Open Access Article
Open Access ArticleScalable and sustainable production of Ti3C2Tz MXene and fluorine recovery from wastewater through cryolite precipitation
Michael S. Carey and
Michel W. Barsoum *
*
Department of Materials Science and Engineering, Drexel University Philadelphia, PA 19104, USA. E-mail: barsoumw@drexel.edu
First published on 27th October 2022
Abstract
Treatment of HF or HCl/LiF etched Ti3C2Tz with 0.05 M NaHCO3 before water washing reduces the wastewater generated by 75%. When etched with HF, cryolite (Na3AlF6) precipitation from spent etching waste effectively removes fluorine from this waste stream, offers insight into the etching chemistry of MAX to MXene, and provides an effective analytical tool for optimization of MXene production. Additionally, washing HF etched multilayered Ti3C2Tz with 0.05 M NaHCO3 allows for the production of delaminated Ti3C2Tz colloidal suspensions, which typically requires the use of TBAOH or DMSO for intercalation and subsequent delamination. Ti3C2Tz made with HCl/LiF and washed with 0.05 M NaHCO3 yields a colloidal suspension with a concentration of 18 mg mL−1 and a film conductivity of 1150 S cm−1.
Introduction
Traditional synthesis of Ti3C2Tz MXene, most commonly obtained by the selective etching of Al from a Ti3AlC2 MAX phase1–3 with hydrofluoric acid, HF, requires a large amount of water to obtain a colloidal suspension of few, to single, flake Ti3C2Tz.3,4 Typically, the amount of water needed is at least 640 mL per gram of MXene as this water is thought to both wash away residual salts formed in the etching process, and to bring the solution pH to neutral at which point a stable colloidal MXene suspension forms. Herein we show that by modifications made to the synthesis process, we can obtain Ti3C2Tz colloidal suspensions with 75% less water utilized in the washing procedure. The fluorine, F, in the remaining wastewater can also be recovered through the precipitation of cryolite.While MXenes have been shown to be quite promising in terms of their performance across a wide variety of applications, their widescale production has lagged.5–7 One of the limiting steps at this time is the large cost of their production and the need for F in the etching medium. Additionally, the treatment of HF containing wastewater is both a major industrial and environmental concern.8
For MXene production to occur at industrial scales, the large amount of acidic wastewater that is currently a byproduct of its methods of production must be addressed. Furthermore, the presence of F− in this wastewater is problematic as this treatment is quite costly and produces a large amount of industrial waste sludge that becomes difficult to dispose of due to environmental regulations.9 It was long believed that the main product of the selective etching of Al from Ti3AlC2 to yield Ti3C2Tz is AlF3,10 which has limited solubility in water (0.5 g/100 g H2O at 25 °C).11 However, Cockreham et al. showed that AlF3 formation only occurs under a range of ionic strength from 8.5 to 10 M and other aluminum/fluorine complex ions are dominant outside this range.12 It should be noted that this work explored the use of cobalt fluorides (CoF2/CoF3) as a source of F, and the applicability to HF or HCl/LiF etching methods has not been demonstrated.
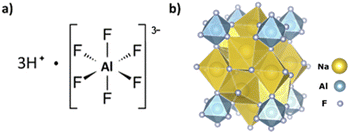
The Ti3AlC2 MAX phase is known to form a protective oxide layer which is composed of Al2O3 and TiO2.13 A product of the reaction between Al-containing species such as alumina comprising this oxide layer, or the aluminum in the MAX structure, and HF is hexafluoroaluminic acid (H3AlF6).14,15 If H3AlF6 is present, then a route to recovering F from the wastewater exists through the addition of sodium cations to form sodium hexafluoroaluminate, otherwise known as cryolite, which can be readily isolated from the spent etching liquid.14,15 The yield of precipitated cryolite allows for the calculation of Al etching efficiency, while also being a product of commercial value. In the reaction of the Al in Ti3AlC2 with HF, a route for the formation of hexafluoroaluminic acid and cryolite may be as follows:
| 2Al + 6HF(aq) → 2AlF3(aq) + 3H2(g) | (1) |
| AlF3(aq) + 3HF → H3AlF6(aq) | (2) |
| 2H3AlF6(aq) + 3Na2CO3 → 2Na3AlF6 + 3H2O + 3CO2(g) | (3) |
With the typical etching procedures using HF16 or HCl/LiF3 there is enough HF to fully convert AlF3 to H3AlF6. Furthermore, AlF63− is believed to be a stable species as the ionic strength of the solution increases.12,17 In this reaction, the formation of H3AlF6 may provide a driving force for the continued extraction of Al from Ti3AlC2, potentially explaining why HF is the only acid capable of etching the MAX phase to yield MXenes.5,18 The structures of hexafluoroaluminic acid and cryolite are shown in Fig. 1.
Sodium hexafluoroaluminate, Na3AlF6, more commonly known as cryolite, is insoluble in water, but soluble in sulfuric acid, H2SO4, with the evolution of HF. Synthetic cryolite finds widespread industrial use as a flux agent to dissolve alumina, Al2O3, in the electrolytic metal extraction process. It is also consumed in the abrasives, ceramic and glass industries.15,19 Many research articles and patents have appeared over the years regarding the production of cryolite, suggesting that if cryolite can be isolated and repackaged as a by-product of MXene production, the economics associated with the scale-up of MXene synthesis may be partially offset. By our calculations, assuming complete etching of Al from Ti3AlC2 and complete conversion to cryolite, 1.07 g may be produced from 1 g of Ti3AlC2. This may be an attractive method to lower the cost of wastewater treatment and produce a high purity, high value compound, while also providing a quantitative tool for the determination of etching efficiency. The feasibility of this method is supported by the work of Kumar et al. which showed that the recovery of synthetic cryolite from the treatment of synthetic HCl/HF leach liquors comprising H3SiF6 and H3AlF6 with 3 M Na2CO3 was 97.5%.15
In this work we demonstrate that by washing the acidified MXene sediment once with a mild solution of low-cost and environmentally benign sodium bicarbonate, BC, colloidal suspensions can be produced using 75% less deionized water than otherwise needed. This reduction in wastewater is achieved independent of the etching method (HF vs. HCl/LiF). By similarly treating the waste HF etching liquid with 3 M sodium carbonate, cryolite can easily be formed and precipitated, according to reaction (3), providing a useful method for the determination of Al extraction efficiency as well as providing a value-added product which effectively removes F from the waste stream.
Results & discussion
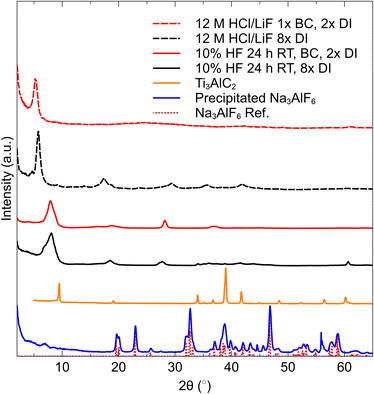
The sediments produced by this method were first investigated by XRD to ensure that etching of the MAX phase was complete, and to characterize the obtained MXene. In Fig. 2, XRD patterns of multilayer (ML) MXene obtained by normal deionized (DI) water washing, with 8 washes of DI (middle, black), ML MXene after 1 wash with 40 mL 0.05 M NaHCO3 and 2 washes of DI, (top, red) and the precipitated Na3AlF6 from the spent etching liquid. A reference profile for Na3AlF6, is also presented.It is evident from these patterns that the MXene obtained by the neutralization method is the same as the MXene obtained by the normal washing method. In fact, the HF-etched ML MXene pattern obtained after BC and DI washing appears to be cleaner than the water only washed sample, as many peaks associated with Ti3AlC2 in the range of ∼35–45° 2θ are still present in that sample. Interestingly, the 110 peak present at ∼61° 2θ is not present in the BC washed specimen, suggesting that there is a complete absence of non-basal ordering in this sample. Furthermore, the HCl/LiF, BC washed film does not display the same number of 00l reflections as the HCl/LiF, DI washed film.
The cryolite precipitated from the waste etching liquid matches nearly perfectly to the reference pattern (bottom red dotted lines in Fig. 2). The amount of cryolite was measured to be 0.89 g, indicating an etching efficiency of 84%. We believe that this method can prove to be an effective tool for optimizing the etching efficiency, and this value can be pushed closer to 100% by variations in etching conditions such as time, pH of solution, temperature, or reagent concentrations.
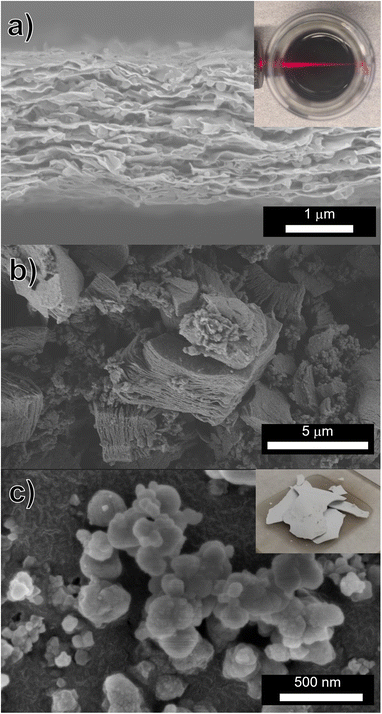
Complete washing of Ti3C2Tz MXene (indicated by a near-neutral pH and stable supernatant after centrifugation) requires between 8 and 10 washing cycles when only washing with only DI H2O. However after treatment with 0.05 M NaHCO3 (regardless of HF or HCl/LiF etching method), this stability occurred within two rounds of washing, as seen in Fig. 3. Interestingly, a stable suspension is found after just the first wash (Fig. 3a). However in our washing procedure, an additional wash step was carried out that resulted in a darker, more concentrated suspension (Fig. 3b). Washing with DI H2O only, required 640 mL. However with the incorporation of one 40 mL 0.05 M NaHCO3 wash before subsequent DI washes, the volume of water necessary to produce a dark colloidal suspension was 160 mL per gram of Ti3C2Tz sediment. An SEM micrograph of the Ti3C2Tz filtered film after exposure to NaHCO3 can be seen in Fig. 4a and b along with an SEM micrograph of the obtained Na3AlF6 in Fig. 4c.
 | ||
| Fig. 3 Digital images of post-neutralization washing procedure with NaHCO3, (a) After washing once, and (b) twice with DI water. | ||
Typically, Ti3C2Tz produced by etching with HF, only do not yield delaminated flakes (referred to as d-Ti3C2Tz, to insinuate delamination) without the use of a chemical exfoliation agent such as TBAOH or DMSO.5,20–22 However, with our approach, a colloidal suspension was obtained after NaHCO3 and DI water washing, and a film of d-Ti3C2Tz was produced by vacuum filtration of this suspension (Fig. 4a). A photograph of this colloidal suspension, and the Tyndall effect produced by the d-Ti3C2Tz flakes, is shown as an inset of Fig. 4a. Conductivity measurements were conducted on this film with a four-point probe, and a DC conductivity of 180 ± 10 S cm−1 was measured.
A film was attempted to be produced by the DMSO assisted intercalation and delamination of HF etched, water only washed, Ti3C2Tz, however the concentration of this suspension was so slow that a continuous film was unable to be obtained. Conductivity measurements were attempted on a section of this ‘film’ and a value of 2.25 ± 0.05 S cm−1 was measured.
Conductivity measurements were similarly conducted on HCl/LiF etched films, one washed first with 0.05 M NaHCO3, and the other washed only with water. In the BC washed case, the colloidal suspension had a concentration of 18 mg mL−1 and a film produced from this suspension had conductivity of 1150 ± 27 S cm−1 was measured, while the H2O washed colloidal suspension had a concentration of 15.6 mg mL−1 and the corresponding film had a conductivity of 1330 ± 54 S cm−1. These are comparable to the conductivity of Ti3C2Tz clay reported by Ghidiu et al.2
Conclusions
In summary, a simple yet effective method of obtaining Ti3C2Tz MXene is presented where the isolated etched sediment is neutralized with a mild solution of sodium bicarbonate. In so doing, the pH can directly be adjusted to neutral without the need of large volumes of DI water. This drastically reduces the amount of wastewater generated, specifically from 640 mL g −1 to 160 mL g −1, a reduction of 75%. This holds for both HF only and HCl/LiF etched Ti3C2Tz. Furthermore, when etched with HF, delaminated films of Ti3C2Tz could be produced, provided they were treated with 0.05 M NaHCO3 before washing with deionized water. Furthermore, films produced by etching with HCl/LiF and then washed once with 0.05 M NaHCO3, followed by two washes with deionized water resulted in a colloidal suspension with a concentration of 18 mg mL−1 Filtered films made from this suspension had a conductivity of 1,150 ± 27 S cm−1. Additionally, cryolite (Na3AlF6) can be formed from this process, which indicates that the etching media used for the extraction of Al from Ti3AlC2 must contain hexafluoroaluminic acid, H3AlF6. This provides insight into the chemistry of the etching reaction and allows for the recovery of fluorine from the spent etching liquid. The cryolite obtained from this neutralization can be precipitated by treatment with sodium carbonate, further offsetting the cost of MXene production, and providing a quantitative tool for calculating etching efficiency which would allow for the optimization of the etching parameters such as time, temperature, pH and reagent concentrations.Materials & methods
The experimental approach in this work is largely separated into two segments which are focused on: (1) neutralization of the MXene sediment to obtain high quality Ti3C2Tz MLs with less water, and (2) precipitation of cryolite from the spent etching waste.Two etching methods are explored in this work. Namely, the use of HF acid only as well as the method first reported by Ghidiu et al. utilizing hydrochloric acid and lithium fluoride to produce HF in situ.2 The latter method is more widely adopted due to the improved handling safety and ability to directly yield delaminated suspensions. However, when considering the precipitation of cryolite through neutralization of the spent etching liquid, the HF only method is a much cleaner system, as there are fewer ionic species present. For the purposes of this work, we demonstrate that cryolite, Na3AlF6, can be precipitated from the spent etching media obtained from the HF-only method.
The neutralization of the MXene sediment with 40 mL 0.05 M NaHCO3 is applicable to both methods of MXene production. Given that the HCl/LiF method is more widely adopted by those that work with MXene, we felt it was important to demonstrate the effectiveness of this washing procedure when working with HCl/LiF etched MXene.
HF etching of Ti3AlC2 to produce HF–Ti3C2Tz
MXene etched by HF acid only, (henceforth referred to as HF–Ti3C2Tz) was obtained by etching 1 g of Ti3AlC2 MAX phase in 10 mL of 10% HF for 24 h at RT.Sodium bicarbonate washing of HF–Ti3C2Tz
After the etching period, the HF–Ti3C2Tz ML sediment is isolated from the etching liquid through centrifugation. The spent liquid portion is set aside and used for cryolite precipitation. After separation, the sediment in each centrifuge tube (0.5 g per tube) was treated with 40 mL of 0.05 M NaHCO3 so that the pH was adjusted to 7.0. After the initial wash with 0.05 M NaHCO3, the sediment was washed two times with 40 mL of DI water.Delamination of NaHCO3 washed HF–Ti3C2Tz
20 mL of DI water was added to the sediment obtained after washing with 0.05 M NaHCO3 and DI water, which was then bath sonicated for 1 h under argon flow. After sonication, the colloidal suspension was isolated by centrifugation at 5000 rpm for 10 minutes. This suspension was then filtered to obtain a film.HF-etched, H2O washed Ti3C2Tz
For comparison to the NaHCO3 method, a separate 1 g batch of HF–Ti3C2Tz was washed only with DI water. To produce a film of the HF–Ti3C2Tz washed only with water, 12 mL of dimethyl sulfoxide (DMSO) was added to the washed sediment and allowed to mix for 24 h at room temperature (RT). After mixing, the DMSO was separated by centrifugation at 3500 rpm for 5 minutes. 20 mL of DI H2O was added to the DMSO intercalated sediment, and bath sonicated for 1 h under flowing argon. After sonication, the colloidal suspension was isolated by centrifugation at 5000 rpm for 10 minutes. This suspension was then filtered to obtain a film.Precipitation of cryolite from HF–Ti3C2Tz etching media
Cryolite was precipitated by adding 3 M Na2CO3 to the isolated etching liquid until a pH of 4.0. This solution was then kept in a 55 °C oil bath for 1 h, after which the gelatinous liquid was vacuum filtered over a Celgard polypropylene membrane where a white crystalline solid was formed.HCl/LiF-etched Ti3C2Tz
To demonstrate the applicability of this method to the more widely used method first reported by Ghidiu et al.,2 two grams of Ti3C2Tz were prepared by etching two grams of Ti3AlC2 in 20 mL of 12 M HCl along with two grams of lithium fluoride. This mixture was allowed to mix at 35 °C for 48 h at 300 rpm.After etching, the contents of the etching bottle were split equally into four centrifuge tubes so that 0.5 g of sediment was in each centrifuge tube. Two of the tubes were washed only with deionized water, while the other two were first washed with 40 mL of 0.05 M NaHCO3, followed by two washes with deionized water.
After the washing period, the contents of the tubes were combined so that roughly 1 g of each separately washed powders of Ti3C2Tz were in each tube. 20 mL of DI H2O was added to each tube, and these mixtures were bath sonicated for 1 h under flowing argon. After sonication, the colloidal suspensions were collected by centrifugation at 5000 rpm for 10 minutes. These colloidal suspensions were filtered into films by vacuum filtration.
Author contributions
M. C. conducted all experiments and wrote the manuscript along with M. W. B. who oversaw the project.Conflicts of interest
There are no conflicts to declare.References
- M. W. Barsoum, MAX phases: properties of machinable ternary carbides and nitrides, John Wiley & Sons, 2013 Search PubMed.
- M. Naguib, M. Kurtoglu, V. Presser, J. Lu, J. Niu, M. Heon, L. Hultman, Y. Gogotsi and M. W. Barsoum, Adv. Mater., 2011, 23, 4248–4253 CrossRef CAS PubMed.
- M. Ghidiu, M. R. Lukatskaya, M.-Q. Zhao, Y. Gogotsi and M. W. Barsoum, Nature, 2014, 516, 78–81 CrossRef CAS PubMed.
- L. Verger, V. Natu, M. Ghidiu and M. W. Barsoum, J. Phys. Chem. C, 2019, 123, 20044–20050 CrossRef CAS.
- L. Verger, V. Natu, M. Carey and M. W. Barsoum, Trends Chem, 2019, 1, 656–669 CrossRef CAS.
- L. Verger, C. Xu, V. Natu, H. Cheng, W. Ren and M. W. Barsoum, Curr. Opin. Solid State Mater. Sci., 2019, 1–15 Search PubMed.
- M. Naguib, V. N. Mochalin, M. W. Barsoum and Y. Gogotsi, Adv. Mater., 2014, 26, 992–1005 CrossRef CAS PubMed.
- C. H. Won, J. Choi and J. Chung, J. Hazard. Mater., 2012, 239–240, 110–117 CrossRef CAS PubMed.
- A. Toyoda and T. Taira, IEEE Trans. Semicond. Manuf., 2000, 13, 305–309 CrossRef.
- O. Mashtalir, M. Naguib, B. Dyatkin, Y. Gogotsi and M. W. Barsoum, Mater. Chem. Phys., 2013, 139, 147–152 CrossRef CAS.
- D. R. Lide, CRC Handbook of Chemistry and Physics, CRC press, 2004, vol. 85 Search PubMed.
- C. B. Cockreham, X. Zhang, H. Li, E. Hammond-Pereira, J. Sun, S. R. Saunders, Y. Wang, H. Xu and D. Wu, ACS Appl. Energy Mater., 2019, 2, 8145–8152 CrossRef CAS.
- M. W. Barsoum, N. Tzenov, A. Procopio and M. Ali, J. Electrochem. Soc., 2001, 148, 551–562 CrossRef.
- V. Sćepanović, S. Radosavljević and J. Misović, J. Fluor. Chem., 1974, 3, 403–408 CrossRef.
- M. Kumar, M. N. Babu, T. R. Mankhand and B. D. Pandey, Hydrometallurgy, 2010, 104, 304–307 CrossRef CAS.
- M. Naguib, M. Kurtoglu, V. Presser, J. Lu, J. Niu, M. Heon, L. Hultman, Y. Gogotsi and M. W. Barsoum, Adv. Mater., 2011, 23, 4248–4253 CrossRef CAS PubMed.
- M. S. Corbillon, M. A. Olazabal and J. M. Madariaga, J. Solution Chem., 2008, 37, 567–579 CrossRef CAS.
- V. D. Jovic, B. M. Jovic, S. Gupta and T. El-raghy, Corr. Sci., 2006, 48, 4274–4282 CrossRef CAS.
- Z. Akdeniz and M. P. Tosi, Chem. Phys. Lett., 1999, 308, 479–485 CrossRef CAS.
- O. Mashtalir, M. Naguib, V. N. Mochalin, Y. Dall’Agnese, M. Heon, M. W. Barsoum and Y. Gogotsi, Nat. Commun., 2013, 4, 1716 CrossRef PubMed.
- J. Halim, S. Kota, M. R. Lukatskaya, M. Naguib, M. Zhao, E. J. Moon, J. Pitock, J. Nanda, S. J. May, Y. Gogotsi and M. W. Barsoum, Adv. Funct. Mater., 2016, 26, 3118–3127 CrossRef CAS.
- P. Urbankowski, B. Ansori, T. Makaryan, D. Er, S. Kota, P. L. Walsh, M. Zhao, V. B. Shenoy, M. W. Barsoum and Y. Gogotsi, Nanoscale, 2016, 8, 11385–11391 RSC.
| This journal is © The Royal Society of Chemistry 2022 |