Open Access Article
Open Access ArticleValue-added materials recovered from waste bone biomass: technologies and applications
Abarasi Hart
 *a,
Komonibo Ebiundu
b,
Ebikapaye Peretomode
c,
Helen Onyeaka
*a,
Komonibo Ebiundu
b,
Ebikapaye Peretomode
c,
Helen Onyeaka
 *d,
Ozioma Forstinus Nwabor
e and
KeChrist Obileke
f
*d,
Ozioma Forstinus Nwabor
e and
KeChrist Obileke
f
aDepartment of Chemical and Biological Engineering, The University of Sheffield, Sheffield, S1 3JD, UK. E-mail: Abarasi.hart@sheffield.ac.uk; hartabarasi@yahoo.com
bDepartment of Chemical Engineering, Niger Delta University, Wilberforce Island, Nigeria
cSchool of Engineering, Robert Gordon University, Aberdeen, AB10 7GJ, UK
dSchool of Chemical Engineering, University of Birmingham, Edgbaston, Birmingham, B15 2TT, UK. E-mail: h.onyeaka@bham.ac.uk; Tel: +44 (0)1214145292
eNatural Product Research Center of Excellence, Faculty of Science, Prince of Songkla University, Hat Yai, Songkhla 90110, Thailand
fDepartment of Physics, University of Fort Hare, PMB X1314, Alice 5700, South Africa
First published on 10th August 2022
Abstract
As the world population increases, the generation of waste bones will multiply exponentially, increasing landfill usage and posing health risks. This review aims to shed light on technologies for recovering valuable materials (e.g., alkaline earth material oxide such as CaO, hydroxyapatite, beta tri-calcium phosphate, phosphate and bone char) from waste bones, and discuss their potential applications as an adsorbent, catalyst and catalyst support, hydroxyapatite for tissue engineering, electrodes for energy storage, and phosphate source for soil remediation. Waste bone derived hydroxyapatite and bone char have found applications as a catalyst or catalyst support in organic synthesis, selective oxidation, biodiesel production, hydrocracking of heavy oil, selective hydrogenation and synthesis of bioactive compounds. With the help of this study, researchers can gather comprehensive data on studies regarding the recycling of waste bones, which will help them identify material recovery technologies and their applications in a single document. Furthermore, this work identifies areas for further research and development as well as areas for scaling-up, which will lead to reduced manufacturing costs and environmental impact. The idea behind this is to promote a sustainable environment and a circular economy concept in which waste bones are used as raw materials to produce new materials or for energy recovery.
1 Introduction
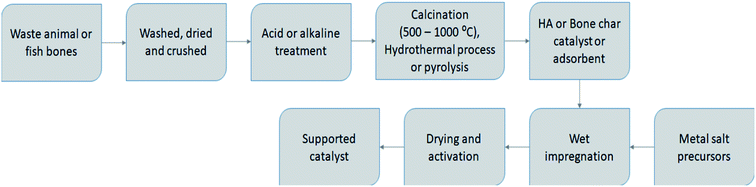
Fresh meat consumption is increasing with the world population, which means slaughterhouses, and meat and fish processing plants, are producing enormous amounts of beef, pork, poultry, and fish waste bones as a by-product. In the food industry today, discarded waste bones pose significant environmental challenges. Worldwide, waste animal bones (WABs) are a large waste from the food industry and domestic waste, with an estimated annual global slaughter production of WABs exceeding 130 million metric tons.1 Rather than being utilized for economic purposes, bone residues are generally considered and treated as slaughterhouse and domestic waste, then disposed of in landfills. In addition to posing a waste management challenge, improper disposal creates more environmental burdens and may lead to health issues.2,3 Landfills are usually the only hygienically and ecologically acceptable disposal method for bone waste. Despite being one of the growing economic and environmental challenges facing the globe, waste is also an incredibly valuable resource today. However, waste management has grown progressively in size and value, waste disposal methods have evolved in response to policies and legislation. In addition to waste collection, recycling and reuse of materials, treatment and disposal which are the basic waste management method, utilizing waste for energy and materials recovery is a more sustainable approach.4 Innovation, policies and legislation, most notably the landfill and carbon taxes, drive changes in the waste management approach, from landfill disposal to waste treatment through reduction, recycling and reuse to energy and resource recovery from waste.5 Underutilization of WABs leads to an increase in disposal costs and environmental pollution. By utilizing WABs more efficiently, the livestock and fish processing industries would make a much greater contribution to world economy.6 Waste-to-energy is commonly accomplished by incinerating WABs for energy recovery and to reduce the volume of the waste for landfill. The valuable materials in the WABs are lost as ashes when this method is used. As a consequence, recovering resources from waste can reduce the burden of environmental management of landfills to prevent groundwater contamination and air pollution.As modern society relies on materials of finite supply on earth, it is difficult to ensure circular economy, sustainability, and waste management without recycling at the end-of-life stage. Due to a massive increase in the exploitation and utilisation of natural resources, waste burden and natural resources scarcity have reached a critical point.3,7 A transition to sustainable economic systems that use resources more efficiently is necessary. As a consequence, the use of waste bones as a renewable resource as a means of waste management is gaining more attention.8 To achieve this, resource recovery from waste is at the heart of the sustainability to close the loop in the supply chain. This study focuses on recovering materials from waste bones and their applications. The goal is to make the most efficient use of WABs' environmental and economic costs before they are permanently disposed of in a landfill. Animal waste bones can be categorized into soft and hard bones based on their hardness. Small animals such as fish and birds produce soft bones that are easier to use. But hard bone wastes are from bigger animals like pigs, goats, and cattle. Today, the use of animal derived products for cattle feed is severely restricted.9 WABs can be used to produce high-value-added materials, which can be used in various sectors, including agrochemical, biomedical, food, and pharmaceutical industries. In recent times, attention has been shifted to the application of sorbents from biomaterials such as waste animal bones, seashells, snail shells and eggshells sources, since they are low-cost materials compared to the cost of commercial adsorbents, such as activated carbon, ion-exchange resins, and titanium oxide.10–12
Compositionally, animal bones consist of 30–35% organic components (collagen (95%) and proteins) and 65–70% inorganic components.13 The effect of using bone powder and bone ash, as a cement additive, on the mechanical properties of cement mortar have been reported in the literature.14,15 WABs are a natural apatite-rich material composed of inorganic substance calcium phosphate (about 65–70%), mainly hydroxyapatite with the chemical composition of Ca10(PO4)6(OH)2. The elemental analysis showed that apatite contents are thus; phosphate at about 56.3% and calcium 36.8%.16 However, bovine and pig bones were found to contain 38% calcium and 18% phosphorus according to titrations and spectrophotometer measurements, respectively.17 In biomedical applications, hydroxyapatite material derived from waste bone biomass has proven to be highly bioactive, high in biocompatibility, and highly osteoconductive.18 The recovery of materials from waste bones as a resource can help promote environmental and resource sustainability, designing value-added products and materials more reusable and recyclable. The physicochemical properties of bone derived materials such as bone char and hydroxyapatite have shown that they possess high surface area, mesoporous microstructure, acid–base properties, good ion-exchange characteristics, and the presence of surface functional groups such as hydroxyl (OH−), carbonate (CO32−) and phosphate (PO42−). As a result of these textural features, materials derived from waste bone have found applications in diverse fields, which the present study will explore critically. They include adsorbent for wastewater and flue gas treatment, catalyst and catalyst support, hierarchical porous carbon for energy storage electrode, phosphate source for soil remediation and hydroxyapatite material for biomedical applications. Using the following valorization techniques calcination, pyrolysis, gasification, hydrothermal hydrolysis processes, subcritical water process and solvent extraction, value-added products and materials such as hydroxyapatite, calcium oxide, bone char, bone ash, β-tricalcium phosphate, and phosphate can be recovered from WABs.
The current linear economic model will put pressure on scarce natural resources, and increase the quantity of domestic waste due to population growth and industrialization, adversely affecting the ecosystem, the environment and human health. As a result, the management of waste bone requires strategies that take into account increased disposal costs, pathogen propagation risks, unpleasant odours, and limited disposal sites. For this reason, converting WABs into value-adding materials with industrial applications would be advantageous economically and environmentally. It has also become increasingly popular to use WABs as inorganic materials, as they are non-toxic and can be safely stored and handled.19 Mohd Pu'ad et al.18 reported a detailed overview of a series of methods for producing hydroxyapatite from natural sources such as fish bone and fish scale, animal bones and shell sources (e.g., cockle, oyster, clam, eggshell, and seashell). Consequently, a review on the application of materials recovered from waste (e.g., fish bone, fish scale, seashells, etc.) for tissue engineering has been reported in the literature.20 Both reviews were only limited to extraction technologies and recovery of hydroxyapatite material. The presence of hydroxyapatite (HA) in animal bone wastes has made them attractive as a catalyst and catalyst support, a review on eco-friendly catalysts derived from bone waste and some of their catalyzed chemical reactions such as transesterification, oxidation, and biofuel production was reported by Nasrollahzadeh et al.21 In 2021, a mini-review focused merely on catalysts recovered from WABs and their utilization in biodiesel production was published by Hussain et al.22 There is no comprehensive review covering resource recovery from waste animal and fish bones and applying these derived materials.
This study highlights bone waste-to-materials, valorization methods, and applications. It is obvious previous reviews focus on a specific application or a valorization technique, but this review is comprehensive as it provides researchers with an overview of the most relevant studies relating to the valorization of bones, identification of appropriate technology, the properties of recovered materials based on method and the synthesis of a perspective that will help guide further research in the field. The methods for extracting hydroxyapatite from waste bone biomass and the processes for producing bone char are also described in this review. This review showcases real examples from published articles, demonstrating how materials, hydroxyapatite, energy storage electrode, adsorbents and catalyst can be recovered from animal and fish bones which are currently viewed as waste. It provides comprehensive insight on material recovery technologies, and the applications of the recovered materials in tissue engineering, adsorbent for wastewater treatment, soil remediation, energy storage material and several catalytic applications. Additionally, this work examines the effects of the valorization method and process conditions on the derived materials' morphology, particle size, crystallinity, textural properties, such as surface area, pore volume, and pore size, as well as the Ca/P ratio. For energy storage applications, bone-derived carbon has a hierarchical porous structure, which is critically explored and discussed. Consequently, future perspectives were also provided to guide further research in the field.
2 Materials recovery from bone waste
Methodologically, the study was carried out by analysing published articles, focusing on valorization techniques employed, the properties of the recovered materials, and the range of applications reported. In literature, excluding the organic components, WABs contain calcium phosphate and varied carbonates depending on the valorization technique the following materials can be recovered bone char, hydroxyapatite (HA), bone ash, phosphate and phosphorus (P). Phosphorus (P) can, however, be recovered from bone char, HA and bone ash using acidic or suitable solvent extracting method. In this context, the valorization techniques can be classified into thermochemical conversion (calcination, combustion/incineration, gasification and pyrolysis), hydrolysis (hydrothermal, solvo-hydrothermal and subcritical water) and solvent leaching. The valorization of WABs demonstrated in this study proves the simultaneous use of resources more efficiently and the recovery of the resources after use. In recent years, pyrolysis and gasification have been adopted as valorization methods to generate energy carriers from WABs and to use the produced bone char as an adsorbent, a catalyst support, for environmental remediation and soil amendment. The mineralogical composition of calcined bone powder, bone char and bone ash are presented in Table 1, which shows that calcium oxide and phosphorus pentaoxide (P2O5) are major components.| Component | Cattle bone ash (wt%)14 | Calcined bone powder (wt%)15 | Cow bone char powder (wt%)23 |
|---|---|---|---|
| a LOI denotes the loss on ignition. | |||
| CaO | 43.26 | 52.45 | 49.80 |
| SiO2 | <0.01 | 1.34 | 0.89 |
| Al2O3 | <0.01 | 0.35 | 0.44 |
| Fe2O3 | <0.01 | 0.25 | — |
| Na2O | <0.01 | 1.6 | 0.96 |
| MgO | 0.54 | 1.3 | 0.78 |
| K2O | <0.01 | 0.3 | — |
| MnO | <0.01 | <0.03 | — |
| P2O5 | 44.67 | 36.85 | 32.90 |
| SO3 | 0.08 | 0.41 | — |
| TiO2 | — | <0.01 | — |
| Cl | — | 0.4 | 0.11 |
| C | — | — | 1.00 |
| LOI | 2.36 | 1.2 | 13.13 |
2.1 Bone char
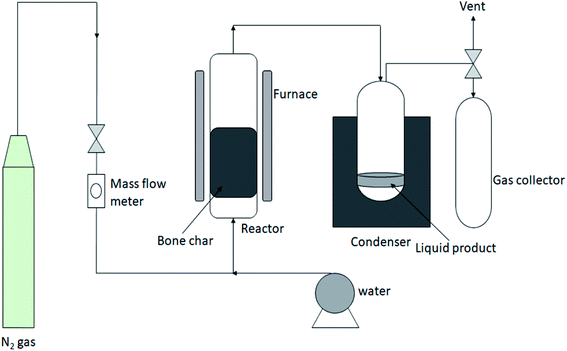
Bone char (or bone charcoal) is a product of WABs carbonization, which consists mostly of carbon and calcium phosphate. Due to the nature of the raw material, bone char is an exclusive form of activated carbon. The physicochemical properties of bone char can be summarized as follows: carbon content (8–11%), ash (around 3%), calcium carbonate, CaCO3 (6–9%), calcium sulphate (0.1–0.2%), phosphate soluble, P2O5 (about 16.5%), iron, Fe2O3 (0.1%), beta tri-calcium phosphate (70–76%), and surface area (80–120 m2 g−1). Animal waste bones can be converted into high-value products such as bone char and hydroxyapatite materials.24 The stages involved in bone char production include bone collection, washing and drying, charring via carbonization either by pyrolysis, gasification or incineration, crushing and sieving, and finally washing, drying, and packaging.The gasification of meat and bone meal followed by thermal cracking of the produced tar under atmospheric pressure using a two-stage fixed-bed reaction system in series, produced the following products: char, tar, and gas including syngas and gaseous energy carriers.26 The animal meat and bone meal undergone gasification in the first stage reactor, while the produced tar was further cracked in the second stage reactor. In the reactions, nitrogen was used as an inert carrier gas, while pure oxygen was used as an oxidant. A laboratory reactor has been used to vacuum pyrolyze a sample of animal meat and bone meal flour as an alternative to incineration and cement kilns.9 The results showed that combustible gas (15.1 wt%), oil (35.1 wt%), char residue (39.1 wt%), and aqueous phase rich in organics (10.7 wt%) were produced. An example of a fixed-bed reactor system for steam gasification of waste bones to produce bone char and syngas is shown in Fig. 2. The production of syngas can compensate for the energy used for bone char production during gasification. However, pyrolysis of bone waste for the production of bone char can be carried out with a fluidized bed reactor and the products are presented in Fig. 1. The produced oil can be used as a fuel in boilers or gas turbines by itself or mixed with petroleum products.
Furthermore, combustion (or incineration) is used as a means of recovering energy from WABs. In addition to improving energy security, the process will reduce landfill disposal costs and produce bone char or ash. In the presence of heat, bone inorganic material undergoes a process of calcination in the combustion zone, where the bone calcium carbonate component (CaCO3) is converted to calcium oxide (CaO). As a result of incineration, the organic and inorganic components of the bones are broken down to release energy for electricity generation. The average calorific value of meat and bone meal (MBM) is about 16.18 MJ kg−1, which indicates a good fuel property in comparison with conventional fuels.20 McDonnell and colleagues29,30 reported the combustion of MBM in a fluidized bed combustor for the purpose of energy recovery at a temperature 880 °C. It was found that 100% MBM pellets showed the most intense volatile activity, as evidenced by the relatively long and intense flames. The inorganic part of the bones is converted into bone ash accounting for about 30 wt% of the original weight,29,31 which a valuable material. There are, however, certain risks associated with incineration, mostly linked to emissions and combustion features.
The common by-product of incineration, pyrolysis or gasification of WABs is bone char/ash. During charring, the amount of oxygen in the environment usually determines the quality of the bone char. On the other hand, one of the most popular methods for removing heavy metals from wastewater is adsorption, in which the pollutant adsorbs onto the solid adsorbent surface.32 Bone char has been reported to be an effective adsorbent by many researchers (this will be explored further in Section 3.1). Utilizing bone char is a cost-effective and environmentally friendly method for removing fluoride, heavy metals, colour, environmental remediation and soil amendment applications. Conversely, bone char adsorbent is considered one of the most cost-effective and less impactful on human health than other adsorbents, such as activated carbon and alumina. In 2019, Alkurdi et al.25 published a review on the application of bone char as a green adsorbent for removing fluoride from drinking water, it was found that the bone char produced in the temperature range of 500–700 °C and a residence time of 2 h gave an optimum removal of fluoride. When used in drinking water treatment, the quality of bone chars is defined by its capacity to hold pollutants, a low content of organic matter that would change the colour or taste of the treated water, and the absence of fecal contamination during the production or storage of the chars.
| Temperature (°C) | Surface area (m2 g−1) | Total pore volume (cm3 g−1) | Pore size (nm) | Ca/P |
|---|---|---|---|---|
| Effect of pyrolysis temperature for 2 h residence time | ||||
| 25 | 0.461 | 0.004 | 32.34 | 2.03 |
| 400 | 114.15 | 0.294 | 9.633 | 3.37 |
| 450 | 83.95 | 0.302 | 12.238 | 2.50 |
| 500 | 69.79 | 0.321 | 15.878 | 2.28 |
| 600 | 50.37 | 0.305 | 21.72 | 2.14 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Effect of residence at 400 °C | ||||
| 1 | 98.63 | 0.291 | 10.798 | 2.13 |
| 2 | 114.15 | 0.294 | 9.633 | 3.37 |
| 3 | 92.40 | 0.315 | 11.736 | |
| Source | Process and conditions | Yield (wt%) | Ref. |
|---|---|---|---|
| Cow bone | Pyrolysis, 650 °C, 5 °C min−1, N2 flow 400 mL min−1 and time 2 h | Bone char: 75.33 | 33 |
| Cow bone | Pyrolysis, 700 °C, 5 °C min−1, N2 flow 400 mL min−1 and time 2 h | Bone char: 75.38 | |
| Cow bone | Pyrolysis, 800 °C, 5 °C min−1, N2 flow 400 mL min−1 and time 2 h | Bone char: 75.41 | |
| Cow bone | Pyrolysis, 900 °C, 5 °C min−1, N2 flow 400 mL min−1 and time 2 h | Bone char: 73.59 | |
| Cow bone | Pyrolysis,1000 °C, 5 °C min−1, N2 flow 400 mL min−1 and time 2 h | Bone char: 72.73 | |
| Meat and bone meal | Two-stage gasification, 650–850 °C, O2 an oxidant, and time 30 min | Bone char: ≈18 | 26 |
| Tar: 18–27 | |||
| Gas: 40–50 | |||
| Meat and bone meal | Two-stage steam gasification, 650–850 °C, steam/MBM (wt/wt) 0.4–0.8, N2 flow 45 mL min−1, and time 30 min | Bone char: 14.1–21.7 | 35 |
| Tar + water: 52.2–57.9 | |||
| Gas: 8.7–18.1 | |||
| Cattle animal bone | Pyrolysis, 500–600 °C at 20 K min−1 | Bone char: 70 | 36 |
| Tar: 5 | |||
| Gas: 18 | |||
| Pyrolysis water: 7 | |||
| Swine bone | Fixed-bed pyrolysis, 450–650 °C at 10 °C min−1 and time 30 min | Bone char: 45.3–47.5 | 37 |
| Bio-oil: 27–33.4 | |||
| Gas: 21.3–25.5 | |||
| Bone char surface area (23.5–48.2 m2 g−1), pore volume (0.031–0.046 cm3 g−1) and pore size (3.86–5.28 nm) |
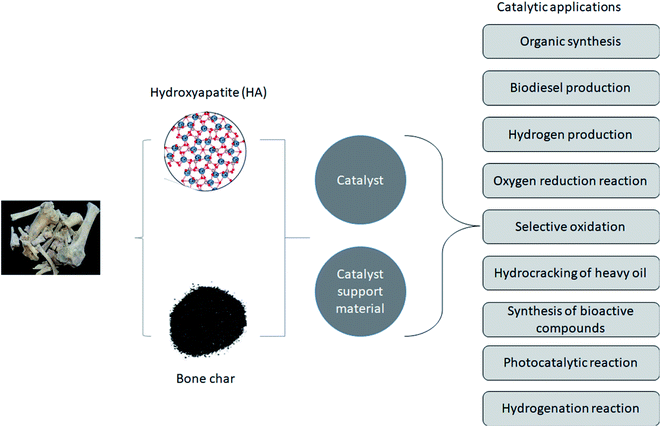
2.2 Hydroxyapatite (HA)
Hydroxyapatite, HA [Ca10(PO4)6(OH)2], is the major mineralogical component of bones. Based on Fourier-transform infrared spectroscopy (FTIR) technique, the bone natural apatite contains trace elements (Na+, Mg2+, and K+) and major functional groups, such as hydroxyl (OH−), carbonate (CO32−) and phosphate (PO42−) in its complex molecules.13,39 But calcium carbonate and carbonate apatite are the two phases of carbonate found in bones. Between 60 and 70 wt% of bones are composed of HA, depending on the animal and the type of bone.40 Specifically, its biocompatibility allows it to be widely used as a biomaterial in tissue engineering and drug delivery agents.11,41,42 Other applications of this material include bioceramics, adsorbents, catalyst and catalyst support, powder carriers, chromatographic lighting materials, and chemical sensors.21 The molecular structure of the calcium apatite [Ca10(PO4)6(OH)2] is shown in Fig. 3. Depending on the thermal treatment conditions, calcination of animal bones can result in a mono-phase HA or a bi-phasic (hydroxyapatite–tricalcium phosphate) bioceramic.43 However, calcination is the conventional approach of producing HA and β-tricalcium phosphate (β-TCP) from waste animal bones. The crystallographic structure of HA determines its soluble and bioactive properties.44 | ||
| Fig. 3 Hydroxyapatite (HA) structure: (a) the unit cell in the (001) plane (source: ref. 45, https://www.mdpi.com/2073-4352/10/9/806/htm. CC BY 4.0) and (b) scanning electron microscope (SEM) image of bone calcined at 950 °C for 6 h (source: ref. 13, https://www.hindawi.com/journals/ijbm/2020/1690178/. CC BY 4.0). | ||
 | ||
| Fig. 4 Methodological pathway for preparing hydroxyapatite powder from waste animal bones: (a) calcination method, (b) hydrothermal method, and (c) subcritical water process. | ||
On the other hand, hydrothermal processing is an approach to producing HA material with a high-temperature (130 to 250 °C) aqueous solution of the bone minerals at high vapour pressure (0.3–4 MPa) in a sealed autoclave reactor (Fig. 4b). The process can be carried out in the presence of water or organic solvent or a mixture of water and organic solvent. When water is used, it is known as hydrothermal, for organic solvent, it is called solvothermal, and a mixture of water and organic solvent is known as solvo-hydrothermal.42 Depending on the pressure and temperature, it can be operated in subcritical or supercritical conditions. Under this condition the interaction between the bone minerals and solvent determine the crystalline, morphology and porosity of the HA produced. Moreover, the solvent type, temperature and time are known process variables that influence the properties of the produced HA. In the pre-treatment stage the bones can be heated to 350 °C for 2–3 h to remove the organic components, dirt, fats, protein, and other components such as bone marrow and soft tissues. According to some literature, boiling water for 8 hours or more or surfactant and alkali solutions can also be used to remove organic components from bone in the pre-treatment stage.18 An alternative version of a hydrothermal treatment method for producing HA from WABs is alkali hydrolysis hydrothermal processing. It involves the crushed bones being treated with an alkaline solution (e.g., NaOH, KOH, etc.) at a temperature in the range of 80–250 °C for a given duration of time (5–20 h depending on the temperature). The resultant material is filtered and washed with distilled water and subsequently oven or freeze-dried.50,51 In contrast, in subcritical water or pressurized low polarity water (PLPW) process, water is heated under pressure to extract and fractionate the crushed bones, as shown in Fig. 4c. Barakat et al.52 investigated and reported thermal decomposition, subcritical water and alkaline hydrothermal methods of extracting HA from bovine bones bio-waste. They observed that by thermal decomposition of bovine bones at 750 °C and time 6 h, HA powder exhibited nanorods morphology with an average length of 300 nm, whereas alkaline hydrothermal approach with a 25 wt% NaOH concentration, temperature of 250 °C and time 5 h results in HA nanoparticles, while a subcritical water method dissolves collagen in the bones at 275 °C and time 1 h forming HA powder with nanoflakes morphology. Although the thermal decomposition method produces good crystallinity hydroxyapatite material, alkaline hydrothermal method and subcritical water method produce nano-sized HA particles.52 The three methods offer high yields, simplicity and cost-effectiveness, making them well-suited for industrial scalability. This suggests that the calcination method may be suitable HA material for heterogeneous catalyst applications, while HA produced via hydrothermal and subcritical water methods for biomedical applications.
The calcination temperature, the treatment time, the extraction method, and the type of bone all play a part in determining the final properties of calcium phosphates, including the Ca/P ratio, morphology, phase purity, surface area, and particle size distribution.43 Hence, there is a need to control and optimize process factors to produce the desired HA material. The produced HA powder is a weak alkaline calcium phosphate having very low water solubility. In recent years, animal bones derived HA materials have specifically interested researchers because of their versatility and their valuable properties as catalysts and catalyst supports, such as excellent ion-exchange capacity, high porosity, very low water solubility, controlled basicity/acidity, and good thermal stability up to 1000 °C.2,42 Also, a variety of transition metals can form synergistic interactions with HA due to its high ion-exchange ability. This means metals such as Cu2+, Pb2+, Sr2+ and Ni2+ can be used as substitutes for the Ca2+ cation, allowing tailored performance, selectivity and design of catalyst to favour desired reactions.
WABs also contain phosphate, which can be recovered. Phosphate recovery from hydroxyapatite, bone char and bone ash can be achieved through extraction method. The process consists of two stages: firstly, reaction of HA/bone char/ash with phosphoric acid (H3PO4) resulting in monocalcium phosphate (Ca(H2PO4)2) formation, which dissolution depends on concentration, temperature, mixing and reaction time.53 In the next stage, monocalcium phosphate filtrate is converted into calcium sulphate (CaSO4) or calcium chloride (CaCl2) using concentrated sulphuric or hydrochloric acid.53,54 The precipitated calcium sulphate/chloride [(CaSO4)/(CaCl2)] is separated. Using a gravimetric method or titration, the amount of P2O5 in the filtrate at the end of the reaction can be determined. Alternatively, bone char or ash can be used to extract phosphate by directly reacting with sulphuric acid or hydrochloric acid.54
 | ||
| Fig. 5 SEM photomicrographs of: (a) crushed raw animal bones, (b) calcined animal bone at 1000 °C for 2 h in air (source: ref. 39, https://www.sciencedirect.com/science/article/pii/S2352340919308406. CC BY 4.0), (c) HA synthesized from bovine bone via subcritical water method at 160 °C, pressure 6.22 bar and duration 2 h (source: ref. 57, https://www.mdpi.com/1996-1944/15/7/2504. CC BY 4.0), and (d) nanohydroxyapatite synthesized from bovine bone powder using 5 M NaOH solution at 80 °C and stirred for 1 h (source: ref. 56, https://iopscience.iop.org/article/10.1088/1742-6596/1082/1/012005. CC BY 3.0). | ||
Fig. 6 shows the thermal response of bone as a function of temperature studied with aid of Thermogravimetric Analyser (TGA) and the X-Ray Diffraction (XRD) patterns of calcined goat bone at 800, 900 and 1000 °C. The weight loss from 25–200 °C is due to water evaporation and the removal of any other contaminants (Fig. 6a). Weight loss ranging from 20 to 32 wt% occurred from 200 °C to 600 °C due to degradation of organic substances, fats and collagen.34,58 Beyond 600 °C is the transformation of carbonate apatite, the decomposition of carbonate and the release of carbon dioxide and other gases from the material. The pore size reduces as a result of microstructure sintering at temperatures above 900 °C.59 As expected from the XRD pattern shown in Fig. 6b, all the peaks corresponding to the standard hydroxyapatite following calcination of the bone can be identified. However, the XRD pattern of calcined bone at 900 °C indicates more crystallinity, as shown by the sharper peaks compared to 800 °C and 1000 °C.
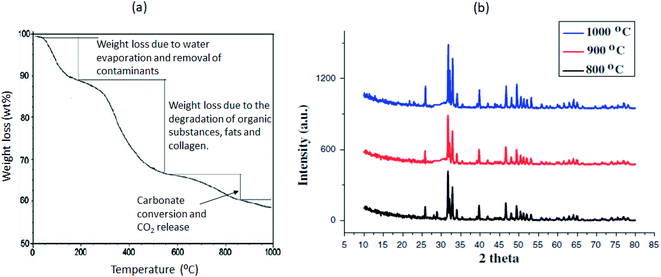 | ||
| Fig. 6 (a) Thermograph of duck-bone using TGA (source: ref. 58, https://iopscience.iop.org/article/10.1088/1757-<?pdb_no 899X?>899X<?pdb END?>/226/1/012073. CC BY 3.0) and (b) XRD of the calcined goat bone catalyst at 800, 900 and 1000 °C (source: ref. 60, https://link.springer.com/article/10.1007/s40243-013-0011-4. CC BY 4.0). | ||
Decomposition, dissolution, or hydrolysis of the organic compounds in WABs can be achieved through one or combination of these processes: calcination, subcritical water, and alkaline hydrothermal. Barakat et al.52 examined the crystallinity of HA produced by the three processes from bovine bone, and found that it can be summarized as follows: calcination > subcritical water > alkaline hydrothermal process. Consequently, the HA is dehydroxylated at temperatures ranging from 1000 °C to 1360 °C during calcination, resulting in the formation of oxyhydroxyapatite (Ca10(PO4)6(OH)2−2xOx), which can readily decompose into tricalcium phosphate (TCP) and tetracalcium phosphate. As a result of thermal sintering at high temperatures (>850 °C), porosity and surface area decreased. A significant feature of HA that distinguishes it from other catalyst supports is its ability to exchange cations with various metallic cations such as alkalis, alkaline earth metals, and transition metals in aqueous solutions.11 Generally, the acidic sites are predominantly ascribed to PO–H from HPO42− species (Brønsted sites) and Ca2+ cations (Lewis sites), while basic sites are attributed to surface PO43− and OH− groups (and possibly other phases such as CaO, Ca(OH)2, and CaCO3).2,11 A second interesting advantage of HA-derived material from WABs is their ability to be tuned in terms of their acid–base properties.
Table 5 summarises valorization methods, conditions and properties of extracted HA from different bone sources. Based on the results, the calcination method appears to be the most commonly used method for producing HA from waste bones. The reason for this is its simplicity compared to others. The common morphology of extracted HA includes spherical, flat plates, rod-like, nanoflakes, and needle-like. It has been observed that crystal size of the bone is preserved when the alkaline hydrolysis method is used, whereas crystal agglomeration occurs for the calcination method (Fig. 5).50 The textural properties of HA material, such as surface area, pore volume and pore size extracted by calcination method is shown in Table 6. Clearly, the bone source has a significant impact on the textural characteristics of the HA.
| Source | Production method | Condition | Ca/P | Particle size | Shape | Ref. |
|---|---|---|---|---|---|---|
| Bovine bones | Calcination | 750 °C, 6 h | 1.65 | Mean length 300 nm | Nanorod | 52 |
| Bovine bones | Subcritical water | 275 °C, 1 h | 1.56 | Nanosize | Nanoflakes | 52 |
| Bovine bones | Alkaline hydrothermal process | 25 wt% NaOH, 250 °C, 5 h | 1.86 | Nanosize | Nanoflakes | 52 |
| Horse bones | Calcination | 700 °C, 2 h | 2.131 | 28 nm | Irregular | 61 |
| Bovine bones | Calcination and vibro-milling | 800 °C, 3 h and ball milled 2 h | 1.66 | 100 nm | Nanoneedle-like | 62 |
| Pig bones | Alkaline treatment and calcination | 4 M NaOH at 100 °C, 4 h. 800 °C and 1200 °C | 1.709 (800 °C) | 70–180 nm (800 °C) and 200–700 nm (1000 °C) | Irregular | 59 |
| 1.675 (1200 °C) | ||||||
| Fish bone | Calcination | 1200 °C, 1 h | 1.62 | 55 nm | Rod-like | 63 |
| Thunnus obesus bone | Alkaline hydrolysis | 2 M NaOH, 250 °C, 5 h | 1.76 | Length 17–71 nm and width 5–10 nm | Nano-rod | 50 |
| Thunnus obesus bone | Calcination | 900 °C, 5 h | 1.65 | 0.3 to 1.0 μm | Irregular | 50 |
3 Applications of bone derived materials
Resource recovery is critical to dealing with bone waste management and their contribution to the economy. The resources recovered from WABs include alkaline earth metal oxides such as CaO, hydroxyapatite (HA), beta tri-calcium phosphate, phosphate or phosphorus, bone char or ash through thermal, alkaline hydrothermal hydrolysis, subcritical water and solvent extraction valorization techniques. These materials can be applied as an adsorbent, a catalyst and catalyst support, a hierarchical carbon source for energy storage applications, HA for biomedical applications, and phosphate source for soil remediation or fertilizer. In this context, phosphate, adsorbent, and HA sources market could be strained, causing chaos in supply chains, highlighting the importance of WABs' circular economy. By using examples from recent state of the art articles, this section explores the applications of these materials. From the results reported in these studies on the applications of these recovered materials, it can be concluded that the scalability of the processes and recycling of WABs would result in economic, sustainability, and environmental benefits. Notably, bone char, which can be used as a mesoporous adsorbent, is mainly made up of hydroxyapatite (70–76%), calcium carbonate (7–9%), and amorphous carbon (9–11%).653.1 Adsorbent material for wastewater treatment
For many organic compounds and metal ions common pollutants found in wastewater, activated carbon has traditionally been the most popular adsorbent, but its cost-intense nature is causing attention to be shifted to low-cost alternatives. Now, wastewater treatment methods are selected based on the concentration of pollutants and the cost of treatment.32 Depending on the source, wastewater could contain a range of pollutants, including dyes, especially from the textile industry, suspended solids, toxic heavy metals, mineral oils, phenolic compounds, and surfactants; and is commonly high in biochemical oxygen demand (BOD) and chemical oxygen demand (COD). Therefore, several methodologies have been developed over the years to treat industrial wastewater, including chemical precipitation–filtration, electrochemical recovery, ion exchange, reverse osmosis, membrane filtration, or adsorption.16,66 One of the more popular methods in wastewater treatment is adsorption, in which pollutants from the effluent adsorb onto the adsorbent surface, with the amount of pollutants removed determined by the adsorption capacity ratio of the adsorbate to the adsorbent.32 With proper adsorbent, the adsorption process can be a promising method for the removal of heavy metals from industrial wastewater. This is because adsorption offers some advantages over chemical precipitation, which include the ability to meet strict effluent discharge standards, permit metal recovery, and produce less sludge.67 It is well known that adsorbents such as activated carbon, titanium oxide, zeolite, etc., usually have high metal adsorption capacities, but they are cost intensive. WABs offers a simple and low-cost alternative to these materials. Consequently, bone char possesses a high porous microstructure with high surface area and pore volume, which makes it a plausible adsorbent material. Furthermore, bone char porous matrix is rich in surface ions, which can be easily replaced by anions like fluoride and other heavy metal pollutants in portable water or industrial wastewater treatment. In fact, the P–OH functional groups on hydroxyapatite surfaces act as sorption sites.21 In other words, in addition to the porous structure and surface area of bone chars, the adsorption capacity is strongly governed by the surface functional groups. There are various mechanisms by which bone char adsorbent surfaces can interact with pollutants molecules, including van der Waals forces, hydrogen bonding, ion exchange, and electrostatic attraction.65The bones of animals can be used to develop green technology that can be used to treat contaminated water. For instance, bone char is widely used for decolorizing cane sugar and defluoridating drinking water as an adsorbent. As a result of using chicken bones to remove phosphates from wastewater, it was found that raw chicken bone removal efficiency was 58.78% for 0.2 g of bone and calcined chicken bone achieved 30.40% for 0.1 g of bone.68 Likewise, HA material derived from cow bone had demonstrated effectiveness in textile wastewater treatment, in the study, the removal of colour was about 99.9%, chemical oxygen demand, COD (80.1%), turbidity (99.4%) and conductivity (30.1%) were achieved with cow bone sintered at 1200 °C.66 Depending on pH and temperature, WABs have been found to be an efficient sorbent with experimentally determined sorption capacity ranging from 29 to 194 mg g−1.10 It was discovered that for chromium(III), the sorption capacity increased with an increase in Cr(III) concentration, temperature and initial pH of metal solution.10 These examples prove that WABs source produced HA can accept a large number of anionic and cationic components, making it a plausible candidate for several applications such as adsorbent and catalysis.2,69 The adsorption of chromium(III) on WABs, was found to follow the pseudo-second-order pattern.10 The mechanism of sorption postulated for HA derived from WABs is ion exchange with Ca2+ ions.10 Deydier et al.16 reported that metal ions binding to HA derived from WABs comprises three successive steps, which are surface complexation and calcium hydroxyapatite of metal ions, Ca10(PO4)6(OH)2 dissolution and then precipitation of slow metal diffusion/substitution of Ca. It has been reported that the dissolution/precipitation produces first a solid solution (Ca/MHA) until all the Ca the heavy metal M has substituted atoms.
Fig. 7 shows a typical heavy metal removal profile from contaminated water as a function of dosage and agitation time. It can be observed that metal ions removal increased rapidly as the cow derived biochar dosage increased from 0.01 g to 0.02 g and plateaued at 0.03 g (Fig. 7a). Likewise, the removal of Mn2+, Fe2+, Ni2+ and Cu2+ ions increases with time and plateaus at 40 min of agitation (Fig. 7b). It is likely that the cow bone derived biochar porous structure dictates the adsorption affinity relative to the heavy metals ions size, which follows this order of Mn2+ > Fe2+ > Ni2+ > Cu2+.
 | ||
| Fig. 7 Typical heavy metals Mn, Fe, Ni and Cu removal using cow bone biochar: (a) dosage effect on Mn2+, Fe2+, Ni2+ and Cu2+ removal at initial concentration, C0, 20 mg L−1; contact time 60 min; pH 5.1 and temperature 298 K and (b) contact time effect at initial concentration, C0, 20 mg L−1; cow bone-derived biochar dosage 0.02 g; pH 5.1 and temperature 298 K (source: ref. 70, https://www.mdpi.com/1996-1944/3/1/452. CC BY 4.0). | ||
The percentage removal (%R) of the metal ions can be calculated using eqn (1). Whereas the amount of metal adsorbed per unit mass of the adsorbent, qe (mg g−1), can be estimated using eqn (2).
 | (1) |
 | (2) |
The adsorption capacity of bone char and hydroxyapatite materials could be improved by increasing the surface area, pore volume or modifying surface functional groups in a way that increases the selectivity of the adsorbent toward specific pollutants. Bio-waste bones can be ground into a powder and calcined to be applied as a biofilter media to remove odours and pollutants from industrial exhaust gas.11 Textile wastewater is known to contain recalcitrant colour pigments and toxic heavy metals. In the study by Hubadillah et al.,66 they found that membrane produced with waste cow bone sintered at temperature 1200 °C characterized by long rod-shaped HA particles and pore size of 0.013 μm demonstrated high removals of colour (99.9%), COD (80.1%), turbidity (99.4%), heavy metals (100%; Cu, Fe, Zn, Cr and Cd), and conductivity (30.1%) from industrial textile wastewater. It has been discovered that adsorbate metal affinity for the bone char adsorbent is dependent on the size of the ion and the bone char pore size distribution.70 Table 7 shows some applications of bone waste derived adsorbents for wastewater treatment. It is obvious that both metal ions, non-metal, organic compounds and colour pollutants can be effectively removed from wastewater through adsorption using bone derived adsorbent. This is because bone char or hydroxyapatite material is a mesoporous adsorbent associated with functional groups. Consequently, by measuring the Fourier transform infrared spectra of bone char before and after As(V) adsorption, it was discovered that Ca–OH plays a critical role in As(V) ion removal.71 In summary, pollutant molecular interactions with bone char depend on the solution properties (temperature, pH, ionic strength/concentration) and the properties of the bone char itself (surface chemistry, surface charge, and textural characteristics).
| Adsorbent source | Pollutants | Results | Ref. |
|---|---|---|---|
| Cow bone charcoal | Mn, Fe, Ni and Cu | (1) The maximum removal was between 75% and 98% from Mn to Cu at dosages between 0.02 g and 0.03 g of cow bone charcoal | 70 |
| (2) The adsorbate affinity for the cow bone charcoal is as follows: Cu > Ni > Fe > Mn | |||
| Cow-bone ash | Fe2+, Zn2+, Pb2+, and Mn2+ | (1) The maximum removal efficiency are as follows: 99%, 97%, 93% and 98% for Fe2+, Zn2+, Pb2+, and Mn2+ | 72 |
| 500–710 μm particle size bone char | Removal of copper(II), zinc(II) and cadmium(II) ions from wastewater | (1) Based on the sum of squares errors (SSE) analysis, the sips isotherm fit the experimental data more accurately than the Langmuir isotherm | 73 and 74 |
| (2) Cd ion reaches equilibrium with the bone char after 72 h achieving about 45% removal | |||
| Swine bone char (particle size less than 180 μm) | Cobalt | (1) The sorption kinetics was described by pseudo-second-order equation | 75 |
| (2) The equilibrium sorption isotherm suggested Freundlich isotherm model | |||
| (3) 99% removal at 800 mg L−1 dosage | |||
| Cattle bone char (particle size 0.79 mm) | Fluoride | (1) The maximum adsorption occurs at pH 3 and the adsorption capacity decreased almost 20 times, increasing the pH from 3 to 12 | 76 |
| Cow bone char (particle size ≈ 1 mm) | (2) The adsorption capacity of the bone char was 2.8 and 36 times greater than those of commercial activated alumina (F-1) and a commercial activated carbon (F-400) | 33 | |
| (3) The best fluoride removal properties are obtained from cow bone char samples synthesized at 700 °C | |||
| Cattle and sheep bone char (particle size range 10–16 mesh) | Endotoxin (also called pyrogens) | (1) The maximum adsorption efficiency achieved is 98% at an adsorbent dose of 40 g L−1 with an initial endotoxin concentration of 80 Eu mL−1 | 77 |
| (2) The Langmuir isotherm adsorption model accurately predicts the experimental data | |||
| Bone char (500–710 μm particle size) | Arsenic(V) | (1) The adsorption of As(V) followed a first-order kinetics model | 71 |
| Cow bone char (size 425–850 μm) | (2) The As(V) removal was strongly dependent on pH and adsorbent dosage | 78 | |
| (3) Langmuir isotherm describes well the adsorption behaviour, while the adsorption process follows a pseudo-second-order kinetic model | |||
| ZnO/bone char (particle size of 0.5–0.8 mm) | Photocatalytic degradation of alkaline methylene blue dye | ZnO/bone char catalyst completely utilize visible light to decompose methylene blue dye | 79 |
| Sheep bone charcoal (particle size in range of 0.1–0.5 mm) | Phosphorous | (1) The maximum adsorption capacity was 30.21 mg g−1 at 100 mg L−1 initial phosphate concentration and 0.2 g dosage | 80 |
| (2) At pH in this study greater than 4, adsorption rate decreased significantly | |||
| Camel bone charcoal (particle sizes ≈ 50 mesh size) | Hg(II) from wastewater effluent | (1) Maximum removal of 10 mg L−1 Hg(II), a minimum adsorbent dosage of 0.03 g | 81 |
| (2) Langmuir isotherm fits the data, and optimum removal conditions are pH 2, contact time 30 min and temperature 25 °C |
Based on reviewed studies, the adsorption capacity of WABs derived adsorbent depends on crystallinity, surface functional groups and properties, and pore size distribution (micro- meso- and macro-pores). Notably, the pore size distribution of bone char is mostly dominated by mesoporous structure.82 Dehydroxylation of hydroxyapatite component of bone char due to charring at high temperatures greater 500 °C decreases adsorption capacity, especially for anion pollutants such as fluorine and phosphorus.83 Recently, Azeem et al.28 published a detailed review outlining the key benefits of using bone waste to produce bone char as well as bone-derived biochar, utilizing them as low-cost and effective methods for excluding potentially toxic elements (such as As, Cd, Cr, Cu, Ni, Pb, Hg, Sb, Se, V, and Zn) from contaminated water and soils. It has also been shown that the adsorption capacity of bone char closely correlates with the characteristics of its pores for metallic or cationic pollutants.84 Thus, an increase in specific surface area and a decrease in average pore size, the adsorption amount increases. In the study by Medellin-Castillo et al.,76 they showed that bone char derived from cattle bones, compared to activated alumina (F-1) and activated carbon (F-400), exhibited adsorption capacities that were 2.8 and 36 times more significant. However, it has been observed that as pyrolysis temperatures rise above 700 °C, the hydroxyapatite of bone char is dehydroxylated, thereby decreasing its fluoride adsorption capacity.33 In the bone char adsorption mechanism, ligand exchange is affected by the degree of hydroxyapatite dehydroxylation.85 In literature, it was reported that the hydroxyl and carboxyl groups on the surface of cattle and sheep derived bone char modified with acetic acid played a significant role in the adsorption of formaldehyde from polluted air.86 In other words, the bone source, method and the conditions in which bone char is produced are crucial to obtaining an effective adsorbent. In 2019, a reported review critically examined the impact of experimental conditions on the adsorption capacity of bone char, as well as the properties and binding affinity of fluoride ions from different water sources.25 The removal of F− ion by the bone char adsorbent was due to ion-exchange with OH− functional group in the structure to form the fluorapatite (Ca10(PO4)6F2), which is enhanced by the large surface area and pore volume. Also, the electrostatic interactions between the bone char surface and the F− ions play a crucial role in the removal process depending on the pH induced surface charges (positively charged surface will increase affinity for F−). By using bone char, fluoride can be removed via ion exchange, precipitation, electrostatic interaction, or a combination of these mechanisms.25 In this context, the surface properties, as well as the conditions of bone char production, such as temperature and residence time, appear to be important factors in designing an efficient pollutants removal adsorbent. Based on the findings from these studies, bone char or HA were characterized as low-cost adsorbents with excellent ion-exchange properties, high adsorption capacity, mesoporous structure and a large surface area in the range of 80 to 120 m2 g−1. Alternatively, by coating the outer layer of the WABs adsorbent with photocatalyst titanium dioxide, with ultraviolet (UV) light or sunlight irradiation exposure, the derived adsorbent can be used to degrade organic pollutants contaminated wastewater more effectively.
3.2 Catalyst and catalyst support
The focus of science and technology is shifting toward circular economies of resources and environmental sustainability. Consequently, researchers seek to recover from bio-waste materials such as WABs catalysts as well as solid support material for catalytic applications. In response to the high price of heterogeneous catalysts, researchers now focus their efforts on synthesizing low-cost, eco-friendly and renewable alternatives to frequently used catalysts.21 The synthesis of heterogeneous catalysts from bio-wastes such as animal bones, eggshells and seashells has become increasingly attractive in recent times.2,11,22 Catalysts made from animal or fish bones can typically be prepared easily by calcining them and then impregnating them with the desired metal precursors shown in Fig. 8. Converting bones from meat- and fish-processing industries into valuable and useful catalysts is another method of managing waste and controlling land pollution. In general, bones contain oxides of alkaline earth metals (e.g., Ca, Mg, etc.) and other non-metals such as P and CO32−.2,87The inorganic elements domicile in bone powder include P, Ca, Mg, B, Fe, Mn, K, Cu, and Zn, which are known catalytic components in addition to the material's surface and physicochemical properties. In addition to Ca5(PO4)3OH, the bone-derived material for catalyst also contain metal oxides such as CaO, MgO, SiO2, Na2O, K2O and MnO. Hence, bone-derived hydroxyapatite material has recently attracted attention due to its versatility, low water solubility, high pore connectivity, controlled basicity/acidity, and excellent thermal stability at high temperatures. As a result, bone-derived catalysts have demonstrated effectiveness as green catalysts for several reactions such as organic synthesis, oxidation of volatile organic compounds, transesterification for biodiesel production and hydrogen production, organic reduction reaction (ORR), synthesis of bioactive compounds, and degradation of pollutants.21,88 Notably, the ratio of Ca/P determines the strength, number and distribution of acid and basic sites on the surface of a bone waste derived catalyst. Incorporating cobalt into hydroxyapatite from bone showed good dispersibility and stability.89 This suggests that WABs derived HA and bone char can be used as a catalyst or catalyst support, as shown in Fig. 9. In Fig. 9, different chemical reactions reported in the literature catalyzed by bone derived catalyst or used as support material are also highlighted from organic synthesis to hydrogenation.
As a catalyst support or carrier, both bone char and HA provide a large surface area for dispersing a specific amount of catalytically active material. As catalyst support, they are responsible for its physical form, texture, mechanical properties, and certain activity, especially for bifunctional catalysts. Hence, the development of a new generation of heterogeneous catalysts is possible using animal bones, such as hydroxyapatite with hierarchical porous structure, which has been reviewed and reported on by Fihri et al.,42 as a catalyst or catalyst support for several chemical reactions. Other catalytic materials from WABs include alkaline earth metal oxides such as CaO, beta tri-calcium phosphate and bone char. They may be an inert component, interact with active materials or adsorb reactant and contribute to the reaction process when utilized as a catalyst support. As a result, bone derived materials can be used as a bulk catalyst (e.g., catalytically active CaO and calcium phosphate) or supported catalyst in the industry. With the use of an animal bone meal (ABM) catalyst, α,α′-bis(substituted benzylidene)cycloalkanones had been synthesized from cycloalkanones and benzaldehydes in water based on crossed-aldol condensation reaction.90 Also, animal bones calcined for 2 h at a temperature of 800 °C with particle sizes in the range 45–200 μm, and then impregnated with an aqueous solution of sodium nitrate was used as a ABM catalyst and in NaNO3/ABM as a catalyst support in the synthesis of oximes from aromatic aldehydes or ketones with hydroxylamine hydrochloride.91 It was found that the conversion of benzaldehyde decreased from 94% to 88% using NaNO3/ABM supported catalyst and from 84% to 79% with just ABM after 8 times. Furthermore, a hydroxyapatite derived from lamb bone using infrared radiation (IRR) or conventional heating during hydrothermal activation was utilized to support cobalt (Co) as solid acid catalyst for glycerol esterification with oleic acid to produce monoglyceride, which has many applications in pharmaceutical.89 About 98% conversion of oleic acid was achieved in a shorter time of 80 min. IRR activation resulted in superior catalyst compared to conventional heating in terms of catalytic properties, while conventionally prepared gave 68 ± 1% yield and 83 ± 1% selectivity, the IRR activated catalyst exhibited significantly higher monooleate selectivity (95 ± 4%) and yield (87 ± 1%).
In the literature, pyrolyzed cow bone (PCB) has been reported as a catalyst for ozone aqueous decomposition in a fluidized bed reactor.92 PCB and ozone were combined in aqueous solutions and the results demonstrated that this combination greatly accelerated the decay rate of ozone compared to ozone decomposing alone. Also, the results indicated that the PCB surface chemistry played a crucial role in the mechanism, and the ozone decomposition reactions occurred mainly on the surface of the catalyst. A Co–Mo catalyst supported on mesoporous bovine bone char was reportedly used to hydrocrack waste lubricant into gasoline fractions.93 An optimum gasoline yield of 58.09% was achieved at temperature of 475 °C and lubricant/catalyst weight ratio 300. Based on the results of the study, in addition to hydrocracking conditions, Co–Mo/bone char catalyst acidity and specific surface area were critical factors that have significant effects on performance. Similarly, a hydroxyapatite derived from cuttlefish bone was utilized as a TiO2 catalyst support by vacuum impregnating the support with a suspension of commercial TiO2 nano-sized powder in isopropanol and also by in situ synthesis of TiO2 on the support by a sol–gel method.94 By using salicylic acid as a model water pollutant, the studied experiments demonstrated the applicability of the synthesized HA/TiO2 catalyst for photocatalytic activity since the result was comparable to that of suspended TiO2 nanoparticles. This suggests that bone derived materials can be used to immobilize metal oxide catalysts as a carrier, and possibly immobilize enzymes for biochemical reactions.
Equally, natural hydroxyapatite synthesized from cow bones was used to support MnO2 (MnO2/HA), and the resultant catalyst was investigated for oxidation of alkylarenes and alcohols.95 By means of this catalyst, alkylarenes and alcohols were oxidized to their carbonyl compounds without an additional oxidizing agent being used. One of the most fundamental transformations in organic chemistry is oxidation reaction. As a result, synthesized Co–Mn catalyst supported on calcined cow bone was studied for selective oxidation of diphenylmethane to benzophenone.96 Upon testing, the Co–Mn/cow bone catalyst exhibited high activity of about 87%, 90% selectivity toward diphenyl ketone and excellent stability under solvent-free conditions. Correspondingly, a N-doped porous carbon material with high surface area is synthesized by carbonizing chicken bones was tested as an oxygen reduction electrocatalyst.97 The experimental results showed a high oxygen reduction reaction activity (ORR) comparable to that of Pt/C catalysts was obtained.
Another type of reaction bone waste-derived catalysts can catalyze is the transesterification reaction between vegetable oil and alcohol, such as methanol, to produce biodiesel. Although homogeneous catalyst produces a higher yield of biodiesel compared to its heterogeneous counterpart, it suffers from separation and purification challenges, making the application of heterogeneous catalyst more attractive.98,99 However, the utilization of a heterogeneous catalyst produced from waste bones is advantageous as it decreases the cost of biodiesel production. Depending on the calcination temperature, bones can be converted into a material composed of alkaline earth material oxide such as CaO, hydroxyapatite, and beta tri-calcium phosphate, which can be catalytically active and used as catalysts for biodiesel production from vegetable oil and methanol.22 As a result, the use of waste animal bones has been studied extensively by many researchers as a source of low-cost, eco-friendly catalysts for biodiesel production. These animal bones include chickens, fishes, goats, bovines, pigs, cows, ostriches, sheep, and turkeys. Fig. 10 shows the trend of publication on the application of bone derived catalyst for biodiesel production. The search criterion was article with title contain any of the keywords “animal bone*” or bone* or “fish bone*” using Scopus database. It is clear that interest in using WABs derived catalysts for biodiesel production from transesterification of vegetable oil and alcohol began in 2014. As evidenced by the increasing number of publications since 2015, bone-derived catalysts are gaining in popularity. Thus, scalability is crucial for meeting industry standards and scale.
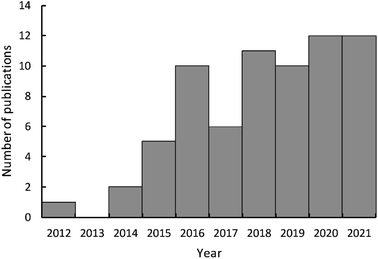 | ||
| Fig. 10 Publication trend on the application of bone-derived catalyst in biodiesel production from 2015 to 2020 using Scopus database. | ||
For instance, crushed Ox bone derived catalyst produced by calcination at temperature 600 °C for 3 h and KOH treated was utilized as a heterogeneous catalyst for Jatropha curcas oil (JCO) conversion into biodiesel.100 The produced biodiesel was found to be 96.82% yield at the temperature of 338 K, while the energy required for the activation (Ea) was 38.55 kJ mol−1 and frequency factor (A) of 7.03 × 106 h−1. Likewise, calcined bovine bone was used to catalyze the transesterification reaction between soybean oil and methanol.19 By using the catalyst prepared by calcination of the bone at 750 °C, the results showed an optimum yield of methyl ester of 97% at temperature of 65 °C for reaction time of 3 h with a catalyst loading of 8 wt% and 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 methanol-to-soybean oil ratio. It is believed that at calcination temperature above 650 °C the calcium carbonate components are converted into calcium oxide and other metal oxides, which the main crystalline component of hydroxyapatite responsible for the catalytic activity of the bone-derived catalyst.11 In addition to the type of bone used, the strength of the active sites determines the catalytic activity. The specific surface area, pore size distribution, particle size and Ca/P ratio are some of the critical factors that could explicitly affect the catalytic activity.
1 methanol-to-soybean oil ratio. It is believed that at calcination temperature above 650 °C the calcium carbonate components are converted into calcium oxide and other metal oxides, which the main crystalline component of hydroxyapatite responsible for the catalytic activity of the bone-derived catalyst.11 In addition to the type of bone used, the strength of the active sites determines the catalytic activity. The specific surface area, pore size distribution, particle size and Ca/P ratio are some of the critical factors that could explicitly affect the catalytic activity.
Through a close reactor system, the dissolution of an active bone catalyst in methanol was studied, and it was found to have a very low solubility of 0.0014%.19 This suggests that the catalyst a stable in organic solvent. In addition to having high catalytic activity, the calcined waste bones have proven to sustain activity over several transesterification reaction cycles. However, the formation of calcium hydroxide or leaching of the catalyst as a result of soluble calcium methoxide formed due to produced water from and over successive transesterification reactions, changes the surface structure in addition to glycerol coverage, resulting in activity decrease over time.11,101,102 Nevertheless, oxidative combustion at 600 °C can regenerate the catalytic activity. Similarly, in another study, a novel layered heterogeneous catalyst of hydroxyapatite and phases of calcium phosphate was prepared by two-step processes, calcination of beef bone followed by hydrothermal reaction, and used to catalyze the synthesis of biodiesel from transesterification of honge oil and methanol.103 The results showed that the yield achieved for the calcined and hydrothermally prepared bone-catalyst as 88% and 96%, respectively. This suggests that the physicochemical properties of the bone-derived catalyst which determine the catalytic activity depends on the method of production. Compared with the calcination method, the hydrothermally processed bone demonstrated greater surface area.103 Basically, the formation of layer structure causes the size of the material to increase with time for hydrothermal approach. The review of the literature shows that irrespective of the bone type (i.e., soft and hard bone) and source, the derived catalyst can achieve an average of 94% yield of biodiesel. Recently, Hussain et al.22 reviewed the current state of several WABs derived catalysts and their ability to catalyze biodiesel production, while also discussing the key properties of the catalysts needed to maximize their catalytic activity and yield of biodiesel from different feedstock. Categorically, by doping various metals and heteroatoms into the porous microstructure of materials produced from WABs, new and effective catalysts for other reactions may be developed since the porous bone structure contains hydroxyapatite with high cation exchange capabilities. However, in catalysts and adsorbents production processes from WABs, the energy requirements can be justified by the possibility of regeneration and the reduction of solid waste. As another advantage, WABs derived catalyst and adsorbent can be regenerated using a chemical method either inorganic chemicals (e.g., NaOH) and organic solvents (e.g., isopropyl, ethyl alcohols, etc.),82 or through thermal oxidation.11 Consequently, through appropriate activation or modification methods, WABs derived catalyst can be enhanced in terms of their performance by increasing textural characteristics, altering surface functional groups or by adding dopants in a way that increases selectivity of the catalyst toward a specific reaction or product. A summary of the use of bone-derived materials as catalysts or catalyst supports for various types of catalytic reactions, the yields and selectivity is shown in Table 8. This wide applications can be attributed to the different inherent functional structures possessed by bone char and HA that play crucial role in catalysis, including pore structure, oxygen-containing groups which are beneficial for radical pathway, and acid-base active sites.104
| Catalytic reaction | Bone source and reaction condition | Results | Ref. |
|---|---|---|---|
| Organic synthesis | |||
| Etherification of glycerol to polyglycerol | Duck-bone HA, 240 °C, 2 wt% catalyst, and time 2 h | Glycerol conversion (99%) and polyglycerol yield (68%) | 58 |
| Synthesis of benzimidazoles, benzoxazoles and benzothiazoles | Animal bone meal (ABM) HA, ZnBr2/ABM, dioxane (solvent), 5 min, and 101 °C | Yield (79%) | 105 |
| Crossed-aldol condensation | HA from ABM, Na/ABM, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mole ratios of cycloalkanones and aldehydes in water, 20–75 min 2 mole ratios of cycloalkanones and aldehydes in water, 20–75 min |
Yields ABM (76%) and Na/ABM (96%) | 106 |
| Suzuki coupling reaction in water | ABM-Pd0, water, sunlight, and time 7 h | Yield (96%) | 107 |
| Esterification of oleic acid with ethanol | Cu/HA derived from fish bone, 0.8 mL min−1 ethanol flow rate, 1000 rpm, 70 °C and 1 h | Conversion (91.86%) | 108 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Energy carriers | |||
| Biodiesel (transesterification reaction) | Bovine bone HA, 65 °C, 3 h, catalyst 8 wt% and 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 methanol to soybean oil ratio 1 methanol to soybean oil ratio |
Yield of methyl ester (97%) | 19 |
| Biodiesel (transesterification reaction) | Chicken bone HA, catalyst 3 wt%, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 methanol to waste cooking oil ratio, 80 °C and 3 h 1 methanol to waste cooking oil ratio, 80 °C and 3 h |
Biodiesel yield (96.31%) | 99 |
| Hydrogen (water–gas shift reaction) | M/HA (pork bone) where M = Ni, Cu, Co and Fe, metal loading 0.2–1.9 wt%, and 150–450 °C | Yields of H2 (43–85%) and CH4 (0.3–9%) | 109 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Selective oxidation | |||
| Oxidation of diphenylmethane | Co–Mn/HA (cow bone), catalyst 100 mg, 17 bar, time 3 h, and 200 °C | Conversion (87%), benzoic acid selectivity (7%) and selectivity to benzophenone (90%) | 96 |
Ozone aqueous decomposition,![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
Cow bone char, catalyst 0.5 g L−1, initial O3 12 mg L−1, and pH 8.5 | Complete conversion (100%) in 400 s | 92 |
| Oxidation of 2,6-diisopropyl naphthalene | Co–Mn/HA (cow bone), oxygen (99.98%) 17 bar, 0.8 g catalyst, 190 °C, and 12 h | Conversion (95%) and selectivity to 2,6-naphthalene dicarboxylic acid (30%) | 110 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Catalytic hydrogenation | |||
| Hydrogenation of aliphatic nitroalkanes | Polysilane/bone charcoal-supported Pd catalyst (600 mg), H2 flow mL min−1 (15–20), 0.2 M solvent ethanol at 0.2 mL min−1 and 30 °C | Yield of primary amine (42 to 99%) and hydroxylamine (12 to 47%) depending on Pd concentration | 111 |
| Hydrogenation of 2-butyne-1,4-diol | Pt nanoparticles supported on bovine-bone powder, H2 (6 bar), 55 °C, 550 rpm and 30 min | Conversion (≈100%) and selectivity towards 2-butene-1,4-diol (83%) | 112 |
| Glucose-to-5-hydroxymethylfurfural (HMF) | Bone char (0.03 g), solvent water, 90 °C, and 3 h | Glucose conversion (78%), fructose yield (12%) and HMF selectivity (63%) | 113 |
3.3 Phosphate source for soil remediation
It can be problematic to manage the large amounts of bone wastes generated every year because they are generally disposed at landfills that emit odours and promote the growth of microbes. Rather, turning bone waste into new value-added materials can bring significant benefits to the environment and economy.114 It aligns with reduce, reuse and recycle, which are three key components of a circular economy toward sustainability and adequate waste management strategies. Several studies have demonstrated that hydroxyapatite, bone char, and ash recovered from WABs can be used for the remediation of toxic metal contaminated water and soil. When WABs are used to remediate toxic metals from soils/water and for soil fertilization, the recovery of phosphate source may reduce the impact of mining natural reserves of phosphate rock that are listed as critical raw materials by the European Commission.114Hazardous substances such as heavy metals accumulate in soils and are transported through food chains, posing a threat to human health. Immobilization of heavy metals diminishes both leaching and plant bioavailability.115 On the other hand, it is possible to mitigate the problem of depleting non-renewable phosphate rock resources for phosphorus fertilizer production by using WABs rich in phosphorus as part of a recycling strategy. Adding a soluble phosphorus source to contaminated soil can cause it to form pyromorphite, which immobilizes due to its very low solubility. On a field scale, synthetic hydroxyapatite is economically unfavourable. Therefore, WABs rich in phosphate can be applied in the remediation of contaminated soil. Bone char, for instance is known to contain about 15% phosphorus.116 It is believed that phosphate-containing amendments are added to soil to improve its fertility or to remediate contaminated soil.117 However, mineral phosphate fertilizers contain up to 556 mgCd kg−1 and are capable of accumulating in soils over time, which inevitably leads to Cd inputs into food chains.116 Adsorption onto the solid material is the main mechanism for Cd immobilization after phosphate fertilizer application. Bone char has been found as a possible candidate for metal immobilization due to its ability to undergo ion exchange. Thus, bone char was studied for immobilization of cadmium (Cd) from phosphate fertilizer and other anthropogenic and geogenic sources to avoid soil contamination.116 It was found that Cd immobilization positively correlated with soil pH. In the study, bone char and triple superphosphate (TSP) were incubated in 11 soils containing between 0.3 and 19.6 mgCd kg−1 and the kinetics of phosphate (P) dissolution was determined over a period of 145 days. It was discovered that the embedded bone char increased the concentration of labile phosphate for most the incubated soils immediately after application, reaching a maximum after 34 days. Since bones contains hydroxyapatite with similar characteristics to phosphate rock, it is plausible that bone char dissolution in soil could help trap some soil contaminating metals via the precipitation of the metal apatite-group mineral. A review of soil amendments for immobilizing potentially toxic elements in contaminated soils has been reported elsewhere.117
Soil remediation with a phosphate may significantly reduce metal bioavailability and leaching to ground and surface waters. By means of commercially available bone meal as a phosphate source, bone apatite [Ca5(PO4)3OH] was tested on a mine contaminated soil with high levels of Pb, Zn and Cd.118 With bone meal to soil ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 and 1
25 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, and with columns irrigated daily with a synthetic rain solution with pH in the range of 2 to 4.4, it was discovered that after 100 days, all treated columns had significantly reduced Pb, Zn, and Cd concentrations. The formation of precipitated metal phosphates or precipitation of pyromorphites [M5(PO4)3OH, where M is metal or Ca5(PO4)3X, where X = F, Cl, Br, or OH] of Zn, Cu, As, Cd and Pb contaminants due to the presence of bone char immobilize them. The removal mechanism is likely to be a combination of adsorption effect and also a type of ion-exchange reaction between the ions in solution and the calcium ions of the bone char apatite. It is obvious bone char possesses the potential to adsorb both cationic and anionic species. Similarly, another study investigated with column experiments the capacity of commercial bonemeal (particle size 90–500 μm; poor crystalline apatite; Ca10(PO4)6OH2) to immobilize Pb and Zn and reduce metal bioavailability in soils with pH ranging from 2.7 to 7.1 via the formation of metal phosphates.119 At the end of the experiment, it was observed that Pb and Zn were associated with phosphorus by analytical scanning electron microscopy of the bone meal treated soil. In 2006, Chen et al.115 studied the effects of bone char application on the bioavailability of Pb in a polluted soil from Hunan Province, China. By determining the amount of Pb taken up by Chinese cabbage, bioavailability was studied. The results showed that as bone char amounts were increased, the Pb concentrations in both shoots and roots decreased, while the Pb concentrations in both shoots and roots decreased by 56.0% and 75.9%, respectively. These results demonstrate the potential for bone char amendments to treat soils contaminated with metals. These results establish the ability of apatite derived from bone char to remediate soils contaminated with metals with the advantage of being a readily available, and cost-effective source of phosphate.
10, and with columns irrigated daily with a synthetic rain solution with pH in the range of 2 to 4.4, it was discovered that after 100 days, all treated columns had significantly reduced Pb, Zn, and Cd concentrations. The formation of precipitated metal phosphates or precipitation of pyromorphites [M5(PO4)3OH, where M is metal or Ca5(PO4)3X, where X = F, Cl, Br, or OH] of Zn, Cu, As, Cd and Pb contaminants due to the presence of bone char immobilize them. The removal mechanism is likely to be a combination of adsorption effect and also a type of ion-exchange reaction between the ions in solution and the calcium ions of the bone char apatite. It is obvious bone char possesses the potential to adsorb both cationic and anionic species. Similarly, another study investigated with column experiments the capacity of commercial bonemeal (particle size 90–500 μm; poor crystalline apatite; Ca10(PO4)6OH2) to immobilize Pb and Zn and reduce metal bioavailability in soils with pH ranging from 2.7 to 7.1 via the formation of metal phosphates.119 At the end of the experiment, it was observed that Pb and Zn were associated with phosphorus by analytical scanning electron microscopy of the bone meal treated soil. In 2006, Chen et al.115 studied the effects of bone char application on the bioavailability of Pb in a polluted soil from Hunan Province, China. By determining the amount of Pb taken up by Chinese cabbage, bioavailability was studied. The results showed that as bone char amounts were increased, the Pb concentrations in both shoots and roots decreased, while the Pb concentrations in both shoots and roots decreased by 56.0% and 75.9%, respectively. These results demonstrate the potential for bone char amendments to treat soils contaminated with metals. These results establish the ability of apatite derived from bone char to remediate soils contaminated with metals with the advantage of being a readily available, and cost-effective source of phosphate.
Bone char has thus far proved to be effective in the remediation of heavy metals contaminated soil and water.120 However, increasing populations result in a growing need for phosphorus (P) for agricultural fertilizer. Additionally, for commercial scale crop production to be sustainable, fertile soils are essential. But the geological and mineralogical nature of phosphate rocks source confer on them a wide variety of environmentally hazardous elements, such as cadmium (Cd), uranium (U), lead (Pb), mercury (Hg) and arsenic (As).121 However, bone char or ash phosphate from WAB contains high levels of phosphorus about 30% and calcium (37%), making it a cost-effective, environmentally-safe, and naturally renewable source of phosphorus for plants. On the one hand, high phosphorous content of bone char and ash produced during the incineration or pyrolysis of WABs make them potential candidate for fertilisers. Unlike phosphate rocks source, WABs contain a large amount of phosphorus, calcium, and trace amount of other mineral elements such as K, Mg, Zn, Fe, etc., all of which crops require for proper growth. Empirically, based on dry weight basis, bovine and poultry bones have proven to hold over 10.5 wt% phosphorous.121 Therefore, from sustainability and environmental point of view, phosphate recovery from WABs will be of critical importance in fertilizer production. It is well known that WABs contain an inorganic calcium phosphate, from which phosphorus pentoxide (P2O5) can be recovered. With the use of bone char/ash for soil amendment, agricultural production is likely to increase due to improved nutrient retention in the soil, improved soil fertility, pH improvement in acidic soils, and soil cation exchange capacity and improved microbial activity. In a study, Du et al.122 reported that they used a hydrothermal process at 200 °C to extract 6.36% of the total phosphorus in chicken bones and pork bones waste for fertilizer application with the aid of artificial humic acid. Conversely, acidic extraction of bone ash recovered about 95% of P using 2 M H2SO4 and 1.25 kg H2SO4/kg ash.123 Furthermore, a pyrolysis carbonization process was used to extract phosphorus from WABs (pig, cattle, and chicken bones), the produced bone char yielded 19.5–23.9 percent P2O5 content, while the concentrated product resulted in 28.9–31.9% P2O5 content suitable for fertilizers.121 The yields of phosphorus from bone char/ash were reported to be 53–100 g from incineration, 48–73 g from pyrolysis, and 62 g from gasification per kg of WABs.124 As the results show, bone ash produced by incineration was the most effective process in producing phosphate. In the concept of circular economy, the extraction of phosphate from WABs will contribute towards closing the phosphate loop and its use in agriculture for fertilizer and remediation of heavy metals contaminated soil and water. Based on the summary presented in Table 9, it is evident bone-derived materials such as bone char, HA and bone ash can be used to remediate contaminated soil and surface/ground water, and a source P fertilizer to improve soil fertility. By implementing bone char/ash in agriculture, one of the primary benefits that may be achieved is improved crop yield as a result of enhanced soil quality. In contrast, mining the earth for phosphate resources to make fertilizer is unsustainable. At the same time, WABs recycling and reuse will reduce the environment hazards associated with the exploitation of natural phosphate rocks as a source of P while decreasing landfill burdens.
| Source | Treatment | Results | Ref. |
|---|---|---|---|
| Pig bones | Bone char produced by pyrolysis at 400–800 °C and solvent extraction | P fertilizer, P2O5 (19.8–33.1 wt%), Ca (20.3–32.6 wt%), Mg (7–11.4 g kg−1), K (5–6 g kg−1) | 38 |
| (1) Swine bone | (1) Pyrolysis at 450–650 °C, initial concentration of Pb 100 mg L−1, and pH 10.49 | (1) Soil remediation, Pb removal efficiency 80.5%, and formation of Pb-phosphate and Pb-carbonate precipitates on bone char | 37 and 125 |
| (2) Meat and bone meal (MBM) | (2) Combustion at 850 °C, bone ash, 1 mg Pb per litre, and 100 mg of ashes per litre | (2) Ashes are calcium (31.0 wt%) and phosphate (57.5 wt%) and the genotoxic result showed that Pb is more efficiently immobilized in pyromorphite [Pb10(PO4)6(OH)2] and lead carbonate dihydrate [PbCO3·2H2O] | |
| Catfish bones | HA used for the remediation of uranium-contaminated groundwater, pH 5.5–7, and 100–300 °C | About 3.9 mg of U(VI) was removed per gram of HA | 126 |
| Sheep bone | Bone char produced by pyrolysis at 500 and 800 °C for 2 h. It was used for soil quality improvement, maize growth, and fractionation and phytoavailability of Cd and Zn in a mining-contaminated soil at 2, 5, and 10% (w/w) | (1) In soils amended with 10% bone char, organic C, N2, and P increased, as did residual Zn and Cd fractions and the oxidizable fractions | 127 |
| (2) In comparison to unamended controls, 10% bone char reduced Zn and Cd content in maize roots (by 57 and 60%) as well as shoots (by 42 and 61%) | |||
| (3) Also, the urease (98%) and phosphates (107%) activities increased | |||
| Cow bones | Production of phosphate fertilizer | (1) Produced 30.7% of P2O5 with the addition of H2SO4 | 128 |
| (2) 45% of P2O5 with H3PO4 |
3.4 Bone tissue engineering
The ideal bone repair material would mimic the composition and microstructure of natural bones. Due to its excellent bioactivity, biocompatibility, and osteoconductive properties, HA biomaterial extracted from fish and animal bones is widely used in biomedical applications.18 HA can be synthesized chemically or recovered from natural sources such as eggshells, seashells, fish bones and animal bones using the methods highlighted in Fig. 4. Chemically synthesized HA materials are prone to bottlenecks such as requiring a high level of purity chemicals as well as prolonged synthesis approach. Biogenic sources, such as animal bones and fish bones, can be used to synthesize biologically desirable HA for biomedical applications and also to avoid the lengthy chemical synthesis process. Hence, WABs can be utilized to engineer bone scaffold (i.e., an interconnected and three-dimensional macroporous network) or as a regenerative material for biomedical implants. A bone-scaffold should have an appropriate porosity, pore size, and pore structure for cell growth and the transport of nutrients and metabolic wastes.13,48 The waste bones must undergo pre-cleaning to remove fat, some fluids, the deproteination, and then, thermal decomposition and cooling to produced HA. The methodologies for producing HA from waste bones can be categorized into three: calcination (i.e., thermal decomposition), subcritical water, and alkaline hydrothermal processes (Fig. 4). A range of physicochemical properties of the HA is influenced by the production method, including crystallinity, porosity, crystalline quality, and mineral composition.129 It has been demonstrated that HA material, synthesized at atomic stoichiometric ratio 1.67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by a calcium and a phosphate precursor, is biologically unsafe upon application due to its inability to replicate natural bone that contains other trace elements such as Fe2+, Na+, Mg2+, K+, and Al3+ and anions such as F−, Cl−, SO42−, and CO32−.13,48,49,130 Also, the produced scaffold is expensive and does not possess a well-defined internal porous architecture. As a consequence, HA produced from natural sources such as WABs, fish scales, fish bones, eggshells, etc., is considered a better alternative. In addition to containing the essential inorganic minerals and other trace elements, they are biologically safe, economical, environment friendly and address waste management challenges. On the other hand, as trace element contents of HA produced from natural sources increase, the Ca/P ratio decreases. Sobczak et al.17 reported HA prepared with two stages thermal calcination process at the temperatures of 650 °C and 950 °C, respectively by means of bovine and pig bones source. While the Ca/P molar ratio of the prepared HA exhibited non-stoichiometry due to the presence of carbonate groups and the presence of a few microelements, the pure crystalline phase of HA demonstrated plausible potential for clinical application. In like manner, Ivankovic et al.48 demonstrated that 3D structures of HA obtained based on the natural morphology of cuttlefish bones can be used for bone tissue engineering. This is because the interconnecting porous network assembly of the cuttlefish bones was preserved during the hydrothermal treatment. Table 10 shows the bone sources, preparation methods and the characteristics of the derived HA powder. Obviously, the source, the preparation method, and process conditions greatly impact the properties of the produced HA, such as crystallinity, composition, morphology, particle shape and size, and porosity. Fig. 11 shows typical scaffold of HA synthesized from bovine bone through hydrothermal method at 160 °C. It shows a highly interconnected and three-dimensional macroporous network for the growth of cells and the movement of nutrients and wastes.
1 by a calcium and a phosphate precursor, is biologically unsafe upon application due to its inability to replicate natural bone that contains other trace elements such as Fe2+, Na+, Mg2+, K+, and Al3+ and anions such as F−, Cl−, SO42−, and CO32−.13,48,49,130 Also, the produced scaffold is expensive and does not possess a well-defined internal porous architecture. As a consequence, HA produced from natural sources such as WABs, fish scales, fish bones, eggshells, etc., is considered a better alternative. In addition to containing the essential inorganic minerals and other trace elements, they are biologically safe, economical, environment friendly and address waste management challenges. On the other hand, as trace element contents of HA produced from natural sources increase, the Ca/P ratio decreases. Sobczak et al.17 reported HA prepared with two stages thermal calcination process at the temperatures of 650 °C and 950 °C, respectively by means of bovine and pig bones source. While the Ca/P molar ratio of the prepared HA exhibited non-stoichiometry due to the presence of carbonate groups and the presence of a few microelements, the pure crystalline phase of HA demonstrated plausible potential for clinical application. In like manner, Ivankovic et al.48 demonstrated that 3D structures of HA obtained based on the natural morphology of cuttlefish bones can be used for bone tissue engineering. This is because the interconnecting porous network assembly of the cuttlefish bones was preserved during the hydrothermal treatment. Table 10 shows the bone sources, preparation methods and the characteristics of the derived HA powder. Obviously, the source, the preparation method, and process conditions greatly impact the properties of the produced HA, such as crystallinity, composition, morphology, particle shape and size, and porosity. Fig. 11 shows typical scaffold of HA synthesized from bovine bone through hydrothermal method at 160 °C. It shows a highly interconnected and three-dimensional macroporous network for the growth of cells and the movement of nutrients and wastes.
| Source | Conditions | Result | Ref. |
|---|---|---|---|
| Bovine bone | Calcination temperatures: 520–620 °C (each 20 °C) at 7.4 °C min−1, and from 700 to 1100 °C (each 100 °C) at three heating rates: 7.4, 9.9, and 11.1 °C min−1 | (1) Ca/P molar ratio decreased for samples calcined up to 900 °C as a result of the dehydroxylation process | 129 |
| (2) All calcined samples from 700 to 1100 °C at the three high heating rates are composed only by inorganic phase | |||
| Cuttlefish bones | Hydrothermal process in the temperature range from 140 to 220 °C for 20 min to 48 h | (1) 3–8 μm diameter dandelion-like HA spheres were observed on the surface of lamellae | 48 and 130 |
| (2) Further conversion into radially oriented nanoplates and nanorods with an average diameter of about 200–300 nm and length of about 8–10 μm was observed | |||
| Aragonitic cuttlefish bones | Hydrothermal treatment at 200 °C (heating and cooling rates were 5 K min−1) for 9 h | (1) The AB-type carbonated hydroxyapatite with similar composition to human bones was observed | 131 |
| (2) Conservation of cuttlefish bone structure with ideal pore size and interconnectivity properties for supporting biological activities | |||
| (3) The osteoblasts test results showed high biocompatibility | |||
| Ostrich bone | Samples dried in a hot air oven for 12 h at 120 °C. Calcined at 650 °C temperature for 6 h, heating rate 5 °C min−1. Thermal decomposition at 950 °C for 6 h under a similar heating and cooling rate | (1) The HA possesses functional groups such as phosphate (PO43−), hydroxyl (OH−), and carbonate (CO32−) | 13 |
| (2) Plate-like texture of the nanosized HA crystals was observed | |||
| Thunnus obesus bone | Alkaline hydrolysis treated with 2 M NaOH at 250 °C for 5 h, and the resultant material dried in an oven at 100 °C. Thermal calcination methods the bones were subjected to a temperature of 900 °C in an electrical muffle furnace under air for 5 h | (1) The thermal calcination method produces good crystallinity with dimensions 0.3–1.0 μm | 50 |
| (2) The alkaline hydrolysis method produces nanorod HA crystals with 17–71 nm length and 5–10 nm width | |||
| (3) It observed that the crystallinity of HA synthesized with thermal calcination is higher than that produced with alkaline hydrolysis method | |||
| Bovine bone | Alkali-heat-treatment method using sodium hydroxide (20 wt%) and heated to 80 °C for 10 h in a water bath. The resultant product filtered and washed with distilled water and freeze-dried | (1) The HA retained major and trace elements of bone | 51 |
| (2) Biocompatibility studies showed that HA exhibited good bioactivity and biological cellular responses |
 | ||
| Fig. 11 SEM micrograph of osteoclasts cultured on bovine bone HA scaffold (source: ref. 57, https://www.mdpi.com/1996-1944/15/7/2504. CC BY 4.0). | ||
Structurally, HA scaffolds provide the necessary support for the proliferation of cells by acting as temporary substrates or templates. As a result, scaffold performance is crucial to the success of bone tissue engineering. With a temperature of 200 °C and 9 h treatment, aragonitic cuttlefish bones were effectively hydrothermally converted into carbonated hydroxyapatite scaffolds of the AB type with comparable composition to human bones.131 It was observed that the scaffolds manufactured sustained the original network structure of the cuttlefish bone, featuring good biocompatibility in osteoblast tests and ideal pore sizes (≈80 μm in width and ≈100 μm in height) and interconnectivity that would support biological activity, such as bone tissue growth and vascularization. In summary, by synthesizing HA from WABs, we can retain some nanoscale properties of the precursor material, such as chemical composition and structure. Consequently, the results of HA synthesized from pure chemicals and bovine bone showed that the hydroxyapatite extracted from bovine bones contained the major and trace elements found in natural bone useful for biomedical application, but these elements were absent in HA powder produced by chemical synthesis.51 Additionally, in vitro testing showed that HA powder obtained from bovine bone stimulated the proliferation and adhesion of MC3T3-E1 cells. In 2018, Terzioğlu et al.43 published a detailed review on the biomedical applications of natural calcium phosphates from fish bones. It has been reported that thermal decomposition method leads to a higher crystallinity of HA than alkaline hydrolysis hydrothermal approach.50 However, the alkaline hydrolysis hydrothermal method is a more effective approach for producing and maintaining HA with nanostructure arrangements and carbonate groups than thermal calcination. Based on the study by Veremeev et al.,132 HA and demineralised bone matrix powder obtained from bovine bone tissue showed excellent repair capability similar to autografts for bone defects and demonstrated sufficient potential for biomedical applications.
3.5 Energy storage material
Batteries and supercapacitors are becoming crucial storage devices as the world transitions from fossil fuels to emissions-free electric power, suggesting significant increase in demand for manufacturing materials' demand. Also, vehicles powered by internal combustion engines emit large amounts of carbon emissions, which has stimulated the move to electric vehicles. The performance of batteries and supercapacitors is largely determined by the inherent characteristics of their electrodes, such as large surface areas and electrical conductivity.133 Carbon is a conductive additive in batteries and an electrode in electrochemical double layer capacitors (EDLCS) because of its large specific surface area is suitable for charge storage, remarkable porosity, and excellent electronic conductivity.134 Therefore, a lot of progress is being made in the development of electrodes made out of carbon. Also, carbon is needed to induce electrical conductivity since many metal oxides materials exhibit high resistivity. Carbon black, activated carbon, carbon nanotubes, graphene, carbide-derived carbon, and carbon aerogels are non-sustainable carbon sources that consume energy, and time, and are non-renewable for manufacturing. The majority of this carbon is produced from coal, petroleum, and their derivatives, which are depleting and linked to climate change.135 Hence, on the other hand, carbon materials from biomass, such as WABs are low-cost, renewable, and environmentally friendly. It has been reported that bone derived carbons provide suitable pore size distribution for ions mobility as well as appropriate network for heteroatom dopants.136,137 The main process used in producing porous carbon from WABs is carbonization at high temperatures in the range of 450–1000 °C in an inert atmosphere such as nitrogen or argon, details of the various methods have been reported in Fig. 1.Tarimo et al.135 reported the conversion of chicken bone into porous carbon materials by pre-carbonizing at a temperature of 400 °C for 4 h in the presence of argon gas, activated with KOH (weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and carbonized at the following temperature 600, 700 and 800 °C for 2 h under argon environment with a specific surface area of 2235.8 m2 g−1. The chicken bone derived carbon was tested in a three-electrode setup using 3 M KOH basic aqueous electrolyte and achieved a capacitance of 218 F g−1 at 0.5 A g−1 in a negative potential window of 1.05 V vs. Ag/AgCl. At 0.5 A g−1, the respective maximum specific energy and power were found to be 17.1 W h kg−1 and 425 W kg−1, and coulombic efficiency was 99.8%, and capacitance retention was 90.1% after 20
1), and carbonized at the following temperature 600, 700 and 800 °C for 2 h under argon environment with a specific surface area of 2235.8 m2 g−1. The chicken bone derived carbon was tested in a three-electrode setup using 3 M KOH basic aqueous electrolyte and achieved a capacitance of 218 F g−1 at 0.5 A g−1 in a negative potential window of 1.05 V vs. Ag/AgCl. At 0.5 A g−1, the respective maximum specific energy and power were found to be 17.1 W h kg−1 and 425 W kg−1, and coulombic efficiency was 99.8%, and capacitance retention was 90.1% after 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. In another study, hierarchical porous carbon was obtained through carbonization of pig bone at 850 °C for 1 h and KOH-activated with the following characteristics abundant micropores with the size of 0.5–0.8 and 1–2 nm, mesopores and macropores with the size of 2–10 and 10–100 nm, high surface area 2157 m2 g−1 and high total pore volume 2.26 cm3 g−1, showed excellent capacitive performance of 185 F g−1 at a current density of 0.05 A g−1.136 In a nutshell, due to low-cost and satisfactory performance, the use of WABs provides an attractive and high quality carbon material that is critical for the development of the supercapacitor industry. Consequently, Goodman et al.138 employed the pyrolysis method to transform cortical bone of bovines into a network of carbon encrusted with native hydroxyapatite, which retains its macroscopic structure. By removing the hydroxyapatite with acid, self-supporting conductive carbon monoliths were created. The carbon monoliths as working electrodes in three-electrode cells, and two-electrode devices, produced specific capacitances of 134 ± 11 F g−1 in aqueous solutions of potassium nitrate and 108 ± 9 F g−1 in the ionic liquid 1-ethyl-3-methylimidizolium bis(trifluoromethylsulfonyl)imide.
000 cycles. In another study, hierarchical porous carbon was obtained through carbonization of pig bone at 850 °C for 1 h and KOH-activated with the following characteristics abundant micropores with the size of 0.5–0.8 and 1–2 nm, mesopores and macropores with the size of 2–10 and 10–100 nm, high surface area 2157 m2 g−1 and high total pore volume 2.26 cm3 g−1, showed excellent capacitive performance of 185 F g−1 at a current density of 0.05 A g−1.136 In a nutshell, due to low-cost and satisfactory performance, the use of WABs provides an attractive and high quality carbon material that is critical for the development of the supercapacitor industry. Consequently, Goodman et al.138 employed the pyrolysis method to transform cortical bone of bovines into a network of carbon encrusted with native hydroxyapatite, which retains its macroscopic structure. By removing the hydroxyapatite with acid, self-supporting conductive carbon monoliths were created. The carbon monoliths as working electrodes in three-electrode cells, and two-electrode devices, produced specific capacitances of 134 ± 11 F g−1 in aqueous solutions of potassium nitrate and 108 ± 9 F g−1 in the ionic liquid 1-ethyl-3-methylimidizolium bis(trifluoromethylsulfonyl)imide.
Heteroatom (N, S, B, P, etc.) doping and functionalization are believed to be effective methods capable of improving the specific capacitance of carbon materials and boosting the ability of electrolyte ions diffusion in the electrode surface.139 The porous carbon skeleton obtained from animal bones via the carbonization process can accommodate these heteroatoms without losing its structural integrity while surface polarity is improved. In 2019, Niu et al.139 synthesized nitrogen-doped activated carbon using simultaneous pyrolysis and KOH-activation method from three species of bio-waste bones (Pork bone, Blackfish bone and Eel bone), carbonized at temperatures 500–800 °C with the highest specific surface area of 1522 m2 g−1. The results showed that it delivered specific capacitance of 263, 302, 264 F g−1 in 6 M KOH electrolyte depending on the bone source combination carbonized at 600 °C. This suggests that the bone source influences the properties and performance of the derived carbon. In a similar vein, bio-carbon derived from chicken bone co-doped with nitrogen and sulfur with 1615.5 m2 g−1 specific surface area and 2.17 nm pore size, applied as electrodes for supercapacitor was found to deliver specific capacitance of 493.9F g−1 and 97.6% capacitance retention, and also exhibited a low electrode resistance of 0.82 and 0.98 Ωcm2 before and after 6000 cycles of testing.134 Additionally, the N-doped porous carbon (specific surface area 1337 m2 g−1) produced from fishbone by means of a single stage carbonization (850 °C), generated a maximum specific capacitance of 476 F g−1 in a 1 M H2SO4 electrolyte at a 1 A g−1 current density in a three-electrode system.140 On the other hand, the microstructure of bone-derived carbon is usually described as a hierarchical porous carbon, since it is composed of porous carbon networks in three dimensions, as shown in Fig. 12. This remarkable feature is critical for energy storage applications. The performance recorded thus far calls for synthesis method scale-up.
 | ||
| Fig. 12 Typical hierarchical porous carbon structures (source: ref. 141, https://www.mdpi.com/2079-4991/9/3/405. CC BY 4.0). | ||
Additionally, the hierarchical porous structures of bone-derived carbons reduce ionic diffusion resistance at the electrode/electrolyte interface. Consequently, chemical activators (e.g., KOH) can be used to improve porosity and surface area, thereby enhancing ionic storage sites. Recently, commercial Solabiol bone granules used to synthesize porous carbon electrodes (≈879 m2 g−1) were found to deliver an excellent specific capacitance of about 804 F g−1 at 1 A g−1 due to its large specific surface area.137 In terms of application in battery, Xie et al.142 studied animal bone pre-carbonized at 500 °C under a nitrogen environment, pulverized using a ball mill, carbonized at 800 °C for 2 h under a nitrogen environment, KOH-activated and then vacuum dried at 120 °C overnight, which produced a hierarchical porous carbon (specific surface area of 1244.8 m2 g−1 and pore volume of 0.6 cm3 g−1). It was applied as a matric to encapsulate selenium (Se) for high-performance Li–Se battery. The results revealed that the Se/bone porous carbon achieved high reversible capacity of 705 mA h g−1 in the second cycle and 591 mA h g−1 after 98 cycles at 0.1C. Therefore, in terms of economics, porous carbon materials derived from WABs are inexpensive, abundant, and sustainable, with adjustable dimensions, and have demonstrated exceptional electrical conductivity, good surface area, and excellent electrochemical stability as energy storage electrodes in both supercapacitors and batteries. If scaled up, waste animal and fish bones will be harnessed as a cost-effective, abundant and renewable, and natural carbon source while at the same time their burden on the environment will be reduced.
4 Conclusion and future outlook
Growing world populations and urbanization contribute to the increased production of meat, fish, and generation of waste bones. Therefore, the environmental impacts of discarded WABs and fish bones as well as their economic value can no longer be overlooked. Meanwhile, the growing shortage of resources and materials as a result of over-consumption and unsustainable production processes, has shifted attention to resource-efficiency and recovery of resources from waste. This study has provided an overview of methods for processing waste animal bones into new materials and their applications, which would otherwise be dumped into landfills. The recovery of useful materials or energy from WABs is economically and environmentally preferable. Based on the results of this study, significant progress has been made in the field of synthesis and application of valuable materials from bone waste over the last decade. It was found, that waste bones need low-energy to recycle and process them into valuable materials for different applications except for calcination process, as pyrolysis and gasification produced energy carriers such as bio-oil and syngas. This is supported by the synthesis of hydroxyapatite from bone waste with a wide range of properties suitable for catalytic applications, drug delivery and bone tissue engineering, and adsorbent, while its char is characterized by hierarchical porous carbon structure which has shown plausible potential in energy storage electrodes. There is a growing need for new, sustainable, and renewable heterogeneous catalysts and hierarchical porous carbon microstructure developed from waste bones; the search for reactions they can catalyze should be expanded. In addition, the effect of bone hardness on the properties of the derived material needs to be evaluated further. Therefore, several application areas, including adsorbents, catalysts for transesterification reactions used in biodiesel production, and soil remediation, have been analyzed with a view to developing scale-up strategies and stimulating new fields of applications so that natural resources and the environment are conserved. Consequently, research and development is required in extracting phosphate from waste bone for use in phosphoric acid production, phosphate sources for other industrial uses, as an enrichment for agricultural soils, and in immobilizing heavy metals in the soil so that they do not pollute groundwater. It is also critical to investigate how the valorization method and process conditions impact the physicochemical and textural properties of the derived material in relation to its application. By expanding the use of waste bones and the scaling up of valorisation methods to match with applications, it will indirectly supplement dwindling natural resources, such as phosphate rock. Therefore, for a notable reduction of the environmental impact and economic benefits, it is imperative to further investigate the technicalities surrounding the collection of waste bones. Bones derived adsorbents can also be modified into photocatalysts, and then tested for their ability to degrade organic pollutants from industrial wastewater. In respect to this view, material such as TiO2 can be coated onto bone char or HA to induce photocatalytic activity. Similarly, the benefits of cation doped HA synthesized from WABs for adsorption processes and catalysts also need to be studied further. Additionally, further studies can be conducted to evaluate the cost implications of valorizing waste bones on a large scale, including life-cycle assessment analysis and techno-economic modelling.Funding
The authors did not receive financial support from any organization for this work.Data availability
Enquiries about data availability should be directed to the authors.Conflicts of interest
The authors declare no conflict of interest.References
- H. N. Nassar, et al., Animal bone affluence in environmental reclamation: Biodiesel production, petro-diesel biodesulfurization and wastewater photo-treatment, Biofuels, Bioprod. Biorefin., 2021, 15, 770–792 CrossRef CAS.
- A. Hart, Circular economy: closing the catalyst loop with metal reclamation from spent catalysts , industrial waste , waste shells and animal bones, Biomass Convers. Biorefin., 2021 DOI:10.1007/s13399-021-01942-8.
- A. Hart and H. Onyeaka, Eggshell and Seashells Biomaterials Sorbent for Carbon Dioxide Capture, ed. S. A. Rehman Khan, IntechOpen, 2020, ch 6, pp. 83–94, DOI:10.5772/intechopen.93870.
- A. G. Adeniyi, et al., Metal oxide rich char from muffle furnace and retort heated reactor treated cow bone, Clean. Eng. Technol., 2022, 8, 100485 CrossRef.
- S. Aulakh and L. Thrope, From Waste Management to Resource Recovery: A Developing Sector, 2011, https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/31750/11-1088-from-waste-management-to-resource-recovery.pdf, accessed 04/02/2022 Search PubMed.
- K. Jayathilakan, K. Sultana, K. Radhakrishna and A. S. Bawa, Utilization of byproducts and waste materials from meat, poultry and fish processing industries: A review, J. Food Sci. Technol., 2012, 49, 278–293 CrossRef CAS PubMed.
- A. J. Lag-Brotons, et al., Editorial: Resource Recovery From Waste, Front. Environ. Sci., 2020, 8, 1–3 CrossRef.
- P. P. Biswas, et al., Sustainable phosphorus management in soil using bone apatite, J. Environ. Manage., 2022, 305, 114344 CrossRef CAS PubMed.
- A. Chaala and C. Roy, Recycling of meat and bone meal animal feed by vacuum pyrolysis, Environ. Sci. Technol., 2003, 37, 4517–4522 CrossRef CAS PubMed.
- K. Chojnacka, Equilibrium and kinetic modelling of chromium(III) sorption by animal bones, Chemosphere, 2005, 59, 315–320 CrossRef CAS PubMed.
- A. Hart, Mini-review of waste shell-derived materials' applications, Waste Manage. Res., 2020, 38, 514–527 CrossRef PubMed.
- R. C. Ferreira, et al., Multi-component adsorption study by using bone char: modelling and removal mechanisms, Environ. Technol., 2022, 43, 789–804 CrossRef CAS PubMed.
- K. P. Malla, et al., Extraction and Characterization of Novel Natural Hydroxyapatite Bioceramic by Thermal Decomposition of Waste Ostrich Bone, Int. J. Biomater., 2020, 2020, 1690178 Search PubMed.
- S. Getahun and B. Bewket, A Study on Effect of Partial Replacement of Cement by Cattle Bone Ash in Concrete Property, J. Civ. Environ. Eng., 2021, 11, 1–6 Search PubMed.
- M. Kotb, M. Assas and H. Abd-Elrahman, Effect of grounded bone powder addition on the mechanical properties of cement mortar, WIT Trans. Ecol. Environ., 2010, 138, 201–212 Search PubMed.
- E. Deydier, R. Guilet and P. Sharrock, Beneficial use of meat and bone meal combustion residue: ‘An efficient low cost material to remove lead from aqueous effluent’, J. Hazard. Mater., 2003, 101, 55–64 CrossRef CAS PubMed.
- A. Sobczak, Z. Kowalski and Z. Wzorek, Preparation of hydroxyapatite from animal bones, Acta Bioeng. Biomech., 2009, 11, 23–28 Search PubMed.
- N. A. S. Mohd Pu'ad, P. Koshy, H. Z. Abdullah, M. I. Idris and T. C. Lee, Syntheses of hydroxyapatite from natural sources, Heliyon, 2019, 5, e01588 CrossRef PubMed.
- S. M. Smith, et al., Transesterification of soybean oil using bovine bone waste as new catalyst, Bioresour. Technol., 2013, 143, 686–690 CrossRef CAS PubMed.
- M. F. M. A. Zamri, et al., Waste to health: A review of waste derived materials for tissue engineering, J. Cleaner Prod., 2021, 290, 125792 CrossRef CAS.
- M. Nasrollahzadeh, et al., Low-cost and sustainable (nano)catalysts derived from bone waste: catalytic applications and biofuels production, Biofuels, Bioprod. Biorefin., 2020, 14, 1197–1227 CrossRef CAS.
- F. Hussain, et al., Review waste animal bones as catalysts for biodiesel production; a mini review, Catalysts, 2021, 11, 1–15 Search PubMed.
- A. AbdulRahman, et al., Preparation and Characterization of Activated Cow Bone Powder for the Adsorption of Cadmium from Preparation and Characterization of Activated Cow Bone Powder for the Adsorption of Cadmium from Palm Oil Mill Effluent, IOP Conf. Ser.: Mater. Sci. Eng., 2016, 136, 012045 Search PubMed.
- Y. Yang, C. Sun, B. Lin and Q. Huang, Surface modified and activated waste bone char for rapid and efficient VOCs adsorption, Chemosphere, 2020, 256, 127054 CrossRef CAS PubMed.
- S. S. A. Alkurdi, R. A. Al-Juboori, J. Bundschuh and I. Hamawand, Bone char as a green sorbent for removing health threatening fluoride from drinking water, Environ. Int., 2019, 127, 704–719 CrossRef CAS PubMed.
- C. G. Soni, Z. Wang, A. K. Dalai, T. Pugsley and T. Fonstad, Hydrogen production via gasification of meat and bone meal in two-stage fixed bed reactor system, Fuel, 2009, 88, 920–925 CrossRef CAS.
- E. Cascarosa, A. Boldrin and T. Astrup, Pyrolysis and gasification of meat-and-bone-meal: Energy balance and GHG accounting, Waste Manag., 2013, 33, 2501–2508 CrossRef CAS PubMed.
- M. Azeem, et al., Removal of potentially toxic elements from contaminated soil and water using bone char compared to plant- and bone-derived biochars: A review, J. Hazard. Mater., 2022, 427, 128131 CrossRef CAS PubMed.
- K. McDonnell, J. Desmond, J. J. Leahy, R. Howard-Hildige and S. Ward, Behaviour of meat and bonemeal/peat pellets in a bench scale fluidised bed combustor, Energy, 2001, 26, 81–90 CrossRef CAS.
- K. McDonnell, E. J. Cummins, C. C. Fagan and M. Orjala, Co-fuelling of peat with meat and bone meal in a pilot scale bubbling bed reactor, Energies, 2010, 3, 1369–1382 CrossRef CAS.
- E. Deydier, R. Guilet, S. Sarda and P. Sharrock, Physical and chemical characterisation of crude meat and bone meal combustion residue: ‘Waste or raw material?’, J. Hazard. Mater., 2005, 121, 141–148 CrossRef CAS PubMed.
- C. W. Cheung, J. F. Porter and G. Mckay, Sorption kinetic analysis for the removal of cadmium ions from effluents using bone char, Water Res., 2001, 35, 605–612 CrossRef CAS PubMed.
- C. K. Rojas-Mayorga, et al., Optimization of pyrolysis conditions and adsorption properties of bone char for fluoride removal from water, J. Anal. Appl. Pyrolysis, 2013, 104, 10–18 CrossRef CAS.
- S. Patel, J. Han, W. Qiu and W. Gao, Journal of Environmental Chemical Engineering Synthesis and characterisation of mesoporous bone char obtained by pyrolysis of animal bones , for environmental application, Biochem. Pharmacol., 2015, 3, 2368–2377 CAS.
- C. G. Soni, A. K. Dalai, T. Pugsley and T. Fonstad, Steam gasification of meat and bone meal in a two-stage fixed-bed reactor system, Asia-Pac. J. Chem. Eng., 2011, 6, 71–77 CrossRef CAS.
- B. Purevsuren, B. Avid, J. Narangerel, T. Gerelmaa and Y. Davaajav, Investigation on the pyrolysis products from animal bone, J. Mater. Sci., 2004, 39, 737–740 CrossRef CAS.
- D. Vamvuka, S. Dermitzakis, D. Pentari and S. Sfakiotakis, Food and Bioproducts Processing Valorization of Meat and Bone Meal through pyrolysis for soil amendment or lead adsorption from wastewaters, Food Bioprod. Process., 2018, 109, 148–157 CrossRef CAS.
- C. D. Piccolla, D. Hesterberg, T. Muraoka and E. Henrique, Optimizing pyrolysis conditions for recycling pig bones into phosphate fertilizer, Waste Manag., 2021, 131, 249–257 CrossRef PubMed.
- J. K. Abifarin, D. O. Obada, E. T. Dauda and D. Dodoo-Arhin, Experimental data on the characterization of hydroxyapatite synthesized from biowastes, Data Brief, 2019, 26, 104485 CrossRef CAS PubMed.
- C. Piccirillo, et al., Extraction and characterisation of apatite- and tricalcium phosphate-based materials from cod fish bones, Mater. Sci. Eng., C, 2013, 33, 103–110 CrossRef CAS PubMed.
- A. Haider, S. Haider, S. S. Han and I. K. Kang, Recent advances in the synthesis, functionalization and biomedical applications of hydroxyapatite: a review, RSC Adv., 2017, 7, 7442–7458 RSC.
- A. Fihri, C. Len, R. S. Varma and A. Solhy, Hydroxyapatite: A review of syntheses, structure and applications in heterogeneous catalysis, Coord. Chem. Rev., 2017, 347, 48–76 CrossRef CAS.
- P. Terzioğlu, H. Öğüt and A. Kalemtaş, Natural calcium phosphates from fish bones and their potential biomedical applications, Mater. Sci. Eng., C, 2018, 91, 899–911 CrossRef PubMed.
- M. Boutinguiza, et al., Biological hydroxyapatite obtained from fish bones, Mater. Sci. Eng., C, 2012, 32, 478–486 CrossRef CAS.
- V. Paterlini, et al., Characterization and Luminescence of Eu3+- and Gd3+-Doped Hydroxyapatite Ca10(PO4)6(OH)2, Crystals, 2020, 10, 806 CrossRef CAS.
- U. Iriarte-Velasco, I. Sierra, L. Zudaire and J. L. Ayastuy, Conversion of waste animal bones into porous hydroxyapatite by alkaline treatment: effect of the impregnation ratio and investigation of the activation mechanism, J. Mater. Sci., 2015, 50, 7568–7582 CrossRef CAS.
- J. Brzezińska-Miecznik, K. Haberko, M. Sitarz, M. M. Bućko and B. Macherzyńska, Hydroxyapatite from animal bones - Extraction and properties, Ceram. Int., 2015, 41, 4841–4846 CrossRef.
- H. Ivankovic, E. Tkalcec, S. Orlic, G. Gallego Ferrer and Z. Schauperl, Hydroxyapatite formation from cuttlefish bones: Kinetics, J. Mater. Sci.: Mater. Med., 2010, 21, 2711–2722 CrossRef CAS PubMed.
- E. Barua, A. B. Deoghare, P. Deb, S. D. Lala and S. Chatterjee, Effect of pre-treatment and calcination process on micro-structural and physico-chemical properties of hydroxyapatite derived from chicken bone bio-waste, Mater. Today: Proc., 2019, 15, 188–198 CAS.
- J. Venkatesan, Z. J. Qian, B. Ryu, N. V. Thomas and S. K. Kim, A comparative study of thermal calcination and an alkaline hydrolysis method in the isolation of hydroxyapatite from Thunnus obesus bone, Biomed. Mater., 2011, 6, 035003 CrossRef PubMed.
- R. X. Sun, et al., Physicochemical and biological properties of bovine-derived porous hydroxyapatite/collagen composite and its hydroxyapatite powders, Ceram. Int., 2017, 43, 16792–16798 CrossRef CAS.
- N. A. M. Barakat, M. S. Khil, A. M. Omran, F. A. Sheikh and H. Y. Kim, Extraction of pure natural hydroxyapatite from the bovine bones bio waste by three different methods, J. Mater. Process. Technol., 2009, 209, 3408–3415 CrossRef CAS.
- K. Krupa-Zuczek, Z. Kowalski and Z. Wzorek, Manufacturing of phosphoric acid from hydroxyapatite, contained in the ashes of the incinerated meat-bone wastes, Pol. J. Chem. Technol., 2008, 10, 13–20 CrossRef CAS.
- A. Demirbaş, Y. Abali and E. Mert, Recovery of phosphate from calcinated bone by dissolution in hydrochloric acid solutions, Resour., Conserv. Recycl., 1999, 26, 251–258 CrossRef.
- N. Tri, et al., Hydrothermal and Calcination Regimes and Characteristics of Nanohydroxyapatite Synthesized from Salmon bones, J. Biochem. Technol., 2020, 11, 82–87 CAS.
- N. H. Adenan, I. Zainol, N. A. Rahim and C. N. A. Jaafar, Extraction of Nanohydroxyapatite from Waste Bovine Bone Using Alkaline Digestion Method, J. Phys.: Conf. Ser., 2018, 1082, 012005 CrossRef.
- G. C. Porter, D. Abdelmoneim, K. C. Li, W. J. Duncan and D. E. Coates, The Effect of Low-Temperature Thermal Processing on Bovine Hydroxyapatite Bone Substitutes, toward Bone Cell Interaction and Differentiation, Materials, 2022, 15, 2504 CrossRef CAS PubMed.
- M. Ayoub, S. Sufian, S. M. Hailegiorgis, S. Ullah and Y. Uemura, Conversion of glycerol to polyglycerol over waste duck-bones as a catalyst in solvent free etherification process, IOP Conf. Ser.: Mater. Sci. Eng., 2017, 226, 012073 Search PubMed.
- A. M. Janus, M. Faryna, K. Haberko, A. Rakowska and T. Panz, Chemical and microstructural characterization of natural hydroxyapatite derived from pig bones, Microchim. Acta, 2008, 161, 349–353 CrossRef CAS.
- A. A. Jazie, H. Sinha and A. S. Pramanik, Transesterification of peanut and rapeseed oils using waste of animal bone as cost effective catalyst, Mater. Renew. Sustain. Energy, 2013, 2, 11 CrossRef.
- S. S. Rahavi, O. Ghaderi, A. Monshi and M. H. Fathi, A Comparative Study on Physicochemical Properties of Hydroxyapatite Powders Derived from Natural and Synthetic Sources, Russ. J. Non-Ferr. Met., 2017, 58, 276–286 CrossRef.
- A. Ruksudjarit, K. Pengpat, G. Rujijanagul and T. Tunkasiri, Synthesis and characterization of nanocrystalline hydroxyapatite from natural bovine bone, Curr. Appl. Phys., 2008, 8, 270–272 CrossRef.
- A. Pal, et al., Synthesis of hydroxyapatite from Lates calcarifer fish bone for biomedical applications, Mater. Lett., 2017, 203, 89–92 CrossRef CAS.
- H. Satraidi, et al., Development of heterogeneous catalyst from chicken bone and catalytic testing for biodiesel with simultaneous processing, IOP Conf. Ser.: Mater. Sci. Eng., 2019, 509, 012125 CAS.
- H. E. Reynel-Avila, D. I. Mendoza-Castillo and A. Bonilla-Petriciolet, Relevance of anionic dye properties on water decolorization performance using bone char: Adsorption kinetics, isotherms and breakthrough curves, J. Mol. Liq., 2016, 219, 425–434 CrossRef CAS.
- S. K. Hubadillah, et al., Novel hydroxyapatite-based bio-ceramic hollow fiber membrane derived from waste cow bone for textile wastewater treatment, Chem. Eng. J., 2020, 379, 122396 CrossRef CAS.
- R. Slimani, et al., Adsorption of copper (II) and zinc (II) onto calcined animal bone meal. Part I: Kinetic and thermodynamic parameters, Chem. Data Collect., 2017, 9–10, 184–196 CrossRef.
- N. Z. Kasim, N. A. A. Abd Malek, N. S. Hairul Anuwar and N. H. Hamid, Adsorptive removal of phosphate from aqueous solution using waste chicken bone and waste cockle shell, Mater. Today: Proc., 2020, 31, A1–A5 CAS.
- S. Pai, S. M Kini, R. Selvaraj and A. Pugazhendhi, A review on the synthesis of hydroxyapatite, its composites and adsorptive removal of pollutants from wastewater, J. Water Process. Eng., 2020, 38, 101574 CrossRef.
- J. C. Moreno, R. Gómez and L. Giraldo, Removal of Mn, Fe, Ni and Cu ions from wastewater using cow bone charcoal, Materials, 2010, 3, 452–466 CrossRef CAS.
- Y. N. Chen, L. Y. Chai and Y. D. Shu, Study of arsenic(V) adsorption on bone char from aqueous solution, J. Hazard. Mater., 2008, 160, 168–172 CrossRef CAS PubMed.
- R. A. Olaoye, O. D. Afolayan, K. A. Adeyemi, L. O. Ajisope and O. S. Adekunle, Adsorption of selected metals from cassava processing wastewater using cow-bone ash, Sci. Afr., 2020, 10, e00653 Search PubMed.
- D. C. K. Ko, C. W. Cheung, K. K. H. Choy, J. F. Porter and G. McKay, Sorption equilibria of metal ions on bone char, Chemosphere, 2004, 54, 273–281 CrossRef CAS PubMed.
- D. C. K. Ko, J. F. Porter and G. McKay, Optimized correlations for the fixed-bed adsorption of metal ions on bone char, Chem. Eng. Sci., 2000, 55, 5819–5829 CrossRef CAS.
- X. Pan, J. Wang and D. Zhang, Sorption of cobalt to bone char: Kinetics, competitive sorption and mechanism, Desalination, 2009, 249, 609–614 CrossRef CAS.
- N. A. Medellin-Castillo, et al., Adsorption of fluoride from water solution on bone char, Ind. Eng. Chem. Res., 2007, 46, 9205–9212 CrossRef CAS.
- A. Rezaee, G. Ghanizadeh, G. Behzadiyannejad, A. Yazdanbakhsh and S. D. Siyadat, Adsorption of endotoxin from aqueous solution using bone char, Bull. Environ. Contam. Toxicol., 2009, 82, 732–737 CrossRef CAS PubMed.
- J. Liu, et al., Adsorption of arsenic(V) on bone char: Batch, column and modeling studies, Environ. Earth Sci., 2014, 72, 2081–2090 CrossRef CAS.
- P. Jia, H. Tan, K. Liu and W. Gao, Enhanced photocatalytic performance of ZnO/bone char composites, Mater. Lett., 2017, 205, 233–235 CrossRef CAS.
- M. T. Ghaneian, G. Ghanizadeh, M. T. H. Alizadeh, M. H. Ehrampoush and F. M. Said, Equilibrium and kinetics of phosphorous adsorption onto bone charcoal from aqueous solution, Environ. Technol., 2014, 35, 882–890 CrossRef CAS PubMed.
- S. S. M. Hassan, N. S. Awwad and A. H. A. Aboterika, Removal of mercury(II) from wastewater using camel bone charcoal, J. Hazard. Mater., 2008, 154, 992–997 CrossRef CAS PubMed.
- D. S. d. C. Coltre, et al., Study of dye desorption mechanism of bone char utilizing different regenerating agents, SN Appl. Sci., 2020, 2, 1–14 Search PubMed.
- B. Sawangjang, et al., Evaluation of fluoride adsorption mechanism and capacity of different types of bone char, Int. J. Environ. Res. Public Health, 2021, 18, 6878 CrossRef CAS PubMed.
- M. Wang, Y. Liu, Y. Yao, L. Han and X. Liu, Comparative evaluation of bone chars derived from bovine parts: Physicochemical properties and copper sorption behavior, Sci. Total Environ., 2020, 700, 134470 CrossRef CAS PubMed.
- L. G. Elvir-padilla and D. I. Mendoza-castillo, Adsorption of dental clinic pollutants using bone char : Adsorbent preparation , assessment and mechanism analysis, Chem. Eng. Res. Des., 2022, 183, 192–202 CrossRef CAS.
- A. Rezaee, H. Rangkooy, A. Jonidi-Jafari and A. Khavanin, Surface modification of bone char for removal of formaldehyde from air, Appl. Surf. Sci., 2013, 286, 235–239 CrossRef CAS.
- A. F. Lee, J. A. Bennett, J. C. Manayil and K. Wilson, Heterogeneous catalysis for sustainable biodiesel production via esterification and transesterification, Chem. Soc. Rev., 2014, 43, 7887–7916 RSC.
- J. A. Bennett, K. Wilson and A. F. Lee, Catalytic applications of waste derived materials, J. Mater. Chem. A, 2016, 4, 3617–3637 RSC.
- P. Mukhopadhyay and R. Chakraborty, Infrared radiation promoted preparation of cost-effective lamb bone supported cobalt catalyst: Efficacy in semi-batch monoolein synthesis, Catal. Commun., 2017, 94, 73–76 CrossRef CAS.
- Y. Riadi, et al., Animal bone meal as an efficient catalyst for crossed-aldol condensation, Tetrahedron Lett., 2010, 51, 6715–6717 CrossRef CAS.
- M. Ait Taleb, et al., Animal bone meal as new recyclable and ecological catalyst for the oximes synthesis in solvent-free conditions, J. Mater. Environ. Sci., 2016, 7, 4580–4588 Search PubMed.
- G. Asgari, A. Seid Mohammadi, S. B. Mortazavi and B. Ramavandi, Investigation on the pyrolysis of cow bone as a catalyst for ozone aqueous decomposition: Kinetic approach, J. Anal. Appl. Pyrolysis, 2013, 99, 149–154 CrossRef CAS.
- M. Pongsendana, W. Trisunaryanti, F. W. Artanti, I. I. Falah and S. Sutarno, Hydrocracking of waste lubricant into gasoline fraction over CoMo catalyst supported on mesoporous carbon from bovine bone gelatin, Korean J. Chem. Eng., 2017, 34, 2591–2596 CrossRef CAS.
- D. Milovac, I. Weigand, M. Kovačić, M. Ivanković and H. Ivanković, Highly porous hydroxyapatite derived from cuttlefish bone as TiO2 catalyst support, Process. Appl. Ceram., 2018, 12, 136–142 CrossRef CAS.
- A. Shaabani, H. Afaridoun and S. Shaabani, Natural hydroxyapatite-supported MnO2: a green heterogeneous catalyst for selective aerobic oxidation of alkylarenes and alcohols, Appl. Organomet. Chem., 2016, 30, 772–776 CrossRef CAS.
- B. H. Monjezi, M. E. Yazdani, M. Mokfi and M. Ghiaci, Liquid phase oxidation of diphenylmethane to benzophenone with molecular oxygen over nano-sized Co-Mn catalyst supported on calcined Cow bone, J. Mol. Catal. A: Chem., 2014, 383–384, 58–63 CrossRef CAS.
- H. Song, et al., Chicken bone-derived N-doped porous carbon materials as an oxygen reduction electrocatalyst, Electrochim. Acta, 2014, 147, 520–526 CrossRef CAS.
- B. Kayode and A. Hart, An overview of transesterification methods for producing biodiesel from waste vegetable oils, Biofuels, 2019, 10, 419–437 CrossRef CAS.
- P. Suwannasom, P. Tansupo and C. Ruangviriyachai, A bone-based catalyst for biodiesel production from waste cooking oil, Energy Sources, Part A, 2016, 38, 3167–3173 CrossRef CAS.
- M. Jayakumar, et al., Waste Ox bone based heterogeneous catalyst synthesis, characterization, utilization and reaction kinetics of biodiesel generation from Jatropha curcas oil, Chemosphere, 2022, 288, 132534 CrossRef CAS PubMed.
- Y. H. Tan, et al., Biodiesel production from used cooking oil using green solid catalyst derived from calcined fusion waste chicken and fish bones, Renewable Energy, 2019, 139, 696–706 CrossRef CAS.
- A. A. Ayodeji, I. E. Blessing and F. O. Sunday, Data on calcium oxide and cow bone catalysts used for soybean biodiesel production, Data Brief, 2018, 18, 512–517 CrossRef PubMed.
- C. Chingakham, C. Tiwary and V. Sajith, Waste Animal Bone as a Novel Layered Heterogeneous Catalyst for the Transesterification of Biodiesel, Catal. Lett., 2019, 149, 1100–1110 CrossRef CAS.
- X. Zhou, et al., Insight into the mechanism of persulfate activated by bone char : Unraveling the role of functional structure of biochar, Chem. Eng. J., 2020, 401, 126127 CrossRef CAS.
- Y. Riadi, et al., An efficient and reusable heterogeneous catalyst Animal Bone Meal for facile synthesis of benzimidazoles, benzoxazoles, and benzothiazoles, Tetrahedron Lett., 2011, 52, 3492–3495 CrossRef CAS.
- Y. Riadi, et al., Animal bone meal as an efficient catalyst for crossed-aldol condensation, Tetrahedron Lett., 2010, 51, 6715–6717 CrossRef CAS.
- Y. Riadi, et al., Novel animal-bone-meal-supported palladium as a green and efficient catalyst for Suzuki coupling reaction in water , under sunlight, Green Chem. Lett. Rev., 2017, 10, 101–106 CrossRef CAS.
- R. Chakraborty and D. Roychowdhury, Fish bone derived natural hydroxyapatite-supported copper acid catalyst : Taguchi optimization of semibatch oleic acid esterification, Chem. Eng. J., 2013, 215–216, 491–499 CrossRef CAS.
- U. Iriarte-Velasco, J. L. Ayastuy, Z. Boukha, R. Bravo and M. Á. Gutierrez-Ortiz, Transition metals supported on bone-derived hydroxyapatite as potential catalysts for the Water-Gas Shift reaction, Renewable Energy, 2018, 115, 641–648 CrossRef CAS.
- A. M. Ghahfarrokhi, P. Moshiri and M. Ghiaci, Studies on calcined cow bone and pyrolyzed wood , suitable supports for immobilizing hybrid nano particles of Co-Mn as new catalysts for oxidation of 2,6-diisopropyl naphthalene, Appl. Catal., A, 2013, 456, 51–58 CrossRef.
- Y. Saito, H. Ishitani and S. Kobayashi, Catalytic Hydrogenation of Aliphatic Nitro Compounds with Polysilane/Bone Charcoal-Supported Palladium Catalysts under Continuous-Flow Conditions, Asian J. Org. Chem., 2016, 5, 1124–1127 CrossRef CAS.
- S. A. Gama-Lara, et al., Synthesis, Characterization, and Catalytic Activity of Platinum Nanoparticles on Bovine-Bone Powder: A Novel Support, J. Nanomater., 2018, 2018, 6482186 Search PubMed.
- B. M. Matsagar, et al., Glucose isomerization catalyzed by bone char and the selective production of 5-hydroxymethylfurfural in aqueous media, Sustainable Energy Fuels, 2018, 2, 2148–2153 RSC.
- S. Mignardi, L. Archilletti, L. Medeghini and C. De Vito, Valorization of Eggshell Biowaste for Sustainable Environmental Remediation, Sci. Rep., 2020, 10, 2436 CrossRef CAS PubMed.
- S. B. Chen, Y. G. Zhu, Y. B. Ma and G. McKay, Effect of bone char application on Pb bioavailability in a Pb-contaminated soil, Environ. Pollut., 2006, 139, 433–439 CrossRef CAS PubMed.
- N. Siebers and P. Leinweber, Bone Char: A Clean and Renewable Phosphorus Fertilizer with Cadmium Immobilization Capability, J. Environ. Qual., 2013, 42, 405–411 CrossRef CAS PubMed.
- K. N. Palansooriya, et al., Soil amendments for immobilization of potentially toxic elements in contaminated soils: A critical review, Environ. Int., 2020, 134, 105046 CrossRef CAS PubMed.
- I. R. Sneddon, M. Orueetxebarria, M. E. Hodson, P. F. Schofield and E. Valsami-Jones, Use of bone meal amendments to immobilise Pb, Zn and Cd in soil: A leaching column study, Environ. Pollut., 2006, 144, 816–825 CrossRef CAS PubMed.
- M. E. Hodson, E. Valsami-Jones and J. D. Cotter-Howells, Bonemeal additions as a remediation treatment for metal contaminated soil, Environ. Sci. Technol., 2000, 34, 3501–3507 CrossRef CAS.
- J. Qu, et al., Effective lead passivation in soil by bone char/CMC-stabilized FeS composite loading with phosphate-solubilizing bacteria, J. Hazard. Mater., 2022, 423, 127043 CrossRef CAS PubMed.
- E. Someus and M. Pugliese, Concentrated phosphorus recovery from food grade animal bones, Sustain, 2018, 10, 1–17 CrossRef.
- Q. Du, S. Zhang, M. Antonietti and F. Yang, Sustainable Leaching Process of Phosphates from Animal Bones to Alleviate the World Phosphate Crisis, ACS Sustainable Chem. Eng., 2020, 8, 9775–9782 CrossRef CAS.
- M. Darwish, A. Aris, M. H. Puteh, M. N. H. Jusoh and A. Abdul Kadir, Waste bones ash as an alternative source of P for struvite precipitation, J. Environ. Manage., 2017, 203, 861–866 CrossRef CAS PubMed.
- J. W. Bujak, New insights into waste management - Meat industry, Renewable Energy, 2015, 83, 1174–1186 CrossRef.
- E. Deydier, et al., Evaluation of meat and bone meal combustion residue as lead immobilizing material for in situ remediation of polluted aqueous solutions and soils: “Chemical and ecotoxicological studies”, J. Hazard. Mater., 2007, 146, 227–236 CrossRef CAS PubMed.
- S. A. Chattanathan, T. P. Clement, S. R. Kanel, M. O. Barnett and N. Chatakondi, Remediation of Uranium-contaminated Groundwater by Sorption onto Hydroxyapatite Derived from Catfish Bones, Water, Air, Soil Pollut., 2013, 224, 1429 CrossRef.
- M. Azeem, et al., Effects of sheep bone biochar on soil quality, maize growth, and fractionation and phytoavailability of Cd and Zn in a mining-contaminated soil, Chemosphere, 2021, 282, 131016 CrossRef CAS PubMed.
- N. W. Sari, F. A. Putri and D. S. Perwitasari, Manufacture of Phosphate Fertilizer from Cow Bones Waste, Int. J. Eco-Innovation Sci. Eng., 2020, 01, 26–29 Search PubMed.
- S. M. Londoño-Restrepo, R. Jeronimo-Cruz, E. Rubio-Rosas and M. E. Rodriguez-García, The effect of cyclic heat treatment on the physicochemical properties of bio hydroxyapatite from bovine bone, J. Mater. Sci.: Mater. Med., 2018, 29, 52 CrossRef PubMed.
- H. Ivankovic, G. Gallego Ferrer, E. Tkalcec, S. Orlic and M. Ivankovic, Preparation of highly porous hydroxyapatite from cuttlefish bone, J. Mater. Sci.: Mater. Med., 2009, 20, 1039–1046 CrossRef CAS PubMed.
- J. H. G. Rocha, et al., Hydrothermal growth of hydroxyapatite scaffolds from aragonitic cuttlefish bones, J. Biomed. Mater. Res., Part A, 2006, 77, 160–168 CrossRef CAS PubMed.
- A. Veremeev, R. Bolgarin, V. Nesterenko, A. Andreev-Andrievskiy and A. Kutikhin, Native Bovine Hydroxyapatite Powder, Demineralised Bone Matrix Powder, and Purified Bone Collagen Membranes Are Efficient in Repair of Critical-Sized Rat Calvarial Defects, Materials, 2020, 13, 3393 CrossRef CAS PubMed.
- G. S. Dos Reis, S. H. Larsson, H. P. de Oliveira, M. Thyrel and E. C. Lima, Sustainable biomass activated carbons as electrodes for battery and supercapacitors–a mini-review, Nanomaterials, 2020, 10, 1–22 CrossRef PubMed.
- A. A. N. Oladipo, S co-doped biocarbon for supercapacitor application: Effect of electrolytes concentration and modelling with artificial neural network, Mater. Chem. Phys., 2021, 260, 124129 CrossRef CAS.
- D. J. Tarimo, et al., Waste chicken bone-derived porous carbon materials as high performance electrode for supercapacitor applications, J. Energy Storage, 2022, 51, 104378 CrossRef.
- W. Huang, H. Zhang, Y. Huang, W. Wang and S. Wei, Hierarchical porous carbon obtained from animal bone and evaluation in electric double-layer capacitors, Carbon, 2011, 49, 838–843 CrossRef CAS.
- Y. Al Haj, et al., Biowaste-derived electrode and electrolyte materials for flexible supercapacitors, Chem. Eng. J., 2022, 435, 135058 CrossRef CAS.
- P. A. Goodman, et al., Preparation and characterization of high surface area, high porosity carbon monoliths from pyrolyzed bovine bone and their performance as supercapacitor electrodes, Carbon, 2013, 55, 291–298 CrossRef CAS.
- L. Niu, et al., Waste bones derived nitrogen–doped carbon with high micropore ratio towards supercapacitor applications, J. Colloid Interface Sci., 2019, 547, 92–101 CrossRef CAS PubMed.
- J. Mu, et al., Fishbone-derived N-doped hierarchical porous carbon as an electrode material for supercapacitor, J. Alloys Compd., 2020, 832, 154950 CrossRef CAS.
- C. Liu, et al., Fast microwave synthesis of hierarchical porous carbons from waste palm boosted by activated carbons for supercapacitors, Nanomaterials, 2019, 9, 405 CrossRef CAS PubMed.
- L. S. Xie, et al., Hierarchical porous carbon derived from animal bone as matric to encapsulated selenium for high performance Li–Se battery, Rare Met., 2017, 36, 434–441 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |