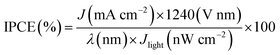Open Access Article
Open Access ArticleSeed layer-free hydrothermal synthesis of porous tungsten trioxide nanoflake arrays for photoelectrochemical water splitting
Yongtao Wanga,
Xinlei Li b and
Yuhua Yang
b and
Yuhua Yang *a
*a
aGuangzhou Key Laboratory of Flexible Electronic Materials and Wearable Devices, Nanotechnology Research Center, School of Materials Science & Engineering, Sun Yat-Sen University, Guangzhou 510275, China. E-mail: yangyuh3@mail.sysu.edu.cn
bMOE Key Laboratory of Laser Life Science & Guangdong Provincial Key Laboratory of Laser Life Science, College of Biophotonics, South China Normal University, Guangzhou 510631China
First published on 15th September 2022
Abstract
The simple preparation of efficient nano-photoanodes has been a key issue in the development of photoelectrochemical water splitting. In this work, a convenient and seed layer-free hydrothermal approach has been developed to synthesize vertically aligned porous WO3 nanoflakes on a fluorine-doped tin oxide conductive glass substrate. The morphology of WO3 nanoflakes could be manipulated by changing the annealing time, which further affected the performance of WO3 nanoflakes as photoanodes. Under optimum conditions, the obtained photoanode can lead to a high photocurrent density of 2.34 mA cm−2 at 1.4 V vs. Ag/AgCl under one sun irradiation (100 mW cm−2) and an incident photon to current conversion efficiency of 60% at 300 nm. The excellent photoelectrochemical performance can be mainly attributed to the larger active surface area, single crystal structure with an optimum thickness and the exposed highly active facets.
1. Introduction
Unlike fossil fuels, hydrogen (H2) is one of the most attractive and environmentally friendly fuels for energy application.1 Photoelectrochemical (PEC) water splitting is a scalable and cost-effective approach for the generation of H2, which converts solar energy into chemical energy and stores it in H2.2,3 In the PEC reaction, a semiconductor with a suitable band gap is used as the photoelectrode, which can harvest solar energy by absorbing photons and produce photogenerated carriers to realize water splitting.4–6 Among them, the water reduction reaction for the extraction of H2 and the water oxidation reaction for the extraction of O2 occur in the photocathode and photoanode, respectively. Moreover, because the water oxidation reaction is an energetically uphill reaction with the four-electron and four-proton transfer process, the exploration of an efficient photoanode material is key for high performance PEC water splitting. Metal oxides such as TiO2, ZnO, BiVO4 and WO3 have been investigated as photoelectrodes owing to their favorable band gap and excellent chemical stability.7–15 Among them, WO3 is a prospective photoanode material, which has a higher oxidizing capacity because of its higher valence band potential (3.1–3.2 eV vs. NHE),16,17 suitable bandgap (2.6–2.8 eV),18 moderate hole diffusion length (∼150 nm),19,20 good electron mobility (∼12 cm2 V−1 s−1)21 and low cost. However, although WO3 possesses impressive advantages as a photoanode material, its practical conversion efficiency is lower than the theoretical value (approximately 10%).22 The reason is that WO3 is an indirect bandgap semiconductor, and a relatively thick film is needed for adequate light absorption. However, the thicker WO3 film usually causes significant recombination of electron–hole pairs.Compared with the bulk materials, the nanostructured WO3 photoanode has the advantages of greatly increasing the density of active redox sites, enlarging the semiconductor–electrolyte interface and enhancing the kinetics parameters of the water oxidation reactions through the reduction of bulk recombinations.22 For example, based on the seed layer-assisted solvothermal growth, Grimes et al. synthesized WO3 nanowire arrays on a fluorine-doped tin oxide (FTO) substrate as a photoanode, which showed the saturation photocurrent of 1.43 mA cm−2 under AM 1.5 G illumination.23 Zhang et al. proposed a postgrowth modification method of the WO3 nanoflakes by a simultaneous solution etching and reducing process, and the modified WO3 photoanodes showed enhanced PEC performance with a photocurrent density of 1.10 mA cm−2 at 1.0 V vs. Ag/AgCl.24 Moreover, Gong et al. synthesized the monoclinic multilayered WO3 photoanode with a photocurrent density of 1.62 mA cm−2 at 1.25 V versus Ag/AgCl via the seed layer-assisted growth.25 Recently, by employing the 2-step hydrothermal process, Wang et al. prepared WO3 plate-like arrays on a FTO substrate with an unprecedented photocurrent density of 3.7 mA cm−2 at 1.23 V vs. RHE under AM 1.5 G illumination.26 However, although great progress in the nanostructured WO3 photoanode has been made in recent years, the common synthesis method refers to either the seed layer-assisted growth or multi-step hydrothermal reaction, which is time-consuming and energy-consuming. Moreover, the seed layer containing a large number of nanocrystalline particles can introduce numerous grain boundaries, which eventually results in the increase of interfacial carrier recombination.27,28 Thus, it is crucial for the development of a simple and seed layer-free synthetic method to grow a nanostructured WO3 photoanode for PEC water splitting.
Herein, we demonstrate a seed layer-free, one-step hydrothermal synthesis method to grow vertically aligned porous WO3 nanoflakes on a FTO substrate. The WO3 nanoflakes comprise a single crystal structure with a thickness of ∼45 nm. The surface morphology and crystalline oriented facets of the WO3 nanoflakes will evolve with different annealing times. More importantly, the optimal WO3 nanoflakes exhibit excellent PEC performance without the use of oxygen evolution cocatalysts, which may open up new opportunities in the design of practical photoanodes for PEC water splitting.
2. Experimental
Materials
All chemicals were used without further purification. H2WO4 (Sinopharm Chemical Reagent Co., Ltd, GR, ≥99.5%) was used as a tungsten source. H2O2 (AR, 30%), HCl (AR, 35%), acetonitrile (AR, ≥99.0%), urea (AR, ≥99.0%), and oxalic acid dihydrate (H2C2O4·H2O) (AR, ≥99.5%), were used in the precursor solution. The FTO conductive glasses (14 Ω, light transmission rate is 90%) were used as substrates that had been cleaned with acetone, ethanol and deionized water in an ultrasonic bath for 15 min and dried in a nitrogen stream. A Na2SO4 solution of 0.1 M (pH 6.8) was used as the electrolyte for testing the photocurrent density, incident-photon-to-current conversion efficiency (IPCE), and the electrochemical impedance spectroscopy (EIS) measurements.Preparation of the precursor for the hydrothermal reaction
A H2WO4 solution as one of the components of the precursor solution was prepared by means of dissolving 1.25 g H2WO4 into 20 ml deionized water by adding 20 ml H2O2 (30 wt%), which was stirred until it became clear in a hot-water-bath at 95 °C. Then, the H2WO4 solution was cooled to room temperature in air. The transparent H2WO4 solution was then diluted by deionized water to 100 ml with a molar concentration of 0.05 M. The precursor consists of 3 ml of the above H2WO4 solution (0.05 M), 0.15 ml HCl (35%), 0.35 ml deionized water, and 12.5 ml acetonitrile with 0.02 g oxalic acid and 0.02 g urea. Then, the mixture was evenly stirred using a magnetic stirring apparatus until there were no longer solid grains of oxalic acid or urea.Hydrothermal growth of the WO3 films
The mixture was placed into a teflon-lined stainless-steel autoclave with a volume of 25 ml. A piece of bare FTO-glass substrate with the conductive layer facing down was immersed and leaned against the wall of the teflon-vessel, which had been previously cleaned. Then, the stainless-steel autoclave was sealed and maintained at 180 °C for 2 h. The as-prepared sample was then rinsed with ethanol and dried in a nitrogen stream. Subsequently, the as-prepared sample was annealed at 500 °C for a certain time in air. In order to study the relationship between the performance and annealing time, the as-prepared samples were annealed for 1 hour, 2 hours and 4 hours in this work, and named as 1 H-WO3, 2 H-WO3 and 4 H-WO3, respectively.Photoelectrochemical test
The photoelectrochemical (PEC) performance was tested at room temperature by using the typical three-electrode PEC cell with a round flat quartz window. A FTO-glass with the as-prepared samples (1.5 × 1.5 cm2) was clamped with a Pt electrode holder as the working electrode and a saturated-potassium-chloride silver chloride electrode (Ag/AgCl) as a reference electrode. A platinum foil (1.5 × 1.5 cm2) as a counter electrode was fixed in the PEC cell with a 0.1 M of Na2SO4 solution (pH 6.8). A 300 W Xenon arc lamp (Newport, MODEL 67005) as a light-source simulator provided an illumination intensity of one sun (AM 1.5 G, 100 mW cm−2). The current/potential curves were scanned from −0.2 V to +1.4 V (vs. Ag/AgCl @ 25 °C) at a scan rate of 50 mV s−1 using an electrochemical station (IVIUM). During the test, the light was vertically illuminated on the working electrode through a quartz window that was parallel with the working electrode and the counter electrode.In the whole test process, the potential conversion between the Ag/AgCl electrode and reversible hydrogen electrode (RHE) can be expressed by the Nernst equation:
| ERHE = EAg/AgCl + 0.059PH + E0Ag/AgCl |
| EAg/AgCl = ERHE − 0.5982 |
IPCE was measured in a Na2SO4 solution. A simulator (Newport 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 911
911![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 W Xenon lamp) coupled to an aligned monochromator (Oriel Cornerstone 260 1/4 m) was used as a simulated light source to provide light at a certain wavelength. A power meter was used to obtain the power density at the certain wavelength, and an electrochemical workstation was used to apply a constant potential (0.63 V vs. Ag/AgCl @ 25 °C). IPCE can be expressed as:29–32
300 W Xenon lamp) coupled to an aligned monochromator (Oriel Cornerstone 260 1/4 m) was used as a simulated light source to provide light at a certain wavelength. A power meter was used to obtain the power density at the certain wavelength, and an electrochemical workstation was used to apply a constant potential (0.63 V vs. Ag/AgCl @ 25 °C). IPCE can be expressed as:29–32
The EIS spectral measurements of the annealed as-prepared samples were performed under an illumination intensity of one sun (AM 1.5 G, 100 mW cm−2) between the frequency of 0.01 Hz to 10 MHz in Na2SO4 solution with an AC 0.63 V vs. Ag/AgCl bias.
3. Results and discussion
Morphology and characterization
The typical XRD pattern of the unannealed as-prepared sample is given in Fig. 1(a). Peaks marked with an asterisk originate from the FTO substrate. The other diffraction peaks agree well with those of H0.23WO3 with the lattice spacing of a = 7.546 Å, b = 7.546 Å, c = 7.546 Å (α = 90°) (PDF#42–1260). The typical SEM of the unannealed as-prepared sample is shown in Fig. 1(b). It can be seen that the nanoflakes vertically grew on the FTO substrate with the uniform thickness of ∼45 nm.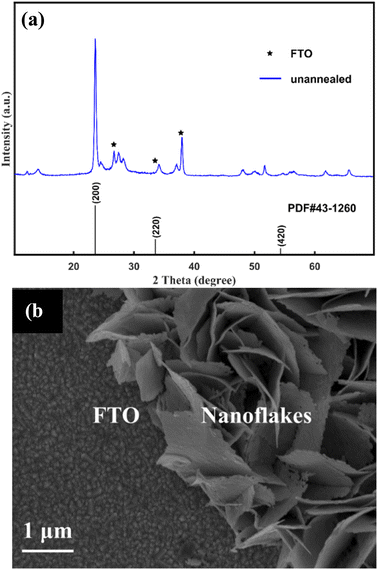 | ||
| Fig. 1 XRD pattern (a) and SEM image (b) of the unannealed samples. Peaks marked with an asterisk originate from the FTO substrate. | ||
After annealing, the XRD pattern of the as-prepared sample changed, as shown in Fig. 2(a). All of the diffraction peaks agree well with those of the monoclinic WO3 with the lattice spacing of a = 7.297 Å, b = 7.539 Å, c = 7.688 Å (α = 88.83°, β = 90.91°, γ = 90.93°) (PDF#43-1305). According to the XRD patterns of the three samples, the (002), (020) and (200) planes are exposed in γ-monoclinic WO3. In addition to the FTO signals, no other peak can be observed, indicating that all samples consisted of pure WO3. Moreover, the X-ray diffraction peaks become sharper and more intense through increasing the annealed time, which indicated that the 2 H-WO3 and 4 H-WO3 samples had better crystallinity than the 1 H-WO3 sample. The peak of the (002) facets was strengthened with the annealing time increasing from 1 hour to 2 hours, but it almost disappeared when the annealing time was increased to 4 hours. It has been reported that the (002) facets are the most active facets when compared with the other facets of γ-monoclinic WO3, and have superior activity in the PEC reaction.25 Therefore, the above results of the XRD patterns suggest that the 2 H-WO3 sample could be a promising photoanode in PEC water splitting, owing to it having more active facets than the other two as-prepared samples.
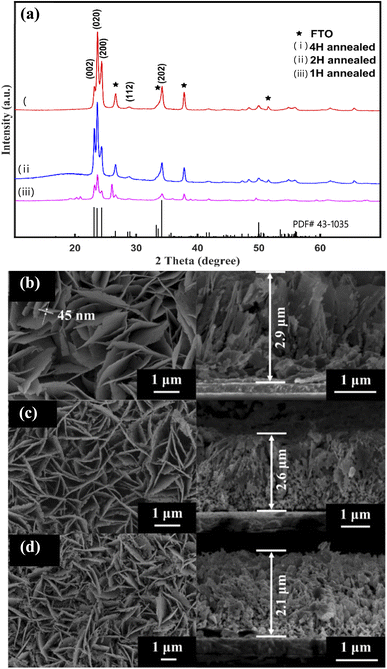 | ||
| Fig. 2 XRD patterns of the annealed WO3 nanoflakes (a). SEM images of the WO3 nanoflakes for different annealed times: (b) 1 hour, (c) 2 hours, (d) 4 hours. | ||
Furthermore, the SEM images revealed that the annealing as-prepared sample is composed of well-aligned WO3 nanoflakes, as shown in Fig. 2(b–d). The morphology of the nanoflakes is obviously different with different annealing times. The 1 H-WO3 sample has an intact flake-like morphology, which is the same as that of the as-prepared sample before annealing (Fig. 2(b)). When the annealing time increased to 2 hours, numerous pores were observed in the nanoflakes (Fig. 2(c)). The size and number of the pores increased with increasing annealing time. Furthermore, even the nanoflakes of the 4 H-WO3 sample were smashed (Fig. 2(d)). The porous structure obtained by annealing is favorable to high PEC performance because the porous structure can afford a higher specific surface area and active sites for electrochemical energy conversion.33 The thickness of the nanofilms of the 1 H-WO3, 2 H-WO3 and 4 H-WO3 samples also gradually decreased with the annealing time from 2.9 μm to 2.1 μm. Combined with the above XRD results, the crystal transformation may be responsible for the morphological changes of the as-prepared sample via annealing in air.34 The dehydration will result in volume shrinkage, which causes the decrease of the nanofilm thickness and the formation of a porous structure.
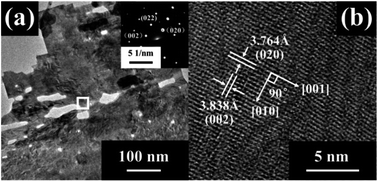
Fig. 3 presents the TEM images and SAED patterns of the 2 H-WO3 nanoflakes. The porous structure was further confirmed by the low-magnification TEM image. The clear SAED patterns reveal that the porous nanoflake is a single crystalline with high crystallinity. Furthermore, the HRTEM image of the nanoflake shows fringe spacings of 0.3764 nm and 0.3838 nm with an angle of 90°, which is consistent with the d-spacing values of the (020) and (002) facets of the monoclinic WO3, respectively. This excellent single-crystallinity structure of the 2 H-WO3 nanoflakes can offer direct conduction paths for the photogenerated electrons, which is beneficial for the PEC performance of the sample.22
Photoelectrochemical properties
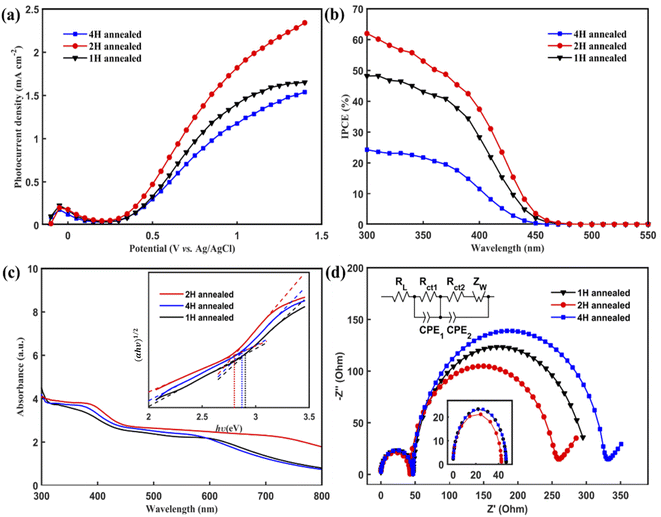
The PEC performances of the WO3 nanoflakes electrode were investigated using linear sweep voltammetry conducted from −0.2 to +1.4 V vs. Ag/AgCl in a Na2SO4 solution (pH 6.8) at room temperature. Fig. 4(a) shows the current density vs. potential (I–V) curves of 1 H-WO3, 2 H-WO3 and 4 H-WO3. As expected, the 2 H-WO3 sample exhibits the best PEC performance with the photocurrent density of 2.34 mA cm−2 at 1.4 V vs. Ag/AgCl at room temperature. Meanwhile, the 1 H-WO3 and 4 H-WO3 samples only could reach to 1.65 mA cm−2 and 1.54 mA cm−2 at the same potential, respectively. Compared with the other reported works,22–25 our WO3 nanoflakes synthesized by simple seed layer-free and one-step hydrothermal method have comparable or even better photocurrent density than the other nanostructured WO3 synthesized by other complex methods. Here, the morphology of the as-prepared sample is suggested to have an important impact on the overall PEC performance. As to the 2H-WO3 sample, it is a porous structure and more pores exist in the nanoflakes, which can afford higher specific surface area and more reactive active sites for electrochemical energy conversion. The well-aligned WO3 nanoflakes facilitate the random reflection of light in the array, which further promotes the absorption of solar energy. As to the 1H-WO3 sample, there are fewer pores in it, so it has fewer reactive active sites compared to the 2H-WO3 samples. For the 4H-WO3 sample, due to the long annealing time, its structure was smashed and collapsed. The flakes appeared to be damaged, and then its PEC performance was weakened. In addition, the more active facet (002) of the 2H-WO3 samples plays a crucial role in its excellent PEC performance.To obtain a quantitative correlation of the nanoflakes, we performed IPCE measurements as a means of studying the photoactive wavelength regime for the nanostructured WO3 films. As shown in Fig. 4(b), the IPCE results of the three types of films were consistent with the I–V curves, with the 2 H-WO3 sample giving the highest efficiency value. At 300 nm, the 2 H-WO3 sample gave IPCE values higher than 60%. However, the 1 H-WO3 and 4 H-WO3 samples gave IPCE values of about 48% and 24%, respectively. The data reveal that the 2 H-WO3 sample had the most outstanding conversion efficiency.
To further explore the origin of the excellent photocatalytic activity, the light absorption of the WO3 nanoflakes photoanodes was investigated, as shown in Fig. 4(c). The absorption edges of 1 H-WO3, 2 H-WO3 and 4 H-WO3 are located at 340 nm, 380 nm and 360 nm, respectively. The 2 H-WO3 sample had higher light absorption intensities compared to the other two samples, which indicates the superior PEC efficiency. The optical band gap, Eg, can be estimated using the following equation:35
| αhυ = A(hυ − Eg)n |
The EIS Nyquist curves of the samples consist of two semi-circles and a line, which are assigned to the electrochemical reaction at the Pt counter electrode at high frequencies (Rct1), charge transfer at the WO3/electrolyte interface at medium frequencies (Rct2), and the Warburg diffusion process of the electrolyte at low frequencies (Zw).36 As shown in Fig. 4(d), the arc radii of the three samples at high frequencies are similar, which means they have the same electrochemical reaction at the Pt counter electrode. However, 2 H-WO3 has the smallest arc radius at medium frequencies, indicating a faster interfacial change transfer and a more effective separation of the photogenerated electron–hole pairs during light illumination.37 The result clearly demonstrates that the 2 H-WO3 photoanode with a modest pore density and more exposure of the (002) and (020) facets remarkably increases the electron mobility by reducing the recombination of electron–hole pairs, which results in a higher reaction efficiency.
Finally, the photocurrent–time curves were measured using the typical three-electrode PEC cell at 0.63 V vs. Ag/AgCl in a Na2SO4 solution (pH 6.8) under AM 1.5 G illumination for 600 s. The 4 H-WO3 and 1 H-WO3 samples exhibit poor stability, and the initial photocurrent density eventually decreased by 83% and 66%, respectively. The photocurrent density of the 2 H-WO3 sample only decreased by 56% for the initial value, and the enhanced stability can be attributed to the good crystallinity and stable crystal exposed facet.21 Fig. 5 shows that all photocurrent densities had a rapid jump between zero and saturation values following the light being switched on and off, which indicates that all samples had an efficient charge separation, migration and surface reaction, i.e., high sensitivity.
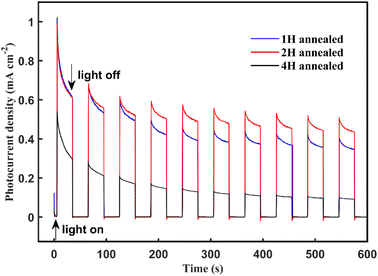 | ||
| Fig. 5 Photocurrent density vs. time curves of the annealing WO3 nanoflakes at 0.63 V vs. Ag/AgCl bias in the Na2SO4 solution under AM 1.5 G illumination at room temperature. | ||
4. Conclusion
The vertically aligned porous WO3 nanoflakes have been synthesized by a convenient and seed layer-free hydrothermal approach. The morphology and crystal facet can be manipulated by adjusting the annealing time. The sample with 2 hours annealing time exhibited much better PEC performance, which shows an enhanced photocurrent density of 2.34 mA cm−2 at 1.4 V vs. Ag/AgCl under AM 1.5 G light irradiation and an IPCE value of more than 60% at 300 nm wavelength without the use of any cocatalyst. The synergistic effect of the porous morphology, good crystallinity and the exposure of high activity (002) facets obtained under 2 hours annealing process effectively enhances the charge transfer efficiency, thereby improving the PEC performance with better photostability.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors thank the National Natural Science Foundation of China (51002192) and the Open fund of the Guangdong Provincial Key Laboratory of Laser Life Science.References
- Y. Li and J. Z. Zhang, Hydrogen Generation from Photoelectrochemical Water Splitting based on Nanomaterials, Laser Photonics Rev., 2010, 4, 517–528 CrossRef CAS.
- F. E. Osterloh, Inorganic Nanostructures for Photoelectrochemical and Photocatalytic Water Splitting, Chem. Soc. Rev., 2013, 42, 2294–2320 RSC.
- P. Zhang, J. J. Zhang and J. L. Gong, Chem. Tantalum-Based Semiconductors for Solar Water Splitting, Soc. Rev., 2014, 43, 4395–4422 RSC.
- R. E. Blankenship, et al., Comparing Photosynthetic and Photovoltaic Efficiencies and Recognizing the Potential for Improvement, Science, 2011, 332, 805–809 CrossRef CAS PubMed.
- A. Z. Weber, C. X. Xiang, A. Berger, J. Newman, M. Singh, K. Fountaine, J. Stevens, K. Chen, N. Lewis and S. Hu, II.G.20 Joint Center for Artificial Photosynthesis: Modeling and Simulation Team, Energy Environ. Sci., 2013, 6, 2984 RSC.
- Q. Wang, et al, Scalable Water Splitting on Particulate Photocatalyst Sheets with a Solar-to-Hydrogen Energy Conversion Efficiency Exceeding 1, Nat. Mater., 2016, 15, 611–615 CrossRef CAS PubMed.
- M. D. Batt and J. S. Lee, Recent theoretical progress in the development of photoanode materials for solar water splitting photoelectrochemical cells, J. Mater. Chem., 2015, 3, 10632–10659 RSC.
- D. K. Lee, D. Lee, M. A. Lumley and K.-S. Choi, Progress on ternary oxide-based photoanodes for use in photoelectrochemical cells for solar water splitting, Chem. Soc. Rev., 2019, 48, 2126–2157 RSC.
- Y. X. Wang, D. M. Chen, J. N. Zhang, M. S. Balogun, P. S. Wang, Y. X. Tong and Y. C. Huang, Charge relays via dual carbon-actions on nanostructured BiVO4 for high performance photoelectrochemical water splitting, Adv. Funct. Mater., 2022, 32, 2112738 CrossRef CAS.
- Y. Y. Wang, J. N. Zhang, M.-S. Balogun, Y. X. Tong and Y. C. Huang, Oxygen vacancy-based metal oxides photoanodes in photoelectrochemical water splitting, Materials Today Sustainability, 2022, 18, 100118 CrossRef.
- X. Y. Lu, K. H. Ye, S. Q. Zhang, J. N. Zhang, J. D. Yang, Y. C. Huang and H. B. Ji, Amorphous type FeOOH modified defective BiVO4 photoanodes for photoelectrochemical water oxidation, Chem. Eng. J., 2022, 428, 131027 CrossRef CAS.
- J. N. Zhang, Y. C. Huang, X. Y. Lu, J. D. Yang and Y. X. Tong, Enhanced BiVO4 photoanode photoelectrochemical performance via Vorate treatment and a NiFeOx cocatalyst, ACS Sustainable Chem. Eng., 2021, 9, 8306–8314 CrossRef CAS.
- M. A. Butler, Photoelectrolysis and Physical Properties of the Semiconducting Electrode WO3, J. Appl. Phys., 1977, 48, 1914–1920 CrossRef CAS.
- W. Morales, M. Cason, O. Aina, N. R. de Tacconi and K. Rajeshwar, Combustion Synthesis and Characterization of Nanocrystalline WO3, J. Am. Chem. Soc., 2008, 130, 6318–6319 CrossRef CAS PubMed.
- G. Hodes, D. Cahen and J. Manassen, Tungsten Trioxide as a Photoanode for a Photoelectrochemical cell (PEC), Nature, 1976, 260, 312–313 CrossRef CAS.
- Q. X. Mi, A. Zhanaidarove, B. S. Brunschwig, H. B. Gray and N. S. Lewis, A Quantitative Assessment of the Competition between Water and Anion Oxidation at WO3 Photoanodes in Acidic Aqueous Electrolytes, Energy Environ. Sci., 2012, 5, 5694–5700 RSC.
- M. Miyauchi, Photocatalysis and Photoinduced Hydrophilicity of WO3 Thin Films with Underlying Pt Nanoparticles, Phys. Chem. Chem. Phys., 2008, 10, 6258–6265 RSC.
- C. Santato, M. Ulmann and J. Augustynski, Photoelectrochemical Properties of Nanostructured Tungsten Trioxide Films, J. Phys. Chem. B, 2001, 105, 936–940 CrossRef CAS.
- J. Y. Zheng, Z. Haider, T. K. Van, A. U. Pawer, M. J. Kang, C. W. Kim and Y. S. Kang, Tuning of the Crystal Engineering and Photoelectrochemical Properties of Crystalline Tungsten Oxide for Optoelectronic Device Applications, CrystEngComm, 2015, 17, 6070–6093 RSC.
- F. F. Abdi, T. J. Savenije, M. M. May, B. Dam and R. van de Krol, The Origin of Slow Carrier Transport in BiVO4 Thin Film Photoanodes: A Time-Resolved Microwave Conductivity Study, J. Phys. Chem. Lett., 2013, 4, 2752–2757 CrossRef CAS.
- C. A. Bignozzi, S. Caramori, V. Cristino, R. Argazzi, L. Meda and A. Tacca, Nanostructured Photoelectrodes Based on WO3: Applications to Photooxidation of Aqueous Electrolytes, Chem. Soc. Rev., 2013, 42, 2228–2246 RSC.
- M. Park, et al., Enhanced Visible Light Activity of Single-Crystalline WO3 Microplates for Photoelectrochemical Water Oxidation, J. Phys. Chem. C, 2016, 120, 9192–9199 CrossRef CAS.
- J. Z. Su, X. J. Feng, J. D. Sloppy, J. L. Guo and C. A. Grimes, Vertically Aligned WO3 Nanowire Arrays Grown Directly on Transparent Conducting Oxide Coated Glass: Synthesis and Photoelectrochemical Properties, Nano Lett., 2011, 11, 203–208 CrossRef CAS PubMed.
- W. J. Li, P. Da, Y. Y. Zhang, Y. C. Wang, X. Lin, X. Gong and G. F. Zheng, WO3 Nanoflakes for Enhanced Photoelectrochemical Conversion, ACS Nano, 2014, 8, 11770–11777 CrossRef CAS PubMed.
- J. J. Zhang, P. Zhang, T. Wang and L. J. Gong, Monoclinic WO3 Nanomultilayers with Preferentially Exposed (002) Facets for Photoelectrochemical Water Splitting, Nano Energy, 2015, 11, 189–195 CrossRef CAS.
- S. C. Wang, H. J. Chen, G. P. Gao, T. Butburee, M. Lyu, S. Thaweesak, J.-H. Yun, A. J. Du, G. Liu and L. Z. Wang, Synergistic Crystal Facet Engineering and Structural Control of WO3 Films Exhibiting unprecedented Photoelectrochemical Performance, Nano Energy, 2016, 24, 94–102 CrossRef CAS.
- D.-D. Qin, C.-L. Tao, S.-I. In, Z.-Y. Yang, T. E. Mallouk, N. Bao and C. A. Grimes, Facile Solvothermal Method for Fabricating Arrays of Vertically Oriented α-Fe2O3 Nanowires and Their Application in Photoelectrochemical Water Oxidation, Energy Fuels, 2011, 25, 5257–5263 CrossRef.
- F. Amano, D. Li and B. Ohtani, Fabrication and Photoelectrochemical Property of Tungsten(VI) Oxide Films with a Flake-Wall Structure, Chem. Commun., 2010, 46, 2769–2771 RSC.
- Z. B. Chen, T. F. Jaramillo, T. G. Deutsch, A. Kleiman-Shwarsctein, A. J. Forman, N. Gaillard, R. Garland, K. Takanabe, C. Heske, M. Sunkara, K. McFarland, E. W. Domen, E. L. Miller, J. A. Turner and H. N. Dinh, Accelerating materials development for photoelectrochemical hydrogen production: Standards for methods, definitions, and reporting protocols, J. Mater. Res., 2010, 25, 3–16 CrossRef CAS.
- J. Zhang, J. H. Bang, C. Tang and P. V. Kamat, Tailored TiO2-SrTiO3 Heterostructure Nanotube Arrays for Improved Photoelectrochemical Performance, ACS Nano, 2010, 4, 387–395 CrossRef CAS PubMed.
- S. Hoang, S. W. Guo, N. T. Hahn, A. J. Bard and C. B. Mullins, Visible Light Driven Photoelectrochemical Water Oxidation on Nitrogen-Modified TiO2 Nanowires, Nano Lett., 2012, 12, 26–32 CrossRef CAS.
- Y. H. Ling, G. M. Wang, D. A. Wheeler, J. Z. Zhang and Y. Li, Sn-Doped Hematite Nanostructures for Photoelectrochemical Water Splitting, Nano Lett., 2011, 11, 2119–2125 CrossRef CAS PubMed.
- B. W. Ren, D. Q. Li, Q. Y. Jin, H. Cui and C. X. Wang, Novel Porous Tungsten Carbide Hybrid Nanowires on Carbon Cloth for High-Performance Hydrogen Evolution, J. Mater. Chem. A, 2017, 5, 13196–13203 RSC.
- J. Yang, W. Z. Li, J. Li, D. B. Sun and Q. Y. Chen, Hydrothermal Synthesis and Photoelectrochemical Properties of Vertically Aligned Tungsten Trioxide (hydrate) Plate-like Arrays Fabricated Directly on FTO Substrates, J. Mater. Chem., 2012, 22, 17744–17752 RSC.
- J. Tauc, R. Grigorovici and A. Vancu, Optical Properties and Electronic Structure of Amorphous Germanium, Phys. Status Solidi, 1966, 15, 627–637 CrossRef CAS.
- Y. Bai, H. Yu, Z. Li, R. Amal, G. Q. Lu and L. Z. Wang, In Situ Growth of a ZnO Nanowire Network within a TiO2 Nanoparticle Film for Enhanced Dye-Sensitized Solar Cell Performance, Adv. Mater., 2012, 24, 5850–5856 CrossRef CAS.
- M. X. Li, W. J. Luo, D. P. Cao, X. Zhao, Z. S. Li, T. Yu and Z. G. Zou, A Co-catalyst-Loaded Ta3N5 Photoanode with a High Solar Photocurrent for Water Splitting upon Facile Removal of the Surface Layer, Angew. Chem., Int. Ed. Engl., 2013, 52, 11016–11020 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |