 Open Access Article
Open Access ArticleStructure, magnetism and magnetocaloric effect in a new triangular lattice compound Gd3Cu9(OH)19Br8
Dong-Er Chenga,
Yi-Yan Wanga,
Yan Suna,
Hui Lianga,
Dan-Dan Wua,
Qiuju Lic,
Xuefeng Sunb and
Xiao-Yu Yue *a
*a
aInstitute of Physical Science and Information Technology, Anhui University, Hefei 230601, P. R. China. E-mail: xyyue@ahu.edu.cn
bDepartment of Physics and Key Laboratory of Strongly-Coupled Quantum Matter Physics (CAS), University of Science and Technology of China, Hefei 230026, P. R. China
cSchool of Physics & Material Science, Anhui University, Hefei 230601, P. R. China
First published on 12th September 2022
Abstract
A new triangular lattice compound Gd3Cu9(OH)19Br8 has been synthesized by the hydrothermal method. The structure, magnetism and magnetocaloric effect of Gd3Cu9(OH)19Br8 have been studied by X-ray diffraction, magnetic susceptibility, isothermal magnetization and specific heat measurements. In Gd3Cu9(OH)19Br8, the Cu2+ ions form a Kagome lattice along the ab plane, and Gd3+ ions are located in the center of hexagonal holes of the Kagome layer. The Cu-sublattice and Gd-sublattice overlap and constitute a magnetic triangular lattice. The temperature dependence of susceptibility and specific heat curves indicate no magnetic transition down to 2 K, suggesting a paramagnetic-like behavior at low temperature. The magnetocaloric effect (MCE) at low temperature has been calculated according to Maxwell's equations. The maximum value of magnetic entropy change −ΔSM is 26.04 J kg−1 K−1 and adiabatic temperature change ΔTad is 13.79 K, for a field change of 0–7 T, indicating a potential application of this compound in the field of magnetic refrigeration at low temperature. The influence of 4f–3d interaction on magnetism and MCE is also discussed.
Introduction
In recent years, spin frustrated antiferromagnets have attracted intensive attention in theory and experiment due to their exotic magnetic phenomena, such as spin ice, spin glass and quantum spin liquid states.1–4 Among them, S = 1/2 Heisenberg antiferromagnets with a Kagome lattice have been considered as potential quantum spin liquid (QSL) candidates due to their strong spin frustration.5,6 The typical QSL candidates include ZnCu3(OH)6Cl2 and ZnCu3(OH)6FBr, in which the Cu2+ ions constitute a geometrical perfect Kagome lattice.7–9 However, the existence of anti-site disorder between Cu2+ and Zn2+ ions induces the bond randomness and plays significant influence on the magnetic ground state in the above compounds.10,11To avoid anti-site disorder, the trivalent yttrium ion (Y3+) has been tried to substitute the nonmagnetic Zn2+ ions. A new compound YCu3(OH)6Cl3 without any Cu2+/Y3+ mixing has been successfully synthesized and used to investigate the possible QSL state.12–14 Following the study of YCu3(OH)6Cl3, a series of similar systems, such as YCu3(OH)6Br2[Brx(OH)1−x] (x ≈ 0.51),15 Y3Cu9(OH)19Cl8,16 LnCu3(OH)6Cl3 (Ln = Gd, Tb, Dy),17 and RCu3(OH)6Cl3 (R = Nd and Sm),18 have been synthesized and further used to study the magnetic ground state. By replacing the Y3+ ions into magnetic lanthanides Ln3+ ions, the magnetic topological structure of these compounds became a triangular lattice constituted by Cu2+ and Ln3+ ions and a significant influence of lanthanides on Cu-Kagome lattice has been revealed in magnetic studies. Especially for compounds with heavy lanthanides, the existence of heavy lanthanides ions can suppress the strong antiferromagnetic (AFM) spin interaction and lead to the reduction of Weiss temperature.17
Compared with other lanthanides, heavy lanthanide Gd3+ ion owns a large spin quantum number (S = 7/2) and no orbital angular momentum. With applying magnetic field, the Gd-based compounds usually release a considerable magnetic entropy change and exhibit remarkable performance in giant magnetocaloric effect (MCE), which is benefit for the potential application in magnetic refrigeration technology.19–22 Among them, the 4f–3d interaction plays an important role in magnetism and MCE. For example, the AFM 4f–3d interactions in compound MCE.23,24 While, the existence of weak 4f–3d interactions in GdCrO4 promotes the spin polarization and enhances the MCE.25
In this work, we have synthesized high-quality single crystals of Gd3Cu9(OH)19Br8 by a traditional hydrothermal method. The X-ray diffraction (XRD) results of Gd3Cu9(OH)19Br8 reveal that the Cu2+ ions form a two-dimensional Kagome lattice and Gd3+ ions are located in the center of hexagonal hole in the Kagome layer. The magnetic ground state and magnetocaloric effect have been studied by the magnetic susceptibility, magnetization and specific heat measurements. No magnetic transition is observed as the temperature down to 2 K, accompanying with a small value of Weiss temperature. A paramagnetic-like state is expected at 2 K in this system, different from the magnetic behavior of LnCu3(OH)6Cl3 (Ln = rare earth ions) reported previously.17,18 This peculiar magnetic phenomenon can be attributed to a significant influence of Gd3+ ions on the Cu-Kagome lattice.
Experimental details section
Synthesis
Gd3Cu9(OH)19Br8 was synthesized by a traditional hydrothermal method. After several iterations of experiment, high-quality single crystals of Gd3Cu9(OH)19Br8 were grown from a mixture of 0.4514 g Gd(NO3)3·6H2O (1 mmol), 0.7248 g Cu(NO3)2 (3 mmol), 0.7203 g NaBr (7 mmol) and 1 ml deionized water. All the starting chemical reactants were analytical grade purity and used as-received without any further purification. These starting materials were sequentially added into a 25 ml Teflon-lined and stirred thoroughly. The Teflon liner was sealed in a stainless steel vessel and heated at 503 K for 3 days, then cooled to room temperature for 3 days. The green single crystals with hexagonal shape (maximum size ≈ 1.0 mm in diameter and 0.2 mm in thickness) were obtained by washing with deionized water.Crystal structure and thermal analysis
The crystal structure of Gd3Cu9(OH)19Br8 were checked by powder X-ray diffraction at room temperature. The relative Rietveld powder diffraction profile fitting software FULLPROF was used to refine the crystal structure by compared with Y3Cu9(OH)19Cl8.16 Thermogravimetric analysis of Gd3Cu9(OH)19Br8 was performed in the TA TGA5500 instruments under a nitrogen atmosphere with a heating rate of 10 K min−1. The samples were placed in an Al2O3 crucible and heated from room temperature to 1173 K.Magnetic and specific heat measurements
The magnetic properties were measured using the superconducting quantum interference device vibrating sample magnetometer (SQUID-VSM; Quantum Design). The single crystals were placed in a quartz sample holder. Before each measurement, the samples were zero-field cooled from 300 K to the desired temperature. Specific heat in the temperature range from 2 to 30 K was measured using the relaxation method on a Physical Property Measurement System (PPMS; Quantum Design).Results and discussion
Crystal structure
Fig. 1 shows the Rietveld refinement of powder XRD pattern for Gd3Cu9(OH)19Br8 by compared with isostructural Y3Cu9(OH)19Cl8.16 The XRD analysis exhibits that Gd3Cu9(OH)19Br8 crystallizes in trigonal crystal system with space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) (no. 148). The unit cell parameters of Gd3Cu9(OH)19Br8 are a = b = 11.6525(2) Å and c = 17.9962(1) Å. The refined atomic coordinates are shown in Table 1. Compared with GdCu3(OH)6Cl3 and YCu3(OH)6Br2[Brx(OH)1−x] (x ≈ 0.51), the crystal symmetry of Gd3Cu9(OH)19Br8 reduces and the size of unit cell becomes larger.15,17 In an asymmetric unit, there are two Gd atoms, two Cu atoms, four O atoms and two Br atoms. Cu atoms have two independent crystallographic sites. Each Cu2+ ion was surrounded by four O2− ions and two Br− ions with Cu–O bond lengths ranging from 1.84(3) to 2.23(4) Å and Cu–Br bond lengths ranging from 2.866(7) to 2.902(13) Å, forming a distorted [CuO4Br2] octahedron. Gd atoms have two independent crystallographic sites, among them Gd1 is coordinated by seven atoms, forming a [GdO7] polyhedron with Gd–O bond lengths ranging from 2.301(6) to 2.36(19) Å, while Gd2 is coordinated by six oxygen atoms and two bromine atoms, forming a [GdO6Br2] polyhedron with the identical Gd–O bond length of 2.52(4) Å and Gd–Br bond length of 2.887(11) Å.
(no. 148). The unit cell parameters of Gd3Cu9(OH)19Br8 are a = b = 11.6525(2) Å and c = 17.9962(1) Å. The refined atomic coordinates are shown in Table 1. Compared with GdCu3(OH)6Cl3 and YCu3(OH)6Br2[Brx(OH)1−x] (x ≈ 0.51), the crystal symmetry of Gd3Cu9(OH)19Br8 reduces and the size of unit cell becomes larger.15,17 In an asymmetric unit, there are two Gd atoms, two Cu atoms, four O atoms and two Br atoms. Cu atoms have two independent crystallographic sites. Each Cu2+ ion was surrounded by four O2− ions and two Br− ions with Cu–O bond lengths ranging from 1.84(3) to 2.23(4) Å and Cu–Br bond lengths ranging from 2.866(7) to 2.902(13) Å, forming a distorted [CuO4Br2] octahedron. Gd atoms have two independent crystallographic sites, among them Gd1 is coordinated by seven atoms, forming a [GdO7] polyhedron with Gd–O bond lengths ranging from 2.301(6) to 2.36(19) Å, while Gd2 is coordinated by six oxygen atoms and two bromine atoms, forming a [GdO6Br2] polyhedron with the identical Gd–O bond length of 2.52(4) Å and Gd–Br bond length of 2.887(11) Å.
| Atom | Wyckoff position | x/a | y/b | z/c |
|---|---|---|---|---|
| Cu(1) | 18f | 0.6641(10) | 0.8244(16) | 0.5022(4) |
| Cu(2) | 9d | 0.5000 | 1.0000 | 0.5000 |
| Gd(1) | 6c | 0.3333 | 0.6667 | 0.5388(3) |
| Gd(2) | 3b | 1.0000 | 1.0000 | 0.5000 |
| Br(1) | 18f | 0.6616(12) | 0.9953(14) | 0.6187(1) |
| Br(2) | 6c | 1.0000 | 1.0000 | 0.3396(6) |
| O(1) | 3a | 0.3333 | 0.6667 | 0.6667 |
| O(2) | 18f | 0.793(4) | 0.797(4) | 0.5439(8) |
| O(3) | 18f | 0.529(15) | 0.658(16) | 0.559(4) |
| O(4) | 18f | 0.490(5) | 0.842(5) | 0.4671(11) |
Fig. 2(a) and (b) show the two-dimensional (2D) structure of Gd3Cu9(OH)19Br8 viewed along the ab plane and c axis, respectively. The [CuO4Br2] octahedra are connected with each other by edge-sharing, forming a slight distorted 2D Kagome lattice along the ab plane. Within ab plane, the direct spatial distances between the two neighboring Cu2+ ions are 3.274(8) Å, 3.392(11) Å and 3.429(8) Å. For each layer, the Gd3+ ions are located in the central position of hexagonal hole in the Cu-Kagome lattice. Whereas, the Gd3+ ions are not coplanar with the Cu-Kagome layer, when viewed along the ab plane. Fig. 2(c) exhibits the magnetic topological structure composed by Cu2+ and Gd3+ ions by removing the nonmagnetic ions. The Cu-sublattice and Gd-sublattice overlap and form a 2D magnetic triangular lattice.
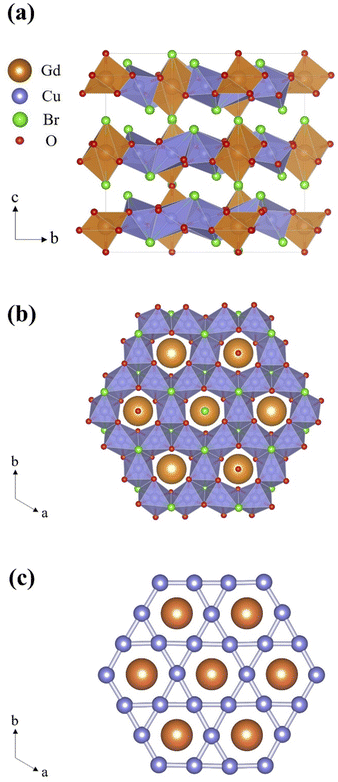 | ||
| Fig. 2 Two-dimensional layered structure of Gd3Cu9(OH)19Br8 viewed along the ab plane (a) and c axis (b). (c) Magnetic topological structure formed by Cu2+ and Gd3+ ions. | ||
Thermal analysis
The thermogravimetric analysis can be used to investigate the thermal stability of Gd3Cu9(OH)19Br8. Fig. 3 exhibits the thermogravimetric (TG) and the corresponding differential of thermogravimetric (DTG) curves from room temperature to 1173 K. The TG curve can be divided into two main stages, similar to that of YCu3(OH)6Br2[Brx(OH)1−x] (x ≈ 0.51).15 Below 600 K, Gd3Cu9(OH)19Br8 is found to be stable. With increasing temperature to 622 K, the first loss of mass starts, then a plateau can be observed between 700 and 763 K. The first mass loss is 17.44% from 622 K to 700 K, which is close to the calculated value 16.50% for the removal of 9.5 water molecules and two bromine ions per formula. The second step occurred from 763 K to 996 K with a mass loss of 38.56%, which can be attributed to the volatilization of two GdBr3 molecules per formula with a theoretical value of 39.57% in Gd3Cu9(OH)19Br8. The final residuals of thermal analysis are not further characterized because the residuals were melted with the bucket.Magnetic properties
Fig. 4(a) and (d) show the temperature-dependent zero-field-cooled (ZFC) heating and field-cooled (FC) magnetic susceptibility curves measured at 0.1 T for magnetic fields perpendicular (χ⊥) and parallel (χ∥) to the crystallographic c axis, respectively. With decreasing temperature to 2 K, the susceptibility increases gradually and no obvious magnetic transition is observed at two field-directions. The inverse of susceptibility above 50 K can be well fitted by Curie–Weiss law. The deduced Weiss temperatures are θ⊥ = −2.79(2) K and θ∥ = −3.99(4) K, respectively. Compared with Y3Cu9(OH)19Br8, the much smaller absolute value of Weiss temperature existed in Gd3Cu9(OH)19Br8 indicates a net weak AFM spin coupling in this compound, suggesting that the existence of Gd3+ ions play significant influence on magnetism. The slight difference between χ⊥ and χ∥ demonstrate a weak magnetic anisotropy of this compound. The calculated effective magnetic moments are μ⊥eff = 15.39 μB and μ∥eff = 15.95 μB. The values are close to the theoretical effective moment 14.70 μB of Gd3Cu9(OH)19Br8 estimated by . Fig. 4(b) and (e) exhibit the ZFC and FC curves measured at 0.01 T, 0.02 T and 0.05 T below 50 K. No ZFC-FC splitting is observed, which excludes the existence of ferromagnetic moment and indicates a paramagnetic state at 2 K.
. Fig. 4(b) and (e) exhibit the ZFC and FC curves measured at 0.01 T, 0.02 T and 0.05 T below 50 K. No ZFC-FC splitting is observed, which excludes the existence of ferromagnetic moment and indicates a paramagnetic state at 2 K.
Fig. 4(c) and (f) show the magnetic hysteresis loops measured at 2 K and 300 K for applied magnetic fields perpendicular and parallel to c axis, respectively. The magnetization increases smoothly with the increasing magnetic field and no field hysteresis is observed. As the increase of applied magnetic field, these weakly coupled spins can easily rotate towards the direction of magnetic field. The magnetization curves seem to saturate with a magnetic moment of 25 μB per f.u. at 7 T and 2 K, which is smaller than the expected saturated magnetic moment 30 μB per f.u. (Gd3+: S = 7/2, g = 2; Cu2+: S = 1/2, g = 2). The dashed lines represent the fitting curves of Brillouin function, 3B7/2(y) + 9B1/2(y), for isolated Gd3+ and Cu2+ ions. At 2 K, the experimental curves do not follow the Brillouin function. The difference between experimental and fitting data should originate from the existence of AFM spin correlation in this system.
Specific heat
Fig. 5(a) shows the temperature dependence of specific heat between 2 K and 30 K measured under different applied magnetic fields perpendicular to the crystallographic c axis. No λ anomaly is observed in zero-field specific heat curve, in agreement with the susceptibility data and further supporting the absence of magnetic order at low temperatures. As the decrease of temperature, the zero-field specific heat data exhibits an upturn below 3 K. With increasing magnetic field, a broad peak occurred and moved to high temperature gradually. Such behavior can be attributed to the formation of short-range spin correlation at low temperature. In order to obtain more information on the nature of magnetic ground state, the contribution of magnetic specific heat Cmag is calculated by subtracting the phonon contribution from the total specific heat data. The phonon contribution is estimated by fitting the zero-field specific heat data above 10 K to the polynomial formula Cbg = aT3 + bT5 + cT7 with a = 9.56 × 10−3 J K−4 mol−1, b = −9.20 × 10−6 J K−6 mol−1, and c = 3.67 × 10−9 J K−9 mol−1. The temperature dependence of magnetic entropy Smag is shown in Fig. 5(b). The calculated values of magnetic entropy are 9.88 J mol−1 K−1 at H = 0 T and 44.69 J mol−1 K−1 at H = 7 T, which are much smaller than the theoretically maximum value of magnetic entropy 103.73 J mol−1·K−1 for the compound, indicating the existence of spin correlation below 2 K. | ||
| Fig. 5 (a) Temperature dependence of specific heat at different applied magnetic fields. (b) The magnetic specific heat and magnetic entropy from 2 to 30 K. | ||
Magnetocaloric effect
Materials with giant magnetocaloric effect can be used to achieve magnetic refrigeration technology with the advantage of higher energy efficiency and environment friendly.19,21,22 The rare-earth Gd3+ ions have a large spin quantum number and no orbital angular moment, which benefits for realizing a large magnetocaloric effect in Gd-based compounds.24–28 In Gd3Cu9(OH)19Br8, no magnetic transition and a paramagnetic-like behavior are observed at low temperature. Under the applied magnetic field, these weakly coupled spins can easily rotate towards the direction of magnetic field, accompanying with a considerable magnetic entropy change in this process. The isothermal magnetic entropy change ΔSM and adiabatic temperature change ΔTad with changing magnetic field are two main parameters to character the magnetocaloric effect.In order to calculate the magnetic entropy change ΔSM, a series of isothermal magnetization curves are measured from 2 to 40 K, as shown in Fig. 6(a). With increasing temperature, the magnitude and curvature of the magnetization curves decrease gradually. Integrating the Maxwell's equation  , the temperature dependence of −ΔSM was obtained and shown in Fig. 6(b). With decreasing temperature, the value of −ΔSM increases monotonously. At 2 T, the maximum value of −ΔSM reaches 23.78 J kg−1 K−1 and 26.04 J kg−1 K−1 for the field changes of 0–3 T and 0–7 T, respectively. Fig. 7 exhibits the temperature dependence of Adiabatic temperature change ΔTad, calculated by the equation
, the temperature dependence of −ΔSM was obtained and shown in Fig. 6(b). With decreasing temperature, the value of −ΔSM increases monotonously. At 2 T, the maximum value of −ΔSM reaches 23.78 J kg−1 K−1 and 26.04 J kg−1 K−1 for the field changes of 0–3 T and 0–7 T, respectively. Fig. 7 exhibits the temperature dependence of Adiabatic temperature change ΔTad, calculated by the equation 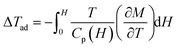 . With decreasing temperature, the value of ΔTad increases gradually and reaches maximum around 5 K for a field change of 0–1 T. With increasing magnetic field, the maximum values of ΔTad reach 7.72 K and 13.79 K under the field changes of 0–3 T and 0–7 T, respectively.
. With decreasing temperature, the value of ΔTad increases gradually and reaches maximum around 5 K for a field change of 0–1 T. With increasing magnetic field, the maximum values of ΔTad reach 7.72 K and 13.79 K under the field changes of 0–3 T and 0–7 T, respectively.
 | ||
| Fig. 6 (a) Magnetization curves measured at different temperatures. (b) Temperature dependence of magnetic entropy change −ΔSM. | ||
Table 2 shows the maximum values of magnetic entropy change −ΔSM and adiabatic temperature change ΔTad of Gd3Cu9(OH)19Br8, compared with some other Gd-based compounds.29–32 As shown in Table 2, the obtained values of Gd3Cu9(OH)19Br8 are much larger than the values of other Gd-based compounds, indicating this compound can be considered as a potential magnetic refrigerant material at low temperature. The enhancement of magnetocaloric effect in frustrated systems with pyrochlore and Kagome lattice have been predicated in theory and further confirmed in experiment.33,34 The frustrated framework can promote the disorder of spin at low temperature and release a considerable magnetic entropy change under the applied magnetic field.
Discussion
The analyses of crystal structure reveal that the crystal symmetry of Gd3Cu9(OH)19Br8 is isostructural with Y3Cu9(OH)19Cl8.16 By replaced the nonmagnetic Y3+ ions into Gd3+ ions, there are three kinds of magnetic coupling existing in Gd3Cu9(OH)19Br8, including the Cu–Cu (3d–3d) spin coupling, Gd–Cu (4f–3d) spin coupling and Gd–Gd (4f–4f) spin coupling. Among them, the direct spatial distances between two neighboring Gd3+ ions are 6.7367(6) Å and 6.8710(16) Å with the exchange path Gd–O–Cu–O–Gd in ab plane. Along the c axis, the direct spatial distances between two neighboring Gd3+ ions is 4.602(8) Å with the exchange path Gd–O–Gd along the c axis. Compared with the super-exchange Cu–Cu spin coupling [Cu–O–Cu exchange path] and Gd–Cu spin coupling [Cu–O–Gd exchange path], the spin coupling between two neighboring Gd3+ ions should be relatively weaker. Thus, the dominant spin correlation in Gd3Cu9(OH)19Br8 can be attributed to the Cu–Cu (3d–3d) spin coupling and Gd–Cu (4f–3d) spin coupling.Firstly, we consider the Cu–Cu (3d–3d) spin coupling. Within the Kagome layer, the direct spatial distances between two neighboring Cu2+ ions are d1 = 3.274(8) Å with the corresponding Cu–O–Cu bond angle 107.5(7)°, d2 = 3.392(11) Å with the corresponding Cu–O–Cu bond angle 120.8(5)° and d3 = 3.429(8) Å with the corresponding Cu–O–Cu bond angle 112.8(5)°. According to the Goodenough–Kanamori rules, the magnetic coupling between two neighboring Cu2+ ions is AFM.35–37 As reported in Y3Cu9(OH)19Cl8, the strong AFM interaction is observed due to the frustrated Cu-Kagome lattice.16 However, the magnetic analyses of Gd3Cu9(OH)19Br8 exhibit a small value of Weiss temperature and suggest a weak spin coupling in this system, which is inconsistent with the strong AFM interaction derived from the Cu-Kagome lattice.
Considering the existence of Gd3+ ions, the magnetic topological lattice of title compound is a triangular lattice constituted by magnetic Cu2+ and Gd3+ ions. Thus, a considerable Gd–Cu (4f–3d) spin coupling exists in this system and plays significant influence on the magnetic ground state. The influence of 4f–3d interaction on magnetism and magnetocaloric effect is an open subject.38–40 For the light lanthanides system LnCu3(OH)6Cl3 (Ln = Nd and Sm), the influence of lanthanide ions on the magnetism of Cu-Kagome lattice is relatively small. These compounds undergo AFM order below 20 K.18 For the heavy lanthanides systems LnCu3(OH)6Cl3 (Ln = Gd, Tb, Dy), the heavy lanthanides ions may modulate Dzyaloshinskii–Moriya (DM) interaction and further induce a ferromagnetic correlation, which mask the strong AFM interaction originated from the Cu-Kagome lattice, but do not prevent the AFM order at low temperature.17 In Gd3Cu9(OH)19Br8, no obvious magnetic transition is observed in magnetic susceptibility and specific heat data. The small value of Weiss temperature indicates that the 4f–3d interaction is probably ferromagnetic and compete with the AFM interaction between Cu2+ ions. The existence of 4f–3d interactions suppress the occurrence of AFM order and enhance the spin disorder at low temperature in our compound. These weakly coupled spins can easily rotate towards the direction of magnetic field and generate a considerable magnetic entropy under a small magnetic field.
Conclusions
In this work, we reported the structure, magnetism and magnetocaloric effect of Gd3Cu9(OH)19Br8. The basic magnetic lattice of Gd3Cu9(OH)19Br8 is a two-dimensional triangular lattice formed by magnetic Cu2+ and Gd3+ ions, in which the Cu2+ ions constitute a Kagome lattice and Gd3+ ions are located in the center of the hexagonal hole. No magnetic transition is observed in magnetic susceptibility curve, which is further confirmed in zero-field specific heat curve. Compared with isostructural Y3Cu9(OH)19Cl8, the strong spin frustration in the Cu-Kagome lattice is obviously suppressed and a paramagnetic-like ground state is observed in Gd3Cu9(OH)19Br8 due to the existence of Gd3+ ions. Under the applied magnetic field, these weakly coupled spins rotate towards the direction of applied magnetic field and yield a considerable MCE. Thus, Gd3Cu9(OH)19Br8 can be considered as a potential candidate for magnetic refrigeration at low temperature.Author contributions
Dong-Er Cheng synthesized samples and data analysis. Yi-Yan Wang helped experiments and interpreted data. Yan Sun, Hui Liang, Dan-Dan Wu and Qiuju Li funded this work. Xuefeng Sun supervised and reviewed this work. Xiao-Yu Yue performed conceptualization and wrote the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 12104010, U1832209, 11874336, 11904003, 52102333 and 12104011) and the Natural Science Foundation of Anhui Province (Grant No. 1908085MA09 and 2108085MA16).References
- M. J. P. Gingras and P. A. McClarty, Rep. Prog. Phys., 2014, 77, 056501 CrossRef CAS PubMed.
- J. A. Mydosh, Rep. Prog. Phys., 2015, 78, 052501 CrossRef CAS PubMed.
- P. W. Anderson, Science, 1987, 235, 1196 CrossRef CAS PubMed.
- O. Tanaka, Y. Mizukami, R. Harasawa, K. Hashimoto, K. Hwang, N. Kurita, H. Tanaka, S. Fujimoto, Y. Matsuda, E. G. Moon and T. Shibauchi, Nat. Phys., 2022, 18, 429 Search PubMed.
- L. Balents, Nature, 2010, 464, 199–208 CrossRef CAS PubMed.
- M. Yang, S. Y. Zhang, W. B. Guo, Y. Y. Tang and Z. Z. He, Dalton Trans., 2015, 44, 15396 RSC.
- J. S. Helton, K. Matan, M. P. Shores, E. A. Nytko, B. M. Bartlett, Y. Yoshida, Y. Takano, A. Suslov, Y. Qiu, J. H. Chung, D. G. Nocera and Y. S. Lee, Phys. Rev. Lett., 2007, 98, 107204 CrossRef CAS PubMed.
- T. H. Han, J. S. Helton, S. Y. Chu, D. G. Nocera, J. A. Rodriguez-Rivera, C. Broholm and Y. S. Lee, Nature, 2012, 492, 406–410 CrossRef CAS PubMed.
- Z. Feng, Z. Li, X. Meng, W. Yi, Y. Wei, J. Zhang, Y. C. Wang, W. Jiang, Z. Liu, S. Li, F. Liu, J. Luo, S. Li, G. Q. Zheng, Z. Y. Meng, J. W. Mei and Y. Shi, Chin. Phys. Lett., 2017, 34, 077502 CrossRef.
- M. Rozenberg and R. Chitra, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 78, 132406 CrossRef.
- Y. Y. Huang, Y. Xu, L. Wang, C. C. Zhao, C. P. Tu, J. M. Ni, L. S. Wang, B. L. Pan, Y. Fu, Z. Hao, C. Liu, J. W. Mei and S. Y. Li, Phys. Rev. Lett., 2021, 127, 267202 CrossRef CAS PubMed.
- W. Sun, Y. X. Huang, S. Nokhrin, Y. Pan and J. X. Mi, J. Mater. Chem., 2016, 4, 8772 CAS.
- A. Zorko, M. Pregelj, M. Klanjšek, M. Gomilšek, Z. Jagličić, J. S. Lord, J. A. T. Verezhak, T. Shang, W. Sun and J. X. Mi, Phys. Rev. B, 2019, 99, 214441 CrossRef CAS.
- A. Zorko, M. Pregelj, M. Gomilšek, M. Klanjšek, O. Zaharko, W. Sun and J. X. Mi, Phys. Rev. B, 2019, 100, 144420 CrossRef CAS.
- X. H. Chen, Y. X. Huang, Y. Pan and J. X. Mia, J. Magn. Magn. Mater., 2020, 512, 167066 CrossRef CAS.
- P. Puphal, M. Bolte, D. Sheptyakov, A. Pustogow, K. Kliemt, M. Dressel, M. Baenitz and C. Krellner, J. Mater. Chem. C, 2017, 5, 2629 RSC.
- Y. Fu, L. L. Huang, X. F. Zhou, J. Chen, X. Y. Zhang, P. Y. Chen, S. M. Wang, C. Liu, D. P. Yu, H. F. Li, L. Wang and J. W. Mei, Chin. Phys. B, 2021, 30, 100601 CrossRef CAS.
- W. Sun, Y. X. Huang, Y. Pan and J. X. Mi, Dalton Trans., 2017, 46, 9535 RSC.
- D. Matsunami, A. Fujita, K. Takenaka and M. Kano, Nat. Mater., 2015, 14, 73 CrossRef CAS PubMed.
- L. H. Yin, J. Yang, P. Tong, X. Luo, W. H. Song, J. M. Dai, X. B. Zhu and Y. P. Sun, Appl. Phys. Lett., 2017, 110, 192904 CrossRef.
- V. Franco, J. S. Bláquez, J. J. Ipus, J. Y. Law, L. M. Moreno-Ramíez and A. Conde, Prog. Mater. Sci., 2018, 93, 112 CrossRef.
- J. R. Gómez, R. F. Garcia, A. D. M. Catoira and M. R. Gómez, Renewable Sustainable Energy Rev., 2013, 17, 74 CrossRef.
- J. K. Murthy, K. D. Chandrasekhar, S. Mahana, D. Topwal and A. Venimadhav, J. Phys. D: Appl. Phys., 2015, 48, 355001 CrossRef.
- D. D. Lei, Z. W. Ouyang, X. Y. Yue, L. Yin, Z. X. Wang, J. F. Wang, Z. C. Xia and G. H. Rao, J. Appl. Phys., 2018, 124, 233904 CrossRef.
- A. Midya, N. Khan, D. Bhoi and P. Mandal, J. Appl. Phys., 2014, 115, 17E114 CrossRef.
- Y. Y. Tang, W. B. Guo, S. Y. Zhang, M. Yang, H. P. Xiang and Z. Z. He, Dalton Trans., 2015, 44, 17026 RSC.
- F. S. Guo, Y. C. Chen, J. L. Liu, J. D. Leng, Z. S. Meng, P. Vrábel, M. Orendáč and M. L. Tong, Chem. Commun., 2012, 48, 12219 RSC.
- M. Balli, B. Roberge, P. Fournier and S. Jandl, Crystals, 2017, 7, 44 CrossRef.
- S. Mahana, U. Manju and D. Topwal, J. Phys. D: Appl. Phys., 2018, 51, 305002 CrossRef.
- L. L. Li, X. Y. Yue, W. J. Zhang, H. Bao, D. D. Wu, H. Liang, Y. Y. Wang, Y. Sun, Q. J. Li and X. F. Sun, Chin. Phys. B, 2021, 30, 077501 CrossRef CAS.
- S. Mahana, U. Manju and D. Topwal, J. Phys. D: Appl. Phys., 2017, 50, 035002 CrossRef.
- L. W. Li, K. Nishimura, H. Igawa and D. X. Huo, J. Alloys Compd., 2011, 509, 4198 CrossRef CAS.
- M. Semjan and M. Žukovic, Acta Phys. Pol., A, 2020, 137, 622 CrossRef CAS.
- R. K. Li, G. J. Li and C. Greaves, J. Mater. Chem. A, 2018, 6, 5230 Search PubMed.
- J. B. Goodenough, Phys. Rev., 1955, 100, 564 CrossRef CAS.
- J. Kanamori, J. Phys. Chem. Solids, 1959, 10, 87–98 CrossRef CAS.
- W. Geertsma and D. Khomskii, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 3011 CrossRef CAS PubMed.
- E. Palacios, C. Tomasi, R. Sáez-Puche, A. J. Dos santos-García, F. Fernández-Martínez and R. Burriel, Phys. Rev. B, 2016, 93, 064420 CrossRef.
- A. A. Wagh, K. G. Suresh, P. S. Anil Kumar and S. Elizabeth, J. Phys. D: Appl. Phys., 2015, 48, 135001 CrossRef.
- M. Das, S. Roy and P. Mandal, Phys. Rev. B, 2017, 96, 174405 CrossRef.
| This journal is © The Royal Society of Chemistry 2022 |




