Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Magnetoelectric interaction in molecular multiferroic nanocomposites†
Alireza Jalouli *a and
Shenqiang Ren
*a and
Shenqiang Ren abc
abc
aDepartment of Mechanical and Aerospace Engineering, University at Buffalo, The State University of New York, Buffalo, New York 14260, USA. E-mail: alirezaj@buffalo.edu
bDepartment of Chemistry, University at Buffalo, The State University of New York, Buffalo, New York 14260, USA
cResearch and Education in Energy Environment & Water Institute, University at Buffalo, The State University of New York, Buffalo, New York 14260, USA
First published on 24th August 2022
Abstract
Incorporation of magnetic and electric orders in a form of multiferroics is an interesting topic in materials science. Making a molecular heterogeneous composite by incorporating the molecular magnet vanadium–chromium Prussian blue analogue (V–Cr PBA) and a molecular ferroelectric imidazolium chloride C3N2H5-ClO4 (ImClO4) provides a pathway towards achieving the room temperature magnetoelectric effect. The change of magnetization of about 6% is shown as a result of applying an electric field (21 kV cm−1) to the composite made of the aforementioned molecular crystals at room temperature. In the ferromagnetic resonance measurement (FMR) under the effect of an applied electric field, a shift of the resonance magnetic field is also observed in the nanocomposites. This work provides a pathway towards molecular multiferroic nanocomposites with magnetoelectric coupling interactions at room temperature.
Introduction
The search for multifunctional materials with coupling effects between electric and magnetic properties is an interesting topic in materials engineering. However, in the realm of molecular chemistry and physics, there is an equal interest in exploring molecular structures that can be tuned or respond to external stimuli manipulations. In that respect, multiferroic materials that simultaneously exhibit both electrical and magnetic properties are an active field of research.1 Some of the potential applications of multiferroic materials include microwave devices,2,3 energy harvesting, and memory storage devices.4,5 Molecular ferroic materials6,7 have the flexibility of incorporating their structures8 for exploring new compositions that demonstrate coupling between electric and magnetic responses.9–11One of the challenges in the path of achieving molecular multiferroic is incorporating suitable ferromagnetic and ferroelectric materials together in such a way that both phases exist simultaneously under the specific temperature, particularly room-temperature multiferroic orders. One of the promising molecular ferrimagnet families are Prussian blue analogues (PBA) with general formula MA(MB(CN)6)b. nH2O (MA MB = transition metals, CN = cyanide ligand).12 In this context, vanadium–chromium PBA (V–Cr PBA) molecular ferrimagnets show room temperature magnetic ordering transition.6,13,14 In Prussian blue analogues V–Cr PBA, the water molecules coordinate with vanadium cations to compensate the vacancies in the PBA lattice. This create zeolitic water molecules existing in the PB lattice,15 leading to the formation of hydrogen bonding networks that are capable to take part in a charge transfer mechanism of proton transfer.16 On the other hand, the water molecules are also connected with some of the vanadium atoms which by themselves are responsible for the magnetic properties of PBA. There are two promising strategies to change the magnetic properties of a molecular magnet material: the pressure and electric field stimuli.10,17 In some PBA, the pressure up to 0.8 GPa can shift magnetization up to 10%, at temperatures well below the transition temperature.17 In both scenarios, the stretching in the MAII![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N
N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) MBIII structure is the responsible for the change of symmetry and the modification of the magnetic property consequently.
MBIII structure is the responsible for the change of symmetry and the modification of the magnetic property consequently.
In this work, room temperature molecular ferroelectric crystal ImClO418 is selected with V–Cr PBA magnet for the preparation of heterogeneous composites.
At room temperature, ImClO4 phase exhibits high polarization of 8 μC cm−1.19,20
In the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio between ImClO4 and V–Cr PBA, we demonstrate a noticeable response at room temperature with six percent of magnetization tunability when the bias electric field is in the order of 20 kV cm−1.
1 ratio between ImClO4 and V–Cr PBA, we demonstrate a noticeable response at room temperature with six percent of magnetization tunability when the bias electric field is in the order of 20 kV cm−1.
Main text
Material characterization
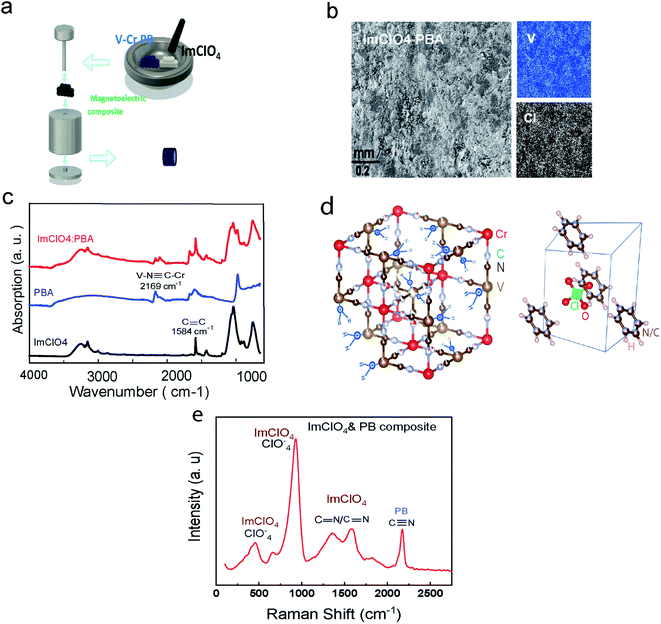
Magnetic V–Cr PBA and ferroelectric ImClO4 crystal structure are displayed in Fig. 1(d). In crystal phase, ImClO4 exhibits a hexagonal symmetry in contrast to the five-member ring symmetry of its molecular structure.18,21To prepare PB powder crystals, the power of potassium hexacyanochromate(III) and vanadium(II) chloride are mixed with 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 weight ratio in water and is left in a centrifuge tube for one day to react completely. The product is washed for a few times and then centrifuged to remove the unreacted agents. The achieved substance with a dark blue color is dried for one day in a vacuum chamber at room temperature.7,22 The product is a dark blue powder of PB nanocrystals. Since the PB powder is sensitive to oxidation, it is necessary to be kept in the glove box. To synthesize ImClO4, equal molar amounts of imidazolium (C3N2H4) with perchloric acid (HClO4) are mixed. The solvent is dried slowly in several days to achieve a white transparent powder crystal.18 Different ratios of the synthesized crystals (PB and ImClO4) were ground together and then pressed to have round palettes with 6 mm diameter and about 1 mm thickness. To contact a thin copper wire to the top and bottom of the composite palette sample, silver epoxy was used (Fig. 1(a), S1†). The Scanning electron Microscopy (SEM) along with the elements scanning of the composite (Fig. 1(b), S1†) show an even distribution of Cl atoms as representative of ImClO4 and V atoms as exist in V–Cr PBA component. The HitachiSU70 SEM/EDS microscope was used for the surface image and also elements scan. In Fig. 1(e), the Raman shift for in-plane-deformation, symmetric and antisymmetric stretching modes of ClO4− at 669 cm−1, 929 cm−1 and 1069 cm−1 are depicted. The other peak depicted is assigned to C
2 weight ratio in water and is left in a centrifuge tube for one day to react completely. The product is washed for a few times and then centrifuged to remove the unreacted agents. The achieved substance with a dark blue color is dried for one day in a vacuum chamber at room temperature.7,22 The product is a dark blue powder of PB nanocrystals. Since the PB powder is sensitive to oxidation, it is necessary to be kept in the glove box. To synthesize ImClO4, equal molar amounts of imidazolium (C3N2H4) with perchloric acid (HClO4) are mixed. The solvent is dried slowly in several days to achieve a white transparent powder crystal.18 Different ratios of the synthesized crystals (PB and ImClO4) were ground together and then pressed to have round palettes with 6 mm diameter and about 1 mm thickness. To contact a thin copper wire to the top and bottom of the composite palette sample, silver epoxy was used (Fig. 1(a), S1†). The Scanning electron Microscopy (SEM) along with the elements scanning of the composite (Fig. 1(b), S1†) show an even distribution of Cl atoms as representative of ImClO4 and V atoms as exist in V–Cr PBA component. The HitachiSU70 SEM/EDS microscope was used for the surface image and also elements scan. In Fig. 1(e), the Raman shift for in-plane-deformation, symmetric and antisymmetric stretching modes of ClO4− at 669 cm−1, 929 cm−1 and 1069 cm−1 are depicted. The other peak depicted is assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C/C
C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N symmetric stretching at 1448, 1586 cm−1 respectively. The Raman spectra system, Renishaw with diode laser (488 nm), was used for the previous part.
N symmetric stretching at 1448, 1586 cm−1 respectively. The Raman spectra system, Renishaw with diode laser (488 nm), was used for the previous part.
In order to confirm the presence of both materials (PBA and ImClO4) in the composite, we measured the Fourier-Transform infrared (FTIR) spectra of each of the constituents before mixing and the as-produced composite.
In Fig. 1(c), the FTIR spectra of the ferroelectric ImClO4, PBA, and the composition of them is displayed. The CC absorption in ImClO4 near 1600 cm−1 is displayed.23 In V–Cr PBA, the peaks located at 2113 and 2169 are assigned to stretching bonds, V(II)–N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–Cr(III) and, VIVO–N
C–Cr(III) and, VIVO–N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–Cr(III) respectively.6,12 The presence of VIV
C–Cr(III) respectively.6,12 The presence of VIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O with the assigned peak at 981 cm−1 is due to being exposed to air for a short time during the synthesis process.6 The ratio of V(III) to V(II) can have a slight shift in TC ranges between 296 to 310 ± 8 K. This will not be very significant change for the suggested properties here. The Agilent Cary 630 FTIR spectrometer which is suitable for dry-powder samples was used for the previous part.
O with the assigned peak at 981 cm−1 is due to being exposed to air for a short time during the synthesis process.6 The ratio of V(III) to V(II) can have a slight shift in TC ranges between 296 to 310 ± 8 K. This will not be very significant change for the suggested properties here. The Agilent Cary 630 FTIR spectrometer which is suitable for dry-powder samples was used for the previous part.
Magnetoelectric coupling
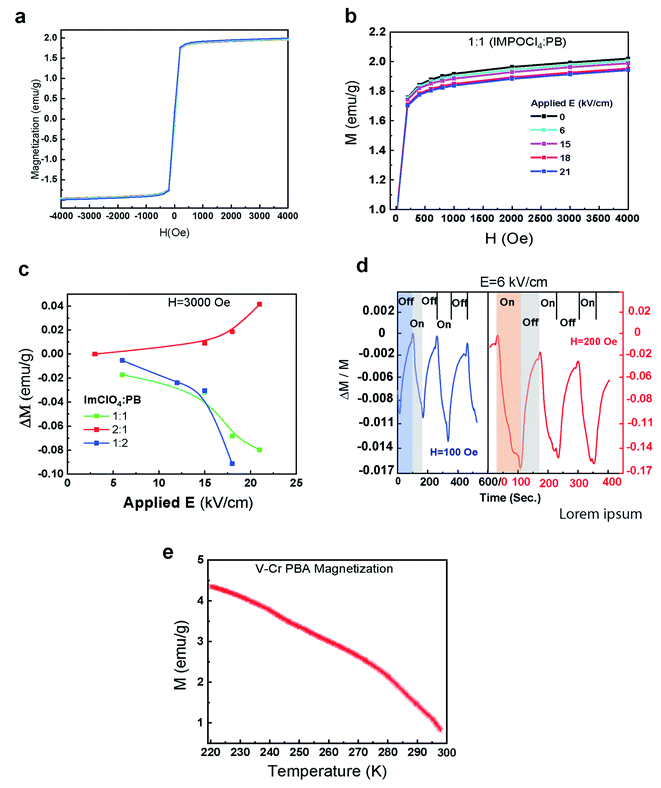
The interplay between the applied electric field and magnetization of PBA-ImClO4 show the splitting of the saturation part of magnetization hysteresis curves. In the Fig. 2(a), the magnetization of such composites can experience a change of about 6% as a result of increasing the electric field from 0 to 21 (kV cm−1). The Microsense EZ7-380V vibrational sample magnetometer (VSM) was used to measure room-temperature magnetization as well as magnetization versus temperature. The composition ratio between PBA and ImClO4 plays an important role in the magnitude of the magnetization change. In Fig. 2(b), the magnetic response to the applied electric field in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 composite at two different magnetic field biases is demonstrated. The amplitude of the response increases by increasing the magnetic field bias. The relative magnetization at H = 100 and 200 Oe changes 0.011 and 0.017 respectively under the on/off cycle of electric field bias E = 6 kV cm−1. It is observed that 2
1 composite at two different magnetic field biases is demonstrated. The amplitude of the response increases by increasing the magnetic field bias. The relative magnetization at H = 100 and 200 Oe changes 0.011 and 0.017 respectively under the on/off cycle of electric field bias E = 6 kV cm−1. It is observed that 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in contrast to 1
1 in contrast to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratios, exhibit an opposite magnetic behavior under the electric field biases (Fig. 2(c), S2†).
2 ratios, exhibit an opposite magnetic behavior under the electric field biases (Fig. 2(c), S2†).
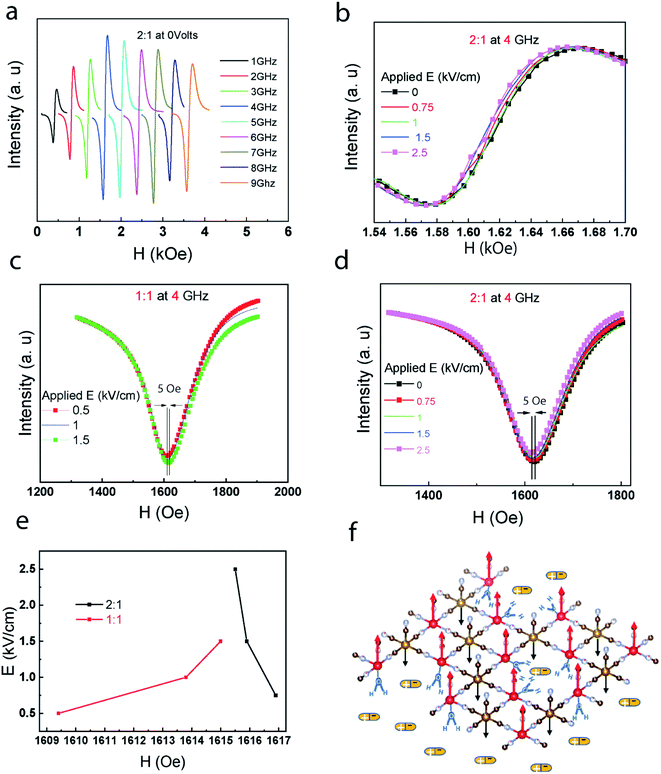
The magneto-electric interaction can be further investigated through FMR.24 The FMR shift could be resulted from the E-field induced change of magnetic symmetry of the structure.25
In the absence of an external electric field, the change of the resonance frequency of composites as a result of the applied magnetic field intensity shows a similar trend regardless of the ratio compositions (Fig. 3(a) and S3†). In the FMR measurement, the magneto-electric coupling is tested at specific frequencies for the composites. In Fig. 3(b), there is an asymmetrical shift in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 FMR curve at 4 GHz. The shift in the position of the resonance field is more recognizable in the integrated curve of the FMR (Fig. 3(c) and (d)). These confirms the same opposite patterns of magnetic response to the electric bias that was shown in the VSM section (Fig. 3(e)).
1 FMR curve at 4 GHz. The shift in the position of the resonance field is more recognizable in the integrated curve of the FMR (Fig. 3(c) and (d)). These confirms the same opposite patterns of magnetic response to the electric bias that was shown in the VSM section (Fig. 3(e)).
In Fig. 3(f), one of the suggested mechanisms based on proton-transfer mechanism is demonstrated. The applied electric field through the electric dipoles of the ferroelectric molecules of ImClO4 influence protons connected to the vanadium atoms. The electric field manipulation to this collective spin–spin interaction26 can lead to the FMR frequency shifts. To explain the opposite pattern observed in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 compared to 2
1 compared to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, there is a possibility here. While the spin alignment is enhanced in 2
1, there is a possibility here. While the spin alignment is enhanced in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 due to the strained is applied by ImClO4 dipoles, this might be detrimental to the spin alignment in 1
1 due to the strained is applied by ImClO4 dipoles, this might be detrimental to the spin alignment in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. To run the FMR measurement we used a home-made setup. It is quite similar to magnetic-resonance system such as NMR and EPR. The principle is based on applying a constant magnetic field to make the ferromagnetic regions undergo precession with Larmor frequencies proportional to the applied H.
1 ratio. To run the FMR measurement we used a home-made setup. It is quite similar to magnetic-resonance system such as NMR and EPR. The principle is based on applying a constant magnetic field to make the ferromagnetic regions undergo precession with Larmor frequencies proportional to the applied H.
The alternative magnetic field which is perpendicular to the constant magnetic field orientation will provide the resonance condition if the frequencies match is met.
Conclusions
Prussian blue analogues are molecular ferrimagnetic insulator with a bandgap 3 eV and conductivity of 10−3 S cm−1.16,27 It has not been reported of sign of electric field effect on bulk material of PBAs to date. Room-temperature molecular multiferroic nanocomposites comprised of molecular ferromagnet V–Cr PBA and ferroelectric ImClO4 suggest a pathway to incorporate magnetic and electric coupling effects at room temperature. The molecular structure of the constituents and the capability of changing the ratio allow us to tailor the characteristics in order to achieve on-demand magnetoelectric properties. The change of magnetization for various ratios of ImClO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PBA can lead to the change of magnetization up to about 6% under the electric field bias. The FMR measurement shows a slight shift of resonance position by applying a relatively weaker electric field (6 kV cm−1).
PBA can lead to the change of magnetization up to about 6% under the electric field bias. The FMR measurement shows a slight shift of resonance position by applying a relatively weaker electric field (6 kV cm−1).
Author contributions
A. Jalouli conducted the project and wrote the manuscript. Dr S. Ren designed the project.Conflicts of interest
There are no conflicts to declare.References
- N. A. Spaldin, Proc. Math. Phys. Eng. Sci., 2020, 476, 20190542 Search PubMed.
- A. Jalouli, S. Khuje, A. Sheng, A. Islam, M. Di Luigi, D. Petit, Z. Li, C.-G. Zhuang, L. Kester, J. Armstrong, J. Yu and S. Ren, ACS Appl. Nano Mater., 2021, 4, 11841–11848 CrossRef CAS.
- C. Li, S. Khuje, D. Petit, Y. Huang, A. Sheng, L. An, M. Di Luigi, A. Jalouli, M. Navarro, A. Islam and S. Ren, Nanotechnology, 2021, 33, 115601 CrossRef PubMed.
- M. M. Vopson, Crit. Rev. Solid State Mater. Sci., 2015, 40, 223–250 CrossRef CAS.
- N. A. Spaldin and R. Ramesh, Nat. Mater., 2019, 18, 203–212 CrossRef CAS PubMed.
- R. Garde, F. Villain and M. Verdaguer, Journal of the American Chemical Society, 2002, 124, 10531–10538 CrossRef CAS PubMed.
- Y. Hu, S. Broderick, Z. Guo, A. T. N'Diaye, J. S. Bola, H. Malissa, C. Li, Q. Zhang, Y. Huang, Q. Jia, C. Boehme, Z. V. Vardeny, C. Zhou and S. Ren, Nat. Commun., 2021, 12, 4602 CrossRef CAS PubMed.
- E. Pardo, C. Train, H. Liu, L. M. Chamoreau, B. Dkhil, K. Boubekeur, F. Lloret, K. Nakatani, H. Tokoro, S. Ohkoshi and M. Verdaguer, Angew. Chem., Int. Ed. Engl., 2012, 51, 8356–8360 CrossRef CAS PubMed.
- W. Liu, M. R. Osanloo, X. Wang, S. Li, N. Dhale, H. Wu, M. L. Van de Put, S. Tiwari, W. G. Vandenberghe and B. Lv, Phys. Rev. B, 2021, 104, 024507 CrossRef CAS.
- Q. Kong, R. Qin, D. Li, H. Zhao, Y. Ren, L. Long and L. Zheng, RSC Adv., 2019, 9, 41832–41836 RSC.
- Y. Zhou and S.-T. Han, Science, 2020, 367, 627–628 CrossRef CAS PubMed.
- L. Hedley, N. Robertson and J. O. Johansson, Electrochim. Acta, 2017, 236, 97–103 CrossRef CAS.
- S.-i. Ohkoshi and K. Hashimoto, The Electrochemical Society Interface, 2002, 11, 34–38 CrossRef CAS.
- S.-i. Ohkoshi, T. Iyoda, A. Fujishima and K. Hashimoto, Phys. Rev. B, 1997, 56, 11642–11652 CrossRef CAS.
- H. Tokoro and S.-i. Ohkoshi, Dalton Trans., 2011, 40, 6825–6833 RSC.
- S.-i. Ohkoshi, K. Nakagawa, K. Tomono, K. Imoto, Y. Tsunobuchi and H. Tokoro, Journal of the American Chemical Society, 2010, 132, 6620–6621 CrossRef CAS PubMed.
- M. Zentkova and M. Mihalik, Crystals, 2019, 9, 112 CrossRef.
- W. Li, H. M. Jafri, C. Zhang, Y. Zhang, H. Zhang, H. Huang, S. Jiang and G. Zhang, J. Mater. Chem. A, 2020, 8, 16189–16194 RSC.
- H. Ma, W. X. Gao, J. L. Wang, T. Wu, G. L. Yuan, J. M. Liu and Z. G. Liu, Adv. Electron. Mater., 2016, 2, 1600038 CrossRef.
- H.-Y. Liu, H.-Y. Zhang, X.-G. Chen and R.-G. Xiong, Journal of the American Chemical Society, 2020, 142, 15205–15218 CrossRef CAS PubMed.
- Z. Pajak, P. Czarnecki, B. Szafranska, H. Maluszynska and Z. Fojud, J. Chem. Phys., 2006, 124, 144502 CrossRef CAS PubMed.
- Y. Hu, T. Zhu, Z. Guo, H. Popli, H. Malissa, Y. Huang, L. An, Z. Li, J. N. Armstrong, C. Boehme, Z. V. Vardeny, A. T. N'Diaye, C. Zhou, M. Wuttig, J. C. Grossman and S. Ren, Nano Lett., 2022, 22, 545–553 CrossRef CAS PubMed.
- J. Coates, Encyclopedia of Analytical Chemistry, 2006, DOI:10.1002/9780470027318.a5606.
- G. Lawes and G. Srinivasan, J. Phys. D: Appl. Phys., 2011, 44, 243001 CrossRef.
- M. Liu and N. X. Sun, Philos. Trans. A Math. Phys. Eng. Sci., 2014, 372, 20120439 Search PubMed.
- A. Jalouli, M. Kilinc, A. Marga, M. Bian, T. Thomay, A. Petrou and H. Zeng, J. Chem. Phys., 2022, 156, 134704 CrossRef CAS PubMed.
- K. Takegahara and H. Harima, Phase Transitions, 2002, 75, 799–805 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra04060c |
| This journal is © The Royal Society of Chemistry 2022 |