Open Access Article
Open Access ArticleGold-catalyzed synthesis of oxazoles from alkynyl triazenes and dioxazoles†
Zhenjun Mao *a and
Hao Zengb
*a and
Hao Zengb
aDepartment of Chemistry, Zhejiang University, Hangzhou 310058, China. E-mail: maozhenjun@zju.edu.cn
bCollege of Biosystems Engineering and Food Science, Zhejiang University, Hangzhou 310058, China
First published on 31st August 2022
Abstract
A gold-catalyzed regioselective [3 + 2] cycloaddition of alkynyl triazenes with 1,2,4-dioxazoles was developed. The triazene group in the products could be replaced to obtain iodo-oxazoles, providing potential transformations to diverse oxazole structures. This protocol features readily available starting materials, mild reaction conditions and scalability. A plausible mechanism involving a nitrene transfer process was proposed.
Introduction
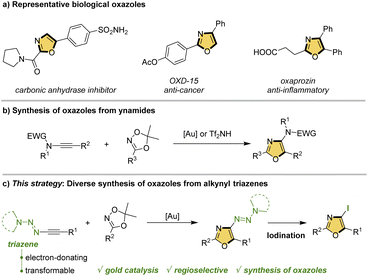
Oxazoles are a ubiquitous structural motif, which is widely found in a broad range of drugs, natural products, functional materials, agrochemicals and ligands (Scheme 1a).1 The presence of the oxazole moiety in these molecules plays important roles in respective processes. For example, the oxazole structures in drug molecules could mimic biological interactions, providing increased metabolic stability.2 Considering the significance of this type of aromatic five-membered nitrogen-containing heterocycle, the construction of oxazole skeletons has attracted considerable attention.3 Typically, amine and carbonyl analogs are classical synthons for the construction of oxazoles,4 and these methods mostly rely on bimolecular cycloaddition processes. Despite recent advances, the exploration of modular and practical routes to trisubstituted oxazoles with structural diversity is less explored,5 and thus remains understudied.The alkynyl triazene is a novel type of electron-rich alkynes, whose structure bears a triazenyl group attached to the triple bond directly. Originally prepared and demonstrated as ynamide analogs by Severin and co-workers,6 the alkynyl triazenes have received considerable attentions in organic synthesis, especially in the construction of heterocycles and biologically valuable skeletons.7 Typically, a variety of elegant works have been successively reported by Severin and Cramer,8 and Cui.9 The versatility of triazenyl group was subsequently realized by the diverse transformations of products in these reactions, thus representing modular, efficient and practical methods toward promising structures. However, compared to the ynamide,10 the flagship of electron-rich alkynes, alkynyl triazenes show vast space to be explored. In this context, we presented intense interests in the development of novel reactions based on alkynyl triazenes. Previously, Liu and Wan independently revealed an Au-catalyzed and a Tf2NH-promoted synthesis of oxazoles from ynamides and dioxazoles (Scheme 1b).11 Based on these studies, we envisioned that alkynyl triazenes and dioxazoles might undergo a regioselective [3 + 2] cycloaddition to assemble oxazoles as well, and more importantly, the electron-donating and transformable properties of triazenyl group could realize the further derivations of products for accessing diverse fully substituted oxazoles. To this end, we attempt to explore the reaction under gold-catalysis, due to the multi-advantages, such as high efficiency and excellent reactive selectivity.12 Herein, we report a gold-catalyzed synthesis of oxazoles from alkynyl triazenes and dioxazoles (Scheme 1c). It is noteworthy that the expected transformations of triazenyl group in the oxazole products are difficult. To our delight, iodination of the oxazole product could be successfully realized, demonstrating the potential for the further derivatization. This protocol features readily available starting materials, high regioselectivity, mild reaction conditions.
Results and discussion
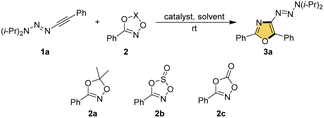
We successfully prepared the alkynyl triazene 1a and dioxane 2a as the model substrates to investigate our hypothesis. The optimizations were listed as Table 1. Initially, numerous gold catalysts, such as AuCl, PPh3AuCl, IPrAuCl and XPhosAuCl, were tested, and no distinct products formed, due to scarce consumption of both starting materials (entries 1–4). Then we tried to replace the chlorine in these catalysts to other anions. For PPh3AuCl, adding AgOTf or AgNTf2 could yield a new product successfully, albeit in low yields (entries 5–6). NMR spectroscopy, mass spectrometry as well as the X-ray crystal analysis13 showed that 3a was a 3-triazenyl-2,5-diphenyloxazole, indicating that a regioselective [3 + 2] cycloaddition occurred. The similar replacement was used in IPrAuCl, XPhosAuCl and JohnPhosAuCl, and to our delight, the yields of 3a were significantly increased (entries 7–12). Of note, using JohnPhosAuCl in combination of AgOTf could furnish 3a in a satisfactory 81% yield (entry 11). The utilize of AgOTf or Zn(OTf)2, or without any catalyst could suppress the reaction completely (entries 13–15). Subsequently, a solvent survey showed that the DCE was effective as well, giving the corresponding 3a in 66% yield (entry 16), whist the use of THF, MeCN or toluene led to the inefficient reactions (entries 17–19).| Entry | 2 | Catalyst | Solvent | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), 2 (0.24 mmol), catalyst (5 mol%), solvent (2 mL), rt, 24 h, argon.b Yield refers to isolated product. DCM, dichloromethane; DCE, 1,2-dichloroethane; THF, tetrahydrofuran. | ||||
| 1 | 2a | AuCl | DCM | 0 |
| 2 | 2a | PPh3AuCl | DCM | 0 |
| 3 | 2a | IPrAuCl | DCM | Trace |
| 4 | 2a | XPhosAuCl | DCM | 0 |
| 5 | 2a | PPh3AuCl/AgOTf | DCM | 24 |
| 6 | 2a | PPh3AuCl/AgNTf2 | DCM | 22 |
| 7 | 2a | IPrAuCl/AgOTf | DCM | 72 |
| 8 | 2a | IPrAuCl/AgNTf2 | DCM | 67 |
| 9 | 2a | XPhosAuCl/AgOTf | DCM | 76 |
| 10 | 2a | XPhosAuCl/AgNTf2 | DCM | 76 |
| 11 | 2a | JohnPhosAuCl/AgOTf | DCM | 81 |
| 12 | 2a | JohnPhosAuCl/AgNTf2 | DCM | 78 |
| 13 | 2a | AgOTf | DCM | Trace |
| 14 | 2a | ZnOTf | DCM | Trace |
| 15 | 2a | None | DCM | 0 |
| 16 | 2a | JohnPhosAuCl/AgOTf | DCE | 66 |
| 17 | 2a | JohnPhosAuCl/AgOTf | THF | 19 |
| 18 | 2a | JohnPhosAuCl/AgOTf | MeCN | 20 |
| 19 | 2a | JohnPhosAuCl/AgOTf | Toluene | 23 |
| 20 | 2b | JohnPhosAuCl/AgOTf | DCM | 0 |
| 21 | 2c | JohnPhosAuCl/AgOTf | DCM | 0 |
Finally, when replaced 2a with 2b or 2c, the reaction would completely suppressed (entries 20–21), demonstrating the exclusive reactivity of 2a in this oxazole synthesis process.
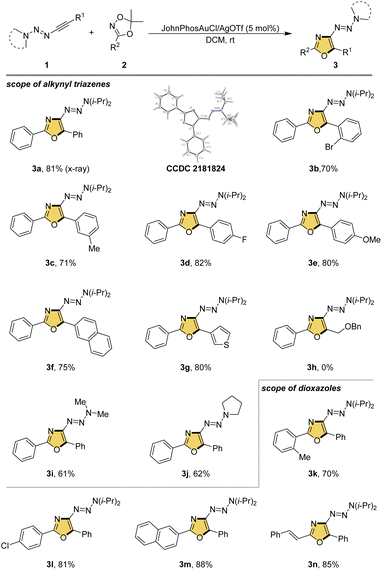
Having the optimized reaction conditions in hand, we next set out to study the substrate scope of alkynyl triazenes and dioxazoles (Scheme 2). For the alkynyl triazene component, the substitution at the β-position of the alkyne was first screened. Benzene rings bearing the bromo, methyl, fluoro or methoxy in different positions were compatible with this reaction, resulting in corresponding oxazole products in good yields (3b–3e). The functional groups and substituted position did not show significant influence to the yield. Furthermore, terminal 2-naphthyl and 3-thiophenyl substituted alkynyl triazenes were proved suitable to access oxazole 3f and 3g in 75% and 80% yields, respectively. Unfortunately, aliphatic substituted alkynyl triazene was not compatible under the current reaction conditions (3h). The variation of triazenyl group was also studied. For example, dimethyl amine derived and tetrahydropyrrole derived alkynyl triazenes were participated well in this cycloaddition, leading to the products in slightly lower yields (3i and 3j). The oxazoles derived from several aromatic acids including 2-methyl benzoic acid, 4-chlorobenzoic acid and 2-naphthoic acid provided the corresponding oxazole products in satisfactory yields (3k–3m). Notably, cinnamic acid derived dioxazole was well tolerated with this process to furnish oxazole 3n in 85% yield.
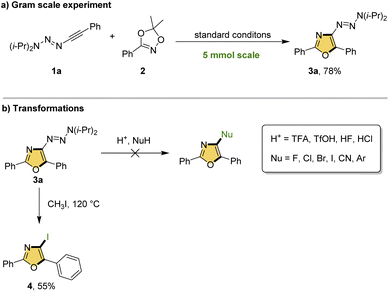
To demonstrate the scalability of this cycloaddition, a 5 mol scale reaction was performed to give the oxazole 3a in 78% yield (Scheme 3a). Next, we studied the synthetic transformation of triazenyl group. According to the reported methods, we attempted to use acid to remove the triazenyl group to form the cation, which could be captured by various nucleophilic reagents.8,9 Unfortunately, all these attempts were failed, probably due to the instability of 3-oxazole cation to trigger the ring opening. To our delight, heating 3a in CH3I at high temperature could obtain a 3-iodo oxazole in moderate yield, which provide an opportunity to further derivation via coupling reactions.14
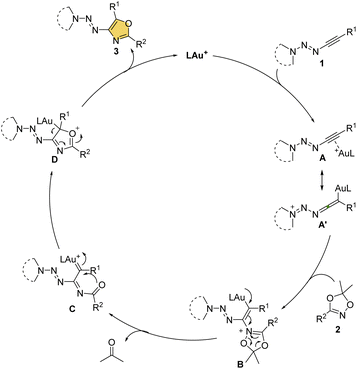
On the basis of the reaction results and literature,8a,11a a plausible reaction mechanism was depicted in Scheme 4. First, the gold catalyst coordinates to alkynyl triazenes 1 to form the complex A or A′, which is regioselectively attacked by dioxazole 2 at the carbon adjacent to the triazenyl group due to the polarity of the triple bond, resulting in the intermediate B. Subsequently, B transforms to the gold carbene species C along with the elimination of acetone via a ring fragmentation. Next an intramolecular nucleophilic cyclization occurs between acyl oxygen and gold carbene to form the intermediate D, which collapses to the product 3 and the gold catalyst, thus completing the catalytic cycle.
Conclusions
In conclusion, we have developed a gold-catalyzed synthesis of fully-substituted oxazoles from alkynyl triazenes and dioxazoles. This protocol features readily available starting materials, mild reaction conditions and scalability. The triazene moiety in products could be transformed to iodo-oxazole derivative. A plausible mechanism involving a nitrene transfer process was proposed.Author contributions
Z. M. conceived the project, Z. M. and H. Z. performed the experiments, analysed the data, and wrote the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for financial support from Science Technology Department of Zhejiang Province (LGG18B020001).Notes and references
- For selective reviews and examples, see: (a) N. David, R. Pasceri, R. R. A. Kitson, A. Pradal and C. J. Moody, Chem.–Eur. J., 2016, 22, 10867–10876 CrossRef CAS PubMed; (b) J. R. Davies, P. D. Kane, C. J. Moody and A. M. Z. Slawin, J. Org. Chem., 2005, 70, 5840–5851 CrossRef CAS PubMed; (c) M. D. Delost, D. T. Smith, B. J. Anderson and J. T. Njardarson, J. Med. Chem., 2018, 61, 10996–11020 CrossRef CAS PubMed; (d) F. Grundmann, V. Dill, A. Dowling, A. Thanwisai, E. Bode, N. Chantratita, R. Ffrench-Constant and H. B. Bode, Beilstein J. Org. Chem., 2012, 8, 749–752 CrossRef CAS PubMed; (e) V. T. T. Huong, T. B. Tai and M. T. Nguyen, J. Phys. Chem. A, 2014, 118, 3335–3343 CrossRef CAS PubMed; (f) Z. Jin, Nat. Prod. Rep., 2013, 30, 869–915 RSC; (g) P. Wipf and S. Venkatraman, J. Org. Chem., 1996, 61, 6517–6522 CrossRef CAS PubMed; (h) V. S. C. Yeh, Tetrahedron, 2004, 60, 11995–12042 CrossRef CAS; (i) X. X. Zhang, X. P. Sun, H. Fan, C. Lyu, P. Li, H. F. Zhang and W. D. Rao, RSC Adv., 2016, 6, 56319–56322 RSC; (j) Q. Zhao, S. Liu, Y. Li and Q. Wang, J. Agric. Food Chem., 2009, 57, 2849–2855 CrossRef CAS PubMed.
- For selective examples, see: (a) D. J. S. Jean and C. Fotsch, J. Med. Chem., 2012, 55, 10315 CrossRef; (b) D. J. St Jean and C. Fotsch, J. Med. Chem., 2012, 55, 6002–6020 CrossRef CAS PubMed; (c) R. N. Gaykar, S. Deswal, A. Guin, S. Bhattacharjee and A. T. Biju, Org. Lett., 2022, 24, 4145–4150 CrossRef CAS PubMed; (d) Y. Liu, K. Zhu, Y. Kong, X. Li, J. Cui, Y. Xia, J. Zhao, S. Duan and P. Li, J. Org. Chem., 2021, 86, 18247–18256 CrossRef CAS PubMed; (e) S. Mai, C. Rao, M. Chen, J. Su, J. Du and Q. Song, Chem. Commun., 2017, 53, 10366–10369 RSC; (f) W. Rodphon, K. Jaithum, S. Linkhum, C. Thongsornkleeb, J. Tummatorn and S. Ruchirawat, J. Org. Chem., 2022 DOI:10.1021/acs.joc.2c00940; (g) Z. Tu, B. Zhao, J. Wan, C. Wang and Y. Liu, Chin. J. Org. Chem., 2021, 41, 4780–4788 CrossRef.
- For selective reviews and examples, see: (a) A. V. Gulevich, A. S. Dudnik, N. Chernyak and V. Gevorgyan, Chem. Rev., 2013, 113, 3084–3213 CrossRef CAS PubMed; (b) A. S. K. Hashmi, Chem. Rev., 2007, 107, 3180–3211 CrossRef CAS PubMed; (c) Y. Hu, X. Xin and B. Wan, Tetrahedron Lett., 2015, 56, 32–52 CrossRef CAS.
- For selective reviews and examples, see: (a) O. Possel and A. M. Vanleusen, Heterocycles, 1977, 7, 77–80 CrossRef CAS; (b) R. H. Prager, J. A. Smith, B. Weber and C. M. Williams, J. Chem. Soc., Perkin Trans. 1, 1997, 2665–2672 RSC; (c) I. J. Turchi and M. J. S. Dewar, Chem. Rev., 1975, 75, 389–437 CrossRef CAS; (d) R. H. Wiley, Chem. Rev., 1945, 37, 401–442 CrossRef CAS PubMed.
- (a) S. Bresciani and N. C. O. Tomkinson, Heterocycles, 2014, 89, 2479–2543 CrossRef CAS; (b) P. Querard, S. A. Girard, N. Uhlig and C. J. Li, Chem. Sci., 2015, 6, 7332–7335 RSC; (c) T. T. Zeng, J. Xuan, W. Ding, K. Wang, L. Q. Lu and W. J. Xiao, Org. Lett., 2015, 17, 4070–4073 CrossRef CAS PubMed; (d) Z. Qi and S. Wang, Org. Lett., 2021, 23, 8549–8553 CrossRef CAS PubMed.
- (a) G. Kiefer, T. Riedel, P. J. Dyson, R. Scopelliti and K. Severin, Angew. Chem., Int. Ed., 2015, 54, 302–305 CrossRef CAS PubMed; (b) F. G. Perrin, G. Kiefer, L. Jeanbourquin, S. Racine, D. Perrotta, J. Waser, R. Scopelliti and K. Severin, Angew. Chem., Int. Ed., 2015, 54, 13393–13396 CrossRef CAS PubMed.
- A. A. Suleymanov and K. Severin, Angew. Chem., Int. Ed., 2021, 60, 6879–6889 CrossRef CAS PubMed.
- For selective examples, see: (a) L. N. Jeanbourquin, R. Scopelliti, F. F. Tirani and K. Severin, Org. Lett., 2017, 19, 2070–2073 CrossRef CAS PubMed; (b) D. Kossler, F. G. Perrin, A. A. Suleymanov, G. Kiefer, R. Scopelliti, K. Severin and N. Cramer, Angew. Chem., Int. Ed., 2017, 56, 11490–11493 CrossRef CAS PubMed; (c) I. R. Landman, E. Acuna-Bolomey, R. Scopelliti, F. Fadaei-Tirani and K. Seyerin, Org. Lett., 2019, 21, 6408–6412 CrossRef CAS PubMed; (d) A. A. Suleymanov, M. Doll, A. Ruggi, R. Scopelliti, F. Fadaei-Tirani and K. Severin, Angew. Chem., Int. Ed., 2020, 59, 9957–9961 CrossRef CAS PubMed; (e) A. A. Suleymanov, R. Scopelliti, F. F. Tirani and K. Severin, Adv. Synth. Catal., 2018, 360, 4178–4183 CrossRef CAS; (f) J. F. Tan, C. T. Bormann, F. G. Perrin, F. M. Chadwick, K. Seyerin and N. Cramer, J. Am. Chem. Soc., 2019, 141, 10372–10383 CrossRef CAS PubMed; (g) J. F. Tan, C. T. Bormann, K. Severin and N. Cramer, Chem. Sci., 2021, 12, 9140–9145 RSC.
- For selective examples, see: (a) C. Wang, Z. Lai, H. Xie and S. Cui, Angew. Chem., Int. Ed., 2021, 60, 5147–5151 CrossRef CAS PubMed; (b) S. Xu, L. Zeng and S. Cui, Org. Lett., 2022, 24, 2689–2693 CrossRef CAS PubMed; (c) L. Zeng, Z. Lai, C. Zhang, H. Xie and S. Cui, Org. Lett., 2020, 22, 2220–2224 CrossRef CAS PubMed.
- For recent reviews of ynamides, see: (a) R. H. Dodd and K. Cariou, Chem.–Eur. J., 2018, 24, 2297–2304 CrossRef CAS PubMed; (b) G. Evano, B. Michelet and C. Zhang, C. R. Chim., 2017, 20, 648–664 CrossRef CAS; (c) B. Prabagar, N. Ghosh and A. K. Sahoo, Synlett, 2017, 28, 2539–2555 CrossRef CAS; (d) L. Li, T.-D. Tan, Y.-Q. Zhang, X. Liu and L.-W. Ye, Org. Biomol. Chem., 2017, 15, 8483–8492 RSC; (e) F. Pan, C. Shu and L.-W. Ye, Org. Biomol. Chem., 2016, 14, 9456–9465 RSC; (f) G. Evano, C. Theunissen and M. Lecomte, Aldrichimica Acta, 2015, 48, 59–70 CAS; (g) X.-N. Wang, H.-S. Yeom, L.-C. Fang, S. He, Z.-X. Ma, B. L. Kedrowski and R. P. Hsung, Acc. Chem. Res., 2014, 47, 560–578 CrossRef CAS PubMed; (h) K. A. DeKorver, H. Li, A. G. Lohse, R. Hayashi, Z. Lu, Y. Zhang and R. P. Hsung, Chem. Rev., 2010, 110, 5064–5106 CrossRef CAS PubMed; (i) G. Evano, A. Coste and K. Jouvin, Angew. Chem., Int. Ed., 2010, 49, 2840–2859 CrossRef CAS PubMed; (j) B. Zhou, T.-D. Tan, X.-Q. Zhu, M.-Z. Shang and L.-W. Ye, ACS Catal., 2019, 9, 6393–6406 CrossRef CAS.
- (a) M. Chen, N. Sun, H. Chen and Y. Liu, Chem. Commun., 2016, 52, 6324–6327 RSC; (b) Y. Zhao, Y. Hu, C. Wang, X. Li and B. Wan, J. Org. Chem., 2017, 82, 3935–3942 CrossRef CAS PubMed.
- (a) L. Zhang, Acc. Chem. Res., 2014, 47, 877–888 CrossRef CAS PubMed; (b) Z. Zheng, X. Ma, X. Cheng, K. Zhao, K. Gutman, T. Li and L. Zhang, Chem. Rev., 2021, 121, 8979–9038 CrossRef CAS PubMed.
- CCDC 2181824.
- For selective examples of coupling of iodo-oxazoles, see: E. F. Flegeau, M. E. Popkin and M. F. Greaney, J. Org. Chem., 2008, 73, 3303–3306 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2181824. For ESI and crystallographic data in CIF or other electronic format see https://doi.org/10.1039/d2ra04559a |
| This journal is © The Royal Society of Chemistry 2022 |