Open Access Article
Open Access ArticleComparison of pharmaceutical removal in two membrane bioreactors with/without powdered activated carbon addition
Ya-Ting Liu ,
Qing Xia
,
Qing Xia ,
Wei-Wei Huang
,
Wei-Wei Huang ,
Xue-Song Yi
,
Xue-Song Yi ,
Li-Li Dong
,
Li-Li Dong and
Fei Yang
and
Fei Yang *
*
Department of Environmental Science and Engineering, Hainan University, Renmin Avenue 58, 570228 Haikou, Hainan Province, P. R. China. E-mail: fei.yang@hainanu.edu.cn; 648409771@qq.com; xsky1028@126.com; huang05106114@163.com; cedar401@163.com; donglili0569@126.com
First published on 21st July 2022
Abstract
The present study investigates the removal of six selected pharmaceuticals from municipal wastewater in two membrane bioreactors (MBRs) with and without powdered activated carbon (PAC) addition. Two approaches were carried out for obtaining different carbon dosages related to the influent: (1) with a fixed solids retention time (SRT) and varying PAC concentrations; (2) with varying SRTs and a fixed PAC concentration. The results reveal that a PAC dosage related to influent of 21 mg L−1 and SRT of 20 d are optimal. The first approach achieved a better removal performance than the second. The removal of amidotrizoic acid (up to 46%), bezafibrate (>92%) and iopromide (around 85%) were mainly caused by biological process, but were also enhanced by PAC addition. Efficient removal (>95%) of sulfamethoxazole, carbamazepine and diclofenac were highly dependent on the PAC dosage. However, carbamazepine shows re-metabolization properties during biological processing. Decreasing the SRT as done in the second approach, not only increased the PAC amount, but also decreased the mass of activated sludge and reduced the capability to degrade complex organic matter. Consequently, biodegradability and adsorbability played decisive roles in the removal of each compound.
1 Introduction
The ubiquitous presence of micropollutants (MPs) such as endocrine disrupting compounds (EDCs), pharmaceuticals and personal care products (PPCPs) in wastewater is emerging as an environmental risk because of evidential or potential adverse effects on exposed wildlife and humans. Therefore, the removal of MPs is becoming an international concern for safe water reuse and disposal of treated wastewater.1–3 However, conventional wastewater treatment systems such as the activated sludge process (CAS) are not designed to remove MPs. Additional and/or novel processes have to be developed and implemented for MP removal in wastewater treatment plants. Previous studies have approved adsorption by powdered activated carbon (PAC) to be one of the cost-effective processes for MPs removal.4,5 The challenge could be the separation of PAC from the sludge or liquid phase due to its micro size. Membrane bioreactor (MBR) processes commonly use ultra- or micro-filtration to separate the sludge phase from the liquid phase. Therefore, it might be an interesting alternative to combine with a PAC adsorption process. Because of the small pore size of membranes (<0.2 μm), PAC particles are retained completely within the system. An additional advantage of MBR application is the potentially higher removal rate for MPs due to its longer solids retention time (SRT) benefiting biodegradation processes.6,7 The addition of activated carbon (AC), especially PAC (because of its large surface area for adsorption), has greatly improved the removal of organics in MBRs.8,9 In general, the efficiency of AC adsorption is expressed and compared by means of the carbon dosage related to the treated water volume.10 However, in combination with a biological process such as an MBR, due to the separation of the hydraulic retention time (HRT) and the SRT in an MBR, the carbon dosage can either be related to the concentration in the reactor, or to the concentration of the treated influent water volume.11 This complicates the comparison of the results of different research works. The SRT expresses the average residence time of a sludge floc in the system. The change of SRT influences the composition, diversity and activity of microorganisms, and consequently is a key factor for the biological degradation capacity of a system. Usually, the diversity of the biocenosis increases with the increase of SRT and improves degradation of organic matter. In a conventional system (without PAC addition), mixed liquor suspended solids (MLSS) consists of an inorganic fraction due to the mineral particles present in the influent and an organic fraction due to the growth of microorganisms and organic matter adsorbed from the influent. Even in case the inorganic fraction is not identical, the biological activity can be compared to other systems by using only the organic fraction (mixed liquor volatile suspended solids (MLVSS) concentration). However, adding PAC to a biological system not only increases the suspended solids fraction in the influent, but also increases the inorganic fraction of the MLSS concentration in the reactor, which cannot be related to the biological fraction. Consequently, two systems operated at the same SRT with and without PAC addition might have significantly different biological retention times. Therefore, in the combination of PAC adsorption and MBR processes, the PAC particles will be combined with activated sludge flocs. The removal mechanisms may occurred by the adsorption of the fresh PAC, aged PAC, sludge flocs (with and without PAC inside), and degradation of the activated sludge. A short SRT will have more fresh PAC which should be good for MPs' removal. The PAC dosage and SRT will play two key factors in the combination system for its cost-effective application.There are two approaches that are able to supply different carbon dosages related to the influent in an MBR: (1) with a fixed SRT and varied PAC concentrations in the reactor and; (2) with varied SRTs and a fixed PAC concentration in the reactor. The objectives of the present study are to evaluate the removal performance of selected pharmaceuticals from municipal wastewater by an MBR with PAC addition via the two approaches and to compare the results to a reference MBR system operated in parallel without PAC addition. The removal mechanisms are discussed by the biodegradability and adsorbability for each MP, and optimal PAC dosage and SRT operating condition are also extracted.
2 Materials and methods
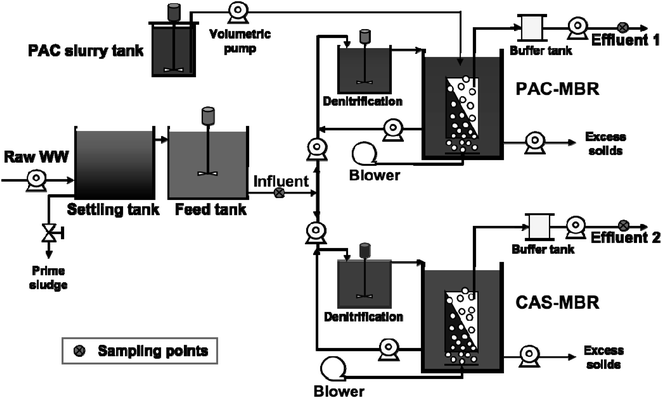
The schematic diagram of the experimental setup is shown in Fig. 1. The system consists of two identical pilot-scale submerged MBRs, each with a working volume of 1 m3. The system also includes a 600 L denitrification (DN) tank for each MBR used for pH control (as a compensation for alkalinity consumption due to nitrification). Each MBR is equipped with a UF flat-sheet membrane module (effective filtration area 10 m2) (BIO-CEL®-BC10-C10-UP150). During the experimental period, the filtration flux was set around 10 LMH, which was below the critical flux for avoiding the effect of fouling accumulation. One MBR was operated with PAC addition (named PAC-MBR); the other was a conventional activated sludge MBR used as a reference (named CAS-MBR). The PAC (QH-200, Shanghai Quanhu Active Carbon Co., Ltd., China) was made from stone coal with a particle size smaller than 40 μm at a proportion of 70%, ash ration around 23%, inner surface area of 1300 m2 g−1 (BET method), iodine adsorption 1250 mg g−1, and molasses number of 0.9 ± 0.2. For each SRT control, the excess MLSS was discharged automatically by a peristaltic pump two times per day. After the discharge, within 10 minutes the stirred PAC slurry was pumped into the PAC-MBR to supplement the PAC wastage from the excess MLSS discharge as well as to refresh the aged PAC.The supplement of the daily PAC amount for the wastage by the excess solids discharge was calculated according to the eqn (1):
| MPAC = CPAC × VEMLSS | (1) |
The PAC dosage related to the influent was calculated according to the eqn (2):
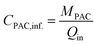 | (2) |
The two MBRs were operated in parallel with a mode of 5 min filtration/1 min relaxation for more than three months before the study started. This offered an HRT of around 12.4 h and a net flux of 8.6 LMH. The feed was pre-settled raw wastewater from a municipal wastewater treatment plant in Jiujiang, Jiangxi Province, China. A recirculating rate of 200% between the MBR and denitrification tank was used for a pH compensation. The different operating conditions during the study are shown below:
• Phase 1 (year 2019): with a fixed SRT of 41 d and varied PAC dosages in the reactor (0.2, 0.4, 0.8, 1.6 and 3.1 g L−1, in turn). The PAC dosage range related to the influent was 2.6, 5.3, 10.6, 21 and 41 mg L−1, respectively. The PAC dosage shift in the reactor was carried out by manually adding the required PAC both in the MBR and denitrification tank. The SRT was kept constant by discharging a fixed volume of MLSS (about 25 L) per day.
• Phase 2 (year 2020): with varied SRTs (60, 35, 20 and 10 d, in turn) and a fixed PAC concentration of 0.5 g L−1 in the reactor (PAC concentration was based on the results from Phase 1). The different SRTs were obtained by discharging defined volumes of MLSS (16.7, 29.6, 51.6 and 102 L, respectively) per day. The influent flux was kept constant. Therefore, the PAC dosage range related to the influent was 4.4, 7.6, 13.2 and 26.5 mg L−1, respectively. The SRT shift to the next step in the reactor was carried out by discharging a defined MLSS volume and subsequently adding tap water and PAC for regain the required MLSS and PAC dosage, then waiting for a certain adaption period before sampling.
Six pharmaceuticals were selected for this research work: amidotrizoic acid (ATA, a radiocontrast agent), sulfamethoxazole (SMX, an antibiotic), bezafibrate (BZF, a blood lipid control drug), carbamazepine (CBZ, an antiepileptic), diclofenac (DCF, an anti-inflammatory drug), and iopromide (IPM, a contrast medium). The samples were taken from the influent and effluents of the two MBRs as the composite samples collected during every 7 days. The influent composite sample was acidified to pH = 2–3 by adding 1 M H2SO4 at the beginning of the sampling (in the empty bottle). Analyses of the pharmaceuticals were conducted by an extraction process followed by HPLC/MS/MS. The limit of quantification (LOQ) for each compound is 50 ng L−1 in the present study.
The daily monitoring was done automatically by the control panels for temperature, pH, dissolved oxygen (DO), MLSS, water level, permeate flow rate and filtration pressure. Routine physico-chemical monitoring on pH, conductivity (EC), MLSS, MLVSS, capillary suction time (CST), COD, filtered COD (fCOD), NH4+–N and NO3−–N was conducted three times per week.
Two kinds of biological retention time namely sludge age (SA) were defined for the PAC-MBR: one including PAC (apparent SA) and the other excluding PAC (normalized SA) from the MLSS. The sludge age was calculated using the eqn (3):
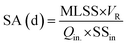 | (3) |
The removal efficiency (RE) was calculated by the eqn (4):
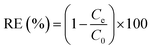 | (4) |
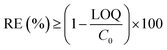 | (5) |
3 Results
3.1 General performance of the two MBRs
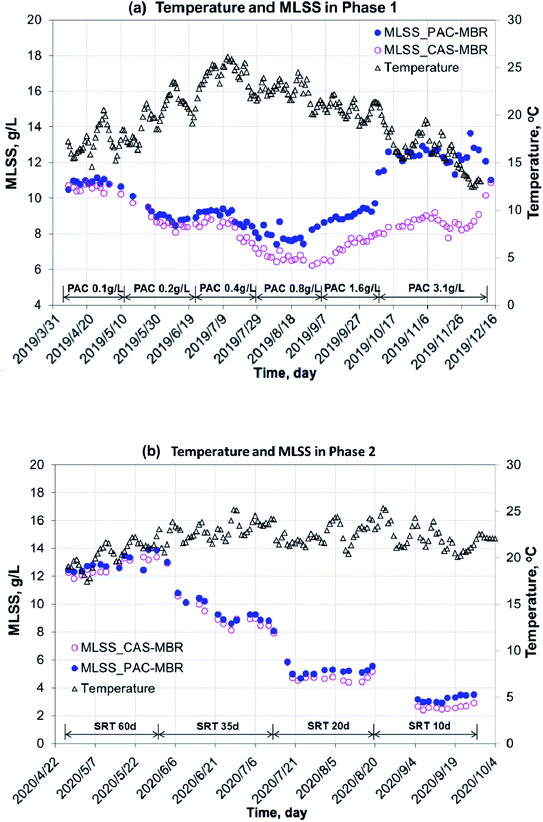
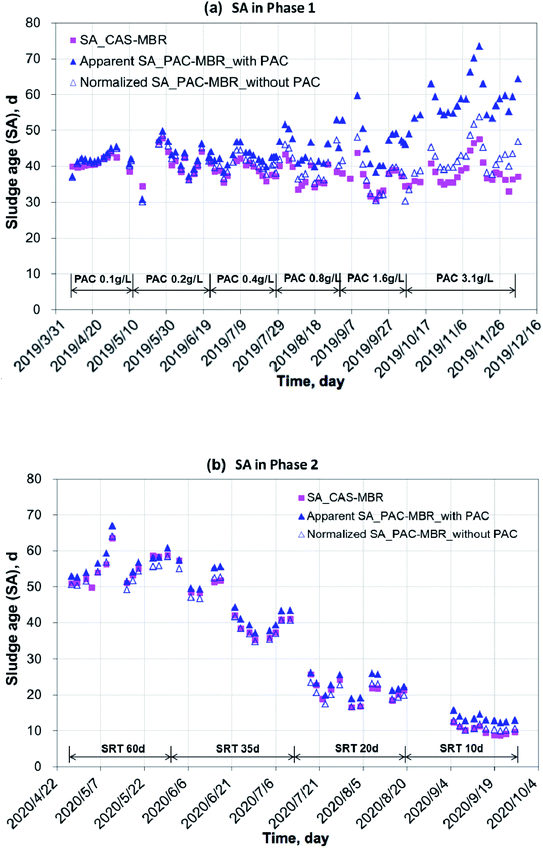
Under the experimental conditions during the year 2019 to 2020, the two MBRs worked stable with an influent COD 439 ± 115 mg L−1 and NH4+–N 61 ± 26 mg L−1. The COD removal rates (the effluent concentrations: 18 ± 5 mg L−1 for the PAC-MBR and 24 ± 4 mg L−1 for the CAS-MBR) and nitrification efficiency (above 98%) were excellent. However, in 2020, with an SRT of 60 d, the DO concentrations in the two MBRs were below 1.0 mg L−1 in most cases. Under a high biomass (average MLSS 12.3 g L−1) with long SRT, the oxygen consumption was very high due to endogenous respiration and deteriorated oxygen transfer as a consequence.13 It did not affect the COD removal, but slightly decreased the ammonia conversion and might affect the biodegradation of the pharmaceuticals.The variations of temperature, MLSS concentration and calculated sludge age in the two MBRs are shown in Fig. 2 and 3. In the PAC-MBR, the microscope check clearly showed that the PAC particles were combined with the activated sludge flocs. In both experimental phases, the MLSS concentrations for the biomass and sludge ages for the sludge activity were the most important parameters, due to their direct concerns to the MPs' removal in the CAS-MBR. Due to the PAC addition in Phase 1 at SRT of 41 d, the MLSS concentrations in the PAC-MBR were higher than that in the CAS-MBR, and the differences were increased from 5% to 34% with the increase of the PAC dosage from 0.4 g L−1 to 3.1 g L−1 in the reactor (Fig. 2a). The same trend was observed for the apparent SA in the PAC-MBR, while the normalized SA was slightly higher than the SA in the CAS-MBR (Fig. 3a). In Phase 2, with varied SRTs from 60 d to 10 d, the MLSS concentration and apparent SA were slightly higher than that in the CAS-MBR, due to the relatively low PAC dosage (0.5 g L−1) in the PAC-MBR, whereas the normalized SA was very close to the SA in the CAS-MBR (Fig. 2b and 3b). The results indicate that although the two systems operated either at fixed or varied SRTs, the biologic conditions were comparable.
3.2 Micropollutants removal of the two MBRs
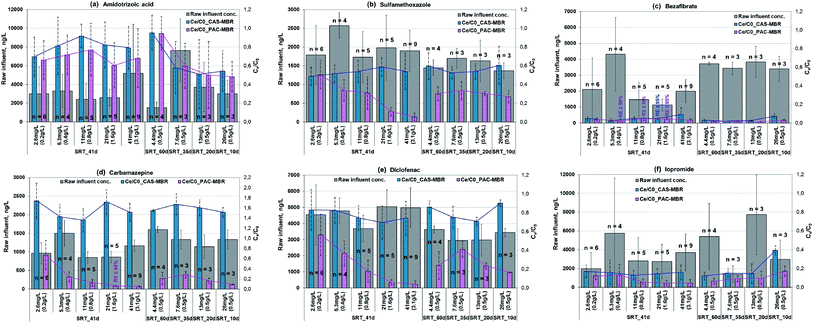
In generally, concentrations of micropollutants especially pharmaceuticals fluctuate strongly in wastewater.14 The removal performance for the six selected pharmaceuticals is shown in Fig. 4a–f. In Phase 2, two parameters affecting the overall removal performance were changed in an opposite direction in the PAC-MBR, i.e. a decrease of SRT and thereby an increase of PAC dosage related to influent. However, it can be assumed that the biologic degradation in the PAC-MBR was comparable to the CAS-MBR, since the normalized sludge ages in the two reactors were almost identical (Fig. 3). Therefore, the removal by adsorption alone in the PAC-MBR can be calculated via the differences in the removal efficiencies of the PAC-MBR and CAS-MBR. Fig. 5 shows the differences, which can be interpreted as the net “additional” elimination via the PAC adsorption.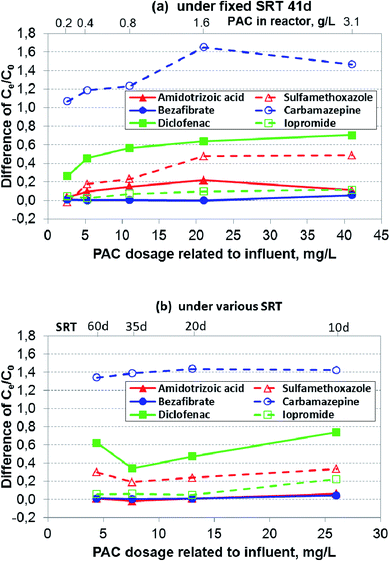 | ||
| Fig. 5 Differences of Ce/C0 between PAC-MBR and CAS-MBR of the six selected pharmaceuticals with the fixed and varied SRTs and varied carbon dosages. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ng L−1. At the SRT of 41 d, the CAS-MBR had a removal fluctuation between 8% and 30%, while at the varied SRTs from 35 d to 10 d, the removal was between 42% and 46% (Fig. 4a). The very low removal (only 5%) at the SRT of 60 d was probably because (1) the aged PAC caused by the long SRT resulted in an adsorption decline for ATA, (2) the insufficient DO level (below 1.0 mg L−1) led to a reduction of ATA biodegradation and, (3) the relatively low influent concentration during this period (Fig. 4a) lowered the relative elimination rate.
000 ng L−1. At the SRT of 41 d, the CAS-MBR had a removal fluctuation between 8% and 30%, while at the varied SRTs from 35 d to 10 d, the removal was between 42% and 46% (Fig. 4a). The very low removal (only 5%) at the SRT of 60 d was probably because (1) the aged PAC caused by the long SRT resulted in an adsorption decline for ATA, (2) the insufficient DO level (below 1.0 mg L−1) led to a reduction of ATA biodegradation and, (3) the relatively low influent concentration during this period (Fig. 4a) lowered the relative elimination rate.In comparison, the PAC addition in the PAC-MBR resulted in an additional elimination, which increased up to 22% with the increase of PAC dosage at an SRT of 41 d (Fig. 5a), while with the varied SRTs from 60 d to 10 d almost no additional removal by the PAC adsorption was observed (Fig. 4a and 5b).
The removal of SMX was enhanced by the PAC addition (Fig. 4b). At the SRT of 41 d, the lowest PAC dosage (2.6 mg L−1) did not enhance the SMX removal. However, when the PAC dosage was increased to 4.4 mg L−1 and higher, the removal performance was enhanced significantly (by additional 18–49%) (Fig. 4b), indicating the existence of a threshold PAC dosage. With the varied SRTs from 60 d to 10 d, the SMX removal was relatively stable (70–73%) (Fig. 4b). In comparison with the removal in the CAS-MBR, the PAC addition brought an additional SMX removal with the increased rates from 19% to 34% (Fig. 5b). The results show that the adsorption rate ultimately not only depends on the PAC concentration (related to the influent) but also on the total mass of PAC in the system.
The addition of PAC in the MBR enhanced the BZF removal efficiency, especially at low temperatures. The BZF concentration of the PAC-MBR effluent was mostly below the LOQ (50 ng L−1) in all experimental phases (Fig. 4c).
CBZ can be removed efficiently by the PAC adsorption (Fig. 4d) due to its relatively good adsorption properties.19 At the SRT of 41 d, the removal efficiency increased exponentially from 33% to >95% with the increase of the PAC dosage from 2.6 to 41 mg L−1 (Fig. 4d). With the varied SRTs from 60 d to 10 d, the CBZ removal increased from 72 to 91% with the increase of the PAC dosage from 4.4 to 26 mg L−1 (Fig. 4d). However, the difference in the CBZ removal between the PAC-MBR and CAS-MBR was almost constant at approximate 140%, regardless of the PAC dosage (Fig. 5b). The removal differences above 100% (Fig. 5) are due to the increased CBZ concentration in the CAS-MBR. This means that even very low carbon dosage is able to prevent the transformation of the CBZ metabolites/conjugates back to the parent form.
The removal of DCF was greatly enhanced by the PAC addition. At the SRT of 41 d, the PAC dosage 2.6 mg L−1 already gave an additional removal of 26% (total removal 44%) in comparison with that in the CAS-MBR. The removal rates increased exponentially from 44% to >95% with the increase of the PAC dosage from 2.6 to 41 mg L−1; at the PAC dosage of 21 mg L−1 it reached its maximum removal (the effluent concentration ≤ LOQ) (Fig. 4e). At the varied SRTs from 60 d to 10 d, the PAC dosage gave an additional removal of 34–74% (total removal 59–83%) with the decrease of SRT (thereby the increase of PAC dosage) (Fig. 5b), indicating a considerable dependence of the DCF removal by the carbon adsorption. However, in comparison with the removal at the fixed SRT the removal efficiency was less and not consistent. It indicates that the overall removal not only depends on the carbon dosage related to the influent but also on the PAC mass in the system.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ng L−1. In the CAS-MBR, the removal of IPM was relatively constant around 85% with the SRTs above 20 d (Fig. 4f). When the SRT decreased from 20 to 10 d, the IPM removal decreased quickly from 85% to 61%, indicating a low biodegradation of IPM at a short SRT.
000 ng L−1. In the CAS-MBR, the removal of IPM was relatively constant around 85% with the SRTs above 20 d (Fig. 4f). When the SRT decreased from 20 to 10 d, the IPM removal decreased quickly from 85% to 61%, indicating a low biodegradation of IPM at a short SRT.The PAC addition enhanced the IPM removal to a moderate extent. At the SRT of 41 d, a low PAC dosage (≤5.3 mg L−1) almost did not enhance its removal, indicating the existence of a threshold PAC dosage for the IPM adsorption. The additional removal was increased by 11% at the PAC dosages ≥10.6 mg L−1 with a total IPM removal around 95% (Fig. 4f and 5a). With the varied SRTs from 60 d to 10 d, the additional removal by PAC adsorption was smaller than 5% for the PAC dosages up to 13 mg L−1; while at the highest PAC dosage (26 mg L−1) the additional removal increased by 22% (Fig. 4f and 5b). Due to the decrease of biological degradation caused by the decrease of SRT, the total IPM removal in the PAC-MBR decreased from 93.5% to 83% (Fig. 4f). The results are in accordance with the previous study that found IPM has very bad adsorbability but relatively good biodegradability behavior.20
A summary of the removal characteristics of the selected MPs is listed in Table 1. The removal of amidotrizoic acid, bezafibrate and iopromide were mainly caused by biological process, although amidotrizoic acid was mainly adsorbed by sludge flocs. Efficient removal of sulfamethoxazole, carbamazepine and diclofenac were highly dependent on the PAC dosage. However, carbamazepine shows re-metabolization properties during biological process. Finally, the results reveal that an PAC dosage related to influent of 21 mg L−1 and SRT of 20 d are optimal.
| MP | Removal by biological degradation | Removal by adsorption alone |
|---|---|---|
| ATA | • Low to moderate (8–46%) – surprising results | • Low (0–22%) |
| • No reliable correlation with SRT change | • At SRT of 41 d: additional removal by PAC addition up to 22% | |
| • At varied SRTs: almost no additional removal (<6%) | ||
| SMX | • Moderate (40–51%) | • Low to moderate (1–50%) (max. total removal >95%) |
| • Slightly dependent on SRT | • Highly dependent on PAC dosage | |
| • May slightly depend on DO level | • Moderately dependent on carbon retention time | |
| • Low PAC dosage with high SRT seems to be favored | ||
| BEZ | • Very high (>92%) | • Very low (0–6%) in general, but at low water temperatures increased to 10–20% (max. total removal >95%) |
| • Independent on SRT | ||
| • Significant decrease at low water temperatures (<15 °C) | ||
| CBZ | • Negative removal indicates significant concentration increase (up to 74%) due to the transformation of conjugates | • 107–165% (max. total removal >95%) |
| • No correlation with SRT change | • Highly dependent on the PAC dosage related to the influent (which implies varied carbon concentrations in the reactor at SRT of 41 d) | |
| • Moderately dependent on the PAC retention time | ||
| • Adsorption at low PAC dosages with high SRT seems to be favored | ||
| DCF | • Low (10–30%) | • Moderate (26–74%) (max. total removal >95%) |
| • Slightly dependent on SRT | • Highly dependent on PAC dosage | |
| • May slightly depend on DO level | • Moderately dependent on carbon retention time | |
| • Low PAC dosage with high SRT seems to be favored | ||
| IPM | • High (60–88%, mostly ca. 85%) | • Low (5–22%) (max. total removal >95%) |
| • Slightly dependent on SRT | • Slightly dependent on PAC dosage |
4 Discussion
The operation of a direct addition of PAC into an MBR for MP removal only requires simple operation procedure, no need of additional reactors and can be implemented with very low equipment cost in existing facilities. Previous study has indicated that a regular replenishment of used PAC with fresh one is important for improving the system performance.21 In a PAC-MBR with a fixed operating flux (therefore the HRT is constant), varying PAC dosages related to the influent can be obtained (1) by changing PAC dosage in the reactor at a fixed SRT and, (2) by changing SRT at a fixed PAC concentration in the reactor. In the first approach, higher PAC concentrations in the reactor offer higher surface areas for the adsorption (therefore the adsorption is expected to be more efficient), while the carbon retention time (CRT, equal to SRT) remains constant. In the second approach, the PAC surface area for the adsorption in the reactor remains constant. On the one hand, this causes a shorter reaction time for the PAC and dissolved compounds in the liquid phase; on the other hand, it increases the replenishment rate of the PAC because of the higher sludge wastage rate. It was expected that the capability of microorganisms to degrade complex organic matter decreases with the decrease of SRT.During Phase 1 (year 2019) the SRT was fixed at 41 d to assure a high biological degradation rate and was varied in turn from 60, 35, 20 to 10 d during Phase 2 (year 2020). The PAC concentration adjusted in the reactor during Phase 2 was selected on the basis of the results from Phase 1. The dosages related to the influent were comparable to those of Remy et al.,22 but lower than those used in other studies (50–80 mg PAC per L).23
In Phase 1 with the fixed SRT of 41 d, the increase of the carbon dosage in the PAC-MBR increased the removal of SMX, CBZ, DFC and IPM (Fig. 4b and d–f). The results indicate that for SMX and IPM in particular there is a kind of threshold PAC dosage existed: below the threshold almost no MP adsorption occurred – firstly for those compounds with higher Freundlich coefficients (better adsorbable) followed by the compounds with lower Freundlich coefficients. A similar threshold PAC dosage was observed by Miehe.15 With the increase of the PAC dosage, the removal of SMX, CMZ and DCF increased almost exponentially.
In Phase 2 with various SRTs, however the decrease of SRT under a constant PAC level in the reactor brought four simultaneous changes: (1) decreased sludge concentration, (2) decreased bacteriological diversity of the biocenoses, (3) increased PAC dosing rate and, (4) reduced carbon retention time in the system. The first two factors negatively affect the biological degradation of MPs and decrease the available surface area for the adsorption onto the sludge flocs. The other two factors affect the adsorption in an opposite direction: the increase of the PAC dosing rate resulted in a higher adsorption ratio of MPs to PAC, while the decrease of the CRT reduced the reaction time of the PAC in the liquid phase, which negatively affected adsorption. The overall removal performance was therefore a compromise between the four factors (or a result of their competition).
As mentioned before, the biological system of the two reactors were comparable. Therefore the removal by adsorption alone was calculated via the differences in the removal rates between the PAC-MBR and CAS-MBR (Fig. 5). The removal by the adsorption was affected by both the increased PAC dosage and reduced CRT. In most cases the removal by the adsorption (at least for the compounds SXM, CMZ and DCF) increased with the increase of the PAC dosage, indicating that the increased carbon dosages had a stronger positive influence on the adsorption process than the decrease in CRT. It was also observed that the increase of the MPs' removal by the adsorption with the increase of the PAC dosage in Phase 2 was lower than that in Phase 1, indicating that the decrease of the CRT has a negative influence on the adsorption process. It was also noticed that the removal behavior of SXM, CMZ and DCF at the lowest PAC dosage of 4.4 mg L−1 (corresponding to the highest SRT) does not follow the trend of the other dosages as the removal is slightly better than that at a higher carbon dosage (7.6 mg L−1) (Fig. 5b). The explanation might be that at the highest SRT of 60 d the biocenoses degraded the organic matter to a larger extent, leading to a lower competitive adsorption on the PAC, which in turn causes a higher adsorption rate of MPs with lower adsorption affinities than the organic matrix. For a better comparison, the salient evidences regarding the removal of the six pharmaceuticals under investigation by biological degradation and adsorption are shown in Table 1.
The results obtained in this study, i.e. the biodegradability and adsorbability of the six selected pharmaceuticals, have been compared with other published data. Previous studies experimentally calculated the kinetic degradation constants (kbiol) for a variety of micropollutants20,24 and can be used to compare the biodegradability rates under similar conditions. Other studies experimentally calculated Freundlich coefficients (KF,S) for the quantification of the adsorbability.25,26 Table 2 summarizes the kinetic degradation constants (kbiol) and Freundlich coefficients (KF,S) of the six selected pharmaceuticals from previous studies. The selected pharmaceuticals can be considered as the representative examples. Comparing the data in Tables 1 and 2, it becomes evident that the biological degradation in the CAS-MBR reflects well for the biodegradability expressed by the kinetic degradation constants (kbiol). In particular, the ranking of the biological degradation of the compounds under the investigation (Table 1) is exactly the same as the ranking based on kbiol (Table 2). Regarding the PAC adsorption performance, the results of the present study basically reflect the adsorbability expressed by the Freundlich coefficients given in the literature. There are some deviations from the published data regarding ATA and SMX. Despite their very low Freundlich coefficients, in the present study they were removed by up to 22% to 48% at 21 mg L−1 PAC dosage (Fig. 5a).
| Pharmaceutical | Biodegradability | Adsorbability |
|---|---|---|
| a According to Joss et al. (2006):20 kbiol < 0.1 L (g sludge d)−1: non-biodegradable substances, removal rate <10%; 0.1 < kbiol <10 L (g sludge d)−1: partially biodegradable substances, removal rate is variable between 10–90%. This group includes the majority of medicines and personal care products; kbiol > 10 L (g sludge d)−1: readily biodegradable substances, removal rate >95%. | ||
| Amidotrizoic acid (ATA) | Extremely low | Extremely low |
| Sulfamethoxazole (SMX) | Low to moderate (kbiol ≤ 0.15 L (g sludge d)−1) | Low |
| Bezafibrate (BZF) | Moderate (kbiol = 2.55 L (g sludge d)−1) | Moderate (KF,S = 160) |
| Carbamazepine (CBZ) | Very low (kbiol = 0.006 L (g sludge d)−1) | Good (KF,S = 393–476) |
| Diclofenac (DCF) | Very low (kbiol = 0.035 L (g sludge d)−1) | Moderate (KF,S = 245–278) |
| Iopromide (IPM) | Moderate (kbiol = 2 L (g sludge d)−1) | Extremely low (KF,S = 2.4) |
It was noticed that the ATA removal performance by the CAS-MBR alone is very surprising in comparison with other observations where almost no removal of ATA via biological processes occurred.20 According to Table 2, ATA shows extremely low biodegradability and adsorbability. However, in the present study ATA was mostly biodegraded to a moderate extent (8–46%) in the CAS-MBR. It indicates that the removal of ATA might be caused by the biodegradation or adsorption onto the sludge flocs. With the change of SRT from 35 d to 10 d, the removal of ATA in the CAS-MBR was quite similar, despite of the change of the biocoenosis caused by the SRT change. It indicates that the removal of ATA was mostly caused by adsorption onto the sludge flocs, rather than by the biodegradation.
Overall, the conditions of organic load, temperature and carbon mixing intensity in the reactors were similar, whereas the carbon dosages and SRTs/CRTs were changed. The results show that for getting a PAC dosage related to the influent, substantially increasing the carbon level in the reactor with a fixed SRT had some advantages in comparison with decreasing SRTs at a fixed carbon level in the reactor (which concomitantly increasing the PAC dosage related to the influent), since the latter approach also changed the sludge properties. Looking at the Ce/C0 values under the similar conditions in the two reactors, i.e. 0.4 g PAC per L and SRT 41 d in 2019 (5.3 mg L−1 related to the influent) and 0.5 g PAC per L and SRT 35 d in 2020 (7.6 mg L−1 related to the influent), the MPs' removal efficiencies were similar (Fig. 4). It can also be concluded that an SRT of 20 d seems to be optimal for the MPs' removal. However, to differentiate the biodegradation and adsorption to sludge for the MPs' removal is difficult. Further research work may focus on tracking the metabolite(s) of each MP to deeply investigate the mechanism of the biodegradation process.
5 Conclusions
The study investigated two approaches for getting the carbon dosages related to the influent for the removal of six selected pharmaceuticals from municipal wastewater under different operating conditions. The approach with a fixed SRT and varied PAC dosages achieved better performance than the approach with varied SRTs/CRTs and a fixed PAC concentration in the reactor, due to the change of the sludge properties and consequently reduced the biodegradation capacity. The adsorption performance itself is affected by the increase of the PAC dosage and the decrease of the CRT in an opposite direction.The removal of the six selected pharmaceuticals depends on its biodegradability and adsorbability. The results reveal a moderate removal of amidotrizoic acid which could be mainly caused by the adsorption on the sludge. The PAC addition slightly enhanced its removal efficiency. Bezafibrate was removed very effectively by the biological degradation, however with water temperatures below 15 °C the performance decreased sharply; PAC addition enhanced the removal and effluent concentrations were mostly below the LOQ. Iopromide was also removed very efficiently by biological degradation; PAC addition enhanced the removal slightly. However, decreased STR affected the overall removal of IPM in both of the MBRs, as the reduced biological degradation under these conditions was stronger than the increased adsorption at the increased carbon dosages. Carbamazepine shows re-metabolization properties during biological treatment, but can be sufficiently removed by the PAC adsorption. Sulfamethoxazole and diclofenac showed moderate removal efficiency in the CAS-MBR, but can be effectively removed by the PAC addition. This means, the removal of sulfamethoxazole, carbamazepine and diclofenac were highly dependent on the PAC dosage in the reactor. Finally, an PAC dosage related to influent of 21 mg L−1 and SRT of 20 d are optimal for the MPs' removal. Temperature and DO concentration also play important roles.
Other factors such as temperature, pH, DO, filtration flux (or HRT), the frequencies of the sludge wastage and PAC addition per day were not well controlled or in limited condition. They could affect the MPs' removal process and make the explanation of the removal mechanisms difficulty in the pilot-scale reactors. Further research work may be done by tracking the metabolite(s) of each MP to differentiate the biodegradation and adsorption to sludge, as well as to investigate the contribution of the denitrification tank on MPs' removal.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work was financially supported by the Key R&D Project of Hainan Province (ZDYF2019198) and The Wastewater Treatment and Reuse Project of Jiujiang Tinci Materials Technology Co. Ltd. (TC-MR-RES-218A0), China. The municipal wastewater treatment plant in Jiujiang, Jiangxi Province, China.References
- W. Ben, B. Zhu, X. Yuan, Y. Zhang, M. Yang and Z. Qiang, Water Res., 2018, 130, 38–46 CrossRef CAS PubMed
.
- A. Kruglova, A. Gonzalez-Martinez, M. Kråkström, A. Mikola and R. Vahala, Sci. Total Environ., 2017, 605–606, 291–299 CrossRef CAS PubMed
.
- Z. Li, L. Ren, Y. Qiao, X. Li, J. Zheng, J. Ma and Z. Wang, Bioresour. Technol., 2022, 343, 126139 CrossRef CAS PubMed
.
- M. Alameddine, Z. T. How and M. Gamal El-Din, Sci. Total Environ., 2021, 770, 144679 CrossRef CAS PubMed
.
- F. Sher, K. Hanif, A. Rafey, U. Khalid, A. Zafar, M. Ameen and E. C. Lima, J. Environ. Manage., 2021, 278, 111302 CrossRef CAS PubMed
.
- N. López-Vinent, A. Cruz-Alcalde, J. Giménez and S. Esplugas, Sci. Total Environ., 2021, 786, 147416 CrossRef PubMed
.
- M. Golgoli, M. Khiadani, A. Shafieian, T. K. Sen, Y. Hartanto, M. L. Johns and M. Zargar, Chemosphere, 2021, 283, 131185 CrossRef CAS PubMed
.
- Y. Jiang, Y. Liu, D. Shi, W. Fu, P.-F. Sun, J. Li and S. Shao, J. Cleaner Prod., 2020, 277, 122341 CrossRef CAS
.
- Y. Zhang, X. Wang, H. Ye, L. Zhou and Z. Zhao, Water Sci. Technol., 2021, 83, 1005–1016 CrossRef CAS PubMed
.
- A. Rostvall, W. Zhang, W. Dürig, G. Renman, K. Wiberg, L. Ahrens and P. Gago-Ferrero, Water Res., 2018, 137, 97–106 CrossRef CAS PubMed
.
- G. Skouteris, D. Saroj, P. Melidis, F. I. Hai and S. Ouki, Bioresour. Technol., 2015, 185, 399–410 CrossRef CAS PubMed
.
- X.-S. Miao, J.-J. Yang and C. D. Metcalfe, Environ. Sci. Technol., 2005, 39, 7469–7475 CrossRef CAS PubMed
.
- B. E. L. Baêta, H. J. Luna, A. L. Sanson, S. Q. Silva and S. F. Aquino, J. Environ. Manage., 2013, 128, 462–470 CrossRef PubMed
.
- M. Ashfaq, Y. Li, Y. Wang, W. Chen, H. Wang, X. Chen, W. Wu, Z. Huang, C.-P. Yu and Q. Sun, Water Res., 2017, 123, 655–667 CrossRef CAS PubMed
.
- U. Miehe, Efficiency of technical barriers for removal of anthropogenic micropollutants - biological wastewater treatment and tertiary filtration, Faculty III - Process Sciences, Technical University of Berlin, 2010 Search PubMed
.
- B. Tiwari, B. Sellamuthu, S. Piché-Choquette, P. Drogui, R. D. Tyagi, M. A. Vaudreuil, S. Sauvé, G. Buelna and R. Dubé, Bioresour. Technol., 2019, 286, 121362 CrossRef CAS PubMed
.
- M. Wu, W. Tang, S. Wu, H. Liu and C. Yang, Sci. Total Environ., 2021, 757, 143902 CrossRef CAS PubMed
.
- S. Kharel, M. Stapf, U. Miehe, M. Ekblad, M. Cimbritz, P. Falås, J. Nilsson, R. Sehlén, J. Bregendahl and K. Bester, Sci. Total Environ., 2021, 759, 143989 CrossRef CAS PubMed
.
- Z. Yu, S. Peldszus and P. M. Huck, Water Res., 2008, 42, 2873–2882 CrossRef CAS PubMed
.
- A. Joss, S. Zabczynski, A. Göbel, B. Hoffmann, D. Löffler, C. S. McArdell, T. A. Ternes, A. Thomsen and H. Siegrist, Water Res., 2006, 40, 1686–1696 CrossRef CAS PubMed
.
- S. Zhang, J. Xiong, X. Zuo, W. Liao, C. Ma, J. He and Z. Chen, Biochem. Eng. J., 2019, 145, 10–17 CrossRef CAS
.
- M. Remy, P. van der Marel, A. Zwijnenburg, W. Rulkens and H. Temmink, Water Res., 2009, 43, 345–350 CrossRef CAS PubMed
.
- S. Baumgarten, H. Fr. Schröder, C. Charwath, M. Lange, S. Beier and J. Pinnekamp, Water Sci. Technol., 2007, 56, 1–8 CrossRef CAS PubMed
.
- M. R. Abargues, J. B. Giménez, J. Ferrer, A. Bouzas and A. Seco, Chem. Eng. J., 2018, 334, 313–321 CrossRef CAS
.
- A. Mojiri, R. Andasht Kazeroon and A. Gholami, Water, 2019, 11, 551 CrossRef CAS
.
- S.-W. Nam, D.-J. Choi, S.-K. Kim, N. Her and K.-D. Zoh, J. Hazard. Mater., 2014, 270, 144–152 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2022 |