Open Access Article
Open Access ArticleTemperature-sensitive foaming agent developed for smart foam drainage technology
Wenfeng Jia *ab,
Chenggang Xian*ab and
Junwen Wu*c
*ab,
Chenggang Xian*ab and
Junwen Wu*c
aState Key Laboratory of Petroleum Resources and Prospecting, China University of Petroleum, Beijing 102249, China. E-mail: jiawf@cup.edu.cn; xianchang@cup.ecu.cn
bUnconventional Oil and Gas Institute, China University of Petroleum, Beijing, 102249, PR China
cSinopec Research Institute of Petroleum Exploration and Development, Beijing 102206, PR China. E-mail: wujunwen@iccas.ac.cn
First published on 17th August 2022
Abstract
The conventional foam drainage technology needs to be defoamed, which is not convenient for its popularization and application. In view of this problem, from the point of molecular design, a temperature-responsive surfactant was designed and synthesized. In the synthetic process, polyoxyethylene alkyl ether carboxylic acid, diethanolamine and sodium chloroacetate were used as raw materials. First, polyoxyethylene alkyl ether carboxylic acid reacted with diethanolamine to generate a tertiary amine with hydroxyl catalyzed by sulfoxide chloride, and the intermediate product then reacted with sodium chloroacetate by the quaternary amine reaction to afford the target temperature-responsive surfactant. The foaming agent can achieve conformational transformation in the temperature range of 20 °C to 120 °C, resulting in the structural change of the self-assembly and regulating the stability of the foam, which makes the formed foam burst rapidly at low temperatures and be super-stable at high temperatures. The indoor evaluations show that the foaming height of the foaming agent is basically unchanged at the same temperature after 4 temperature-changing cycles, and the temperature-controlled defoaming rate reaches 90%, indicating that it has the intelligent temperature response switching performance of “high-temperature defoaming, low-temperature defoaming”. Its preparation process is simple, low-cost, and environmentally friendly. It is expected to be popularized and applied in the field of gas fields, expand the application scope of foam drainage technology, reduce the cost of foam drainage, and help the efficient development of gas fields.
1. Introduction
In the later stage of natural gas exploitation, due to the low pressure of the gas well and the decrease in drainage capacity, the large amount of liquid produced by the gas well leads to a decrease in exploitation efficiency. Foam drainage technology is widely used in natural gas recovery because of its simple operation and excellent drainage effect.1 In this process, the foaming agent is added to the bottom of the well, so that the bottom hole fluid is stirred by natural gas and fully mixed with the foaming agent to form a large amount of foam, reducing the friction loss and gravity gradient in the tubing during the self-flowing stage to effectively reduce the bottom hole back pressure and make the liquid be continuously lifted.2 The regeneration capacity of the foam is very strong, and when its aqueous solution is carried to the ground pipelines and separation equipment, it is repeatedly agitated, resulting in foam accumulating in the separator. When the surfactant is excessive or the foam produced is too stable, this phenomenon is particularly serious. A large amount of foam will be brought to the gathering and transportation pipeline, causing blockage and resulting in an increase in the collection and transportation pressure.3 Therefore, it is necessary to inject a defoamer at the entrance of the separator to achieve defoaming, inhibit the regeneration of foam and facilitate the separation of gas and water.4 However, the addition of defoaming agents will limit the recycling of foaming agents, and the introduction of a defoaming device will increase the production cost.5Foam drainage technology has entered the personalized application stage, endowing foam with intelligent responsiveness, making it foam at wellbore temperature and defoam at ground temperature, removing the defoaming process of conventional foam drainage technology and simplifying the foam drainage process. It is of great research value to reduce the cost and expand the application range of foam drainage gas production.
Temperature-sensitive foam is foam whose stability can be controlled by temperature.6,7 The foaming agent is the most important and basic component of the foam system, so the construction of a temperature-sensitive foaming agent is the key to building a temperature-sensitive foam system. In 2011, the Fameau group first carried out research on temperature-sensitive foam, and an ultra-stable temperature-responsive foam was first reported by the co-assembly of 12-hydroxystearic acid (12-HSA) and ethanolamine or hexanolamine salt, and reversible change could be achieved in the temperature range of 20 °C to 50 °C.8–11 However, the upper limit of the response temperature of this foam cannot meet the requirements of the gas well drainage temperature. Raghavan's group investigated the viscosity of the C22 cationic surfactant, erucic acid dihydroxyethyl methyl ammonium chloride (EHAC), in saline solutions using steady-state and dynamic rheological methods.12,13 The surfactant (EHAC) self-assembles into large worm-like micelles upon heating (∼90 °C), generating an unusually strong viscoelasticity (viscosity > 10 Pa s). The upper limit of the response temperature is high, but its foaming and foaming stabilization ability has not been systematically studied.
Novel smart foam is currently the main research direction, and a variety of ideas have been proposed for the development of temperature-sensitive foam.14–16 Regarding the development of temperature-sensitive foaming agents, the basic principle is regulating the breaking and recombination of self-assemblies in the solution with temperature changes.17–19
From the perspective of molecular design, the two surfactants are connected by linking groups, which reduces the electrostatic repulsion between the head groups and enables them to align closely at the interface,20 making it easier to self-assemble micelles. Additionally, the temperature-sensitive ethoxy chain segment is introduced into Gemini surfactants;21 with the increase in temperature, the ethoxy chain dehydrates, the hydrophilic head group area decreases, and the hydrophobic chain segment length increases, promoting micelle growth. Based on this idea, a novel surfactant has been synthesized to achieve conformational transformation in the temperature range of 20 °C to 120 °C, which leads to changing the assembly structure and adjusting the stability of the foam. Temperature-sensitive foam bursts quickly at low temperatures, and has super stability at high temperatures, which is of great significance to simplifying the foam defoaming process and effectively improving the recovery of gas reservoirs.
2. Experimental section
2.1. Chemicals
Polyoxyethylene alkyl ether carboxylic acid, diethanolamine, acetone, sulfoxide chloride (SOCl2), ethanol (95%), sodium chloroacetate, and ether were purchased from Beijing Chemical Reagent Plant, China; these chemicals were of analytical grade and were not purified further before use. The water used in the tests was deionized water.All the glassware was cleaned by scouring powder and then rinsed with ultrapure water before the experiments.
2.2. Synthesis of the temperature-sensitive Gemini surfactant GACB
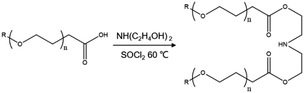
The detailed preparation method of the temperature-sensitive Gemini surfactant molecule is as follows:In a typical procedure, polyoxyethylene alkyl ether carboxylic acid (30.0 g) and diethanolamine (6.2 g) were dissolved in acetone (100 mL) at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 (mol mol−1). SOCl2 (5 mL) as a catalyst was added dropwise at low temperature using a constant pressure drip funnel. After the completion of dropping, the mixture was heated to 60 °C for the reaction with circulating water cooling. The reaction time was 6 h, and the resulting liquid was distilled under reduced pressure to remove the solvent and unreacted diethanolamine to afford the intermediate.
1.5 (mol mol−1). SOCl2 (5 mL) as a catalyst was added dropwise at low temperature using a constant pressure drip funnel. After the completion of dropping, the mixture was heated to 60 °C for the reaction with circulating water cooling. The reaction time was 6 h, and the resulting liquid was distilled under reduced pressure to remove the solvent and unreacted diethanolamine to afford the intermediate.
The intermediate product (25 g) and sodium chloroacetate (7 g) were dissolved in a mixture of ethanol and water (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), and the reaction was carried out by heating the system to 80 °C in a round-bottom flask and cooled by circulating water. The reaction time was 12 h. After the end of the reaction, the solvent was removed by reduced pressure distillation, and the mixture was washed and filtered with ethanol. Finally, the remaining product was recrystallized with a mixed solution of ethanol and ether to afford the target product. The synthetic route of the temperature-sensitive Gemini surfactant GACB is shown in Fig. 1.
1, v/v), and the reaction was carried out by heating the system to 80 °C in a round-bottom flask and cooled by circulating water. The reaction time was 12 h. After the end of the reaction, the solvent was removed by reduced pressure distillation, and the mixture was washed and filtered with ethanol. Finally, the remaining product was recrystallized with a mixed solution of ethanol and ether to afford the target product. The synthetic route of the temperature-sensitive Gemini surfactant GACB is shown in Fig. 1.
2.3. Experimental instruments
The 1H NMR spectra were recorded on a Bruker Advance III 400WB NMR. The surface tension of the surfactant solutions was studied using a KRÜSS K100 surface tensiometer. A Ross–Miles foaming device (Model 2151) and a high-temperature and high-pressure visualization foam instrument (Jiangsu Baobo Machinery Manufacturing Co., LTD) were used to investigate the foaming properties and temperature-sensitive characteristics. The glassware used in the experiment was washed with detergent and deionized water.2.4. Molecular structure characterization
A small amount of the sample was dissolved in D2O, injected into a glass NMR tube with a diameter of 5 mm, and the 1H NMR spectrum of the product at room temperature was recorded on a Bruker AV III HD (400 MHz, magnetic field strength: 9.4 T), and the map data were peaked and integrated with the MestReNova software to determine the product structure characteristics.2.5. Equilibrium surface tension
The equilibrium surface tension was determined by the platinum ring method using a KRÜSS K100 surface tensiometer. The maximum uncertainty on γ values is ±0.05 mN m−1. All the measurements were performed at 25.0 ± 0.1 °C and repeated at least three times to obtain an average value.2.6. Foam performance evaluation methods
The Ross–Miles test (suitable for evaluation at 20–90 °C) was applied to determine the foam properties, including the foaming and stability properties. The experimental procedures are as follows:(1) Put the cylindrical glass container (50 mm internal diameter, 1100 mm height) in a water bath at a constant temperature of 80.0 ± 0.1 °C. (2) Wash the Ross–Miles foaming device with ultrapure water, and then wash it two to three times with the fluid to be tested. (3) Put 50 cm3 of the solution to be tested into the Ross–Miles foaming device and heat to 80.0 ± 0.1 °C. (4) Inject 200 cm3 of the preheated solution into the dropping funnel. (5) Put the dropping funnel onto the pipe rack and make it vertical to the cylindrical glass container. (6) Open the dropping funnel piston and keep the solution flowing into the center of the calibration tube. When the solution flows out of the dropping funnel, start the stopwatch immediately and record the maximum foaming height marked as Hmax, which is used to evaluate the foaming property, and the time when the foam height reduces to half of the original height, which is called half-life period. The stability of foam is evaluated by the half-life period, marked as t1/2.
The high-temperature and high-pressure visualization test (suitable for evaluation at 20–160 °C) is as follows: (1) wash the high-temperature and high-pressure visualization device with ultrapure water, and then wash it two to three times with the fluid to be tested. (2) Set the test temperature of the high-temperature and high-pressure foam device and wait for the temperature to rise to the operating temperature. (3) Pour 100 mL of the test solution into a graduated cylinder and pump it into the testing kettle. (4) Turn on the stirring device at a constant speed of 1000 rpm for 3 min to generate foam. (5) After the end of the stirring, record the maximum height of the foam Hmax and the half-life period t1/2.
The experiments were repeated at least three times, and the average value was collected.
3. Results and discussion
3.1. Molecular structure characterization of GACB
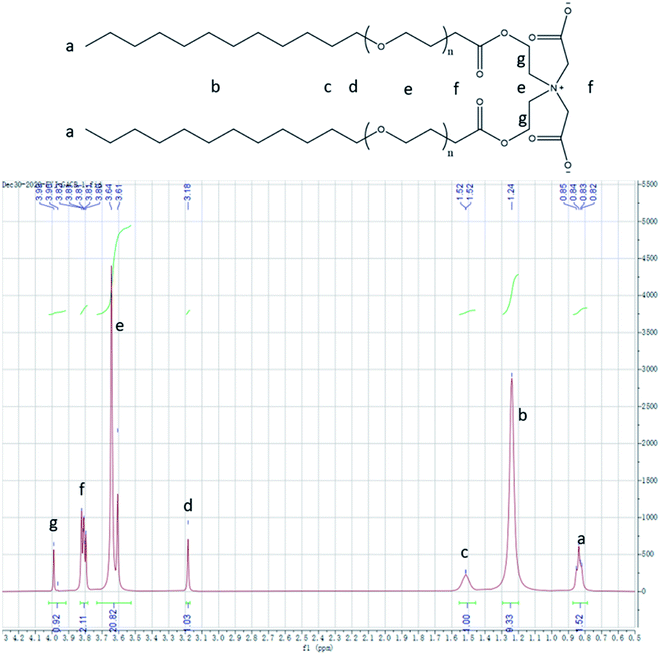
The 1H NMR spectra of the temperature-sensitive Gemini surfactant GACB dissolved in D2O are shown in Fig. 2. The peaks at δ 0.83 ppm and 1.24 ppm came from the CH3 and CH2 groups at the tail of the surfactant [CH3–(CH2)n–], respectively. The chemical shift of methylene [–CH2–CH2–(O–CH2–CH2)n–] linked to the ethoxy group shifts to the left, with characteristic peaks occurring at δ 1.52 ppm and δ 3.17 to 3.20 ppm. Characteristic peaks of methylene (–O–CH2–CH2–O–CH2–CH2–CH2–) adjacent to quaternary ammonium groups [–(CH2)2–N–(CH2–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O–O)2] appear at δ 3.61 to 3.64 ppm. At δ 3.81 to 3.82 ppm is the characteristic peak of the methylene group (–O
O–O)2] appear at δ 3.61 to 3.64 ppm. At δ 3.81 to 3.82 ppm is the characteristic peak of the methylene group (–O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH2–) connected to the carbonyl group. The peak at δ 3.81 to 3.82 ppm is caused by methylene (–C
C–CH2–) connected to the carbonyl group. The peak at δ 3.81 to 3.82 ppm is caused by methylene (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O–O–CH2–) connected to the ester group. The integration area of each peak (a–g) and the ratio of different hydrogen atoms in the sample are 3
O–O–CH2–) connected to the ester group. The integration area of each peak (a–g) and the ratio of different hydrogen atoms in the sample are 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42
42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The results show that the structure of the product coincided with the molecular structure of the target compound.
1. The results show that the structure of the product coincided with the molecular structure of the target compound.
Table 1 shows the weight of the raw materials and products and yield at each step in the above synthetic process of the temperature-sensitive Gemini surfactant GACB. The yield of the single-step reaction reached more than 80%, and the total yield of GACB was 79.12%.
3.2. Performance evaluation of GACB
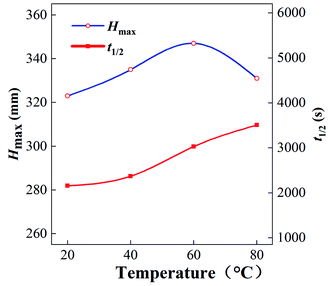 | ||
| Fig. 4 Half-life period t1/2 and maximum foaming height Hmax of foams produced with 0.2% GACB solution at different temperatures. | ||
The high-temperature and high-pressure visualization test was used to determine the foam properties at 20–120 °C with 0.2% GACB solution. As shown in Fig. 5, when the temperature was above 100 °C, the foaming performance increased while the foam stability decreased. Compared with that at 40 °C, the maximum foam height of GACB increased by about 1 time at 120 °C, and the half-life of foam decreased from 40 min to 10–15 min. This is mainly due to the following changes when the temperature rises: (1) the surface viscosity of the liquid film decreases, and the liquid film drainage rate increases; (2) the molecular movement in the foam increases, the liquid film becomes thinner, and the “gas channeling” increases; (3) the liquid vapor pressure increases, and the rapid evaporation of the liquid film makes it thin. However, the foaming ability of the surfactant is still high at 120 °C, which proves that the foaming agent selected in this paper has a good temperature response and the liquid film has high strength and elasticity at high temperatures and can resist the dispersion caused by the intense Brownian motion of molecules at high temperatures and the foam coalescence and disproportionation caused by the thinning of the foam liquid film.
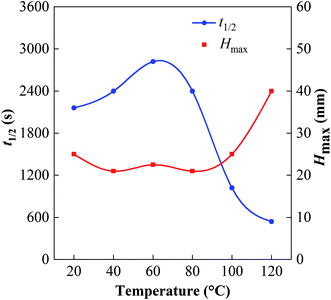 | ||
| Fig. 5 The maximum height of the foam Hmax and the half-life period t1/2 of 0.2% GACB solution under different temperatures determined by the high-temperature and high-pressure visualization test. | ||
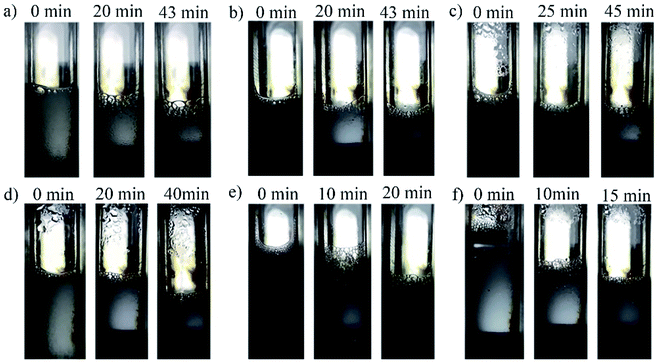
As seen by the observation window of the high-temperature and high-pressure foam apparatus, it was found that the water phase started to accelerate evaporation or even boil under high temperature, as shown in Fig. 6. The evaporated gas can continuously stir up the solution to form new foam and the rapid loss of the liquid phase also leads to a decrease in the foam volume, which was the reason that the foam performance changed in the high-temperature environment.
3.3. Temperature response performance
In order to further verify the temperature response performance of the foaming agent, the high-temperature and high-pressure foam apparatus was used to investigate the same foaming agent at various temperatures from 25 to 120 °C. The heating rate was about 0.5 °C min−1, and the cooling rate was about 0.1 °C min−1. The change of the foam height after every heating or cooling was recorded, and the test results are shown in Fig. 7. During the heating process, the foaming height of the temperature-sensitive foaming agent increased significantly with the increase in temperature. During the cooling process, the foaming height decreased gradually. The foam at 120 °C even exceeded the imaging range of the foam apparatus window. | ||
| Fig. 7 Images of the foaming height of 0.2% GACB solution at a single heating procedure (from 25 to 120 °C) and cooling procedure (from 120 °C to 25 °C) at atmospheric pressure. | ||
The system was further heated and cooled, repeated many times, and the foaming height after the temperature change was recorded, as shown in Fig. 8. The foaming height of GACB at room temperature was less than 30 mm, and after heating, the foaming height of the foaming agent reached more than 40 mm. After four temperature-changing cycles, the foaming height at the same temperature was basically unchanged. Therefore, it is proved that GACB has temperature response performance and can realize multiple cycles of foaming height variation with temperature.
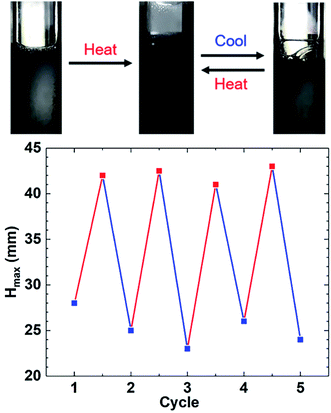 | ||
| Fig. 8 Foaming height of 0.2% GACB solution after many heating (120 °C) and cooling (25 °C) procedures at atmospheric pressure. | ||
The temperature response mechanism was explained as follows: hydrogen bonds are formed in the low-temperature environment between the ethoxy groups and water molecules in GACB, which enhances its water solubility and shows the characteristics of hydrophilic groups. The ethoxy groups are dehydrated because the hydrogen bonds break with the increase in temperature, which results in the ethoxy groups showing the characteristics of hydrophobic groups. According to the theory of stacking parameters, this change can promote the growth of micelles and make the original spherical micelles gradually grow into rod micelles with higher viscosity. Gemini surfactants consist of two single-chain surfactants through the linking groups in the molecules, which greatly reduces the electrostatic repulsion between the head groups, so that molecules can be arranged more closely at the interface, lowering the surface tension and critical micellar concentration, and are more easily self-assembled to form micelles in the solution, thus counteracting the negative effects of high temperature on the foam system. The change of micellar morphology caused by ethoxy groups with temperature, or the micelle growth due to the Gemini structure, will make the liquid drainage rate of the foam system decrease significantly at high temperatures, so that the foam will be more stable at high temperature and burst at low temperature, giving the foam temperature response performance.
4. Conclusions
The current conventional foam drainage technology needs defoaming treatment, which is not convenient for its popularization and application. Based on ethoxy groups with temperature stimulus response, a temperature-responsive Gemini surfactant molecule GACB was designed and synthesized. Conformational transitions of the surfactant result in a change of the self-assembly structure in the temperature range of 20 to 120 °C, regulating the stability of the foam, rapidly breaking the foam at low temperature, and affording superb stability at high temperature. The indoor evaluations show that the foaming height of the foaming agent at the same temperature is basically unchanged after four temperature-changing cycles, indicating that it has the intelligent temperature response performance of “high-temperature foam stabilization and low-temperature foam elimination”. It is of great significance to simplify the foam drainage procedure, expand the application scope of foam drainage technology, reduce the foam drainage cost and effectively improve the recovery of gas reservoirs.Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This study was supported by the Strategic Cooperation Technology Projects of CNPC and CUPB (ZLZX2020-01).References
- S. Yuan, Y. Hu and K. Luo, State of the art, challenges and countermeasures of natural gas development in China, Petrol. Explor. Dev., 2005, 32(6), 1–6 Search PubMed.
- J. F. Lea and H. V. Nickens, Solving Gas-Well Liquid-Loading Problems, SPE 72092, 2004 Search PubMed.
- M. Solesa, S. S. Borets, et al., Production Optimization Challenges of Gas Wells with Liquid Loading Problem Using Foaming Agents, SPE 101276, 2006 Search PubMed.
- Stephenson, R. Rouen and M. Rosenzweig, Gas-Well Dewatering: A Coordinated Approach, SPE 58984, 2000 Search PubMed.
- Lea and R. E. Tighe, Gas Well Operation with Liquid Production, SPE 11583, 1983 Search PubMed.
- M. J. Greenhill-Hooper, T. P. O'Sullivan and P. A. Wheeler, The aggregation behavior of Octadecylphenylalkoxysulfonates: I. Temperature dependence of the solution behavior[J], J. Colloid Interface Sci., 1988, 124(1), 77–87 CrossRef CAS.
- P. M. Claesson, R. Kjellander and P. Stenius, et al., Direct measurement of temperature-dependent interactions between non-ionic surfactant layers, J. Chem. Soc., 1986, 82(9), 2735–2746 CAS.
- A. L. Fameau, A. Carl, A. Saint-Jalmes and R. von Klitzing, Responsive Aqueous Foams, ChemPhysChem, 2015, 16, 66–75 CrossRef CAS PubMed.
- A. L. Fameau, S. Lam and A. Arnould, et al., Smart Non-Aqueous Foams from Lipid-Based Oleogel, Langmuir, 2015, 31(50), 13501–13510 CrossRef CAS PubMed.
- A. L. Fameau, A. Saint-Jalmes and F. Cousin, et al., Smart Foams: Switching Reversibly between Ultrastable and Unstable Foams, Angew. Chem., Int. Ed., 2011, 50(36), 8264–8269 CrossRef CAS.
- J. P. Douliez, L. Navailles and F. Nallet, et al., Self-Assembly of Unprecedented Swollen Multilamellar Twisted Ribbons from a Racemic Hydroxy Fatty Acid, ChemPhysChem, 2008, 9, 74–77 CrossRef CAS.
- S. R. Raghavan and E. W. Kaler, Highly Viscoelastic Wormlike Micellar Solutions Formed by Cationic Surfactants with Long Unsaturated Tails, Langmuir, 2001, 17(2), 300–306 CrossRef CAS.
- T. S. Davies, A. M. Ketner and S. R. Raghavan, Self-Assembly of Surfactant Vesicles That Transform into Viscoelastic Wormlike Micelles upon Heating, J. Am. Chem. Soc., 2006, 128(20), 6669–6675 CrossRef CAS.
- S. M. S. Hussain, M. S. Kamal and L. T. Fogang, Synthesis and Physicochemical Investigation of Betaine Type Polyoxyethylene Zwitterionic Surfactants Containing Different Ionic Head-groups, J. Mol. Struct., 2019, 1178, 83–88 CrossRef CAS.
- A.-L. Fameau and S. Fujii, Stimuli-Responsive Liquid Foams: From Design to Applications, Curr. Opin. Colloid Interface Sci., 2020, 50, 101380 CrossRef CAS.
- Y. Horiguchi, H. Kawakita, K. Ohto and S. Morisada, Temperature-Responsive Pickering Foams Stabilized by Poly(N-Isopropylacrylamide) Nanogels, Adv. Powder Technol., 2018, 29(2), 266–272 CrossRef CAS.
- M. M. S. Lencina, E. Fernández Miconi, M. D. Fernández Leyes, C. Domínguez, E. Cuenca and H. A. Ritacco, Effect of Surfactant Concentration on the Responsiveness of a Thermo responsive Copolymer/Surfactant Mixture with Potential Application on “Smart” Foams Formulations, J. Colloid Interface Sci., 2018, 512, 455–465 CrossRef CAS PubMed.
- T. S. Davies, A. M. Ketner and S. R. Raghavan, Self-Assembly of Surfactant Vesicles That Transform into Viscoelastic Wormlike Micelles upon Heating, J. Am. Chem. Soc., 2006, 128(20), 6669–6675 CrossRef CAS.
- S. R. Raghavan and E. W. Kaler, Highly Viscoelastic Wormlike Micellar Solutions Formed by Cationic Surfactants with Long Unsaturated Tails, Langmuir, 2001, 17(2), 300–306 CrossRef CAS.
- R. Zana, Dimeric and oligomeric surfactants. Behavior at interfaces and in aqueous solution: a review, Adv. Colloid Interface Sci., 2002, 97, 205–253 CrossRef CAS.
- M. J. Greenhill-Hooper, T. P. O'Sullivan and P. A. Wheeler, The Aggregation Behavior of Oc-tadecylphenylalkoxysulfonates: I. Temperature Dependence of the Solution Behavior, J. Colloid Interface Sci., 1988, 124(1), 77–87 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |