability for the oxidation of toluene, endowing it the possibility of future industrialization. | ||
| Fig. 9 Catalytic stability without (a) and with (b) water (10 vol%) of CeMnOx-3 for toluene oxidation at 280 °C. | ||
In practical application, moisture tolerance of the catalyst is another important factor to be considered as VOCs gas in the industry often contains water. Therefore, the influence of vapor on the long-term catalytic activity of CeMnOx-3 under the feed gas stream including 5 vol% of water at 280 °C was investigated and the result was given in Fig. 9b. In the absence of water vapor, the toluene conversion maintained 100%, consistent with the results in Fig. 9a. After introducing water vapor into the reaction stream, the toluene conversion decreased to 95% directly and remained stable afterward. Once the water vapor was stopped, the conversion recovered to 97%. For the subsequent two cycles, the catalytic activity showed a similar trend and the toluene conversion was above 90% throughout the test. Finally, the toluene conversion could recover to 97% after the water vapor was out. Therefore, the catalyst owned excellent water vapor tolerance, which is a favorable factor for achieving industrial application.
4 Conclusions
In this work, we combined the template method and interfacial reaction to prepare a series of CeMnOx binary oxide hollow spheres with uniform morphology, diameter and thickness. Mn was incorporated by an interfacial redox reaction between KMnO4 and Ce elements with low valence on the surface of SiO2@CeO2. The as-prepared binary oxides exhibit better catalytic activities than CeO2 hollow spheres. CeMnOx-3 exhibits the lowest T90 (nearly 100 °C lower than that of CeO2), excellent stability and H2O resistance even with WHSV of 120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1. Redox properties and surface oxygen vacancies of the binary oxide catalysts are improved as the addition of Mn would cause strong interaction between Ce and Mn, increasing of surface-adsorbed oxygen species, which are critical to the enhanced catalytic performance.
000 h−1. Redox properties and surface oxygen vacancies of the binary oxide catalysts are improved as the addition of Mn would cause strong interaction between Ce and Mn, increasing of surface-adsorbed oxygen species, which are critical to the enhanced catalytic performance.
Author contributions
Yuhua Zheng: methodlogy, analysis, writing and editing. Jing Zhou: synthesis. Xi Zeng and Dandan Hu, investigation and characterization. Fang Wang: data curation. Yanbin Cui: supervision, funding acquisition and review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is financially supported by the financial support from National Natural Science Foundation of China (No. 22076189) and the Open Research Fund Program of Key Laboratory of Cleaner Production and Integrated Resource Utilization of China National Light Industry (No. CP2021YB06).References
- B. J. FinlaysonPitts and J. N. Pitts, Science, 1997, 276, 1045–1052 CrossRef CAS PubMed.
- S. Kim, R. Vermeulen, S. Waidyanatha, B. A. Johnson, Q. Lan, M. T. Smith, L. Zhang, G. Li, M. Shen, S. Yin, N. Rothman and S. M. Rappaport, Cancer Epidemiol., Biomarkers Prev., 2006, 15, 2246–2252 CrossRef CAS.
- M. Amann and M. Lutz, J. Hazard. Mater., 2000, 78, 41–62 CrossRef CAS.
- Y. Guo, M. Wen, G. Li and T. An, Appl. Catal., B, 2021, 281, 119447 CrossRef CAS.
- G. R. Parmar and N. N. Rao, Crit. Rev. Environ. Sci. Technol., 2009, 39, 41–78 CrossRef CAS.
- X. Zhang, B. Gao, A. E. Creamer, C. Cao and Y. Li, J. Hazard. Mater., 2017, 338, 102–123 CrossRef CAS PubMed.
- C. He, J. Cheng, X. Zhang, M. Douthwaite, S. Pattisson and Z. Hao, Chem. Rev., 2019, 119, 4471–4568 CrossRef CAS PubMed.
- S. Scire and L. F. Liotta, Appl. Catal., B, 2012, 125, 222–246 CrossRef CAS.
- L. F. Liotta, Appl. Catal., B, 2010, 100, 403–412 CrossRef CAS.
- H. Liu, X. Li, Q. G. Dai, H. L. Zhao, G. T. Chai, Y. L. Guo, Y. Guo, L. Wang and W. C. Zhan, Appl. Catal., B, 2021, 282, 9 Search PubMed.
- K. W. Zha, W. J. Sun, Z. Huang, H. L. Xu and W. Shen, ACS Catal., 2020, 10, 12127–12138 CrossRef CAS.
- X. Y. Wang, Y. Liu, T. H. Zhang, Y. J. Luo, Z. X. Lan, K. Zhang, J. C. Zuo, L. L. Jiang and R. H. Wang, ACS Catal., 2017, 7, 1626–1636 CrossRef CAS.
- H. C. Wang, W. Q. Guo, Z. Jiang, R. O. Yang, Z. Jiang, Y. Pan and W. F. Shangguan, J. Catal., 2018, 361, 370–383 CrossRef CAS.
- S. C. Kim and W. G. Shim, Appl. Catal., B, 2010, 98, 180–185 CrossRef CAS.
- X. L. Meng, L. K. Meng, Y. J. Gong, Z. H. Li, G. Mo and J. Zhang, RSC Adv., 2021, 11, 37528–37539 RSC.
- S. Y. Feng, J. D. Liu and B. Gao, Chem. Eng. J., 2022, 429, 12 Search PubMed.
- J. Y. Yun, L. K. Wu, Q. L. Hao, Z. H. Teng, X. Gao, B. J. Dou and F. Bin, J. Environ. Chem. Eng., 2022, 10, 8 Search PubMed.
- Y. Q. Zeng, K. G. Haw, Z. G. Wang, Y. A. Wang, S. L. Zhang, P. Hongmanorom, Q. Zhong and S. Kawi, J. Hazard. Mater., 2021, 404, 7 Search PubMed.
- Q. Y. Wang, K. L. Yeung and M. A. Banares, Catal. Today, 2020, 356, 141–154 CrossRef CAS.
- M. A. Salaev, A. A. Salaeva, T. S. Kharlamova and G. V. Mamontov, Appl. Catal., B, 2021, 295, 38 CrossRef.
- S. Zhang, H. Z. Wang, H. Y. Si, X. Q. Jia, Z. Y. Wang, Q. Li, J. Kong and J. B. Zhang, ACS Appl. Mater. Interfaces, 2020, 12, 40285–40295 CrossRef CAS PubMed.
- Z. Wang, G. L. Shen, J. Q. Li, H. D. Liu, Q. Wang and Y. F. Chen, Appl. Catal., B, 2013, 138, 253–259 CrossRef.
- L. L. Zhao, Z. P. Zhang, Y. S. Li, X. S. Leng, T. R. Zhang, F. L. Yuan, X. Y. Niu and Y. J. Zhu, Appl. Catal., B, 2019, 245, 502–512 CrossRef CAS.
- W. Stober, A. Fink and E. Bohn, J. Colloid Interface Sci., 1968, 26, 62–69 CrossRef.
- Z. H. Li, V. Ravaine, S. Ravaine, P. Garrigue and A. Kuhn, Adv. Funct. Mater., 2007, 17, 618–622 CrossRef CAS.
- W. Y. Ko, L. J. Chen, Y. H. Chen, W. H. Chen, K. M. Lu, J. R. Yang, Y. C. Yen and K. J. Lin, J. Phys. Chem. C, 2013, 117, 16290–16296 CrossRef CAS.
- S. S. Acharyya, S. Ghosh and R. Bal, ACS Appl. Mater. Interfaces, 2014, 6, 14451–14459 CrossRef CAS PubMed.
- W. Liu, S. N. Wang, R. Y. Cui, Z. X. Song and X. J. Zhang, Microporous Mesoporous Mater., 2021, 326, 111370 CrossRef CAS.
- C. Zhu, S. N. Guan, W. Z. Li, A. T. Ogunbiyi, K. Chen and Q. Zhang, ACS Omega, 2021, 6, 35404–35415 CrossRef CAS PubMed.
- J. Zhang, Y. D. Cao, C. A. Wang and R. Ran, ACS Appl. Mater. Interfaces, 2016, 8, 8670–8677 CrossRef CAS.
- L. Li, C. Y. Zhang, F. Chen, Y. T. Xiang, J. L. Yan and W. Chu, Catal. Today, 2021, 376, 239–246 CrossRef CAS.
- L. H. Hu, Q. Peng and Y. D. Li, J. Am. Chem. Soc., 2008, 130, 16136–16137 CrossRef CAS PubMed.
- L. F. Liotta, H. J. Wu, G. Pantaleo and A. M. Venezia, Catal. Sci. Technol., 2013, 3, 3085–3102 RSC.
- S. S. Acharyya, S. Ghosh, R. Tiwari, B. Sarkar, R. K. Singha, C. Pendem, T. Sasaki and R. Bal, Green Chem., 2014, 16, 2500–2508 RSC.
- B. Fazio, L. Spadaro, G. Trunfio, J. Negro and F. Arena, J. Raman Spectrosc., 2011, 42, 1583–1588 CrossRef CAS.
- P. Venkataswamy, D. Jampaiah, K. N. Rao and B. M. Reddy, Appl. Catal., A, 2014, 488, 1–10 CrossRef CAS.
- F. Y. Hu, J. J. Chen, S. Zhao, K. Z. Li, W. Z. Si, H. Song and J. H. Li, Appl. Catal., A, 2017, 540, 57–67 CrossRef CAS.
- X. T. Lin, S. J. Li, H. He, Z. Wu, J. L. Wu, L. M. Chen, D. Q. Ye and M. L. Fu, Appl. Catal., B, 2018, 223, 91–102 CrossRef CAS.
- J. Mondal, P. Borah, S. Sreejith, K. T. Nguyen, X. G. Han, X. Ma and Y. L. Zhao, ChemCatChem, 2014, 6, 3518–3529 CrossRef CAS.
- S. Ghosh, S. S. Acharyya, S. K. Sharma and R. Bal, Catal. Sci. Technol., 2016, 6, 4644–4654 RSC.
- J. M. Chalmers and P. R. Griffiths, Handbook of Vibrational Spectroscopy, John Wiley & Sons Ltd., West Sussex, 2002 Search PubMed.
- S. H. Xie, Y. X. Liu, J. G. Deng, X. T. Zhao, J. Yang, K. F. Zhang, Z. Han and H. X. Dai, J. Catal., 2016, 342, 17–26 CrossRef CAS.
- G. L. Zhou, H. R. Liu, K. K. Cui, A. P. Jia, G. S. Hu, Z. J. Jiao, Y. Q. Liu and X. M. Zhang, Appl. Surf. Sci., 2016, 383, 248–252 CrossRef CAS.
- K. Wang, Y. Q. Chang, L. Lv and Y. Long, Appl. Surf. Sci., 2015, 351, 164–168 CrossRef CAS.
- C. J. Ma, Y. Y. Wen, Q. Q. Yue, A. Q. Li, J. L. Fu, N. W. Zhang, H. J. Gai, J. B. Zheng and B. H. Chen, RSC Adv., 2017, 7, 27079–27088 RSC.
- R. L. Mi, D. Li, Z. Hu and R. T. Yang, ACS Catal., 2021, 11, 7876–7889 CrossRef CAS.
- X. W. Liu, K. B. Zhou, L. Wang, B. Y. Wang and Y. D. Li, J. Am. Chem. Soc., 2009, 131, 3140–3141 CrossRef CAS PubMed.
- H. Pan, Y. F. Jian, C. W. Chen, C. He, Z. P. Hao, Z. X. Shen and H. X. Liu, Environ. Sci. Technol., 2017, 51, 6288–6297 CrossRef CAS PubMed.
- P. Venkataswamy, K. N. Rao, D. Jampaiah and B. M. Reddy, Appl. Catal., B, 2015, 162, 122–132 CrossRef CAS.
- T. Y. Li, S. J. Chiang, B. J. Liaw and Y. Z. Chen, Appl. Catal., B, 2011, 103, 143–148 CrossRef CAS.
- S. Y. Ding, C. Zhu, H. Hojo and H. Einaga, J. Hazard. Mater., 2022, 424, 11 CrossRef.
- H. Sun, X. L. Yu, X. Y. Ma, X. Q. Yang, M. Y. Lin and M. F. Ge, Catal. Today, 2020, 355, 580–586 CrossRef CAS.
- P. F. Zhang, H. F. Lu, Y. Zhou, L. Zhang, Z. L. Wu, S. Z. Yang, H. L. Shi, Q. L. Zhu, Y. F. Chen and S. Dai, Nat. Commun., 2015, 6, 10 Search PubMed.
- J. Kirchnerova, M. Alifanti and B. Delmon, Appl. Catal., A, 2002, 231, 65–80 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, XRD of SiO2@CeO2, SEM images of SiO2 and CeMnOx. See https://doi.org/10.1039/d2ra04678d |
| This journal is © The Royal Society of Chemistry 2022 |