Open Access Article
Open Access ArticleAdvances in functional guest materials for resistive gas sensors
Ze Wang
 a,
Lei Zhu
ab,
Jingzhao Wanga,
Rui Zhuangc,
Pengfei Muc,
Jianan Wang
a,
Lei Zhu
ab,
Jingzhao Wanga,
Rui Zhuangc,
Pengfei Muc,
Jianan Wang
 *a and
Wei Yan
*a and
Wei Yan
 a
a
aDepartment of Environmental Science and Engineering, Xi'an Key Laboratory of Solid Waste Recycling and Resource Recovery, State Key Laboratory of Multiphase Flow in Power Engineering, School of Energy and Power Engineering, Xi'an Jiaotong University, 28 Xianning West Road, Xi'an 710049, China. E-mail: wangjn116@xjtu.edu.cn
bSchool of Physics and Electrical Engineering, Weinan Normal University, Chaoyang Street, Weinan, 714099, China
cChambroad Chemical Industry Institute Co.,Ltd, Boxing Economic Development Zone, 256500, Shandong Province, China
First published on 30th August 2022
Abstract
Resistive gas sensors are considered promising candidates for gas detection, benefiting from their small size, ease of fabrication and operation convenience. The development history, performance index, device type and common host materials (metal oxide semiconductors, conductive polymers, carbon-based materials and transition metal dichalcogenides) of resistive gas sensors are firstly reviewed. This review systematically summarizes the functions, functional mechanisms, features and applications of seven kinds of guest materials (noble metals, metal heteroatoms, metal oxides, metal–organic frameworks, transition metal dichalcogenides, polymers, and multiple guest materials) used for the modification and optimization of the host materials. The introduction of guest materials enables synergistic effects and complementary advantages, introduces catalytic sites, constructs heterojunctions, promotes charge transfer, improves carrier transport, or introduces protective/sieving/enrichment layers, thereby effectively improving the sensitivity, selectivity and stability of the gas sensors. The perspectives and challenges regarding the host–guest hybrid materials-based gas sensors are also discussed.
1. Introduction
There are various types of gases existing in the human environment that affect the environmental quality and human safety, such as toxic, flammable, explosive, and indicator gases.1–3 High-performance gas detecting devices are needed to monitor environmental pollution, realize the early warnings about the dangerous gases, assist in the early diagnosis of disease, and aid in food industry production.4–7 Gas sensor technology is one of the most promising candidates for gas detection and has become a hotspot in monitoring the environment, safety, and health in recent years, benefiting from the advantages of their small size, ease of fabrication, low power consumption and easy operation.8–11To date, many types of gas sensors with different working mechanisms have been developed, e.g., catalytic combustion gas sensors,12 electrochemical gas sensors,13 infrared absorption gas sensors14 and resistive gas sensors.15–17 Among them, the resistive gas sensor exhibits attractive advantages, including higher sensitivity, faster response/recovery times, greater stability/repeatability, ease of operation and integration into portable devices.18–20 Based on these advantages, the resistive gas sensor has been widely studied and occupies a large market share.21 At present, resistive gas sensors with various types of metal oxide semiconductors (MOSs, e.g., SnO2,22 ZnO (ref. 8) and In2O3 (ref. 4)) as the host sensing component have been commercialized, and sensing materials like conductive polymers (CPs) and carbon-based materials have also been widely studied.23–25 However, just adopting single-component materials still faces some severe challenges. For example, MOSs suffer from high operating temperatures and poor selectivity; CPs suffer from poor stability and low sensitivity; carbon-based materials have the problems of poor reversibility.9,26 To date, numerous previously reported studies have used optimization methods like morphology modulation,27 UV irradiation,28 and low-temperature plasma treatment29 for better gas-sensing performance. Although these methods can improve the sensitivity of the material to a limited extent, it is difficult to change the characteristic defects of the materials themselves and the available sensing performance still cannot meet the requirements of practical application. A reliable solution for these drawbacks is the introduction and utilization of functional guest materials.
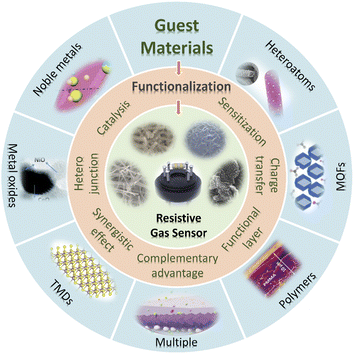
Guest materials are functional components that can optimize the gas sensing properties of the host materials. There are many ways for different types of guest materials to functionalize and optimize host materials. For example, noble metals can introduce catalytic sites and contribute to fast response, high sensitivity and low operating temperature via chemical/electronic sensitization.30 Metal oxides can construct heterojunctions with semiconductor host materials, thus changing the interface potential energy barriers as well as regulating the transfer of electrons and holes.31 Functional membranes (metal–organic frameworks (MOFs) and polymers) can act as protective/sieving/enrichment layers, thereby effectively improving the stability/selectivity/sensitivity of the sensor.26,32–34 In addition, the hybridization of host–guest materials is usually accompanied by synergistic effects and complementary advantages.30 Therefore, the introduction of guest materials is an effective way to improve the sensing performance and overcome the defects of the host materials.
This article firstly concentrates on the development and configuration types of resistive gas sensors, and the gas sensing mechanism and the advantages/disadvantages of various host materials are briefly introduced. Furthermore, this article presents a comprehensive review of the recent research efforts and developments on various functional guest materials used in resistive gas sensors, referring to the enhancement mechanism of gas sensing performance, preparation and design of host–guest hybrid materials and their application in resistive gas sensors (Fig. 1).
2. Introduction to resistive gas sensors
A resistive gas sensor is a device that can effectively transform the gas changes in the surrounding environment into resistance signals. When the sensing material reacts with or adsorbs the gas molecules, the electrons or holes will be generated and transferred in the material, and further change the resistance of the sensing material. To develop high-performance resistive gas sensors, before selecting guest materials for functionalization, it is necessary to choose appropriate substrate devices and host materials as well as to deeply understand the sensing mechanisms of various host materials. Herein, the development history, performance indexes, devices and host materials for resistive gas sensors are introduced.2.1. Development history of resistive gas sensors
In the early 1950s, Brattain and Bardeen35 demonstrated for the first time that some semiconductor materials (such as Ge) could change their electrical resistance depending on their exposed environment, which laid the foundation for the birth of resistive gas sensors. In 1962, Seiyama et al.36 reported the first resistive gas sensing device that adopted the ZnO film as the sensing layer and could operate at 485 °C. In 1967, following Seyama's work, Shaver37 further demonstrated that modifying the MOSs with the appropriate noble metals (Pt, Pd, Rh, etc.) could significantly improve their inherent gas-sensing properties, benefiting from their excellent catalytic properties. Since then, various guest materials used in resistive gas sensors have been continuously explored to greatly enhance the sensing performance of resistive gas sensors.In 1971, Taguchi patented the first resistive gas sensor with tin dioxide (SnO2) serving as the sensing material for practical applications. The primary application of these commercialized devices was only employed as alarms to prevent accidents and fires by monitoring the presence of hazardous levels of explosive gases.21 In the 1980s, the field of resistive gas sensors experienced a significant expansion, becoming one of the most active research areas in the sensor community. In 1983, Nylander et al.38 firstly reported an ammonia sensor based on the conductive polymer (polypyrrole (PPy)) as the sensing material. Since then, a series of CPs such as PPy, polythiophene (PTh) and polyaniline (PANI), have been widely used as sensing materials in resistive gas sensors. With the increasingly developed nanotechnologies and the emergence of various carbon-based materials, carbon nanotubes and graphene have also been gradually applied in resistive gas sensors.39,40 In addition to MOSs, CPs and carbon-based materials, other host sensing materials with high sensing responses have also been developed recently, such as nitrides (g-C3N4,41 h-BN,42 etc.), transition metal dichalcogenides (TMDs, such as MoS2,43 MoSe2,44 WS2,45 etc.), ferrites (ZnFe2O4,46 CuFe2O4,47 LaFeO3 (ref. 19) etc.), black phosphorous,48 MXenes49 and organic frameworks.50
2.2. Performance index for the resistive gas sensors
The four most critical indexes for evaluating sensor performance are defined as ‘4S’, i.e. sensitivity, speed (response/recovery times), selectivity and stability. Sensitivity reflects the change rate of sensor resistance in a certain concentration of the target gas. It is defined as Ra/Rg for n-type semiconductors and Rg/Ra for p-type semiconductors. Ra is the resistance of the gas sensor in air, and Rg is the resistance of the gas sensor in the gas to be measured.18 A higher response means a higher sensitivity of the gas sensor to the gas to be measured. Response/recovery time is a parameter that reflects the response speed of the gas sensor to detect the target gas. The time it takes for the sensor to achieve 90% of the total resistance change during adsorption and desorption is defined as response time and recovery time, respectively. Selectivity refers to the sensor's ability to identify the measured gas and to suppress the interference gas, also known as cross sensitivity. In mixed gas detection, the selectivity is very important; poor selectivity will affect the qualitative identification of the gas to be measured, and further limit the quantitative analysis; stability represents the long-term reliability and service life of the gas sensor, aiming at evaluating the resistance of the sensor to various influencing factors other than gas concentration.In addition to the above 4 basic indexes, the operating temperature, repeatability, lower limit of detection, and linearity are also essential parameters for measuring its sensing performance. An ideal gas sensor has low operating temperature, high sensitivity, short response/recovery times, outstanding selectivity and excellent stability.51,52 It is almost impossible to combine all properties for a single material. Only by introducing functional guest materials, overcoming the defects of single materials and improving the performance indexes of the sensor can the resistive gas sensor be better commercialized and applied. Therefore, the study of guest material is of great significance.
2.3. Devices of resistive gas sensors
The type of resistive gas sensor device is also an important factor that affects the gas sensing performance. Different resistive gas sensor devices always have different characteristics, and their suitable sensing materials and application scenarios are also different. The common resistive gas sensor devices can be divided into the following four categories: tubular gas sensors, interdigitated electrode (IDE)-based gas sensors, flexible/wearable gas sensors and Micro-Electro-Mechanical System (MEMS)-based gas sensors.53–58As shown in Fig. 2a, a tubular gas sensor consists of a ceramic tube, a Ni–Cr heater, gold signal electrodes, Pt wires, a sensing film and a base.53 At present, the ceramic tube type is the dominant type of resistive gas sensor, mainly because of its advanced preparation technology and low cost.59,60 The circuit diagram of the tubular gas sensor is shown in Fig. 2b. The heater placed in the ceramic tube can provide a high operating temperature. The high thermal resistance of the ceramic allows the gas sensor to work at a higher temperature. However, the tubular gas sensors always suffer from a limited electrode area to load the sensing materials, which makes its available sensing response not high enough. Therefore, tubular sensors are more suitable for sensing materials simultaneously with high sensitivity, high operating temperature and good thermal stability.
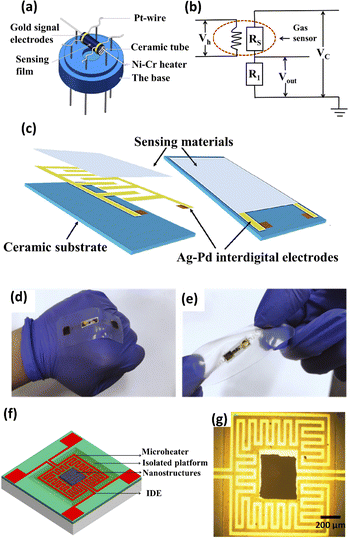 | ||
| Fig. 2 (a) Schematic illustration of the structure of a tubular gas sensor. (b) Circuit diagram of a tubular gas sensor. This figure has been reproduced with permission from ref. 53, Elsevier, Copyright 2020. (c) Schematic illustration of the structure of an IDE gas sensor. This figure has been reproduced with permission from ref. 54, Elsevier, Copyright 2020. Demonstration of the flexible gas sensor deformed by (d) making a fist, (e) twisting. This figure has been reproduced with permission from ref. 55, Elsevier, Copyright 2020. (f) Schematic diagram and (g) photographic image of a MEMS-based gas sensor. This figure has been reproduced with permission from ref. 58, Elsevier, Copyright 2016. | ||
The IDE-based gas sensors are characterized by their miniaturization, fast response and low-cost mass fabrication.2,61 The IDE-based gas sensors have electrodes with periodic patterns in the finger-like or comb-like surface, which consists of a ceramic substrate, interdigital electrodes and sensing materials (Fig. 2c).54 The structure of the IDE can significantly increase the contact area between the electrode and gas sensing materials, enabling the high sensitivity of the gas sensors even at a low temperature.
The flexible/wearable gas sensors are mainly composed of two parts: a flexible substrate and electrode (Fig. 2d and e).55 The flexible/wearable gas sensor is one of the most popular gas sensors in recent years due to its being lightweight and portable with excellent electrical performance and high integration.55 Its gas sensing properties will not change significantly when stretched, tilted or bent, but it exhibits low resistance under high temperature.62 Different flexible substrates, such as polyethylene-terephthalate (PET), polyimide (PI) and Kapton, have been used for the flexible and wearable gas sensors.63–66 Carbon-based materials, such as graphene, graphene oxide (GO), reduced graphene oxide (rGO), single-wall carbon nanotubes (SWCNT), etc., have been reported to be ideal candidates for flexible/wearable gas sensors due to their high mechanical strength, good stability, high carrier mobility and good flexibility.23,67
MEMS is an electromechanical system with three-dimensional (3D) geometry (Fig. 2f and g).58 It is constructed on a silicon-wafer platform based on photolithography microelectronics manufacturing and post-processing techniques.66 These processes allow designers to accumulate different sensor arrays on a sensor platform to meet the requirements of integration, intelligence and multifunction. Nanoscale MOS materials combined with MEMS technology are widely used in the field of gas sensors due to their greatly reduced size, low cost and low power consumption.68 Typically, compared with a conventional tubular gas sensor equipped with a heater consuming about 1–5 W during its operation, MEMS-based gas sensors consuming less than 30–50 mW can reach a working temperature of up to 500 °C.69
2.4. Host materials and sensing mechanisms
The sensing mechanism of a host–guest hybrid material is generally governed by the host material.26 For the same kind of guest material, the gas-sensing enhancement mechanisms and the host–guest interactions may be different when it is hybridized with different host materials. Therefore, it is necessary to understand the material type, advantages/disadvantages and the sensing mechanisms of the host materials before studying the guest materials, so as to select appropriate guest materials for functionalization according to the characteristics of the host materials. Herein, we mainly introduce four types of host materials: MOSs, CPs, carbon-based materials and TMDs.The gas-sensing mechanism of the MOS-based resistive gas sensor is highly dependent on the surface reactions between target gas molecules and chemisorbed oxygen species (O2−, O−, and O2−).70 At an elevated temperature, oxygen molecules in air are chemisorbed on the surfaces of metal oxides by trapping electrons in MOSs, generating either electron depletion regions in the case of n-type MOSs or hole accumulation layers for p-type MOSs. Reducing or oxidizing gas molecules will react with the chemisorbed oxygen and are adsorbed on MOSs, modulating the thickness of the electron depletion layers or hole accumulation layers, resulting in a resistance change of the gas sensors.26
MOSs applied in resistive gas sensors are mainly divided into n-type (SnO2,71 ZnO,72 TiO2,73 In2O3,4 etc.) and p-type (CuO,74 NiO,75 etc.) MOSs. In general, MOSs possess wide band gaps, excellent physical/chemical properties and unique structures (small grain size and high porosity), enabling the superiorities of high sensitivity, fast response and good stability when used as gas-sensitive materials.22 However, they also suffer from some disadvantages including high working temperature, low gas selectivity and serious baseline drift.76 Therefore, the main purpose of the introduction of guest materials to optimize MOSs in resistive gas sensors is to reduce the operating temperature and improve the gas selectivity of MOSs.
The sensing mechanism of CPs can be divided into the following three different types according to the target gases: those that (1) change the resistance between conducting polymer chains by REDOX reactions (e.g. NO2, CO, NH3);77 (2) adjust the distance between the molecular chains of the conductive polymer by forming hydrogen bond/dipole moment (e.g. H2, NH3), thus changing the resistance of the sensing layers;78 (3) affect the carrier concentration in the CPs through the interaction between the adsorbed gas molecules and the charges in the conductive polymer sensitive layer, thus changing the resistance of the CPs (e.g. various volatile organic compounds (VOCs), H2O).79,80 Various CPs such as PANI, PPy and PTh have been reported for application in gas sensors due to their excellent electronic properties, low operating temperatures and intrinsic redox reactivity.18,81 However, unsatisfactory sensitivity and poor long-term stability are two main challenges for the pure CPs as host sensing materials in resistive gas sensors, which need to be further improved by the appropriate guest modifiers.
The gas-sensing mechanism of the resistive sensors with carbon-based host materials is mainly based on their conductance changes caused by the adsorption of target analytes on their surfaces. Depending on the position of adsorption sites, the adsorbed gas molecules (1) directly interact with carbon-based materials by donating electrons or depriving electrons (intra-carbon) or (2) change the electron hopping currents among the sensing materials (inter-carbon) by the swelling effect.26 Carbon-based materials possess large theoretical surface areas and hence can provide a large sensing area for the adsorption of gas molecules. Besides, they exhibit high carrier mobility and low resistance at room-temperature conditions but endure low response, poor selectivity and low reproducibility.23 Therefore, the introduction of guest materials is necessary to optimize the sensing performance factors (stability, repeatability and response/recovery time) of carbon-based materials.
TMDs are an emerging class of two-dimensional (2D) inorganic compounds with a general formula MX2 (X–M–X), where M represents a layer of transition metal element from group 4 to group 10, and X represents a chalcogen element (S, Se, and Te).9,82 TMDs are layered graphene-like materials and their sensing mechanism is similar to the intra-carbon-sensing mechanism, mainly based on the direct interaction between target analytes and sensing materials.20,26,83 TMDs with excellent electronic, optical, and catalytic properties can realize a room-temperature gas sensing response.13 However, their adjacent layers are connected by weak intermolecular forces, leading to the special aggregation and self-stacking effects, hence reducing the permeability and surface active sites of TMDs. Another serious problem for TMDs-based host materials is that they are easily oxidized in an air environment.
3. Functional guest materials in resistive gas sensors
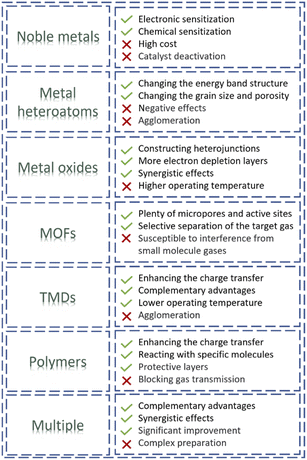
As mentioned above, single gas-sensitive materials, such as MOSs, CPs, carbon-based materials and TMDs, generally have their advantages and disadvantages. It is necessary to introduce functional guest materials to improve the gas sensing properties of the sensors. Herein, seven typical guest materials (noble metals, metal heteroatoms, metal oxides, metal–organic frameworks (MOFs), TMDs, polymers, and multiple guest materials) used in resistive gas sensors are reviewed according to functions, advantages/disadvantages and mechanisms.3.1. Noble metals
The common noble metals in resistive gas sensors mainly include Pd,84 Pt,85 Ag,86 Au,87 etc. When used as the guest materials, these noble metals are generally modified onto host materials to form heterostructural nanoparticles, improving the sensitivity and reducing the working temperature of gas sensors through catalytic and adsorption effects. The functional mechanisms of the noble metals as the guest materials mainly depend on electronic sensitization or chemical sensitization.88 Under normal circumstances, the work function of the noble metals is higher than that of the MOS gas-sensitive materials, and requires equilibrium and charge redistribution. At the interface of the noble metal and MOS, the conduction band will bend to form a Schottky barrier, and the electrons in the conduction band of the MOS sensing material further are transferred to the noble metal nanoparticles to form an interface dipole layer. This process prevents the recombination of separated electron–hole pairs, thereby increasing the gas response of the MOS. This phenomenon is called electronic sensitization (Fig. 3a). Chemical sensitization refers to the adsorption and dissociation enhancement of gases on the surface of sensing materials (Fig. 3b). Noble metal nanoparticles can promote the adsorption of oxygen molecules and the formation of oxygen ions through chemical reduction, and then the oxygen ions spill over to the surface of sensing materials, thus increasing the concentration of oxygen ions. The target gas molecules can also be directly attached to the noble metal nanoparticles, migrating to the surface of the host MOS and further reacting with oxygen ions. This spillover effect can significantly improve the gas sensing performance (Fig. 3c).8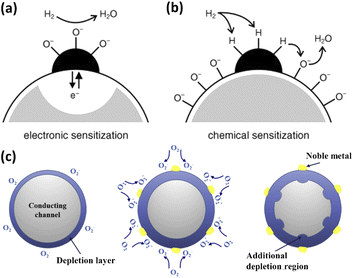 | ||
| Fig. 3 Mechanisms of (a) electronic sensitization and (b) chemical sensitization of noble metals. This figure has been reproduced with permission from ref. 88, Wiley, Copyright 2005. (c) Schematic illustration of the spillover effect. This figure has been reproduced with permission from ref. 8, Elsevier, Copyright 2017. | ||
Jaroenapibal et al.89 fabricated Ag-doped WO3 by the electrospinning method. Porous mats of nanofibers comprising monoclinic WO3 nanoparticles (28–39 nm) incorporated with small Ag particles were observed. At 225 °C, the response sensitivity of 3 mol% Ag-doped WO3 nanofibers to 5 ppm NO2 gas was 90.3, more than 9 times higher than that of undoped WO3 nanofibers. The Ag–WO3 also showed high selectivity to NO2 gas as compared with other interfering gases (CH4, NH3, SO2 and H2S). The enhancement of the sensing performance is mainly attributed to the catalytic effect, enhanced electron sensitization and increased oxygen vacancy content caused by Ag nanoparticles (Fig. 4a and b). Significant decreases in gas responses were observed in the samples with higher Ag dopant levels (5 and 10 mol%), where the crystallite size of Ag nanoparticles became too large and hindered their catalytic effects (Fig. 4c). Li et al.90 synthesized pure and Au-loaded Co3O4 porous hollow nanocages using ZIF-67 as a sacrificial template. Due to the spillover effect of Au, the content of adsorbed oxygen was increased from 9.1% of pure Co3O4 to 27.5% of Au/Co3O4, enhancing the catalytic activities for acetone adsorption and the reactions were also enhanced. The response of Au/Co3O4 to 100 ppm acetone at 190 °C was 14.5, and the limit of detection was 1 ppm. Meanwhile, the addition of Au significantly changed the original morphology of Co3O4 and formed coarser surfaces and openings, which improved the transport of gas molecules through Au/Co3O4. This improved gas accessibility could also account for the significantly increased recovery speed of Au/Co3O4 (280 s) as compared to pure Co3O4 (7559 s).
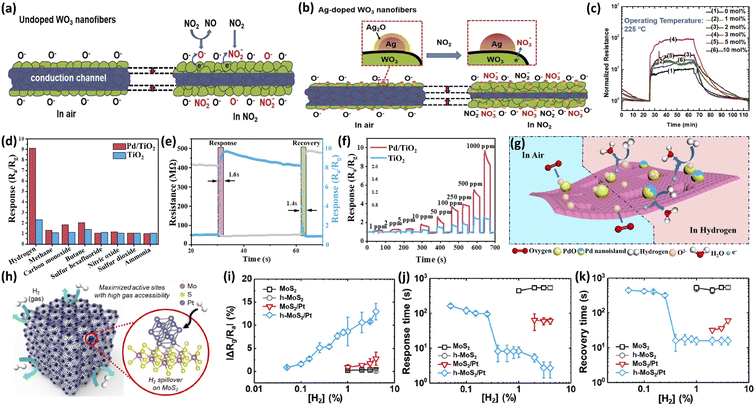 | ||
| Fig. 4 Schematic diagram of the NO2 sensing mechanism of (a) WO3 and (b) Ag-loaded WO3 nanofibers. (c) Resistance plots of the WO3 and Ag-loaded WO3 nanofibers measured in air and 5 ppm NO2 at operating temperatures of 225 °C. This figure has been reproduced with permission from ref. 89, Elsevier, Copyright 2018. (d) Selectivity of the TiO2 and Pd/TiO2 toward different reference gases (1000 ppm) at 230 °C. (e) Response/recovery curve of the sensor based on Pd/TiO2 toward 1000 ppm H2 at 230 °C. (f) Dynamic response curves of the sensors based on TiO2 and Pd/TiO2 toward 1 ppm to 1000 ppm H2 at 230 °C. (g) Schematic illustration of the H2 gas-sensing mechanism of Pd/TiO2. This figure has been reproduced with permission from ref. 93, Elsevier, Copyright 2021. (h) Schematic illustration of the structure and sensing mechanisms of h-MoS2/Pt. (i) Response, (j) response time, and (k) recovery time of sensors based on MoS2, h-MoS2, MoS2/Pt, and h-MoS2/Pt. This figure has been reproduced with permission from ref. 97, American Chemical Society, Copyright 2020. | ||
The introduction of Pd can greatly improve the sensitivity and selectivity of the H2 sensor.91 Pd is widely used as the gas sensing material for H2 because of its superior capacity to absorb and catalyze H2.26,92 Wang et al.93 prepared 2D porous TiO2 nanosheets by a graphene oxide template method and further introduced Pd nanoparticles onto these nanosheets by an impregnation technique to obtain the final Pd–TiO2 nanosheets. The H2 sensor based on Pd–TiO2 nanosheets exhibited an instantaneous response, low detection limit (1 ppm), good selectivity and linear response (Fig. 4d–f), which were mainly attributed to the synergistic effect between Pd and TiO2 (including the high adsorption ability of Pd–TiO2 toward both O2 and H2 as well as the effective catalysis ability of Pd toward H2) and the unique porous 2D structure (Fig. 4g).
Noble metals-decorated carbon-based materials-based gas sensors can reveal the effective gas sensing capability at low temperature.94 This is mainly due to the synergy of the catalytic effect of noble metals and the prompt electron transfer of carbon-based materials between the target gas and metal electrodes.95 Kwon et al.85 prepared high-performance toluene (C7H8) gas sensor by using the Pt-multiwalled carbon nanotubes (MWCNTs) composite materials. The size of the Pt nanoparticles can be optimized by controlling the predeposited Pt thickness, thus obtaining the highest sensing performance. The Pt-functionalization drastically enhanced the sensing behavior of the Pt-MWCNTs composite sensor. The response of this sensor at 150 °C could reach 3.91 to 1 ppm C7H8 gas (237.1% higher than pure MWCNTs) and the response/recovery times (55/70 s) were decreased by 89.2% and 92.7%, respectively. This Pt-MWCNTs sensor also showed high selectivity for C7H8 in comparison with other gases, including C2H5OH, CO, H2, and C6H6. Johnson et al.96 decorated Pd nanoparticles on graphene nanoribbon porous film for H2 detection. Gas measurement indicated that the catalytic noble metal plays an important role in H2 sensing and showed fast recovery/response times (6/23 s) to 40 ppm H2 at room temperature (RT).
Noble metals loaded on TMDs can also achieve room temperature gas sensing. Park et al.97 coated MoS2 nanofilms on polystyrene particles by a one-step Pickering emulsification method and then loaded Pt nanoparticles on MoS2 films. By further pyrolyzing this compound, the highly porous hollow hybrid materials (h-MoS2/Pt) were formed. The obtained h-MoS2/Pt possessed a high specific surface area to guarantee abundant permeable paths for H2 molecules and maximized the active site of MoS2 (Fig. 4h). Besides, the spillover effect of Pt nanoparticles also significantly improved the gas-sensitive performance of the original h-MoS2. As a result, h-MoS2/Pt achieved fast response/recovery times (8.1/16 s) and high sensitivity to H2 at RT (Fig. 4i–k). Zhang et al.98 demonstrated a high-performance CO sensor based on Pd-decorated WSe2 (Pd–WSe2) hexagonal nanosheet nanostructures. The Pd–WSe2 thin film sensor had excellent sensing performance for CO gas, including high sensitivity (the response to 5 ppm CO at RT was 9.25), excellent repeatability, good selectivity and fast response/recovery speeds (52/97 s).
In summary, precious metals can significantly enhance the sensing performance, including sensitivity, response recovery speed, and operating temperature. However, the addition of noble metals will increase the cost, and the catalyst poisoning will cause the failure of gas sensor components. Therefore, noble metals as guest sensing materials still have certain limitations in practical application.
3.2. Metal heteroatoms
In resistive gas sensors, metal heteroatoms as the sensing guest materials are generally doped into MOSs in the form of functional ions (such as Ni2+, Zn2+, Al3+, Ca2+ and Co3+).22,99–102 The doping of metal heteroatoms is widely considered a simple and effective way to elevate the performance of gas sensors. The introduction of these metal heteroatoms will change the grain size, porosity and specific surface area of the host MOSs, in turn modifying the adsorption sites and diffusion paths of the gas molecules.103 Most importantly, it can change the energy band structure of the material. When the grain size is less than twice the Debye length, the electron depletion layer will occupy the entire grain, thereby improving the gas sensing properties of the host MOS.17 A higher specific surface area and larger porosity provide more adsorption sites and abundant transport channels to promote gas diffusion and improve the sensing sensitivity of the host MOS. To verify the influence of heteroatomic doping on the material energy band structure, Chen et al.104 prepared Al-doped In2O3, and the changes in the In2O3 band gap and Fermi level caused by the addition of Al atoms were revealed by DFT calculations (Fig. 5a and b). The calculated band gap increased from 0.94 eV to 0.99 eV upon the addition of Al atoms, and the Fermi level increased from 5.17 eV to 5.24 eV. Due to the elevated Fermi level, Al-doped In2O3 exhibited a higher response. However, not all metal heteroatoms can improve the sensing performance of gas sensors. As shown in Fig. 5c, Chen et al.104 also studied the doping of In2O3 with other metal heteroatoms and found that some dopants (such as Al, Ga and Zr) raised the Fermi level of In2O3, while the others (such as Ti, V, Cr, Mo, W and Sn) reduced the Fermi level. Only the former can improve the response of gas sensors.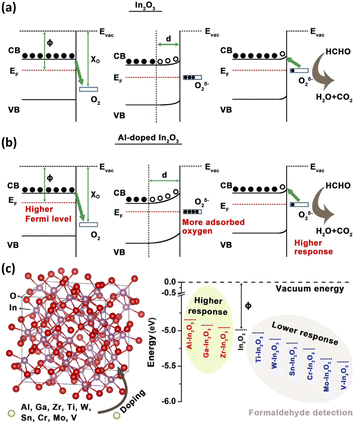 | ||
| Fig. 5 Schematic energy diagram of (a) In2O3- and (b) Al-doped In2O3; (c) lattice model of 9 kinds of metal atoms doped In2O3 and the comparison of the Fermi energy levels of In2O3 materials before and after doping. This figure has been reproduced with permission from ref. 104, American Chemical Society, Copyright 2018. | ||
Zhao et al.105 synthesized Ca-doped In2O3 nanotubes via electrospinning and annealing technology. The average size of In2O3 grains initially decreased following an increase in Ca content from ≤3 mol% to ≥7 mol%. Ca doping also increased the oxygen vacancy concentration and was beneficial to the selective catalysis of ethanol (Fig. 6a and b). In particular, the sensor based on 3% Ca–In2O3 showed the best sensing performance toward ethanol, with high sensitivity, good selectivity, long-term stability and excellent reproducibility (Fig. 6c and d). Hjiri et al.106 prepared Al-doped ZnO nanoparticles (Al–ZnO) by sol–gel technology for application in the CO gas sensor (the response to 50 ppm CO at 300 °C was 80, the response time was only 7 s, and the detection limit was 250 ppb). They found that Al could promote the adsorption and chemical inter-reaction of CO and O2 to improve the gas-sensing response.
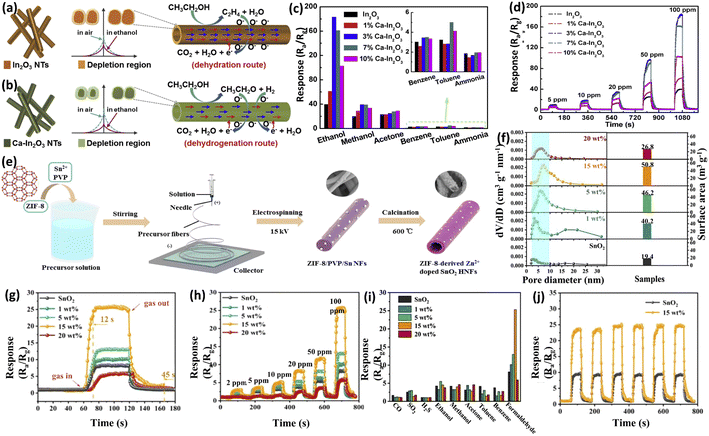 | ||
| Fig. 6 Schematic diagrams of (a) pure In2O3 and (b) Ca–In2O3 exposed to air and ethanol. (c) Selectivity of pure and Ca-doped In2O3 for various gases (100 ppm) at 240 °C. (d) Dynamic response curves of pure and Ca-doped In2O3 to 5–100 ppm ethanol at 240 °C. This figure has been reproduced with permission from ref. 105, Elsevier, Copyright 2019. (e) Schematic illustration of the synthetic procedure for ZZS HNFs. (f) Pore size distribution and surface areas of pristine SnO2 and ZZS HNFs with different Zn doping amounts. Sensing properties of the sensors based on pristine SnO2 and ZZS HNFs with different Zn doping amounts at 400 °C: (g) response/recovery curves to 100 ppm HCHO and (h) dynamic response curves to different concentrations of HCHO. (i) Responses of sensors to various 100 ppm gases at 400 °C. (j) Six dynamic response–recovery cycles of the sensors based on pristine SnO2 and 15 wt% ZZS HNFs for the detection of 100 ppm HCHO. This figure has been reproduced with permission from ref. 22, Elsevier, Copyright 2019. | ||
In addition, the doped nanoparticles are prone to agglomerate and reduce the specific surface area and the exposed active sites of sensitive materials, consequently resulting in the reduced gas sensing response of resistive gas sensors. To overcome this problem, our group22 synthesized a series of MOF-derived Zn2+-doped SnO2 hollow nanofibers (ZZS HNFs) via the facile electrospinning method and annealing treatment for smart HCHO monitoring (Fig. 6e). Compared with the original SnO2, all ZZS HNFs possessed a smaller pore size and larger specific surface area, indicating that MOFs can be used as a potential metal-ion precursor to realize the homogeneous doping of nanofibers. Among the as-prepared ZZS HNFs, the 15 wt% ZZS HNFs exhibited the highest response, fastest response/recovery time (12/45 s), best selectivity and repeatability, and a detection limit as low as 500 ppb towards HCHO (Fig. 6g–j). The enhancement in the sensing properties of 15 wt% ZZS HNFs could be attributed to the high specific surface area and the increase in oxygen vacancies and chemisorbed oxygen species, benefiting from the homogeneous Zn2+ doping and the one-dimensional (1D) nanostructures.
Based on the above cases, the enhanced gas sensing performance caused by metal heteroatoms in resistive gas sensors is mainly attributed to their positive effects on the optimization of the physicochemical properties (including grain size, oxygen vacancy concentration and catalytic activity, etc.) of the host materials. Metal heteroatoms can form a large number of surface defects on the surface of the host sensing material and increase the number of chemically active adsorption sites, thereby improving the chemical adsorption capacity toward the targeted gas. Hence, metal heteroatoms can effectively improve the sensitivity of the sensor to the target gas.
3.3. Metal oxides
The introduction of metal oxides into host semiconductor materials has many positive effects, such as introducing more electron depletion layers, improving catalytic activity, increasing adsorption sites, changing the energy band structure and accelerating electron transport, thus achieving high sensitivity and favourable selectivity.107 Heterojunctions can be formed when metal oxides are combined with other host semiconductor materials, enabling the complementary and synergistic effects of host–guest materials. According to the different conduction types, the heterojunctions can be divided into p–n, n–n and p–p heterojunctions.31 For the p–n heterojunction, the electrons at the conduction band move from the n-type semiconductor to the p-type semiconductor, and holes transfer from the p-type semiconductor to the n-type semiconductor until the Fermi level of the nanocomposite reaches equilibrium. Due to the recombination of electrons and holes, an electron depletion layer will be formed at the p–n heterojunction (Fig. 7a). For the n–n heterojunction, electrons will transfer from the high-Fermi level material to the low-Fermi level material at the n–n heterojunction interface, where the semiconductor with a high Fermi level creates a depletion layer and the semiconductor with a low Fermi level forms an accumulation layer (Fig. 7b). For the p–p heterojunction, the holes transfer from a p-type semiconductor with higher valence band energy to another p-type semiconductor with lower valence band energy. Therefore, as shown in Fig. 7c, a cavity depletion zone is formed on the surface of the former (with high valence band energy), while a cavity accumulation zone is formed on the surface of the latter (with low valence band energy).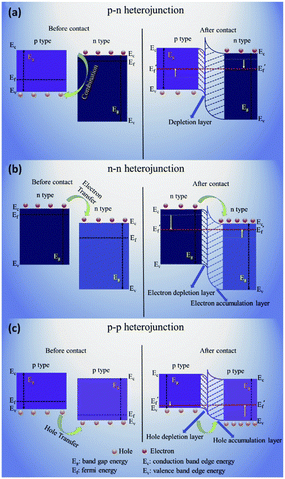 | ||
| Fig. 7 Schematic illustrations of the energy band structures of different types of heterojunctions: (a) p–n junction, (b) n–n junction and (c) p–p junction. This figure has been reproduced with permission from ref. 31, Royal Society of Chemistry, Copyright 2019. | ||
Kumaresan et al.108 synthesized nanofibers with the n-WO3/n-TiO2 heterostructure by uniformly loading WO3 nanoparticles (guest) on the TiO2 nanofiber (host) surface through a simple spin coating method. Compared to the original TiO2, n-WO3/n-TiO2 exhibited a more excellent H2 sensing performance. Its response to 1000 ppm H2 at RT was greatly improved from 52.34 to 78.21, and the response/recovery times were also reduced from 42/47 s to 20/23 s. The improved H2-sensing performance of n-WO3/nTiO2 was mainly due to the increase in the gas adsorption sites caused by the heterojunction, the high specific surface area of nanofiber structure, the excellent catalysis effect of WO3 nanoparticles and the Fermi-level effect.
Jayababu et al.109 synthesized NiO-decorated CeO2 nanostructures via a co-precipitation technique and sol–gel process. The highest response of 1570 (nearly 11 times higher than that of the pure CeO2) was observed for the NiO/CeO2-based gas sensor and sharp response/recovery times (15/19 s) towards 100 ppm isopropanol at RT. The band diagram of the NiO/CeO before and after junction formation is presented in Fig. 8a. After decorating the surface of CeO2 with NiO nanoparticles, the diffusion of electrons and holes from one side to the other side takes place until their Fermi levels become equal. This whole phenomenon develops an internal potential at the interface of the two materials, which is called the built-in potential. The built-in potential at the p-NiO and n-CeO2 interface, as well as the highly catalytic nature and chemical sensitization of decorated NiO, played a vital role in the superior sensing performance of the NiO/CeO2 sensor (Fig. 8b).
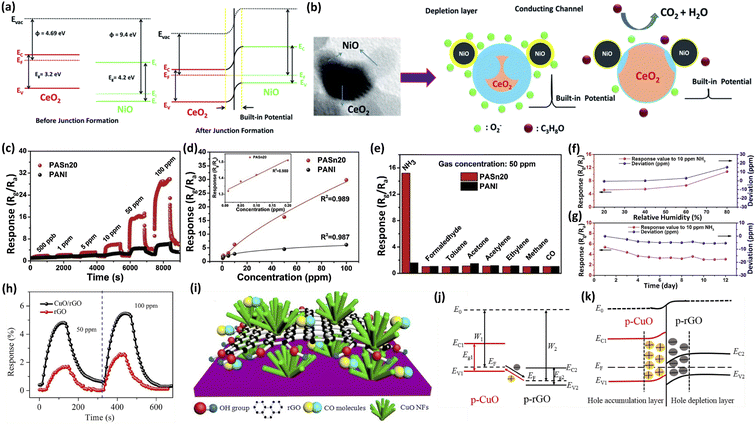 | ||
| Fig. 8 (a) Schematic diagram demonstrating the band gap alignment of NiO/CeO2. (b) Schematic illustration of the isopropanol gas sensing mechanism of NiO/CeO2 gas sensors with surface adsorption and desorption reactions. This figure has been reproduced with permission from ref. 109, Royal Society of Chemistry, Copyright 2019. (c) Dynamic response curves of the sensors based on PANI and PASn20 to various concentrations of NH3 at 28 °C. (d) The fitting curves of the PANI sensor and PASn20 sensor to NH3 at 28 °C. (e) The cross-sensitivity of the sensors based on PANI and PASn20 to various testing gases of 50 ppm at 28 °C. (f) The response value of PASn20 at 20–80% RH at 28 °C. (g) Long-term stability of PASn20 at 28 °C. This figure has been reproduced with permission from ref. 111, Elsevier, Copyright 2019. (h) Performance comparison of CO gas sensing between the CuO/rGO nanocomposite sensor and pure rGO film. (i) Schematic of the hybrid nanostructure of CuO/rGO. (j) The energy band diagram of CuO and rGO. (k) The energy band diagram for the p–p junction of the CuO/rGO heterostructure. This figure has been reproduced with permission from ref. 113, Elsevier, Copyright 2019. | ||
Lou et al.60 designed a highly sensitive and selective formaldehyde sensor based on SnO2/ZnO heterospheres synthesized by atomic layer deposition (ALD). By optimizing the loading of ZnO through changing ALD cycles, the electronic properties at the SnO2/ZnO heterointerface can be modulated. The SnO2/ZnO sensor with ZnO ALD of 10 cycles had the best response/recovery speeds (12/24 s). The SnO2/ZnO sensor also registered a low detection limit of 70 ppb, which allows for the reliable detection of sub-ppm formaldehyde. This remarkable sensor performance indicated that the decoration of metal oxides by ALD surface engineering is promising for the design of host–guest materials.
To date, CPs have been widely used as sensitive materials for the detection of NH3 at RT.110 Adding metal oxides as guest materials is an effective way to solve the low sensitivity and poor stability of CPs. Li et al.111 synthesized SnO2-decorated PANI via a combined approach of hydrothermal and in situ polymerization. Compared with the PANI sensor, the 20 mol% SnO2-polyaniline (PASn20) sensor exhibited a 6.2 times higher response (29.8) to 100 ppm NH3 at RT. Furthermore, the PASn20 sensor possessed the ability to detect low NH3 concentrations of 10–200 ppb and a linear response, outstanding selectivity, as well as favorable stability (Fig. 8c–g). The enhanced sensing performance was attributed to the micro-structure with large specific surface area and the formation of the p–n heterojunctions at the surface between PANI and SnO2.
Adopting metal oxides to decorate carbon-based materials can effectively improve the gas sensitivity and achieve fast response/recovery times even under low-temperature conditions, due to the abundance of active sites and a quicker electron transfer benefiting from the well-matched work functions of metal oxides and carbon-based materials.112 Zhang et al.113 reported a sub-ppm-level CO gas sensor based on a CuO-decorated rGO hybrid nanocomposite. The CuO/rGO hierarchical nanocomposite was successfully deposited on a substrate with interdigital microelectrodes via a layer-by-layer self-assembly technique. The gas sensing experiment revealed that the CuO/rGO nanocomposite sensor is capable of sub-ppb-level CO gas detection, good selectivity, fast response/recovery times, acceptable repeatability and stability, which outstripped that of the pure rGO film sensor (Fig. 8h). The enhanced gas sensing properties of the CuO/rGO nanocomposite is ascribed to its hierarchical porous nanostructure and electronic modulation at the interfaces between CuO nanoflowers and rGO nanosheets (Fig. 8i–k).
At RT, the critical drawbacks of the pristine TMD-based gas sensors were their sluggish response and incomplete recoverability due to their lower stability in air or target gas.114 Decorating MOS on TMDs has attracted significant attention; they not only increase the number of adsorption sites but also provide numerous gas channels and accelerate electron transport. As a result, they have improved sensitivity, stability and recoverability with short response/recovery times. Ikram et al.114 developed rod-like p–n MoS2–ZnO heterostructures. The ZnO was converted from ZIF-8 by a hydrothermal method. At room temperature, the sensor showed an over 30-fold enhancement in the response compared to the pristine MoS2 sensor and displayed short response/recovery times while lowering the detection limit of NO2 to 10 ppb. The sensor retained high stability upon sensing repetition for 10 consecutive weeks. This work demonstrated a facile strategy for the synthesis of p–n MoS2–ZnO heterostructures for reliable NO2 gas sensing at RT.
Although the construction of heterostructures will lead to an increased operating temperature in some cases due to a higher activation barrier at the material interfaces, it may be conducive to some specific high-temperature gas sensors.
3.4. Metal–organic frameworks (MOFs)
MOFs are a unique crystalline and porous solid materials composed of metal nodes (metal ions or clusters) and functional organic ligands.50 MOFs have been investigated extensively for numerous applications (such as gas storage,115,116 catalysis,117 and chemical sensors118–120) in recent years, due to their large surface areas, tunable pore sizes and abundant functionalizable sites.50 Nevertheless, many MOFs have relatively low conductivity, meaning that single MOF materials cannot act as host sensing materials for resistive gas sensors. However, the introduction of MOF membranes as guest materials can be considered an ideal solution to solve the issue associated with the poor selectivity of resistive gas sensors.26,30,121 MOFs with plenty of micropores and active sites can be used as gas molecular probes. Gas molecules can be easily adsorbed and enriched on metal nodes and functional groups in organic ligands, thus improving the sensitivity and reducing the detection limit of gas sensors.26,122 In addition, MOFs can also be used as molecular sieves due to their adjustable pore structures. The selective separation of the target gas molecule from the interfering gas can be achieved by a properly designed pore structure.34As shown in Fig. 9a, our group34 reported an “in situ enrichment amplification” (IEA) strategy for constructing the highly sensitive ppb-level HCHO sensor (Fig. 9a). ZIF-8 was coated on electrospun SnO2 nanofibers by solvothermal reaction and core–shell ZIF-8-SnO2 nanofibers were obtained (Fig. 9b). The screening effect of ZIF-8 helps the small kinetic diameter of formaldehyde (2.43 Å) to easily pass through the small pore aperture of ZIF-8 (3.4 Å), benefiting the selective adsorption for HCHO gas. The IEA-based gas sensors exhibit high sensitivity and selectivity toward the detection of HCHO gas (Fig. 9c and d). The calculated detection limit of the IEA sensor to HCHO gas is 63 ppb, much lower than that of the conventional HCHO sensor (183 ppb). In addition. The validity and universal applicability of the IEA strategy for enhancing gas-sensing properties were verified by using three different materials (SnO2, In2O3, and LaFeO3) as the host materials. All their responses improved significantly after IEA functionalization (Fig. 9e). Therefore, using guest MOFs as enrichment layers is an effective strategy for enhancing the sensitivity of gas sensors.
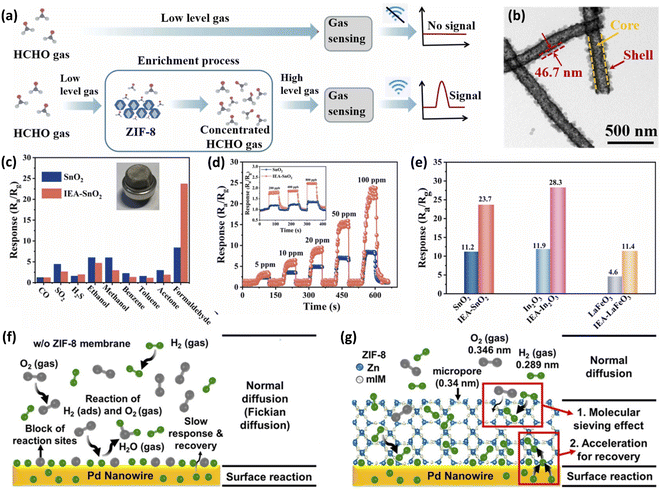 | ||
| Fig. 9 (a) Schematic illustration of the IEA-based gas sensor. (b) Transmission electron microscope (TEM) image of ZIF-8-SnO2. (c) Responses of sensors to various 100 ppm gases at 300 °C. (d) Dynamic response curves of sensors for different concentrations of HCHO at 300 °C. (e) Responses of different conventional HCHO gas sensors (SnO2, In2O3, and LaFeO3) and the IEA-based sensors (IEA-SnO2, IEA-In2O3, and IEA-LaFeO3) to 100 ppm HCHO. This figure has been reproduced with permission from ref. 34, Elsevier, Copyright 2022. Sensing model for (f) Pd nanowires and (g) Pd nanowires with ZIF-8 membrane. This figure has been reproduced with permission from ref. 119, American Chemical Society, Copyright 2017. | ||
As mentioned before, Pd is a good H2-sensitive material, but it has the disadvantage of easy poisoning and deactivation. In addition, the O2 in air interferes with the detection of H2 by Pd-based H2 sensor, depressing the sensitivity and retarding the response/recovery speed. In this regard, Koo et al.123 synthesized ZIF-8 polyhedron particles on lithographically patterned Pd nanowires to construct ZIF-8/Pd NW H2 sensors. ZIF-8, as a molecular sieving layer, allows the selective penetration of H2 into Pd-based sensors and effective screening of the relatively larger gas molecules including O2 (0.346 nm) and N2 (0.364 nm) in air, thus improving the selectivity of the sensor and significantly reducing the negative effects of air on the sensor (Fig. 9f and g). In addition, the H2-sensing speed of Pd NWs was dramatically accelerated after the ZIF-8 deposition; the response/recovery times of the ZIF-8/Pd NW sensor to 1% of H2 were reduced from 164/229 s to 7/10 s.
MOFs can also be composited with a variety of carbon-based materials, and the integration of MOF and carbon-based materials can improve the stability and electrical conductivity. Tung et al.124 used pristine graphene (pG) as the host material and compounded it with three MOF materials (Cu–BTC, UiO-66 and ZIF-8). Graphene with high carrier mobility provided low electrical noise and low power consumption, and the MOFs with a high surface area and adsorption capacity provided enhanced sensitivity and selectivity for specific VOCs. All three gas sensors could detect chloroform vapors at the ppm level at RT, and pG–Cu–BTC sensors showed the highest sensitivity and selectivity to chloroform. This was due to the largest specific surface area of Cu–BTC among the three MOFs and the interaction of chloroform molecules with Cu–BTC leads to increased selectivity.
MOFs can be used as molecular probes and molecular sieves to enhance gas sensing properties (e.g. selectivity, stability and limit of detection). However, some tough problems still exist in the practical application of MOFs. For example, (1) MOFs are generally selective adsorbents only for small molecules of gases. (2) Unmatched design (e.g., an excessively thick MOF-membrane) may block the gas diffusion paths and thus greatly reduce the sensitivity and response recovery speed of gas sensors. (3) When the molecular size of the interfering gases is smaller than or close to the target molecule, it will be difficult to realize high selectivity for the target gases.
3.5. Transition metal dichalcogenides (TMDs)
TMDs are a new kind of semiconductor with high electronic activity, high adsorption capacity and large specific surface area, showing great application prospects in RT gas sensors. The functional mechanism of TMDs guest materials is mainly based on the construction of heterojunctions, enhancement of charge transfer, synergistic effects and complementary advantages, similar to that of metal oxides. 2D TMDs suffer from the disadvantage of easy agglomeration, however, it can be effectively overcome after appropriate modification by some functional guest materials. MOSs modified with 2D TMDs can suppress the agglomeration of the MOSs and improve electrical conductivity, thereby improving the gas sensing performance.125MoS2 is the leading application in the field of gas sensing, and its composites with MOSs are also widely used in gas detection applications. Ding et al.126 synthesized Cu2O nanoparticles decorated with MoS2 nanosheets via a facile hydrothermal and wet chemical method. Under an optimal low-operating temperature of 75 °C, the response of the p–p Cu2O/MoS2 sensor (872%) with an optimized composition for 100 ppm NH3 increased by more than 8 times as compared with the pristine Cu2O (103%). The Cu2O/MoS2 sensor also exhibited excellent selectivity for NH3 against other interferent gases. The enhanced sensing property of the nanohybrid benefits from the superimposed effect of the p–p heterojunction formation and the elevated specific surface area at the interface between Cu2O and MoS2.
Chang et al.127 introduced MoS2 nanosheets on the surface of p-type ZnO derived from ZIF-8 to produce ZnO@MoS2 core/shell heterojunctions as a novel acetone sensor. The ZnO@MoS2 exhibited an enhancement of about 80 times in response to 100 ppb acetone compared that of pure ZnO (Fig. 10a). More importantly, this ZnO@MoS2 heterojunction sensor exhibited an ultra-fast response/recovery (60/40 s) to acetone of ultra-low concentration (5 ppb). Moreover, the acetone sensing performance is negligibly affected by humidity and other gases, which is suitable for exhaled acetone detection (Fig. 10b and c). The sharp increase in the negative heterojunction interface resistance, ultra-fast gas diffusion rates in MoS2 nanosheets and strong interaction energy are key factors for the excellent acetone sensing properties of ZnO@MoS2.
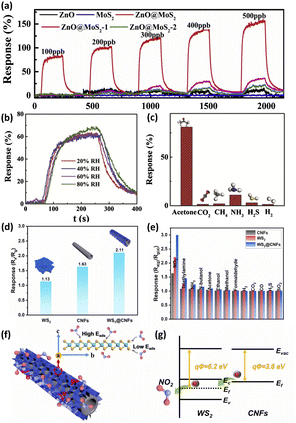 | ||
| Fig. 10 (a) Dynamic response curves of the ZnO and MoS2-based sensor to 100–500 ppb acetone at 350 °C. (b) The sensing characteristics of the ZnO@MoS2 sensor towards 100 ppb acetone under different relative humidity levels. (c) The response of the ZnO@MoS2 sensor to 100 ppb acetone, 250 ppm CO2, 250 ppm CH4, 5 ppm NH3, 5 ppm H2S, and 250 ppm H2. This figure has been reproduced with permission from ref. 123, Elsevier, Copyright 2020. (d) The response of CNFs, WS2, and WS2@CNFs to 10 ppm of NO2 at RT. (e) Selectivity of CNFs, WS2, and WS2@CNFs to 50 ppm of various gases. (f) Schematic illustration of the sensing mechanism of WS2@CNFs. (g) Band diagram for WS2@CNFs. This figure has been reproduced with permission from ref. 124, Elsevier, Copyright 2021. | ||
The edges of TMDs have high adsorption capability and electronic activity. The exposure of the edges of TMDs remains a great obstacle to achieving high sensor sensitivity. Loading TMDs onto carbon-based materials is an effective method. In an attempt to increase the exposure of the edges of TMDs to improve the gas sensing properties, Xu et al.128 demonstrated a high-performance RT NO2 gas sensor based on WS2 nanosheets/carbon nanofibers (CNFs) composite few-layer WS2 nanosheets anchored on CNFs through a hydrothermal process. This process achieved a coating presenting an optimized active surface area and accessibility of the sensing layers. The exposure of WS2 edges remarkably improved the sensing properties. Consequently, the WS2@CNFs composite exhibited excellent selectivity for NO2 at RT with improved response, good selectivity and much lower detection limit in comparison to the CNFs (Fig. 10d and e). The improved response and selectivity are due to the synergistic contribution from the WS2 edge-rich structure and high conductivity of CNFs (Fig. 10f and d). Density functional theory (DFT) calculations verified that the edge sites of WS2 were more beneficial for NO2 adsorption with improved electron transfer as compared to the basal surface of WS2.
3.6. Polymers
CPs, as guest materials used in resistive gas sensors, have many unique advantages benefiting from their excellent electronic properties, low operating temperatures and intrinsic redox reactivity. CPs can form heterojunctions with the host gas sensing materials to enhance electron transport. In addition, some groups on CPs can react with specific gas molecules, thus increasing selectivity and sensitivity to those gases.129For example, Guo et al.57 developed a hierarchically nanostructured rGO–PANI composite film. The assembled flexible, transparent electronic gas sensor exhibited favorable performance, such as high sensitivity and linear responses towards NH3 gas concentrations ranging from 100 ppb to 100 ppm, as well as outstanding selectivity (Fig. 11a). The fast electron transfer between hybrids and NH3, assisted by π–π interactions of PANI and rGO with a low electron transfer energy barrier, led to more electron transfer from PANI to rGO, thus effectively improving the responsivity and response speed (Fig. 11c). In addition, the rGO–PANI sensor showed satisfactory flexibility and stability (Fig. 11d). Sonker et al.130 synthesized TiO2–PANI nanofilm by spin-coating technology for enabling the CO2 gas sensor to operate at RT conditions. In addition to the formation of enough active sites on the surface of TiO2, the uniformly dispersed 1% PANI particles in TiO2 (TiO2–PANI (1%)) could also change the electron Debye length and enhance the chemical adsorption, hence realizing the excellent CO2 sensing performance (53–1000 ppm CO2 gas) at RT. Han et al.129 fabricated a polyethyleneimine (PEI)-functionalized CNT sensor for CO2 detection at RT. Uniform CNT thin films prepared by a filtration method were used as resistive networks. The abundant amino group in PEI could react with CO2 to form carbamate at RT, giving PEI-CNT a better sensing performance than the original CNT.
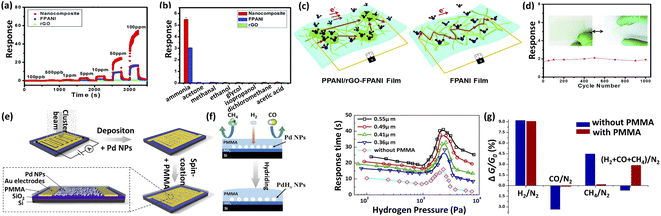 | ||
| Fig. 11 (a) Sensing performances with different concentrations of NH3 ranging from 100 ppb to 100 ppm for the rGO film, the PANI and the rGO–PANI. (b) Gas sensing selectivity (averaged results with error bars) of the rGO, PANI and the rGO–PANI for various volatile gases. (c) The proposed mechanisms of charge transport for the rGO–PANI and PANI. (d) The investigation of the flexibility of the transparent sensors by measuring the sensing performances in the bent and extended states (5 ppm NH3). This figure has been reproduced with permission from ref. 57, Royal Society of Chemistry, Copyright 2016. (e) Schematic illustration of the procedure used to fabricate the hydrogen sensor based on a PMMA-membrane-coated Pd NP film. (f) Schematic illustration of the sensing kinetics of PMMA-Pd and plots of the response time versus the thickness of the PMMA membrane layer. (g) The response of sensors with and without a PMMA membrane layer to target gas mixtures including CO/N2, CH4/N2, H2/N2, and (H2 + CO + CH4)/N2. This figure has been reproduced with permission from ref. 127, American Chemical Society, Copyright 2017. | ||
In addition to CPs, non-conductive polymers can also serve as functional guest materials for use in resistive gas sensors. Coating with a polymeric membrane for screening out interfering gases is a feasible approach to fabricating gas sensors with high selectivity. They can also act as protective layers against the poisoning and inactivation of the host materials, thereby improving the stability of gas sensors. Chen et al.131 fabricated polymethyl methacrylate (PMMA) membrane-coated Pd nanoparticle films for high-performance H2 gas sensors by carrying out gas-phase cluster deposition and PMMA spin coating (Fig. 11e). After PMMA coating, the sensor response decreased slightly, but the selectivity for hydrogen increased significantly (Fig. 11f and g). However, the device sensing kinetics were strongly affected by the thickness of the PMMA layer, with the devices with thicker PMMA membrane layers showing a slower response to H2 gas (Fig. 11f).
3.7. Multiple guest materials
Although the above guest materials (noble metals, metal heteroatoms, metal oxides, MOFs, TMDs and polymers) have many advantages and can significantly improve the gas sensing properties, there are still some problems (e.g. poor stability, easy poisoning and inactivation, insufficient sensitivity and selectivity) that hinder the practical application of these materials. In many cases, adding two or more guest materials can ameliorate these defects and dramatically improve the sensitivity.30To solve the toxic inactivation of Pd-based sensors, Xie et al.92 proposed a hybrid H2 sensor consisting of Pd nanocluster film, MOF, and polymer (Fig. 12a–c). The PMMA coating, as a protection layer, endows the sensor with excellent H2 selectivity and CO poisoning resistance. The ZIF-67 serves as an interface layer between the Pd film and the polymer layer, which alters the nature of the interaction with hydrogen and leads to significant sensing performance improvements, owing to the interfacial electronic coupling between Pd NCs and the MOF. The strategy overcomes the shortcomings of retarded response speed and degraded sensitivity induced by the polymer coating of a Pd film-polymer hybrid system. Pd/ZIF-67/PMMA had both lower adsorption and analytical energy, thus exhibiting faster response/recovery (Fig. 12d–f). As shown in Fig. 12g, compared with the pristine Pd sensor, Pd/ZIF-67 showed a higher response but poor poisoning resistance, while Pd/PMMA had good poisoning resistance but a reduced response. Pd/ZIF-67/PMMA exhibited both high sensitivity and poisoning resistance even with excellent selectivity (Fig. 12h).
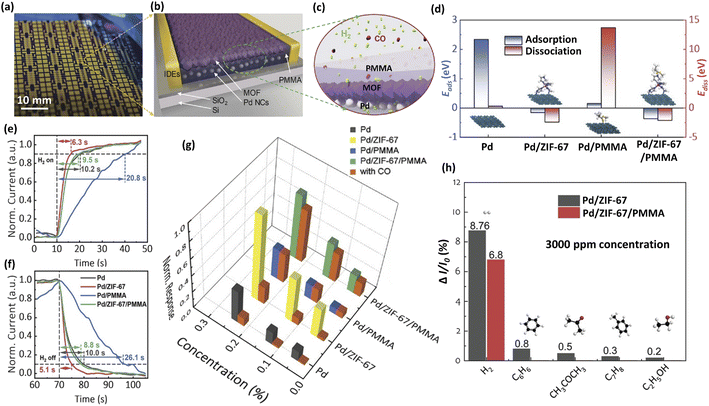 | ||
| Fig. 12 (a–c) The prototype and concept of the Pd/MOF/PMMA nanocomposite-based H2 sensor. (d) Calculated adsorption and dissociation energies for Pd, Pd/ZIF-67, Pd/PMMA, and Pd/ZIF-67/PMMA. (e) Response and (f) recovery curves of Pd, Pd/ZIF-67, Pd/PMMA, and Pd/ZIF-67/PMMA-based sensors. (g) Comparison of normalized response amplitudes at 0.05%, 0.13%, and 0.24% H2 concentrations with and without 1% CO for all four samples. (h) Selective sensing performance of the Pd/ZIF-67 and Pd/ZIF-67/PMMA sensors against various VOCs at 3000 ppm. This figure has been reproduced with permission from ref. 92, Wiley, Copyright 2012. | ||
When the heterojunction was combined with noble metal catalysts, its sensing properties can be greatly enhanced. Zhang et al.132 prepared a new ternary nanocomposite of Ag–ZnO/MoS2 via a layer-by-layer (LbL) self-assembly method for carbon monoxide (CO) sensing application. The Ag–ZnO/MoS2 nanocomposite sensor has excellent response, swift response/recovery characteristics, as well as acceptable repeatability and selectivity, which outstripped the pure ZnO and ZnO/MoS2 sensors. The underlying sensing mechanism of the Ag–ZnO/MoS2 nanocomposite film was attributed to the catalytic activity of Ag and the synergistic effect of ZnO and MoS2. Liu et al.133 fabricated a high-response formaldehyde gas sensor based on Ag–ZnO/In2O3 nanofibers. The Ag–ZnO/In2O3 exhibited superior sensitivity, low detection limit (9 ppb), excellent selectivity and durable stability (the deviation value ≤ 3%). Particularly, an ultra-high response value of about 186 towards 100 ppm of formaldehyde at 260 °C was achieved. The enhanced gas sensing properties can be mainly attributed to multi-level heterojunctions (n–n heterojunction and ohmic junction) and the spill-over effect of Ag, ultimately increasing the adsorption of gas molecules on the surface of the sensing material.
Wang et al.134 synthesized Au@ZnO@ZIF-8 via an anisotropic growth method for the simultaneous detection and removal of formaldehyde at RT. Due to the synergistic effects of the high conductivity of ZnO, the superior gas adsorption capability of ZIF-8, the clean interface between ZnO and ZIF-8, and the plasmonic resonance of gold nanorods, the Au@ZnO@ZIF-8 demonstrated an excellent sensing performance with a selective detection toward formaldehyde at RT. Au@ZnO@ZIF-8 hybrids had enhanced selective adsorption, detection and oxidation of HCHO and prevented interference from gases such as H2O and toluene, where Au helped to generate charge carriers on a ZnO surface under visible-light irradiation.
Herein, we have further summarized the previously reported gas sensors based on different kinds of guest materials (noble metals, metal heteroatoms, metal oxides, MOF, TMD, polymers and multiple guest materials), as shown in Table 1.
| Type of guest material | Host–guest material | Target gas | Performance | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| Concentration | Response | Tresponse/Trecvoery (s) | Temperature (°C) | Limit of detection | ||||
| Noble metal | WO3–Ag | NO2 | 5 ppm | 90.3 | — | 225 | 0.5 ppm | 89 |
| WO3–Pd | H2 | 500 ppm | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 867 867 |
1.2/77 | 50 | 5 ppm | 135 | |
| WO3–Ru | Ethanol | 100 ppm | 120 | — | 200 | 221 ppb | 136 | |
| Co3O4–Au | Acetone | 100 ppm | 14.5 | 319/280 | 190 | 1 ppm | 90 | |
| Co3O4–Pd | Ethanol | 100 ppm | 24 | 12/25 | 150 | — | 67 | |
| TiO3–Pd | H2 | 1000 ppm | 9 | 1.6/1.4 | 230 | 1 ppm | 93 | |
| CNT–Pt | Toluene | 5 ppm | 5.06 | 80/90 | 150 | 1 ppm | 85 | |
| Graphene–Pd | H2 | 40 ppm | 55% | 6/23 | RT | — | 96 | |
| MoS2–Pt | H2 | 1% | 8 | 8.1/16 | RT | — | 97 | |
| WSe2–Pd | CO | 5 ppm | 9.25 | 52/97 | RT | 1 ppm | 98 | |
| Metal heteroatom | In2O3–Al | HCHO | 100 ppm | 60.3 | 2/— | 150 | 60 ppb | 104 |
| In2O3–Ca | Ethanol | 100 ppm | 183.3 | 2/56 | 240 | 5 ppm | 105 | |
| ZnO–Al | CO | 80 ppm | 74 | 21/70 | 250 | 250 ppb | 106 | |
| SnO2–Zn | HCHO | 100 ppm | 25.7 | 12/45 | 400 | 500 ppb | 22 | |
| In2O3–Sn | HCHO | 100 ppm | 24.5 | 2.6/79.4 | 160 | — | 137 | |
| Metal oxide | TiO2–WO3 | H2 | 1000 ppm | 78.21 | 20/23 | RT | — | 108 |
| CeO2–NiO | Isopropanol | 100 ppm | 1570 | 15/19 | RT | 1 | 109 | |
| SnO2–ZnO | HCHO | 1 ppm | 9.7 | 12/24 | 200 | 70 ppb | 60 | |
| SnO2–CuO | Toluene | 75 ppm | 540 | 100/36 | 400 | — | 138 | |
| Bi2Mo3O12–Co3O4 | Ethanol | 100 ppm | 30.25 | 34/26 | 170 | 1 ppm | 139 | |
| PANI–SnO2 | NH3 | 100 ppm | 29.8 | 125/167 | RT | 10 ppb | 111 | |
| rGO/CuO | CO | 1 ppm | 2.56 | 70/160 | RT | 250 ppb | 113 | |
| MWCNT/SnO2 | H2S | 50 ppm | 108 | 23/44 | 70 | 43 ppb | 140 | |
| MoS2–ZnO | NO2 | 50 ppm | 35 | 1.5/30.9 | RT | 10 ppb | 114 | |
| MOF | SnO2-ZIF-8 | HCHO | 100 ppm | 23.7 | 2/26 | 300 | 63 ppb | 34 |
| ZnO-ZIF-8 | HCHO | 100 ppm | 12.5 | 16/9 | 300 | 5.6 ppm | 141 | |
| ZnO-ZIF-71 | Ethanol | 50 ppm | 320% | — | 250 | — | 142 | |
| SnO2-ZIF-67 | CO2 | 5000 ppm | 16.5 | 10/25 | 205 | — | 143 | |
| Pd-ZIF-8 | H2 | 1% | 3.5 | 7/10 | RT | 0.06% | 123 | |
| Graphene–Cu–BTC | Chloroform | 22.6 ppm | 2.5 | — | RT | 2.82 ppm | 124 | |
| TMD | Cu2O–MoS2 | NH3 | 100 ppm | 872% | — | RT | — | 126 |
| ZnO–MoS2 | Acetone | 500 ppb | 150% | 9/17 | 350 | 5 ppb | 127 | |
| ZnO–MoSe2 | H2 | 500 ppm | 60 | 19/40 | RT | 1 ppm | 144 | |
| CNF–WS2 | NO2 | 10 ppm | 6.9 | 23/94 | 150 | 31 ppb | 128 | |
| Polymer | rGO–PANI | NH3 | 10 ppm | 8 | 36/18 | RT | 100 ppb | 57 |
| TiO2–PANI | CO2 | 1000 ppm | 53 | 552/342 | RT | — | 130 | |
| CNT–PEI | CO2 | 1000 ppm | 4.2 | — | RT | — | 129 | |
| GO–PPy | CO | 300 | 45 | 89/95 | RT | — | 145 | |
| Pd–PMMA | H2 | 1000 ppm | 9 | 22.84/— | RT | 50 ppm | 131 | |
| Multiple | Pd-ZIF-67-PMMA | H2 | 1% | 25 | 9.5/8.8 | RT | — | 92 |
| ZnO–Ag–MoS2 | CO | 100 ppm | 5 | 40/50 | RT | 1 ppm | 132 | |
| ZnO–Au-ZIF-8 | HCHO | 100 ppm | 70 | — | RT | 250 ppb | 134 | |
| ZnO–Ag–In2O3 | HCHO | 100 ppm | 186 | 10/67 | 260 | 9 ppb | 133 | |
| TiO2–CoTiO3–Pd | Benzene | 50 ppm | 33.46 | 49/9 | RT | 100 ppb | 73 | |
4. Conclusions and perspectives
In this review, the functions, working mechanisms, and advantages/disadvantages of seven commonly-used guest materials (noble metals, metal heteroatoms, metal oxides, MOFs, TMDs, polymers and multiple guest materials) in resistive gas sensors are discussed in detail. Resistive gas sensors have been considered attractive candidates for gas detection due to their fast response/recovery, ease of operation, low cost, and good portability. To date, plenty of gas-sensitive materials (represented by MOSs) have been developed. The shortcomings of these materials make it difficult for them to meet the needs of practical applications. For example, MOSs endure high operating temperatures and poor selectivity; CPs suffer from poor stability and low sensitivity; carbon-based materials have the problems of poor reversibility; TMDs tend to agglomerate and self-stack, and oxidize easily in air. To solve these problems and obtain better gas-sensitive properties, it is necessary to introduce functional guest materials. Guest materials can functionalize the host material by introducing catalytic sites, promoting charge transfer, improving carrier transport, and/or introducing protective/sieving/enrichment layers. Guest materials can also produce synergistic effects and complementary advantages with the host materials, thereby effectively improving the sensitivity, selectivity and stability of the gas sensors. However, the introduction of guest materials will also cause some negative problems in some cases. Fig. 13 shows the merits and demerits of the functional guest materials in detail.With the development of industrialization, environmental problems are receiving more and more attention. To improve the performance of resistive gas sensors, the introduction of functional guest materials has become an inevitable trend. Although some progress has been made in the preparation of high-performance resistive gas sensors based on various guest materials, current research still faces some challenges that need to be further addressed:
(1) Gas sensors that operate under low energy consumption and room-temperature conditions are receiving ever-increasing attention. Some emerging 2D materials (such as TMDs, rGO, 2D-conductive-MOFs, and MXenes) show great potential in this area and have great application prospects after optimization by guest materials.
(2) For most host–guest hybrid materials, the control of the synthesis process (such as the doping amount, thickness of the load film, aperture control, etc.) has a great influence on the gas sensor performance. Hence, it may be necessary to focus more attention on the influencing mechanism of these key parameters rather than focusing only on gas-sensitive materials themselves.
(3) The modification of guest materials can greatly improve the performance of the host materials but the current research on the mechanism is not in-depth. Interpretations of their sensing mechanisms are mainly based on the speculation of experimental results. It is necessary to explore the gas sensitivity mechanisms at a deeper level through in situ characterization tests combined with theoretical calculations and simulation.
(4) It is difficult for a single gas sensor to meet the requirements of multi-component mixed gas detection in the fields of human exhaled disease diagnosis and atmospheric gas pollutant monitoring. Therefore, a sensor array (e-nose) composed of multiple sensor units is needed to further improve the accuracy of gas detection combined with intelligent algorithms.
Author contributions
Jianan Wang conceived and designed the work; Ze Wang wrote the manuscript; Jianan Wang, Lei Zhu, Rui Zhuang, Pengfei Mu and W. Yan revised the manuscript. Ze Wang and Jingzhao Wang arranged the figures. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Abbreviations
| MOSs | Metal oxide semiconductors |
| CPs | Conductive polymers |
| MOFs | Metal–organic frameworks |
| PPy | Polypyrrole |
| PTh | Polythiophene |
| PANI | Polyaniline |
| TMDs | Transition metal dichalcogenides |
| IDE | Interdigitated electrode |
| MEMS | Micro-Electro-Mechanical System |
| PET | Polyethylene-terephthalate |
| PI | Polyimide |
| GO | Graphene oxide |
| rGO | Reduced graphene oxide |
| CNT | Carbon nanotube |
| SWCNT | Single wall carbon nanotube |
| MWCNTs | Multi-walled carbon nanotubes |
| ZZS HNFs | Zn2+-doped SnO2 hollow nanofibers |
| CNFs | Carbon nanofibers |
| 1D | One-dimensional |
| 2D | Two-dimensional |
| 3D | Three-dimensional |
| IEA | In situ enrichment amplification |
| PMMA | Polymethyl methacrylate |
| pG | Pristine graphene |
| TEM | Transmission electron microscope |
| RT | Room temperature |
| VOCs | Volatile organic compounds |
| ALD | Atomic layer deposition |
| LbL | Layer-by-layer |
| DFT | Density functional theory |
| PEI | Polyethyleneimine |
Acknowledgements
We thank the financial support from the National Natural Science Foundation of China (52172097 and 51978569), Key Research and Development Program of Shaanxi Province (2022GY-301), Basic and Public Projects of Zhejiang Province (LGF21E020001), China Postdoctoral Science Foundation (2020M683467), China Scholarship Council foundation (201906285020) and Shaanxi Association for Science and Technology Youth Talent Promotion Project (20220401).References
- J. van den Broek, S. Abegg, S. E. Pratsinis and A. T. Guntner, Nat. Commun., 2019, 10, 4220 CrossRef CAS PubMed.
- F. Zhang, Q. Lin, F. Han, Z. Wang, B. Tian, L. Zhao, T. Dong and Z. Jiang, Microsyst. Nanoeng., 2022, 8, 40 CrossRef CAS PubMed.
- Y. Zhang, J. Zhao, T. Du, Z. Zhu, J. Zhang and Q. Liu, Sci. Rep., 2017, 7, 1960 CrossRef PubMed.
- W. Liu, L. Xu, K. Sheng, X. Zhou, B. Dong, G. Lu and H. Song, NPG Asia Mater., 2018, 10, 293–308 CrossRef CAS.
- G. S. Kulkarni, K. Reddy, Z. Zhong and X. Fan, Nat. Commun., 2014, 5, 4376 CrossRef CAS PubMed.
- S. Jiang and Y. Liu, TrAC, Trends Anal. Chem., 2020, 126, 115877 CrossRef CAS.
- P. F. M. Pereira, P. H. de Sousa Picciani, V. Calado and R. V. Tonon, Trends Food Sci. Technol., 2021, 118, 36–44 CrossRef CAS.
- L. Zhu and W. Zeng, Sens. Actuators, A, 2017, 267, 242–261 CrossRef CAS.
- R. Malik, V. K. Tomer, Y. K. Mishra and L. Lin, Appl. Phys. Rev., 2020, 7, 021301 CAS.
- H. Nazemi, A. Joseph, J. Park and A. Emadi, Sensors, 2019, 19, 1258 CrossRef PubMed.
- S. Akshya and A. V. Juliet, Sci. Rep., 2021, 11, 970 CrossRef CAS PubMed.
- D. Del Orbe Henriquez, I. Cho, H. Yang, J. Choi, M. Kang, K. S. Chang, C. B. Jeong, S. W. Han and I. Park, ACS Appl. Nano Mater., 2020, 4, 7–12 CrossRef.
- D. Tyagi, H. Wang, W. Huang, L. Hu, Y. Tang, Z. Guo, Z. Ouyang and H. Zhang, Nanoscale, 2020, 12, 3535–3559 RSC.
- D. Popa and F. Udrea, Sensors, 2019, 19, 2076 CrossRef CAS PubMed.
- J. Y. Monter-Guzmán, X. Chu, E. Comini, M. Epifani and R. Zanella, Chemosensors, 2021, 9, 193 CrossRef.
- Z. Yang, L. Jiang, J. Wang, F. Liu, J. He, A. Liu, S. Lv, R. You, X. Yan, P. Sun, C. Wang, Y. Duan and G. Lu, Sens. Actuators, B, 2021, 326, 128828 CrossRef CAS.
- T. Lin, X. Lv, Z. Hu, A. Xu and C. Feng, Sensors, 2019, 19, 233 CrossRef PubMed.
- Z. Wang, L. Zhu, S. Sun, J. Wang and W. Yan, Chemosensors, 2021, 9, 198 CrossRef CAS.
- L. Zhu, J. Wang, J. Liu, X. Chen, Z. Xu, Q. Ma, Z. Wang, J. Liang, S. Li and W. Yan, Appl. Surf. Sci., 2022, 590, 153085 CrossRef CAS.
- Z. Wang, L. Zhu, J. Liu, J. Wang and W. Yan, Energy Fuels, 2022, 36, 6038–6057 CrossRef CAS.
- G. Neri, Chemosensors, 2015, 3, 1–20 CrossRef CAS.
- L. Zhu, J. Wang, J. Liu, M. S. Nasir, J. Zhu, S. Li, J. Liang and W. Yan, Sens. Actuators, B, 2021, 326, 128819 CrossRef CAS.
- V. Schroeder, S. Savagatrup, M. He, S. Lin and T. M. Swager, Chem. Rev., 2019, 119, 599–663 CrossRef CAS PubMed.
- H. Cruz-Martinez, H. Rojas-Chavez, F. Montejo-Alvaro, Y. A. Pena-Castaneda, P. T. Matadamas-Ortiz and D. I. Medina, Sensors, 2021, 21, 1992 CrossRef CAS PubMed.
- C.-C. Lin, S. Gupta, C. Chang, C.-Y. Lee and N.-H. Tai, Mater. Lett., 2021, 297, 129941 CrossRef CAS.
- W.-T. Koo, J.-S. Jang and I.-D. Kim, Chem, 2019, 5, 1938–1963 CAS.
- C. Dong, R. Zhao, L. Yao, Y. Ran, X. Zhang and Y. Wang, J. Alloys Compd., 2020, 820, 153194 CrossRef CAS.
- D. Zhang, S. Yu, X. Wang, J. Huang, W. Pan, J. Zhang, B. E. Meteku and J. Zeng, J. Hazard. Mater., 2022, 423, 127160 CrossRef CAS PubMed.
- Z. Li, X. Liu, M. Zhou, S. Zhang, S. Cao, G. Lei, C. Lou and J. Zhang, J. Hazard. Mater., 2021, 415, 125757 CrossRef CAS PubMed.
- Y. Jian, W. Hu, Z. Zhao, P. Cheng, H. Haick, M. Yao and W. Wu, Nano-Micro Lett., 2020, 12, 71 CrossRef CAS PubMed.
- Z. Li, H. Li, Z. Wu, M. Wang, J. Luo, H. Torun, P. Hu, C. Yang, M. Grundmann, X. Liu and Y. Fu, Mater. Horiz., 2019, 6, 470–506 RSC.
- H. Yuan, N. Li, W. Fan, H. Cai and D. Zhao, Adv. Sci., 2022, 9, 2104374 CrossRef CAS PubMed.
- F. A. A. Nugroho, I. Darmadi, L. Cusinato, A. Susarrey-Arce, H. Schreuders, L. J. Bannenberg, A. B. da Silva Fanta, S. Kadkhodazadeh, J. B. Wagner, T. J. Antosiewicz, A. Hellman, V. P. Zhdanov, B. Dam and C. Langhammer, Nat. Mater., 2019, 18, 489–495 CrossRef CAS PubMed.
- L. Zhu, J. Wang, J. Liu, Z. Xu, M. S. Nasir, X. Chen, Z. Wang, S. Sun, Q. Ma, J. Liu, J. Feng, J. Liang and W. Yan, Sens. Actuators, B, 2022, 354, 131206 CrossRef CAS.
- W. H. Brattain and J. Bardeen, Bell Syst. Tech. J., 1954, 32, 1–41 CrossRef.
- T. Seiyama and A. Kato, Anal. Chem., 1962, 34, 1502–1503 CrossRef CAS.
- P. J. Shaver, Appl. Phys. Lett., 1967, 11, 255–257 CrossRef CAS.
- C. Nylander, M. Armgarth and L. Lundstrom, Anal. Chem. Symp. Ser., 1983, 17, 203–207 CAS.
- F. Schedin, A. K. Geim, S. V. Morozov, E. W. Hill, P. Blake, M. I. Katsnelson and K. S. Novoselov, Nat. Mater., 2007, 6, 652–655 CrossRef CAS PubMed.
- Y. P. Sun, K. F. Fu, Y. Lin and W. J. Huang, Acc. Chem. Res., 2002, 35, 1096–1104 CrossRef CAS PubMed.
- D. Wang, S. M. Huang, H. J. Li, A. Y. Chen, P. Wang, J. Yang, X. Y. Wang and J. H. Yang, Sens. Actuators, B, 2019, 282, 961–971 CrossRef CAS.
- J. N. Chang, H. J. Zhang, J. L. Cao and Y. Wang, Adv. Powder Technol., 2022, 33, 103432 CrossRef CAS.
- J. S. Kim, H. W. Yoo, H. O. Choi and H. T. Jung, Nano Lett., 2014, 14, 5941–5947 CrossRef CAS PubMed.
- K. Rathi and K. Pal, Adv. Mater. Interfaces, 2020, 7, 2000140 CrossRef CAS.
- J. H. Kim, J. Y. Kim, A. Mirzaei, H. W. Kim and S. S. Kim, Sens. Actuators, B, 2021, 332, 129493 CrossRef CAS.
- H. J. Zhang, F. N. Meng, L. Z. Liu, Y. J. Chen and P. J. Wang, J. Alloys Compd., 2018, 764, 147–154 CrossRef CAS.
- X. L. Yang, S. F. Zhang, Q. Yu, P. Sun, F. M. Liu, H. Y. Lu, X. Yan, X. Zhou, X. S. Liang, Y. Gao and G. Y. Lu, Sens. Actuators, B, 2018, 270, 538–544 CrossRef CAS.
- B. Y. Zong, Q. K. Xu, Q. J. Li, X. Fang, X. Y. Chen, C. B. Liu, J. B. Zang, Z. Bo and S. Mao, J. Mater. Chem. A, 2021, 9, 14411–14421 RSC.
- D. Wang, D. Zhang, Y. Yang, Q. Mi, J. Zhang and L. Yu, ACS Nano, 2021, 15, 2911–2919 CrossRef CAS PubMed.
- H. Y. Li, S. N. Zhao, S. Q. Zang and J. Li, Chem. Soc. Rev., 2020, 49, 6364–6401 RSC.
- J. Zhang, X. H. Liu, G. Neri and N. Pinna, Adv. Mater., 2016, 28, 795–831 CrossRef CAS PubMed.
- J. F. Fennell, S. F. Liu, J. M. Azzarelli, J. G. Weis, S. Rochat, K. A. Mirica, J. B. Ravnsbaek and T. M. Swager, Angew. Chem., Int. Ed., 2016, 55, 1266–1281 CrossRef CAS PubMed.
- S. Li, L. Xie, M. He, X. Hu, G. Luo, C. Chen and Z. Zhu, Sens. Actuators, B, 2020, 310, 127828 CrossRef CAS.
- J. Wang, Q. Zhou, Z. Wei, L. Xu and W. Zeng, Ceram. Int., 2020, 46, 29222–29232 CrossRef CAS.
- N. Yi, Z. Cheng, H. Li, L. Yang, J. Zhu, X. Zheng, Y. Chen, Z. Liu, H. Zhu and H. Cheng, Mater. Today Phys., 2020, 15, 100265 CrossRef.
- R. You, D.-D. Han, F. Liu, Y.-L. Zhang and G. Lu, Sens. Actuators, B, 2018, 277, 114–120 CrossRef CAS.
- Y. Guo, T. Wang, F. Chen, X. Sun, X. Li, Z. Yu, P. Wan and X. Chen, Nanoscale, 2016, 8, 12073–12080 RSC.
- B. Behera and S. Chandra, Sens. Actuators, B, 2016, 229, 414–424 CrossRef CAS.
- D. Meng, J. P. Si, M. Y. Wang, G. S. Wang, Y. B. Shen, X. G. San and F. L. Meng, Chin. Chem. Lett., 2020, 31, 2133–2136 CrossRef CAS.
- C. Lou, C. Yang, W. Zheng, X. Liu and J. Zhang, Sens. Actuators, B, 2021, 329, 129218 CrossRef CAS.
- M. K. Filippidou, M. Chatzichristidi and S. Chatzandroulis, Sens. Actuators, B, 2019, 284, 7–12 CrossRef.
- A. Nag, S. C. Mukhopadhyay and J. Kosel, IEEE Sens. J., 2017, 17, 3949–3960 CAS.
- Z. Q. Zheng, J. D. Yao, B. Wang and G. W. Yang, Sci. Rep., 2015, 5, 11070 CrossRef CAS PubMed.
- Z. Q. Zheng, J. D. Yao, B. Wang and G. W. Yang, Nanotechnology, 2017, 28, 415501 CrossRef PubMed.
- P. M. Perillo and D. F. Rodriguez, J. Alloys Compd., 2016, 657, 765–769 CrossRef CAS.
- S. M. Majhi, A. Mirzaei, H. W. Kim, S. S. Kim and T. W. Kim, Nano Energy, 2021, 79, 105369 CrossRef CAS PubMed.
- G. Yuan, Y. Zhong, Y. Chen, Q. Zhuo and X. Sun, RSC Adv., 2022, 12, 6725–6731 RSC.
- T. J. Hsueh and S. S. Wu, Sens. Actuators, B, 2021, 329, 129201 CrossRef CAS.
- L. F. Zhu, J. C. She, J. Y. Luo, S. Z. Deng, J. Chen, X. W. Ji and N. S. Xu, Sens. Actuators, B, 2011, 153, 354–360 CrossRef CAS.
- I. D. Kim, A. Rothschild and H. L. Tuller, Acta Mater., 2013, 61, 974–1000 CrossRef CAS.
- T. He, W. Liu, T. Lv, M. Ma, Z. Liu, A. Vasiliev and X. Li, Sens. Actuators, B, 2021, 329, 129275 CrossRef CAS.
- X. Yang, S. Zhang, Q. yu, L. Zhao, P. Sun, T. Wang, F. Liu, X. Yan, Y. Gao, X. Liang, S. Zhang and G. Lu, Sens. Actuators, B, 2019, 281, 415–423 CrossRef CAS.
- D. Wang, D. Zhang and Q. Mi, Sens. Actuators, B, 2022, 350, 130830 CrossRef CAS.
- M. Liu, Z. Wang, P. Song, Z. Yang and Q. Wang, Sens. Actuators, B, 2021, 340, 129946 CrossRef CAS.
- J. Lee, Y. Jung, S.-H. Sung, G. Lee, J. Kim, J. Seong, Y.-S. Shim, S. C. Jun and S. Jeon, J. Mater. Chem. A, 2021, 9, 1159–1167 RSC.
- J. H. Lee, Sens. Actuators, B, 2009, 140, 319–336 CrossRef CAS.
- S. Virji, R. B. Kaner and B. H. Weiller, J. Phys. Chem. B, 2006, 110, 22266–22270 CrossRef CAS PubMed.
- K. H. Hong, K. W. Oh and T. J. Kang, J. Appl. Polym. Sci., 2004, 92, 37–42 CrossRef CAS.
- N. V. Bhat, A. P. Gadre and V. A. Bambole, J. Appl. Polym. Sci., 2001, 80, 2511–2517 CrossRef CAS.
- O. S. Kwon, S. J. Park, H. Yoon and J. Jang, Chem. Commun., 2012, 48, 10526–10528 RSC.
- X. X. Xing, L. L. Du, D. L. Feng, C. Wang, Y. Y. Tian, Z. X. Li, H. G. Liu and D. C. Yang, Sens. Actuators, B, 2022, 351, 130944 CrossRef CAS.
- Y. Huang, E. Sutter, J. T. Sadowski, M. Cotlet, O. L. A. Monti, D. A. Racke, M. R. Neupane, D. Wickramaratne, R. K. Lake, B. A. Parkinson and P. Sutter, ACS Nano, 2014, 8, 10743–10755 CrossRef CAS PubMed.
- C. Anichini, W. Czepa, D. Pakulski, A. Aliprandi, A. Ciesielski and P. Samori, Chem. Soc. Rev., 2018, 47, 4860–4908 RSC.
- L. Yang, W. Xiao, J. Wang, X. Li and L. Wang, RSC Adv., 2021, 11, 37120–37130 RSC.
- Y. J. Kwon, H. G. Na, S. Y. Kang, S.-W. Choi, S. S. Kim and H. W. Kim, Sens. Actuators, B, 2016, 227, 157–168 CrossRef CAS.
- A. Vahl, O. Lupan, D. Santos-Carballal, V. Postica, S. Hansen, H. Cavers, N. Wolff, M.-I. Terasa, M. Hoppe, A. Cadi-Essadek, T. Dankwort, L. Kienle, N. H. de Leeuw, R. Adelung and F. Faupel, J. Mater. Chem. A, 2020, 8, 16246–16264 RSC.
- S. Kumar, S. Kumar, M. Sengar and P. Kumari, RSC Adv., 2021, 11, 13674–13699 RSC.
- M. E. Franke, T. J. Koplin and U. Simon, Small, 2006, 2, 36–50 CrossRef CAS PubMed.
- P. Jaroenapibal, P. Boonma, N. Saksilaporn, M. Horprathum, V. Amornkitbamrung and N. Triroj, Sens. Actuators, B, 2018, 255, 1831–1840 CrossRef CAS.
- Z. Li, Y. Zhang, H. Zhang and J. Yi, Sens. Actuators, B, 2021, 344, 130182 CrossRef CAS.
- X. Xing, Z. Li, X. Chen, L. Du, Y. Tian, D. Feng, C. Wang, G. Liu and D. Yang, ACS Appl. Mater. Interfaces, 2022, 14, 17911–17919 CrossRef CAS PubMed.
- B. Xie, B. Ding, P. Mao, Y. Wang, Y. Liu, M. Chen, C. Zhou, H. M. Wen, S. Xia, M. Han, R. E. Palmer, G. Wang and J. Hu, Small, 2022, 18, 2200634 CrossRef CAS PubMed.
- D. Wang, J. Yang, L. Bao, Y. Cheng, L. Tian, Q. Ma, J. Xu, H. J. Li and X. Wang, J. Colloid Interface Sci., 2021, 597, 29–38 CrossRef CAS PubMed.
- Z. Chen, J. Wang and Y. Wang, Talanta, 2021, 235, 122745 CrossRef CAS PubMed.
- U. Latif and F. L. Dickert, Sensors, 2015, 15, 30504–30524 CrossRef CAS PubMed.
- J. L. Johnson, A. Behnam, S. J. Pearton and A. Ural, Adv. Mater., 2010, 22, 4877–4880 CrossRef CAS PubMed.
- C. H. Park, W. T. Koo, Y. J. Lee, Y. H. Kim, J. Lee, J. S. Jang, H. Yun, I. D. Kim and B. J. Kim, ACS Nano, 2020, 14, 9652–9661 CrossRef CAS PubMed.
- D. Zhang, D. Wang, W. Pan, M. Tang and H. Zhang, Sens. Actuators, B, 2022, 360, 131634 CrossRef CAS.
- H. Jun, W. Tao, W. Yanjie, H. Da, H. Guili, H. Yutong, H. Nantao, S. Yanjie, Z. Zhihua, Z. Yafei and Y. Zhi, Sens. Actuators, B, 2018, 263, 120–128 CrossRef.
- C. S. Reddy, L. W. Zhang, Y. J. Qiu, Y. N. Chen, A. S. Reddy, P. S. Reddy and S. R. Dugasani, J. Ind. Eng. Chem., 2018, 63, 411–419 CrossRef.
- S. Palimar, S. D. Kaushik, V. Siruguri, D. Swain, A. E. Viegas, C. Narayana and N. G. Sundaram, Dalton Trans., 2016, 45, 13547–13555 RSC.
- Z. H. Wang, C. L. Hou, Q. M. De, F. B. Gu and D. M. Han, ACS Sens., 2018, 3, 468–475 CrossRef CAS PubMed.
- D. Degler, U. Weimar and N. Barsan, ACS Sens., 2019, 4, 2228–2249 CrossRef CAS PubMed.
- H. Chen, Y. Zhao, L. Shi, G. D. Li, L. Sun and X. Zou, ACS Appl. Mater. Interfaces, 2018, 10, 29795–29804 CrossRef CAS PubMed.
- C. Zhao, H. Gong, G. Niu and F. Wang, Sens. Actuators, B, 2019, 299, 126946 CrossRef CAS.
- M. Hjiri, F. Bahanan, M. S. Aida, L. El Mir and G. Neri, J. Inorg. Organomet. Polym. Mater., 2020, 30, 4063–4071 CrossRef CAS.
- S. Yang, G. Lei, H. Xu, Z. Lan, Z. Wang and H. Gu, Nanomaterials, 2021, 11, 1026 CrossRef CAS PubMed.
- M. Kumaresan, M. Venkatachalam, M. Saroja and P. Gowthaman, Inorg. Chem. Commun., 2021, 129, 108663 CrossRef CAS.
- N. Jayababu, M. Poloju, J. Shruthi and M. V. R. Reddy, RSC Adv., 2019, 9, 13765–13775 RSC.
- J. G. Ibanez, M. E. Rincon, S. Gutierrez-Granados, M. Chahma, O. A. Jaramillo-Quintero and B. A. Frontana-Uribe, Chem. Rev., 2018, 118, 4731–4816 CrossRef CAS PubMed.
- S. Li, A. Liu, Z. Yang, J. He, J. Wang, F. Liu, H. Lu, X. Yan, P. Sun, X. Liang, Y. Gao and G. Lu, Sens. Actuators, B, 2019, 299, 126970 CrossRef CAS.
- H. Jin, T. P. Huynh and H. Haick, Nano Lett., 2016, 16, 4194–4202 CrossRef CAS PubMed.
- D. Zhang, C. Jiang, J. Liu and Y. Cao, Sens. Actuators, B, 2017, 247, 875–882 CrossRef CAS.
- M. Ikram, H. Lv, Z. Liu, K. Shi and Y. Gao, J. Mater. Chem. A, 2021, 9, 14722–14730 RSC.
- W. Wang, X. H. Xiong, N. X. Zhu, Z. Zeng, Z. W. Wei, M. Pan, D. Fenske, J. J. Jiang and C. Y. Su, Angew. Chem., Int. Ed., 2022, 61, e202201766 CAS.
- D. F. Lv, P. J. Zhou, J. H. Xu, S. Tu, F. Xu, J. Yan, H. X. Xi, W. B. Yuan, Q. Fu, X. Chen and Q. B. Xia, Chem. Eng. J., 2022, 431, 133208 CrossRef CAS.
- C. S. Diercks, Y. Z. Liu, K. E. Cordova and O. M. Yaghi, Nat. Mater., 2018, 17, 301–307 CrossRef CAS PubMed.
- L. H. Shi, N. Li, D. M. Wang, M. K. Fan, S. L. Zhang and Z. J. Gong, TrAC, Trends Anal. Chem., 2021, 134, 116131 CrossRef CAS.
- J. Xue, Q. Sun, Q. Li and J. Qian, CrystEngComm, 2022, 24, 3649–3655 RSC.
- J. Ding, L. Zhong, X. Wang, L. Chai, Y. Wang, M. Jiang, T.-T. Li, Y. Hu, J. Qian and S. Huang, Sens. Actuators, B, 2020, 306, 127551 CrossRef CAS.
- R. Zhang, L. Lu, Y. Chang and M. Liu, J. Hazard. Mater., 2022, 429, 128321 CrossRef CAS PubMed.
- X. L. Zhang, Z. Y. Zhai, J. N. Wang, X. K. Hao, Y. X. Sun, S. Y. Yu, X. Q. Lin, Y. Qin and C. J. Li, Chemnanomat, 2021, 7, 1117–1124 CrossRef CAS.
- W. T. Koo, S. Qiao, A. F. Ogata, G. Jha, J. S. Jang, V. T. Chen, I. D. Kim and R. M. Penner, ACS Nano, 2017, 11, 9276–9285 CrossRef CAS PubMed.
- T. T. Tung, M. T. Tran, J.-F. Feller, M. Castro, T. Van Ngo, K. Hassan, M. J. Nine and D. Losic, Carbon, 2020, 159, 333–344 CrossRef CAS.
- D. Zhang, Z. Yang, S. Yu, Q. Mi and Q. Pan, Coord. Chem. Rev., 2020, 413, 213272 CrossRef CAS.
- Y. Ding, X. Guo, B. Du, X. Hu, X. Yang, Y. He, Y. Zhou and Z. Zang, J. Mater. Chem. C, 2021, 9, 4838–4846 RSC.
- X. Chang, X. Li, X. Qiao, K. Li, Y. Xiong, X. Li, T. Guo, L. Zhu and Q. Xue, Sens. Actuators, B, 2020, 304, 127430 CrossRef CAS.
- Y. Xu, J. Xie, Y. Zhang, F. Tian, C. Yang, W. Zheng, X. Liu, J. Zhang and N. Pinna, J. Hazard. Mater., 2021, 411, 125120 CrossRef CAS PubMed.
- M. Han, S. Jung, Y. Lee, D. Jung and S. H. Kong, Micromachines, 2021, 12, 1053 CrossRef PubMed.
- R. K. Sonker, S. R. Sabhajeet and B. C. Yadav, J. Mater. Sci.: Mater. Electron., 2016, 27, 11726–11732 CrossRef CAS.
- M. Chen, P. Mao, Y. Qin, J. Wang, B. Xie, X. Wang, D. Han, G. H. Wang, F. Song, M. Han, J. M. Liu and G. Wang, ACS Appl. Mater. Interfaces, 2017, 9, 27193–27201 CrossRef CAS PubMed.
- D. Zhang, Y. e. Sun, C. Jiang, Y. Yao, D. Wang and Y. Zhang, Sens. Actuators, B, 2017, 253, 1120–1128 CrossRef CAS.
- J. Liu, L. Zhang, B. Cheng, J. Fan and J. Yu, J. Hazard. Mater., 2021, 413, 125352 CrossRef CAS PubMed.
- D. Wang, Z. Li, J. Zhou, H. Fang, X. He, P. Jena, J. B. Zeng and W. N. Wang, Nano-Micro Lett., 2018, 10, 4 CrossRef PubMed.
- Z. Zhu, X. Xing, D. Feng, Z. Li, Y. Tian and D. Yang, Nanoscale, 2021, 13, 12669–12675 RSC.
- J. Li, Q. Ding, X. Mo, Z. Zou, P. Cheng, Y. Li, K. Sun, Y. Fu, Y. Wang and D. He, RSC Adv., 2021, 11, 39130–39141 RSC.
- J. Y. Zhou, J. L. Bai, H. Zhao, Z. Y. Yang, X. Y. Gu, B. Y. Huang, C. H. Zhao, L. Cairang, G. Z. Sun, Z. X. Zhang, X. J. Pan and E. Q. Xie, Sens. Actuators, B, 2018, 265, 273–284 CrossRef CAS.
- A. Hermawan, Y. Asakura, M. Inada and S. Yin, J. Mater. Sci. Technol., 2020, 51, 119–129 CrossRef.
- S. U. Din, M. U. Haq, R. Khatoon, X. Chen, L. Li, M. Zhang and L. Zhu, RSC Adv., 2020, 10, 21940–21953 RSC.
- H. Liu, W. Zhang, H. Yu, L. Gao, Z. Song, S. Xu, M. Li, Y. Wang, H. Song and J. Tang, ACS Appl. Mater. Interfaces, 2016, 8, 840–846 CrossRef CAS PubMed.
- H. Tian, H. Fan, M. Li and L. Ma, ACS Sens., 2015, 1, 243–250 CrossRef.
- T. Zhou, Y. Sang, X. Wang, C. Wu, D. Zeng and C. Xie, Sens. Actuators, B, 2018, 258, 1099–1106 CrossRef CAS.
- D. M. ME, N. G. Sundaram and S. B. Kalidindi, Chemistry, 2018, 24, 9220–9223 CrossRef PubMed.
- A. Abun, B.-R. Huang, A. Saravanan, D. Kathiravan and P.-D. Hong, ACS Appl. Nano Mater., 2020, 3, 12139–12147 CrossRef CAS.
- M. A. Farea, H. Y. Mohammed, P. W. sayyad, N. N. Ingle, T. Al-Gahouari, M. M. Mahadik, G. A. Bodkhe, S. M. Shirsat and M. D. Shirsat, Appl. Phys. A, 2021, 127, 681 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |