Open Access Article
Open Access ArticleEffect of Pr/Zn on the anti-humidity and acetone-sensing properties of Co3O4 prepared by electrospray†
Xiangxiang Fan‡
 *ab,
Junfeng Wang‡a,
Chuanlong Suna,
Chun Huangab,
Yujie Luab,
Pan Daiab,
Yajuan Xua and
Wuming Heab
*ab,
Junfeng Wang‡a,
Chuanlong Suna,
Chun Huangab,
Yujie Luab,
Pan Daiab,
Yajuan Xua and
Wuming Heab
aSchool of Information Engineering, Huzhou University, Huzhou 313000, China. E-mail: fanxiangxiang@zjhu.edu.cn
bZhejiang Province Key Laboratory of Smart Management & Application of Modern Agricultural Resources, Huzhou University, Huzhou 313000, China
First published on 4th July 2022
Abstract
Co3O4 is a P-type metal-oxide semiconductor which can realize acetone detection at a lower temperature, but the lower working temperature brings the enhanced humidity effect. In order to solve the problem of a Co3O4 gas sensor being easily affected by humidity, an acetone-sensing material of Co3O4 mixed with Pr/Zn was prepared by electrospray in this work. The optimal working temperature of Pr/Zn–Co3O4 is 160 °C, and the detection limit can reach 1 ppm. The fluctuation of the acetone response is about 7.7% in the relative humidity range of 30–90%. Compared with pure Co3O4, the anti-humidity property of this material is obviously enhanced, but the gas-sensing response deteriorates. Compared with Pr–Co3O4, the anti-humidity and acetone sensing properties of Pr/Zn–Co3O4 were both improved. The morphology, composition, crystal state and energy state of the material were analyzed by SEM, EDS, XRD and XPS. The material of Pr/Zn–Co3O4 is a multi-component mixed material composed of PrCoO3, ZnO, Pr6O11 and Co3O4. The improved anti-humidity and acetone sensing properties exhibited by this material are the result of the synergistic effect of ZnO and Pr3+.
1. Introduction
Acetone is an important respiratory marker of diabetes. The accurate and stable detection of acetone in breathing gas is of great significance to changing the existing medical diagnosis model for diabetes. As a low-cost and easy-to-manufacture gas sensor, the metal oxide sensor has been widely used in research of acetone detection.1–5 However, the main problems currently faced in practical application are the low concentration of markers and high humidity in the breathing gas, which requires the sensor to have a lower detection limit and better anti-humidity properties.Co3O4 is a p-type metal-oxide semiconductor, which was widely used in the detection of acetone due to catalytic activity for volatile organic compounds (VOCs) and lower working temperature.6–10 Su et al. prepared flower-shaped Co3O4, and the detection limit of acetone reached 0.2 ppm.6 Qiao et al. prepared 3D radial Co3O4 nanorod clusters, and the detection limit of acetone reached 0.1 ppm.7 Because the acetone concentration in breathing gas is more than 0.3–0.9 ppm, Co3O4 is a promising material for acetone sensors for breathing gas. The acetone sensitivity of Co3O4 can be further improved by constructing composed materials.11–14 The composed materials of Co3O4 nanorod and ZnO nanosheet prepared by Jang et al. responded to 5 ppm acetone as high as 29, and the theoretical detection limit could reach 5 ppb.15 However, the sensitivity and stability of Co3O4 for acetone sensing deteriorate in a high-humidity environment.16–18 Srinivasan et al. have shown that high humidity conditions could affect the acetone sensitivity of Co3O4, and the acetone response dropped by 16.59% under a relative humidity of 89%.16 Zhou et al. found that the increase of relative humidity would also reduce the long-term stability of the Co3O4 acetone sensor, where the sensor response dropped by 10% in 20 days under a relative humidity of 33%.17 Therefore, humidity is a negative factor restricting the application of Co3O4 for respiration detection, but there are still few reports on the research that actually proposes to solve the influence of humidity on the Co3O4 acetone sensor.
Doping is a commonly used strategy to improve the performance of metal oxide gas sensors.19,20 The elements of Ce, Tb and Pr all have two valence states of 3+ and 4+. Because of the valence states, Ce, Tb and Pr all have reversible oxidation–reduction reactions, which can remove hydroxyl groups and promote the formation of ionized oxygen. These elements have been doped into the sensing materials to realize the anti-humidity property of the gas sensors.21–24 Yoon et al. used layer-by-layer coating to modify CeO2 nanoclusters on the surface of In2O3 hollow spheres, which exhibited humidity independence.21 Kwak et al. prepared Tb-doped SnO2 yolk–shell spheres by ultrasonic spray pyrolysis, which exhibited similar gas responses in dry condition and relative humidity of 80%.22 The Pr-doped In2O3 prepared by Kim et al. also exhibited similar gas responses in different humid conditions.23 It can be seen that doping of Ce, Tb and Pr provide a new way for the development of gas sensors with anti-humidity property. However, these sensors all worked at a high temperature of 450 °C, and the higher operating temperature promotes the humidity independence of the sensor. In addition, although the doping of Ce, Tb and Pr improved the humidity independence, the gas responses of the sensors reduced to a greater extent. The anti-humidity performance of a sensor with the doping of Ce, Tb and Pr at a low operating temperature and the method to enhance sensitivity of the sensor with the doping of Ce, Tb and Pr still need to be further investigated.
In this work, multi-element hybrid material of Co3O4 and the element of Pr/Zn was prepared by electrospray. This work tried to synergize the reversible redox reaction characteristics of Pr and the n-type semiconductor characteristics of ZnO to achieve anti-humidity and acetone enhanced-sensing properties. The mechanism of anti-humidity and enhanced-sensing was initially investigated through the characterization and analysis of the hybrid material.
2. Experimental
2.1 Preparation of materials
Firstly, the electrospray solution was prepared. The solvent was made up of ethanol and water in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. A mmol of cobalt acetate tetrahydrate (Co(CH3COO)2·4H2O, AR), B mmol of praseodymium nitrate hexahydrate (Pr(NO3)3·6H2O, AR) and C mmol of zinc acetate dihydrate (Zn(CH3COO)2·2H2O, AR) were dissolved in 10 mL of the above mixed solvent and fully stirred with a magnetic stirrer to obtain a uniform solution. Next, the configured solution was transferred into a syringe for electrospray. The schematic diagram of electrospray system has been shown in our previous report.25 The liquid supply flow of the syringe pump was 0.6 mL h−1. The voltage and distance between the nozzle and the collector were 8 kV and 7 cm respectively. The collector was kept at the temperature of 200 °C. Finally, the precursor materials were calcined. The calcination process was carried out in a muffle furnace with a calcination temperature of 600 °C for 3 h. The heating rate of the muffle furnace was set as 2 °C min−1. All materials were used in the purchased state without any pretreatment.
1. A mmol of cobalt acetate tetrahydrate (Co(CH3COO)2·4H2O, AR), B mmol of praseodymium nitrate hexahydrate (Pr(NO3)3·6H2O, AR) and C mmol of zinc acetate dihydrate (Zn(CH3COO)2·2H2O, AR) were dissolved in 10 mL of the above mixed solvent and fully stirred with a magnetic stirrer to obtain a uniform solution. Next, the configured solution was transferred into a syringe for electrospray. The schematic diagram of electrospray system has been shown in our previous report.25 The liquid supply flow of the syringe pump was 0.6 mL h−1. The voltage and distance between the nozzle and the collector were 8 kV and 7 cm respectively. The collector was kept at the temperature of 200 °C. Finally, the precursor materials were calcined. The calcination process was carried out in a muffle furnace with a calcination temperature of 600 °C for 3 h. The heating rate of the muffle furnace was set as 2 °C min−1. All materials were used in the purchased state without any pretreatment.
According to the above process, seven samples with different parameters were prepared, and the parameters are shown in Table 1.
| Samples | A | B | C |
|---|---|---|---|
| Co100 | 2 | 0 | 0 |
| Pr5Co95 | 1.9 | 0.1 | 0 |
| Pr10Co90 | 1.8 | 0.2 | 0 |
| Pr20Co80 | 1.6 | 0.4 | 0 |
| Pr10Zn10Co80 | 1.6 | 0.2 | 0.2 |
| Pr10Zn20Co70 | 1.4 | 0.2 | 0.4 |
| Pr10Zn40Co50 | 1 | 0.2 | 0.8 |
2.2 Characterization of materials
The morphology and element composition of the samples were analyzed by scanning electron microscope and energy dispersive X-ray spectroscopy (SEM and EDS, SU8010, Hitachi, Japan). X-ray diffraction spectroscopy (XRD, Ultima IV, Rigaku) was used to analyze the crystal composition and crystal quality of the samples. X-ray photoelectron spectroscopy (XPS, Escalab Xi+, Thermo Fisher Scientific) was used to analyze the chemical state of the Pr, Co, Zn and O elements in the sample.2.3 Gas sensing measurement
In order to detect the acetone responses of all the samples in different humidity environments, a test system was built as shown in Fig. 1. The test system was consisted of an air pump, a filter, a saturated salt solution and a test chamber. The size of the test chamber in this work was 300 mm × 300 mm × 200 mm with the volume of 18 L. The air was passed into the filter through the air pump to filter out the macromolecular substances and the water molecules in the air. Then the gas was passed into the saturated salt solution to generate the air with the required humidity. Finally, the air was passed into the test chamber. The final humidity in the test chamber was calibrated by a humidity sensor, and the relative humidity in the test chamber could be adjusted between 30% and 90%.During gas sensing test, the saturated acetone steam was injected into the test chamber to obtain the corresponding concentration of acetone test gas. The volume of saturated acetone steam injected into the test chamber could be calculated according to our previous report.26 The formula for calculating the volume of saturated acetone vapor was Vg = P0·Cg·Vc/Pg, where P0 is the ambient atmospheric pressure, Cg is the required concentration of acetone, Vc is the volume of the test chamber, and Pg is the saturated vapor pressure of acetone. After the gas sensing test was complete, the acetone-containing gas was removed from the test chamber and replaced with fresh air.
The resistance of the sensor was measured and saved by the source meter (B2902A, Keysight). The response was defined as (Rg − Ra)/Ra, where Rg and Ra is the resistance of the sensor in acetone atmosphere and in air respectively. The response and recovery time was defined as the time required for the resistance to change to 90% of the change amplitude.
3. Results and discussion
3.1 Gas sensing properties
Fig. 2(a) shows the responses variation of Co100, Pr5Co95, Pr10Co90 and Pr20Co80 with temperature. The responses of the four samples all show a trend of first becoming larger and then smaller as the temperature increases. Among them, the optimal working temperature of Co100 is 190 °C, and the optimal working temperature of the hybrid samples with Pr reduced to 160 °C. Fig. 2(b) shows the responses comparison of the four samples to 50 ppm acetone at the optimal working temperature. The response of Co100 is the largest, and the responses of the hybrid samples with Pr are greatly weakened. The response of Pr5Co95 is only 0.56, which is only a quarter of Co100. With the Pr concentration further increase, the response of the samples is further reduced, but the change is not significant. It can be seen that the addition of Pr will reduce the sensitivity of sensing materials.Fig. 2(c) shows the responses variation of Co100, Pr5Co95, Pr10Co90 and Pr20Co80 with relative humidity. During the relative humidity increases from 30% to 90%, the responses of all the samples decrease. But, the response of Co100 is still larger than those of the hybrid samples in different relative humidity. Fig. 2(d) shows the responses ratios of the four samples at 90% and 30% relative humidity. As figure shown, the ratio of Pr10Co90 is the largest. Compared with the response in 30% relative humidity, the response in 90% relative humidity only drops by 9%. The ratio of Co100 is the smallest. Compared with the response in 30% relative humidity, the response of Co100 in 90% relative humidity drops by 28%. It can be seen that the suitable incorporation of Pr can greatly improve anti-humidity property of Co3O4.
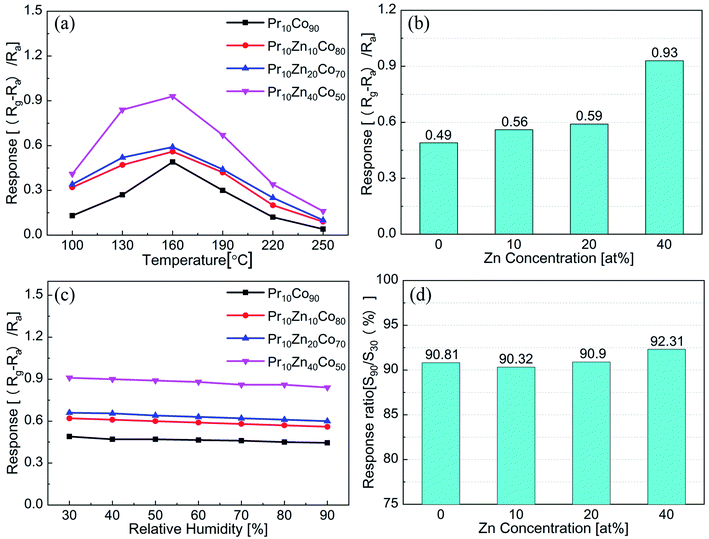
Fig. 3(a) shows the responses variation of Pr10Co90, Pr10Zn10Co80, Pr10Zn20Co70 and Pr10Zn40Co50 with temperature. The responses of the four samples have the same trend with temperature increases, and the largest responses of the samples are achieved at 160 °C. As shown in Fig. 3(b), the incorporation of Zn can enhance the response of Pr10Co90. The response of Pr10Zn40Co50 to 50 ppm acetone is 0.93, which is about twice that of Pr10Co90. Fig. 3(c) shows the responses variation of Pr10Co90, Pr10Zn10Co80, Pr10Zn20Co70 and Pr10Zn40Co50 with relative humidity. In the range of 30–90% relative humidity, the responses of the four samples maintain good stability. As Fig. 3(d) shown, the ratios of the four samples at 90% and 30% relative humidity are all greater than 90%. Among them, the response of Pr10Zn40Co50 in 90% relative humidity is only 7.7% lower than that in 30% relative humidity. This indicates that the incorporation of Zn could enhance the acetone sensitivity of Pr–Co3O4 and maintain a good anti-humidity property.
Fig. 4(a) shows the comparison of the anti-humidity and acetone sensing properties of Co100, Pr10Co90 and Pr10Zn40Co50. Co100 exhibits the largest gas sensing response, but poor anti-humidity property. Pr10Co90 exhibits better anti-humidity property, but the gas sensing response is greatly deteriorated. Pr10Zn40Co50 maintains excellent anti-humidity property while shows a good gas sensing property. It indicates that the multiple incorporation of Pr and Zn into Co3O4 can synergistically improve the anti-humidity and acetone sensing properties.
Fig. 4(b) shows the responses of Pr10Zn40Co50 to different concentrations of acetone. The sample shows a good response–recovery to 1–50 ppm acetone, and the response to 1 ppm acetone is 0.14. Fig. 4(c) shows the response–recovery curve of Pr10Zn40Co50 to 50 ppm acetone. The response and recovery time of the sensor is 70 and 330 s, respectively. Fig. 4(d) shows the responses comparison of Pr10Zn40Co50 to 50 ppm acetone, ethanol, triethylamine, methanol, benzene, SO2, CO and NO2. Among them, the response to acetone is the highest.
Fig. 5(a) shows the cycle test of Pr10Zn40Co50 to 50 ppm acetone. The response curves of the sample maintain a good consistency during the four-cycle tests. Fig. 5(b) shows the response curves of Pr10Zn40Co50 to 50 ppm acetone over two weeks. The sensor shows good stability with a relative standard deviation of 3.08% for the response values.
 | ||
| Fig. 5 (a) Cycle tests curves to 50 ppm acetone for Pr10Zn40Co50. (b) Responses to 50 ppm acetone over two weeks. | ||
The anti-humidity comparison of Co3O4-based acetone sensor between this work and the previous reported work is listed in Table 2. All the sensors in the references show high acetone responses under low humidity conditions. However, under high humidity conditions greater than 80%, the acetone responses of the sensors show a drop of more than 15%, and some even drop by 66.7%.16–18,27–29 The sensor of Pr10Zn40Co50 in this work shows good agreement in low and high humidity, and the relative deviation is much smaller than the reported.
| Materials | Temp. (°C) | Con. (ppm) | Res./low hum. (%) | Res./high hum. (%) | Relative deviation (%) | Ref. |
|---|---|---|---|---|---|---|
| a Res. was defined as (Rg − Ra)/Ra. Relative deviation was defined as (Res(low) − Res(high))/Res(low). Temp.: temperature; Con.: concentration; res.: response; hum.: humidity; ref.: reference. | ||||||
| Co3O4 | 500 | 50 | 242/32 | 195/89 | 19.4 | 16 |
| Co3O4 | 240 | 500 | 3.16/33 | ∼1.3/∼95 | 58.9 | 17 |
| Co3O4 | 150 | 100 | 34.2/30 | 15.3/80 | 55.3 | 27 |
| Co3O4/graphene | 190 | 1 | 54.3/30 | 45.6/90 | 16 | 18 |
| Co3O4/graphene | 160 | 50 | 5.4/20 | 4/90 | 25.9 | 28 |
| Pt–Co3O4 | 200 | 0.5 | 2.1/0 | 0.7/80 | 66.7 | 29 |
| Pr/Zn–Co3O4 | 160 | 50 | 0.91/30 | 0.84/90 | 7.7 | This work |
3.2 Materials characterization
Fig. 6 shows the SEM images and EDS spectra of Co100, Pr10Co90 and Pr10Zn40Co50. As SEM images shown, all three samples are composed of nanoparticles, which include bottom and surface layer. The bottom is the particle layer and the surface is dispersed with aggregated particle clusters. Among them, Co100 has a higher agglomeration density and a rougher surface, while Pr10Zn40Co50 has a flatter surface with smaller and more dispersed clusters. The elementals proportions of Co, Pr and Zn in the samples were analyzed by EDS. The molar proportion of Co element in Co100 is 100%. The molar proportions of Co and Pr in Pr10Co90 are approximately 91% and 9%, respectively. The molar proportions of Co, Pr and Zn in Pr10Zn40Co50 are approximately 50%, 9% and 41%, respectively. The molar proportions of the elements are almost the same as the experimental design.Fig. 7 shows the XRD spectra of Co100, Pr10Co90 and Pr10Zn40Co50. The diffraction peaks of Co100 are located at 31.28°, 36.86°, 36.58°, 44.8°, 55.62°, 59.38° and 65.24°, corresponding to [2 2 0], [3 1 1], [2 2 2], [4 0 0], [4 2 2], [5 1 1] and [4 4 0] crystal planes of spinel Co3O4 (JCPDS 43-1003). As the spectra shows that Co100 is composed of Co3O4 without other impurities. In addition to the diffraction peaks of Co3O4, Pr10Co90 has three new diffraction peaks located at 33.3°, 41.26° and 47.9°, respectively. These three peaks correspond to [2 2 0], [2 2 2] and [4 0 0] crystal planes of PrCoO3 (JCPDS 25-1069), which indicate that Pr10Co90 is a hybrid material of Co3O4 and PrCoO3. For Pr10Zn40Co50, the other five new diffraction peaks appear on the spectrum, which are located at 31.7°, 34.4°, 36.24, 56.62° and 62.8°. These five peaks correspond to [1 0 0], [0 0 2], [1 0 1], [1 1 0] and [1 0 3] crystal planes of ZnO (JCPDS 36-1451). This indicates that Pr10Zn40Co50 contains at least three kinds of crystals including Co3O4, PrCoO3 and ZnO.
Fig. 8(a)–(c) show the high-resolution XPS spectra of Co 2p in Co100, Pr10Co90 and Pr10Zn40Co50. The peak near 794.7 eV corresponds to Co 2p1/2, which can be divided into the peaks of 795.9 eV and 794.6 eV by Gaussian fitting. The peak near 779.7 eV corresponds to Co 2p3/2, which can be divided into the peaks of 780.5 eV and 779.5 eV. Among these peaks, the fitting peaks of 795.9 eV and 780.5 eV are derived from Co2+, while the fitting peaks of 794.6 eV and 779.5 eV are derived from Co3+. By analyzing the peak areas, the ratios of the Co2+ and Co3+ in the three samples of Co100, Pr10Co90 and Pr10Zn40Co50 are 78.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21.4, 74.3
21.4, 74.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25.7 and 71.2
25.7 and 71.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28.8, respectively. Due to the incorporation of Pr, the proportion of Co3+ in the hybrid material increases, which is attributed to the formation of PrCoO3.
28.8, respectively. Due to the incorporation of Pr, the proportion of Co3+ in the hybrid material increases, which is attributed to the formation of PrCoO3.
 | ||
| Fig. 8 High-resolution XPS spectra of (a and g) Co100, (b, d and h) Pr10Co90 and (c, e, f and i) Pr10Zn40Co50. | ||
Fig. 8(d) and (e) show the high-resolution XPS spectra of Pr 3d5/2 in Pr10Co90 and Pr10Zn40Co50. This peak is located near 933 eV, and can be divided into the peaks of 935 eV, 933 eV, 930.6 eV and 927.8 eV. The fitting peaks of 935 eV and 930.6 eV are derived from Pr4+, while the fitting peaks of 933 eV and 927.8 eV are derived from Pr3+. By analyzing the area of the peaks, the ratios of the peaks area of Pr3+ and Pr4+ are 57.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42.5 and 59
42.5 and 59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41 for Pr10Co90 and Pr10Zn40Co50, respectively. The proportions of Pr3+ and Pr4+ in the hybrid samples are consistent with that in Pr6O11 reported in the previous literature.23 It indicates that the samples of Pr10Co90 and Pr10Zn40Co50 also contain Pr6O11.
41 for Pr10Co90 and Pr10Zn40Co50, respectively. The proportions of Pr3+ and Pr4+ in the hybrid samples are consistent with that in Pr6O11 reported in the previous literature.23 It indicates that the samples of Pr10Co90 and Pr10Zn40Co50 also contain Pr6O11.
Fig. 8(f) shows the high-resolution XPS spectrum of Zn in Pr10Zn40Co50. The peak near 1044.6 eV is derived from Zn 2p1/2, and the peak near 1021.5 eV is derived from Zn 2p3/2.
Fig. 8(g)–(i) show the high-resolution XPS spectra of O 1s in Co100, Pr10Co90 and Pr10Zn40Co50. The O 1s peaks of the three samples can be divided into 4 peaks, which are located at 531.9 eV (OIV), 530.9 eV (OIII), 529.5 eV (OII) and 528.5 eV (OI). OI, OII, OIII and OIV are derived from Pr3+–O, crystal lattice oxygen, adsorbed oxygen and Pr4+–O. The proportions of different oxygen components are analyzed based on the area ratio. Since Co100 does not contain Pr, its spectrum only contains OII and OIII, and the components proportions are 50.2% and 49.8%. The proportions of OI, OII, OIII and OIV in Pr10Co90 are 3.6%, 41.3%, 31% and 24.1%, while the proportions of OI, OII, OIII and OIV in Pr10Zn40Co50 are 3.9%, 39%, 38.8% and 18.3%. Among the oxygen components, OIII is the key to the gas sensitivity. In the three samples, Co100 has the highest proportion of OIII, while Pr10Co90 has the lowest proportion.
3.3 Gas sensing mechanism
According to the response curves in Fig. S1,† the samples of Co100, Pr10Co90 and Pr10Zn40Co50 all exhibit the characteristics of p-type semiconductor. For p-type semiconductor, the gas sensitivity depends on the thickness of the hole accumulation layer on the surface. Due to the chemical adsorption of oxygen, oxygen traps electrons from the sensing material to form oxygen ions when a sample is exposed to the air, so that the hole accumulation layer on the surface will increase and the resistance will decrease. When the sample is exposed to acetone, oxygen ions react with acetone and release electrons back to the sensing material, resulting in a reduction of the hole accumulation layer and an increase of resistance. The process can be expressed by the following reactions:30| O2(ads) + e− → O−2(ads) |
| O−2(ads) + e− → 2O−(ads) |
| CH3COCH3(g) + 8O−(ads) → 3CO2 + 3H2O + 8e− |
| CH3COCH3(g) + 4O−2(ads) → 3CO2 + 3H2O + 4e− |
Since the working temperatures of the sensors are relatively low, water molecules will adsorb on the surface and react with the adsorbed oxygen ions to form hydroxyl groups and electronics in a high-humidity environment, resulting in water poisoning of the sensing material and reducing of gas sensitivity. Because Pr has reversible oxidation–reduction reaction property, Pr3+ can promote the reverse reaction process of water poisoning. The hydroxyl groups could be effectively removed and the oxygen ions are regenerated with the transition from Pr3+ to Pr4+. Pr4+ recaptures the electrons brought by the water molecule reaction and turns into Pr3+. During the above cyclic redox reaction, the reactants and products are equal. When the hydroxyl groups on the materials surface are completely removed, the resistance and response of the sensitive material are hardly affected. Therefore, Pr10Co90 and Pr10Zn40Co50 shows better consistency of gas responses than Co100 in different relative humidity. The process can be expressed by the following reactions:23
| H2O + O− ↔ 2OH + e− |
| Pr3+ + 2OH → Pr4+ + H2O + O−(ads) |
| Pr4+ + e− → Pr3+ |
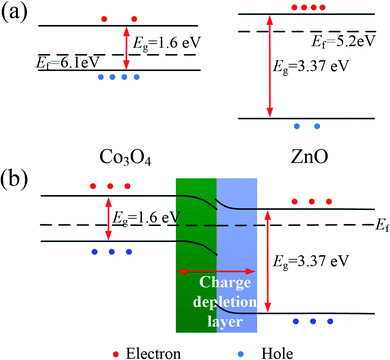
The gas sensitivity of Co100, Pr10Co90 and Pr10Zn40Co50 mainly depend on the surface reaction. Co3O4 contains two kinds of Co ions (Co2+ and Co3+), in which Co2+ is the reaction sites.31 Therefore, the concentration of Co2+ on surface will affect the surface reaction. From XPS analysis, it can be found that the concentration of Co2+ on surface of the hybrid samples is reduced because of the formation of PrCoO3, which results in a reduction of reaction sites and a decrease of the sensitivity. Although the Co2+ concentration of Pr10Zn40Co50 is also reduced, the gas sensitivity is improved because of the addition of ZnO. The enhanced sensitivity could be contributed to two reasons. First, ZnO in the hybrid sample brings more electrons, which promotes the increase of oxygen ions adsorbed on the surface. This can be confirmed from the XPS diagram. The concentration of adsorbed oxygen on the surface of Pr10Zn40Co50 is relatively higher than that of Pr10Co90. Second, p–n heterojunctions are formed in Pr10Zn40Co50. As shown in Fig. 9(a), the Fermi level of Co3O4 is located at 6.1 eV, while that of ZnO is at 5.2 eV. When the two metal oxides are in contact, ZnO transfers electrons to Co3O4 in order to maintain the unity of the Fermi level at the interface. Therefore, the p–n heterojunction is formed with the appearance of a charge depletion layer at the interface, as shown in Fig. 9(b). Heterojunction can adjust the barrier of the material, causing a greater change of resistance during the gas reaction.32,33
4. Conclusions
An acetone sensing material of Pr10Zn40Co50 was prepared in this work, which is a hybrid material of PrCoO3, ZnO, Pr6O11 and Co3O4. Compared with pure Co3O4, the optimal working temperature of Pr10Zn40Co50 decreases, and the hybrid material exhibits more stable responses to acetone in different humidity conditions. The detection limit for acetone could reach 1 ppm. The anti-humidity property of Pr10Zn40Co50 result from the reverse reaction of Pr to water poisoning, which weakens the reaction of water molecules on the surface of the material to generate hydroxyl groups. Compared with Pr10Co90 containing only Pr addition, the sensitivity of Pr10Zn40Co50 is also enhanced, which depends on the increase of adsorbed oxygen on the surface and the adjustment of the potential barrier by the p–n heterojunction. This work shows that synergistic effects of each material in hybrid material can improve the gas sensing properties.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by Zhejiang Provincial Natural Science Foundation (LQ20F010001).References
- J. Cao, N. Zhang, S. Wang, C. Chen and H. Zhang, Researching the crystal phase effect on gas sensing performance in In2O3 nanofibers, Sens. Actuators, B, 2020, 305, 127475 CrossRef CAS.
- H.-J. Cho, S.-J. Choi, N.-H. Kim and I.-D. Kim, Porosity controlled 3D SnO2 spheres via electrostatic spray: selective acetone sensors, Sens. Actuators, B, 2020, 304, 127350 CrossRef CAS.
- P. Wang, T. Dong, C. Jia and P. Yang, Ultraselective acetone-gas sensor based ZnO flowers functionalized by Au nanoparticle loading on certain facet, Sens. Actuators, B, 2019, 288, 1–11 CrossRef CAS.
- N. Chen, Y. Li, D. Deng, X. Liu, X. Xing, X. Xiao and Y. Wang, Acetone sensing performances based on nanoporous TiO2 synthesized by a facile hydrothermal method, Sens. Actuators, B, 2017, 238, 491–500 CrossRef CAS.
- D. Han, Y. Ji, F. Gu and Z. Wang, Cobalt oxide nanorods with special pore structure for enhanced ethanol sensing performance, J. Colloid Interface Sci., 2018, 531, 320–330 CrossRef CAS PubMed.
- C. Su, L. Zhang, Y. Han, X. Chen, S. Wang, M. Zeng, N. Hu, Y. Su, Z. Zhou, H. Wei and Z. Yang, Glucose-assisted synthesis of hierarchical flower-like Co3O4 nanostructures assembled by porous nanosheets for enhanced acetone sensing, Sens. Actuators, B, 2019, 288, 699–706 CrossRef CAS.
- X. Qiao, C. Ma, X. Chang, X. Li, K. Li, L. Zhu, F. Xia and Q. Xue, 3D radial Co3O4 nanorod cluster derived from cobalt-based layered hydroxide metal salt for enhanced trace acetone detection, Sens. Actuators, B, 2021, 327, 128926 CrossRef CAS.
- D. Tang, L. Jia, Z. Zhao, R. Yang, X. Wang and X. Guo, EDTA assistant preparation and gas sensing properties of Co3O4 nanomaterials, J. Inorg. Mater., 2020, 35, 1214–1222 Search PubMed.
- J. Cao, S. Wang, H. Zhang and T. Zhang, Constructing one dimensional Co3O4 hierarchical nanofibers as efficient sensing materials for rapid acetone gas detection, J. Alloys Compd., 2019, 799, 513–520 CrossRef CAS.
- X. Chen, S. Wang, C. Su, Y. Han, C. Zou, M. Zeng, N. Hu, Y. Su, Z. Zhou and Z. Yang, Two-dimensional Cd-doped porous Co3O4 nanosheets for enhanced room-temperature NO2 sensing performance, Sens. Actuators, B, 2020, 305, 127393 CrossRef CAS.
- C. Zhang, L. Li, L. Hou and W. Chen, Fabrication of Co3O4 nanowires assembled on the surface of hollow carbon spheres for acetone gas sensing, Sens. Actuators, B, 2019, 291, 130–140 CrossRef CAS.
- K. Xu, W. Zhao, X. Yu, S. Duan and W. Zeng, MOF-derived Co3O4/Fe2O3 p–n hollow cubes for improved acetone sensing characteristics, Phys. E, 2020, 118, 113869 CrossRef CAS.
- T. Akamatsu, T. Itoh, Y. Masuda, W. Shin, I. Matsubara and M. Kida, Gas sensor properties of nanopore-bearing Co3O4 particles containing Pt or Pd particles, J. Asian Ceram. Soc., 2020, 8, 138–148 CrossRef.
- F. Qu, N. Zhang, S. Zhang, R. Zhao, D. Yao, S. Ruan and M. Yang, Construction of Co3O4/CoWO4 core-shell urchin-like microspheres through ion-exchange method for high-performance acetone gas sensing performance, Sens. Actuators, B, 2020, 309, 127711 CrossRef CAS.
- J.-S. Jang, W.-T. Koo, D.-H. Kim and I.-D. Kim, In situ coupling of multidimensional MOFs for heterogeneous metal-oxide architectures: Toward sensitive chemiresistors, ACS Cent. Sci., 2018, 4, 929–937 CrossRef CAS PubMed.
- P. Srinivasan, A. J. Kulandaisamy, G. K. Mani, K. J. Babu, K. Tsuchiya and J. B. B. Rayappan, Development of an acetone sensor using nanostructured Co3O4 thin films for exhaled breath analysis, RSC Adv., 2019, 9, 30226 RSC.
- T. Zhou, T. Zhang, J. Deng, R. Zhang, Z. Lou and L. Wang, P-type Co3O4 nanomaterials-based gas sensor: Preparation and acetone sensing performance, Sens. Actuators, B, 2017, 242, 369–377 CrossRef CAS.
- Y. Xiong, X. Chang, X. Qiao, K. Li, L. Zhu, F. Xia, X. Li, Q. Zheng, W. Xing and Q. Xue, Co-MOF-74 derived Co3O4/graphene heterojunction nanoscrolls for ppb-level acetone detection, Sens. Actuators, B, 2019, 300, 127011 CrossRef CAS.
- Z. Yang, W. Cao, C. Peng, T. Wang, B. Li, H. Ma, Y. Su, Z. Zhou, J. Yang and M. Zeng, Construction, application and verification of a novel formaldehyde gas sensor system based on Ni-doped SnO2 nanoparticles, IEEE Sens. J., 2021, 21, 11023–11030 CAS.
- J. Hu, T. Wang, Y. Wang, D. Huang, G. He, Y. Han, N. Hu, Y. Su, Z. Zhou, Y. Zhang and Z. Yang, Enhanced formaldehyde detection based on Ni doping of SnO2 nanoparticles by one-step synthesis, Sens. Actuators, B, 2018, 263, 120–128 CrossRef CAS.
- J.-W. Yoon, J.-S. Kim, T.-H. Kim, Y. J. Hong, Y. C. Kang and J.-H. Lee, A new strategy for humidity independent oxide chemiresistors: Dynamic self-refreshing of In2O3 sensing surface assisted by layer-by-layer coated CeO2 nanoclusters, Small, 2016, 12, 4229–4240 CrossRef CAS PubMed.
- C.-H. Kwak, T.-H. Kim, S.-Y. Jeong, J.-W. Yoon, J.-S. Kim and J.-H. Lee, Humidity-independent oxide semiconductor chemiresistors using terbium-doped SnO2 yolk-shell spheres for real-time breath analysis, ACS Appl. Mater. Interfaces, 2018, 10, 18886–18894 CrossRef CAS PubMed.
- J.-S. Kim, C. W. Na, C.-H. Kwak, H.-Y. Li, J. W. Yoon, J.-H. Kim, S.-Y. Jeong and J.-H. Lee, Humidity-independent gas sensors using Pr-doped In2O3 macro-porous spheres: Role of cyclic Pr3+/Pr4+ redox reactions in suppression of water poisoning effect, ACS Appl. Mater. Interfaces, 2019, 11, 25322–25329 CrossRef CAS PubMed.
- H.-Y. Li, C.-S. Lee, D. H. Kim and J.-H. Lee, Flexible room-temperature NH3 sensor for ultrasensitive, selective, and humidity-independent gas detection, ACS Appl. Electron. Mater., 2018, 10, 27858–27867 CrossRef CAS PubMed.
- X. Fan, Y. Xu, C. Ma and W. He, In-situ growth of Co3O4 nanoparticles based on electrospray for an acetone gas sensor, J. Alloys Compd., 2021, 854, 157234 CrossRef CAS.
- X.-X. Fan, X.-L. He, J.-P. Li, X.-G. Gao and J. Jia, Ethanol sensing properties of hierarchical SnO2 fibers fabricated with electrospun polyvinylpyrrolidone template, Vacuum, 2016, 128, 112–117 CrossRef CAS.
- F. Dang, Y. Wang, L. Xu, P. Cheng, Z. Weng, T. Wang, L. Lv, C. Wang, X. Li and B. Zhang, Solvent-dependent synthesis of okra-shaped Co3O4 for acetone gas detection at low operation temperatures, ACS Appl. Electron. Mater., 2021, 3, 3400–3410 CrossRef CAS.
- Y. Gao, D. Chen, X. Hou, Y. Zhang, S. Yi, H. Ji, Y. Wang, L. Yin and J. Sun, Microwave-assisted synthesis of hierarchically porous Co3O4/rGO nanocomposite for low-temperature acetone detection, J. Colloid Interface Sci., 2021, 594, 690–701 CrossRef CAS PubMed.
- A. Ma, S. Y. Baek, J. H. Seo, S. A. Abbas, J.-H. Kwon, S. J. Ahn and K. M. Nam, Photodeposition of Pt nanoparticles on Co3O4 nanocubes for detection of acetone at part-per-billion levels, ACS Appl. Nano Mater., 2021, 4, 2752–2759 CrossRef CAS.
- S. Deng, X. Liu, N. Chen, D. Deng, X. Xiao and Y. Wang, A highly sensitive VOC gas sensor using p-type mesoporous Co3O4 nanosheets prepared by a facile chemical coprecipitation method, Sens. Actuators, B, 2016, 233, 615–623 CrossRef CAS.
- V. Amiri, H. Roshan, A. Mirzaei, G. Neri and A. I. Ayesh, Nanostructured metal oxide-based acetone gas sensors: A review, Sensors, 2020, 20, 3096 CrossRef CAS PubMed.
- N. Han, G. Pan, J. Zheng, R. Wang and Y. Wang, Co3O4-ZnO P-N heterostructure nanomaterials film and its enhanced photoelectric response to visible lights at near room temperature, Mater. Res.–Ibero-Am. J., 2019, 22, e20180689 CAS.
- X. Chang, X. Qiao, K. Li, P. Wang, Y. Xiong, X. Li, F. Xia and Q. Xue, UV assisted ppb-level acetone detection based on hollow ZnO/MoS2 nanosheets core/shell heterostructures at low temperature, Sens. Actuators, B, 2020, 317, 128208 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra03321f |
| ‡ The authors contribute equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |