Open Access Article
Open Access ArticleIodine-catalyzed synthesis of benzoxazoles using catechols, ammonium acetate, and alkenes/alkynes/ketones via C–C and C–O bond cleavage†
Jasem Aboonajmia,
Farhad Panahi *a,
Mina Aali Hosseinia,
Mahdi Aberib and
Hashem Sharghi
*a,
Mina Aali Hosseinia,
Mahdi Aberib and
Hashem Sharghi *a
*a
aDepartment of Chemistry, College of Sciences, Shiraz University, Shiraz 71454, Iran. E-mail: Panahi@shirazu.ac.ir; shashem@chem.susc.ac.ir; hashemsharghi@gmail.com; Fax: +98 7132280926; Tel: +98 7136137136
bDepartment of Chemical and Materials Engineering, Faculty of Shahid Rajaee, Technical and Vocational University (TVU), Shiraz Branch, Shiraz, Iran
First published on 21st July 2022
Abstract
An efficient metal-free synthesis strategy of benzoxazoles was developed via coupling catechols, ammonium acetate, and alkenes/alkynes/ketones. The developed methodology represents an operationally simple, one-pot and large-scale procedure for the preparation of benzoxazole derivatives using molecular iodine as the catalyst.
Introduction
The development of metal-free methods for the efficient synthesis of valuable organic compounds is an exciting area of research, due to their capability to produce non-toxic metal-free drugs and medicinally important compounds.1–4 In this regard, molecular iodine was used as an efficient catalyst in the synthesis of many heterocyclic compounds via multi-component reactions.5–8 Benzoxazoles are one of the important classes of nitrogen-containing heterocycles with extensive utilization especially in medicinal chemistry.9,10 Moreover, the benzoxazole heterocycle is one of the widely found structures in nature, and a range of related natural products are known as potent pharmacophores (Fig. 1).9,11–15 | ||
| Fig. 1 Chemical structure of some naturally occurring and biologically important benzoxazole-based compounds. | ||
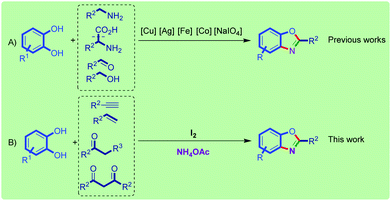
Considering their utmost importance, various synthetic strategies have been developed to synthesize benzoxazoles.16–25 However, extensive efforts have been devoted toward the progress of clean and efficient preparation of benzoxazole derivatives.26–28 Metal-free syntheses of benzoxazoles have recently received attention due to the increasing demands for their metal-contamination-free synthesis. However, the synthesis of benzoxazole derivatives via oxidation of catechols has gained intense interest. For all of the reported methods for the synthesis of benzoxazoles using catechols, a transition metal-catalyzed process was applied. Coupling reaction of catechols with amines using copper29,30/iron31/Co,32 with amino acids using silver33/iron,31 with aldehydes and ammonium acetate using iron34/NaIO435 and with benzyl alcohol using iron36 are the available methods for the metal-catalyzed synthesis of benzoxazoles using catechols (Scheme 1A).
In continuation of our program on the synthesis of benzoxazole derivatives,31,32,34,36 we would like to introduce a metal-free one-pot multi-component method for the efficient synthesis of 2-aryl benzoxazoles.
Results and discussion
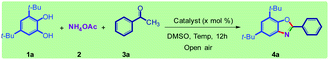
The study was started by the reaction of catechol (1a), ammonium acetate (2), and acetophenone (3a) in the absence of catalyst in a dimethyl sulfoxide (DMSO) solvent at 140 °C; however, no benzoxazole product (4a) was observed (Table 1, entry 1). In another experiment, under solvent-free conditions, iodine (I2, 10 mol%) was used as the catalyst, and no product was observed again (Table 1, entry 2). To our delight, 75% of desired product 4a was produced when DMSO was used as the solvent besides the use of 10 mol% of I2 as the catalyst (Table 1, entry 3). Considering the important role of solvent here, other solvents were also tested (Table S1†). Except in DMF (about 30%), no product was observed in other solvents. The high yield in DMSO confirmed the I2–DMSO hybrid catalysis role, which is known.7,8| Entry | Catalyst (x mol%) | Temp. (°C) | Yield 4a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (%) b (%) |
|---|---|---|---|
| a Reaction condition: 1a (1.0 mmol), 2 (1.2 mmol), 3a (1.0 mmol), DMSO (5.0 mL), open air.b Isolated yield.c No solvent was used.d N2. | |||
| 1 | None | 140 | 0 |
| 2 | I2 (10) | 140 | 0c |
| 3 | I2 (10) | 140 | 75 |
| 4 | I2 (10) | 150 | 92 |
| 5 | I2 (10) | 100 | Trace |
| 6 | I2 (5) | 150 | 61 |
| 7 | I2 (15) | 150 | 87 |
| 8 | I2 (10) | 150 | 81d |
By selecting DMSO as the solvent, different sources of iodine were checked, and among them, I2 showed high activity (Table S2†). It was found that an elevated temperature of 150 °C furnished the product in a high yield of 92% (Table 1, entry 4).
At lower temperatures, the yield decreased dramatically. For example, at 100 °C, a trace amount of product was detected (Table 1, entry 5). The catalytic loading evaluation revealed that 10 mol% of I2 is suitable to obtain high yields of product (Table 1, entries 6 and 7). Moreover, the reaction was investigated under N2 conditions and 81% of product was obtained (Table 1, entry 8). Among different nitrogen sources tested, ammonium acetate was recognized as the best one (Table S3†).
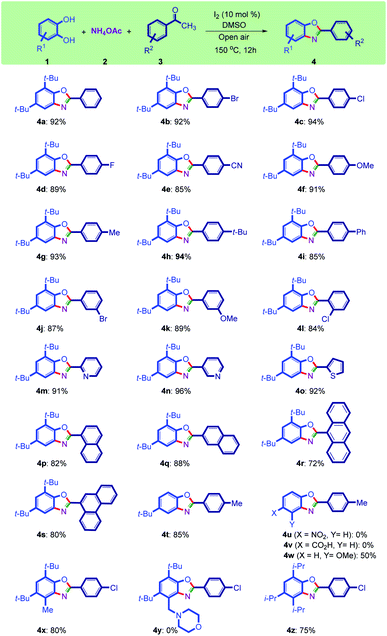
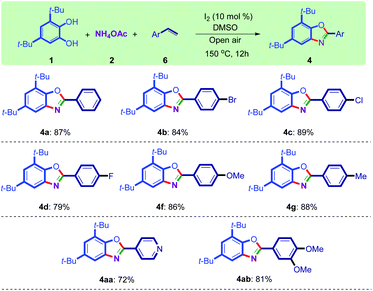
With the optimized reaction conditions in hand (Table 1, entry 4, bolded), the benzoxazole synthesis was then conducted using different ketones (Scheme 2). To illustrate the substrate scope and show the generality of the new method, differently substituted ketones were transformed to their benzoxazoles fruitfully (Scheme 2). Halogen-substituted acetophenone afforded the corresponding benzoxazoles (4b, c, d, j, l) in high yields. The synthesis of halogen-substituted benzoxazoles is important because they are attachable to organic backbones using coupling reactions such as Heck, Suzuki, and Sonogashira.37,38
Other acetophenones bearing functional groups such as cyano (4e), alkoxy (4f, k), aryl (4i), and alkyl (4g, h, t) moieties are working well under optimized conditions. The substituents in ortho and meta positions also tolerated the reaction conditions (4j–l).
As sterically hindered substrates, 2-chloroacetophenone and 1-acetonaphthone gave 4l and 4p in 84% and 82% yields, respectively. It seems that both electron-poor and electron-rich acetophenones worked well with this method. Heterocyclic substrates including pyridine and thiophene can be used in this protocol to synthesize bis(heterocycles) 4m–o as useful ligands and/or biologically important molecules39–41 in more than 90% yields. Polyaromatic substrates also worked well with this methodology, and benzoxazoles containing naphthalene (4p, q), anthracene (4r), and phenanthrene (4s) were synthesized successfully in high yields.
Other commercially available catechols including tert-butyl (4t), nitro (4u), carboxyl (4v), and methoxy (4w) groups were used in this reaction. However, only the electron-rich catechol was productively converted into benzoxazole derivatives by this procedure. The formation of compounds 4x and 4z represent the high efficiency of this method in the synthesis of poly-substituted benzoxazoles. Synthesis of compound 4y was not successful using its corresponding catechol containing a morpholine group.42
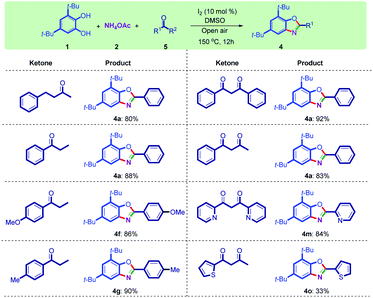
This method was also checked with other ketones, and the results indicated that the method is also working with other 1,3-dicarbonyl compounds and aryl–alkyl ketones (Scheme 3).
Due to the course of our research on the development of this method for benzoxazole synthesis, it was found that styrenes can be used instead of ketones (Scheme 4).
 | ||
| Scheme 4 Synthesis of benzoxazoles from styrenes. Reaction conditions: 1 (1.0 mmol), 2 (1.2 mmol), 6 (1.0 mmol), I2 (10 mol%), DMSO (5.0 mL), open air. All yields are isolated. | ||
The reaction of styrenes, catechols, and ammonium acetate was carried out in the presence of I2 as the catalyst in DMSO at 150 °C to furnish benzoxazoles in good to excellent yields (Scheme 4). The results indicated that styrenes with electron-donating and electron-withdrawing groups are working with this procedure. Next, the applicability of this method was evaluated in the synthesis of benzoxazoles using phenylacetylenes instead of acetophenone, to further show the utility of this protocol in organic synthesis. Interestingly, the reaction of catechols, ammonium acetate, and phenylacetylenes also resulted in the production of benzoxazole 4a in 81% yield under our optimized conditions. The reaction was also checked with other phenylacetylene derivatives, and a satisfactory yield of products was obtained (Scheme 5).
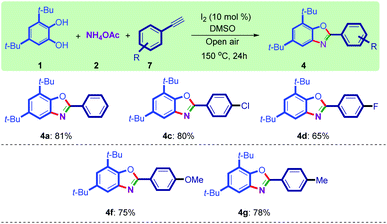 | ||
| Scheme 5 Synthesis of benzoxazoles from phenylacetylenes. Reaction conditions: 1 (1.0 mmol), 2 (1.2 mmol), 7 (1.0 mmol), I2 (10 mol%), DMSO (5.0 mL), open air. All yields are isolated. | ||
In order to highlight the utility of this methodology, a large-scale synthesis (50 mmol) of compound 4a was accomplished, and 70% of the product was isolated (Scheme 6).
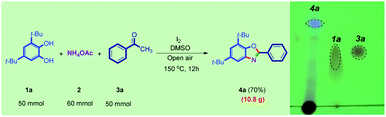 | ||
| Scheme 6 Large-scale synthesis of benzoxazole 4a by a new method. The TLC of the reaction is shown beside. | ||
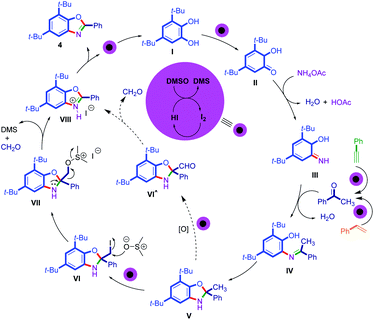
In agreement with the previously reported catalytic cycle for benzoxazole synthesis and also I2–DMSO catalysis, a possible reaction mechanism for this protocol is proposed (Scheme 7).7,18,20,29 According to the previous reports, phenylacetylenes and styrenes could be converted into acetophenone, thus the catalytic cycle was presented only for acetophenone.43–47 Moreover, we checked the reaction of styrenes and phenylacetylenes in the presence of iodine/DMSO, and the GC-mass analysis of the reaction mixture showed the presence of acetophenone. It seems that catechol (I) is converted into mono-quinone (II) in the presence of the I2–DMSO catalyst system.32,35 The condensation reaction of II with ammonium acetate generates o-iminocyclohexadiene alcohol (III).35 The generation of intermediate (IV) is possible by another condensation reaction of the imine group and carbonyl group of acetophenone.35 The intermediate (V) is generated by an intramolecular cyclization reaction of intermediate (IV).29,31 The iodination of methyl group in intermediate (V) can produce the intermediate of VI.8,48 The generation of intermediate VII is assumed by the attack of DMSO to SP3 carbon via a SN2 reaction.8,48 The elimination of formaldehyde and dimethyl sulfide (DMS) in intermediate VII resulted in the generation of intermediate VIII.8,48 Eventually, oxidation of intermediate VIII using I2–DMSO and aerobic oxidative as an oxidant system leads to the final benzoxazole product and the catalyst system is regenerated to start the next cycle.8
Another plausible pathway is suggested based on the converted methyl group V to oxo derivative VI^ based on the reported evidence. Recently, Bathula and co-workers have monitored the oxidation of a methyl group to an oxo derivative under I2–DMSO by NMR spectroscopy.7 Finally, 2-aryl benzoxazole 4 was generated via loss of formaldehyde from intermediate VI^ and then deprotonation of VIII.7
Conclusions
We reported a new and practical method for the efficient synthesis of benzoxazole derivatives via the reaction of catechols, ammonium acetate, and alkenes/alkynes/ketones in the presence of an I2–DMSO catalyst system. This reaction occurs via some C–C and C–O bond cleavage processes to afford the final product under metal-free conditions in high yields.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are thankful to the Iran's Science Elites Federation for their support. Also, the financial support from the research councils of Shiraz University is gratefully acknowledged.Notes and references
- Y. Zhang, Y. Jin, L. Wang, Q. Zhang, C. Meng and C. Duan, Green Chem., 2021, 23, 6926–6930 RSC.
- M. J. Yi, H. X. Zhang, T. F. Xiao, J. H. Zhang, Z. T. Feng, L. P. Wei, G. Q. Xu and P. F. Xu, ACS Catal., 2021, 11, 3466–3472 CrossRef CAS.
- P. Ghosh, S. Mondal and A. Hajra, J. Org. Chem., 2018, 83, 13618–13623 CrossRef CAS PubMed.
- Y. Takeda, R. Kajihara, N. Kobayashi, K. Noguchi and A. Saito, Org. Lett., 2017, 19, 6744–6747 CrossRef CAS PubMed.
- X. Wang, F. Yan and Q. Wang, Synth. Commun., 2021, 51, 1763–1781 CrossRef CAS.
- Y. M. Ren, C. Cai and R. C. Yang, RSC Adv., 2013, 3, 7182–7204 RSC.
- O. Ravi, A. Shaikh, A. Upare, K. K. Singarapu and S. R. Bathula, J. Org. Chem., 2017, 82, 4422–4428 CrossRef CAS PubMed.
- S. Mohammed, R. A. Vishwakarma and S. B. Bharate, J. Org. Chem., 2015, 80, 6915–6921 CrossRef CAS PubMed.
- C. S. Demmer and L. Bunch, Eur. J. Med. Chem., 2015, 97, 778–785 CrossRef CAS PubMed.
- X. K. Wong and K. Y. Yeong, ChemMedChem, 2021, 16, 3237–3262 CrossRef CAS PubMed.
- W. H. Jiao, J. Li, D. Wang, M. M. Zhang, L. Y. Liu, F. Sun, J. Y. Li, R. J. Capon and H. W. Lin, J. Nat. Prod., 2019, 82, 2586–2593 CrossRef CAS PubMed.
- R. N. Brown, R. Cameron, D. K. Chalmers, S. Hamilton, A. Luttick, G. Y. Krippner, D. B. McConnell, R. Nearn, P. C. Stanislawski, S. P. Tucker and K. G. Watson, Bioorg. Med. Chem., 2005, 15, 2051–2055 CrossRef CAS PubMed.
- F. Muro, S. Iimura, Y. Yoneda, J. Chiba, T. Watanabe, M. Setoguchi, G. Takayama, M. Yokoyama, T. Takashi, A. Nakayama and N. Machinaga, Bioorg. Med. Chem., 2009, 17, 1232–1243 CrossRef CAS PubMed.
- Ö. Temiz-Arpaci, I. Yildiz, S. Özkan, F. Kaynak, E. Aki-Şener and I. Yalçin, Eur. J. Med. Chem., 2008, 43, 1423–1431 CrossRef PubMed.
- S. Sunny, S. E. John and N. Shankaraiah, Asian J. Org. Chem., 2021, 10, 1986–2009 CrossRef CAS.
- R. Sattar, R. Mukhtar, M. Atif, M. Hasnain and A. Irfan, J. Heterocycl. Chem., 2020, 57, 2079–2107 CrossRef CAS.
- T. B. Nguyen, L. Ermolenko and A. Al-Mourabit, J. Am. Chem. Soc., 2013, 135, 118–121 CrossRef CAS PubMed.
- C. Yu, X. Guo, Z. Xi, M. Muzzio, Z. Yin, B. Shen, J. Li, C. T. Seto and S. Sun, J. Am. Chem. Soc., 2017, 139, 5712–5715 CrossRef CAS PubMed.
- T. B. Nguyen and P. Retailleau, Org. Lett., 2017, 19, 3887–3890 CrossRef CAS PubMed.
- S. Gao, L. Gao, H. Meng, M. Luo and X. Zeng, Chem. Commun., 2017, 53, 9886–9889 RSC.
- G. Chakraborty, R. Mondal, A. K. Guin and N. D. Paul, Org. Biomol. Chem., 2021, 19, 7217–7233 RSC.
- S. Su, J. Li, M. Sun, H. Zhao, Y. Chen and J. Li, Chem. Commun., 2018, 54, 9611–9614 RSC.
- T. T. T. Nguyen, L. A. Nguyen, Q. A. Ngo, M. Koleski and T. B. Nguyen, Org. Chem. Front., 2021, 8, 1593–1598 RSC.
- L. A. Nguyen, Q. A. Ngo, P. Retailleau and T. B. Nguyen, Adv. Synth. Catal., 2021, 363, 2098–2103 CrossRef CAS.
- R. Zhang, Y. Qin, L. Zhang and S. Luo, Org. Lett., 2017, 19, 5629–5632 CrossRef CAS PubMed.
- E. Niknam, F. Panahi, F. Daneshgar, F. Bahrami and A. Khalafi-Nezhad, ACS Omega, 2018, 3, 17135–17144 CrossRef CAS PubMed.
- L. Tang, X. Guo, Y. Yang, Z. Zha and Z. Wang, Chem. Commun., 2014, 50, 6145–6148 RSC.
- F. Chen, C. Zhu and H. Jiang, Adv. Synth. Catal., 2021, 363, 2124–2132 CrossRef CAS.
- X. Chen, F. Ji, Y. Zhao, Y. Liu, Y. Zhou, T. Chen and S. F. Yin, Adv. Synth. Catal., 2015, 357, 2924–2930 CrossRef CAS.
- X. Meng, Y. Wang, Y. Wang, B. Chen, Z. Jing, G. Chen and P. Zhao, J. Org. Chem., 2017, 82, 6922–6931 CrossRef CAS PubMed.
- H. Sharghi, J. Aboonajmi, M. Aberi and M. Shekouhy, Adv. Synth. Catal., 2020, 362, 1064–1083 CrossRef CAS.
- H. Sharghi, M. Aali Hosseini, J. Aboonajmi and M. Aberi, ACS Sustain. Chem. Eng., 2021, 9, 11163–11170 CrossRef CAS.
- M. W. B. McCulloch, F. Berrue, B. Haltli and R. G. Kerr, J. Nat. Prod., 2011, 74, 2250–2256 CrossRef CAS PubMed.
- H. Sharghi, J. Aboonajmi and M. Aberi, J. Org. Chem., 2020, 85, 6567–6577 CrossRef CAS PubMed.
- S. Wu, D. Zhou, F. Geng, J. Dong, L. Su, Y. Zhou and S. F. Yin, Adv. Synth. Catal., 2021, 363, 3607–3614 CrossRef CAS.
- J. Aboonajmi, F. Panahi and H. Sharghi, ACS Omega, 2021, 6, 22395–22399 CrossRef CAS PubMed.
- S. S. Malunavar, S. M. Sutar, H. M. Savanur, R. G. Kalkhambkar and K. K. Laali, Tetrahedron Lett., 2020, 61, 151509 CrossRef CAS.
- K. Seth, P. Purohit and A. K. Chakraborti, Org. Lett., 2014, 16, 2334–2337 CrossRef CAS PubMed.
- M. Cui, X. Wang, P. Yu, J. Zhang, Z. Li, X. Zhang, Y. Yang, M. Ono, H. Jia, H. Saji and B. Liu, J. Med. Chem., 2012, 55, 9283–9296 CrossRef CAS PubMed.
- S. K. Subran, S. Banerjee, A. Mondal and P. Paira, New J. Chem., 2016, 40, 10333–10343 RSC.
- K. L. Walker, L. M. Dornan, R. N. Zare, R. M. Waymouth and M. J. Muldoon, J. Am. Chem. Soc., 2017, 139, 12495–12503 CrossRef CAS PubMed.
- Y. A. Sayapin, I. O. Tupaeva, V. V. Tkachev and G. V. Shilov, Russ. J. Org. Chem., 2016, 52, 214–218 CrossRef CAS.
- A. Shaikh, O. Ravi, S. Pushpa Ragini, N. Sadhana and S. Reddy Bathula, Tetrahedron Lett., 2020, 61, 151356 CrossRef CAS.
- T. Mitsudome, T. Umetani, N. Nosaka, K. Mori, T. Mizugaki, K. Ebitani and K. Kaneda, Angew. Chem., 2006, 118, 495–499 CrossRef.
- J. Park, J. Yeon, P. H. Lee and K. Lee, Tetrahedron Lett., 2013, 54, 4414–4417 CrossRef CAS.
- M. Bassetti, S. Ciceri, F. Lancia and C. Pasquini, Tetrahedron Lett., 2014, 55, 1608–1612 CrossRef CAS.
- T. Nobuta, S. I. Hirashima, N. Tada, T. Miura and A. Itoh, Org. Lett., 2011, 13, 2576–2579 CrossRef CAS PubMed.
- Y. Liu, X. Yuan, X. Guo, X. Zhang and B. Chen, Tetrahedron, 2018, 74, 6057–6062 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, spectral data and copy of NMRs. See https://doi.org/10.1039/d2ra03340b |
| This journal is © The Royal Society of Chemistry 2022 |