 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A review on recent advances in the electrochemical reduction of CO2 to CO with nano-electrocatalysts
Kee Chun Poon
 *a,
Wei Yang Wan
*a,
Wei Yang Wan
 a,
Haibin Su
b and
Hirotaka Sato
*a
a,
Haibin Su
b and
Hirotaka Sato
*a
aSchool of Mechanical & Aerospace Engineering, Nanyang Technological University, 50 Nanyang Avenue, Singapore 639798. E-mail: hirosato@ntu.edu.sg; keechun.poon@ntu.edu.sg
bDepartment of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Hong Kong, China
First published on 15th August 2022
Abstract
The electrochemical reduction (ECR) of CO2 is a powerful strategy to reduce the world's carbon footprint by converting CO2 to useful products such as CH3OH and CO. Recent techno-economic analysis has found that for the electro-conversion of CO2 to be adapted for practical use, the main products formed from this reaction need to be low-order, such as CO. This review summarizes recent progress in the ECR of CO2 to CO on nano-electrocatalysts (noble, non-noble metals and carbon nanomaterials) and provides the limitations and challenges that each electrocatalyst faces. It discusses the mechanism behind the performance of the electrocatalysts and offers the potential future prospects of the ECR process.
 Wei Yang Wan | Wan Wei Yang received his B.E. from Nanyang Technological University in 2019. He carried out his research studies in the field of electrochemistry, electroless plating and electroplating. |
1. Introduction
The increase in energy demand over the past few centuries has led to a surge in the utilization of fossil fuel-based power sources such as petroleum, coal and natural gas.1,2 The result of this has been a surge in greenhouse emissions, causing severe environmental effects such as global warming, ocean acidification and desertification.3–5 The leading source of greenhouse emissions is carbon dioxide (CO2), which the Intergovernmental Panel on Climate Change (IPCC) has contributes up to 76% of all greenhouse gases.6 Therefore, there is an increasing need to solve the crisis of the extensive build-up of CO2 that the world has generated over the years. Additionally, carbon capture and utilization have been established by the work of Ghiat et al. to help with CO2 abatement.69 In another study done by Sun et al., integrated CO2 capture and utilization (ICCU) reduced the path of CO2 usage and its conversion.70 Concepts and models have also been established by Garcia et al. for heterogeneous catalysts employed in CO2 conversion.71 Progress in electrochemical CO2 reduction on copper (Cu) in water-based electrolytes has also been established, together with a comprehensive study on the utilization and conversion of CO2.72,73There are two common methods that researchers and industries have been focused on to tackle this problem. First would be a solution to the CO2 emission problem at the production level. This would involve a reduction in the dependence on fossil fuel and the search for alternative energy sources. These alternative energy sources include solar, wind, biomass, geothermal, hydro and biofuels, which are clean, renewable and most importantly do not produce any harmful CO2 by-products.7–11 However, despite the recent increase of these alternative sources from 5% in 2008 to almost 28% in 2020, their high production cost and limitations such as geographical constraints have limited the full switch to such alternatives.12–14 Furthermore, the effective use of such renewable energy relies heavily on energy conversion and storage technologies, which are limited by low energy density conversions and high production costs, which makes the large-scale production of such facilities difficult to accomplish.15–17 Therefore, an approach to overcome the CO2 production problem should be established.
The second approach would involve the carbon capture and utilization (CCU) method. The idea behind this is simple. The CO2 products are retrieved and converted into useful materials such as carbon monoxide (CO), formic acid (HCOOH), methanol (CH3OH), and methane (CH4).18–21 This strategy can effectively reduce the CO2 in the air, and also create fuel to produce useful and desirable industrial products. One example of this conversion is CO2 hydrogenation, where CO2 is converted to CH3OH.22–24 However, the main problem with combustion-based conversion is that the heat comes from fossil fuel, which substantially lowers the net amount of CO2 being converted.25,26
Recently, electrochemical method has become an increasingly popular method in the conversion of CO2.27–30 This method, which is called the electrochemical reduction (ECR) of CO2, is particularly appealing for multiple reasons. Firstly, using the ECR technique, high selectivity of the desired products can be obtained, as seen in Fig. 1.31 The selectivity of the products can be actively controlled by simply adjusting the voltage (energy) supplied to the reaction. Furthermore, the energy supplied in the electrochemical reduction can be obtained from renewable sources, which means additional CO2 is not generated during this process.32,33 It should be noted that this process can be conducted at ambient pressure and temperature with little complications for its scaling up. Moreover, the reactor setup for the electroreduction process is modular, increasing the ease of its large-scale application.34–36 Lastly, the reactants consumed in the electroreduction process are simple such as water and CO2, while the other components that are not consumed such as the electrolyte and electrodes are reusable, which reduces both the cost and pollution.34–36
 | ||
| Fig. 1 Thermodynamic potential difference of the products made by the electrochemical reduction of carbon dioxide (CO2). Reprinted with permission from ref. 31. Copyright 2016 Wiley. | ||
However, CO2 ECR still exhibits some drawbacks. Firstly and importantly, based on the recent techno-economic analysis conducted for industrial use (TEA survey), it was found that the ECR method is competitive for industrial application, where lowered order products such as carbon monoxide (CO) and formate ion (HCOO−) are preferred over high-order products such as CH3OH.32,33 The faradaic efficiency (FE) of CO, which corresponds to the high current density, is higher than that of the other intermediates. This is due to the high activation barrier requirement of the ECR process, which is related to the next point, i.e., the structure of CO2. Due to the linear nature of the CO2 molecule, its conformation needs to be changed to the bent CO2˙− to become activated towards the electroreduction process, as seen in Fig. 3.31,37 This requirement results in a high energy barrier for the formation of the CO2˙− intermediate, which translates to a large overpotential requirement (the difference between the equilibrium and onset potential). Ultimately, this results in poor energy efficiency for the electroreduction process. Furthermore, the kinetics of the electroreduction reaction is slow with the limited mass transfer process of CO2, which results in sluggish reaction rates for the reduction of CO2, and thus a long processing time is needed.38,39 Another drawback is the CO2 electroreduction in aqueous solvents, where the competing reaction of the hydrogen evolution reaction (HER) decreases the overall production efficiency of the reaction.40–42 Also, the electrocatalyst used for the electroreduction process can be poisoned by the intermediates formed during the process, which can deactivate the catalyst and incur greater labour and replacement costs. Finally, mixed products can be formed during the ECR process, which adds another overhead on the production line due to the need for product separation.
2. Understanding the fundamentals and evaluation of electrochemical reduction of CO2 to CO
The ECR of CO2 to CO is a multi-step reaction, which involves a two-electron transfer process, as seen in Fig. 2.31 Overall, for the ECR to CO, there are 3 main steps. Firstly, the adsorption of CO2 on the surface of the electrocatalyst. This is followed by the reduction of CO2 to form the CO2˙− intermediate. The final step is the reduction (typically with another CO2 molecule) to produce CO.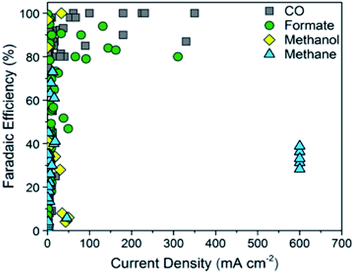 | ||
| Fig. 2 Faradaic efficiency versus current density for carbon monoxide. Reprinted with permission from ref. 32. Copyright 2018, ACS Publications. | ||
To understand and evaluate the electrocatalyst performance towards ECR to CO, there are certain definitions and comparison methods that need to be clarified beforehand, as follows:
2.1 Onset potential and overpotential
The onset potential is the potential applied to the electrocatalyst (vs. the reference electrode) for CO to be detected. The overpotential is the difference between the onset potential and the equilibrium potential of the ECR to CO reaction. Therefore, a desirable electrocatalyst should require the greatest positive onset potential, while having the smallest possible overpotential (the best value is the same as that for equilibrium).2.2 Current density
This is obtained by normalizing the current by either the surface area of the electrode or the electrocatalyst. This value indicates the amount of reaction occurring during the ERC to CO. It is also used in the calculation of the faradaic efficiency (FE), which is discussed in the following point. Also, this value is important especially from an industrial application point of view given that it will determine the production and operation cost of running the ECR setup.2.3 Faradaic efficiency (FE)
This is defined as the percentage of CO formed by the amount of electrons used in the ECR. The FE is calculated using eqn (1), as follows:
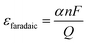 | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 485 C mol−1) and Q is the amount of charge passed during the ECR. The FE enables the understanding of the percentage of CO produced in the ECR process.
485 C mol−1) and Q is the amount of charge passed during the ECR. The FE enables the understanding of the percentage of CO produced in the ECR process.
3. Electrocatalysts
Recently, a variety of electrocatalysts has been used for the ECR to CO process. They can be classified into 2 main categories, i.e., metal and non-metal electrocatalysts, each with its own advantages and disadvantages. The metal electrocatalysts can be further split into 2 sub-categories, i.e., noble metals and non-noble metals. This review explores the recent advances in each of these categories together with its shortcomings and potential applications.3.1 Noble metals
In the case of noble metals, in the past decade, gold (Au), silver (Ag) and palladium (Pd) have been the three most commonly explored metals for the electro-conversion of CO2 to CO. This is due to several factors, which will be explained in detail later.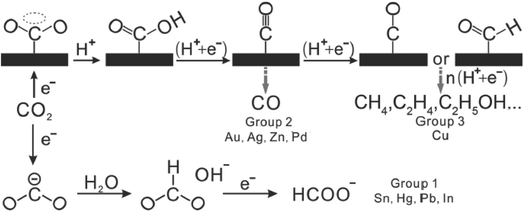 | ||
| Fig. 3 Reaction pathway for the electrochemical reduction of CO2. Reprinted with permission from ref. 31. Copyright 2016, Wiley. | ||
One of the most effective ways to reduce the cost of Au catalysts is by using nanoparticles (NPs). In the study conducted by Mistry et al., they found that the catalytic activity in terms of current density was improved significantly when the size of Au NPs was reduced from 7.7 nm to 1.1 nm over a wide potential range, as seen in Fig. 4.28 However, this resulted in a lower FE for the formation of CO with the FE of H2 increasing. This was ascribed to the reduction in the size of the Au NPs, which led to an increase in the abundance of both edge and corner sites. The edges were theorized to enable the formation of CO, while the corners were the key sites for the formation of H2.28 Therefore, although the overall ECR performance of Au NPs for CO increased as the particle size became smaller, the lower FE of CO was not ideal. This theory was also supported by Zhu et al., who observed the same trend in their study on Au NPs and confirmed their observation by improving the FE of CO from 90% to 97% by embedding the Au NPs in an ionic matrix to prevent the HER reaction.44 Furthermore, their DFT calculations supported both Mistry et al. and their hypothesis that the edges promote CO evolution, while the corner sites are active for H2 evolution. Interestingly, in a follow up work by Back et al., their DFT study on Au NPs showed contradictory results, where their findings suggested that the corners promote CO evolution, while edges are for H2 evolution.45 They attributed the discrepancy in their findings in comparison to Zhu et al. to the quantum size effect, given that their modelling was achieved using large Au NPs (309 atoms or more), which would give more accurate results. The quantum size effect can be defined as strong binding energies as a result of the size of the Au NPs. In the case of Au NPs with size less than 2 nm, the sizing model was affected. Therefore, for correct prediction of Au NPs, this theorized quantum size effect should be considered to acquire Au NPs with the optimal size, which is strongly correlated with their catalytic activity.
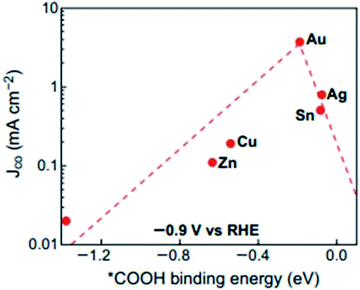 | ||
| Fig. 4 Volcano plot of different metals towards the electrochemical reduction (ECR) of CO2. Reprinted with permission from ref. 43. Copyright 2017, American Chemical Society. | ||
Another way to improve the catalytic performance of Au NPs is by shape modification. In a recent study conducted by Yang et al., they found with DFT calculations that the (2 2 1) facet of Au had a lower potential barrier compared to the traditional (1 1 1), which was the focus of studies in the past, as seen in Fig. 5.46 The size effect of the Au trisoctahedron (TOH) NP was found to have a direct correlation with the faradaic efficiency (FE), where an increase in particle size to more than 100 nm caused the FE for CO to decrease by more than 60%. The SEM image of the 50 nm Au TOH also showed the clear edge features of the NP, which improved its performance for the electrocatalytic reduction of CO2. Considering this, they designed and synthesized Au trisoctahedron for the ECR of CO2 to CO. They discovered that Au TOH could increase the FE for CO by 1.5 times compared to colloidal Au. Furthermore, they found that the performance of the Au TOH decreased with an increase in particle size. They hypothesized that with an increase in the Au TOH particle size, there was a systematic decrease in the edge to face ratio, which resulted in a decline in the performance of Au TOH given that the edge sites of Au TOH were found to be the most active catalytic sites due to their good balance between COOH stability and CO desorption free energy.
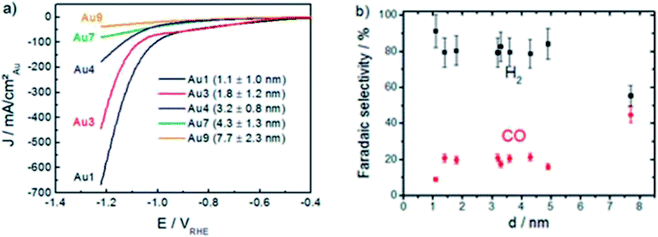 | ||
| Fig. 5 (a) Linear sweep voltammetry (LSV) curves of the ECR of CO2 on gold (Au) nanoparticles (NPs) of various sizes. (b) Effect of Au NP size on the faradaic efficiency (FE). Reprinted with permission from ref. 28. Copyright 2013, American Chemical Society. | ||
In conclusion, Au NPs are theoretically the best electrocatalyst for the ECR of CO2 to CO. Recent studies have explored different ways such as shape and morphology manipulation to control and improve their catalytic activity. However, their underlying mechanism is still under debate, with both Zhu and Back et al. both showing contradictory DFT results. Furthermore, the optimization to improve the cost efficiency by reducing the size of Au NPs has not yielded favorable results. Therefore, there is still much need for further research in both mechanism study and optimization for Au to be deemed a suitable candidate for the large-scale ECR process.
Similar to Au, researchers have tried to improve the performance (to enhance its cost benefit) of Ag by using NPs with modification of their size. Both Salehi-Khojin et al. and Kim et al. found that Ag NPs performed better in terms of current density and FE for the ECR of CO2 to CO compared to bulk Ag films.47,48 They both reported that they found 5 nm to be the optimal size for the best activity by Ag NPs, with Salehi-Khojin et al. reporting a 10-times increment in activity, while Kim et al. reported a 4.8-times enhancement with reference to Ag film/foil, as seen in Fig. 6. Interestingly, both groups reported different reasons for this enhancement. Salehi-Khojin et al., using DFT modelling, found that reducing the Ag NPs size below 5 nm resulted in an increase in the binding energy of the COOH intermediate and decrease in the rate of the desorption of OH, which reduced the overall reaction rate. This is the typical behaviour shown by the volcano plot. Kim et al. attributed their enhanced performance to the cysteamine anchoring agent. According to their DFT calculations, they found that the cysteamine anchoring agent led to the localization of unpaired electrons on the surface of the Ag NPs, which helped to stabilize the key COOH intermediate. Regarding the optimal performance of their 5 nm Ag NPs, they attributed this to the optimal coverage of the anchoring agent on the Ag NPs, with other sizes resulting in suboptimal coverage (too much or too little coverage).
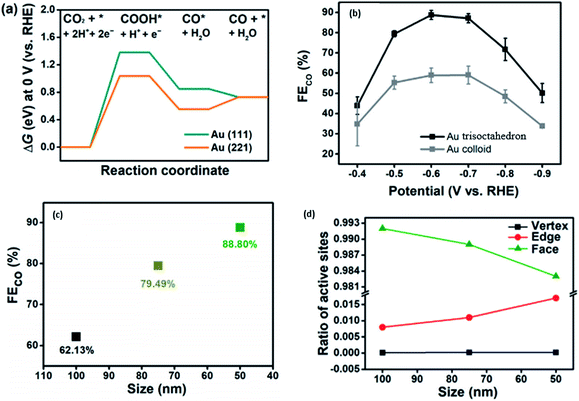 | ||
| Fig. 6 (a) Gibbs free energy diagram for the ECR of CO2 to CO on Au (1 1 1) and Au (2 1 1). (b) FE of Au trisoctahedron (TOH) compared to that of Au NPs (colloid). (c) Effect of the size of Au TOH on FE. (d) Ratio of active sites in Au TOH with respect to particle size. Reprinted with permission from ref. 46. Copyright 2020, Elsevier. | ||
However, in the recent study conducted by Back et al., their DFT calculations discovered that in contrast to Au, the Ag NP edges are the most active sites for the ECR of CO2 to CO.45 They also found that unlike Au, the optimal size of Ag NPs was 2 nm with minimal interference from the unwanted and competing HER process. This was due to the increase in highly active sites, as seen in Fig. 7. It is also worth noting that Back et al. collaborated their findings with the experimental findings observed by Salehi-Khojin et al. They explained that in their calculations, they did not consider the quantum-size effect. Therefore, the trend observed in the Ag NPs by Salehi-Khojin et al. from 1–5 nm was not consistent with their results. However, for the 5–200 nm Ag NPs, the quantum-size effect was not observed, which is consistent with the findings by Salehi-Khojin, where the edge sites were the most active.
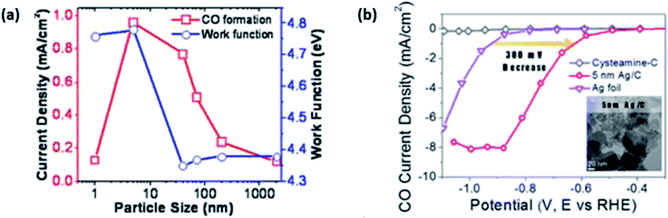 | ||
| Fig. 7 (a) Graph depicting the current density and work function against the particle size of silver (Ag) NPs. Reprinted with permission from ref. 47. Copyright 2012, American Chemical Society. (b) Linear sweep voltammetry (LSV) curves of 5 nm Ag NPs compared to those of Ag foil and cysteamine-C. Reprinted with permission from ref. 48. Copyright 2015, American Chemical Society. | ||
There have also been attempts to increase the performance of Ag NPs by using a variety of different shapes. Hsieh et al. used high surface area Ag nanocoral to improve the performance of Ag NPs.49 As seen in Fig. 8, they reported a 32-fold increment in catalytic activity compared to bulk Ag foil. This enhancement was ascribed to both the nanostructure morphology effect, and importantly the presence of doped chlorine, which inhibited the undesirable HER side reaction. In the recent study by Liu et al., they used Ag nanocubes to achieve this effect.50 They reported a near unity FE (99%) for Ag nanocubes with a length below 25 nm (L25-Ag-NCs), exhibiting an ultra-low overpotential (146 mV vs. RHE), higher energy efficiency (64%) and durability (stability over 18 h) compared to nanocubes with a length of less than 70 nm, Ag NPs and Ag foil, as seen in Fig. 9. They substantiated their results with DFT calculations, which showed that in L25-Ag-NCs, the maximum number of energetic favored sites were introduced through precise synthesis of the nanostructure enclosed by the Ag (1 0 0) facet.
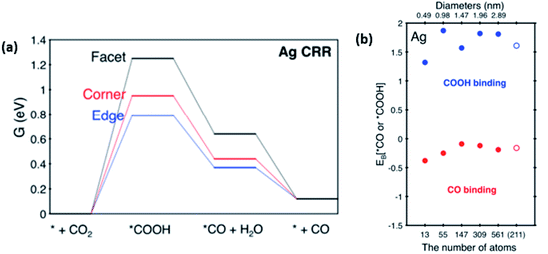 | ||
| Fig. 8 (a) Gibbs free energy diagram on different facets of silver (Ag) NPs for the electrochemical reduction (ECR) of CO2. (b) Binding energies of Ag NPs of different sizes at the (2 1 1) edge. Reprinted with permission from ref. 45. Copyright 2015, American Chemical Society. | ||
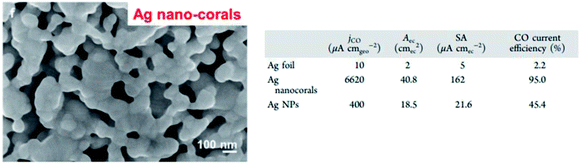 | ||
| Fig. 9 Ag nano-corals with high catalytic activity (38 times better than Ag foil). Reprinted with permission from ref. 49. Copyright 2015, American Chemical Society. | ||
In summary, Ag is seen as a promising alternative to Au, being theoretically the second best electrocatalyst for the ECR of CO2 to CO. Interestingly, unlike Au, Salehi-Khojin and Kim et al. showed that reducing the size of Ag NPs could significantly improve both their catalytic performance and product selectivity although the former attributed the increase to the stabilization of the COOH intermediate, while the latter hypothesized that the cysteine anchoring agent was key in the synthetic process. Overall, this shows the potential of lowering the cost of Au-based electrocatalysts by improving the mass activity. Other groups such as Hsieh and Liu et al. were successful in improving the performance of Ag by its shape manipulation (coral and cubes respectively). Lastly, the DFT calculations by both Salehi-Khojin and Back et al. are in agreement with the edges being the key factor for CO evolution, which can be targeted in the future for further optimization. Hence, Ag has been shown to be a cheaper alternative compared to Au with the possibility of significantly higher mass activity due to the trend of improved electrocatalytic activity with a decrease in its size.
In previous studies on Pd, it was found by Gao et al. that the final product of either CO or HCOO− could be actively determined by the type of Pd surface present, as seen in Fig. 10.51 Based on their in situ XPS and DFT results, they found that with the hydrogen-absorbed Pd surface over a mixture of α- and β-phase palladium-hydride (PdH) core, the formation of HCOO− was more likely via the HCOO* intermediate step at an FE as high as 98%. Alternatively, when the β-phase PdH core was covered with a metallic Pd surface, CO was the major product of the ECR with an FE of 93.4%. This was due to the fact that the formation of CO was promoted via the COOH* intermediate instead.
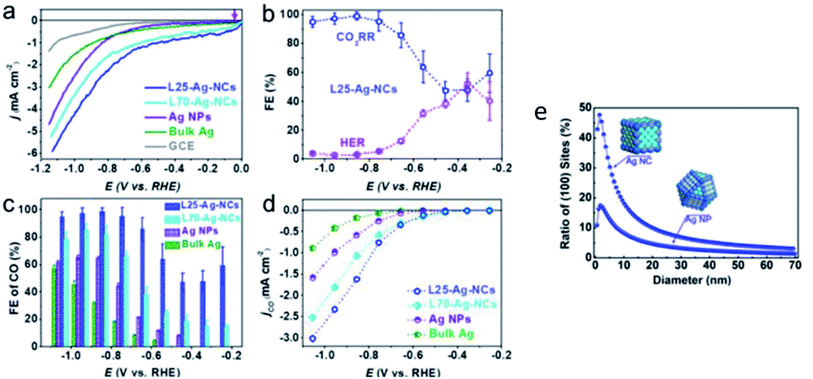 | ||
| Fig. 10 (a) Linear sweep voltammetry (LSV) curves of varying sizes of silver (Ag) nanocubes (NC) compared to those of Ag NPs, bulk Ag and GCE. (b) Overall FE of 25 nm Ag-NCs. (c) FE of CO and (d) normalized LSV curves for 25 and 75 nm Ag-NCs, Au NPs and bulk Ag. (e) Ratio of (1 0 0) active sites of Ag-NC compared to that of Ag NPs. Reprinted with permission from ref. 50. Copyright 2020, American Chemical Society. | ||
However, in the recent study conducted by He et al., they found that the PdH core, which was previously determined to be essential for the formation of the Pd NP catalyst, was not optimal for the ECR process.52 In their study, as seen in Fig. 11, they discovered that the Pd–N4 site on a nitrogen-doped carbon-supported Pd single atom (Pd-NC) was more suitable for the ECR of CO2 to CO. It was shown via in situ XAFS and DFT calculations that the Pd–N4 single atom site can stabilize the formation of the CO2 intermediate, therefore enhancing the electrocatalytic performance of the Pd catalyst at low overpotentials. This translated into a higher mass activity performance of 373.0 mgPd−1 for Pd-NC compared to 28.5 mgPd−1 for the traditional Pd/C. Compared to the active phase engineering conducted by Gao et al., the performance of the Pd-NC was better at a lower potential but suffered at higher overpotentials.
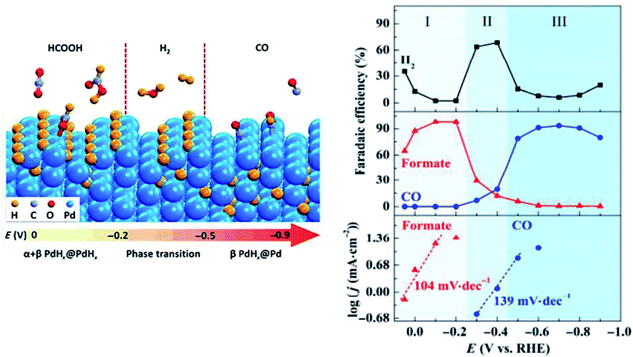 | ||
| Fig. 11 The effect of different phases of palladium (Pd) on the product of the electrochemical reduction (ECR) of CO2. On the β-phase of the palladium–hydrogen (PdH) core, up to 93.4% faradaic efficiency (FE) towards carbon monoxide (CO) can be achieved. Reprinted with permission from ref. 51. Copyright 2017, Springer. | ||
Lastly, in the recent work by Dong et al., they used facet design to enhance the ECR performance of Pd NPs.53 As seen in Fig. 12, Dong et al. synthesized Pd NPs with an equal size but different shapes to represent different facets, including concave cubes, cubes and octahedrons to represent the (3 1 0), (1 0 0) and (1 1 1), respectively. Based on both experimental results and DFT calculations, they found that the concave cubes enclosed with (3 1 0) facet had the best ECR activity with an FE selectivity of 90.6% compared to the octahedrons and cubes with an FE of 71.3% and 68.2%, respectively. This result is in good agreement with the results reported by Gao et al. They further found that the reason for the high activity of the (3 1 0) facet was due to the stabilization of the formed COOH* intermediate and the ease of desorption of CO* from the (3 1 0) facet.
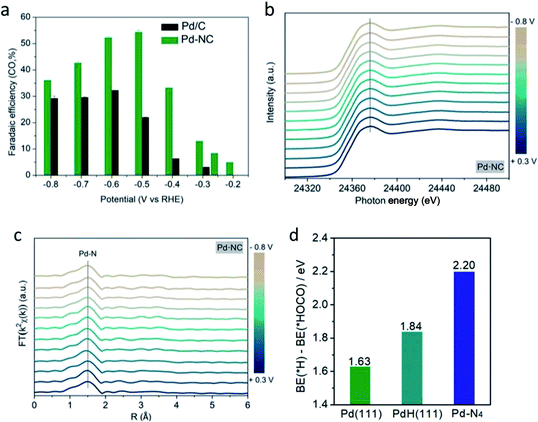 | ||
| Fig. 12 (a) faradaic efficiency (FE) towards CO for Pd-NC and Pd/C, (b) XANES spectra and (c) Fourier-transformed k2-weighted EXAFS profiles of Pd-NC. (d) DFT calculation of the difference in the binding energy of H and HOCO on Pd (1 1 1), PdH (1 1 1) and Pd–N4. Reprinted with permission from ref. 52. Copyright 2020, Wiley. | ||
In summary, recent advances in Pd electrocatalysts have been established via core and surface manipulation. Researchers such as Dong et al. found success in facet design. However, in terms of the design and understanding of optimal Pd NPs, there has been some contention with Gao et al. finding the Pd–H core with pure Pd metal covering its surface to have the optimal results, while He et al. disagreed with their findings, reporting a single Pd atom core with Pd–N4 active surface site having overall better catalytic and mass activity. Also, the current price of Pd metal is 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 USD, which is 28% higher than Au metal and significantly greater than the cheaper Ag metal of 610 USD. Therefore, although Pd is a potential electrocatalyst for the ECR process, there is still much to be understood in terms of mechanism and electrocatalyst design before it can be utilized effectively.
600 USD, which is 28% higher than Au metal and significantly greater than the cheaper Ag metal of 610 USD. Therefore, although Pd is a potential electrocatalyst for the ECR process, there is still much to be understood in terms of mechanism and electrocatalyst design before it can be utilized effectively.
3.2 Non-noble metals
For non-noble metals, zinc (Zn) and bismuth (Bi) are two of the more popular metals, which are actively studied currently. The reason for this is different for each metal and will be discussed in greater detail below.Quan et al. fabricated Zn nanoplates (n-Zn) by electroreducing Zn foil, as seen in Fig. 13.54 n-Zn exhibited superior ECR properties compared to Zn foil. Furthermore, they also found that the Zn electrocatalyst generally performed better for the ECR of CO2 to CO in NaCl solution compared to NaHCO3 solution. Their n-Zn had an FE of 93% in NaCl compared to that of 60% in NaHCO3 over a 10 h operation. They proposed that the reason for this improvement was due to the fact that the Cl− ions in the NaCl solution could greatly suppress the competing HER process. Consequently, this promoted the formation of the CO2˙− radical, which enhanced the conversion efficiency of CO2, as illustrated in Fig. 13. This trend was also observed when they used Ag NPs instead, with the FE of the Ag NPs increasing from 20% to 70% by simply changing the solution from NaHCO3 to NaCl.
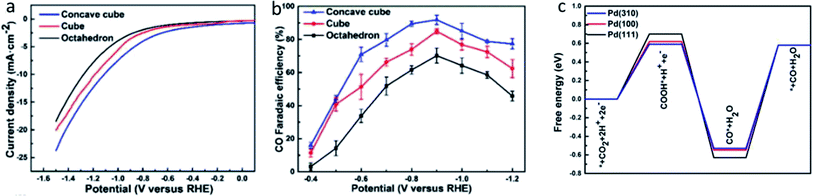 | ||
| Fig. 13 (a) Linear sweep voltammetry (LSV) curves and (b) CO FE of concave cube, cube and octahedron Pd NPs, which are Pd (3 1 0), (1 0 0) and (1 1 1), respectively. (c) DFT calculations of the free energy diagram for the ECR of different facets of Pd NPs. Reprinted with permission from ref. 53. Copyright 2019, Elsevier. | ||
In more recent works, the focus has been increasing the performance of Zn electrocatalysts by using porous Zn, which possessed an increased surface area. In the study by Lu et al., they electrodeposited Zn(NO3)2 on a Zn film, which resulted in a highly porous Zn network, as seen in Fig. 14.55 They found that the roughness of the electrodeposited porous Zn network (ED-Zn) was correlated with the specific capacitance and FE. The roughest ED-Zn with a roughness factor of 17.4 had the highest FE of 80% at −1.1 V vs. RHE.
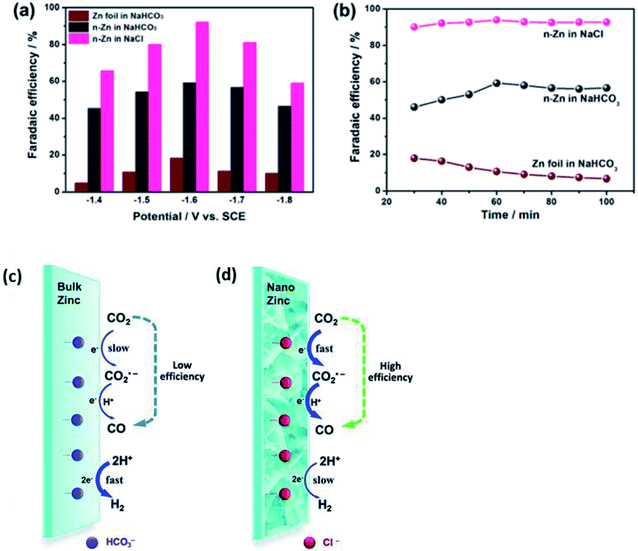 | ||
| Fig. 14 Comparison between zinc (Zn) foil and n-Zn electrodes in CO2 reduction. (a) FECO versus applied potential. (b) FECO versus electrolysis time at −1.6 V. Schematic illustration of CO2 reduction to CO on Zn electrodes in NaHCO3 (c) and NaCl solution (d). Reprinted with permission from ref. 54. Copyright 2015, Royal Society of Chemistry. | ||
A more in-depth study was conducted by Luo et al., where they observed an increased ECR performance for porous Zn.56 In their study, they electrodeposited Zn on Cu mesh to obtain a porous Zn network, as shown in Fig. 15. Their porous Zn (p-Zn) exhibited an excellent FE of 95% at −0.95 vs. RHE. They attributed this superior performance to the local pH effect, where p-Zn had a higher geometric current density compared to Zn foil, which led to a higher proton consumption rate near the electrode, resulting in the suppression of the competing HER. This was supported by their experiment, where they tested p-Zn in buffers with various buffer strengths and found that the FE of p-Zn improved dramatically when the local pH increased. Therefore, they emphasized the importance of both surface area and the impact of local pH to improve the ECR performance of Zn.
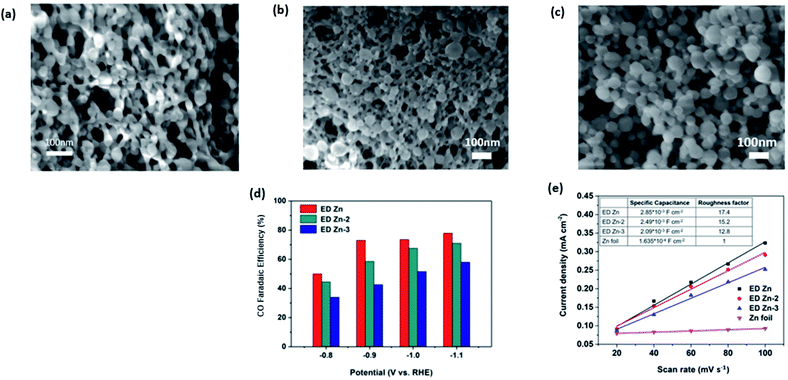 | ||
| Fig. 15 SEM images of (a) ED Zn, (b) ED Zn-2 and (c) ED Zn-3 and (d) FE of CO on ED Zn, ED Zn-2 and ED Zn-3 at various constant potentials ranging from −1.1 to −0.8 V vs. RHE. (e) Specific capacitances and roughness factors of ED Zn, ED Zn-2, ED Zn-3 and Zn foil. Reprinted with permission from ref. 55. Copyright 2018, Elsevier. | ||
In conclusion, Zn is a promising electrocatalyst for the ECR process due to its low cost. Recent advancements have targeted the shape of Zn (nanoplatelets and porous) to improve its performance. However, there is a lack of understanding on the underlying mechanism in the Zn ECR process. There has been some attempt to understand this, with Xiao et al. reporting that the (1 0 0) facet and edge sites are optimal for the ECR to CO but their findings were limited to hexagonal Zn nanoplatelets.57 Therefore, additional studies on more generic Zn NPs are needed before Zn can be applied in the ECR process.
Hu et al. also performed an extensive study on the use of Fe, Co and Ni, which are categorized as transition metals, and found to have great significance to the catalytic activity.74 Not only the catalytic activity was tremendously enhanced, but also the high selectivity for the products was retained. Interestingly, Ni-doped carbon has been established to have an FE as high as 93%. All these metals have a huge correlation with the formation of products that boost the CO2RR activity, while retaining the porosity of carbon electrocatalysts.
In the earlier study conducted by DeMeglio et al., they electrodeposited Bi on a glassy carbon substrate, as seen in Fig. 16.59 They found that the Bi electrocatalyst they synthesized exhibited an FE as high as 95% in 1,3-dialkyl-substituted imidazolium-based ionic liquids (commonly used in carbon sequestration) with almost no activity without the use of the ionic liquids. They theorized that the enhancement was the result of the possible interaction between the Bi0 and Bi3+ sites that stabilizes the key CO2˙− intermediate. However, the role of the ionic liquid is still unclear, where it is suggested that the ionic liquid may act as a mediator or there may be an interaction between the CO2 at the bulk Pt electrodes and imidazolium cations, which can enhance the CO2 electroreduction.
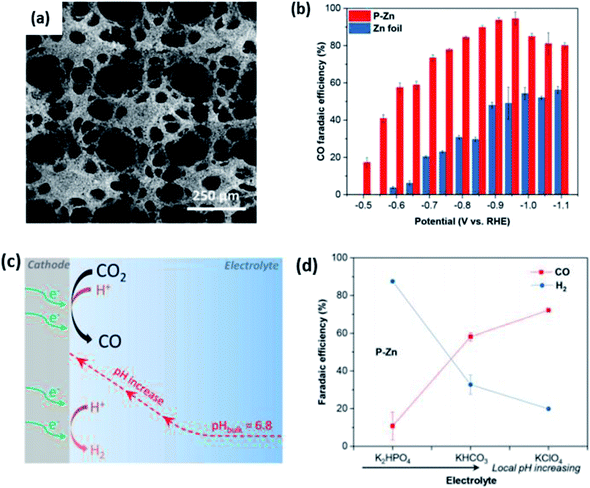 | ||
| Fig. 16 (a) SEM image of porous electrodeposited Zn on Cu mesh (P–Zn). (b) CO FE of P–Zn and Zn foil. (c) Schematic illustration of the local pH effect on the ECR of CO2. (d) FE of P–Zn in different electrolytes. Reprinted with permission from ref. 56. Copyright 2019, American Chemical Society. | ||
In another study conducted by Zhang et al., they reported similar results using Bi NPs in acetonitrile, which was mediated using 1-butyl-3-methylimidazolium trifluoromethanesulfonate ([bmim][OTf]).60 They found that the size of the Bi NPs played a significant role in the ECR of CO2 to CO with a decline in the FE from 87% to 69% as the particle size decreased, as seen in Fig. 17. However, after surface activation via hydrazine treatment (reduction), the particle size had no effect, with both sizes of Bi NPs exhibiting almost similar FE. They speculated that the reason why the size effect was no longer present is due to the direct interaction (proton transfer) between the ionic liquid and the adsorbed CO2, which means that the proton transfer step is no longer affected by the morphology of Bi.
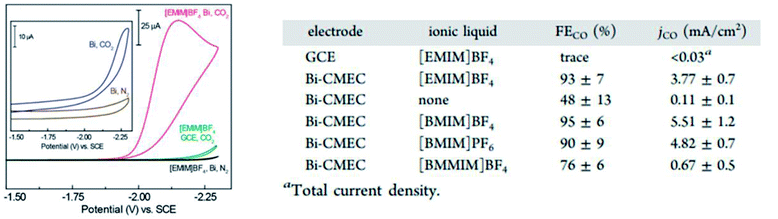 | ||
| Fig. 17 CV traces recorded for Bi-modified and bare GCEs in MeCN containing 20 mM [EMIM]BF4. Inset: Bi-modified GCE in MeCN without ionic liquid (IL). Table summarizing the faradaic efficiencies (FECO) and current densities (jCO) for the ECR of CO2 to CO at an applied potential of −1.95 V vs. SCE in MeCN. Reprinted with permission from ref. 58. Copyright 2013, American Chemical Society. | ||
Briefly, Bi has been considered a viable electrocatalyst for the ECR to CO because it is cheap and environmentally friendly. Research has shown both the high current density and FE with the synthesis process (surface activation) and the type of ionic liquid used are the key factors. However, there is still a lack of understanding on the roles played by ionic liquids in the ECR of CO2 to CO in Bi. This is related to another obvious problem, where Bi is still currently limited to organic solvents and is unstable for use in aqueous solvents. The sizing effect of the Bi NP is more prevalent in organic solvents due to the lack of protons for the reduction of the metal-based oxides. Alternatively, in aqueous solvents, where a higher proton number is present, the sizing effect of the Bi NP is less dominant. Also, this render the aqueous solvents less stable in terms of sizing effect compared to organic solvents, where the sizing effect is more dominant.60 Thus, by discovering the key role that the ionic liquid plays, Bi can be eventually considered as a potential candidate for the ECR process.
3.3 Non-metal-based electrocatalyst
In the case of 0D carbon nanomaterials, graphene quantum dots (GQD) are usually used. GQD are atom-thin (less than 2 nm in thickness) and have minuscule transverse directions (less than 10 nm). In the research conducted by Wu et al., they found that by doping GQD with nitrogen (NGQD), they could improve the FE of the ECR of CO2 to CO from 13% to 43%, as seen in Fig. 18.61 They speculated that this improvement in selectivity was due to the heteroatomic N doping and high exposure of the edge sites of the NGQD but did not present any investigation into this. In a subsequent study by Zou et al., their modelling found that the pyridinic N of NGQD enhanced their bonding with the key COOH* intermediate, which in turn actively promoted the ECR of CO2 to CO, as seen in Fig. 19.62 Therefore, they concluded that heteroatomic N doping can play a key role in the future design and application of 0D carbon nanomaterials.
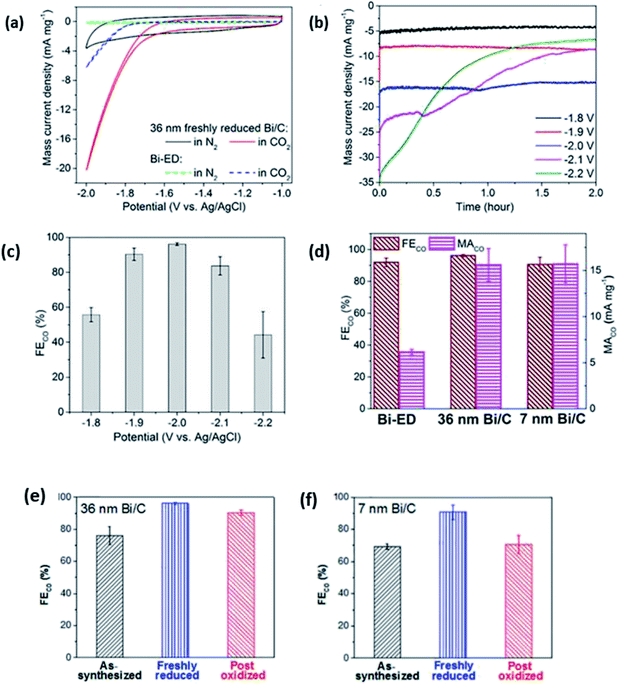 | ||
| Fig. 18 (a) Cyclic voltammograms of 36 nm freshly reduced Bi/C and Bi-ED in MeCN containing 100 mM [bmim][OTf]. (b) Current density transients and (c) FECO under constant potential electrolysis during CO2 reduction at different applied potentials. (d) FECO and MACO on Bi-ED (36 nm and 7 nm freshly reduced Bi/C). FECO of 36 and 7 nm Bi/Cs under different treatment. Reprinted with permission from ref. 59. Copyright 2016, American Chemical Society. | ||
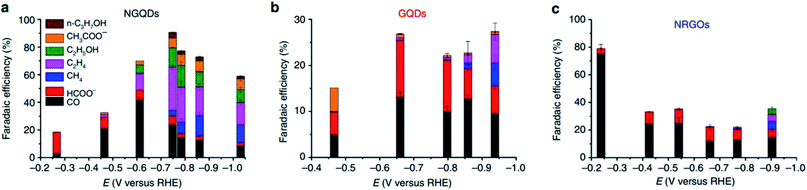 | ||
| Fig. 19 FE of different products of (a) NGQDs, (b) GQDs and (c) NRGOs. Reprinted with permission from ref. 60. Copyright 2016, Nature Research. | ||
In the case of 1D carbon nanomaterials, carbon nanotubes (CNT) are the carbon allotropes that represent this group. In an interesting study by Ma et al., they applied the same principle by heteroatomically doping CNT with nitrogen (NCNT).63 They reported that their NCNT could maintain an FE of CO above 94.5% between −0.6 to −0.9 V vs. RHE over a 40 h long electrolysis process, as seen in Fig. 20. They also found that the pyridinic N was the key factor in their high performance with speculation that there may be weak interaction between CO2 and pyridinic N, which aids in the formation of CO. Lastly, they also reported the use of NCNT for gas-phase CO2 electrolysis, which could actively suppress the competing HER reaction with the FE of CO kept at nearly 100% over a 17 h electrolysis. This shows that gas-phase electrolysis is a potential approach to maximize the efficiency of CO production with easier industrial translation.
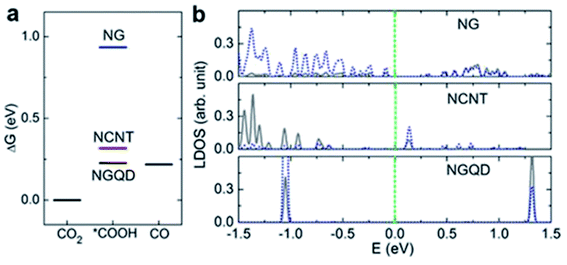 | ||
| Fig. 20 (a) Thermodynamic free energy diagram for the conversion of CO2 to CO on different N-doped carbon materials, with adsorbed *COOH as the intermediate state. (b) LDOS for pyridinic N (blue dotted lines) and C atoms (gray lines) from adsorbed *COOH. These two atoms form N–C bonds in the intermediate states. Reprinted with permission from ref. 61. Copyright 2017, Nature Communications. | ||
Finally, for 2D carbon nanomaterials, graphene is the representative for this group. Han et al. recently performed a study comparing the effects of nitrogen-doped graphene (NG) with other sources of graphene such as regular graphene (G), edge-filled graphene (EG) and defective graphene (DG), as seen in Fig. 21.64 They reported surprising results, where DG had the best performance among the different types of graphene. DG had the highest FE of CO at 84% over a 10 h period. They theorized that this superior result was due to the synthesis process, where the DG was synthesized by the removal of nitrogen in NG. The defects caused by nitrogen doping and subsequent removal of the doped nitrogen were special and resulted in unique defect sites, which were not replicated in EG (plasma process) (Fig. 22).
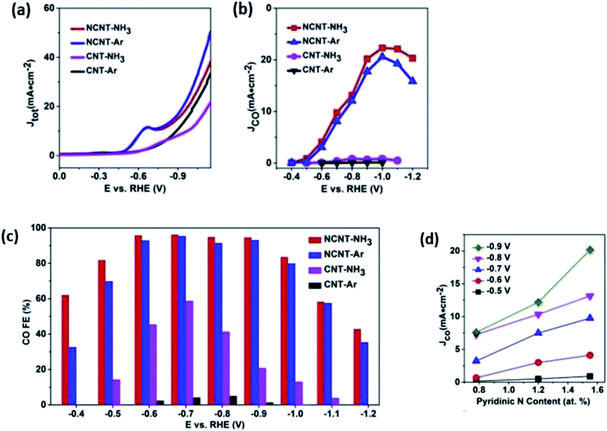 | ||
| Fig. 21 (a) Linear sweep voltammetry (LSV) curves for catalysts in CO2-saturated 0.5 M NaHCO3 aqueous solution at 50 mV s−1. (b) Partial current density of CO vs. applied potential for the catalysts. (c) CO FEs at various applied potentials. (d) CO current density at different applied potentials vs. the content of pyridinic N (at%) Reprinted with permission from ref. 62. Copyright 2019 Elsevier. | ||
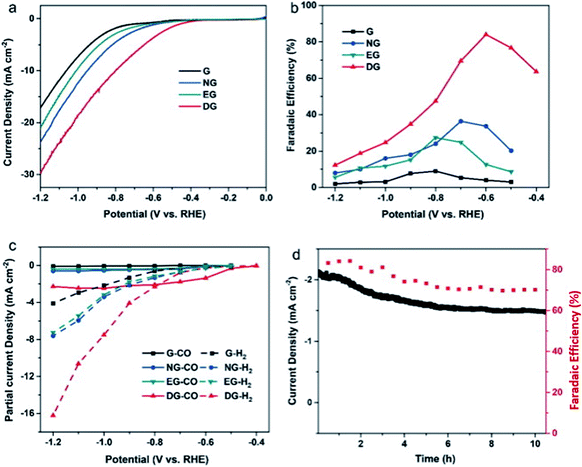 | ||
| Fig. 22 (a) Linear sweep voltammetry (LSV) curves for pristine graphene, nitrogen-doped graphene (NG), edge-filled graphene (EG) and distorted graphene (DG) in CO2-saturated 0.1 M KHCO3 electrolyte. The scan rate was 10 mV s−1. (b) Faradaic efficiencies to CO at different applied potentials on graphene, NG, EG and DG, respectively. (c) Partial current densities of CO and H2 generated on graphene, NG, EG and DG. (d) Time-dependent total current density curve (left y-axis) and faradaic efficiency (right y-axis) for CO of DG in CO2-saturated 0.1 M KHCO3 solution at an applied potential of 0.6 V vs. RHE for >10 h. Reprinted with permission from ref. 63. Copyright 2019, Elsevier. | ||
In summary, carbon nanomaterials are an inexpensive alternative compared to metal-based electrocatalysts for the ECR of CO2 to CO. Unfortunately, carbon nanomaterials are generally inert and need to be activated before they can be utilized as an electrocatalyst. The literature shows the current trend for this activation across all varieties of carbon nanomaterials by the nitrogen doping. However, this gives rise to another issue where the activation process is achieved via hydrothermal treatment (typically), which increases the production cost. Besides, the performance of carbon electrocatalysts is subpar in terms of both catalytic performance and FE compared to their metal counterparts. The one redeeming factor that carbon electrocatalysts have is their enhanced durability (albeit overall lower), which attributed to their inert nature. Therefore, for carbon nanomaterials to be used effectively over metal-based electrocatalysts, extensive studies are still required to improve both their catalytic performance and FE.
4. Conclusion and future prospects
In this review, we summarized the latest developments in nano-based electrocatalysts for the ECR of CO2 to CO. Noble metals, non-noble metals and carbon-based electrocatalysts were discussed and their advantages and problems highlighted.In the case of noble metals, Au, Ag and Pd were the focus in this review. Au is theoretically the best electrocatalyst for the ECR of CO2 to CO because it has optimal binding energy for the key intermediate of COOH. This has translated to it having the highest catalytic activity and FE among the catalysts. However, Au suffers from poor mass activity due to the competitive HER during the optimization, which hampers its performance. Therefore, new ways to optimize the mass activity of Au are needed. Alternatively, Ag is theoretically the second best electrocatalyst. Its overall performance is inverse to that of Au. Researchers have shown the higher mass activity of Ag due to the lower HER as its size is reduced. Unfortunately, Ag has a lower current density, and in some cases lower FE compared to Au. Next, Pd has been the focus of recent research interest due to its high selectivity for the products. Regrettably, the exact mechanism behind the high performance of Pd is still under debate, with studies claiming the PdH core with pure Pd on its surface is key, while others have contested these claims with their own results, showing that the use of a pure Pd core with Pd–N4 active sites is better. Therefore, further studies on Pd are still needed before it can be actively used as an electrocatalyst. Overall, noble metal electrocatalysts have the greatest potential of being adapted and used for industrial applications.
For non-noble metals, Zn and Bi have been evaluated as the top choices in this field. In particular, Zn is cheap and has shown great potential as a suitable replacement for noble metals. Although new developments have shown that the performance of Zn is almost comparable with that of Ag, it still has a debilitating problem where its performance is heavily influenced by the local pH effect. This severely affects the potential application of Zn given that the local pH effect is difficult to control on a macro-scale. Therefore, new or improved optimization is needed for Zn to be considered as a suitable electrocatalyst. Regarding Bi, it has been shown as cheap and environmentally friendly alternative. Bi has also shown the highest FE among the various catalysts. However, these results are only limited to ionic liquids and when it is exposed to aqueous solvents, unwanted side reactions occur. Thus, studies are required to establish the understanding on the usability of enhanced Bi in aqueous solvents before it can be used effectively.
Lastly, for carbon-based electrocatalysts, they are the cheapest electrocatalysts compared to the rest. New research has shown both in terms of theoretical modelling and experiments that nitrogen doping is the most effective method for improving the otherwise inert but cheap carbon electrocatalysts. This was found to be true for 0D, 1D and 2D carbon catalysts. However, their performance in terms of current density and FE is still inadequate compared to traditional metal catalysts. Nevertheless, one key advantage that carbon-based electrocatalyst have is their durability and resistance to poisoning over time, which are superior to that of their metal counterparts.
Here, we highlight future prospects that researchers can explore.
4.1 Amorphous materials
Recent developments in other catalysis fields have shown the potential of amorphous catalysts. Amorphous structures provide abundant highly active sites, which may be useful for the ECR process and should be explored in depth.4.2 Bi-metallic/tri-metallic
Researchers have shown promising results by combining different metals with greater advantages, overcoming the shortcomings of the individual catalysts. Some of these studies have been done on the ECR process but are limited to high-order products such as CH3OH. Therefore, it will be interesting to see the effect it has on the ECR of CO2 to CO.4.3 Doping of metal electrocatalysts
In this review, we highlighted the benefits of doping in improving the performance of carbon-based electrocatalysts. Doping is also commonly used in other catalyst fields in improving the performance of materials. Interestingly, this is not widely explored in terms of the ECR process. This can be due to the high production cost associated with the doping process, which remains a constraint.4.4 Catalyst stability
The particle size of electrocatalysts has a direct influence on their edge to face ratio. Decreasing the particle size results in an increase in active catalytic sites, which contributes to good COOH stability and translates to high durability of high energy efficiency.Finally, this review presented the electrocatalysts that have the greatest potential in improving if not eliminating the CO2 problem plaguing the world today. Despite the numerous challenges, it is believed that focus on the development of these electrocatalysts is crucial for their implementation as a practical solution to reduce CO2 in the near future.
Funding sources
This work is supported by the Singapore Ministry of Education [MOE2017-T2-2-067].Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
The authors declare no competing financial interest.References
- M. S. Dresselhaus and I. L. Thomas, Alternative energy technologies, Nature, 2001, 414(6861), 332–337 CrossRef CAS PubMed.
- S. Chu and A. Majumdar, Opportunities and challenges for a sustainable energy future, Nature, 2012, 488(7411), 294–303 CrossRef CAS PubMed.
- D. A. Lashof and D. R. Ahuja, Relative contributions of greenhouse gas emissions to global warming, Nature, 1990, 344(6266), 529–531 CrossRef CAS.
- S. Dupont, O. Ortega-Martínez and M. Thorndyke, Impact of near-future ocean acidification on echinoderms, Ecotoxicology, 2010, 19(3), 449–462 CrossRef CAS PubMed.
- H. N. Le Houérou, Climate change, drought and desertification, J. Arid Environ., 1996, 34(2), 133–185 CrossRef.
- E. Ottmar, P.-M. Ramón, S. Youba, C. M. Jan, F. Ellie, S. Kadner, S. Kristin, A. Anna, B. Ina, B. Steffen, E. Patrick, K. Benjamin, S. Jussi, S. Steffen, v. S. Christoph and Z. Timm, Climate Change 2014 Mitigation of Climate Change, IPCC, 2014 Search PubMed.
- L. Cameron and B. van der Zwaan, Employment factors for wind and solar energy technologies: A literature review, Renewable Sustainable Energy Rev., 2015, 45, 160–172 CrossRef.
- L. Dong, H. Liu and S. Riffat, Development of small-scale and micro-scale biomass-fuelled CHP systems – A literature review, Appl. Therm. Eng., 2009, 29(11), 2119–2126 CrossRef CAS.
- E. Barbier, Geothermal energy technology and current status: an overview, Renewable Sustainable Energy Rev., 2002, 6(1), 3–65 CrossRef.
- S. Rehman, L. M. Al-Hadhrami and M. M. Alam, Pumped hydro energy storage system: A technological review, Renewable Sustainable Energy Rev., 2015, 44, 586–598 CrossRef.
- J. Y. Zhu and X. S. Zhuang, Conceptual net energy output for biofuel production from lignocellulosic biomass through biorefining, Prog. Energy Combust. Sci., 2012, 38(4), 583–598 CrossRef CAS.
- B. Looney and S. Dale, BP Statistical Review of World Energy 2020, 2020 Search PubMed.
- J. P. Painuly, Barriers to renewable energy penetration; a framework for analysis, Renewable Energy, 2001, 24(1), 73–89 CrossRef.
- A. Verbruggen, M. Fischedick, W. Moomaw, T. Weir, A. Nadaï, L. J. Nilsson, J. Nyboer and J. Sathaye, Renewable energy costs, potentials, barriers: Conceptual issues, Energy Policy, 2010, 38(2), 850–861 CrossRef.
- A. Castillo and D. F. Gayme, Grid-scale energy storage applications in renewable energy integration: A survey, Energy Convers. Manage., 2014, 87, 885–894 CrossRef.
- P. Harsha and M. Dahleh, Optimal Management and Sizing of Energy Storage Under Dynamic Pricing for the Efficient Integration of Renewable Energy, IEEE Trans. Power Syst., 2015, 30(3), 1164–1181 Search PubMed.
- S. Ould Amrouche, D. Rekioua, T. Rekioua and S. Bacha, Overview of energy storage in renewable energy systems, Int. J. Hydrogen Energy, 2016, 41(45), 20914–20927 CrossRef CAS.
- A. Al-Mamoori, A. Krishnamurthy, A. A. Rownaghi and F. Rezaei, Carbon Capture and Utilization Update, Energy Technol., 2017, 5(6), 834–849 CrossRef.
- F. M. Baena-Moreno, M. Rodríguez-Galán, F. Vega, B. Alonso-Fariñas, L. F. Vilches Arenas and B. Navarrete, Carbon capture and utilization technologies: a literature review and recent advances, Energy Sources, Part A, 2019, 41(12), 1403–1433 CrossRef CAS.
- A. Kätelhön, R. Meys, S. Deutz, S. Suh and A. Bardow, Climate change mitigation potential of carbon capture and utilization in the chemical industry, Proc. Natl. Acad. Sci. U. S. A., 2019, 116(23), 11187–11194 CrossRef.
- P. C. Psarras, S. Comello, P. Bains, P. Charoensawadpong, S. Reichelstein and J. Wilcox, Carbon Capture and Utilization in the Industrial Sector, Environ. Sci. Technol., 2017, 51(19), 11440–11449 CrossRef CAS PubMed.
- C. A. Huff and M. S. Sanford, Cascade Catalysis for the Homogeneous Hydrogenation of CO2 to Methanol, J. Am. Chem. Soc., 2011, 133(45), 18122–18125 CrossRef CAS PubMed.
- J. A. Rodriguez, P. Liu, D. J. Stacchiola, S. D. Senanayake, M. G. White and J. G. Chen, Hydrogenation of CO2 to Methanol: Importance of Metal–Oxide and Metal–Carbide Interfaces in the Activation of CO2, ACS Catal., 2015, 5(11), 6696–6706 CrossRef CAS.
- D. S. Marlin, E. Sarron and Ó. Sigurbjörnsson, Process Advantages of Direct CO2 to Methanol Synthesis, Front. Chem., 2018, 6, 446 CrossRef CAS PubMed.
- F. Pontzen, W. Liebner, V. Gronemann, M. Rothaemel and B. Ahlers, CO2-based methanol and DME – Efficient technologies for industrial scale production, Catal. Today, 2011, 171(1), 242–250 CrossRef CAS.
- A. González-Garay, M. S. Frei, A. Al-Qahtani, C. Mondelli, G. Guillén-Gosálbez and J. Pérez-Ramírez, Plant-to-planet analysis of CO2-based methanol processes, Energy Environ. Sci., 2019, 12(12), 3425–3436 RSC.
- R. Reske, H. Mistry, F. Behafarid, B. Roldan Cuenya and P. Strasser, Particle Size Effects in the Catalytic Electroreduction of CO2 on Cu Nanoparticles, J. Am. Chem. Soc., 2014, 136(19), 6978–6986 CrossRef CAS PubMed.
- H. Mistry, R. Reske, Z. Zeng, Z.-J. Zhao, J. Greeley, P. Strasser and B. R. Cuenya, Exceptional Size-Dependent Activity Enhancement in the Electroreduction of CO2 over Au Nanoparticles, J. Am. Chem. Soc., 2014, 136(47), 16473–16476 CrossRef CAS PubMed.
- X. Wang, Z. Chen, X. Zhao, T. Yao, W. Chen, R. You, C. Zhao, G. Wu, J. Wang, W. Huang, J. Yang, X. Hong, S. Wei, Y. Wu and Y. Li, Regulation of Coordination Number over Single Co Sites: Triggering the Efficient Electroreduction of CO2, Angew. Chem., Int. Ed., 2018, 57(7), 1944–1948 CrossRef CAS PubMed.
- Y. Song, W. Chen, C. Zhao, S. Li, W. Wei and Y. Sun, Metal-Free Nitrogen-Doped Mesoporous Carbon for Electroreduction of CO2 to Ethanol, Angew. Chem., Int. Ed., 2017, 56(36), 10840–10844 CrossRef CAS.
- D. D. Zhu, J. L. Liu and S. Z. Qiao, Recent Advances in Inorganic Heterogeneous Electrocatalysts for Reduction of Carbon Dioxide, Adv. Mater., 2016, 28(18), 3423–3452 CrossRef CAS PubMed.
- M. Jouny, W. Luc and F. Jiao, General Techno-Economic Analysis of CO2 Electrolysis Systems, Ind. Eng. Chem. Res., 2018, 57(6), 2165–2177 CrossRef CAS.
- O. G. Sánchez, Y. Y. Birdja, M. Bulut, J. Vaes, T. Breugelmans and D. Pant, Recent advances in industrial CO2 electroreduction, Curr. Opin. Green Sustainable Chem., 2019, 16, 47–56 CrossRef.
- A. S. Agarwal, Y. Zhai, D. Hill and N. Sridhar, The Electrochemical Reduction of Carbon Dioxide to Formate/Formic Acid: Engineering and Economic Feasibility, ChemSusChem, 2011, 4(9), 1301–1310 CrossRef CAS PubMed.
- H. Li and C. Oloman, The Electro-Reduction of Carbon Dioxide in a Continuous Reactor, J. Appl. Electrochem., 2005, 35(10), 955–965 CrossRef CAS.
- B. Endrődi, G. Bencsik, F. Darvas, R. Jones, K. Rajeshwar and C. Janáky, Continuous-flow electroreduction of carbon dioxide, Prog. Energy Combust. Sci., 2017, 62, 133–154 CrossRef.
- E. E. Benson, C. P. Kubiak, A. J. Sathrum and J. M. Smieja, Electrocatalytic and homogeneous approaches to conversion of CO2 to liquid fuels, Chem. Soc. Rev., 2009, 38(1), 89–99 RSC.
- Y. B. Vassiliev, V. S. Bagotsky, N. V. Osetrova, O. A. Khazova and N. A. Mayorova, Electroreduction of carbon dioxide: Part I. The mechanism and kinetics of electroreduction of CO2 in aqueous solutions on metals with high and moderate hydrogen overvoltages, J. Electroanal. Chem. Interfacial Electrochem., 1985, 189(2), 271–294 CrossRef.
- X. Nie, M. R. Esopi, M. J. Janik and A. Asthagiri, Selectivity of CO2 Reduction on Copper Electrodes: The Role of the Kinetics of Elementary Steps, Angew. Chem., Int. Ed., 2013, 52(9), 2459–2462 CrossRef CAS PubMed.
- R. M. Arán-Ais, D. Gao and B. Roldan Cuenya, Structure- and Electrolyte-Sensitivity in CO2 Electroreduction, Acc. Chem. Res., 2018, 51(11), 2906–2917 CrossRef.
- E. R. Cave, C. Shi, K. P. Kuhl, T. Hatsukade, D. N. Abram, C. Hahn, K. Chan and T. F. Jaramillo, Trends in the Catalytic Activity of Hydrogen Evolution during CO2 Electroreduction on Transition Metals, ACS Catal., 2018, 8(4), 3035–3040 CrossRef CAS.
- A. Dutta, M. Rahaman, N. C. Luedi, M. Mohos and P. Broekmann, Morphology Matters: Tuning the Product Distribution of CO2 Electroreduction on Oxide-Derived Cu Foam Catalysts, ACS Catal., 2016, 6(6), 3804–3814 CrossRef CAS.
- J. T. Feaster, C. Shi, E. R. Cave, T. Hatsukade, D. N. Abram, K. P. Kuhl, C. Hahn, J. K. Nørskov and T. F. Jaramillo, Understanding Selectivity for the Electrochemical Reduction of Carbon Dioxide to Formic Acid and Carbon Monoxide on Metal Electrodes, ACS Catal., 2017, 7(7), 4822–4827 CrossRef CAS.
- W. Zhu, R. Michalsky, Ö. Metin, H. Lv, S. Guo, C. J. Wright, X. Sun, A. A. Peterson and S. Sun, Monodisperse Au Nanoparticles for Selective Electrocatalytic Reduction of CO2 to CO, J. Am. Chem. Soc., 2013, 135(45), 16833–16836 CrossRef CAS PubMed.
- S. Back, M. S. Yeom and Y. Jung, Active Sites of Au and Ag Nanoparticle Catalysts for CO2 Electroreduction to CO, ACS Catal., 2015, 5(9), 5089–5096 CrossRef CAS.
- D.-R. Yang, L. Liu, Q. Zhang, Y. Shi, Y. Zhou, C. Liu, F.-B. Wang and X.-H. Xia, Importance of Au nanostructures in CO2 electrochemical reduction reaction, Sci. Bull., 2020, 65(10), 796–802 CrossRef CAS.
- A. Salehi-Khojin, H.-R. M. Jhong, B. A. Rosen, W. Zhu, S. Ma, P. J. A. Kenis and R. I. Masel, Nanoparticle Silver Catalysts That Show Enhanced Activity for Carbon Dioxide Electrolysis, J. Phys. Chem. C, 2013, 117(4), 1627–1632 CrossRef CAS.
- C. Kim, H. S. Jeon, T. Eom, M. S. Jee, H. Kim, C. M. Friend, B. K. Min and Y. J. Hwang, Achieving Selective and Efficient Electrocatalytic Activity for CO2 Reduction Using Immobilized Silver Nanoparticles, J. Am. Chem. Soc., 2015, 137(43), 13844–13850 CrossRef CAS.
- Y.-C. Hsieh, S. D. Senanayake, Y. Zhang, W. Xu and D. E. Polyansky, Effect of Chloride Anions on the Synthesis and Enhanced Catalytic Activity of Silver Nanocoral Electrodes for CO2 Electroreduction, ACS Catal., 2015, 5(9), 5349–5356 CrossRef CAS.
- S. Liu, C. Sun, J. Xiao and J.-L. Luo, Unraveling Structure Sensitivity in CO2 Electroreduction to Near-Unity CO on Silver Nanocubes, ACS Catal., 2020, 10(5), 3158–3163 CrossRef CAS.
- D. Gao, H. Zhou, F. Cai, D. Wang, Y. Hu, B. Jiang, W.-B. Cai, X. Chen, R. Si, F. Yang, S. Miao, J. Wang, G. Wang and X. Bao, Switchable CO2 electroreduction via engineering active phases of Pd nanoparticles, Nano Res., 2017, 10(6), 2181–2191 CrossRef CAS.
- Q. He, J. H. Lee, D. Liu, Y. Liu, Z. Lin, Z. Xie, S. Hwang, S. Kattel, L. Song and J. G. Chen, Accelerating CO2 Electroreduction to CO Over Pd Single-Atom Catalyst, Adv. Funct. Mater., 2020, 30(17), 2000407 CrossRef CAS.
- H. Dong, L. Zhang, P. Yang, X. Chang, W. Zhu, X. Ren, Z.-J. Zhao and J. Gong, Facet design promotes electroreduction of carbon dioxide to carbon monoxide on palladium nanocrystals, Chem. Eng. Sci., 2019, 194, 29–35 CrossRef CAS.
- F. Quan, D. Zhong, H. Song, F. Jia and L. Zhang, A highly efficient zinc catalyst for selective electroreduction of carbon dioxide in aqueous NaCl solution, J. Mater. Chem. A, 2015, 3(32), 16409–16413 RSC.
- Y. Lu, B. Han, C. Tian, J. Wu, D. Geng and D. Wang, Efficient electrocatalytic reduction of CO2 to CO on an electrodeposited Zn porous network, Electrochem. Commun., 2018, 97, 87–90 CrossRef CAS.
- W. Luo, J. Zhang, M. Li and A. Züttel, Boosting CO Production in Electrocatalytic CO2 Reduction on Highly Porous Zn Catalysts, ACS Catal., 2019, 9(5), 3783–3791 CrossRef CAS.
- J. Xiao, M.-R. Gao, S. Liu and J.-L. Luo, Hexagonal Zn Nanoplates Enclosed by Zn(100) and Zn(002) Facets for Highly Selective CO2 Electroreduction to CO, ACS Appl. Mater. Interfaces, 2020, 12(28), 31431–31438 CrossRef CAS.
- L. Peng, L. Shang, T. Zhang and G. Waterhouse, N Recent Advances in the Development of Single-Atom Catalysts for Oxygen Electrocatalysis and Zinc–Air Batteries, Adv. Energy Mater., 2020, 10, 2003018 CrossRef CAS.
- J. L. DiMeglio and J. Rosenthal, Selective Conversion of CO2 to CO with High Efficiency Using an Inexpensive Bismuth-Based Electrocatalyst, J. Am. Chem. Soc., 2013, 135(24), 8798–8801 CrossRef CAS PubMed.
- Z. Zhang, M. Chi, G. M. Veith, P. Zhang, D. A. Lutterman, J. Rosenthal, S. H. Overbury, S. Dai and H. Zhu, Rational Design of Bi Nanoparticles for Efficient Electrochemical CO2 Reduction: The Elucidation of Size and Surface Condition Effects, ACS Catal., 2016, 6(9), 6255–6264 CrossRef CAS.
- J. Wu, S. Ma, J. Sun, J. I. Gold, C. Tiwary, B. Kim, L. Zhu, N. Chopra, I. N. Odeh, R. Vajtai, A. Z. Yu, R. Luo, J. Lou, G. Ding, P. J. A. Kenis and P. M. Ajayan, A metal-free electrocatalyst for carbon dioxide reduction to multi-carbon hydrocarbons and oxygenates, Nat. Commun., 2016, 7(1), 13869 CrossRef CAS.
- X. Zou, M. Liu, J. Wu, P. M. Ajayan, J. Li, B. Liu and B. I. Yakobson, How Nitrogen-Doped Graphene Quantum Dots Catalyze Electroreduction of CO2 to Hydrocarbons and Oxygenates, ACS Catal., 2017, 7(9), 6245–6250 CrossRef CAS.
- C. Ma, P. Hou, X. Wang, Z. Wang, W. Li and P. Kang, Carbon nanotubes with rich pyridinic nitrogen for gas phase CO2 electroreduction, Appl. Catal., B, 2019, 250, 347–354 CrossRef CAS.
- P. Han, X. Yu, D. Yuan, M. Kuang, Y. Wang, A. M. Al-Enizi and G. Zheng, Defective graphene for electrocatalytic CO2 reduction, J. Colloid Interface Sci., 2019, 534, 332–337 CrossRef CAS PubMed.
- F. Y. Gao, Z. Z. Wu and M. R. Gao, Electrochemical CO2 Reduction on Transition-Metal Chalcogenide Catalysts: Recent Advances and Future Perspectives, Energy Fuels, 2021, 35, 12869–12883 CrossRef CAS.
- T. Zheng, K. Jiang and H. Wang, Recent Advances in Electrochemical CO2-to-CO Conversion on Heterogeneous Catalysts, Adv. Mater., 2018, 30, 1802066 CrossRef PubMed.
- F. Y. Gao, R. C. Bao, M. R. Gao and S. H. Yu, Electrochemical CO2-to-CO conversion: electrocatalysts, electrolytes, and electrolyzers, J. Mater. Chem. A, 2020, 8, 15458–15478 RSC.
- M. G. Kibria, J. P. Edwards, C. M. Gabardo, C. T. Dinh, A. Seifitokaldani, D. Sinton and E. H. Sargent, Electrochemical CO2 Reduction into Chemical Feedstocks: From Mechanistic Electrocatalysis Models to System Design, Adv. Mater., 2019, 31, 1807166 CrossRef.
- I. Ghiat and T. Al-Ansari, A review of carbon capture and utilisation as a CO2 abatement opportunity within the EWF nexus, J. CO2 Util., 2021, 45, 101432 CrossRef CAS.
- S. Sun, H. Sun, P. T. Williams and C. Wu, Recent advances in integrated CO2 capture and utilization: a review, Sustainable Energy Fuels, 2021, 5, 4546–4559 RSC.
- A. Morales-García, F. Viñes, J. R. B. Gomes and F. Illas, Concepts, models, and methods in computational heterogeneous catalysis illustrated through CO2 conversion, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2021, 11, e1530 Search PubMed.
- A. Saravanan, P. S. Kumar, D. V. N. Vo, S. Jeevanantham, B. Bhuvaneswari, V. A. Narayanan, P. R. Yaashikaa, S. Swetha and B. Reshma, A comprehensive review on different approaches for CO2 utilization and conversion pathways, Chem. Eng. Sci., 2021, 236, 116515 CrossRef CAS.
- S. Nitopi, E. Bertheussen, S. B. Scott, X. Liu, A. K. Engstfeld, S. Horch, B. Seger, I. E. L. Stephens, K. Chan, C. Hahn, J. K. Nørskov, T. F. Jaramillo and I. Chorkendorff, Progress and Perspectives of Electrochemical CO2 Reduction on Copper in Aqueous Electrolyte, Chem. Rev., 2019, 119, 7610–7672 CrossRef CAS PubMed.
- X. M. Hu, H. H. Hval, E. T. Bjerglund, K. J. Dalgaard, M. R. Madsen, M. M. Pohl, E. Welter, P. Lamagni, K. B. Buhl, M. Bremholm, M. Beller, S. U. Pedersen, T. Skrydstrup and K. Daasbjerg, Selective CO2 Reduction to CO in Water using Earth-Abundant Metal and Nitrogen-Doped Carbon Electrocatalysts, ACS Catal., 2018, 8, 6255–6264 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |



