Open Access Article
Open Access ArticleRecent developments in supramolecular complexes of azabenzenes containing one to four N atoms: synthetic strategies, structures, and magnetic properties
Juhi Singh†
,
Suvam Kumar Panda†
 and
Akhilesh Kumar Singh
and
Akhilesh Kumar Singh
 *
*
School of Basic Sciences, Indian Institute of Technology Bhubaneswar, Bhubaneswar, 752 050, India. E-mail: aksingh@iitbbs.ac.in
First published on 29th June 2022
Abstract
For the last couple of decades, azabenzene-based ligands have drawn much attention from inorganic chemists due to their ability to coordinate with different metal ions to form supramolecular clusters. These azabenzenes are weak σ donors and strong π acceptors and electron-deficient. Metallogrid complexes and non-grid oligomers are well-defined supramolecular clusters, formed by appropriate chelating ligands, and can show interesting optical, magnetic, and electronic properties. Self-assembly of [n × n] metallogrid complexes is dominated by the entropic factor while the formation of oligonuclear metal ion complexes is dominated by other effects like CFSE, electrostatic factors, ligand conformational characters, etc. Herein, the present article gives an overview of six-membered heterocyclic azine-based ligands and their potential for different metal ions to form polynuclear complexes. Moreover, their temperature-dependent magnetic properties and SCO phenomena are well described and tabulated.
1. Introduction
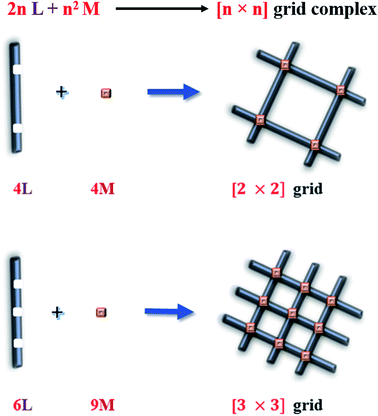
In heterocyclic chemistry, according to Hantzsch–Widman nomenclature, azabenzene (azine) refers to the compounds containing six-membered aromatic rings with one or more N atoms in the heterocyclic ring.1 Heterocyclic azines are named depending on n, the number of N atoms present in the aromatic six-membered heterocyclic ring, e.g. n = 1 (pyridine); n = 2 (diazine: pyrimidine, pyridazine, pyrazine); n = 3 (1,3,5-triazine); and n = 4 (1,2,4,5-tetrazine). These azabenzenes form supramolecular clusters with different transition and inner-transition metal ions and show variable magnetic responses at different temperatures. The roots of modern supramolecular chemistry were first described by Hermann Emil Fischer in 1894.2 After that in 1979, Lehn defined Supramolecular Chemistry as “chemistry beyond the molecule”.3 Supramolecular systems are held together by hydrophobic forces, π–π interactions, or electrostatic effect, i.e., metals don't coordinate by direct covalent forces.4 Molecular self-assembly is the basic concept behind Supramolecular Chemistry.5 Synthetic coordination chemists mainly focus on the preparation of polynuclear transition metal-based self-assembled multimetallic clusters because they show fascinating magnetic, electronic, redox, and photophysical properties.6 Desired supramolecular clusters can be synthesized by an appropriate choice of bridging groups and chelating ligands. For example, [n × n] grids are formed by ligand-directed-assembly process to form polynuclear complexes, in which 2n number of ligands are arranged like poles of a grid with n2 metal ions situated at the joint of the grid (Fig. 1). These types of grids are called homometallic [n × n] grids. When both n values are different then the formed self-assembly grid is called [n × m] heterometallic grid, in which all metal centres are not same. So in molecular magnetism and coordination chemistry, metallogrid complexes hold a special place due to their inventive structure and used for information storage devices, logic and electronic switches.7 Nuclearity of grid assembly or coordination capacity of ligands can be extended by using suitable different bridging groups substituted with different functional groups at terminal ends.8–101.1 Magnetism
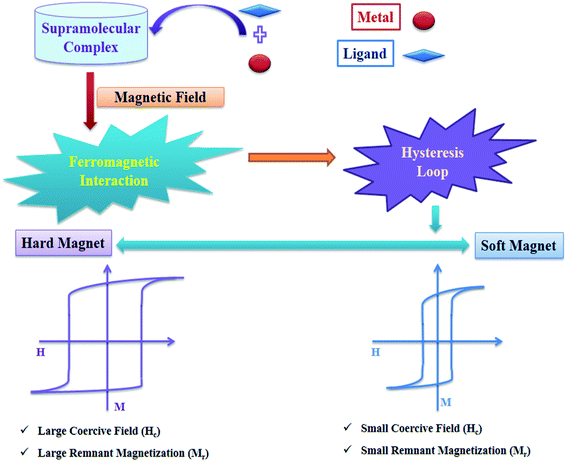
Magnetism in a material largely arises due to a magnetic moment that is generated by the electrons' intrinsic spin.11 In presence of external magnetic field alignment of ground state spins of electrons show attractive or repulsive responses. This results into two kinds of magnetism, diamagnetism and paramagnetism. Diamagnetism arises due to presence of paired electron materials which show repulsive response to applied magnetic fields. Paramagnetism is shown by species having unpaired electrons and their spins align in the same direction as of applied magnetic field. They show attractive response towards applied magnetic fields (Fig. 2).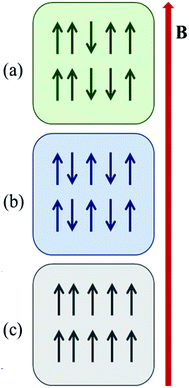 | ||
| Fig. 2 Different arrangementof neighbouring spin; (a) paramagnetism, (b) antiferromagnetism, and (c) ferromagnetism. | ||
1.2 Temperature- and field-dependent magnetic properties
In a complex, paramagnetic metal centres show intramolecular magnetic coupling with each other in presence of an applied magnetic field and below a certain temperature (Curie and Néel points), alignment of neighboring spins change rapidly (Fig. 3b and c).11,12 Alignment of spins in an antiparallel direction give rise to antiferromagnetism and alignment of spins in uniform parallel direction give rise to ferromagnetism (Fig. 2b and c). Paramagnetic substances obey Curie law i.e., χ = C/T, where χ is magnetic susceptibility (degree of magnetization), T is absolute temperature, and C is curie constant. But curie law was unable to describe the behaviour of ferromagnetic and antiferromagnetic substances, so to overcome this problem Curie–Weiss law i.e., χ = C/(T − θ) came into picture where θ is the Curie Weiss constant (For ferromagnetic substances θ > 0 and for antiferromagnetic substances θ < 0) as shown in Fig. 3a.13,14 Ferromagnetic substances show hysteresis behaviour and form a hysteresis loop shown in Fig. 3d. Hysteresis loops give two important terms, coercive field (Hc) and remnant magnetization (Mr). Twice of the coercive field is equal to the maximum width of the hysteresis loop and is defined as the field required demagnetizing the fully magnetized material. Remnant magnetization is defined as the magnetization that remains after removal of the applied magnetic field. Hysteresis loop is used for the characterization of soft and hard magnets (Fig. 4). Soft magnets have less Hc value and approximately zero value of Mr, used in magnetic shielding and electric motors. But hard magnets have higher value of Hc and significant value of Mr, used in data storage devices.10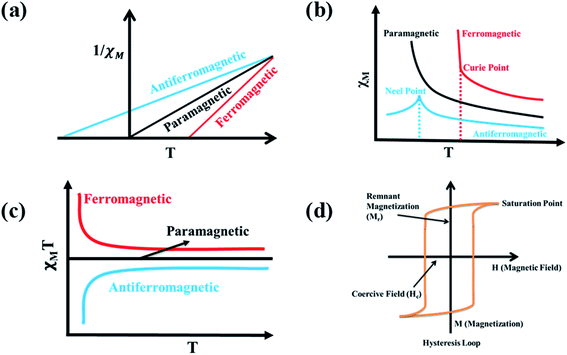 | ||
| Fig. 3 Characteristic graphs of (a) 1/χM vs. T, (b) χM vs. T, (c) χMT vs. T for paramagnetic, ferromagnetic, and antiferromagnetic substances, (d) Hysteresis loop. | ||
1.3 Device for magnetic measurement
The common effective and sensitive device that is used for measuring the magnetic properties is SQUID (Superconducting quantum interference device) magnetometer.15 It is made of superconductors those are separated by thin insulating layers (Josephson junction) and can detect small magnetic fields.16 These SQUID magnetometers are now commonly used in magnetic coupling measurements between two paramagnetic centres. SQUID magnetometers normally operate at low temperature to room temperature i.e., at liquid helium or liquid nitrogen temperature to room temperature and they consist of superconductors that are separated by appropriately thin insulating layers or junctions, known as Josephson junctions, through which electrons can flow.17 When current flows through SQUID, magnetic flux (Φ) is generated by the samples inside the apparatus and the superconducting coils. When the sample is moved up and down inside the SQUID an alternating magnetic flux is generated within the pick-up coil and through a superconducting pick-up coil, the magnetic signal is obtained. The magnetic flux is transferred by this coil from the sample to a radio frequency SQUID device within the liquid helium bath and found far away from the sample. As a result, this can act as a magnetic flux-to-voltage converter device. This voltage is then amplified and gives the information regarding the materials' magnetic properties (magnetic susceptibility and effective magnetic moment).18–20 This is used widely in research, biological studies, electronic measurements, and instruments in which conventional measurement fails to sense the faint signals.21 Characteristic graphs shown in Fig. 3 are produced by the data obtained from a SQUID magnetometer.1.4 Magnetic interaction
There are the two categories of magnetic materials: (a) magnetically concentrated and (b) magnetically dilute substances. In magnetically dilute substances, due to a large distance of separation between individual paramagnetic centres, interaction between them is not possible.22 On the other hand, in magnetically concentrated substances direct exchange or superexchange interaction occurs between total-electron-spin of paramagnetic centres, which is clearly due to the small inter-micromagnetic separation.23 In the direct-exchange, there is an interaction between two nearby cations without involving any intermediate anion; while superexchange refers to a strong magnetic exchange interaction, which involves the coupling between nearby cations through a non-magnetic anion.24,25In metallogrid complexes, spin exchange interaction takes place between the paramagnetic metal centers through the diamagnetic bridging groups. So, to determine the interactions among all spin centers, ligand field effects, and Zeeman splitting terms, a generalized spin Hamiltonian equation can be used for the evaluation of multimetallic cluster assemblies.26,27
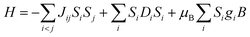 | (1) |
Normally, in isotropic and spin only conditions the ligand field effects, 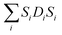 , are ignored and by assuming the Lande splitting factor (g) are identical and isotropic for all spin centres, the Hamiltonian can be written as
, are ignored and by assuming the Lande splitting factor (g) are identical and isotropic for all spin centres, the Hamiltonian can be written as
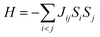 | (2) |
When the Hamiltonian applied on a specific spin-coupled arrangement, it provides a square matrix and on diagonalization it provides the energy of the subsequent spin states (S′) in form of Si, Sj and J.
| E(S′) = −J(S′(S′ + 1) − Si(Si + 1) − Sj(Sj + 1) | (3) |
When J > 0 (Ferromagnetic Substances), (Si + Sj) is the spin states for ground state and (Si − Sj) is the spin states for excited state and vice versa for J < 0 (antiferromagnetic substances).
Based on the resultant spin Hamiltonian, estimation of the magnetic behaviour of metallogrid complexes can be executed for isotropic exchange interaction.28 This can be generalized by Heisenberg-Dirac-Van-Vleck (HDVV) equation:
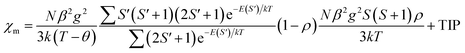 | (4) |
In this review, azabenzene-based ligands such as pyridine, pyridazine, pyrimidine, pyrazine, 1,3,5-triazine, 1,2,4,5-tetrazine and their derivatives are highlighted under the umbrella termed as azabenzene. These azabenzene containing compounds are used to prepare a variety of polytopic ligands which upon metalation lead to the formation of grid complexes. The iron and cobalt complexes of these ligands show interesting magnetic properties and sometimes spin crossover (SCO) phenomena as well. SCO materials show crossover between the low spin and high spin states and they may be used in data storage,29,30 molecular switches,31 and optical displays.32 Generally, in octahedral d4–d7 transition metal complexes, the transition between LS and HS is observed due to the presence of intermediate ligand field and the variation of temperature, pressure, or light.33 There are a few reports of grid complexes of azabenzene-based ligands which show ferromagnetic interaction and form hysteresis loop at particular temperature. Some of these ligands and their magnetic behaviours are described below based on the recent articles. Finally, all the results are highlighted in a tabular manner for the better understanding of the readers (Table 1).
| S.No. | Ligands | Compound | Magnetic order | Grid | Ref. |
|---|---|---|---|---|---|
| a A.F.M. = antiferromagnetic, F.M. = ferromagnetic, D.M. = diamagnetic, S.C.O. = spin crossover. | |||||
| 1 | L1 | [((L1)2Dy4)(μ2-O)4]-(H2O)8·2CH3OH·8H2O | A.F.M. | — | 43 |
| [((L1)2Ho4)(μ2-O)4]-(H2O)8·6CH3OH·4H2O | A.F.M. | — | |||
| 2 | L2 | [Dy4(L2)4(μ2-OH)3(μ2-OMe)]4NO3·2MeOH·4H2O | A.F.M. | [2 × 2] | 44 |
| [Tb4(L2)4(μ2-OH)3(μ2-OMe)]4NO3·2MeOH·4H2O | A.F.M. | [2 × 2] | |||
| [Gd4(L2)4(μ2-OH)3(μ2-OMe)]4NO3·2MeOH·5H2O | A.F.M. | [2 × 2] | |||
| [Er4(L2)4(μ2-OH)3(μ2-OMe)]4NO3·3MeOH·3H2O | A.F.M. | [2 × 2] | |||
| 3 | L3 | [Cu16(L3)8](dmf)3 | A.F.M. | [4 × 4] | 45 |
| 4 | L4 | [Dy4(L4)4(H2O)12](CF3SO3)4·12H2O | A.F.M. | [2 × 2] | 46 |
| [Dy4(L4′)4Cl4(H2O)8]Cl8 | A.F.M. | [2 × 2] | |||
| 5 | L5 | [Dy4Cu4(L5)4Cl8(H2O)4]Cl4·28H2O | F.M. | [2 × 2] | 46 |
| 6 | L6 | [Dy4(L62)4(μ2-OH)4]·xCH3OH·yH2O | A.F.M. | [2 × 2] | 51 |
| [Tb4(L62)4(μ2-OH)4]·xCH3OH·yH2O | A.F.M. | [2 × 2] | |||
| [Ho4(L62)4(μ2-OH)4]·xCH3OH·yH2O | A.F.M. | [2 × 2] | |||
| [Er4(L62)4(μ2-OH)4]·xCH3OH·yH2O | A.F.M. | [2 × 2] | |||
| 7 | L7 | [Mn10(L7′)6(H2O)5][Mn(H2O)6]2·11H2O | A.F.M. | [3 × 3] | 10 |
| 8 | L8 | [Mn11(L8′)6(H2O)8][Mn(H2O)6]·24H2O | A.F.M. | [3 × 3] | 10 |
| 9 | L9 | [FeIII4(L9′)4] | A.F.M. | [2 × 2]] | 52 |
| [FeII2FeIII2(L9)4](BF4)2·2CH3CN | A.F.M. | [2 × 2] | |||
| [Mn4(L9)4]·MeCN | A.F.M. | [2 × 2] | |||
| [Co4(L9)4]·CHCl3 | A.F.M. | [2 × 2] | |||
| [Cu4(L9)4]·CHCl3 | A.F.M. | [2 × 2] | |||
| 10 | L10 | [Dy(L10)(H2O)](ClO4)·2CH3CH2OH | A.F.M. | — | 53 |
| [Dy(L10)(NO3)] | A.F.M. | — | |||
| [Dy2(L10)(dbm)4] | F.M. | — | |||
| 11 | L11 | [Dy(L11)Cl] | — | — | 54 |
| [Dy(L11)Br] | — | — | |||
| 12 | L12 | [Cu2(L12)2] | D.M | — | 55 |
| [Ni2(L12)2] | A.F.M. | — | |||
| 13 | L13 | [FeII(L13)](ClO4)2·H2O | S.C.O | — | 56 |
| [CoII(L13)](ClO4)2·H2O | — | — | |||
| [Fe0.4Co0.6II(L13)](ClO4)2·H2O | S.C.O | — | |||
| 14 | L14 | [Cu(L14)2(ClO4)]ClO4 | A.F.M. | — | 73, 74 ,76 and 77 |
| [Cu2(L14)(OH)-(ClO4)3(H2O)3].H2O | A.F.M. | — | |||
| [Cu2(L14)2(H2O)2](ClO4)4 | A.F.M. | — | |||
| [Cu2(L14)2(ClO4)4] | A.F.M. | — | |||
| [Cu2(L14)(N3)4].H2O/[Cu2(L14)-(N3)4(H2O)])n | A.F.M. | — | |||
| [Cu2(L14)(OH)(dca)3]·H2O)n | A.F.M. | — | |||
| [Ni4(L14)4(μ-N3)4]Cl4.5H2O | F.M. | [2 × 2] | |||
| [CoII4(L14)4(N3)4])·sol | F.M. and A.F.M | [2 × 2] | |||
| [FeII4 (L14)4 (N3)4][BPh4]4.sol | S.C.O | [2 × 2] | |||
| 15 | L15 | [(L15)4 CoII4(N3)4]·sol | F.M. and A.F.M. | [2 × 2] | 76 and 77 |
| [(L15)4FeII4 (N3)4][BPh4]4·sol | A.F.M | [2 × 2] | |||
| 16 | L16 | [Dy4(HL16)2L(DMF)8]·2ClO4CH2Cl2·4DMF(CH3CH2)2O.H2O | F.M. | — | 78 |
| [Dy6 (L16)3(PhCOO)6(CH3OH)6]·11CH3OH.H2O | A.F.M. | — | |||
| [Dy10Na2 (L16)4(μ3-OH)4(DMF)12-(NO3)6]·6NO3.2DMF(CH3CH2)2O·H2O | A.F.M. | — | |||
| 17 | L17 | Fe2L17(H2O)4(BF4)4 | A.F.M. | — | 91 |
| Co2L17(H2O)3(MeCN)2(BF4)4 | A.F.M. | — | |||
| Ni2L17(H2O)4(BF4)4 | A.F.M. | — | |||
| Fe4(L17)2(F)4(BF4)4·52H2O | A.F.M. | — | |||
| Co4(L17)2(F)4(BF4)4·3H2O | A.F.M. | — | |||
| Ni4(L17)2(F)4(BF4)4·4H2O | A.F.M. | — | |||
| 18 | L18 | [Fe4(HL18a)4]Cl4·9H2O | S.C.O | [2 × 2] | 92 and 93 |
| [Fe4(L184a)2(H2L18a)2]·(BF4)4·6H2O | S.C.O | [2 × 2] | |||
| [Fe4(L18b)2(H2L18b)2]ClO4)4·4H2O | D.M. | [2 × 2] | |||
| [Co4(HL18a)4](ClO4)4·8H2O | S.C.O | [2 × 2] | |||
| [Co4(L18b)4](ClO4)4·16H2O | D.M | [2 × 2] | |||
| 19 | L19 | [Fe4(L19)4](BF4)8·xMeCN | S.C.O. | [2 × 2] | 94 |
| [Fe4(H2L19)2(HL19)2](BF4)6·8MeCN | S.C.O. | [2 × 2] | |||
| [Fe4(HL19)4](BF4)4·12MeCN | S.C.O. | [2 × 2] | |||
| [Zn4(HnL194)](n)+ (n = 8 and 4) | D.M. | [2 × 2] | |||
| 20 | L20 | [Ni4(L20)4].2CH3CN·4CH3OH·4H2O | A.F.M. | [2 × 2] | 95 |
| 21 | L21 | [Ni4(H2L21)4](CF3SO3)8·4CH3NO2·8H2O | A.F.M. | [2 × 2] | 95 |
| 22 | L22 | [Dy4(L22)4(H2O)4(NO3)4]·6CH3OH·6H2O | A.F.M. | [2 × 2] | 99 |
| 23 | L23 | [Co4(L23H)4](ClO4)4·6DMF·3H2O | D.M. | — | 104 |
| [Co4(L23Me)4](ClO4)8·4MeCN·H2O | S.C.O. | — | |||
| [Co4(L23Br)4](ClO4)8·CH3OH·2MeCN·5H2O | A.F.M. | — | |||
| 24 | L24 | [Zn3(L24)3](BF4)6 | — | — | 100 |
| [Fe3(L24)3](BF4)6 | — | — | |||
| 25 | L25 | ((H2O)MnIIL25)(μ-1,3-N3)(L21MnII(NO3))](NO3)2 | A.F.M. | — | 110 |
| [(L25MnII)(μ-1,3-N3)]n(NO3)n | A.F.M. | — | |||
| 26 | L26 | [Cu4(L22)4]8+ | F.M. | [2 × 2] | 111 |
| 27 | L27 | [Fe(L27H)2(NCS)2]·2CH2Cl2 | S.C.O. | — | 33 |
| [Fe(L27F)2(NCS)2]·2CH2Cl2 | S.C.O. | — | |||
| 28 | L28 | [Dy4(L28)2(CH3OH)3(NO3)3]·3NO3·2H2O | A.F.M. | — | 122 |
| [Dy4(L28)2(CH3OH)2(SCN)4(OCH3)2]·2CH3OH·2H2O | A.F.M. | — | |||
| ([Dy4(L28)2(CH3OH)(SCN)6(CH3CN)]·3CH3OH·4CH3CN)2 | A.F.M. | — | |||
| [Dy4(L28)2(CH3OH)2(SCN)6]·6CH3OH·2H2O | A.F.M. | — | |||
| [Dy4(L28)2(CH3OH)2(SCN)4(OCH3)2]·5CH3OH·2H2O | A.F.M. | — | |||
| [Dy4(L28)2(CH3OH)(SCN)5(H2O)2]·SCN·4CH3OH·2H2O | A.F.M. | — | |||
| 29 | L29 | [Dy(tmhd)3]2(L29) | A.F.M. | — | 133 |
| (Cp2Co)([Dy(tmhd)3]2(L29)) | A.F.M. | — | |||
| [Co4(L29)4(dbm)4]·4MeCN | A.F.M. | [2 × 2] | |||
| 30 | L30 | [Dy4(L30)4(MeOH)8](NO3)4·aMeOH·bH2O | F.M. | — | 135 |
| [Gd4(L30)4(MeOH)8](NO3)4·aMeOH·bH2O | F.M. | — | |||
| 31 | L31 | [Ni4(L26)Cl6(DMF)8]Cl·1/2(H2O) | F.M. | — | 136 |
| 32 | L32 | [DyIII2(L28)(THMD)6]·4(C6H6) | A.F.M. | — | 139 |
| [DyIII2(L28)2)(THMD)4] | A.F.M. | — | |||
1.5 Rationale for choosing such azabenzene-based ligands
Polytopic pyridine/pyrimidine/pyrazine/pyridazine/triazine/tetrazine and hydrazone-based ligands depict the power of self-assembly as a methodology for the synthesis of polymetallic systems with specific and predetermined organization of metal centers in a closely spaced bridged arrangement. Homometallic, heterometallic, homovalent, heterovalent and mixed-spin-state [n × n] (n ≥ 2) square-grids, [n × m] (n, m ≥ 2) rectangular-grids or non-grid oligomeric structures may be isolated using these ligands to give an interesting structural and magnetic properties. These ligands have potential to provide the supramolecular assembly with interesting magnetic properties having ferromagnetic, antiferromagnetic and ferrimagnetic interactions between the metal centers. The magnetic properties of such supramolecular assembly may be exploited for molecular devices, molecular switches31 and for information storage and processing devices.29,302. Pyridine-based ligands
Pyridine is a six-membered N-heterocyclic compound, discovered by Scottish chemist Thomas Anderson in 1849.34,35 Pyridine is able to form σ bond with metal centre by overlapping the sp2 lone pair orbital of N atom with vacant metal orbitals. Due to this reason pyridine can be utilized as a ligand for the development of metal complexes. Pyridine and its derivatives are widely used in coordination chemistry. Inorganic chemists take very much interest in design and synthesis of polytopic pyridine hydrazone-based ligands. These polytopic pyridine-based ligands are widely utilized in self-assembled [n × n] and [n × m] grid complex formations.36–42 Some of these ligands, based on the recent literature, which have potential towards self-assembly for the syntheses of polymetallic complexes are described in this current review (Scheme 1).In 2013 Chandrasekhar et al. reported the ligand L1, which formed a tetranuclear complexes [((L1)2Dy4)(μ2-O)4]-(H2O)8·2CH3OH·8H2O (DyL1) and [((L1)2Ho4)(μ2-O)4]·(H2O)8·6CH3OH·4H2O (HoL1) with lanthanide salts LnCl3·6H2O (Ln = DyIII or HoIII).43 In tetranuclear complexes, all lanthanide ions were situated in the plane and held together by perpendicularly arranged deprotonated ligands, which were coordinated via their two coordination pockets ONO and ONNO. All these arrangements formed tetranuclear complexes that showed temperature-dependent magnetic susceptibility between 2 and 300 K. Initially, the χmT values were found as 53.4 cm3 mol−1 K for complex DyL1 and 53.0 cm3 mol−1 K for complex HoL1, that slowly decreased as the temperature was lowered and suddenly at below 30 K the χmT values were steeply decreased and reached to 29.6 and 21.0 cm3 mol−1 K at 2 K for DyL1 and HoL1 respectively. These behaviours of complexes suggested the antiferromagnetic coupling among metal centres (Fig. 5).
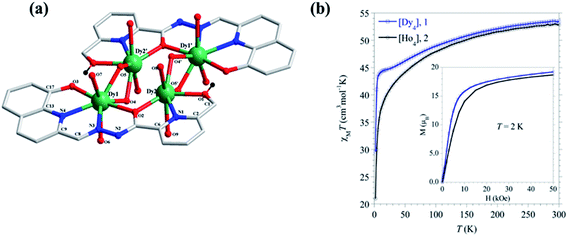 | ||
| Fig. 5 (a) represents structure of complex DyL1 and (b) show magnetic behaviour of complex DyL1 (blue) and HoL1 (black). Reproduced with permission from ref. 43 Copyright© 2013, American Chemical Society. | ||
Again to explore more toward lanthanide complexes, in 2014, the same group reported the ligand L2, which formed tetranuclear [2 × 2] homometallic grid complexes, [Ln4(L2)4(μ2-OH)3(μ2-OMe)]4NO3·xMeOH·yH2O (Ln = Dy, Tb, Gd, and Er), when reaction was done with different hydrated lanthanide(III) salts Ln(NO3)3 xH2O (Ln = Dy, Tb, Gd, and Er).44 The ligand backbone contained two unsymmetrical coordination pockets (NNO & ONO) and was able to connect with two lanthanide ions. So, in DyL2, TbL2, GdL2 and ErL2 lanthanide centres were eight coordinated and satisfied by NNO and ONO coordination pockets of perpendicular ligands and additional two coordinations were fulfilled by μ-OH and μ-OMe ligands. So overall, in grid complexes two LnIII ions were coordinated with 6O, 2N and the remaining two LnIII ions contained coordination environments of 4O, 4N. For analyzing the magnetic property of GdL2, magnetic susceptibility measurement was done. Initially at 290 K, the χmT value was 30.9 cm3 mol−1 K, that continuously decreased on lowering the temperature, indicated the dominating nature of antiferromagnetic interactions among the GdIII-centers. Same type of behaviour was shown by other lanthanide complexes i.e., at 290 K, χmT values were observed as 55.0 (DyL2), 46.6 (TbL2) and 44.1 (ErL2) cm3 mol−1 K, that decreased down on lowering the temperature (Fig. 6).
 | ||
| Fig. 6 (a) represents structure of TbL2 and (b) show magnetic behaviour of complex DyL2 (black), TbL2 (red), and ErL2 (blue). Modified and reproduced with permission from ref. 44 Copyright© 2014, American Chemical Society. | ||
Amit Adhikary and coworkers in 2014 reported the potential ligand L3, which had the inclusion of pyridine ring at the center with two o-vanillin groups present at the terminal and both the groups were connected by a carbohydrazide linkage.45 At room temperature when L3 was reacted with methanolic solution of Cu(ClO4)2·6H2O a novel [4 × 4] metallogrid complex [Cu16(L3)8](dmf)3 (CuL3) was formed through self-assembly. The ligand backbone contained four coordination pockets having unsymmetrical coordination modes due to the non-participation of one methoxy group present at terminal side of the ligand. The destabilization factor arisen due to the o-vanillin groups was greater than the stabilization factor between aromatic rings and to avoid the stearic interaction between o-vanillin groups in grid structure ligands were arranged in parallel manner to each other and not in eclipsed form. In CuL3, 8 CuII had distorted square-pyramidal geometry which was stabilized by N1O4 coordination pockets (two hydroxyl O atoms, one methoxy O atoms from o-vanillin moieties and one N atom from the hydrazone moiety) and remaining 8 CuII had distorted octahedral geometry, stabilized by N2O4 coordination pockets (one N atom from hydrazone moiety and one from pyridine ring, and four O atoms from the pyridine hydrazone moieties). The [4 × 4] grid structure contained nine [2 × 2] square fragment in which the central fragment was unique. The molecule had one inversion centre and fourfold rotational axis. Variable temperature magnetic susceptibility in the form of χmT was studied for the complex CuL3. Initially (at room temperature) χmT value of the complex was 6.43 cm3 mol−1 K, which was steadily decreased down to 1.80 cm3 mol−1 K when the temperature was slowly decreased to 20 K and finally at 1.8 K the value of χmT was decreased rapidly to 1.01 cm3 mol−1 K. So, it was confirmed that, in the lower range of temperature, CuL3 showed antiferromagnetic interaction.
To explore the susceptibility response toward heterometallic complexes, Jianfeng Wu and coworkers, in 2015, developed 4f based heterometallic grid-like complexes based on the ligand L4.46 To satisfy higher coordination number of 4f based heterometallic grid, additional ligands viz. NO3−, OH− and OAc− also contribute in the formation of grid complexes.47–50 A [2 × 2] metallogrid complex was formed by the reaction of ligand L4 with Dy(CF3SO3)3·6H2O or DyCl3·6H2O. In [2 × 2] grid complex, all four DyIII ions were joined together through four perpendicular ligands that occupied the corner sites by using terminal N–N–O pockets (Fig. 7). Here one interesting thing was observed that, after the formation of [2 × 2] 4f grid complex, there was also a possibility to form [3 × 3] or [2 × 2] 3d–4f grid complex by using free N–N–N coordination pockets of the ligand. Nevertheless, it was observed that only 4f based grid complex was formed when its further reaction was carried out with 3d and 4f metal ions. So, ligand L4 was modified to a novel ligand L5 with two large O–N–N–O pockets, present in terminal side, instead of two small N–N–O pockets. This made the ligand L5 suitable for coordination with 4f metal ions and the free N–N–N pockets could bind with 3d metal ions. Heterometallic [2 × 2] grid complex [DyIII4CuII4L5], was formed by the reaction of DyCl3·6H2O and CuCl2·2H2O with ligand L5, in which four DyIII placed at the corner site, coordinated through the O–N–N–O pockets of ligand and one coordination sites of DyIII was occupied by H2O molecule. Four CuII ions were coordinated with the middle N–N–N pocket of ligand and two coordination sites were occupied by Cl− ion. At room temperature χmT value for 4f grid-like complexes [Dy4(L4)4(H2O)12](CF3SO3)4·12H2O (DyL4a) was 55.17 cm3 K mol−1, and [Dy4(L4)4Cl4(H2O)8]Cl8 (DyL4b) was 55.29 cm3 mol−1 K that gradually decreased at 2 K to 37.4 cm3 mol−1 K and 37.0 cm3 K mol−1 respectively, suggested the antiferromagnetic interaction between metal centers. But in 3d–4f heterometallic grid complex, [Dy4Cu4(L5)4Cl8(H2O)4]Cl4·28H2O (DyCuL5), χmT value first slowly decreased from 54.9 cm3 K mol−1 and then sharply increased to 91.2 cm3 K mol−1 at 30 K, suggested the ferromagnetic interaction and again decreased at 2 K to a minimum value of 73.5 cm3 K mol−1 suggested the weak antiferromagnetic interaction Fig. 7.
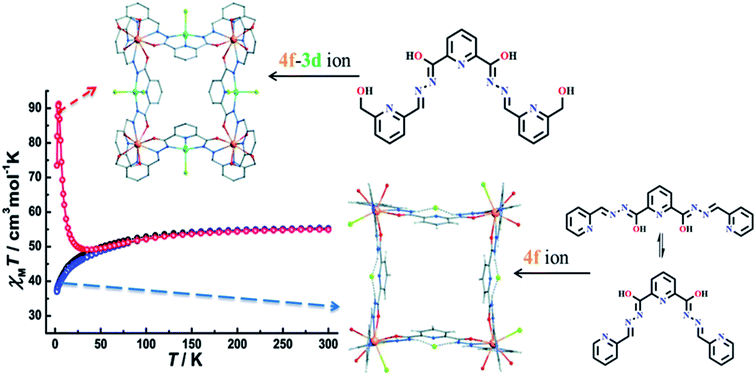 | ||
| Fig. 7 Structures (orange, azure, green, blue, dark and red spheres representing Dy, Cu, Cl, N, C and O) and Magnetic properties of 4f ion (DyL4b) and 4f–3d ion (DyCuL5) based complexes. Modified and reproduced by permission from ref. 46, ©2015, The Royal Society of Chemistry. | ||
In 2016 Biswas et al. designed and synthesized one multidentate flexible ligand L6 which upon reaction with Ln(NO3)3·5H2O formed tetranuclear [2 × 2] homometallic grid complexes, [LnIII4(L62)4(μ2-OH)4]·xCH3OH·yH2O (Ln = Dy, Tb, Ho, Er), with lanthanide metals.51 Reported ligand L6 contained two ONO coordination pockets and was able to bind with two LnIII ions. Lanthanide complexes of ligands composed of four LnIII ions were held together by two coordination pockets of perpendicular dianionic ligands [LH2]2−. In χmT vs. T plot of DyL6, it was observed that the χmT value was 56.0 cm3 mol−1 K at 300 K which further decreased to 43.9 cm3 K mol−1 on lowering down the temperature to 2 K. It was also reported that similar types of magnetic behaviour were shown by the remaining three complexes.
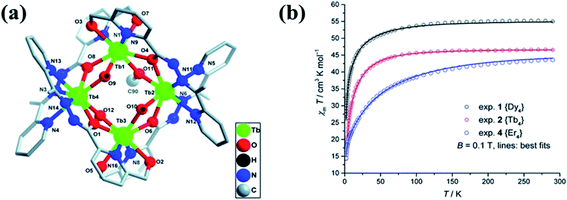
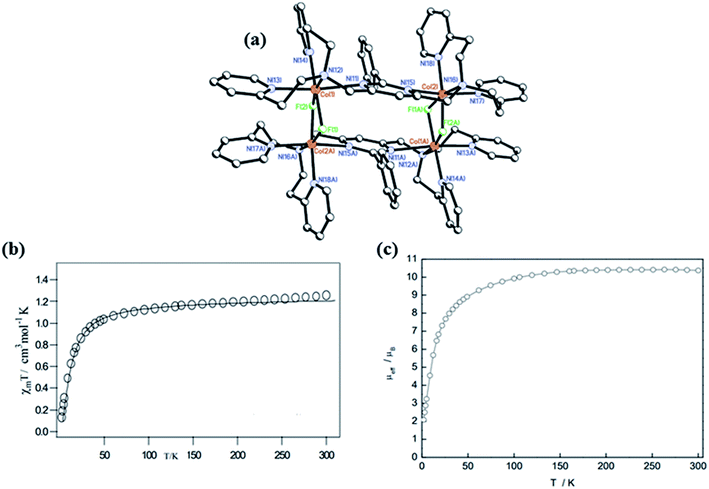
Lakma et al. in 2016 reported [3 × 3] metallogrid complexes with the ligands L7 and L8.10 Both ligands were hydrolysed when the metalation was carried out with MnII(OAc)2·4H2O and formed self-assembled [3 × 3] homometallic grids that contained fully deprotonated form of L7′ and L8′.10 Due to combined effect of steric and electronic factors [3 × 3] grid structure of L7′ i.e., substituted with OCH3 group at para position organized into a 2D polymeric structure when para position was substituted with H. Pseudo-octahedral pentaaquamanganese(II) complex was formed by [Mn10(L7′)6(H2O)5][Mn(H2O)6]2·11H2O (1.11H2O) (Fig. 8). In the lattice of 1.11 H2O, two additional [Mn(H2O)6]2+ complexes were present while in [Mn11(L8′)6(H2O)8][Mn(H2O)6]·24H2O (2.24H2O) two tetraaquamanganese(II) units were present to link between neighboring grids. In the temperature range of 300 K to 3 K, the χmT value of 1.11H2O decreased from 52.3 to 11.4 emu K mol−1, further curve was extrapolated to 9.0 emu K mol−1. This nature of plot showed the antiferromagnetic interaction between metal centers. Same type of behaviour was observed for 2.24H2O, in which χmT value decreased from 45.6 emu K mol−1 (at 300 K) to 10.3 emu K mol−1 (at 3 K) and then extrapolated up to 8.7 emu K mol−1.
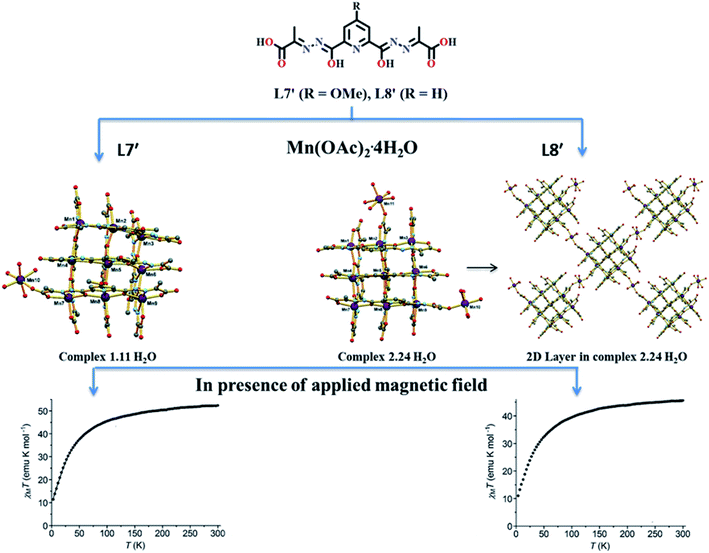 | ||
| Fig. 8 Ligand L7′ and L8′ and their corresponding core structures, 1.11 H2O and 2.24 H2O, with their magnetic properties. Modified and reproduced by permission from ref. 10, ©2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
Hossain et al. in 2017 reported an unsymmetrical ditopic ligand L9, formed tetranuclear homo valent FeIII and mixed valent FeII/FeIII [2 × 2] grid complex.52 Complexes [FeIII4(L9′)4] (FeL9) and [FeII2FeIII2(L9)4](BF4)2·2CH3CN (FeL9a) were synthesized by using the same ligand L9 by changing the reaction condition (Scheme 2). Mixed valent complex was formed under inert condition in which all FeIII2 FeII2 centres were organized in a ‘head-to-head’ fashion while homo valent complex was formed in open air in which all FeIII4 centres were organized in ‘head-to-tail’ fashion. Both complexes showed response in magnetic field, at room temperature the χmT value for FeL9 and FeL9a were found as 11.89 and 12.88 cm3 mol−1 K respectively which upon cooling. At 2 K, the χmT value for FeL9 and FeL9a were dropped to 0.44 and 0.41 cm3 mol−1 K. This indicated the presence of antiferromagnetic coupling. In 2019, with the same unsymmetrical ditopic ligand L9, tetranuclear [2 × 2] grid complexes were synthesized with M = MnII, CoII, CuII and ZnII by using its two coordination pockets (N2O4) and corresponding complexes [M4(L9)4] showed antiferromagnetic interactions (Fig. 9).9
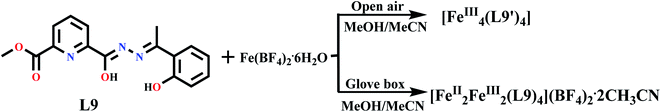 | ||
| Scheme 2 Formation of FeL9 and FeL9a under different conditions.52 | ||
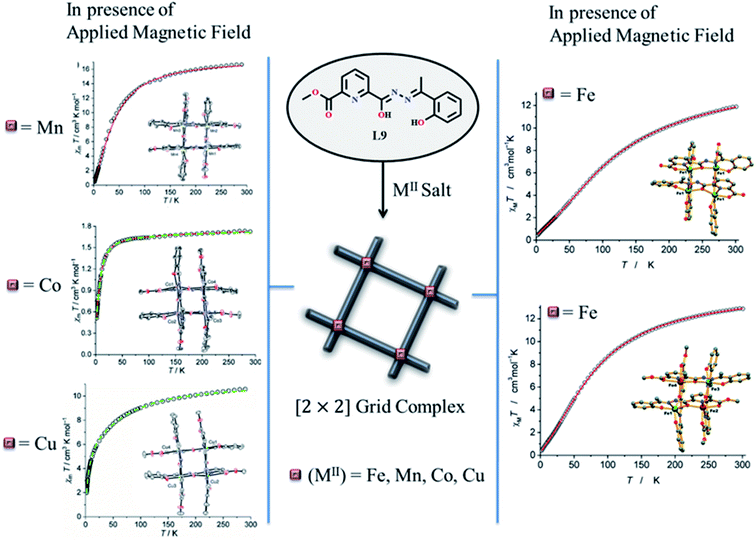 | ||
| Fig. 9 Magnetic properties and the formation of [2 × 2] [M4(L9)4] (M = Fe, Mn, Co, Cu) grid complexes by utilizing the ligand L9. Modified and reproduced by permission from ref. 52 and 9, ©2017 and 2019 respectively, The Royal Society of Chemistry. | ||
It is well-known that pyridine-based ligands, due to their easy functionalization, are the most reported ligands for the syntheses of multimetallic complexes. So, contemplating, designing, and working with such ligands will never stop and some more recently reported potential ligands suitable for the current review are highlighted below.
Shao-Min et al. in 2021 reported one pyridine-based symmetrical ligand L10 and its three dysprosium complexes with different salts of DyIII ion.53 Two of these complexes were mononuclear [Dy(L10)(H2O)](ClO4)·2CH3CH2OH (DyL10a), [Dy(L10)(NO3)] (DyL10b) while one of them was dinuclear [Dy2(L10)(dbm)4] (DyL10c, Hdbm = dibenzoylmethane) in nature. Dy(III) centers in DyL10a and DyL10c were octa-coordinated in nature with triangular dodecahedron geometry around the metal center, while DyL10b was nine coordinated in nature and exhibited a spherical capped square antiprism geometry around the metal center. The dimeric form of DyL10a was attributed to the hydrogen bonding interaction between the inner sphere water molecules, while in DyL10c, it was due to the phenoxy oxygen atoms, which linked two Dy(III) centers. In DyL10a and DyL10b at 300 K, the χmT values were found to be 13.24 and 14.17 cm3 mol−1 K respectively. Further, with decreasing temperature these values smoothly decreased to 12.11 cm3 mol−1 K for DyL10a at 22.56 K and to 13.06 cm3 mol−1 K for DyL10b at 12.07 K. On further cooling, χmT value of DyL10a promptly increased to 13.43 cm3 mol−1 K at 2 K suggested the ferromagnetic interaction between two Dy(III) ions. For DyL10b, on further cooling χmT value slowly increased to 13.17 cm3 mol−1 K at 5.56 K which further rapidly dropped to 10.31 cm3 mol−1 K at 2 K. This increase in the magnetic susceptibility was attributed to the weak intermolecular ferromagnetic couplings and the rapid decrease was attributed to the presence of magnetic anisotropy. Similarly, for DyL10c magnetic susceptibility at room temperature was found to be 27.20 cm3 mol−1 K and on decreasing the temperature χmT value decreased slowly to 23.84 cm3 mol−1 K at 6.35 K and further acquired a value of 24.38 cm3 mol−1 K at 2 K. This initial increase is attributed due to the weak intracluster antiferromagnetic interaction and the slight increase suggested the presence of weak intracluster ferromagnetic interaction.
Two DyIII-based mononuclear complexes, obtained from the potential ligand L11, [Dy(L11)X] (X = Cl, DyL11a; Br, DyL11b), were reported by Li Zhu et.al. in 2021, which were stable in pentagonal bipyramidal geometries and showed SMM behavior.54 Introduction of both electron withdrawing and donating groups in the ligand backbone was the driving force for the regulation of magnetic anisotropy and SMM behaviour. Magnetic susceptibilities of these compounds were determined at 2–300 K temperature range under 1 kOe applied magnetic field. At room temperature the χmT values for DyL11a and DyL11b were found to be 14.05 and 13.77 cm3 mol−1 K respectively. The decrease of temperature resulted a slow decrease of χmT values in both the complexes which decreased sharply around 20 K and at 2 K the χmT values were decreased to 7.64 and 4.77 cm3 mol−1 K respectively for DyL11a and DyL11b. The decrease in the χmT values mainly attributed to the interaction of magnetic anisotropy of the DyIII systems.
Gholamhossein et.al. in 2021 reported an easy-to-synthesize small molecule quinoline-based ligand L12 and its dinuclear CuII and NiII complexes [Cu2(qpyzc)2] (CuL12) and [Ni2(qpyzc)2] (NiL12) respectively.55 The ligand contained one coordination NNN pocket in which either of the metal ions could well-fit and the fourth coordination site was satisfied by the other N of pyrazole moiety from another complex. Both the complexes acquired a distorted square planner geometry and the overall charge of the complexes were balanced from the ligand only. It was observed that the behavior of NiL12 was diamagnetic, as no EPR signal was observed neither at 298 K nor at 110 K, while CuL12 was successfully showed its paramagnetic behavior. The magnetic properties of the CuL12 were inspected in the temperature range of 2–300 K. At 300 K, χmT value was found at 0.73 cm3 mol−1 K, whereas decrease the temperature resulted a decrease in the χmT value and reached a value of 0.012 cm3 mol−1 K at 15 K, which was further saturated at low temperature up to 2 K. This decrease in the χmT value with temperature well-indicated the anti-ferromagnetic coupling behavior between two CuII centers.
A multi-functional cyclam-based ligand L13 and its complexes with FeII and CoII ions were prepared by Bohuslav et.al. in 2021 and their magnetic properties were studied.56 Three complexes [MII(L13)](ClO4)2·H2O (MII = Fe (FeL13), Co (CoL13) or Fe0.4Co0.6 (Fe0.4Co0.6L13)) were prepared and crystal structure revealed that all three had octahedral geometry around the metal center. Magnetic studies revealed the presence of SCO behavior in FeL13, which was also found in the mixed complex Fe0.4Co0.6L13. The CoL13 complex showed a field-induced single-molecule magnet behaviour and surprisingly this SMM behavior was also found in the mixed complex Fe0.4Co0.6L13. So, this mixed system was claimed to be the first system allowing SCO phenomenon in combination with field-induced SMM properties. At room temperature the χmT values of FeL13, CoL13, and Fe0.4Co0.6L13 were mentioned as 3.77, 2.68, and 3.14 cm3 mol−1 K respectively. Lowering the temperature resulted in both FeII contained complexes FeL13 and Fe0.4Co0.6L13 to remain in their high spin state up to 215 and 200 K respectively. Afterwards, the regular decrease of χmT values suggested the presence of SCO behavior in both complexes. T1/2 of FeL13 and Fe0.4Co0.6L13 were found at 141 K and 128 K respectively (Fig. 10). The low spin behavior of the FeL13 is well confirmed by the graph after 50 K and there, the constant magnetic moment of 0.03 cm3 mol−1 K up to 2 K confirmed the completion of high spin to low spin conversion. However, in Fe0.4Co0.6L13 the χmT values suggested the presence of equal amount of both paramagnetic high spin CoII and diamagnetic low spin FeII metal centres and the χmT value was almost half of the CoL13. The magnetic behavior of CoL13 was different from other complexes, here there was no basic differences in χmT values from 300 K to 100 K and further cooling showed a slow decrease in the χmT values, which was reported to be the fingerprint of zero field splitting (ZFS) effect.
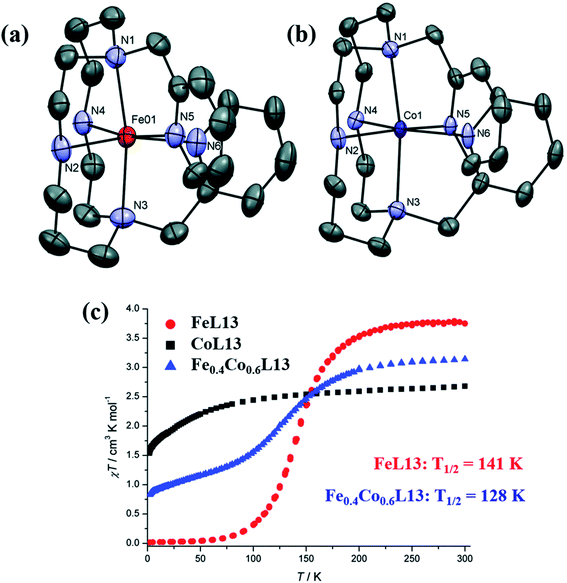 | ||
| Fig. 10 Single crystal XRD structures of (a) FeL13 and (b) CoL13, showing the binding mode of FeII and CoII ions by utilizing the ligand L13 and (c) magnetic properties of FeL13, CoL13, and Fe0.4Co0.6L13. Modified and reproduced by permission from ref. 56, ©2021, The Royal Society of Chemistry. | ||
In coordination chemistry six membered heterocyclic diazine-based ligands have a significant class of applications in the area of magnetism.57–59 Diazine-based ligands can bridge between two metal centres because of their π accepting ability and can withdraw electrons from the metal ion into their aromatic ring to strengthen the metal–ligand interaction by π back bonding. The electrons are withdrawn from the metal ion to an unoccupied π* orbital of the bridging ligand.60–62 Pyridazine, pyrimidine and pyrazine are also known as 1,2-diazine, 1,3-diazine and 1,4-diazine respectively. Among these three, pyridazine is the strongest and Pyrazine is the weakest base. But these six membered heterocyclic diazines act like weaker bases than pyridine, due to the presence of two nitrogen atom in the ring. So overall basicity order is – pyridine > pyridazine > pyrimidine > pyrazine.63 These heterocyclic diazines are weak σ donor and good π acceptor. Order of dipole moments of these diazines are – pyridazine > pyrimidine > pyrazine.64 There are variety of substituted diazine-based ligands present, which act as multidentate and can form binuclear or multinuclear complexes.
3. Pyridazine-based ligands
Pyridazine is a six membered heterocyclic ring, named by scientist Knorr, having direct N–N bond. Broad range of biological and pharmaceutical activities was shown by pyridazine and their derivatives.65 It shows two Kekule structures and also known as 1,2-diazine or o-diaza benzene. It is a colourless liquid at room temperature and has pyridine like odour. It shows a high boiling point (208 °C) and low melting point (−8 °C). Compared to other hydrocarbons these are more easily soluble in water. It shows versatile biological activities for example antiinflammatory,66,67 analgesic,68 anticancer,63 antiaggregative,69 antidepressant,66,67 antihypertensive70,71 etc. 3,6 disubstituted based pyridazine ligands used for the self-assembly grid like metal complex formation,72 some relevant literature are described below (Scheme 3).Teresa F. Mastropietro et al. in 2012 reported pyridazine based six copper complexes [Cu(L14)2(ClO4)]ClO4 (CuL14a), [Cu2(L14)(OH)(ClO4)3(H2O)3]·H2O (CuL14b), [Cu2(L14)2(H2O)2](ClO4)4 (CuL14c), [Cu2(L14)2(ClO4)4] (CuL14d), ([Cu2(L14)(N3)4]·H2O/[Cu2(L14)-(N3)4(H2O)])n (CuL14e) and ([Cu2(L14)(OH)(dca)3]·H2O)n (CuL14f) where L14 = 3,6-bis(2′-pyridyl)pyridazine and dca = dicyanamide.73 L14 when reacts with aqueous solution of copper perchlorate in different ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 2
2, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 2
1, and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 provided different copper based complexes CuL14a–d while additional use of azide and dca gave 1D polymers CuL14e and CuL14f. L14 have two NN coordination pockets, either one or both coordination pockets were used by these complexes and additionally perchlorate, hydroxyl, water, azide and dca groups were used for satisfying the remaining coordination sites of copper. In complex CuL14c and CuL14d both the coordination pockets of L14 were used and formed binuclear complexes in each case. In which four coordination sites of copper were satisfied by L14 ligand in 1
2 provided different copper based complexes CuL14a–d while additional use of azide and dca gave 1D polymers CuL14e and CuL14f. L14 have two NN coordination pockets, either one or both coordination pockets were used by these complexes and additionally perchlorate, hydroxyl, water, azide and dca groups were used for satisfying the remaining coordination sites of copper. In complex CuL14c and CuL14d both the coordination pockets of L14 were used and formed binuclear complexes in each case. In which four coordination sites of copper were satisfied by L14 ligand in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dimeric unit. Variable temperature magnetic measurement explained antiferromagnetic interaction between metal centers in complexes CuL14b, CuL14c, CuL14e and CuL14f (Fig. 11). It is noteworthy to mention that, distance between Cu-Npyr is very less that caused steric hindrance and inhibited the coordination of four copper with four L14 ligand but when FeII, CoII, and NiII were used instead of CuII, these types of steric hindrances were not observed and lead to form very stable grid complexes, which are explained below.
1 dimeric unit. Variable temperature magnetic measurement explained antiferromagnetic interaction between metal centers in complexes CuL14b, CuL14c, CuL14e and CuL14f (Fig. 11). It is noteworthy to mention that, distance between Cu-Npyr is very less that caused steric hindrance and inhibited the coordination of four copper with four L14 ligand but when FeII, CoII, and NiII were used instead of CuII, these types of steric hindrances were not observed and lead to form very stable grid complexes, which are explained below.
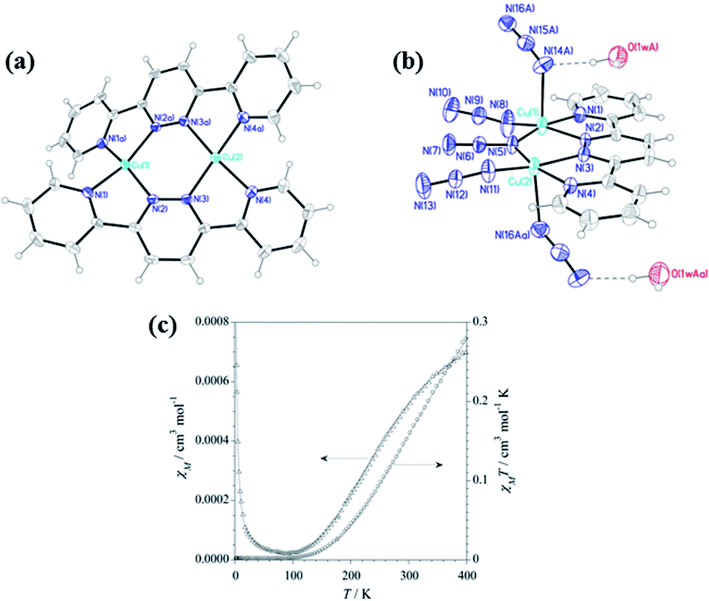 | ||
| Fig. 11 (a) and (b) represents structure of CuL14d and CuL14e, and (c) represents magnetic behavior of CuL14e. Modified and reproduced with permission from ref. 73 Copyright© 2014, American Chemical Society. | ||
In 2020, based on 3,6-bis(2′-pyridyl) pyridazine (L14) ligand four NiII complexes (mono-, di-, tri-, and tetranuclear) are reported by Bruno et al.74 Among all four, tetranuclear NiII complex [Ni4(μ-L14)4(μ-N3)4]Cl4·5H2O (NiL14) has formed [2 × 2] metallogrid complex and shown a distinct ferromagnetic interaction. L14 was synthesized by using a published procedure by Butte and Case in 1961.75 In the structure of tetranuclear NiL14 complex, hexacoordinated metal centres were presented, in which four coordination sites were satisfied by two L14 ligands and two coordination sites were occupied by bridging azido groups and formed [2 × 2] metallogrid complex. In the graph χmT vs. T, on cooling, χmT value continuously increased and reach to maximum of 10.60 cm3 mol−1 K at 70 K from 5.0 cm3 mol−1 K at room temperature and afterwards, it was decreased to 9.40 cm3 mol−1 K at 1.9 K. Zero field splitting effects or intermolecular antiferromagnetic interaction may be the reason behind the decrease of χmT values at 1.9 K.
One more ligand bearing same coordination pockets as L14 was published by guo et al. in 2020 with pyridyl-substituted pyrazine derivative i.e., (L15).76 L14 and L15 both formed tetranuclear [2 × 2] grid complexes with CoII i.e., ([CoII4(L14)4(N3)4])·sol (CoL14) and [(L15)4 CoII4(N3)4])·sol (CoL15). Both pyridyl- and pyrazoyl-substituted metallogrid complexes with CoII have shown satisfactory magnetic properties. At 300 K, the χmT value for both CoL14 and CoL15 are 11.50 and 12.15 cm3 mol−1 K respectively, that sharply increased to 20.26 cm3 mol−1 K (at 12 K) and 25.37 cm3 mol−1 K (at 16 K) respectively, and then dropped to 18.12 and 21.23 cm3 mol−1 K respectively. Such behaviour revealed the presence of ferromagnetic interaction between the metal centers, while the low temperature decrease of χmT revealed the presence of intermolecular antiferromagnetic interaction between metal centers (Fig. 12).
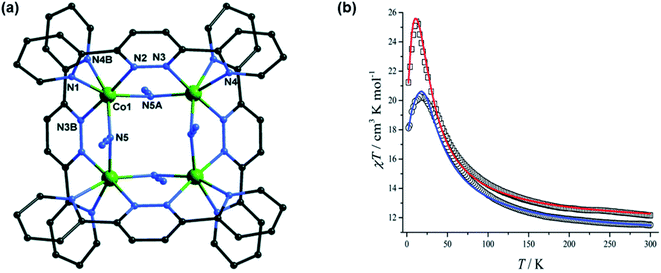 | ||
| Fig. 12 (a) represents the structure of CoL14 and (b) represents magnetic behaviour of CoL15 (red) and CoL14 (blue). Modified and reproduced with permission from ref. 76, ©2020, the Royal Society of Chemistry. | ||
Both ligands L14 and L15 have huge potentials to show various magnetic behaviors as well as they were structurally suitable for many transition metal complexes to provide grid like structures. Again by using the same ligands L14 and L15 and by changing the metal ion to FeII, same group had published two FeII grids ([L14)4FeII4 (N3)4][BPh4]4·sol (FeL14)and [(L15)4FeII4(N3)4][BPh4]4·sol (FeL15)) in 2021.77 Among these supramolecular self-assembled complexes, FeL14 showed spin crossover behavior at 230 K. At 300 K, χmT values for both FeL14 and FeL15 was found to be 12.34 and 12.77 cm3 mol−1 K respectively. Upon lowering the temperature a gradual decrease in the χmT value was identified in FeL15 until 5 K, which was rapidly decreased to 6.66 cm3 mol−1 K at 2 K. This behavior of FeL15 was the indication of ZFS or intermolecular antiferromagnetic coupling. In case of FeL14, the χmT value slowly decreased to 11.5 cm3 mol−1 K upon cooling till 270 K. Then further cooling resulted an abrupt decrease in the χmT value to 5.0 cm3 mol−1 K at 200 K. The SCO temperature was estimated to be 230 K. After 200 K, further cooling resulted a gradual decrease in the χmT value to 0.59 cm3 mol−1 K at 2 K and this was attributed to the continuous transition to the low spin FeII state.
Jingjing Lu et al. in 2019 reported a pyridazine based Schiff-base ligand which formed three novel dysprosium-based complexes; [Dy4(HL16)2L(DMF)8]·2ClO4CH2Cl2·4DMF(CH3CH2)2O·H2O (DyL16a), [Dy6L163(PhCOO)6(CH3OH)6]·11CH3OH·H2O (DyL16b) and [Dy10Na2L164(μ3-OH)4(DMF)12-(NO3)6]·6NO3·2DMF(CH3CH2)2O·H2O (DyL16c).78 Synthesized pyridazine based ligand L16 reacted with dysprosium salts in different reaction conditions with varying molar ratio provided different dysprosium complexes (Fig. 13). Initially, reaction of dysprosium perchlorate with L16 gave linear tetranuclear triple-stranded helicate DyL16a, when this perchlorate was replaced by benzoate ion in methanol with molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 gave a hexanuclear triple-stranded helicate DyL16b. Again, a dual double-stranded helicate DyL16c was obtained when Dy(NO3)3·6H2O, L16, and NaHCO3 react in 2
1 gave a hexanuclear triple-stranded helicate DyL16b. Again, a dual double-stranded helicate DyL16c was obtained when Dy(NO3)3·6H2O, L16, and NaHCO3 react in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 molar ratio in DMF solvent. These lanthanide complexes showed magnetic response in the range of 2–300 K at 1000 Oe magnetic field. Initially at 300 K, 57.31, 84.77, and 140.54 cm3 mol−1 K of χmT values were found for DyL16a, DyL16b, and DyL16c respectively. After decreasing the temperature, χmT value of DyL16a gradually decreased to 48.37 cm3 mol−1 K at 12 K and then suddenly increased to 63.36 cm3 K mol−1 at 2 K. This scenario indicated the possibility of ferromagnetic coupling between the DyIII centers. Moreover, χmT values of DyL16b and DyL16c were decreased to 78.44, and 127.82 cm3 K mol−1 at a decreased temperature of 50 K, which further decreased to 68.61 and 103.53 cm3 K mol−1 at 2 K respectively. These behaviours of DyL16b and DyL16c suggested the weak antiferromagnetic interaction between DyIII centers.
4 molar ratio in DMF solvent. These lanthanide complexes showed magnetic response in the range of 2–300 K at 1000 Oe magnetic field. Initially at 300 K, 57.31, 84.77, and 140.54 cm3 mol−1 K of χmT values were found for DyL16a, DyL16b, and DyL16c respectively. After decreasing the temperature, χmT value of DyL16a gradually decreased to 48.37 cm3 mol−1 K at 12 K and then suddenly increased to 63.36 cm3 K mol−1 at 2 K. This scenario indicated the possibility of ferromagnetic coupling between the DyIII centers. Moreover, χmT values of DyL16b and DyL16c were decreased to 78.44, and 127.82 cm3 K mol−1 at a decreased temperature of 50 K, which further decreased to 68.61 and 103.53 cm3 K mol−1 at 2 K respectively. These behaviours of DyL16b and DyL16c suggested the weak antiferromagnetic interaction between DyIII centers.
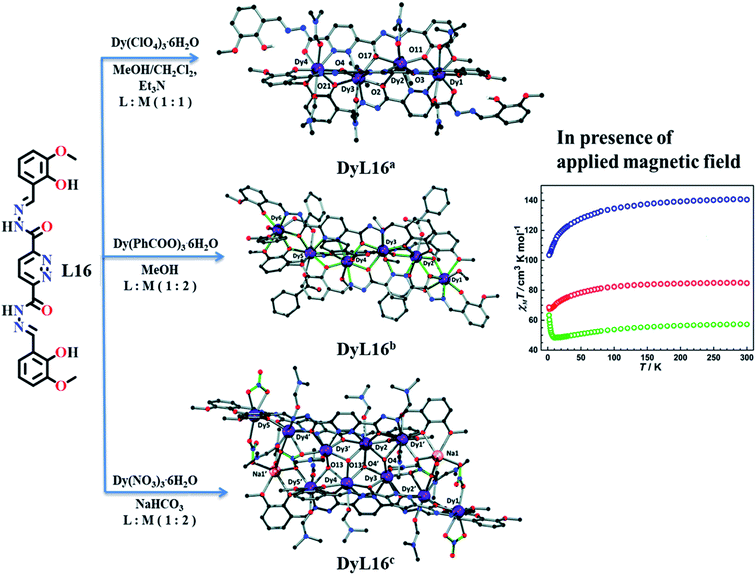 | ||
| Fig. 13 Formation of different dysprosium complexes from ligand L16 and their magnetic properties DyL16a (green), DyL16b (red), and DyL16c (blue). Modified and reproduced from ref. 78, © 2019, with permission from the Centre National de la Recherche Scientifique (CNRS) and the Royal Society of Chemistry. | ||
4. Pyrimidine-based ligands
In natural products, drugs, bioactive molecules, supramolecules, biogenetics, and photophysical materials pyrimidine is used as a fundamental structure.79–85 4,6-disubstituted pyrimidine based ligands can form self-assembly metallogrid complexes and able to show magnetic properties86–90 and some of them are described here based on the recent literature (Scheme 3). | ||
| Scheme 3 Pyrimidine, pyrazine, 1,3,5-triazine, and 1,2,4,5-tetrazine based ligands discussed in the present review. | ||
In 2012 Worku A. Gobeze et al. reported pyrimidine based bis-tetradentate acyclic amine ligand (L17) formed by reaction between 4,6-bis(aminomethyl)-2-phenylpyrimidine and 2-vinylpyridine.91 On the reaction of L17 and metal tetrafluoroborate with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio in MeCN gave dinuclear complexes Fe2L17(H2O)4(BF4)4 (FeL17), Co2L17(H2O)3(MeCN)2(BF4)4 (CoL17), and Ni2L17(H2O)4(BF4)4 (NiL17), while in more protic solvents like MeOH tetranuclear complexes, Fe4(L17)2(F)4(BF4)4·52H2O (FeL17a), Co4(L17)2(F)4(BF4)4·3H2O (CoL17a), and Ni4(L17)2(F)4(BF4)4·4H2O (NiL17a), were formed. In dinuclear and tetranuclear complexes coordination sites of metal centers were satisfied by L17 in bis-tetradentate fashion (two nitrogen of pyrimidine ring, four nitrogen of pyridine ring and two nitrogen of tertiary amines were coordinated to metal from one ligand). Magnetic susceptibility measurements for all complexes were done in the range of 300 to 2 K. Initially, χmT values of FeL17, CoL17, NiL17, FeL17a, and NiL17a were 2.3, 2.82, 1.2, 3.6 and 4.47 cm3 K mol−1 respectively, which gradually decreased down as the temperature was lowered. CoL17a also showed similar type of magnetic behavior in the form of μeff, which attained a minimum value of 2 μB at 2 K. So, plots of χmT vs. T and μeff vs. T of these complexes revealed the dominance of antiferromagnetic interaction between metal centers (Fig. 14).
2 molar ratio in MeCN gave dinuclear complexes Fe2L17(H2O)4(BF4)4 (FeL17), Co2L17(H2O)3(MeCN)2(BF4)4 (CoL17), and Ni2L17(H2O)4(BF4)4 (NiL17), while in more protic solvents like MeOH tetranuclear complexes, Fe4(L17)2(F)4(BF4)4·52H2O (FeL17a), Co4(L17)2(F)4(BF4)4·3H2O (CoL17a), and Ni4(L17)2(F)4(BF4)4·4H2O (NiL17a), were formed. In dinuclear and tetranuclear complexes coordination sites of metal centers were satisfied by L17 in bis-tetradentate fashion (two nitrogen of pyrimidine ring, four nitrogen of pyridine ring and two nitrogen of tertiary amines were coordinated to metal from one ligand). Magnetic susceptibility measurements for all complexes were done in the range of 300 to 2 K. Initially, χmT values of FeL17, CoL17, NiL17, FeL17a, and NiL17a were 2.3, 2.82, 1.2, 3.6 and 4.47 cm3 K mol−1 respectively, which gradually decreased down as the temperature was lowered. CoL17a also showed similar type of magnetic behavior in the form of μeff, which attained a minimum value of 2 μB at 2 K. So, plots of χmT vs. T and μeff vs. T of these complexes revealed the dominance of antiferromagnetic interaction between metal centers (Fig. 14).
 | ||
| Fig. 14 (a) represents structure of NiL17a, (b) and (c) represents magnetic behavior of NiL17 and CoL17a. Reproduced from ref. 91, © 2012, with permission from The Royal Society of Chemistry. | ||
Yi-Tong Wang et al. in 2013 reported [2 × 2] metallogrid FeII complexes with the L18.92 These polynuclear complexes showed spin crossover behaviour. Three [2 × 2] grid-like FeII complexes [Fe4(HL18a)4]Cl4·9H2O (FeL18a), [Fe4(L18a)2(H2L18a)2](BF4)4·6H2O (FeL18b) and [Fe4(L18b)2(H2L18b)2]ClO4)4·4H2O (FeL18c) were formed by ditopic Schiff base ligands H2L18. All these three complexes were formed by using FeCl2·4H2O metal salt in MeOH solution and for FeL18c aqueous solution of NaBF4/NaClO4 was used. All complexes had similar [2 × 2] core grid structure in which all FeII metal centres were planar except FeL18a and hexacoordinated with two imine, pyrimidine and pyridine N atoms of the ligand. Magnetic susceptibility experiments confirmed that above 300 K, FeL18a and FeL18b showed spin transition from LS to HS state. At 400 K, the observed χmT value was 6.56 emu K mol−1 for FeL18a, related to 50% HS FeII that decreased at 100 K as temperature was decreased. It further decreased at 2 K due to the presence of LS FeII ions (Fig. 15). Same behaviour was shown by FeL18b but at 300 K. FeL18c had shown diamagnetic interactions.
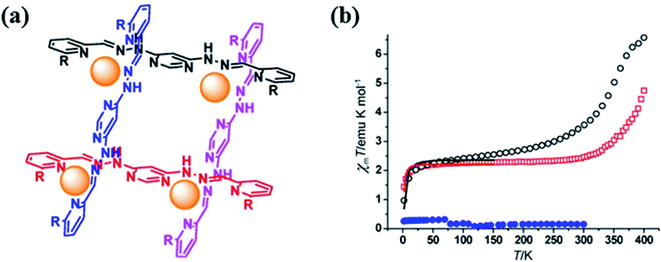 | ||
| Fig. 15 (a) represents structure of L18 and (b) represents plot of χmT vs. T for complex FeL18a (black), FeL18a (red) and FeL18a (blue). Modified and reproduced with permission from ref. 92 Copyright © 2013, American Chemical Society. | ||
Shu-Qi Wu et al. in 2014 reported two tetranuclear CoII metallogrid complexes, and one of them had shown spin crossover behaviour. When the methanolic solution of same ligands (L18a and L18b) were reacted with CoCl2·6H2O in aqueous solution of NaClO4, [2 × 2] metallogrid complexes [Co4(HL18a)4](ClO4)4·8H2O (CoL18a) & [Co4(L18b)4](ClO4)4·16H2O (CoL18b) respectively were formed.93 Core structures of both the complexes is very similar, only the deprotonated ligands were different. [2 × 2] grid of CoL18a possessed four octahedral CoII ions and four partially deprotonated ligands (HL18a)− while CoL18b contained four octahedral CoIII ions (t62g) and four doubly deprotonated ligands (L18b)2−. Both complexes form a regular 3D packing network that stabilize by H bonds, Br–Br contacts and other intramolecular bonds. The magnetic susceptibility measurements were confirmed that CoL18a had shown the SCO phenomenon in the range of temperature from 2–400 K while CoL18b didn't show such type of phenomenon. At 400 K, χmT value of CoL18a was 6.75 emu K mol−1 due to presence of 2HS + 2LS CoII ions that gradually decreased on cooling the temperature. At 130 K, decrease in χmT value by 2.3 emu was observed due to the presence of 1HS + 3LS CoII ions and further decrease in the χmT values attributed to the LS CoII ions. CoL18b complex had shown the temperature independent paramagnetism related to the LS CoIII ions (Fig. 16).
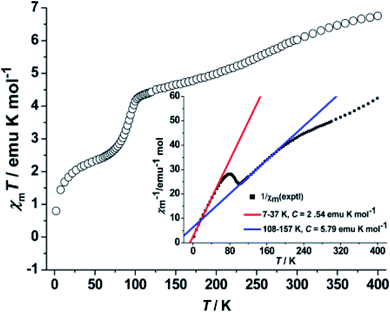 | ||
| Fig. 16 Plots of χmT vs. T and 1/χm vs. T for CoL18a. Modified and reproduced with permission from ref. 93 Copyright© 2014, American Chemical Society. | ||
L19 was reported in 2018 by Sebastien Dhers et al., which contained two NNN coordination pockets and chelated with two FeII and ZnII centers.94 When L19 was reacted with Fe(BF4)2 and Zn(BF4)2 in acetonitrile with required amount of base, [2 × 2] grid complexes were formed, i.e., [Fe4(H2L19)4](BF4)8·xMeCN (FeL19a), [Fe4(H2L19)2(HL19)2](BF4)6·8MeCN (FeL19b), [Fe4(HL19)4](BF4)4·2MeCN (FeL19c), [Zn4(HnL19)4]8+ (ZnL19a), and [Zn4(HnL19)4]4+ (ZnL19a). In these complexes, six coordination sites of each metal center were satisfied by NNN coordination pockets of L19, which was arranged around metal centers in perpendicular manner, resulted in the formation of grid complexes. Deprotonated form of ligand L19 was acted as a strong field ligand and protonated form of H2L19 acted as a weak field ligand, that responsible for SCO behavior of these iron complexes. The magnetic properties of these iron complexes were represented in Fig. 17. During magnetic susceptibility measurement it was observed that null magnetic responses were shown by ZnL19a and ZnL19a represented diamagnetic interaction between Zn centers. While iron complexes show magnetic response in magnetic field and their magnetic susceptibility measurements were done in the range of 300–1.85 K. In χmT vs. T plot, initially at higher temperature χmT value of FeL19a, FeL19b, and FeL19c were 6.6, 4.3, and 3.6 cm3 K mol−1 respectively, which were decreased on lowering the temperature and became plateau below 120 K and reached to a minimum value of 2.4, 1.5, and 2.6 cm3 K mol−1 respectively. These types of plot indicated the presence of SCO phenomenon of iron complexes (Fig. 17).
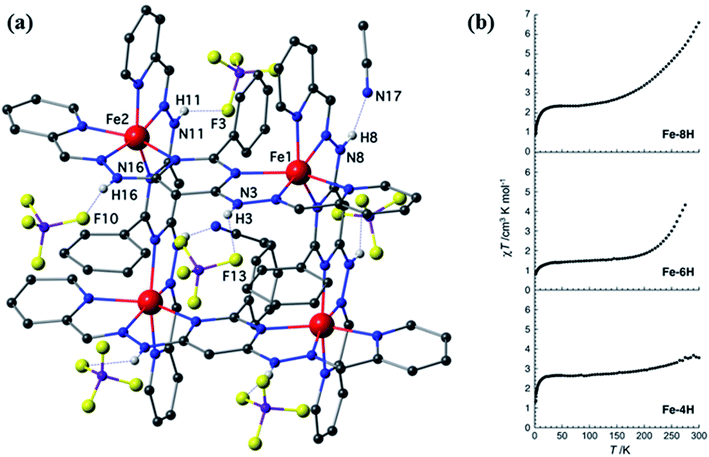 | ||
| Fig. 17 (a) represents structure of FeL19a (Fe–8H), and (b) represents magnetic behavior of FeL19a (Fe–8H), FeL19b (Fe–6H) and FeL19c (Fe–4H). Modified and reproduced with permission from ref. 94 Copyright© 2018, American Chemical Society. | ||
Furthermore, to contribute more towards pyrimidine-based metallogrid complexes, Lakma et al. in 2018, synthesized ligand L20 and L21 by changing the terminal groups of 4,6-dihydrazinylpyrimidine by methyl pyruvate and diacetyl monoxime respectively and both were able to form self-assemble [2 × 2] metallogrid complexes.95 Two coordination pockets of these ligands were attached with two metal centers and act as hexadentate ligands. These ligands were formed tetranuclear [2 × 2] grid complexes with NiII, [Ni4(L20)4]·2CH3CN·4CH3OH·4H2O (NiL20) and [Ni4(H2L21)4](CF3SO3)8·4CH3NO2·8H2O (NiL21). In the crystal structure of these complexes all six coordination sites of NiII centres were occupied by two tridentate coordination pockets of two pyrimidine-based ligands [Fig. 18a and 18b]. Magnetic properties of NiL20 and NiL21 were shown in Fig. 18c and 18d. Both complexes showed similar type of curve in χmT vs. T graph. On cooling χmT values of both the complexes were continuously decreased (from 4.80 cm3 K mol−1 for NiL20 and from 4.43 cm3 K mol−1 for NiL21) and below 100 K both the curves were steeply decreased with reaching a value close to 0 cm3 K mol−1 at 2 K, that signified the antiferromagnetic exchange interactions between the four centres of a complex.
5. Pyrazine-based ligands
It is an attractive molecule and between two metal centres it can be utilized as a bridging ligand.96 Functionalization of pyrazine, at 2 and 5 positions, is able to coordinate with 3d octahedral metal ions.97 2,5 disubstituted pyrazine based ligands (Scheme 3) could easily be synthesized by Schiff base condensation of pyrazine-2,5-dicarbaldehyde with various primary amines that produced a variety of ligands.98,99Wei Huang et al. in 2016 reported two unsymmetrical pyrazine based lanthanide complexes formed via self-assembly.100 Synthesized pyrazine based ligand, pyrazine-2,5-diylbis(ethan1-yl-1-ylidene)di(benzohydrazide)(L22), when reacted with Dy(NO3)·6H2O in different solvents different lanthanide complexes were formed. With two equivalents of Dy(NO3)·6H2O, binuclear complex [(Dy2(L22)(NO3)4(DMF)4] (DyL22a) was formed in presence of NEt3 and DMF/CH2Cl2 and a tetranuclear complex [Dy4(L22)4(H2O)4(NO3)4]·6CH3OH·6H2O (DyL22b) was formed when MeOH was used as solvent. By changing the solvent, both dinuclear complexes can be reversibly convertible (Scheme 4). Tetranuclear complex was arranged as [2 × 2] metallogrid structure and this type of example (polynuclear DyIII) is rarely available. In tetranuclear [2 × 2] DyIII metallogrid complex, nine coordination sites of metal centres are satisfied by one water molecule, one chelating nitrate and remaining by two N2O coordination pockets of the ligand. This tetranuclear grid complex showed magnetic response in 2500 Oe magnetic field in the range of 2–300 K. Initially at 300 K, χmT value was 54.98 cm3 mol−1 K and after decreasing the temperature, χmT value gradually decreased up to 45.61 cm3 mol−1 K at 2 K. This type of behaviour revealed the possibility of antiferromagnetic coupling between the DyIII centres which further confirmed by 1/χm versus T plots that followed Curie–Weiss law with negative value of θ (Fig. 19).
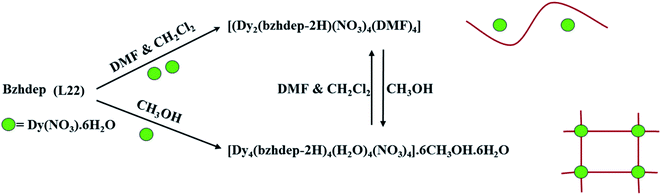 | ||
| Scheme 4 Represents reversible transformation between a Binuclear Fragment (DyL22a) and a tetranuclear metallogrid dysprosium complex (DyL22b) in different solvents. | ||
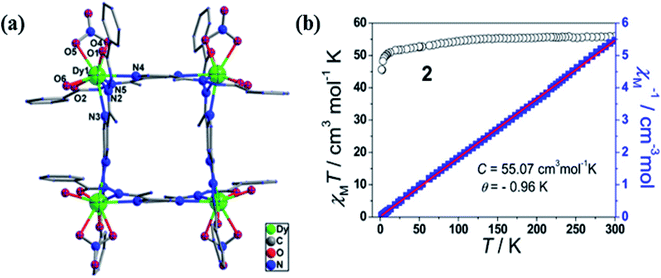 | ||
| Fig. 19 (a) and (b) represents structure and plot of χmT vs. T (black) & 1/χm vs. T (blue) for DyL22b. Modified and reproduced with permission from ref. 100, Copyright© 2016, American Chemical Society. | ||
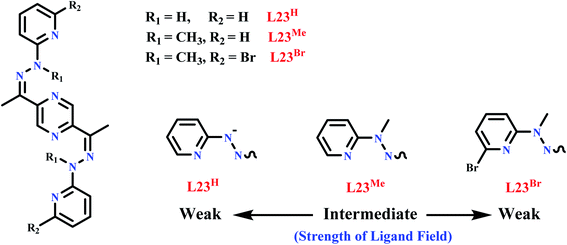
L23 and L24 have two antiparallel coordination pockets and could able to form [2 × 2] grid complexes with transition metals. L24 was reported in 2017 by Hogue et al. in which it was expected to form a [2 × 2] grid complex, but unexpectedly triangular metal complex was formed with FeII and ZnII.101 Conclusion came out from this experiment was that, L24 type of ligands are flexible in nature like bis-terdentate pyrazine-diamide ligands, and can form [2 × 2] metallogrid complexes. But analogous to this, diamine bis-terdentate pyrazine ligand gives trimetallic triangle architectures.102–104 L23 was reported in 2016 by Shen et al., in L23 (LH, LMe and LBr) two NNN coordination pockets were present, that chelated with two CoII centres.105 In complex, each cobalt centre have distorted octahedral environment which formed by using two N atoms of pyrazine, pyridine and hydrazone moiety. So when L23 (LH, LMe and LBr) was reacted with Co(ClO4)2.6H2O, it formed three [2 × 2] grid complexes, [Co4(L23H)4](ClO4)46DMF·3H2O (CoL23H), [Co4(L23Me)4](ClO4)8·4MeCN·H2O (CoL23Me), [Co4(L23Br)4](ClO4)8·CH3OH·2MeCN·5H2O (CoL23Br). In the applied magnetic field, null magnetic susceptibility was observed for L23H complex, that implied low spin CoIII grid complex was formed having d6 configuration. CoL23H was found to be diamagnetic however, CoL23Me and CoL23Br were found to be paramagnetic. In χmT vs. T plot, on cooling χmT value of both complexes were decreased below 40 K that implied the weak antiferromagnetic interaction between the metal centres. So it is clear that the substituents like R1, R2 can affect the magnetic property of a complex (Scheme 5).
6. 1,3,5-Triazine-based ligands
Triazine comes under the class of nitrogen containing heterocycles and among all the well-known triazine is 1,3,5-triazine, also known as s-triazine. Chlorinated derivative of 1,3,5-triazine which is commonly termed as cyanuric chloride (C3N3Cl3). The derivatives of cyanuric chlorides are very attracting as they have interesting biological applications in pharmaceutical industry mainly in antimalaric, antibacterial, antifungal, and antineoplastic.106–108 Various 2,4,6-mono, di and trisubstituted symmetrical and nonsymmetrical derivatives of triazine are formed by swaping of chlorine atoms with different nucleophiles under controlled conditions.109,110 There are many ligands containing 1,3,5-triazine unit, but only a few 1,3,5-triazine based ligands form grid complexes. In Scheme 3, a variety of 2,4,6-substituted triazine based ligands are shown. All these ligands are synthesized by the same general method that coordinate with metal in different coordination modes showing different properties like spin crossover, water adsorption, and magnetic properties.L25 was published by Animesh Das et al. in 2011.111 Three tridentate coordination pockets of L25 were able to coordinate with three metal centres but due to steric interaction of methyl at hydrazone N atom, L25 only was able to bind with one MnII centre by using one N atom of triazine and two binding sites of chelating arms. Corresponding complexes of L25 with MnII showed antiferromagnetic properties but failed to form a grid complex. L26 was reported by Nathalie Parizel et al. in 2011.112 When an equivalent amount of CoII salt was reacted with L26 both, grid and pincer like complexes were produced.109 But when it was reacted with CuII, only the grid complex was formed. So, it was concluded that the self-assembly process can be tuned by changing the respective metal ions. Ferromagnetic exchange interaction was displayed by the square [2 × 2] [Cu4(L26)4]8+ grid complex.
Behaviour of SCO is very interesting in d6, FeII ions because high spin states are related with paramagnetic properties while low spin states are related with diamagnetic properties that show applications in optical displays, molecular switches, and data storage devices.32 Since 2006, for the formation of SCO different types of pyridine based ligands have been designed and synthesized.113–116 After that s-triazine based SCO compounds were developed.117 Various dipyridyl amino-substituted-triazine ligands with different nature of transition behaviour were described and developed by Murray and co-workers.118–121 Again in 2013 Nanthawat Wannarit et al. reported the ligand L27 which was prepared by selective and straightforward substitution of two chlorine atoms of 2,4,6-trichloro-1,3,5-triazine by phenolic compounds and one chlorine atom by 2,2′-dipyridylamine. Surprisingly, its complex with FeII had shown SCO properties.33,122 In this ligand, triazine acted as an ancillary ligand and dipyridine group coordinate with the metal centre by its two N atoms. In [Fe(L27)2 (NCS)2]·2CH2Cl2 (FeL27), FeII centre form octahedral geometry in which axial position was occupied by two trans thiocyanate group and equatorial position was occupied by two L27 ligand shown in Fig. 20. Variable-temperature magnetic susceptibility measurements showed SCO properties of FeL27H and FeL27F in temperature range 300–5 K shown in Fig. 20(c). At 300 K, χmT value of FeL27H was 3.19 cm3 mol−1 K due to the presence of high spin d6, FeII complex which decreased upon cooling and at 100 K it reached nearly 0.11–0.07 cm3 mol−1 K, that implied the low spin character of d6, FeII complex. This SCO behaviour was further supported by its T1/2 = 233 K (at 233 K half of the FeII centres changed their spin state) and ΔT80 = 90 K (within 90 K 80% of the transition occurred) value. In case of FeL27F, initially at 280 K, χmT value was 3.18 cm3 mol−1 K, which immediately reduced to 0.1–0.2 cm3 mol−1 K when temperature was reduced to 200 K. Its T1/2 and ΔT80 value was found to be 238 K and 50 K respectively.
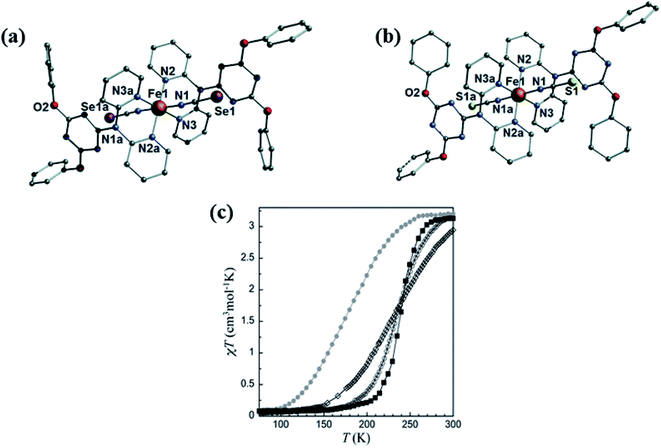 | ||
| Fig. 20 Structural representation (a) FeL27H and (b) FeL27F and (c) variable temperature magnetic susceptibility measurement of (FeL27H empty circle and FeL27F full square). Modified and reproduced with permission from ref. 33, © 2013, The Royal Society of Chemistry. | ||
In 2019 Jingjing Lu et al. reported triazine based six tetranuclear DyIII complexes [Dy4(L28)2(CH3OH)3(NO3)3]·3NO3·2H2O (DyL28a), [Dy4(L28)2(CH3OH)2(SCN)4(OCH3)2]·2CH3OH·2H2O (DyL28b), ([Dy4(L28)2(CH3OH)(SCN)6(CH3CN)]·3CH3OH·4CH3CN)2 (DyL28c), [Dy4(L28)2(CH3OH)2(SCN)6]·6CH3OH·2H2O (DyL28d), [Dy4(L28)2(CH3OH)2(SCN)4(OCH3)2]·5CH3OH·2H2O (DyL28e), and [Dy4(L28)2(CH3OH)(SCN)5(H2O)2]·SCN·4CH3OH·2H2O (DyL28f).123 All these tetranuclear complexes were obtained by reacting the ligand L28 with respective salts of dysprosium using different solvents. In DyL28a–c and DyL28d–f, DyIII centers were octa-coordinated in nature and showed a pseudo-D4d symmetry environment (Scheme 6 and Fig. 21a). For DyL28a–c magnetic susceptibility measurements are shown in Fig. 21b in which initially, the observed χmT values were 56.82, 56.35, and 58.24 cm3 mol−1 K respectively, and those gradually decreased on lowering the temperature and at 18 K these values reached 51.08, 49.20, and 52.33 cm3 mol−1 K respectively. On further cooling, the χmT values suddenly increased at 2 K and reached to 58.05, 62.66, and 61.76 cm3 mol−1 K respectively. These observed values revealed the presence of ferromagnetic interaction and also same type of behaviour was found for the complexes DyL28d–f.
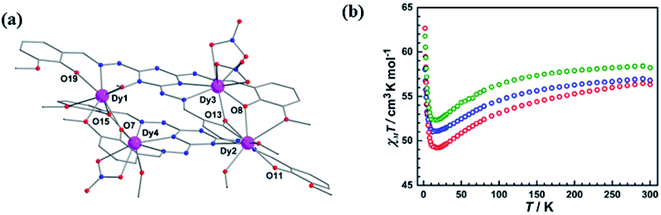 | ||
| Fig. 21 (a) Structure of DyL28a and (b) χmT vs. T plot for DyL28a (green), DyL28b (blue) and DyL28c (red). Modified and reproduced with permission from ref. 122, Copyright© 2019, American Chemical Society. | ||
7. 1,2,4,5-Tetrazine-based ligands
1,2,4,5-tetrazine or s-tetrazine (TTZ) is one of the most interesting N-heterocyclic ligands. The chemistry of 1,2,4,5-tetrazine (TTZ) heterocyclic is interesting due to four sp2 nitrogen atoms present in the ring. It has good electron accepting properties which facilitates the formation of its stable radical anions.124–130 Due to its poor basic property, coordination ability of TTZ is low. It is a weaker base than triazine, and the basicity of tetrazine was never determined but it was estimated that s-tetrazine has pKa value on the order of −6 (for s-triazine pKa = −1.7).131 Tetrazine can be functionalised at 3,6 position which can develop various types of ligands and can coordinate with same or different type of metal ions. On the basis of substituents on 3 and 6 positions TTZ may be classified in two parts, symmetric TTZ ligands and non-symmetric TTZ ligands. Ditopic dipyridyl and dipyrimidyl tetrazines are mostly used as symmetric ligands. Dipyridyl tetrazine is more developed compared to dipyrimidyl tetrazine in coordination chemistry. Excellent photoluminescence properties may be shown by coordination polymers that are incorporated with bis(pyridyl) tetrazine ligands, because tetrazine acts as a typical organic chromophore.132 In Scheme 3, a variety of 1,2,4,5-tetrazine based ligands were highlighted. Dimensionality of the resulting coordination compounds can be influenced by the position of nitrogen atom in the pyridyl substituents. 3,6-bis(4-pyridyl)-1,2,45-tetrazine (4,4′-bptz), 3,6-bis(3-pyridyl)-1,2,45-tetrazine (3,3′-bptz), and 3,6-bis(2-pyridyl)-1,2,45-tetrazine (2,2′-bptz) type ligands can enable coordinations in different manner.133In 2017 Brian S. Dolinar et al. reported L29-based dinuclear lanthanide complexes, [Dy(tmhd)3]2(L29) (DyL29a) and (Cp2Co)([Dy(tmhd)3]2(L29)) (DyL29b) (tmhd = 2,2,6,6-tetramethyl-3,5- heptane dionate).134 DyL29a was formed by the reaction of stoichiometric amount of L29 and Dy(tmhd)3 in DCM solvent and DyL29b was synthesized in toluene by the same method with the extra addition of Cp2Co. Eight coordination sites of DyIII in DyL29a was satisfied by two NN coordination pockets of L29 and remaining six sites were occupied by three tmhd ligands. DyL29b has similar type of coordination environment like the neutral complex DyL29a, except the counter cation part (Cp2Co)+ and formed [DyL29b]− anion. Magnetic susceptibility measurements of both complexes were done in the range of 300–2 K under an applied magnetic field 1000 Oe. Both complexes have similar type of coordination environment but showed different types of magnetic properties. Initially at 300 K, χmT values of DyL29a and DyL29b were found to be 27.0 and 33.4 emu K mol−1 respectively, which decreased on lowering down the temperature to 10 K and 26 K respectively. After that, χmT value for DyL29a sharply decreased to 18.7 emu K mol−1 at 2 K while for DyL29b a sudden increase in the χmT value, 42.8 emu K mol−1 was observed at 2 K. Finally, plot of χmT vs. T graph revealed the presence of antiferromagnetic coupling in DyL29a and ferromagnetic coupling in DyL29b (Fig. 22).
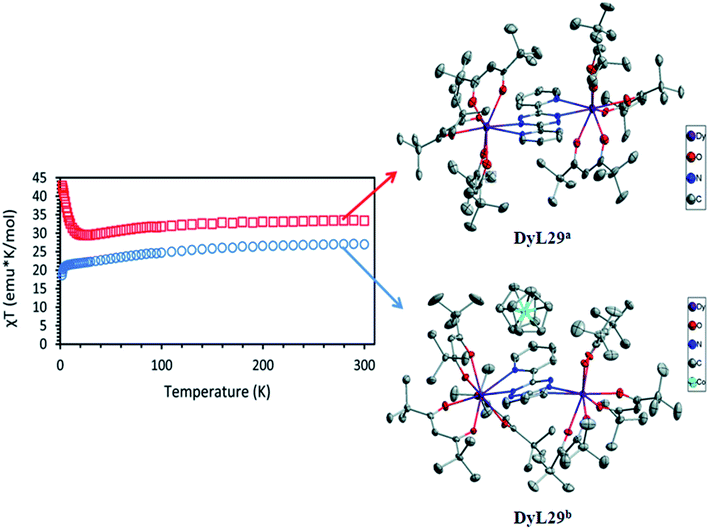 | ||
| Fig. 22 Structure and magnetic behavior of DyL29a (blue circle) and DyL29b (red square). Modified and reproduced by permission from ref. 133, © 2017, The Royal Society of Chemistry. | ||
The first 2,2′-bptz (L29) radical bridged CoII square complex was reported by the same group in 2017.135 In complex [Co4(L29)4(dbm)4]·4MeCN, 1,3-diphenyl-1,3-propanedionate (dbm) was used as auxiliary chelating ligand. 2,2′-bptz formed [2 × 2] grid complex with cobalt in which CoII centres had distorted octahedral environment. Four coordination sites were satisfied by bptz ligand and remaining two sites are satisfied by dbm ligand. Magnetic behaviour of the complex showed that χmT value gradually increased upon cooling the temperature to 60 K, (Fig. 23b), after that it suddenly decreased to 2.72 cm3 K mol−1 at 2 K, which indicated the existence of antiferromagnetic interaction between the bptz radical and CoII ions (Fig. 23).
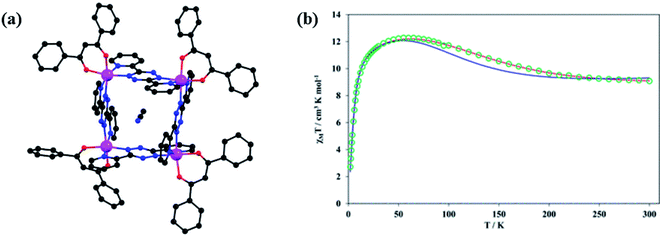 | ||
| Fig. 23 (a) Structural representation (Co = magenta, O = red, N = blue, C = black) and (b) χmT vs. T plot of [2 × 2] grid complex of [Co4(L29)4(dbm)4]·4MeCN. Modified and reproduced with permission from ref. 134, Copyright© 2017, American Chemical Society. | ||
 | ||
| Fig. 24 (a) represents structure of DyL30a and (b) represents magnetic behavior of DyL30a and DyL30b. Modified and reproduced by permission from ref. 135, © 2017, The Royal Society of Chemistry. | ||
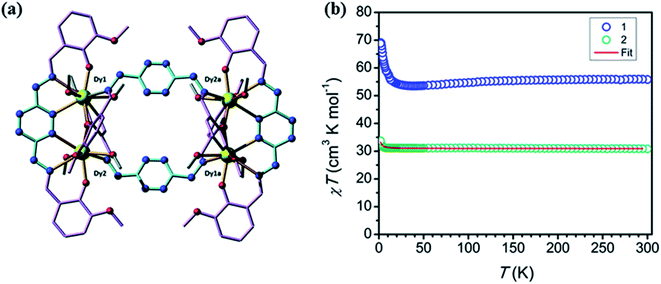
In 2017 T. Lacelle et al. reported two tetranuclear complexes [Ln4(L30)4(MeOH)8](NO3)4·aMeOH·bH2O, where Ln = DyIII (a = 8.07, b = 0.65) (DyL30a), and GdIII (a = 8.19, b = 0.91) (DyL30b), based on 3,6-bis(vanillidenehydrazinyl)-1,2,4,5-tetrazine (L30), which were formed by the reaction between L30 and M(NO3)3·H2O (M = Dy, Gd) in presence of NaN3 in MeOH solvent.136 L30 contained two large NNO coordination pockets with two N of tetrazine, two N of hydrazone moiety and two O from vanilidine moiety suitable for satisfying the coordination sites of lanthanide ions, L30 also contained two small OO coordination pockets from vanilidine moieties. Tetranuclear complex contained two subunits, in which higher coordination pockets of two ligands were used. These two subunits were linked by using another two ligands with distinct coordination pockets consisting oxygen atom of vanillidine moiety and nitrogen of hydrazone moiety. Both complexes DyL30a and DyL30b showed magnetic response under 1000 Oe applied field. Initially at 300 K, χmT values of DyL30a and DyL30b were observed as 55.81 cm3 K mol−1 and 30.81 cm3 K mol−1 respectively and after decreasing the temperature, χmT values of both complexes remained constant up to 12 K and then suddenly increased at 1.9 K with χmT values, 63.86 cm3 K mol−1 and 33.73 cm3 K mol−1 respectively. This indicated the possibility of ferromagnetic coupling between the metal centers (Fig. 24).
3,6-bis(2-pyrimidyl)-1,2,4,5-tetrazine (L31) ligand has four coordination pockets and can coordinate with four metal centres. In 2017 by Lemes et al. first example of L31 radical-based complex [Ni4 (L31˙−)Cl6(DMF)8]Cl·0.5(H2O) (NiL31) was published in which all donor atoms of ligand were coordinated with the metal centre.137 In the complex, each Ni atom had an octahedral environment in which two coordination sites were occupied by two N donor atoms, two axial positions were taken by DMF molecules and remaining sites were occupied by two chlorine atoms. These chlorine atoms acted as bridging ligands between metal centres (Fig. 25a). Magnetic property of NiL31 was investigated and susceptibility data were obtained in the range of 300–1.8 K at 1000 Oe. It was observed that, up to 9 K, χmT value monotonically increased upon lowering the temperature which revealed the presence of ferromagnetic interaction (Fig. 25b) between NiII centres and bridging tetrazinyl radical.138,139
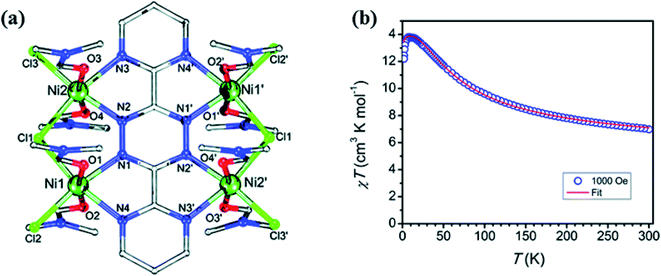 | ||
| Fig. 25 (a) Structural representation (Ni (green), O (red), N (blue), Cl (light green) and C (grey)) and (b) χ.T vs. T plot of NiL31. Modified and reproduced by permission from ref. 136, © 2017, The Royal Society of Chemistry. | ||
In 2020 Maykon A. Lemes et al. reported the ligand L32, 3,6-bis(3,5-dimethyl-pyrazolyl)-1,2,4,5-tetrazine (bpytz), which provided unique type of pancake bonding between metal centers.140 Dinuclear complexes, [DyIII2 (L32)(THMD)6]·4(C6H6) (DyL32a), [DyIII2(L32)2)(THMD)4] (DyL32b), and [YIII2(L32)2)(THMD)4] (YL32) (TMHD = 2,2,6,6-tetramethyl-3,5-heptanedionate) were obtained by the reaction of equivalent amount of L32 and M(TMHD)3 (M = DyIII and YIII) in benzene solvent under different condition (aerobic and anaerobic condition respectively). In these three complexes coordination sites of metal centers were completed by two chelating pockets of L32 and remaining sites were satisfied by TMHD. Complexes DyL32b and YL32 had similar type of structures, in which L32 was in radical anion form and gave intramolecular pancake type bonding, while in DyL32a, L32 existed in neutral form. Magnetic studies of DyL32a and DyL32b were done under 1000 Oe magnetic field. Initially, at 300 K, χmT values of DyL32a and DyL32b were found to be 27.83 and 27.53 cm3 K mol−1 respectively and after lowering down the temperature to 100 K and 50 K, χmT values were not changed and remained constant. This implied the negligible interactions between metal centers. Moreover, further decrease in temperature provided a decrease in χmT values, indicated the weak antiferromagnetic interaction between DyIII centers (Fig. 26).
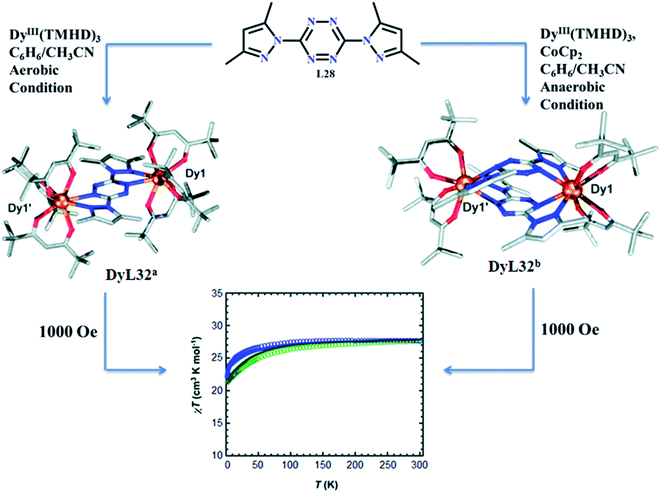 | ||
| Fig. 26 Structure and magnetic properties of DyL32a (blue) and DyL32b (green). Modified and reproduced by permission from ref. 139, © 2020, The Royal Society of Chemistry. | ||
8. Conclusion and future prospects
This review covers maximum reports on structural and magnetic behaviour of different azabenzene-based ligands published in last 10 years. From the above discussion, it is summarized that N and O atoms containing azabenzene-based aromatic ligands show unique chemistry in coordination complexes. Presence of multiple nitrogen atoms in coordination complexes allows them to interact with suitable substrates that generate supramolecular complexes. This review paper restricts to pyridine, diazine, triazine and tetrazine based ligands. In triazine and tetrazine type, only 1,3,5-triazine and 1,2,4,5-tetrazine are focused due to their strong π acceptor ability as well as symmetrical structures. Magnetic behaviour of the complexes of all the discussed ligands have been highlighted and also their structural representation and coordination pockets to form metallogrid complexes are well explained. Compared to pyridine and diazine based ligands, triazine and tetrazine get little attention as bridging ligand, which is further used for the formation of metallogrid complexes. So, by using different substrates, a variety of azine-based ligands can be made to show different topology and have potential to coordinate with different metal centres to give a homometallic and heterometallic grid complex.Recently inorganic chemists focus on the synthesis of the ligand systems that give polynuclear clusters and till date very few ligands have been reported on unsymmetrical azine-based ligands. Hydrazone-based ligands were used as powerful precursors for the synthesis of self-assembled polynuclear complexes. Derivatives of azine based ligands form different types of grid complexes and show interesting properties like magnetic, spin crossover phenomena and water absorption etc. As most of the reported hydrazone-based ligands show antiferromagnetic interaction with 3d metals, so there is a huge scope for the development of ligands which can show ferromagnetic coupling with 3d metals, or with 3d-4f metals, and can show hysteresis loop because it is advantageous for the development of soft or hard magnets and also can be used in data storage devices. We have tried to cover most of the works related to this field. We extend our regrets to the authors whose work we might have missed in the present review. We sincerely hope that this article will make an interesting read, provide insights to future researches and studies.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
AKS acknowledges SERB, India (Grant No. CRG/2020/004334) for funding. JS and SKP acknowledge IIT Bhubaneswar for the fellowship.References
- A. Katoh and R. Saito, J. Synth. Org. Chem., Jpn., 2004, 62, 335–346 CrossRef CAS.
- E. Fischer, Ber. Dtsch. Chem. Ges., 1894, 27, 2985–2993 CrossRef CAS.
- J. M. Lehn, Angew. Chem., Int. Ed. Engl., 1988, 27, 89–112 CrossRef.
- J.-M. Lehn, Angew. Chem., Int. Ed. Engl., 1990, 29, 1304–1319 CrossRef.
- I. P. Parkin, Appl. Organomet. Chem., 2001, 15, 236 CrossRef CAS.
- J. G. Hardy, Chem. Soc. Rev., 2013, 42, 7881–7899 RSC.
- M. Ruben, J. Rojo, F. J. Romero-Salguero, L. H. Uppadine and J. M. Lehn, Angew. Chem., Int. Ed., 2004, 43, 3644–3662 CrossRef CAS PubMed.
- L. N. Dawe, K. V. Shuvaev and L. K. Thompson, Chem. Soc. Rev., 2009, 38, 2334–2359 RSC.
- A. Lakma, S. M. Hossain, J. van Leusen, P. Kögerler and A. K. Singh, Dalton Trans., 2019, 48, 7766–7777 RSC.
- A. Lakma, S. M. Hossain, R. N. Pradhan, D. Topwal, A. Cornia and A. K. Singh, Eur. J. Inorg. Chem., 2016, 2016, 2993–2999 CrossRef CAS.
- J. S. Miller, Chem. Soc. Rev., 2011, 40, 3266–3296 RSC.
- S. Roy, T. N. Mandal, A. K. Barik, S. Gupta, M. S. E. Fallah, J. Tercero, R. J. Butcher and S. K. Kar, Dalton Trans., 2009, 8215–8226 RSC.
- R. L. Carlin, Magnetochemistry, Springer Science & Business Media, 2012 Search PubMed.
- P. W. Selwood, Magnetochemistry, Read Books Ltd, 2013 Search PubMed.
- R. Fagaly, Rev. Sci. Instrum., 2006, 77, 101101 CrossRef.
- C. Granata and A. Vettoliere, Phys. Rep., 2016, 614, 1–69 CrossRef CAS.
- R. Kleiner, D. Koelle, F. Ludwig and J. Clarke, Proc. IEEE, 2004, 92, 1534–1548 CAS.
- K. Enpuku, T. Minotani, M. Hotta and A. Nakahodo, IEEE Trans. Appl. Supercond., 2001, 11, 661–664 CrossRef.
- T. Ryhänen, H. Seppä, R. Ilmoniemi and J. Knuutila, J. Low Temp. Phys., 1989, 76, 287–386 CrossRef.
- H. Weinstock, SQUID Sensors: Fundamentals, Fabrication and Applications, Springer Netherlands, 2012 Search PubMed.
- K. Gramm, L. Lundgren and O. Beckman, Phys. Scr., 1976, 13, 93 CrossRef CAS.
- K. R. Meihaus, J. D. Rinehart and J. R. Long, Inorg. Chem., 2011, 50, 8484–8489 CrossRef CAS PubMed.
- S. Kobayashi, H. Ueda, C. Michioka and K. Yoshimura, Inorg. Chem., 2016, 55, 7407–7413 CrossRef CAS PubMed.
- P. W. Anderson, Phys. Rev., 1950, 79, 350–356 CrossRef.
- P. W. Anderson, Phys. Rev., 1959, 115, 2–13 CrossRef CAS.
- M. Speldrich, J. van Leusen and P. Kögerler, J. Comput. Chem., 2018, 39, 2133–2145 CrossRef CAS PubMed.
- I. de PR Moreira, N. Suaud, N. Guihéry, J.-P. Malrieu, R. Caballol, J. Bofill and F. Illas, Phys. Rev. B, 2002, 66, 134430 CrossRef.
- W. H. Harman, T. D. Harris, D. E. Freedman, H. Fong, A. Chang, J. D. Rinehart, A. Ozarowski, M. T. Sougrati, F. Grandjean and G. J. Long, J. Am. Chem. Soc., 2010, 132, 18115–18126 CrossRef CAS PubMed.
- M. Ruben, E. Breuning, J.-M. Lehn, V. Ksenofontov, F. Renz, P. Gütlich and G. B. M. Vaughan, Chem.–Eur. J., 2003, 9, 4422–4429 CrossRef CAS PubMed.
- A. Bousseksou, G. Molnár, L. Salmon and W. Nicolazzi, Chem. Soc. Rev., 2011, 40, 3313–3335 RSC.
- O. Sato, Proc. Jpn. Acad., Ser. B, 2012, 88, 213–225 CrossRef CAS PubMed.
- P. Gütlich, Y. Garcia and H. A. Goodwin, Chem. Soc. Rev., 2000, 29, 419–427 RSC.
- N. Wannarit, O. Roubeau, S. Youngme, S. J. Teat and P. Gamez, Dalton Trans., 2013, 42, 7120–7130 RSC.
- P. P. Pandey, Pyridine, IntechOpen, 2018 Search PubMed.
- T. L. S. Kishbaugh, Progress in Heterocyclic Chemistry, ed. G. W. Gribble and J. A. Joule, Elsevier, 2012, vol. 24, pp. 343–391 Search PubMed.
- L. N. Dawe, T. S. M. Abedin and L. K. Thompson, Dalton Trans., 2008, 1661–1675, 10.1039/B716114J.
- L. N. Dawe, T. S. M. Abedin, T. L. Kelly, L. K. Thompson, D. O. Miller, L. Zhao, C. Wilson, M. A. Leech and J. A. K. Howard, J. Mater. Chem., 2006, 16, 2645–2659 RSC.
- M. U. Anwar, L. N. Dawe, S. R. Parsons, S. S. Tandon, L. K. Thompson, S. K. Dey, V. Mereacre, W. M. Reiff and S. D. Bunge, Inorg. Chem., 2014, 53, 4655–4668 CrossRef CAS PubMed.
- C. J. Matthews, L. K. Thompson, S. R. Parsons, Z. Xu, D. O. Miller and S. L. Heath, Inorg. Chem., 2001, 40, 4448–4454 CrossRef CAS PubMed.
- L. Zhao, C. J. Matthews, L. K. Thompson and S. L. Heath, Chem. Commun., 2000, 265–266, 10.1039/A909180G.
- O. Waldmann, H. U. Güdel, T. L. Kelly and L. K. Thompson, Inorg. Chem., 2006, 45, 3295–3300 CrossRef CAS PubMed.
- J. Hou, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2008, 64, m1571 CrossRef CAS PubMed.
- V. Chandrasekhar, S. Hossain, S. Das, S. Biswas and J.-P. Sutter, Inorg. Chem., 2013, 52, 6346–6353 CrossRef CAS PubMed.
- S. Biswas, S. Das, J. van Leusen, P. Kögerler and V. Chandrasekhar, Eur. J. Inorg. Chem., 2014, 2014, 4159–4167 CrossRef CAS.
- A. Adhikary, S. Goswami, J. A. Sheikh and S. Konar, Eur. J. Inorg. Chem., 2014, 2014, 963–967 CrossRef CAS.
- J. Wu, L. Zhao, M. Guo and J. Tang, Chem. Commun., 2015, 51, 17317–17320 RSC.
- P. Zhang, L. Zhang, S.-Y. Lin, S. Xue and J. Tang, Inorg. Chem., 2013, 52, 4587–4592 CrossRef CAS PubMed.
- X.-L. Li, H. Li, D.-M. Chen, C. Wang, J. Wu, J. Tang, W. Shi and P. Cheng, Dalton Trans., 2015, 44, 20316–20320 RSC.
- S. Xue, Y.-N. Guo, L. Ungur, J. Tang and L. F. Chibotaru, Chem.–Eur. J., 2015, 21, 14099–14106 CrossRef CAS PubMed.
- J. Wu, X.-L. Li, M. Guo, L. Zhao, Y.-Q. Zhang and J. Tang, Chem. Commun., 2018, 54, 1065–1068 RSC.
- S. Biswas, S. Das, S. Hossain, A. K. Bar, J.-P. Sutter and V. Chandrasekhar, Eur. J. Inorg. Chem., 2016, 2016, 4683–4692 CrossRef CAS.
- S. M. Hossain, A. Lakma, R. N. Pradhan, S. Demeshko and A. K. Singh, Dalton Trans., 2017, 46, 12612–12618 RSC.
- S.-M. Xu, Z.-W. An, W. Zhang, Y.-Q. Zhang and M.-X. Yao, CrystEngComm, 2021, 23, 2825–2834 RSC.
- L. Zhu, Y. Dong, B. Yin, P. Ma and D. Li, Dalton Trans., 2021, 50, 12607–12618 RSC.
- G. Mohammadnezhad, N. Ahfad, S. Meghdadi, H. Farrokhpour, S. Schmitz, A. Haseloer, A. Buchholz, W. Plass and A. Klein, Eur. J. Inorg. Chem., 2021, 2021, 1786–1795 CrossRef CAS.
- B. Drahoš, I. Šalitroš, I. Císařová and R. Herchel, Dalton Trans., 2021, 50, 11147–11157 RSC.
- S. Derossi, M. Casanova, E. Iengo, E. Zangrando, M. Stener and E. Alessio, Inorg. Chem., 2007, 46, 11243–11253 CrossRef CAS PubMed.
- M. Schweiger, S. R. Seidel, A. M. Arif and P. J. Stang, Angew. Chem., 2001, 113, 3575–3577 CrossRef.
- R. P. John, J. Park, D. Moon, K. Lee and M. S. Lah, Chem. Commun., 2006, 3699–3701, 10.1039/B607675K.
- J. S. Gardner, D. P. Strommen, W. S. Szulbinski, H. Su and J. R. Kincaid, J. Phys. Chem. A, 2003, 107, 351–357 CrossRef CAS.
- F. D. Rochon and M. Fakhfakh, Inorg. Chim. Acta, 2009, 362, 1455–1466 CrossRef CAS.
- D. P. Ašanin, M. D. Živković, S. Rajković, B. Warżajtis, U. Rychlewska and M. I. Djuran, Polyhedron, 2013, 51, 255–262 CrossRef.
- R. K. Bansal, Heterocyclic chemistry, New Age International, 2008 Search PubMed.
- W. C. Schneider, J. Am. Chem. Soc., 1948, 70, 627–630 CrossRef CAS PubMed.
- S. Lin, Z. Liu and Y. Hu, J. Comb. Chem., 2007, 9, 742–744 CrossRef CAS PubMed.
- L. Pitarch, R. Coronas and J. Mallol, Eur. J. Pharmacol., 1974, 9, 644–650 CAS.
- S. Mirzoeva, A. Sawkar, M. Zasadzki, L. Guo, A. V. Velentza, V. Dunlap, J.-J. Bourguignon, H. Ramstrom, J. Haiech and L. J. Van Eldik, J. Med. Chem., 2002, 45, 563–566 CrossRef CAS PubMed.
- F. ROHET, C. RUBAT, P. COUDERT, E. ALBUISSON and J. COUQUELET, Chem. Pharm. Bull., 1996, 44, 980–986 CrossRef CAS PubMed.
- A. Montero-Lastres, N. Fraiz, R. Laguna, E. CANO, I. ESTEVEZ and E. RAVINA, Biol. Pharm. Bull., 1999, 22, 1376–1379 CrossRef CAS PubMed.
- R. Slater, W. Howson, G. Swayne, E. Taylor and D. Reavill, J. Med. Chem., 1988, 31, 345–351 CrossRef CAS PubMed.
- R. Buchman, J. A. Scozzie, Z. S. Ariyan, R. D. Heilman, D. J. Rippin, W. J. Pyne, L. J. Powers and R. J. Matthews, J. Med. Chem., 1980, 23, 1398–1405 CrossRef CAS PubMed.
- S. K. Dey, L. K. Thompson and L. N. Dawe, Chem. Commun., 2006, 4967–4969 RSC.
- T. F. Mastropietro, N. Marino, D. Armentano, G. De Munno, C. Yuste, F. Lloret and M. Julve, Cryst. Growth Des., 2013, 13, 270–281 CrossRef CAS.
- R. Bruno, N. Marino, J. Cano, A. P. Alvarez, A. Ben Tama, F. Lloret, M. Julve and G. De Munno, Cryst. Growth Des., 2020, 20, 6478–6492 CrossRef CAS.
- W. A. Butte and F. H. Case, J. Org. Chem., 1961, 26, 4690–4692 CrossRef CAS.
- Z. Guo, Y.-F. Deng, Y. Zhang, Z. Pikramenou and Y.-Z. Zhang, Dalton Trans., 2020, 49, 9218–9222 RSC.
- Z. Guo, M. You, Y.-F. Deng, Q. Liu, Y.-S. Meng, Z. Pikramenou and Y.-Z. Zhang, Dalton Trans., 2021, 50, 14303–14308 RSC.
- J. Lu, X.-L. Li, C. Jin, Y. Yu and J. Tang, New J. Chem., 2020, 44, 994–1000 RSC.
- L. M. De Coen, T. S. Heugebaert, D. Garcia and C. V. Stevens, Chemical Reviews, 2016, 116, 80–139 CrossRef CAS PubMed.
- W. B. Parker, Chemical Reviews, 2009, 109, 2880–2893 CrossRef CAS PubMed.
- S. R. Walker, E. J. Carter, B. C. Huff and J. C. Morris, Chemical Reviews, 2009, 109, 3080–3098 CrossRef CAS PubMed.
- G. S. Feng, M. W. Chen, L. Shi and Y. G. Zhou, Angew. Chem., 2018, 130, 5955–5959 CrossRef.
- N. Aizawa, Y.-J. Pu, H. Sasabe and J. Kido, Org. Electron., 2012, 13, 2235–2242 CrossRef CAS.
- A. S. Duerfeldt and D. L. Boger, J. Am. Chem. Soc., 2014, 136, 2119–2125 CrossRef CAS PubMed.
- J.-Q. Zhang, Y.-J. Luo, Y.-S. Xiong, Y. Yu, Z.-C. Tu, Z.-J. Long, X.-J. Lai, H.-X. Chen, Y. Luo and J. J. Weng, J. Med. Chem., 2016, 59, 7268–7274 CrossRef CAS PubMed.
- A. R. Stefankiewicz, G. Rogez, J. Harrowfield, A. N. Sobolev, A. Madalan, J. Huuskonen, K. Rissanen and J.-M. Lehn, Dalton Trans., 2012, 41, 13848–13855 RSC.
- K. V. Shuvaev, L. N. Dawe and L. K. Thompson, Dalton Trans., 2010, 39, 4768–4776 RSC.
- O. Waldmann, M. Ruben, U. Ziener, P. Müller and J. M. Lehn, Inorg. Chem., 2006, 45, 6535–6540 CrossRef CAS PubMed.
- X.-Y. Cao, J. Harrowfield, J. Nitschke, J. Ramírez, A.-M. Stadler, N. Kyritsakas-Gruber, A. Madalan, K. Rissanen, L. Russo, G. Vaughan and J.-M Lehn, Eur. J. Inorg. Chem., 2007, 2944–2965 CrossRef CAS.
- S. T. Onions, A. M. Frankin, P. N. Horton, M. B. Hursthouse and C. J. Matthews, Chem. Commun., 2003, 2864–2865 RSC.
- W. A. Gobeze, V. A. Milway, B. Moubaraki, K. S. Murray and S. Brooker, Dalton Trans., 2012, 41, 9708–9721 RSC.
- Y.-T. Wang, S.-T. Li, S.-Q. Wu, A.-L. Cui, D.-Z. Shen and H.-Z. Kou, J. Am. Chem. Soc., 2013, 135, 5942–5945 CrossRef CAS PubMed.
- S.-Q. Wu, Y.-T. Wang, A.-L. Cui and H.-Z. Kou, Inorg. Chem., 2014, 53, 2613–2618 CrossRef CAS PubMed.
- S. Dhers, A. Mondal, D. Aguilà, J. Ramírez, S. Vela, P. Dechambenoit, M. Rouzières, J. R. Nitschke, R. Clérac and J.-M. Lehn, J. Am. Chem. Soc., 2018, 140, 8218–8227 CrossRef CAS PubMed.
- A. Lakma, R. N. Pradhan, S. M. Hossain, J. van Leusen, P. Kögerler and A. K. Singh, Inorg. Chim. Acta, 2019, 486, 88–94 CrossRef CAS.
- B. Warżajtis, B. Đ. Glišić, N. S. Radulović, U. Rychlewska and M. I. Djuran, Polyhedron, 2014, 79, 221–228 CrossRef.
- D. P. Ašanin, M. D. Živković, S. Rajković, B. Warżajtis, U. Rychlewska and M. I. Djuran, Polyhedron, 2013, 51, 255–262 CrossRef.
- A. M. Stadler, F. Puntoriero, F. Nastasi, S. Campagna and J. M. Lehn, Chem.–Eur. J., 2010, 16, 5645–5660 CrossRef CAS PubMed.
- J. Ramirez, A.-M. Stadler, G. Rogez, M. Drillon and J.-M. Lehn, Inorg. Chem., 2009, 48, 2456–2463 CrossRef CAS PubMed.
- R. W. Hogue, S. Dhers, R. M. Hellyer, J. Luo, G. S. Hanan, D. S. Larsen, A. L. Garden and S. Brooker, Chem.–Eur. J., 2017, 23, 14193–14199 CrossRef CAS PubMed.
- J. Hausmann and S. Brooker, Chem. Commun., 2004, 1530–1531, 10.1039/B403905J.
- J. Hausmann and S. Brooker, Chem. Commun., 2004, 1530–1531 RSC.
- J. Hausmann, G. B. Jameson and S. Brooker, Chem. Commun., 2003, 2992–2993 RSC.
- J. Klingele, J. F. Boas, J. R. Pilbrow, B. Moubaraki, K. S. Murray, K. J. Berry, K. A. Hunter, G. B. Jameson, P. D. Boyd and S. Brooker, Dalton Trans., 2007, 633–645 RSC.
- N. C. Desai, A. H. Makwana and R. D. Senta, J. Saudi Chem. Soc., 2016, 20, 686–694 CrossRef CAS.
- N. Desai, A. H. Makwana and R. Senta, J. Saudi Chem. Soc., 2016, 20, 686–694 CrossRef CAS.
- R. Shanmugakala, P. Tharmaraj, C. D. Sheela and C. Anitha, Int. J. Inorg. Chem., 2012, 2012, 301086 Search PubMed.
- N. S. Mewada, D. R. Shah, H. P. Lakum and K. H. Chikhalia, J. Assoc. Arab Univ. Basic Appl. Sci., 2016, 20, 8–18 Search PubMed.
- J. Ramírez, A.-M. Stadler, N. Kyritsakas and J.-M. Lehn, Chem. Commun., 2007, 237–239, 10.1039/B612222A.
- P. de Hoog, P. Gamez, W. L. Driessen and J. Reedijk, Tetrahedron Lett., 2002, 43, 6783–6786 CrossRef CAS.
- A. Das, S. Demeshko, S. Dechert and F. Meyer, Eur. J. Inorg. Chem., 2011, 1240–1248 CrossRef CAS.
- N. Parizel, J. Ramírez, C. Burg, S. Choua, M. Bernard, S. Gambarelli, V. Maurel, L. Brelot, J.-M. Lehn, P. Turek and A.-M. Stadler, Chem. Commun., 2011, 47, 10951–10953 RSC.
- M. Quesada, M. Monrabal, G. Aromí, V. A. de la Peña-O'Shea, M. Gich, E. Molins, O. Roubeau, S. J. Teat, E. J. MacLean, P. Gamez and J. Reedijk, J. Mater. Chem., 2006, 16, 2669–2676 RSC.
- J. S. Costa, K. Lappalainen, G. de Ruiter, M. Quesada, J. Tang, I. Mutikainen, U. Turpeinen, C. M. Grunert, P. Gütlich, H. Z. Lazar, J.-F. Létard, P. Gamez and J. Reedijk, Inorg. Chem., 2007, 46, 4079–4089 CrossRef PubMed.
- S. Bonnet, M. A. Siegler, J. S. Costa, G. Molnár, A. Bousseksou, A. L. Spek, P. Gamez and J. Reedijk, Chem. Commun., 2008, 5619–5621, 10.1039/B811746B.
- S. Bonnet, G. Molnár, J. Sanchez Costa, M. A. Siegler, A. L. Spek, A. Bousseksou, W.-T. Fu, P. Gamez and J. Reedijk, Chem. Mater., 2009, 21, 1123–1136 CrossRef CAS.
- M. Quesada, V. A. de la Peña-O’Shea, G. Aromí, S. Geremia, C. Massera, O. Roubeau, P. Gamez and J. Reedijk, Adv. Mater., 2007, 19, 1397–1402 CrossRef CAS.
- T. M. Ross, B. Moubaraki, S. M. Neville, S. R. Batten and K. S. Murray, Dalton Trans., 2012, 41, 1512–1523 RSC.
- T. M. Ross, B. Moubaraki, S. R. Batten and K. S. Murray, Dalton Trans., 2012, 41, 2571–2581 RSC.
- T. M. Ross, B. Moubaraki, K. S. Wallwork, S. R. Batten and K. S. Murray, Dalton Trans., 2011, 40, 10147–10155 RSC.
- T. M. Ross, B. Moubaraki, D. R. Turner, G. J. Halder, G. Chastanet, S. M. Neville, J. D. Cashion, J.-F. Létard, S. R. Batten and K. S. Murray, Eur. J. Inorg. Chem., 2011, 1395–1417 CrossRef CAS.
- N. Wannarit, N. Nassirinia, S. Amani, N. Masciocchi, S. Youngme, O. Roubeau, S. J. Teat and P. Gamez, Inorg. Chem., 2014, 53, 9827–9836 CrossRef CAS PubMed.
- J. Lu, Y.-Q. Zhang, X.-L. Li, M. Guo, J. Wu, L. Zhao and J. Tang, Inorg. Chem., 2019, 58, 5715–5724 CrossRef CAS PubMed.
- H. T. Chifotides and K. R. Dunbar, Acc. Chem. Res., 2013, 46, 894–906 CrossRef CAS PubMed.
- M. Savastano, C. Bazzicalupi, C. Giorgi, C. García-Gallarín, M. D. López de la Torre, F. Pichierri, A. Bianchi and M. Melguizo, Inorg. Chem., 2016, 55, 8013–8024 CrossRef CAS PubMed.
- M. Savastano, C. Bazzicalupi, C. García, C. Gellini, M. D. López de la Torre, P. Mariani, F. Pichierri, A. Bianchi and M. Melguizo, Dalton Trans., 2017, 46, 4518–4529 RSC.
- M. Savastano, C. García-Gallarín, M. D. López de la Torre, C. Bazzicalupi, A. Bianchi and M. Melguizo, Coord. Chem. Rev., 2019, 397, 112–137 CrossRef CAS.
- B. J. Jordan, M. A. Pollier, L. A. Miller, C. Tiernan, G. Clavier, P. Audebert and V. M. Rotello, Org. Lett., 2007, 9, 2835–2838 CrossRef CAS PubMed.
- Q. Zhou, P. Audebert, G. Clavier, R. Méallet-Renault, F. Miomandre, Z. Shaukat, T.-T. Vu and J. Tang, J. Phys. Chem. C, 2011, 115, 21899–21906 CrossRef CAS.
- E. Kurach, D. Djurado, J. Rimarčik, A. Kornet, M. Wlostowski, V. Lukeš, J. Pécaut, M. Zagorska and A. Pron, Phys. Chem. Chem. Phys., 2011, 13, 2690–2700 RSC.
- G. Clavier and P. Audebert, Chem. Rev., 2010, 110, 3299–3314 CrossRef CAS PubMed.
- J. Li, Y. Peng, H. Liang, Y. Yu, B. Xin, G. Li, Z. Shi and S. Feng, Eur. J. Inorg. Chem., 2011, 2712–2719 CrossRef.
- O. Stetsiuk, A. Abhervé and N. Avarvari, Dalton Trans., 2020, 49, 5759–5777 RSC.
- B. S. Dolinar, S. Gómez-Coca, D. I. Alexandropoulos and K. R. Dunbar, Chem. Commun., 2017, 53, 2283–2286 RSC.
- D. I. Alexandropoulos, B. S. Dolinar, K. R. Vignesh and K. R. Dunbar, J. Am. Chem. Soc., 2017, 139, 11040–11043 CrossRef CAS PubMed.
- T. Lacelle, G. Brunet, A. Pialat, R. J. Holmberg, Y. Lan, B. Gabidullin, I. Korobkov, W. Wernsdorfer and M. Murugesu, Dalton Trans., 2017, 46, 2471–2478 RSC.
- M. A. Lemes, G. Brunet, A. Pialat, L. Ungur, I. Korobkov and M. Murugesu, Chem. Commun., 2017, 53, 8660–8663 RSC.
- D. Luneau, F. M. Romero and R. Ziessel, Inorg. Chem., 1998, 37, 5078–5087 CrossRef CAS.
- T. M. Barclay, R. G. Hicks, M. T. Lemaire and L. K. Thompson, Inorg. Chem., 2001, 40, 5581–5584 CrossRef CAS PubMed.
- M. A. Lemes, N. Mavragani, P. Richardson, Y. Zhang, B. Gabidullin, J. L. Brusso, J. O. Moilanen and M. Murugesu, Inorg. Chem. Front., 2020, 7, 2592–2601 RSC.
Footnote |
| † These authors made equal contributions. |
| This journal is © The Royal Society of Chemistry 2022 |