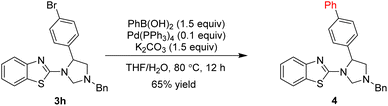Open Access Article
Open Access Article[3 + 2] cycloaddition of nonstabilized azomethine ylides and 2-benzothiazolamines to access imidazolidine derivatives†
Kai-Kai Wang ab,
Yan-Li Lic,
Ming-Yue Wanga,
Jun Jinga,
Zhan-Yong Wang
ab,
Yan-Li Lic,
Ming-Yue Wanga,
Jun Jinga,
Zhan-Yong Wang *a and
Rongxiang Chen
*a and
Rongxiang Chen *a
*a
aSchool of Pharmacy, Xinxiang University, Xinxiang 453000, P. R. China. E-mail: chenhlmei@163.com; zhanyongw@126.com; Fax: +86 373-3683138
bKey Laboratory of Nano-carbon Modified Film Technology Engineering of Henan Province, Xinxiang 453000, P. R. China
cMedical College, Xinxiang University, Xinxiang 453000, P. R. China
First published on 4th October 2022
Abstract
A simple and practical method for the construction of 1,3,5-trisubstituted imidazolidine derivatives via [3 + 2] cycloaddition reaction has been developed. This reaction could smoothly proceed between nonstabilized azomethine ylides generated in situ and 2-benzothiazolamines to deliver a wide scope of differently substituted imidazolidines in high yields (up to 98%). The structure of one example was confirmed by X-ray single-crystal structure analysis.
Heterocyclic compounds are important structural motifs that are frequently discovered in natural products and biologically active molecules, with wide applications and potency in the field of medicinal chemistry.1 Among them, the 2-aminobenzothiazole scaffolds, which contain a core of isothiourea motifs as a privileged scaffold, are ubiquitous in natural products, drugs and bioactive compounds.2 Some examples of biologically active molecules and pharmaceuticals containing benzothiazoles are shown in Fig. 1. These compounds display a variety of important biological activities, such as anti-HIV, anticancer, antineoplastic and anticonvulsant activity, and so on.3
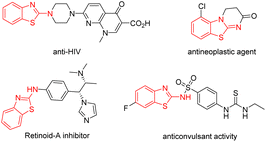 | ||
| Fig. 1 Selected examples of biologically active compounds containing a 2-aminobenzothiazole scaffolds. | ||
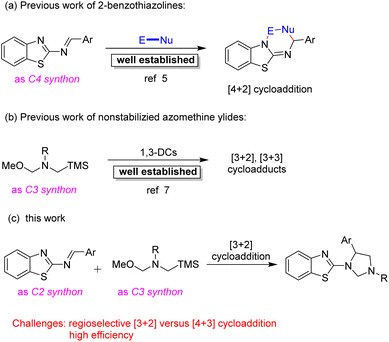
Due to their momentous and potent biological activities and structural diversifications, more organic and medicinal chemists have been significantly attracted to developing effective and advanced methodologies for the construction of benzazole scaffolds.4 The 2-benzothiazolamines could serve as alternative C4 synthons with various reaction partners in [4 + 2] cycloadditions for the straightforward and convenient access to 2-aminobenzothiazole scaffolds, and this type of reaction has been well established (Scheme 1a).5 By contrast, the 2-benzothiazolamines which acted as C2 synthons in cycloaddition reactions have been very limited.6 Therefore, the development of efficient cycloaddition between the 2-benzothiazolamines as C2 synthons and suitable reaction partners to provide diverse functionalized heteroarenes molecules is always in great demand.
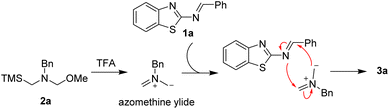
On the other hand, the 1,3-dipole cycloaddition is recognized one of the most efficient and powerful methods for building five membered heterocyclic rings from simple starting materials.7 In particular, the nonstabilized azomethine ylides generated in situ from N-benzyl-substituted compounds were a highly reactive intermediate with unsaturated compounds, such as activated alkenes,8 aromatic ketones,9 aromatic aldehydes,10 phthalic anhydrides,11 imines,12 cyano compounds13 or stable dipoles,14 to build various N-containing heterocycles via [3 + 2] or [3 + 3] cycloaddition reactions (Scheme 1b). Despite the progress were well developed via 1,3-dipolar cycloadditions employing nonstabilized azomethine ylides as the substrates, challenges still remain. Herein, we would like to present an effective process to furnish functionalized 1,3,5-trisubstituted imidazolidine derivatives via [3 + 2] cycloaddition strategy from 2-benzothiazolamines with nonstabilized azomethine ylides generated in situ (Scheme 1c). In contrast, the desired product was not obtained via [4 + 3] cycloaddition reaction.
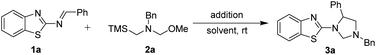
Initially, we used 2-benzothiazolimine 1a and N-(methoxymethyl)-N-(trimethylsilyl-methyl)-benzyl amine 2a which could in situ form azomethine ylide in the presence of acid as model substrates to optimize the reaction conditions. The results were shown in Table 1. The reaction could smoothly proceed to afford the [3 + 2] cycloadduct 3a in 48% yield in CHCl3 for 12 h under room temperature when used AcOH as the acid (entry 1, in Table 1). Subsequently, organic and inorganic acids were screened to improve the yield (entries 2–4, in Table 1). The results were found that the 1,3,5-trisubstituted imidazolidine 3a could be provided in 90% yield using TFA as the addition (entry 3, in Table 1). Whereas the other acids were found to be the poor yields. Furthermore, in order to improve the yield of the product, a series of reactive solvents were also screened (entries 5–13, in Table 1). The reaction could quickly transform and furnish the corresponding product in 98% yield for 1 h in CH2Cl2 (entry 5, in Table 1). Compared with CH2Cl2 solvent, the others solvents, such as DCE, ethyl acetate, toluene, MeOH, CH3CN, dioxane, Et2O and THF, led to decreased yields (entries 5−13).
| Entry | Additive | Solvent | Time | Yield of 3ab (%) |
|---|---|---|---|---|
| a Reaction conditions: 2-benzothiazolimine 1a (0.1 mmol, 1.0 equiv.), N-(methoxymethyl)-N-(trimethylsilyl-methyl)-benzyl-amine 2a (0.12 mmol, 1.2 equiv.), addition (0.01 mmol, 0.1 equiv.) and solvent (1.0 mL) in a test tube at room temperature.b Yield of the isolated product. | ||||
| 1 | AcOH | CHCl3 | 12 | 48 |
| 2 | TfOH | CHCl3 | 12 | 51 |
| 3 | TFA | CHCl3 | 3 | 90 |
| 4 | HCl | CHCl3 | 12 | 20 |
| 5 | TFA | CH2Cl2 | 1 | 98 |
| 6 | TFA | DCE | 3 | 82 |
| 7 | TFA | EtOAc | 6 | 75 |
| 8 | TFA | Toluene | 12 | 70 |
| 9 | TFA | MeOH | 12 | 31 |
| 10 | TFA | CH3CN | 3 | 71 |
| 11 | TFA | Dioxane | 12 | 59 |
| 12 | TFA | Et2O | 12 | 46 |
| 13 | TFA | THF | 12 | 62 |
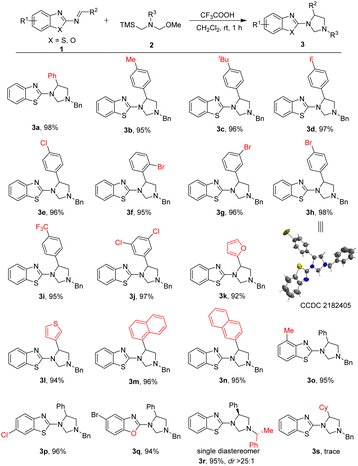
After establishing the optimal reaction conditions, the scope and limitations of this [3 + 2] cycloaddition reaction for formation of 1,3,5-trisubstituted imidazolidines were examined. The results were shown in Table 2. Firstly, a wide range of 2-benzothiazolamines 1 with different electron properties and substitution patterns could be utilized as competent substrates in this [3 + 2] cycloaddition with nonstabilized azomethine ylides 2a. The reaction worked well in the annulation affording the corresponding products 3a–3j in excellent yields (95–98%) bearing either electron-donating or electron-withdrawing functional substituents including the different positions of the substituents on the benzene ring of R2 group. It shows that the steric effect or electronic nature of R2 group hardly effected the reactive efficiency. Additionally, the chemical structure of 3h (CCDC 2182405†)15 was unequivocally confirmed by X-ray crystallographic analysis (see ESI†). On the other hand, when the R2 groups were heteroaromatic substituted group, such as 2-furanyl, 3-thienyl, 1-naphthyl and 2-naphthyl, the cycloaddition reaction were well tolerated and could also proceed smoothly without obvious interference to produce corresponding pyrazoles 3k–3n in 92–96% yields. Meanwhile, substituted substrates at the C4 or C6-position of the 2-aminobenzothiazole, such as Me and Cl were also reacting smoothly to afford the corresponding products in 95%, 96% yields (3o–3p), respectively. Notably, the 2-benzoxazolimine as substrate also reacted smoothly with azomethine ylides 2a to deliver the corresponding product 3q in high yield (94%). Especially, when using (R)-nonstabilized azomethine ylide as the chiral substrate in the cycloaddition reaction, the reaction could proceed smoothly under the standard conditions to afford the corresponding chiral product 3r in 95% yield as single diastereomer only. The chiral center was determined by NOESY analysis (see ESI†). Nevertheless, as for the R2 group, when it was changed from an aryl group to an alkyl group, the reaction did not take place (3s). The possible reason may be due to the low activity of alkyl substituted substrate.
| a Reaction conditions: 2-benzothiazolimine 1 (0.1 mmol, 1.0 equiv.), N-alkyl amine 2 (0.12 mmol, 1.2 equiv.), TFA (0.01 mmol, 0.1 equiv.) and CH2Cl2 (1.0 mL) in a test tube at room temperature for 1 h. Yield of the isolated product. |
|---|
 |
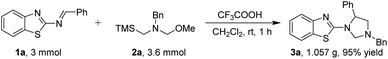
To further illustration and highlight the practical utility of the method for 1,3,5-trisubstituted imidazolidines, the gram scale experiments were performed. The reaction of 3 mmol of 1a with 3.6 mmol 2a proceeded smoothly under optimized condition for 1 h, producing the 1,3,5-trisubstituted imidazolidine 3a (1.057 g) in 95% yield without an obvious loss of efficiency (Scheme 2).
On the other hand, we developed conditions to convert the bromine atom into phenyl group via Suzuki coupling of product 3h with phenylboronic acid, which obtained product 4 in 65% yield (Scheme 3).
Based on the results presented in Table 2, a possible mechanism for this transformation is proposed to explain the reaction process in Scheme 4. First, the nonstabilized azomethine ylide from N-(methoxymethyl)-N-(trimethylsilyl-methyl)-benzyl amine 2a is generated in the presence of TFA. Then, this nonstabilized azomethine ylide could react with 2-benzothiazolimine 1a to obtain the desired product 3a via [3 + 2] cycloaddition reaction.
In conclusion, we have developed a mild [3 + 2] cycloaddition reaction from nonstabilized azomethine ylides generated in situ with 2-benzothiazolamines. In this cycloaddition, 2-benzothiazolamines were used as C2 synthon. The method could offer a broad range of functionalized 1,3,5-trisubstituted imidazolidines in high yields (up to 98%) with high regioselectivity at room temperature. Additionally, the merits of our method are cheap starting materials, wide substrate scope, without metal catalyst. The synthetic utility and practicality were also highlighted by the gram-scale experiment and the synthetic transformation. The potential application of these 1,3,5-trisubstituted imidazolidines is under investigation in this laboratory.
Author contributions
The design of this protocol was carried out by K.-K. Wang and R. X. Chen. The manuscript was written through contributions of R. X. Chen and Z.-Y. Wang. The main experiments including synthesis of the target molecules were performed by K.-K. Wang and Y.-L. Li and J. Jing. The author of M.-Y. Wang conducted the large-scale preparation of the starting materials. All the authors analyzed the results and experimental data. K.-K. Wang participated in the modification of the manuscript. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for financial support from the National Natural Science Foundation of China (21801214), the Program for Youth Backbone Teacher Training in University of Henan Province (2021GGJS163), the Natural Science Foundation of Henan Province (202300410016), Funding of Henan College Students Innovation and Entrepreneurship Training Program (202211071018).Notes and references
- (a) M. Wang and Z. Shi, Chem. Rev., 2020, 120, 7348 CrossRef CAS PubMed; (b) M. M. Heravi and V. Zadsirjan, RSC Adv., 2020, 10, 44247 RSC; (c) N. Kerru, L. Gummidi, S. Maddila, K. K. Gangu and S. B. Jonnalagadda, Molecules, 2020, 25, 1909 CrossRef CAS.
- (a) M. Amir and M. Z. Hassan, Mini-Rev. Med. Chem., 2013, 13, 2060 CrossRef CAS PubMed; (b) S. Bondock, W. Fadaly and M. A. Metwally, J. Sulfur Chem., 2009, 30, 74 CrossRef CAS.
- (a) S. Massari, D. Daelemans, M. L. Barreca, A. Knezevich, S. Sabatini, V. Cecchetti, A. Marcello, C. Pannecouque and O. Tabarrini, J. Med. Chem., 2010, 53, 641 CrossRef CAS PubMed; (b) N. Siddiqui, S. N. Pandeya, S. A. Khan, J. Stables, A. Rana, M. Alam, M. F. Arshad and M. A. Bhat, Bioorg. Med. Chem. Lett., 2007, 17, 255 CrossRef CAS PubMed; (c) R. M. Kumbhare, T. Dadmal, U. Kosurkar, V. Sridhar and J. V. Rao, Bioorg. Med. Chem. Lett., 2012, 22, 453 CrossRef CAS; (d) S. Bondock, W. Fadaly and M. A. Metwally, Eur. J. Med. Chem., 2010, 45, 3692 CrossRef CAS PubMed.
- (a) F. Gan, P. Luo, J. Lin and Q. Ding, Synthesis, 2020, 52, 3530 CrossRef CAS; (b) A. Ammazzalorso, S. Carradori, R. Amoroso and I. F. Fernandez, Eur. J. Med. Chem., 2020, 207, 112762 CrossRef CAS PubMed; (c) X. Liu and Z. B. Dong, Eur. J. Org. Chem., 2020, 2020, 408 CrossRef CAS.
- (a) J.-M. Wang, Y.-X. Chen, C.-S. Yao and K. Zhang, Asian J. Org. Chem., 2022, 11, e202200238 CAS; (b) W.-Y. Ma, E. Montinho-Inacio, B. I. Iorga, P. Retailleau, X. Moreau, L. Neuville and G. Masson, Adv. Synth. Catal., 2022, 364, 1708 CrossRef CAS; (c) Y. Zhang, J.-H. Yang, Y.-Q. Xia, L. Dong and F.-E. Chen, Chem. – Eur. J., 2021, 27, 6183 CrossRef CAS PubMed; (d) C. Ke, Z. Liu, S. Ruan, X. Feng and X. Liu, Org. Chem. Front., 2021, 8, 5705 RSC; (e) X.-P. Chen, K.-Q. Hou, F. Zhou, A. S. C. Chan and X.-F. Xiong, J. Org. Chem., 2021, 86, 1667 CrossRef CAS PubMed; (f) Q. Ni, X. Wang, F. Xu, X. Chen and X. Song, Chem. Commun., 2020, 56, 3155 RSC; (g) D. Lu, J.-H. Wu, J. Pan, X. Chen, X. Ren and T. Wang, Chem. Commun., 2020, 56, 11231 RSC; (h) L. Jarrige, D. Glavač, G. Levitre, P. Retailleau, G. Bernadat, L. Neuville and G. Masson, Chem. Sci., 2019, 10, 3765 RSC.
- (a) S.-J. Liu, Z.-H. Chen, J.-Y. Chen, S.-F. Ni, Y.-C. Zhang and F. Shi, Angew. Chem., Int. Ed., 2022, 61, e202112226 CAS; (b) Ref. 5b..
- (a) M. Breugst and H.-U. Reissig, Angew. Chem., Int. Ed., 2020, 59, 12293 CrossRef CAS PubMed; (b) J. Adrio and J. C. Carretero, Chem. Commun., 2014, 50, 12434 RSC; (c) L. Wei, X. Chang and C.-J. Wang, Acc. Chem. Res., 2020, 53, 1084 CrossRef CAS; (d) A. L. Cardoso and M. I. L. Soares, Curr. Org. Chem., 2019, 23, 3064 CrossRef CAS.
- (a) V. I. Savych, V. L. Mykhalchuk, P. V. Melnychuk, A. O. Isakov, T. Savchuk, V. M. Timoshenko, S. A. Siry, S. O. Pavlenko, D. V. Kovalenko, O. V. Hryshchuk, V. A. Reznik, B. A. Chalyk, V. S. Yarmolchuk, E. B. Rusanov and P. K. Mykhailiuk, J. Org. Chem., 2021, 86, 13289 CrossRef CAS PubMed; (b) D. L. Obydennov, V. D. Steben'kov, K. L. Obydennov, S. A. Usachev, V. S. Moshkin and V. Y. Sosnovskikh, Synthesis, 2021, 53, 2621 CrossRef CAS; (c) K.-K. Wang, Y.-L. Li, G.-Y. Ma, M.-H. Yi and B.-K. Zhu, J. Heterocyclic Chem., 2019, 56, 2274 CrossRef CAS; (d) T. Thierry, C. Lebargy, E. Pfund and T. Lequeux, J. Org. Chem., 2019, 84, 5877 CrossRef CAS PubMed; (e) E. M. Buev, A. A. Smorodina, V. S. Moshkin and V. Y. Sosnovskikh, Tetrahedron Lett., 2019, 60, 649 CrossRef CAS; (f) I. McAlpine, M. Tran-Dube, F. Wang, S. Scales, J. Matthews, M. R. Collins, S. K. Nair, M. Nguyen, J. Bian, L. M. Alsina, J. Sun, J. Zhong, J. S. Warmus and B. T. O'Neill, J. Org. Chem., 2015, 80, 7266 CrossRef CAS PubMed; (g) S. Lee, S. Diab, P. Queval, M. Sebban, I. Chataigner and S. R. Piettre, Chem. – Eur. J., 2013, 19, 7181 CrossRef CAS PubMed; (h) K.-K. Wang, Y.-X. Xie, Y.-L. Li, R. Chen and Z.-Y. Wang, RSC Adv., 2020, 10, 28720 RSC.
- (a) E. M. Buev, V. S. Moshkin and V. Y. Sosnovskikh, Org. Lett., 2016, 18, 1764 CrossRef CAS PubMed; (b) V. S. Moshkin, E. M. Buev and V. Y. Sosnovskikh, Tetrahedron Lett., 2015, 56, 5278 CrossRef CAS.
- E. M. Buev, M. A. Stepanov, V. S. Moshkin and V. Y. Sosnovskikh, Org. Lett., 2020, 22, 631 CrossRef CAS PubMed.
- H. Santos, A. Distiller, A. M. D'Souza, Q. Arnoux, J. M. White, A. G. Meyer and J. H. Ryan, Org. Chem. Front., 2015, 2, 705 RSC.
- (a) Z. Chen, Y. Zhou, T. Hu, H.-Y. Xiong and G. Zhang, J. Org. Chem., 2021, 86, 7714 CrossRef CAS PubMed; (b) S. Zimmermann, M. Akbarzadeh, F. Otte, C. Strohmann, M. G. Sankar, S. Ziegler, A. Pahl, S. Sievers and K. Kumar, Chem. – Eur. J., 2019, 25, 15498 CrossRef CAS PubMed; (c) J. K. Laha and K. P. Jethava, J. Org. Chem., 2017, 82, 3597 CrossRef CAS PubMed; (d) C. Izquierdo, F. Esteban, J. L. G. Ruano, A. Fraile and J. Alemán, Org. Lett., 2016, 18, 92 CrossRef CAS; (e) K.-K. Wang, Y.-L. Li, Z.-Y. Wang, X. Ma, Y.-L. Mei, S.-S. Zhang and R. Chen, J. Heterocycl. Chem., 2020, 57, 1456 CrossRef CAS.
- M. Manneveau, S. Tanii, F. Gens, J. Legros and I. Chataigner, Org. Biomol. Chem., 2020, 18, 3481 RSC.
- (a) K.-K. Wang, Y.-L. Li, Z.-Y. Wang, M.-W. Hu, T.-T. Qiu and B.-K. Zhu, Org. Biomol. Chem., 2019, 17, 244 RSC; (b) S.-n. Li, B. Yu, J. Liu, H.-l. Li and R. Na, Synlett, 2016, 27, 282 CrossRef CAS.
- CCDC 2182405 for 3h.†.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, structural proofs. CCDC 2182405 (3h). For ESI and crystallographic data in CIF or other electronic format see https://doi.org/10.1039/d2ra04616d |
| This journal is © The Royal Society of Chemistry 2022 |