 Open Access Article
Open Access ArticleGold nanocrystals: optical properties, fine-tuning of the shape, and biomedical applications
Meng Li
 *a,
Jianlu Wei
b,
Yang Song
*a,
Jianlu Wei
b,
Yang Song
 a and
Feiyong Chen
*a
a and
Feiyong Chen
*a
aResources and Environment Innovation Institute, Shandong Jianzhu University, Jinan, 250101, P. R. China. E-mail: mengli_ujn@163.com; ctokyo@hotmail.com
bDepartment of Orthopaedic Surgery, Qilu Hospital Shandong University, 107 Wenhua Xi Road, Jinan, 250012, P. R. China
First published on 16th August 2022
Abstract
Noble metal nanomaterials with special physical and chemical properties have attracted considerable attention in the past decades. In particular, Au nanocrystals (NCs), which possess high chemical inertness and unique surface plasmon resonance (SPR), have attracted extensive research interest. In this study, we review the properties and preparation of Au NCs with different morphologies as well as their important applications in biological detection. The preparation of Au NCs with different shapes by many methods such as seed-mediated growth method, seedless synthesis, polyol process, ultrasonic method, and hydrothermal treatment has already been introduced. In the seed-mediated growth method, the influence factors in determining the final shape of Au NCs are discussed. Au NCs, which show significant size-dependent color differences are proposed for preparing biological probes to detect biomacromolecules such as DNA and protein, while probe conjugate molecules serves as unique coupling agents with a target. Particularly, Au nanorods (NRs) have some unique advantages in the application of biological probes and photothermal cancer therapy compared to Au nanoparticles (NPs).
1. Introduction
Nobel metal nanoparticles (NPs) are widely applied in numerous fields. Among various noble metal nanomaterials, Au nanomaterials, which possess high chemical inertness and unique surface plasmon resonance (SPR) have gained extensive interest and research.1–3 Au nanocrystals (NCs) are studied comparatively early and have attracted increasing attention in medical and biological research because of their special physical and chemical properties and bio-affinity.4 It has been proven that Au NCs can be prepared via a simple preparation procedure with good control over particle size, stable chemical properties, good biocompatibility, as well as easy to modify with biomolecules. Therefore, Au NCs have been proposed for applications in genomics,5 biosensors,6–8 clinical chemistry,9 cancer cell photothermal therapy,10–14 optical imaging techniques,15,16 targeted delivery of drugs, antigens, peptides, DNA, and so on.17–19The interesting optical properties of Au NCs result from the excitation of free electron plasmons by the electromagnetic field.1,20–22 The plasmon modes of Au NCs are determined by shape, composition, size, and surrounding media. The resonance frequency is very sensitive to the shape of the NCs. In particular, Au nanorods (NRs) are promising systems for optical studies, as the spectrum is easily tunable by varying the aspect ratio.2,23,24 As such, it is crucial to have tight control over the size and crystal structure of Au NCs.25–27 As a face-centered cubic (fcc) metal, Au NCs with different shapes have been synthesized successively.28–33 In realistic chemical synthesis, many critical parameters including thermodynamic and kinetic parameters are responsible for the final shapes of Au NCs. Shape control synthesis provides one of the most powerful means to tailor the properties of Au NCs.34,35
Due to extensive research and the importance of Au NCs, various reviews related to the preparation and applications of Au NCs have been published over the years.36–43 Most of them provided a specific introduction to various aspects of Au NCs, such as photosynthesis, modification, functionalization, toxicity of Au NCs, as well as the application of Au NCs in therapeutic applications, targeted drug delivery, and energy or environment.36–40,43,44 A few publications are providing a generalized introduction to the synthesis, properties, and applications of Au NCs.4,44,45 In particular, there is still a lack of detailed understanding of the synthesis mechanism or the precise controlling of the reaction parameters, which may prevent us from realizing the full potential of synthetic approaches and accurately controlling the growth of Au NCs. The specific physicochemical and optoelectrical characteristics of Au NCs are closely related to their size and morphology, which can be controlled by the selection of a synthetic approach.38,46,47 The understanding of the reaction process and mechanism is promising to choose the appropriate synthesis route and reaction parameters such as time, temperature, and pH according to the required characteristics. Due to the frequently reported publications on Au NCs, this review may be incomplete and should be regarded as the starting point for understanding the basic principles behind the synthesis, properties, and biomedical applications of Au NCs.
In this review, the properties and preparation of Au NCs with different morphologies as well as their important applications in biological detection are summarized. The preparation of Au NCs with different shapes by many methods such as seed-mediated growth method, seedless synthesis, the polyol process, ultrasonic method, and hydrothermal treatment is introduced. For the widely reported seed-mediated growth method, the influencing factors in determining the final shape of Au NCs were studied. Some of the advantages and disadvantages of chemical synthesis methods are discussed, as well as the green synthesis methods are highlighted. The main objective of the present work is to provide a better basis for the synthesis, properties, and biomedical applications of Au NCs, which is beneficial for the further development of Au NCs.
2. Properties of Au NCs
Seo et al. reported that surface energy has a strong influence on the physical and chemical properties of the materials and there was a significant negative correlation between the ratio of surface atoms versus total volume and surface energy.25 It must be mentioned that bulk Au has distinct yellowish color, and the size dependence of the color of colloidal Au is simply the consequence of how light interacts with matter.20–22 NCs, typically in the size range of 1–100 nm, possess size and shape-dependent properties, which differ from their bulk behavior. The color of the colloidal dispersions of Au NCs in a fluid, typically water, varies from red to blue, depending upon the shape and size of the particles. For example, spherical colloidal Au NCs with a diameter of around 10 nm produce aqueous dispersions with a ruby red color, while increasing their size to nearly 100 nm or changing the morphology to rod-like (length 30 nm, diameter 10 nm) results in making the colloidal dispersion appear bluish.48 Fig. 1(a) shows the photograph of Au NPs with sizes 3, 4, 6, 9, and 35 nm (from left to right).49 | ||
| Fig. 1 (a) Photograph of the colloids 3, 4, 6, 9, and 35 nm (from left to right) of Au NPs. Reproduced from ref. 49 with permission from Elsevier, copyright [2008]; (b) schematic illustrating a localized surface plasmon. Reproduced from ref. 50 with permission from Annual Reviews, copyright [2007]. | ||
The research interest in Au NCs over the past decades has been stemming from their remarkable optical properties related to SPR. SPR comes from the excitation of free electron plasmon by the electromagnetic field.1–3 When light impinges on Au NCs, free electrons of Au NCs interact with the incident light and produce resonance coupling. The oscillation leads to the charge separation between free electrons and the metal core, producing a coulomb force, such that the electrons oscillate around the surface of the particles.50 Fig. 1(b) illustrates the localized surface plasmon.50 SPR is produced when the vibration frequency of the incident light and free electrons are equal. SPR causes a strong absorption peak in UV-vis absorption spectroscopy. Optical absorption peaks of Au NCs appeared in the wavelength range of 510–550 nm, and the resonance frequency is very sensitive to the shape of NPs.
3. Synthesis of Au NPs
Au NCs, as a face-centered cubic (fcc) metal, are known to have enclosed by low-index {100}, {111}, and {110} facets. The relative surface energies are in the order of γ{111} < γ{100} < γ{110}.25 This phenomenon results from the rapid elimination of the high-index facets with the addition of atoms during the Au NC formation process.26 The rate of crystal growth in the direction perpendicular to a high-index facet is generally much faster than that along the normal direction of a low-index facet, which results in the rapid elimination of the high-index facets with the addition of atoms during the formation of NCs.26,51 As a result, in solution chemistry, fcc metal NCs enclosed by low-index {100}, {111} and {110} facets are more commonly observed because of their relatively low surface energy.52The physical and chemical properties were controlled by the shape of Au NCs.25,27 Xia et al. mentioned that Au NCs, which possess high-index facets on their surface, such as trisoctahedral and concave cubic, showed better electro-catalytic activity in comparison to that possessing only low-index facets.53 This phenomenon can be explained by the reason that the high-index facets of a single crystal possess a high density of low-coordinated atoms such as steps, edges, and kinks, which can be served as highly active sites for adsorption and even catalysis.27,54,55
As a symmetric fcc structure, it is not easy for Au to form single-crystal multi-armed NCs in isotropic aqueous solutions. Nevertheless, Chen et al. provided a systematic introduction to the preparation methods of monopod, bipod, tripod, and tetrapod Au NCs.71 To date, Au NCs with different shapes as spheres,28–30 rods,31–33,73 cubes,29,74 triangle,53 polyhedron25,59 and others34,75 have been synthesized successively. Such as those in the TEM or SEM images of Au NCs with different morphologies shown in Fig. 2.
 | ||
| Fig. 2 (a, b, i, j, k and l) TEM images of Au nanostructures with different shapes: (a) spherical (b) triangle (i) rod-like (j) tetrapod (k) starlike (l) nanocage. (c–h) SEM images of Au nanostructures with different shapes: (c) concave cubic (d) hexagonal bipyramid (e) octahedral (f) rhombic dodecahedra (g) decadron (h) icosahedral. (a) Reproduced from ref. 29 with permission from American Chemical Society, copyright [2010]; (b) reproduced from ref. 56 with permission from American Chemical Society, copyright [2014]; (c) reproduced from ref. 57 with permission from American Chemical Society, copyright [2010] (d) reproduced from ref. 58 with permission from American Chemical Society, copyright [2013] (e and f) reproduced from ref. 59 with permission from American Chemical Society, copyright [2008] (g and h) reproduced from ref. 25 with permission from American Chemical Society, copyright [2008] (i) reproduced from ref. 31 with permission from American Chemical Society, copyright [2012] (j) reproduced from ref. 60 with permission from American Chemical Society, copyright [2014] (k) reproduced from ref. 61 with permission from American Chemical Society, copyright [2014] (l) reproduced from ref. 62 with permission from the Royal Society of Chemistry, copyright [2017]. | ||
There are many methods to prepare Au NCs, such as conventional chemical synthesis, polymer-mediated synthesis, UV-induced photochemical synthesis, ultrasound-assisted synthesis, laser ablation synthesis, and microbial-mediated synthesis.76–82 The solution-based chemical route produces a number of structural architectures, from the quasi-spherical, triangle, concave cubic, hexagon, truncated tetrahedra, octahedra, rhombic dodecahedra, trisoctahedra, decahedra, icosahedra, rod-like to tetrapod, star-like, and branched structures in high yield. Fig. 3 shows the general synthetic conditions of different methods for the preparation of Au NCs with typical morphologies.
 | ||
| Fig. 3 The general synthetic conditions of different approaches for the preparation of Au NCs with different morphologies. (*Optional). | ||
The most typical method for preparing colloidal gold is the sodium citrate reduction method. Colloidal gold of different sizes can be prepared by changing the type and concentration of the reducing agent.83 In general, parameters are responsible for the final shapes of noble metal NCs. The synthetic method, reagents used in the growth process, the temperature of synthesis, reaction time, dimensions, and yield of Au NCs with different shapes are illustrated in Table 1.
| Morphology | Synthetic method | Reagents | Temp. (°C) | Reaction time | Dimension (nm) | Yield (%) | Ref. |
|---|---|---|---|---|---|---|---|
| a RT: room temp.; N/A: unmentioned. 4-ATP: 4-aminothiophenol; DDAB: didodecyldimethylammonian bromide; PVP: poly(vinylpyrrolidone); CTAB: cetyltrimethylammonium bromide; CTAC: cetyltrimethylammonium chloride; DEG: diethylene glycol; AA: ascorbic acid. | |||||||
| Sphere | Seedless method | HAuCl4, AA, NaBH4, CTAC | 28 | Overnight | ∼7–27 | 100 | 53 |
| Triangle | Seed-mediated, growth method | CTAB, HAuCl4, AA | RT | N/A | 35 | 80 | 63 |
| Concave cubic | Seedless method | NaBH4, HAuCl4, AA, CTAC, HCl, AgNO3 | 28 | Overnight | ∼62.3–173 | 95 | 53 |
| Cube | Seed-mediated, growth method | CTAB, HAuCl4, AA, (AgNO3) | RT | N/A | 85 (70) | 85 | 63 |
| Seed-mediated, growth method | KBr, HAuCl4, CPC-capped seed, CPC | 30 | 120 h | 55.7 | 96.1 | 59 | |
| Seedless method | CTAB, HAuCl4, AA, NaBH4 | 28 | Overnight | ∼10–65 | N/A | 53 | |
| Thermal method | AuCl3, DDAB, NaBH4, dodecylthiol, octyl ether | 100 | a few minutes | ∼10 | N/A | 64 | |
| Hexagon | Seed-mediated, growth method | CTAB, HAuCl4, AA | RT | N/A | 70 | 90 | 63 |
| Tetrahedral | Polyol process | PVP, HAuCl4, DEG | 200 | 25 min | ∼210–290 | 60 | 25 |
| Octahedral | Seed-mediated, growth method | HAuCl4, AA, CPC-capped seed, CPC | 30 | 120 min | 53.4 | 97.2 | 59 |
| Hydrothermal method | CTAB, HAuCl4 | 160 | 600 min | 50 | 90 | 65 | |
| Rhombic dodecahedral | Seed-mediated, growth method | HAuCl4, AA, CPC-capped seed, CPC | 30 | 120 min | 53.4 | 100 | 59 |
| Seed-mediated, growth method | HAuCl4, NaBH4, CTAB, AA, 4-ATP | 30 | 120 min | 120 | 72 | 27 | |
| Trisoctahedral | Seedless method | CTAC, HAuCl4, AA, NaBH4 | 28 | 180 min | ∼50–250 | 95 | 53 |
| Seed-mediated, growth method | CTAC, HAuCl4, AAs | 28 | N/A | ∼55–120 | 90 | 63 and 66 | |
| Decahedral | Polyol process | PVP, HAuCl4, DEG | 245 | 10 min | ∼48–90 | 80 | 25 |
| Ultrasonic method | HAuCl4, PVP, Au seed | RT | N/A | ∼35–65 | 80–90 | 67 and 68 | |
| Icosahedral | Polyol process | PVP, HAuCl4, DEG | 245 | 10 min | 94 | N/A | 25 |
| Rod-like | Seed-mediated, growth method | CTAB, HAuCl4, AA | 25 | 180 min | Width: 6∼42, length: 22∼475 | 97 | 63, 69 and 70 |
| Seedless method | CTAB, HAuCl4, AA, NaBH4, AgNO3 | 28 | 180 min | Width: ∼11, length: ∼46 | 98 | 53 | |
| Tetrapod | Seed-mediated, growth method | CTAB, HAuCl4, AA, (AgNO3) | RT | N/A | 293(30) | 75(70) | 63 |
| Star-like | Seed-mediated, growth method | CTAB, HAuCl4, AA | 30 | 720 min | 66 | 95 | 63, 71 and 72 |
In solution-based chemical synthesis, thermodynamic and kinetic parameters controlled the growth of NCs.26,84 Thermodynamic parameters include temperature and reduction potential, while kinetic parameters include reactant concentration, diffusion, solubility, and the reaction rate. By controlling these parameters, it is possible to tune the nucleation and growth stages of NCs and achieve crystallographic control.
The seed-mediated growth method is the widely reported method in conventional chemical synthesis.85,86 Since its inception and application, the seed-mediated growth method has made a revolutionary impact on the majority of the reported synthesis of shape-controlled Au NCs. It is typical that the seed-mediated growth process comprises the preparation of small Au NCs and subsequent growth in reaction solutions.87,88 Both, the crystallinity of seeds and the growth rates of different crystallographic facets play a vital role in determining the final shape of the resultant nanostructure.89
The shape of Au NCs could be controlled by tuning nucleation and growth stages in the seed-mediated growth method.26,90,91 In the solution-phase synthesis of NCs, “nucleation” can be broadly defined as the formation of a small cluster from atoms in the solution. Small Au seed particles are generated under conditions of high chemical supersaturation. This leads to the fastest nucleation rate. Once the nuclei have grown past a critical size, they will have relatively stable crystallinity and well-defined crystallographic facets exposed on their surface, which can be termed “seeds”. The shape of a seed is primarily determined by the minimization of the surface energy.85
After the nucleation stage, each type of seed can still grow into an NC with several possible shapes. The reaction conditions are then altered and more Au ions and the reductant are added, together with some form of a shape-templating surfactant or molecule, and the seeds are grown into larger particles of particular morphologies or habits. The final shape is controlled by the growth rates of different facets.89 As the seed grows into a nanocrystal, the growth rates of different facets can be altered with capping agents to control the final shape. Typically, the growth stage is much slower and proceeds under milder reducing conditions than those at the nucleation stage.92 The shaping of these nanostructures can be analyzed by the deposition of metal atoms on the seed surface under reductive environments.25 Grzelczak et al. proclaimed that in comparison to the nucleation stage, the growth stage was under a milder reducing process and the reaction rate was much slower.92,93
Some chemical stabilizers such as poly(vinyl pyrrolidone) (PVP), poly(vinyl alcohol), and sodium dodecyl sulfate are added to reaction mixtures to control the growth of Au NCs.94–96 Polyhedral Au NCs with decahedral, icosahedral, and truncated tetrahedral shapes are synthesized by a simple one-pot polyol process in the presence of PVP.97,98 A high PVP concentration of up to 360 equivalent of the Au precursor, HAuCl4, effectively stabilizes decahedral seeds to yield uniform decahedra with various edge sizes. Decreased PVP concentration subsequently leads to selective formation of icosahedral and truncated tetrahedral.25,99 At relatively low PVP concentration, the internal surface energy of the crystals becomes more important than the interaction energy between surface atoms and PVP, and the particles prefer more rounded shapes to reduce the total surface area. The icosahedral structure is close to spherical and has stable {111} facets on the surface. The edge truncation of the icosahedron helps to diminish its structural strain energy.25
Typical reducing agents such as citric acid, borohydride, tetrafluoroborate, AA are used during the preparation of Au NCs.94,100,101 AA is a commonly used reducing agent during the preparation of Au NCs. However, it has been reported that Au NCs can be obtained in large quantities by the aspartate reduction of Au ions. Suri et al. put forward amines as an attractive class of reducing agents because of their universal presence in biology and the environment.102 Dong et al. described that amino acids can control the size and morphology of the Au NCs. They mentioned that amino acid moieties could form an organic matrix to have an influence on the biomolecules and inorganic materials.103
Conventional synthesis techniques involving the seed-mediated growth method, seedless method, polyol process, hydrothermal method, and ultrasonic method have been developed for the synthesis of Au NCs with different sizes and shapes. Chemical methods can generate Au NCs at a low cost and provide repeatable results using various chemicals. Although the chemical synthesis methods described above are effective, many of them suffer from some drawbacks such as high temperature, harsh reaction conditions, long reaction times, the use of toxic and aggressive chemicals as reducing and/or capping agents to control the size and composition of Au NCs, resulting in the environmental pollution caused by the use of organic solvents.104–107 Besides, the toxic capping agents or chemicals used in the synthesis process tend to adsorb on the surface of Au NCs, which may cause severe threats to living cells while using Au NCs for drug delivery and diagnostic applications.108–112 Consideration of the environmental problems, Firdhouse et al. summarized the detailed advantages and disadvantages of conventional synthesis techniques for Au NPs synthesis.113
In view of the environmental impact, eco-friendly green synthesis methods have currently become popular.114–120 Current strategies of ‘‘green’’ concern include the use of nontoxic chemicals, biodegradable polymers, and environmentally benign solvents.121 In the green synthesis methods, the extracts from living organisms such as leaves, stems, roots, seeds, flowers, fruit, whole plant, fungi, and algae are available as reducing and capping agents.121,122 The synthesis of Au NCs via green routes has the advantages of simplicity, economical friendliness, low cost, moderate reaction conditions, and application of non-toxic chemicals.114,123,124 This technique provides a wide range of applications for functional nanomaterials because ascorbic acid, terpenes, alkaloids, phenols, polyphenols, and flavonoids are coated on the surface of NCs, resulting in Au NCs with lower toxicity than chemically synthesized counterparts. Not only that, Au NCs showed enhanced solubility and stability due to coating with biomolecules.125 Nevertheless, the green synthesis methods are possibly related to some major operational obstacles, such as the need for a longer time, critical downstream processing to purify nanoparticles in high yields, and difficulty to optimize the process parameters and expand the scale.126,127
4. Synthesis of Au NRs
One-dimensional Au nanostructures have received considerable attention due to their size-dependent optical, catalytic, and electronic properties.128,129 The research interest in Au NRs over the past decades has stemmed from their anisotropic configuration and unique optical properties. Since its inception and commercialization, Au NRs have made a revolutionary impact in the field of bioanalysis and have become a powerful tool for bioanalytical chemistry.Shape control provides one of the most powerful tools to tailor the properties of noble metal nanostructures. In Fig. 4(a), L/D is the aspect ratio. Fig. 4(b) is the photograph of the colloidal solution of Au NRs. The color of the colloidal solution is related to the aspect ratios of Au NRs. There are distinct differences between the spherical Au NPs and Au NRs. The distinct difference from spherical NPs is that Au NRs possess two distinct surface plasmon resonances; a transverse SPR corresponding to the short axis of the rod, and a longitudinal SPR corresponding to the long axis (Fig. 4(c)). The energy of the longitudinal SPR can be tuned from the middle of the visible region of the electromagnetic spectrum (∼600 nm) to the infrared region (∼1800 nm), simply by changing the aspect ratio of the Au NRs.23,24
 | ||
| Fig. 4 (a) Au NRs possess two separate plasmon absorbances, one corresponding to the short (“transverse”) axis and the one corresponding to the longitudinal axis. L/D is the aspect ratio. Reproduced from ref. 24 with permission from American Chemical Society, copyright [2013]; (b) photograph of colloidal Au prepared in water. Aspect ratios are 2.6, 4.1, 5.6, and 7.4 (from the left), respectively. Reproduced from ref. 20 with permission from Elsevier, copyright [2009]; (c) schematic representation of transverse and longitudinal plasmon absorbances in Au NRs. Reproduced from ref. 24 with permission from American Chemical Society, copyright [2013]. | ||
Au NRs have been prepared by conventional wet chemistry, photochemical and electrochemical methods.130 Among the reported procedures, the seed-mediated growth has been by far the most efficient and popular approach. Au NRs are prepared by a seed-mediated growth approach, which uses ∼4 nm Au nanoclusters as seeds and subsequent reduction of a metal salt with a weak reducing agent in the presence of a directing surfactant to produce NRs.128 Besides the seed-mediated growth method, seedless synthesis is the other typical synthetic method to prepare Au NRs. The difference between the seed-mediated growth method and seedless synthesis is that the sizes of in situ seeds grown using NaBH4 are about 1.5 nm, while the sizes of the seeds used in the seed-mediated growth method are about 3.5 nm. The seedless method with a size of about 1.5 nm is in favor of the synthesis of anisotropic Au NCs.1,131 The dimension of Au NRs prepared by the seedless synthesis method is about 25 × 6 nm, while about 60 × 15 nm by the seed-mediated growth method.132 The size of Au NRs produced by seedless synthesis is much smaller than that prepared by the seed-mediated growth method.133–135 The seed-mediated growth method is discussed in detail in the following part.
The seed-mediated growth method is a typical growth process that involves the preparation of Au NRs. Many factors affect the growth of Au NRs, such as the concentration of reagents, the pH of the growth solution, and the type of the directing surfactant. Table 2 lists the influencing factors on the growth of Au NRs. In the solution-phase synthesis, impurities or capping agents can change the order of free energies of different facets through their interaction with a metal surface. As a result, the facet with a slower growth rate will be exposed more on the surface.89
| Parameter | Influence factors | Mechanism | Effect on products | Reference |
|---|---|---|---|---|
| a AR: aspect ratio. | ||||
| Directing surfactant | CTAB: concentration | Growth rate in different facets | Morphology, AR, morphology | 20 and 136–138 |
| A binary surfactant mixture | Growth rate in different facets | Uniformity, yield, AR, width | 23 | |
| Au ions | Concentration | Growth rate | Length | 23 |
| Ag ions | Concentration | Interact with surfactant | Length, AR, yield | 23 and 139–141 |
| Seed | Amount | Growth rate | Size, AR | 131 |
| Crystallinity | Binding with surfactant | Morphology, dimension, crystallinity, yield, AR | 1 and 92 | |
| (Citrate-capped: multiply-twinned) | ||||
| (CTAB-capped: single crystal) | ||||
| Size | Growth rate | Size, shape, AR | 131 and 142 | |
| Reducing agent (AA) | The molar ratio of AA to Au | Reaction rate | AR, yield | 20 |
| Temperature | Reduction rate | Morphology, AR | 20 | |
| pH | Reduction rate, reducing power | Morphology, yield, AR | 20, 87 and 142–146 | |
| Halide ions | Molar ratio | Growth rate in different facets | Morphology | 142, 147 and 148 |
| Aromatic additives | Cationic CTAB micelles | CTAB-aromatic additive systems | AR, yield, monodispersity | 31 |
4.1 There are many factors affecting the growth of Au NRs
The original idea was to use micelles formed by the cationic surfactant CTAB as a “soft template” to direct NR formation.20 Previous studies suggested that CTAB formed a bilayer on the surface of NR.136,137 Compared to the ends, the assembly of CTAB bilayers along the long faces of Au NRs is preferred. Besides, CTAB assists in the fine-tuning of the shape because CTAB preferentially binds to the middle of the NRs. This property makes it possible to form Au NRs with a dog-bone shape.128
It has been demonstrated that decreasing the concentration of CTAB yielded the NRs with shorter aspect ratios (1 to 6).142 On the contrary, employing a surfactant with a longer aliphatic surfactant and smaller seed solution obtained longer NRs.142 In 2005, citrate-capped Au NPs were chosen as seeds for Au NRs growth.1 Then, Liu and Guyot-Sionnest observed differences between the crystalline structures of citrate and CTAB-stabilized seeds, confirming the multiply-twinned structure of citrate-capped seeds.1
A careful choice of the experimental conditions allows the growth of the seeds into NRs. Factors including temperature,20 pH,144,146,149,150 crystallinity of seed particles,1,151 concentration of reactants,128,150,151 single-component surfactants other than CTAB,152 binary surfactant mixtures,23 aromatic additives,31 and the presence of iodide ions in the growth solution142,147,148 have been carefully evaluated by several research groups.
First reduction:
| Au3+ → Au+ |
| CTA–AuBr4 + C6H8O6 → CTA–AuBr2+C6H6O6 + 2H+ + 2Br− |
Second reduction:
| Au+ → Au0 |
| CTA–AuBr2 + C6H8O6 → 2Au + C6H6O6 + 2CTA+ + 2H+ + 4Br− |
The overall reaction is:
| 2CTA–AuBr4 + 3C6H8O6 → 2Au + 3C6H6O6 + 2CTA+ + 6H+ + 8Br− |
The first reduction is confined to the metal micelles. The second reduction starts only after the addition of the seed solution. The study on the effect of the concentration of AA on the growth of Au NRs indicated that the reaction rate becomes faster with the increasing in the concentration of the reducing agent. The decrease in the reaction rate was in favor of the isotropic growth, thereby increasing the yield of NRs. The reduction rate affects the morphology of NRs. For example, an insufficient amount of AA yielded fatter Au NRs.20
The pH value affects the reduction rate and reduces the power of the reducing agent. AA has a much weaker reducing power in a strongly acidic solution than in a weakly acidic solution.87,143 Adding a small amount of the HCl solution to the growth solution could decrease the whole reduction rate and increase the aspect ratio.153,154 The feasible formation of Au nanoprisms with an addition of a small amount of NaOH has been observed in a previous report by Mirkin et al.155 This is because the reducing power of AA can be enhanced by eliminating the proton produced by the reduction.145 Therefore, the formation of nanoprisms can be promoted at higher pH values. In other words, the chemical potential of AA is maintained at a higher state after the addition of NaOH.142
The effect of the silver ion content does not always increase the aspect ratio of Au NRs. The negative effects of higher concentrations of silver ions may be due to their interaction with the bromide-resistant ions of the surfactant monomer.23 Babak et al. explored the role of silver in the growth process of Au NRs and reported that Ag+ could first increase and then decrease the length of Au NRs, similar to the effect of Au ions.23
Liu and Guyot-Sionnest proposed an elaborate explanation for the role of Ag+.1 They suggested that the under-potential deposition (UPD) of metallic silver occurs on different crystal facets of Au NCs, leading to symmetry breaking and rod formation. UPD is an important process that occurs during the formation of metallic layers on metallic substrates.156 It is widely observed that when a metal working electrode is slowly cathodically polarized, a second noble metal ion can be deposited on the substrate to form a thin film.157 Crucially, the initial deposition of metal monolayers with potentials much higher than the Nernst potential of the deposited metal is often observed. This deposition of the first and sometimes the second monolayer is called UPD. Ag+ UPD does occur on Au {111} and the UPD process is influenced by the presence of chloride ions.92 In the presence of Cl−, the UPD offset becomes stronger.
The above mechanism does not work in the growth of Au NRs using surfactant templates because the added silver ions are not reduced to atomic silver. Both silver ions and Au ions were contained in the growth solution, and AA as a reducing agent can only reduce the Au ions. Only under a basic pH, AA has the ability to reduce silver ions.140 The deposition of silver on the surface of Au NRs only happens at pH ≥ 8 in the CTAB solution, with AA as the reducing agent.158 Jana et al. reported that the preparation of Au NRs in the absence of silver ions can only obtain Au NPs.139 This result confirmed that Ag+ adsorbed on the surface of Au NPs in the form of AgBr restricts the growth and stabilizes the NR surface.
The question that remains unresolved is why the addition of AgNO3 during the growth of Au NRs leads to an increase in the yield and greatly improves the control of the aspect ratio of Au NRs. If the silver ion has catalytic activity and promotes the formation of NRs, the length should not be improved and only the number of NRs would increase. In fact, Ag+ could combine with the headgroups of the capping material such as CTAB to form Ag–Br pairs. Ag+ as a complexing agent between the monomers assists the template elongation.141 Ag–Br pairs, which are formed between AgNO3 and CTAB adsorbed in the facets of Au NPs cause restriction, limiting their growth, and obtaining Au NRs.23 This combination decreases the charge density on the bromine ion, resulting in less repulsion between adjacent head groups on the gold surface. The repulsion between the neighboring headgroups results in CTAB template elongation.
5. Biomedical applications
Metal and semiconductor NCs show different characteristics, which are usually different from their corresponding bulk materials. NCs with a high surface area can enter cells and interact with intracellular substances. In recent years, metal and semiconductor NCs with new or improved optical, electrical and magnetic properties have attracted wide interest in basic research and biomedical applications.165–168 Semiconductor NPs consist of a few atoms up to several thousand atoms. The electronic energy level will be quantized when its size is small enough (typically less than 10 nm), in this case, they are also named quantum dots (QDs).169 Yang et al. mentioned that QDs possess high photoluminescence efficiency and have unique applications in biological and light-emitting devices.170 QDs have attracted significant interest due to their small size and high fluorescence quantum yield.171 However, possible applications in biomedical fields are limited by their toxicity.Compared to toxic QDs, Au NCs have good biological applications in single cell research due to their good stability, cell permeability, and easy coupling with biological macromolecules. Besides, the diameter of Au NCs is in the range of 1–100 nm, and most biological molecules (such as proteins and nucleic acids) are in this size range.167 Milkin et al. mentioned that nanomaterials are attractive probe candidates because of the following four reasons. First, they have a small size (1–100 nm) and a large specific surface area. Second, physical and chemical properties can be adjusted by controlling the size, composition, and shape of NCs. Then, they unusually target binding properties. Finally, they have overall structural robustness.176 Based on the above properties, Au NCs can be used as probes to detect the physiological functions of biological molecules and reveal the life process at the molecular level. The typically biomedical applications of Au NCs in sensing, imaging, therapy, and immunology are presented in Fig. 5.
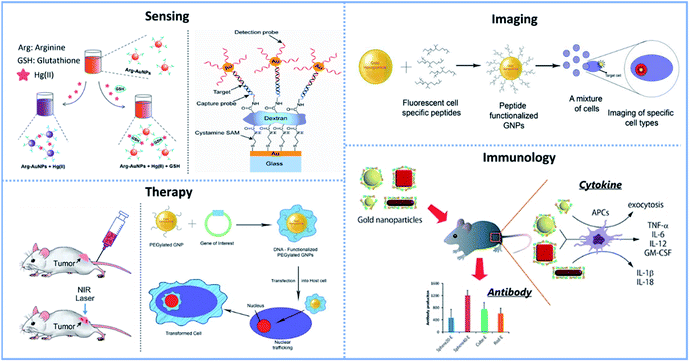 | ||
| Fig. 5 Summary of the typically biomedical applications of Au NCs. Sensing application: (left) arginine-modified Au NPs for GSH sensing in cell cancer are distributed and regulated by a chromogenic approach. Reproduced from ref. 172 with permission from John Wiley and Sons, copyright [2015]; (right) schematic representation of the sandwich DNA detection assay via Au NP-mediated SPR signal amplification. Reproduced from ref. 173 with permission from Elsevier, copyright [2006]. Imaging application: Au NPs functionalized with cell-specific peptides for bioimaging. Reproduced from ref. 174 with permission from MDPI (Basel, Switzerland), copyright [2011]. Therapy application: (left) scheme of the photothermal destruction of an implanted tumor in a mouse after injection of Au NRs functionalized with poly(ethylene glycol). Reproduced from ref. 16 with permission from Royal Society of Chemistry, copyright [2011]; (right) functionalized Au NPs for gene delivery. Reproduced from ref. 174 with permission from MDPI (Basel, Switzerland), copyright [2011]. Immunology application: the antigen-based West Nile Virus Protein (WNVE) is attached to PSS-MA coated Au NPs (different sizes and forms) through electrostatic activity directed to virus vaccinations. Reproduced from ref. 175 with permission from American Chemical Society, copyright [2013]. | ||
5.1 Biomedical applications of Au NPs
The electronic energy levels of Au NPs are split owing to their small size. The distance between the energy levels is related to the size of NPs. Specific wavelength light absorption occurs when the electron transition from a low band gap to a high band gap, resulting in the solution presenting different colors.177 The color of colloidal Au is related to the size of Au NPs.178,179 The color changes from pale orange-yellow, wine red, deep red, blue, and purple with the increase in the particle size. Its unique color change is the basis of biochemical applications.177,180The properties of colloidal Au mainly depend on the diameter and surface characteristics of Au NPs. They have controllable surface chemical properties for modification with various chemical and biologically active molecules.181 It has been reported that the affinity of Au NPs with various sizes and shapes to thiols, disulfides, dithiocarbamates, and amines allows bioconjugation with many kinds of biological molecules.182 The above characteristics provide the possibility for the detection of specific DNA and proteins.183 Due to the good biocompatibility of Au, colloidal Au can be used for biological labels and the determination of protein concentration.
The SPR of Au NPs is extremely sensitive to the environment, size and shape.181,182 The aggregation degree, particle size range, and morphology of Au NPs can be determined by UV-visible absorption spectroscopy. The characteristic absorption peaks of Au NPs appeared at 510–550 nm. Under normal conditions, the surface of Au NPs is negatively charged, and the electrostatic repulsion between particles is greater than the van der Waals force. Therefore, a certain distance between the particles is maintained and the solution remains stable. When the external conditions change, such as lowering the temperature or adding the electrolyte solution, it would lead to aggregation between particles, resulting in the SPR absorption peak red shift.180 For example, the color of colloidal Au changes, and the absorption spectrum red-shifts while Au NPs are absorbed by biomacromolecules and the distances between the particles are smaller than their diameters.16,48 These are the principles for the detection of DNA and proteins.16
With the discovery of immune Au labelling, Au NPs began to be used in biomedical applications in the 1970s.184 All the nano gold probes mentioned here were based on citrate-stabilized anionic Au NPs. Since the 1980s, Au NPs, which are connected with biomacro-molecules, have been used for various analytical methods.16 In 1980, the sol particle immunoassay was proposed by Leuvering et al.185 In 1996, Mirkin et al. proposed to use the assembled Au NPs with thiol-modified oligonucleotides as biological probes to detect DNA.186–188 Subsequently, a large number of reports using colorimetric assay methods to detect DNA, antigen and biological molecules, using Au nanoprobes decorated with oligonucleotides, antibodies, peptides have appeared.
The colorimetric assay method has become an attractive choice for biological detection because it does not require the use of advanced equipment and is easy to operate in many applications. When particles aggregate to a certain extent, the color of the solution changes dramatically. Plasmon resonance absorption peak red-shifts when the target molecule hybridizes with functional groups to cause the colorimetric response, indicating the presence of the target molecule.16,186 Colorimetric methods have high selectivity and sensitivity for particle size, shape, and structure.15,176
In the colorimetric assay method, the surface of Au NPs was functionalized. Glomm W. R. and Khlebtsov et al. referred to “functionalization” as defined as biomacromolecules attached to the surface of Au NPs.189,190 Physical adsorption and coordination bond coupling are the two main methods to combine Au NPs with biological macromolecules, such as single-stranded oligonucleotides, antibodies, peptides, and carbohydrates.16,184,186,191 Au NPs are used as optical markers, as well as probe-conjugated molecules for coupling with the target. Functionalization is the basis of biological applications.16 In general, Au NPs were decorated with intermediate linkers so that they could be functionalized. The most commonly used intermediate linkers are thiol or thiolated derivatives, which are connected by the Au–S bond. In addition to alkylthiols, other ligands such as phosphine, amino, and carboxyl groups can also be used as linkers.192,193
Genetic testing contains two contents including gene sequence identification and point mutation determination. Gene sequence identification may be used for genetic diagnosis. Point mutation determination is of great importance in the diagnosis of genetic diseases and drug resistance.194 There are two strategies for detecting DNA using the colorimetric method: one is based on the conjugation of 10–30 nm Au NPs with thiol-modified single-stranded oligonucleotides (ssDNA), and the other uses the unmodified Au NPs as Au nanoprobes.186,195–198 The main process of preparing Au NPs for DNA detection is as follows: first, a thiol-modified oligonucleotide is synthesized. Then, oligonucleotides are added to the Au solution to form a solid connection with the surface of Au NPs through an Au–S covalent bond. Finally, buffer solution with an appropriate concentration should be added. After centrifugation, the nano-gold probe is obtained.182,195 The complex process is time-consuming. It is unstable in the presence of a buffer solution because of the aggregating effect of salt ions, but Au NPs are readily stabilized by functionalization.182 Rothberg et al. designed a gene detection method without the surface modification of Au NPs.196,199 Colloidal Au remains stable due to electrostatic repulsion between particles. When a small amount of electrolyte solution, such as NaCl solution, is added, the electrostatic repulsion between nanoparticles is shielded by the electrolyte solution, resulting in particle aggregation and color change. There are some different propensities between ssDNA and double-stranded oligonucleotides (dsDNA). Due to van der Waals force, ssDNA can be adsorbed on the surface of Au NPs and remain stable at relatively high electrolyte concentrations. However, dsDNA presents a negatively charged phosphate skeleton, so dsDNA does not adsorb on the surface of negatively charged Au NPs.200 Therefore, after adding ssDNA or dsDNA and a certain concentration of salt solution to nano-gold solution, the color of adding ssDNA remained red, while the color changed from red to blue in the presence of dsDNA.196,201
In addition, nano gold probes can be used to detect the mismatch of base pairs.180,202 Taton et al. created a solid-phase detection mode based on the gold nanoprobe to identify target genes.203 In colorimetric analysis, the solution color changed from red to blue when the growth solution contained the target gene. After the formation of the three-dimensional network structure, the system temperature gradually increased. When the temperature increases to a certain extent, the mismatched base pairs degenerated. As a result, the distance between particles returned to the distance before aggregation, and the color of the solution gradually returned to red. On the contrary, the completely complementary ssDNA remained stable, and the solution color did not change at this temperature. Therefore, this method can be used to detect point mutation.
Nanogold probes can be used for the detection of proteins.204,205 Au NPs were easily decorated using thiolate chemistry and their optical properties depend on the size.206 Citrate-protected Au NPs, which are conjugated with proteins such as antibodies are used in biomolecular detection.207 However, in a salt environment, citrate-protected Au NPs tend to aggregate. It is surprising that they are not easy to aggregate after being connected with the protein layer. This method can be used to confirm the successful connection between Au NPs and proteins.208 Au NPs have strong adsorption with proteins and other biological macromolecules. As a consequence, there is a good prospect in the immune analysis.
5.2 Biomedical applications of Au NRs
In addition to Au NPs, Au NRs also have been used in biological fields due to their strong light scattering efficiency, stable optical characteristics and are easy to process, and they are a good molecular probe because of the polarized light. Because of their anisotropic configuration and unique optical properties, Au NRs have great potential in chemical and biochemical sensing such as for metal ions, amino acids, antibodies, cancer cell imaging, and photothermal therapy.130,166,195,209–214 In addition, Au NRs have the advantages of strong optical signals, high photostability and can be easily synthesized and engineered so that they can be used as single-molecule optical probes. More importantly, Au NRs whose light absorption and scattering are polarized are suitable for probe orientations.A typical Au nano-solution is hydrophobic and thermodynamically unstable, which requires surfactant stability.190 Au NPs synthesized by reducing HAuCl4 usually have negative charges on their surface. In contrast, Au NRs synthesized by the seed-mediated growth method are stabilized by CTAB, which is a widely used cationic surfactant and has a positive charge on its surface. In contrast to Au NPs, it is difficult for Au NRs to be functionalized due to the presence of CTAB surfactant molecules serving as stabilizers. Nevertheless, Dujardin et al. obtained a specific organization of short Au-NRs into anisotropic three-dimensional (3D) aggregates by DNA hybridization, but in this approach, it is needed to remove excess surfactant after the synthesis of Au NRs.215 Although CTAB bilayers hinder the formation of the Au–S bond between thiol-modified DNA and Au NRs, there exists electrostatic interaction between the positively charged ammonium of CTAB and the phosphate backbone of the DNA.216 Since the Au NR surface has a positive charge, it can connect with the negatively charged un-modified ssDNA and dsDNA through electrostatic interactions.217 This is the most fundamental difference between Au NPs and Au NRs.218
There are mainly three methods to displace CTAB from the surfaces of Au NRs or harness the electrostatic interactions between CTAB and DNA.216 One approach is to control the electrostatic interactions between DNA and positively charged surfaces.16,195,219 The second approach is through packing the NRs with a thin film of silica. The outer silica layers were modified with DNA functionalized by amine or thiol. The third approach is using the ligand exchange process to decorate Au NRs with ss-DNA.216,220
CTAB-coated Au NRs were able to withstand high salt concentrations even without decorations and deposits.221,222 He et al. described the use of un-modified CTAB-coated Au NRs for colorimetric assay. In this approach, it is not necessary to remove the excess CTAB from the solution by centrifugation.195 It only needs the addition of target DNA to the mixture of the Au NRs without any modification and the label-free probe DNA in certain buffer salt solutions. The SPR absorption band will red-shift in a high ionic strength buffer after mixing the NR probe and target ssDNA, indicating the aggregation of Au NRs. This phenomenon is ascribed to the formation of dsDNA from hybridization between the target DNA and probe DNA. In contrast, the addition of noncomplementary targets will not cause any spectral changes.16,195 This protocol only experiences one step and is very simple for detecting hybridization. Moreover, it is easy to detect single-base-pair mismatches without temperature control, providing promising applications in the detection of single-nucleotide polymorphisms (SNPs) and disease diagnosis.195,223,224 Although Au NRs have unique advantages in biological application, few studies applied Au NRs as orientation probes. The reason may come from the following reasons. First, there is a lack of good preparation and processing methods to obtain the desired size. Broader functionalities and applications can be achieved by tuning the longitudinal plasmon band of Au NRs.225 Second, there is no suitable imaging technique to decipher its three dimensions.
Au NRs have shown potential in photothermal cancer therapy and optoelectronic technology.134,226,227 In 2004, O'Neill et al. demonstrated that Au NRs with SPR absorption in the near-infrared (NIR) region can target tumors in vivo. After excitation with a NIR laser, Au NRs released heat into the tumor environment, resulting in the rupture of the tumor cell membrane.228 After that, Huang et al. reported that Au NRs with a low aspect ratio can be used as a simultaneous imaging and therapeutic agent to promote tumor cell recognition and photothermal removal in vitro due to their strong scattering and absorption properties in NIR spectroscopy.229 The demonstration greatly increases the interest in the treatment and diagnosis of certain cancers using Au NRs. Compared with traditional chemotherapy, photothermal therapy may become an effective and specific cancer treatment option. Ideally, cell death can only be induced in the region where Au NRs are excited by laser with appropriate wavelengths.229–231 Photothermal therapy has attracted tremendous attention in killing cancer cells, making it a promising biomedical candidate for the treatment of cancer. In recent years, using assembled Au NRs for the photothermal killing of bacteria has also shown promising prospects.232
6. Conclusions and outlook
6.1 Conclusions
In this study, we focused on the properties and preparation of Au NCs with different morphologies as well as their important applications in biological detection. The ability to carefully tailor the physical properties of Au NCs such as sizes, shapes, and composition is essential for their biomedical applications. As a face-centered cubic (fcc) metal, Au NCs can take a variety of geometrical shapes. In realistic chemical synthesis, particularly in the solution phase, many critical parameters, which can be divided into thermodynamic and kinetic parameters are responsible for the final morphologies of Au NCs. The seed-mediated growth method is the widely reported method, both the crystallinity of seeds and the growth rates of different crystallographic facets play a vital role in determining the final shape of a resultant nanostructure.The colorimetric assay method has become an attractive choice for biological detection because it does not require the use of advanced equipment and is easy to operate in many applications. Nanogold probes including citrate-coated Au NPs and CTAB-coated Au NRs are being used for biological detection, such as oligonucleotides, proteins, and enzymes. Citrate-protected Au NPs can be easily decorated via thiolate chemistry and their optical properties depend on their size. In addition to Au NPs, Au NRs also have been used in biological fields. Since the Au NR surface has a positive charge, it can connect with the negatively charged un-modified ssDNA and dsDNA through electrostatic interactions. There are some disadvantages while using Au NCs in the colorimetric assay method. For example, it requires complex experimental procedures and cannot monitor the hybridization process real-time. In addition, it could not be achieved for absolute quantitative analysis. Therefore, it is important to find a simple, real-time, and quantification system.
6.2 Perspective and challenge
The chemical method can generate Au NCs at a low cost and provide repeatable results using various chemicals. Gold NPs are considered an important research field due to their unique and tunable SPR and their application in biomedical science, including drug delivery, tissue/tumor imaging, photothermal therapy, and immunochromatography identification of pathogens in clinical specimens. The synthesis of Au NCs with such extended medical applications should be free from toxic chemicals used during the synthesis process. It is promising to develop a green, effective, simple, air-stable, and cost-effective technique for the synthesis of Au NCs. The tendency of the presented approach is: (i) without the usage of a surfactant, capping agent, or template; (ii) the selection of an environmentally acceptable solvent with the use of eco-friendly reducing and stabilizing agents, for example, replacing toxic chemicals with extracts from living organisms for the synthesis of Au NCs; (iii) excellent yield of the products; (iv) simple maintenance and reuse of the Au NCs. For Au NCs synthesized using chemical methods, functionalizing the surface with more peculiar ligands to regulate and detoxify, which is caused by the toxic capping agents, is encouraged. These are the few future aspects for the generation of NCs via green synthesis and many more are yet to be explored by researchers.As for the application, gold nanoprobes are facing many challenges, such as selectivity and sensitivity decrease, reduction of the catalytic activity, and potential biohazard, in the practical application. To address these challenges, the existing detection technology should be further improved, and new functional nanoprobes should be developed on the basis of practical problems. In addition, more sensitive and easier methods of analysis should be designed. Along with further research, it is our ultimate goal to achieve automatic and intelligent sample testing. While using Au NRs as probes, developing a suitable imaging technique to decipher its three dimensions is expected. We hope that our research efforts in this review will contribute to a better understanding of the synthesis, optical properties, shape tuning, and applications of Au NCs.
Author contributions
Meng Li conceived the study and collected the literature. All authors were involved in writing and revising the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the projects from Shandong Top Talent Special Foundation; National Key Research and Development Program of China (No. 2022YFE0105800); The Introduction and Cultivation Plan for Young Innovative Talents of Colleges and Universities by the Education Department of Shandong Province; Nanxun Collaborative Innovation Center Key Research Project (No. JZ2022ZH01); Doctoral research fund project in Shandong Jianzhu University (X22033Z); the Open-ended Fund of Key Laboratory of Urban Pollutant Conversion, Chinese Academy of Sciences (KLUPC KF-2020-4).References
- M. Liu and P. Guyot-Sionnest, J. Phys. Chem. B, 2005, 109, 22192–22200 CrossRef CAS PubMed.
- D. Yin, X. Li, Y. Ma and Z. Liu, Chem. Commun., 2017, 53, 6716–6719 RSC.
- E. Yeo, U. J. Cheah, D. Neo, W. I. Goh, P. Kanchanawong, K. C. Soo, P. S. Thong and J. Kah, J. Mater. Chem. B, 2017, 5, 254–268 RSC.
- S. Menon, R S. and VK S., Resource-Efficient Technologies, 2017, 3, 516–527 CrossRef.
- X. Liu, Q. Dai, L. Austin, J. Coutts, G. Knowles, J. Zou, H. Chen and Q. Huo, J. Am. Chem. Soc., 2008, 130, 2780–2782 CrossRef CAS PubMed.
- S. Gupta, S. Huda, P. K. Kilpatrick and O. D. Velev, Anal. Chem., 2007, 79, 3810–3820 CrossRef CAS PubMed.
- Q. L. Wang, H. F. Cui, C. L. Li, X. Song, Q. Y. Lv and Z. Y. Li, Sensors and Actuators Reports, 2020, 2, 100021 CrossRef.
- Z. Yang, A. S. Malinick, T. Yang, W. Cheng and Q. Cheng, Sensors and Actuators Reports, 2020, 2, 100023 CrossRef.
- P. Baptista, E. Pereira, P. Eaton, G. Doria, A. Miranda, I. Gomes, P. Quaresma and R. Franco, Anal. Bioanal. Chem., 2008, 391, 943–950 CrossRef CAS PubMed.
- X. Huang, P. K. Jain, I. H. El-Sayed and M. A. El-Sayed, Lasers in Medical Science, 2008, 23, 217–228 CrossRef PubMed.
- S. Lal, S. E. Clare and N. J. Halas, Acc. Chem. Res., 2008, 41, 1842–1851 CrossRef CAS PubMed.
- W. Il Choi, J. Kim, C. Kang, C. C. Byeon, Y. H. Kim and G. Tae, ACS Nano, 2011, 5, 1995–2003 CrossRef PubMed.
- S. Siddique and J. C. L. Chow, Gold Nanoparticles for Drug Delivery and Cancer Therapy, Appl. Sci., 2020, 10, 3824 CrossRef CAS.
- R. A. Barmin, P. G. Rudakovskaya, O. I. Gusliakova, O. A. Sindeeva, E. S. Prikhozhdenko, E. A. Maksimova, E. N. Obukhova, V. S. Chernyshev, B. N. Khlebtsov and A. A. Solovev, Nanomaterials, 2021, 11, 415 CrossRef CAS PubMed.
- N. G. Khlebtsov and L. A. Dykman, J. Quant. Spectrosc. Radiat. Transfer, 2010, 111, 1–35 CrossRef CAS.
- L. Dykman and N. Khlebtsov, Chem. Soc. Rev., 2012, 41, 2256–2282 RSC.
- F. Farzaneh, M. R. Rashidi and O. Yadollah, Talanta, 2018, 192, 118–127 Search PubMed.
- T. Xue, W. Liang, Y. Li, Y. Sun, Y. Xiang, Y. Zhang, Z. Dai, Y. Duo, L. Wu, K. Qi, B. N. Shivananju, L. Zhang, X. Cui, H. Zhang and Q. Bao, Nat. Commun., 2019, 10, 28 CrossRef CAS PubMed.
- A. Graczyk, R. Pawlowska, D. Jedrzejczyk and A. Chworos, Molecules, 2020, 25, 204 CrossRef CAS PubMed.
- V. Sharma, K. Park and M. Srinivasarao, Mater. Sci. Eng., R, 2009, 65, 1–38 CrossRef.
- Z. Yang, Z. Li, X. Lu, F. He, X. Zhu, Y. Ma, R. He, F. Gao, W. Ni and Y. Yi, Nano-Micro Lett., 2017, 9, 5 CrossRef PubMed.
- M. Yang, Y. Liu, W. Hou, X. Zhi, C. Zhang, X. Jiang, F. Pan, Y. Yang, J. Ni and D. Cui, Nanoscale, 2017, 9, 334–340 RSC.
- B. Nikoobakht and M. A. El-Sayed, Chem. Mater., 2003, 15, 1957–1962 CrossRef CAS.
- S. E. Lohse and C. J. Murphy, Chem. Mater., 2013, 25, 1250–1261 CrossRef CAS.
- D. Seo, C. I. Yoo, I. S. Chung, S. M. Park, S. Ryu and H. Song, J. Phys. Chem. C, 2008, 112, 2469–2475 CrossRef CAS.
- W. Niu and G. Xu, Nano Today, 2011, 6, 265–285 CrossRef CAS.
- H. E. Lee, K. D. Yang, S. M. Yoon, H. Y. Ahn and K. T. Nam, ACS Nano, 2015, 9, 8384–8393 CrossRef CAS PubMed.
- A. Henglein and D. Meisel, Langmuir, 1998, 14, 7392–7396 CrossRef CAS.
- Y. Zheng, X. Zhong, Z. Li and Y. Xia, Part. Part. Syst. Charact., 2013, 31, 266–273 CrossRef.
- P. Suchomel, L. Kvitek, R. Prucek, A. Panacek and R. Zboril, Sci. Rep., 2018, 8, 4589 CrossRef PubMed.
- X. Ye, L. Jin, H. Caglayan, J. Chen, G. Xing, C. Zheng, V. Doan-Nguyen, Y. Kang, N. Engheta and C. R. Kagan, ACS Nano, 2012, 6, 2804–2817 CrossRef CAS PubMed.
- M. Censabella, M. G. Grimaldi and F. Ruffino, Mater. Charact., 2019, 147, 101–115 CrossRef CAS.
- M. Brown and B. J. Wiley, Chem. Mater., 2020, 32, 6410–6415 CrossRef CAS.
- A. McLean, M. Kanetidis, T. Gogineni, R. Ukani, R. McLean, A. Cooke, I. Avinor, B. Liu, P. Argyrakis, W. Qian and R. Kopelman, Chem. Mater., 2021, 33, 2913–2928 CrossRef CAS.
- L. Z. Wang, J. Phys. Chem. B, 2012, 104, 1153–1175 CrossRef.
- M. Yaseen, M. Humayun, A. Khan, M. Usman, H. Ullah, A. A. Tahir and H. Ullah, Energies, 2021, 14, 1–88 Search PubMed.
- A. Sani, C. Cao and D. Cui, Biochem. Biophys. Rep., 2021, 26, 100991 CAS.
- N. Sarfraz and I. Khan, Chem.–Asian J., 2021, 16, 720–742 CrossRef CAS PubMed.
- X. Y. Liu, J. Q. Wang, C. R. Ashby Jr, L. Zeng, Y. F. Fan and Z. S. Chen, Drug Discovery Today, 2021, 26, 1284–1292 CrossRef CAS PubMed.
- M. H. Mohd-Zahid, R. Mohamud, C. A. C. Abdullah, J. Lim, H. Alem, W. N. W. Hanaffi and I. Z. Alias, RSC Adv., 2020, 10, 973–985 RSC.
- K. Nejati, M. Dadashpour, T. Gharibi, H. Mellatyar and A. Akbarzadeh, J. Cluster Sci., 2022, 33, 1–16 CrossRef CAS.
- L. Tessaro, A. Aquino, A. P. A. de Carvalho and C. A. Conte-Junior, Sensors and Actuators Reports, 2021, 3, 100060 CrossRef.
- T. Ahmad, J. Iqbal, M. A. Bustam, M. Irfan and H. M. Anwaar Asghar, J. Cleaner Prod., 2021, 309, 127460 CrossRef CAS.
- R. Herizchi, E. Abbasi, M. Milani and A. Akbarzadeh, Artif. Cells, Nanomed., Biotechnol., 2016, 44, 596–602 CrossRef CAS PubMed.
- N. Elahi, M. Kamali and M. H. Baghersad, Talanta, 2018, 184, 537–556 CrossRef CAS PubMed.
- I. Kherbouche, Y. Luo, N. Félidj and C. Mangeney, Chem. Mater., 2020, 32, 5442–5454 CrossRef CAS.
- M. T. Yaraki and Y. N. Tan, Chem.–Asian J., 2020, 15, 3180–3208 CrossRef CAS PubMed.
- C. J. Murphy, A. M. Gole, S. E. Hunyadi, J. W. Stone, P. N. Sisco, A. Alkilany, B. E. Kinard and P. Hankins, Chem. Commun., 2008, 18, 544–557 RSC.
- D. Philip, Spectrochim. Acta, Part A, 2008, 71, 80–85 CrossRef PubMed.
- K. A. Willets and R. P. Van Duyne, Annu. Rev. Phys. Chem., 2007, 58, 267–297 CrossRef CAS PubMed.
- S. Xu, X. Dong, S. Chen, Y. Zhao, G. Shan, Y. Sun, Y. Chen and Y. Liu, Sens. Actuators, B, 2019, 281, 375–382 CrossRef CAS.
- R. Rajendra, P. K. Gangadharan, S. Tripathi, S. Kurungot and N. Ballav, Nanoscale, 2016, 8, 19224–19228 RSC.
- J. Zhang, C. Xi, C. Feng, H. Xia, D. Wang and X. Tao, Langmuir, 2014, 30, 2480–2489 CrossRef CAS PubMed.
- G. Mettela, R. Boya, D. Singh, G. Kumar and G. U. Kulkarni, Sci. Rep., 2013, 3, 1793 CrossRef.
- L. Karuppasamy, C. Y. Chen, S. Anandan and J. J. Wu, Electrochim. Acta, 2017, 246, 75–88 CrossRef CAS.
- L. Chen, F. Ji, Y. Xu, L. He, Y. Mi, F. Bao, B. Sun, X. Zhang and Q. Zhang, Nano Lett., 2014, 14, 7201–7206 CrossRef CAS PubMed.
- J. Zhang, M. R. Langille, M. L. Personick, K. Zhang, S. Li and C. A. Mirkin, J. Am. Chem. Soc., 2010, 132, 14012–14014 CrossRef CAS PubMed.
- M. L. Personick, M. R. Langille, J. Wu and C. A. Mirkin, J. Am. Chem. Soc., 2013, 135, 3800–3803 CrossRef CAS PubMed.
- W. Niu, S. Zheng, D. Wang, X. Liu, H. Li, S. Han, J. Chen, Z. Tang and G. Xu, J. Am. Chem. Soc., 2009, 131, 697–703 CrossRef CAS PubMed.
- C. Kai, S. R. Kothapalli, H. Liu, L. K. Ai, J. V. Jokerst, J. Han, Y. Meng, J. Li, J. Levi and J. C. Wu, J. Am. Chem. Soc., 2014, 136, 3560–3571 CrossRef PubMed.
- D. Dam, R. C. Lee and T. W. Odom, Nano Lett., 2014, 14, 2843–2848 CrossRef CAS PubMed.
- C. Yan, L. Cui, Q. Yang, X. Zhou, L. Pan, X. Zhang, H. Yang, Z. Zhou and S. Yang, J. Mater. Chem. B, 2017, 5, 8761–8769 RSC.
- T. K. Sau and C. J. Murphy, J. Am. Chem. Soc., 2004, 126, 8648–8649 CrossRef CAS PubMed.
- R. Jin, S. Egusa and N. F. Scherer, Thermally-Induced Formation of Atomic Au Clusters and Conversion into Nanocubes, J. Am. Chem. Soc., 2004, 126(32), 9900–9901 CrossRef CAS PubMed.
- Y. Huang, W. Wang, H. Liang and H. Xu, Cryst. Growth Des., 2009, 9, 858–862 CrossRef CAS.
- Y. Yu, Q. Zhang, X. Lu and J. Y. Lee, J. Phys. Chem. C, 2010, 114, 11119–11126 CrossRef CAS.
- A. Sanchez-Iglesias, I. Pastoriza-Santos, J. Perez-Juste, B. Rodriguez-Gonzalez, F. Abajo and L. M. Liz-Marzan, Adv. Mater., 2006, 18, 2529–2534 CrossRef CAS.
- J. Fuentes-García, J. Santoyo-Salzar, E. Rangel-Cortes, G. Goya, V. Cardozo-Mata and J. Pescador-Rojas, Ultrason. Sonochem., 2020, 70, 105274 CrossRef PubMed.
- T. K. Sau and C. J. Murphy, Langmuir, 2004, 20, 6414–6420 CrossRef CAS PubMed.
- H. A. Keul, M. Moeller and M. R. Bockstaller, J. Phys. Chem. C, 2008, 112, 13483–13487 CrossRef CAS.
- S. Chen, Z. L. Wang, J. Ballato, S. H. Foulger and D. L. Carroll, J. Am. Chem. Soc., 2004, 125, 16186–16187 CrossRef PubMed.
- H. L. Wu, C. H. Chen and M. H. Huang, Chem. Mater., 2009, 21, 110–114 CrossRef CAS.
- T. Wen, Z. Hu, W. Liu, H. Zhang, S. Hou, X. Hu and X. Wu, Langmuir, 2012, 28, 17517–17523 CrossRef CAS PubMed.
- X. Xia, M. Yang, Y. Wang, Y. Zheng, Q. Li, J. Chen and Y. Xia, ACS Nano, 2012, 6, 512–522 CrossRef CAS PubMed.
- Y. Wang, K. Sentosun, A. Li, M. Coronado-Puchau, A. Sánchez-Iglesias, S. Li, X. Su, S. Bals and L. M. Liz-Marzán, Chem. Mater., 2015, 27, 8032–8040 CrossRef CAS.
- J. Polte, T. T. Ahner, F. Delissen, S. Sokolov, F. Emmerling, A. F. Thunemann and R. Kraehnert, J. Am. Chem. Soc., 2010, 132, 1296–1301 CrossRef CAS PubMed.
- R. Shenhar, T. B. Norsten and V. M. Rotello, Adv. Mater., 2005, 17, 657–669 CrossRef CAS.
- F. Kim, J. H. Song and P. Yang, J. Am. Chem. Soc., 2002, 124, 14316–14317 CrossRef CAS PubMed.
- K. Okitsu, A. Yue, S. Tanabe, H. Matsumoto and Y. Yobiko, Langmuir, 2001, 17, 7717–7720 CrossRef CAS.
- J. P. Sylvestre, S. Poulin, A. V. Kabashin, E. Sacher, M. Meunier and J. Luong, J. Phys. Chem. B, 2004, 108, 16864–16869 CrossRef CAS.
- P. Prema, P. A. Iniya and G. Immanuel, RSC Adv., 2016, 6, 4601–4607 RSC.
- M. Sengani, A. M. Grumezescu and V. D. Rajeswari, OpenNano, 2017, 2, 37–46 CrossRef.
- S. Mandal, S. Phadtare and M. Sastry, Curr. Appl. Phys., 2005, 5, 118–127 CrossRef.
- X. Huang, S. Li, Y. Huang, S. Wu, X. Zhou, S. Li, C. L. Gan, F. Boey, C. Mirkin and H. Zhang, Nat. Commun., 2011, 2, 292 CrossRef PubMed.
- Y. Cheng, J. Tao, G. Zhu, J. A. Soltis, B. A. Legg, N. Elias, Y. De, M. L. Sushko and J. Liu, Nanoscale, 2018, 10, 11907–11912 RSC.
- Y. Shimasaki, M. Kitahara, M. Shoji, A. Shimojima, H. Wada and K. Kuroda, Chem.–Asian J., 2018, 13, 3935–3941 CrossRef CAS PubMed.
- C. J. Murphy, A. M. Gole, S. E. Hunyadi and C. J. Orendorff, Inorg. Chem., 2006, 45, 7544–7554 CrossRef CAS PubMed.
- J. Tang, Q. Ou, H. Zhou, L. Qi and S. Man, Nanomaterials, 2019, 9, 185 CrossRef CAS PubMed.
- Y. Xiong and Y. Xia, Adv. Mater., 2007, 19, 3385–3391 CrossRef CAS.
- S. G. Kwon, G. Krylova, P. J. Phillips, R. F. Klie, S. Chattopadhyay, T. Shibata, E. E. Bunel, Y. Liu, V. B. Prakapenka and B. Lee, Nat. Mater., 2015, 14, 215–223 CrossRef CAS PubMed.
- S. E. Habas, H. Lee, V. Radmilovic, G. A. Somorjai and P. Yang, Nat. Mater., 2007, 6, 692–697 CrossRef CAS PubMed.
- M. Grzelczak, J. Pérez-Juste, P. Mulvaney and L. Liz-Marzán, Chem. Soc. Rev., 2008, 37, 1783–1791 RSC.
- W. Leng, P. Pati and P. J. Vikesland, Environ. Sci.: Nano, 2015, 2, 440–453 RSC.
- Z. Wei and C. J. Liu, Mater. Lett., 2011, 65, 353–355 CrossRef CAS.
- A. Dzimitrowicz, J. Piotr, G. Krzysztof, N. Piotr and P. Pohl, J. Nanopart. Res., 2015, 17, 185 CrossRef PubMed.
- N. Saito, J. Hieda and O. Takai, Thin Solid Films, 2009, 518, 912–917 CrossRef CAS.
- K. M. Koczkur, S. Mourdikoudis, L. Polavarapu and S. E. Skrabalak, Dalton Trans., 2015, 44, 17883–17905 RSC.
- S. E. Skrabalak, L. Au, X. Li and Y. Xia, Nat. Protoc., 2007, 2, 2182–2190 CrossRef CAS PubMed.
- H. Wang, Z. Shan, K. D. Gilroy, Z. Cai and Y. Xia, Nano Today, 2017, 15, 121–144 CrossRef CAS.
- R. S. Das, B. Singh, S. Mukhopadhyay and R. Banerjee, Dalton Trans., 2012, 41, 4641–4648 RSC.
- J. Olesiak-Banska, M. Gordel, R. Kolkowski, K. Matczyszyn and M. Samoc, J. Phys. Chem. C, 2012, 116, 13731–13737 CrossRef CAS.
- N. Wangoo, K. K. Bhasin, R. Boro and C. R. Suri, Anal. Chim. Acta, 2008, 610, 142–148 CrossRef CAS PubMed.
- S. Yong, Y. Jin and S. Dong, Chem. Commun., 2004, 10, 1104–1105 Search PubMed.
- U. M. Badeggi, E. Ismail, A. O. Adeloye, S. Botha, J. A. Badmus, J. L. Marnewick, C. N. Cupido and A. A. Hussein, Biomolecules, 2020, 10, 452 CrossRef CAS PubMed.
- A. Ahmeda, A. Zangeneh and M. M. Zangeneh, Appl. Organomet. Chem., 2020, 34, e5290 CAS.
- P. Boomi, G. P. Poorani, S. Selvam, S. Palanisamy, S. Jegatheeswaran, K. Anand, C. Balakumar, K. Premkumar and H. G. Prabu, Appl. Organomet. Chem., 2020, 34, e5574 CrossRef CAS.
- S. Ahmad Khan, S. Shahid and C. S. Lee, Biomolecules, 2020, 10, 835 CrossRef PubMed.
- P. Ghosh, G. Han, M. De, C. K. Kim and V. M. Rotello, Adv. Drug Delivery Rev., 2008, 60, 1307–1315 CrossRef CAS PubMed.
- D. Cabuzu, A. Cirja, R. Puiu and A. Mihai Grumezescu, Curr. Top. Med. Chem., 2015, 15, 1605–1613 CrossRef CAS PubMed.
- T. Riddin, M. Gericke and C. Whiteley, Nanotechnology, 2006, 17, 3482 CrossRef CAS PubMed.
- A. L. Ginzburg, L. Truong, R. L. Tanguay and J. E. Hutchison, ACS Nano, 2018, 12, 5312–5322 CrossRef CAS PubMed.
- A. Sasidharan and N. Monteiro-Riviere, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2015, 7, 779–796 CAS.
- M. J. Firdhouse and P. Lalitha, Inorg. Chem. Commun., 2022, 143, 109800 CrossRef.
- P. Quaresma, L. Soares, L. Contar, A. Miranda, I. Osório, P. A. Carvalho, R. Franco and E. Pereira, Green Chem., 2009, 11, 1889–1893 RSC.
- I. Bibi, N. Nazar, M. Iqbal, S. Kamal, H. Nawaz, S. Nourene, Y. Safa, K. Jilani, M. Sultan, S. Ata, F. Rehman and M. Abbas, Adv. Powder Technol., 2017, 28, 2035–2043 CrossRef CAS.
- N. Abdel-Raouf, N. M. Al-Enazi and I. B. M. Ibraheem, Arabian J. Chem., 2017, 10, S3029–S3039 CrossRef CAS.
- B. Khodadadi, M. Bordbar, A. Yeganeh-Faal and M. Nasrollahzadeh, J. Alloys Compd., 2017, 719, 82–88 CrossRef CAS.
- H. Veisi, M. Farokhia, M. Hamelian and S. Hemmati, RSC Adv., 2018, 8, 38186–38195 RSC.
- P. Zhao, A. El-kott, A. E. Ahmed, A. Khames and M. A. Zein, Inorg. Chem. Commun., 2021, 131, 108781 CrossRef CAS.
- T. Ahmad, M. A. Bustam, M. Irfan, M. Moniruzzaman, H. M. A. Asghar and S. Bhattacharjee, Mater. Chem. Phys., 2018, 220, 240–248 CrossRef CAS.
- C. Tamuly, M. Hazarika, S. C. Borah, M. R. Das and M. P. Boruah, Colloids Surf., B, 2013, 102, 627–634 CrossRef CAS PubMed.
- V. Kumar and S. K. Yadav, J. Chem. Technol. Biotechnol., 2009, 84, 151–157 CrossRef CAS.
- K. Cheirmadurai, S. Biswas, R. Muralia and P. Thanikaivelan, RSC Adv., 2014, 4, 19507–19511 RSC.
- B. Khodadadi, M. Bordbar and M. Nasrollahzadeh, J. Colloid Interface Sci., 2017, 490, 1–10 CrossRef CAS PubMed.
- S. Ali, M. Iqbal, A. Naseer, M. Yaseen, I. Bibi, A. Nazir, M. I. Khan, N. Tamam, N. Alwadai, M. Rizwan and M. Abbasf, Environ. Nanotechnol., Monit. Manage., 2021, 16, 100511 CAS.
- K. B. Narayanan and N. Sakthivel, Adv. Colloid Interface Sci., 2010, 156, 1–13 CrossRef CAS PubMed.
- M. Teimouri, F. Khosravi-Nejad, F. Attar, A. A. Saboury, I. Kostova, G. Benelli and M. Falahati, J. Cleaner Prod., 2018, 184, 740–753 CrossRef CAS.
- L. Gou and C. J. Murphy, Chem. Mater., 2015, 17, 3668–3672 CrossRef.
- C. D. Dios, Y. F. Hua, F. García, A. Cebollada and G. Armelles, Plasmonics, 2018, 13, 1–6 CrossRef.
- Y. Y. Yu, S. S. Chang, C. L. Lee and C. Wang, J. Phys. Chem. B, 1997, 101, 6661–6664 CrossRef CAS.
- A. Gole and C. J. Murphy, Chem. Mater., 2004, 16, 3633–3640 CrossRef CAS.
- N. R. Jana, Chem. Commun., 2003, 15, 1950–1951 RSC.
- C. J. Orendorff and C. J. Murphy, J. Phys. Chem. B, 2006, 110, 3990–3994 CrossRef CAS PubMed.
- M. Ali, B. Snyder and M. A. El-Sayed, Langmuir, 2012, 28, 9807–9815 CrossRef CAS PubMed.
- S. Si, C. Leduc, M. H. Delville and B. Lounis, ChemPhysChem, 2012, 13, 193–202 CrossRef CAS PubMed.
- J. Gao, C. M. Bender and C. J. Murphy, Langmuir, 2003, 19, 9065–9070 CrossRef CAS.
- B. Nikoobakht and M. A. El-Sayed, Langmuir, 2001, 17, 6368–6374 CrossRef CAS.
- S. Y. Lee, S. H. Jin, S. M. Kim and J. W. Kim, Met. Mater. Int., 2014, 20, 695–699 CrossRef CAS.
- N. R. Jana, L. Gearheart and C. J. Murphy, Adv. Mater., 2001, 13, 1389–1393 CrossRef CAS.
- A. P. Alivisatos, J. Phys. Chem., 1996, 100, 13226–13239 CrossRef CAS.
- M. Liu, A. Kira and H. Nakahara, Langmuir, 2003, 13, 4807–4809 CrossRef.
- T. H. Ha, H. J. Koo and B. H. Chung, J. Phys. Chem. C, 2007, 111, 1123–1130 CrossRef CAS.
- C. J. Murphy, T. K. Sau, A. M. Gole, C. J. Orendorff, G. Jinxin, G. Linfeng, S. E. Hunyadi and L. Tan, J. Phys. Chem. B, 2005, 109, 13857–13870 CrossRef CAS PubMed.
- B. D. Busbee, S. O. Obare and C. J. Murphy, Adv. Mater., 2003, 15, 414–416 CrossRef CAS.
- C. Wang, T. Wang, Z. Ma and Z. Su, Nanotechnology, 2005, 16, 2555 CrossRef CAS.
- F. Kim, K. Sohn, J. Wu and J. Huang, J. Am. Chem. Soc., 2008, 130, 14442 CrossRef CAS PubMed.
- M. Grzelczak, A. Sanchez-Iglesias, B. Rodriguez-Gonzalez, R. Alvarez-Puebla, J. Perez-Juste and L. M. Liz-Marzan, Adv. Funct. Mater., 2008, 18, 3780–3786 CrossRef CAS.
- J. E. Millstone, W. Wei, M. R. Jones, H. Yoo and C. A. Mirkin, Nano Lett., 2008, 8, 2526–2529 CrossRef CAS PubMed.
- Z. R. Guo, C. R. Gu, X. Fan, Z. P. Bian, H. F. Wu, D. Yang, N. Gu and J. N. Zhang, Nanoscale Res. Lett., 2009, 4, 1428–1433 CrossRef CAS PubMed.
- J. Zhu, K. T. Yong, I. Roy, R. Hu, H. Ding, L. Zhao, M. T. Swihart, G. S. He, Y. Cui and P. N. Prasad, Nanotechnology, 2010, 21, 285106 CrossRef PubMed.
- L. Liu, Z. Guo, L. Xu, R. Xu and L. Xiang, Nanoscale Res. Lett., 2011, 6, 143 CrossRef PubMed.
- X. Kou, S. Zhang, C. K. Tsung, Z. Yang, M. H. Yeung, G. Stucky, L. Sun, J. Wang and C. Yan, Chem.–Eur. J., 2010, 13, 2929–2936 CrossRef PubMed.
- W. Ahmed, A. S. Bhatti and J. V. Ruitenbeek, J. Nanopart. Res., 2017, 19, 115 CrossRef PubMed.
- S. Wang, N. Kristian, S. Jiang and W. Xin, Electrochem. Commun., 2008, 10, 961–964 CrossRef CAS.
- J. E. Millstone, S. Park, K. L. Shuford, L. Qin, G. C. Schatz and C. A. Mirkin, J. Am. Chem. Soc., 2005, 127, 5312–5313 CrossRef CAS PubMed.
- L. B. Rogers, D. P. Krause, J. C. Griess and D. B. Ehrlinger, J. Electrochem. Soc., 1949, 95, 33 CrossRef CAS.
- Z. Yang, Z. Li, X. Lu, F. He, X. Zhu, Y. Ma, R. He, F. Gao, W. Ni and Y. Yi, Nano-Micro Lett., 2017, 9, 5 CrossRef PubMed.
- M. Z. Liu and P. Guyot-Sionnest, J. Phys. Chem. B, 2004, 108, 5882–5888 CrossRef CAS.
- Z. Zhang, H. Li, F. Zhang, Y. Wu, Z. Guo, L. Zhou and J. Li, Langmuir, 2014, 30, 2648–2659 CrossRef CAS PubMed.
- G. Weng, X. Dong, J. Li and J. Zhao, J. Mater. Sci., 2016, 51, 7678–7690 CrossRef CAS.
- Z. Chen, T. Balankura, K. A. Fichthorn and R. M. Rioux, ACS Nano, 2019, 13, 1849–1860 CAS.
- J. H. Lee, K. J. Gibson, C. Gang and Y. Weizmann, Nat. Commun., 2015, 6, 7571 CrossRef CAS PubMed.
- M. J. Schnepf, M. Mayer, C. Kuttner, M. Tebbe, D. Wolf, M. Dulle, T. Altantzis, P. Formanek, S. Förster and S. Bals, Nanoscale, 2017, 9, 9376–9385 RSC.
- A. H. Poghosyan, A. A. Shahinyan and J. Koetz, Colloid Polym. Sci., 2018, 296, 1301–1306 CrossRef CAS.
- A. H. Poghosyan, A. A. Shahinyan and J. Koetz, Colloid Polym. Sci., 2018, 296, 1301–1306 CrossRef CAS.
- C. Song, F. Li, W. Chen, X. Guo, D. Chen, J. Zhang, J. Zhang and L. H. Wang, J. Mater. Chem. B, 2019, 7, 2001–2008 RSC.
- P. Li, Y. Wu, D. Li, X. Su, C. Luo, Y. Wang, J. Hu, G. Li, H. Jiang and W. Zhang, Nanoscale Res. Lett., 2018, 13, 313 CrossRef PubMed.
- S. Bandyopadhyay, B. H. McDonagh, G. Singh, K. Raghunathan, A. Sandvig, I. Sandvig, J. Andreassen and W. R. Glomm, Nanoscale Res. Lett., 2018, 13, 254 CrossRef PubMed.
- G. Q. Tao and J. Wang, Carbon, 2018, 133, 209–217 CrossRef CAS.
- R. Zhang, X. Zhang, L. I. Xiaoyu and P. Yang, Funct. Mater. Lett., 2013, 6, 1330003 CrossRef.
- P. Yang, J. Wang and L. Zhang, J. Cluster Sci., 2013, 24, 399–426 CrossRef CAS.
- V. Biju, T. Itoh, A. Anas, A. Sujith and M. Ishikawa, Anal. Bioanal. Chem., 2008, 391, 2469–2495 CrossRef CAS PubMed.
- Y. Xianyu, Y. Xie, N. Wang, Z. Wang and X. Jiang, Small, 2015, 11, 5510–5514 CrossRef CAS PubMed.
- P. M. Tiwari, K. Vig, V. A. Dennis and S. R. Singh, Nanomaterials, 2011, 1, 31–63 CrossRef CAS PubMed.
- K. Niikura, T. Matsunaga, T. Suzuki, S. Kobayashi, H. Yamaguchi, Y. Orba, A. Kawaguchi, H. Hasegawa, K. Kajino, T. Ninomiya, K. Ijiro and H. Sawa, ACS Nano, 2013, 7, 3926–3938 CrossRef CAS PubMed.
- N. L. Rosi and C. A. Mirkin, Chem. Rev., 2010, 36, 1547–1562 Search PubMed.
- C. L. Haynes and R. V. Duyne, J. Phys. Chem. B, 2001, 105, 5599–5611 CrossRef CAS.
- S. L. Diedenhofen, D. Kufer, T. Lasanta and G. Konstantatos, Light: Sci. Appl., 2015, 4, 234 CrossRef.
- Q. Zhang, Y. N. Tan, J. Xie and J. Y. Lee, Plasmonics, 2009, 4, 9–22 CrossRef CAS.
- R. Elghanian, J. J. Storhoff, R. C. Mucic, R. L. Letsinger and C. Mirkin, Science, 1997, 277, 1078–1081 CrossRef CAS PubMed.
- W. Sun, G. Wang, N. Fang and E. S. Yeung, Anal. Chem., 2009, 81, 9203 CrossRef CAS PubMed.
- E. Boisselier and D. Astruc, Chem. Soc. Rev., 2009, 38, 1759–1782 RSC.
- A. Wang, C. J. Wu and S. H. Chen, J. Proteome Res., 2006, 5, 1488–1492 CrossRef CAS PubMed.
- W. P. Faulk and G. M. Taylor, Immunochemistry, 1971, 8, 1081–1083 CrossRef CAS PubMed.
- J. H. W. Leuvering, P. J. H. M. Thal, M. v. d. Waart and A. H. W. M. Schuurs, Fresenius' Zeitschrift für analytische Chemie, 1980, 301, 132 CrossRef CAS.
- C. A. Mirkin, R. L. Letsinger, R. C. Mucic and J. J. Storhoff, Nature, 1996, 382, 607–609 CrossRef CAS PubMed.
- L. Di, Y. Yan, A. Wieckowska and I. Willner, Chem. Commun., 2007, 34, 3544–3546 Search PubMed.
- X. M. Li, P. Y. Fu, J. M. Liu and S. S. Zhang, Anal. Chim. Acta, 2010, 673, 133–138 CrossRef CAS PubMed.
- N. G. Khlebtsov, Quantum Electron., 2008, 38, 504–529 CrossRef CAS.
- W. R. Glomm, J. Dispersion Sci. Technol., 2005, 26, 389–414 CrossRef CAS.
- Z. Krpetic, P. Nativo, F. Porta and M. Brust, Bioconjugate Chem., 2009, 20, 619–624 CrossRef CAS.
- L. A. Dykman and V. A. Bogatyrev, Russ. Chem. Rev., 2010, 76, 181 CrossRef.
- P. Maity, S. Xie, M. Yamauchi and T. Tsukuda, Nanoscale, 2012, 4, 4027–4037 RSC.
- D. Zhu, D. Xing, X. Shen and G. Yan, Chin. Sci. Bull., 2003, 48, 1741–1744 CrossRef CAS.
- W. He, C. Z. Huang, Y. F. Li, J. P. Xie, R. G. Yang, P. F. Zhou and J. Wang, Anal. Chem., 2008, 80, 8424–8430 CrossRef CAS PubMed.
- H. Li and L. Rothberg, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 14036–14039 CrossRef CAS PubMed.
- Q. Dai, L. Xiong, J. Coutts, L. Austin and Q. Huo, J. Am. Chem. Soc., 2008, 130, 8138–8139 CrossRef CAS PubMed.
- D. A. Gell, R. P. Grant and J. P. Mackay, Adv. Exp. Med. Biol., 2012, 747, 19–41 CrossRef CAS PubMed.
- H. Li and L. Rothberg, Anal. Chem., 2005, 77, 6229–6233 CrossRef CAS PubMed.
- J. D. Watson and S. Devons, Phys. Today, 1968, 21, 71–72 CrossRef.
- E. Papadopoulou, N. Gale, J. F. Thompson, T. A. Fleming, T. Brownc and P. N. Bartlett, Chem. Sci., 2016, 7, 386–393 RSC.
- X. Lin, A. P. Ivanov and J. B. Edel, Chem. Sci., 2017, 8, 3905–3912 RSC.
- T. A. Taton, C. A. Mirkin and R. L. Letsinger, Science, 2000, 289, 1757–1760 CrossRef CAS PubMed.
- H. R. Sim, A. W. Wark and H. J. Lee, Analyst, 2010, 135, 2528–2532 RSC.
- C. Nietzold and F. Lisdat, Analyst, 2012, 137, 2821–2826 RSC.
- E. Yasun, B. Gulbakan, I. Ocsoy, Y. Quan and W. Tan, Anal. Chem., 2012, 84, 6008–6015 CrossRef CAS PubMed.
- Y. Wang, D. Li, W. Ren, Z. Liu and S. Dong, Chem. Commun., 2008, 22, 2520–2522 RSC.
- H. Jans, X. Liu, L. Austin, G. Maes and Q. Huo, Anal. Chem., 2009, 81, 9425–9432 CrossRef CAS PubMed.
- S. Link, M. B. Mohamed and M. A. El-Sayed, J. Phys. Chem. B, 1999, 103, 3073–3077 CrossRef CAS.
- M. Rex, F. E. Hernandez and A. D. Campiglia, Anal. Chem., 2006, 78, 445–451 CrossRef CAS PubMed.
- H. Nakashima, K. Furukawa, Y. Kashimura and K. Torimitsu, Chem. Commun., 2007, 10, 1080–1082 RSC.
- P. K. Sudeep, S. Joseph and K. G. Thomas, J. Am. Chem. Soc., 2005, 127, 6516–6517 CrossRef CAS PubMed.
- M. Li, J. Woongkang, S. Sukumar, R. Raodasari and I. Barman, Chem. Sci., 2015, 6, 3906–3914 RSC.
- X. Huang, I. H. El-Sayed, Q. Wei and M. A. El-Sayed, J. Am. Chem. Soc., 2006, 128, 2115–2120 CrossRef CAS PubMed.
- E. Dujardin, S. Mann, L. B. Hsin and C. Wang, Chem. Commun., 2001, 14, 1264–1265 RSC.
- I. C. Pekcevik, L. Poon, M. Wang and B. D. Gates, Anal. Chem., 2013, 85, 9960–9967 CrossRef CAS PubMed.
- S. J. Zhen, F. L. Guo, L. I. Yuanfang and C. Z. Huang, Sci. China: Chem., 2013, 56, 387–392 CrossRef CAS.
- H. Li and L. Rothberg, J. Am. Chem. Soc., 2004, 126, 10958–10961 CrossRef CAS PubMed.
- H. C. Huang, S. Barua, D. B. Kay and K. Rege, ACS Nano, 2010, 4, 1769–1770 CrossRef CAS.
- A. Wijaya, S. B. Schaffer, I. G. Pallares and K. Hamad-Schifferli, ACS Nano, 2009, 3, 80–86 CrossRef CAS PubMed.
- V. Baumann, P. Röttgermann, F. Haase, K. Szendrei, P. Dey, K. Lyons, R. Wyrwich, M. Gräßel, J. Stehr, L. Ullerich, F. Bürsgensd and J. Rodríguez-Fernández, RSC Adv., 2016, 6, 103724–103739 RSC.
- W. Ma, H. Kuang, L. Xu, L. Ding, C. Xu, L. Wang and N. A. Kotov, Nat. Commun., 2013, 4, 2689 CrossRef PubMed.
- D. Ho, J. A. Kretzmann, M. Norret, P. Toshniwal, J. P. Veder, H. Jiang, P. Guagliardo, A. M. Munshi, R. Chawla and C. W. Evans, Nat. Nanotechnol., 2018, 13, 1148–1153 CrossRef CAS PubMed.
- K. J. Lee, H. M. So, B. K. Kim, D. W. Kim and J. H. Jang, J. Nanomater., 2011, 2, 1–8 CrossRef PubMed.
- Y. Bao, L. Vigderman, E. R. Zubarev and C. Jiang, Langmuir, 2012, 28, 923–930 CrossRef CAS PubMed.
- S. W. Liu, L. Wang, M. Lin, Y. Liu, L. N. Zhang and H. Zhang, Chin. J. Polym. Sci., 2019, 37, 115–128 CrossRef CAS.
- S. Wang, H. Xu and J. Ye, Phys. Chem. Chem. Phys., 2014, 16, 12275–12281 RSC.
- D. P. O'Neal, L. R. Hirsch, N. J. Halas, J. D. Payne and J. L. Westb, Cancer Lett., 2004, 209, 171–176 CrossRef PubMed.
- X. Huang, I. H. El-Sayed, Q. Wei and M. A. El-Sayed, J. Am. Chem. Soc., 2006, 128, 2115–2120 CrossRef CAS PubMed.
- Y. Wang, X. Ji, P. Pang, Y. Shi, J. Dai, J. Xu, J. Wu, T. B. Kirk and W. Xue, J. Mater. Chem. B, 2018, 6, 2481–2488 RSC.
- W. Xu, J. Qian, G. Hou, Y. Wang, J. Wang, T. Sun, L. Ji, A. Suo and Y. Yao, Acta Biomater., 2049, 83, 400–413 CrossRef PubMed.
- T. Yang, D. Wang and X. Liu, Colloids Surf., B, 2019, 173, 833–841 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |




